Abstract
Systematic Lupus Erythematosus (SLE) is an autoimmune syndrome of unclear etiology. While T and B cell abnormalities contribute to disease pathogenesis, recent work suggests that inflammatory M1-like macrophages also play a role. Previous work showed that the TLR2/1 agonist PAM3CSK4 (PAM3) could stimulate normal human monocytes to preferentially differentiate into immunosuppressive M2-like rather than inflammatory M1-like macrophages. This raised the possibility of PAM3 being used to normalize the M1:M2 ratio in SLE. Consistent with that possibility, monocytes from lupus patients differentiated into M2-like macrophages when treated with PAM3 in vitro. Furthermore, lupus-prone NZB x NZW F1 mice responded similarly to weekly PAM3 treatment. Normalization of the M2 macrophage frequency was associated with delayed disease progression, decreased autoantibody and inflammatory cytokine synthesis, reduced proteinuria and prolonged survival in NZB x NZW F1 mice. The ability of PAM3 to bias monocyte differentiation in favor of immunosuppressive macrophages may represent a novel approach to the therapy of SLE.
Keywords: SLE, treatment, M2 macrophages, TLR2/1, PAM3, NZB/W
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune syndrome characterized by periods of intense inflammation affecting multiple organ systems (most notably the kidneys, skin and CNS) in association with the production of autoantibodies targeting nucleic acids and proteins that bind to nucleic acids [1,2]. Lupus is a multifactorial disease whose onset and severity are influenced by both genetic and environmental factors. Current treatments include antimalarials, corticosteroids and various immunosuppressive drugs [3]. These are largely but not universally effective at controlling disease flares and their prolonged use has undesirable side effects. The development and testing of novel therapies for lupus is facilitated by the availability of spontaneous murine models of the disease. In particular, NZB x NZW F1 mice (hereafter NZB/W) develop lupus-like disease whose symptoms and gender bias are similar to those seen in humans [4,5]. The immune abnormalities manifested by female NZB/W mice also mimic those of lupus patients including autoantibody production, lymphocyte activation and changes in monocyte/macrophage frequency [6].
Monocytes constitute 5-10% of peripheral blood leukocytes and function to protect the host from infection by differentiating into macrophages and dendritic cells [7]. Monocytes are typically classified into 3 subtypes based on their phenotype and function. Those that express CD14 but not CD16 are termed ‘classical’ monocytes. These are phagocytic but do not support inflammatory responses. ‘Non-classical’ monocytes express a CD14lowCD16+ phenotype and are inflammatory. An ‘intermediate’ or transitional monocyte population also exists that is CD14+CD16+ and has both phagocytic and inflammatory properties [7]. In response to stimulation, monocytes differentiate into macrophages with diverse phenotypes and functions. These range from ‘classical’ pro-inflammatory M1-like macrophages that protect the host from infection to ‘alternatively activated’ M2-like macrophages that are immunosuppressive, support tissue remodeling, and phagocytose apoptotic cells [8–10].
Recent studies suggest that monocytes and macrophages can contribute to the pathogenesis of SLE. Indeed, lupus flares are associated with a relative increase in the frequency of M1 vs M2 macrophages [11]. The frequency of inflammatory monocytes is also elevated in SLE patients and the concentration of serum cytokines that promote macrophage differentiation (such as CSF-1 and MCP-1) correlates with disease severity [12]. Normalizing the M1:M2 ratio by adoptive transfer of M2 macrophages is of benefit in the treatment of murine lupus [13]. Hence, efforts to increase the frequency of immunosuppressive M2-like macrophages represents a potentially useful strategy for the treatment of SLE.
Our lab previously showed that the TLR2/1 agonist PAM3 was unique among TLR ligands in promoting the generation of M2-like macrophages from normal human monocytes [14,15]. This work extends those findings to show that PAM3 similarly induces monocytes from lupus patients to preferentially differentiate into M2-like macrophages. Weekly in vivo treatment of female NZB/W mice with PAM3 normalized the M1:M2 ratio in those animals. Treatment was accompanied by immunologic changes including a reduction in IgG anti-DNA autoAb and inflammatory cytokine levels and clinically by decreased proteinuria and prolonged survival. The ability of PAM3 to preferentially support the generation of immunosuppressive M2 rather than inflammatory M1 macrophages represents a novel approach to the therapy of SLE.
2. Materials & Methods
2.1. Isolation and differentiation of human monocytes.
Blood was obtained from SLE patients and healthy volunteers after obtaining IRB-approved written consent (NIDDK/NIAMS IRB, Bethesda, MD). PBMC were isolated by density centrifugation over Histopaque-1077 (Millipore Sigma, Merck) at 400 g for 30 min. Monocytes were sorted using human CD14 Microbeads (MACS, Miltenyi Biotec) according to the manufacturers’ protocol. Briefly, 20 μl of CD14 microbeads were added to 107 cells suspended in 80 μl of MACS buffer. After 15 min incubation in the dark at 4° C cells were washed, resuspended in 500 μl of MACS buffer and loaded onto magnetized LS columns. The columns were extensively washed and CD14+ cells collected by removing the magnet and flushing with 5 ml of MACS buffer.
105 purified monocytes/well were seeded into 96-well flat bottom plates (Corning) and stimulated for 5 days at 37°C with 1 μg/ml PAM3CSK4 (PAM3) (Invivogen), 250 ng/ml MCSF (Miltenyi Biotec), 250 ng/ml IFNγ (Miltenyi Biotec) or 1 μl /ml R848 (Invivogen). Previous studies established that these were the optimally stimulatory concentrations of each agent ([14,15] and data not shown). Cells were maintained in medium consisting of RPMI 1640 (Lonza) supplemented with 2% heat-inactivated fetal calf serum 25 nmol/L HEPES, 1 mmol/L sodium pyruvate, non-essential amino acids (NEAAs) and 0.0035% 2-mercaptoethanol (2-ME).
2.2. In vivo Experiments
Female Balb/C, Female NZB and male NZW mice purchased from The Jackson Laboratory (Bar Harbour, ME) were bred. Female NZB/W F1 offspring were housed under pathogen-free conditions in the NCI animal facility. All experiments were reviewed and approved by the NCI Animal Care and Use Committee. Lupus-prone NZB/W mice were injected weekly with 100 μg of PAM3 (Invivogen) i.p. starting early (7 wk of age) or late (13 wk).
The protein content of early morning urine was monitored using Albustix test strips (Siemens Heathineers). BUN and creatinine levels were measured in serum of 6 month old mice by ELISA (Bioassay systems and Thermofischer, respectively) according to the manufacturers’ protocol.
Two long term studies were performed in NZB/W mice. In the first, 5 mice/group were treated with PAM3 starting at either 7 or 13 weeks of age. Surviving mice were sacrificed at 6 months of age to assess the phenotype of macrophages and cytokine production. In the second experiment, 6 mice/group were treated as described above and survival monitored through 45 weeks. An excess of controls (NZB/W mice treated with PBS group) were included in these experiments. Survival was monitored until 45 weeks of age. Mice were sacrificed only when deemed in extremis by veterinary staff.
Single cell suspensions were prepared from peritoneal and spleen cells of sacrificed mice and cultured in medium consisting of RPMI 1640 (Lonza) supplemented with 5% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 mg/ml streptomycin 25 nmol/L HEPES, 1 mmol/L sodium pyruvate, NEAAs and 0.0035% 2-ME. Mice were bled to obtain sera for the evaluation of cytokine and auto-antibody levels.
2.3. Kidney Histology
Kidney damage was evaluated in 2-3 μm formalin fixed paraffin embedded sections using Periodic Acid Shiff (PAS) and Masson trichrome staining. Pathological changes evaluated included glomerular activity (i.e., glomerular proliferation, karyorrhexis /fibrinoid necrosis, cellular crescents and hyaline deposits), tubulointerstitial activity (i.e., tubular dilatation and interstitial inflammation) and chronicity of the lesions (i.e., glomerulosclerosis, tubular atrophy, interstitial fibrosis) as previously reported [16]. Glomerular activity was evaluated on 30 glomeruli /sample and scored using 0-3 scale in which 0= no lesions, 1=lesions in <25% of glomeruli, 2=lesions in 25-50% of glomeruli and 3= lesions in >50% of glomeruli. Tubular activity and chronicity indices were based on a 0-4 scale as 0=no lesions, 1=lesions in 1-10%, 2=11-25%, 3=>25-50% and 4=>50%. Glomerulonephritis was calculated using the mean scores for each pathological feature. The infiltration of M2 macrophages into these kidneys was determined by immunohistochemical staining of formalin fixed sections stained with antibodies specific for F4/80 and CD206.
2.4. Flow Cytometry
Human cells were stained with fluorochrome-conjugated Abs specific for human CD16, 25F9 (eBioscience), CD163, CD40 and/or CD14 (Biolegend) for 20 min in PBS – 2% BSA on ice. Murine cells were first incubated with Fc Block (Biolegend) for 15 min and then stained with fluorochrome conjugated Abs specific for murine CD45, CD11 b, F480 and CD206 under the same conditions. Stained cells were washed and fixed with Fix & Perm Medium A (Invitrogen).
To detect intracellular IL-12, cells were cultured overnight with a 1: 1,000 dilution of GolgiPlug (BD Biosciences), stained in Fix & Perm Medium B for 20 min with fluorochrome-conjugated anti-IL-12 (BDBiosciences). All stained cells were washed, re-suspended in PBS - 2% BSA and analyzed using an LSR Fortessa (BD Biosciences).
2.5. Endocytosis Assay
Human macrophages that were stimulated for 5 days, were then incubated with 25 μg/ml of pH-Rodo Zymosan (Invitrogen) particles or medium (as background control) for 45 min at 37° C. Cells were then washed and stained with 25F9 and CD163. Uptake was calculated by subtracting background from pH-Rodo Zymosan levels determined by LSR Fortessa.
2.6. ELISA
Immunol 2HB-microtiter plates were coated with human anti-TNFα (R&D Systems), mouse anti-IL12 (BD Biosciences), mouse anti-TNFα (R&D Systems) or mouse anti-IL-10 (R&D Systems) and then blocked with 2% BSA-PBS for 2 hr. After washing, culture supernatants or serially diluted sera were added overnight at 4° C. Plates were washed, incubated with biotin-labeled secondary Ab and developed by adding phosphatase-conjugated streptavidin (AKP) followed by p-nitrophenyl phosphatase (pNPP) substrate (Southern Biotech). Optical density was measured using a SpectraMax M5 microplate Reader and SoftMax Pro Acquisition and Analysis software (Molecular Devices).
IgG anti-dsDNA was detected by coating Immunol 2HB-microtiter plates with 4 μg/ml of CT-DNA (Sigma) in DNA binding solution (Abcam) for 4 hr. The plates were blocked with 2% BSA-PBS and incubated with diluted mouse serum as above. Bound Ab was detected with biotin labeled IgG (Invitrogen) followed by phosphate-conjugated streptavidin and pNPP substrate.
2.7. Statistical Analysis
Statistical analysis was done using 2-tailed unpaired Student t-tests or Dunnett corrected One-way ANOVA multiple comparison tests (GraphPad Software Inc.).
3. Results
3.1. Frequency of inflammatory monocytes in lupus patients.
Human monocytes are conventionally classified into 3 subsets based on their activity and phenotype (see Introduction). The literature indicates that an imbalance in the relative frequency of monocyte subpopulations supports the development and persistence of autoimmune disease [6]. To clarify the role of monocytes in patients with SLE, PBMC from patients with both active and inactive disease (Table 1) were examined. Consistent with previous reports, the absolute number of monocytes in the peripheral blood of patients did not differ from normal controls [6] although the frequency of inflammatory monocytes (intermediate and non-classical) was significantly elevated in SLE patients (Fig 1A,B). The frequency of inflammatory versus classical monocytes did not differ between patients with active versus inactive lupus (Supplemental Fig 1).
Table 1:
Characteristics of SLE patients.
| Number of patients | 35 |
| Age | 46±13 |
| Gender | (N and %) |
| Female | 32 (91%) |
| Male | 3 (9%) |
| SLEDAI-2K | |
| <6 | 30 (85%) |
| >6 | 5 (15%) |
| Medications | |
| HCQ | 27 (77%) |
| Prednisone | 26 (74%) |
| AZA | 7 (20%) |
| MMF | 9 (26%) |
Figure 1:
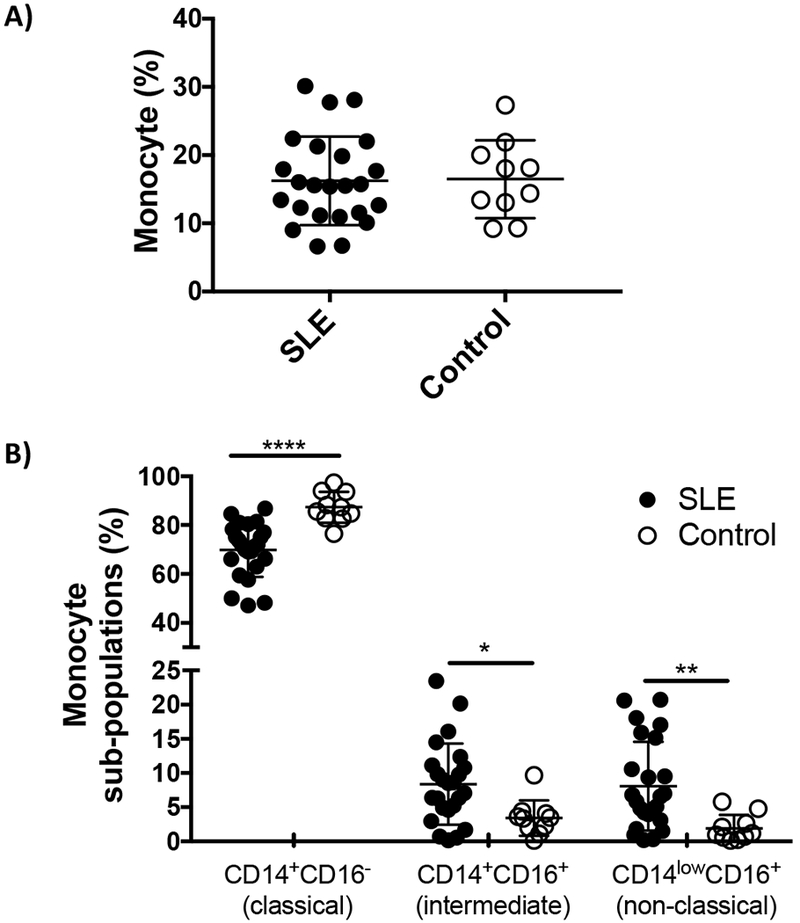
Characterization of monocytes from patients with SLE.
Data show A) the percent of monocytes in PBMC and B) the percent of each monocyte subpopulation in samples from 24 lupus patients and 10 healthy controls. * p<0.05; ** p<0.01; *** p<0.001.
3.2. PAM3 induces lupus monocytes to preferentially mature into phenotypically M2-like macrophages.
Human monocytes can be stimulated to differentiate into macrophages that vary in both phenotype and function depending upon the nature of the stimulus [8,17]. While the best way to categorize these different types of macrophages remains controversial, for simplicity the scheme originally developed to describe murine macrophages will be used in this work [18]. Macrophages that are pro-inflammatory, promote Th1 responses and/or support anti-tumor immunity will be referred to as classically activated “M1-like” whereas those that down-modulate over-exuberant inflammatory responses, clear apoptotic cells and/or support tissue remodeling will be referred to as alternatively activated “M2-like” [19].
Previous studies showed that normal human monocytes preferentially matured into M1-like macrophages when stimulated by most TLR agonists [8,20]. The one exception to this rule was PAM3 which uniquely triggered human monocytes to mature into immunosuppressive M2-like macrophages [14,15]. Given the excess of inflammatory monocytes detected in the blood of lupus patients coupled with evidence from lupus-prone mice that boosting the frequency of M2 macrophages can reduce disease severity [13,21], experiments were undertaken to determine whether PAM3 could stimulate monocytes from lupus patients to mature into M2-like macrophages.
Preliminary experiments identified the optimal conditions for triggering the differentiation of freshly isolated human monocytes ([14,15] and data not shown). The maturation process was characterized by acquisition of 25F9, a surface marker uniquely expressed by mature human macrophage [22]. Consistent with earlier studies of normal human donors, monocytes from lupus patients preferentially differentiated into 25F9+ macrophages when cultured with PAM3 (p <.001, Fig 2 A, B). Other agents that support the generation of M2-like (MCSF) or M1-like (IFNγ or R848) macrophages were included as controls [14]. The majority of macrophages generated by PAM3 and MCSF expressed an M2-like phenotype characterized by the up-regulation of CD163 (a scavenger receptor preferentially expressed by M2-like macrophage) as well as 25F9 (p. <.01, Fig 2 A-D). In contrast, the macrophages generated by R848 and IFNγ predominantly expressed the M1-like CD163− 25F9+ phenotype (p. <.05, Fig 2 A-D). There was no difference in the response of monocytes isolated from lupus patients vs normal controls in their response to PAM3, MCSF, R848 or IFNγ. As previously reported, M1-like macrophages also selectively up-regulated the co-stimulatory molecule CD40 (p <.001, Fig 2E) [23]. It is noteworthy that although unstimulated monocytes generally die during in vitro culture, those from lupus patients survived 3-fold better and these preferentially differentiated into M1-like macrophages (p =.031, Fig 2C and data not shown).
Figure 2:

Effect of PAM3 on the phenotype of lupus monocytes.
Monocytes were MACS purified from the peripheral blood of lupus patients and normal controls and stimulated in vitro for 5 days with optimal concentrations of PAM3, M-CSF, IFNγ or R848. A) Representative histogram showing the expression of 25F9 and CD163 by cultured cells. Note that <1 % of purified monocytes expressed either marker before being placed in culture (day 0). B) Percent of cultured cells that acquired 25F9 expression, C) percent of 25F9+ cells that co-expressed the CD163 M2-like macrophage marker and D) ratio of CD163+25F9+: CD163−25F9+ cells. E) Mean fluorescence intensity of CD40 expression. Data derive from independent studies of 23 lupus samples (solid bars) and 9 normal controls (open bars) where R848 or IFNγ were used as the M1 stimuli.
** p<.01; *** p<.001; **** p<.0001 compared to unstimulated cells from the same donors, whereas ++++ p<.0001 compared to Day 0.
3.3. PAM3 treatment induces lupus monocytes to preferentially mature into functionally M2-like macrophages.
An important feature of M2-like macrophages is their ability to endocytose apoptotic debris [10]. Indeed, defective dead cell clearance reportedly contributes to the inflammation and autoAb production characteristic of lupus patients [24–27]. The ability of PAM3 to generate macrophage with endocytic activity was therefore evaluated. Results show that lupus monocytes treated with PAM3 acquired endocytic activity (reflected by their ability to internalize zymosan particles) whereas those treated with M1 stimuli did not (Fig 3A). This mirrored the effect of PAM3 on monocytes from normal volunteers. In both lupus patients and controls, this gain in function correlated with increased expression of CD163 by PAM3 treated monocytes (R2 = 0.92, Fig 3B).
Figure 3:
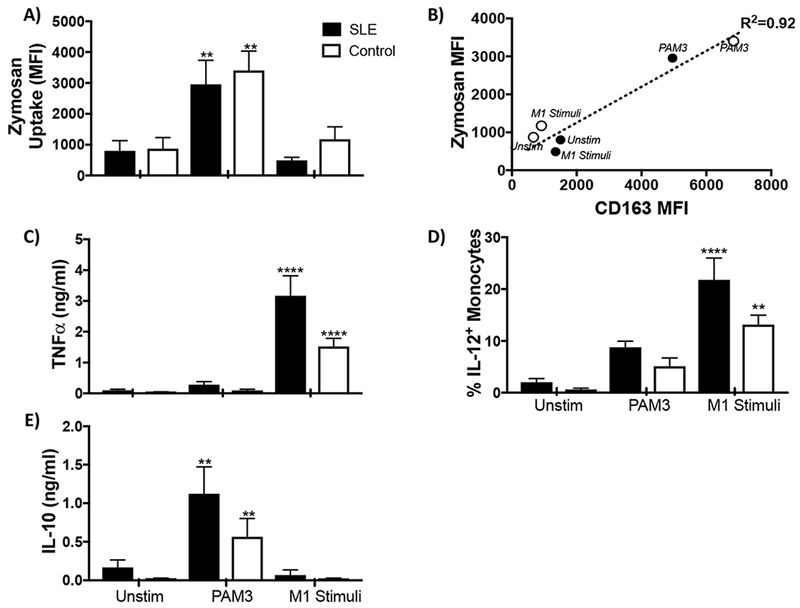
Effect of PAM3 on the function of lupus monocytes.
MACS purified monocytes were cultured as described in Fig 2. A) The uptake of zymosan-labelled particles after 5 days of culture (N= 17 lupus samples and 6 controls). B) Correlation between the expression of CD163 and uptake of zymosan particles. C) Concentration of TNFα in culture supernatants. D) Percent of cultured cells staining for intracytoplasmic IL-12. E) Concentration of IL-10 in culture supernatants (N = 12 lupus samples and controls).
** p<.01; *** p<.001; **** p<.0001 compared unstimulated cells from the same donors.
The production of pro-inflammatory cytokines such as TNFα and IL-12 is characteristic of inflammatory M1-like macrophages [8,28]. The production of these cytokines reportedly worsens disease in lupus patients [29]. The effect of PAM3 on cytokine production was monitored. As expected, macrophage generated by treatment with M1 stimuli secreted large amounts of TNFα and gained the ability to produce IL-12. Those cultured with PAM3 showed no increase in the production of those cytokines when compared to unstimulated controls (Fig 3 C, D). However, they did acquire the ability to produce the immunosuppressive cytokine IL-10 (Fig 3E). In toto, these findings support the possibility that PAM3 might be useful for generating M2-like macrophages for the treatment of SLE. As a precursor to human clinical evaluation, studies were initiated to examine the effect of PAM3 in a relevant murine model.
3.4. The imbalance of M1:M2 macrophages in lupus-prone NZB/W mice is normalized by treatment with PAM3.
Initial experiments examined whether administering PAM3 to female NZB/W mice (a widely accepted model of human lupus) elicited the same alteration in macrophage phenotype in vivo as was observed using human cells in vitro. Towards that end, mice were injected weekly with 100 μg of PAM3 i.p., a schedule and dose previously reported to be effective in the treatment of allergic asthma and cardiac dysfunction [30–32]. Therapy was initiated at either 7 or 13 wk of age, timepoints that were either before or after the onset of detectable kidney damage (increased proteinuria) [33].
To monitor the effect of treatment on the M1:M2 ratio, macrophages were obtained from the peritoneal cavity of 6 month old mice. Murine macrophages were identified by their expression of the F480 surface marker and M2 macrophages by the co-expression of the CD206 mannose receptor [22,34]. The ratio of M1:M2 macrophage was significantly elevated in untreated NZB/W mice when compared to control BALB/c mice (Fig 4). This is consistent with previous reports showing that disease in NZB/W mice is worsen by an excess of inflammatory macrophages [35]. The M1:M2 ratio was normalized in adult mice following PAM3 treatment starting at either 7 or 13 weeks of age (p.< 0.01 for the early and <0.05 for the late treatment group).
Figure 4:
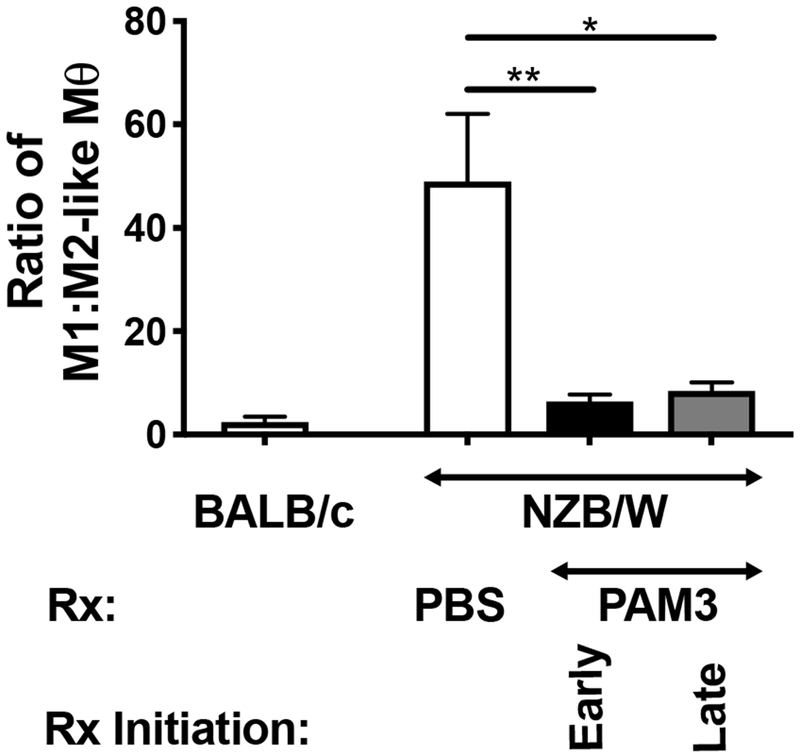
Effect of PAM3 treatment on the M1:M2 ratio of NZB/W mice.
Female NZB/W mice were treated weekly with 100 μg of PAM3 starting either early (7 wk) or late (13 wk). Controls included littermates treated with PBS and normal (BALB/c) mice. The M1:M2 ratio was calculated based on the ratio of CD206−:CD206+ F480+ macrophages collected from the peritoneal cavity at 6 months of age. N = 5 NZB/W mice/group, N = 2 BALB/c controls.
* p<0.05; ** p<0.01; *** p<0.001 for PAM3 vs PBS treated NZB/W mice.
3.5. Effect of PAM3 treatment on the immune milieu of NZB/W mice.
Additional immune manifestations of lupus in NZB/W mice included the production of autoAbs and inflammatory cytokines [5]. Consistent with the hypothesis that PAM3 might be useful in the treatment of SLE, serum IgG anti-DNA autoAb levels were 3-4 fold lower in the PAM3 vs PBS treated groups (p. < 0.04 and <0.01 in the early and late treatment groups respectively, Fig 5A). This was accompanied by a significant decline in serum IL-12 levels and the amount of TNFα produced by spleen cell cultures (p <.001, Fig 5 B, C). Conversely, splenocytes from mice treated from 7 wk of age with PAM3 produced significantly more IL-10 (an immunosuppressive cytokine derived from M2 macrophages) than did untreated controls (p <.01, Fig 5D). Spleen cells from mice whose treatment was delayed until wk 13 also produced more IL-10 but this effect did not reach statistical significance.
Figure 5:
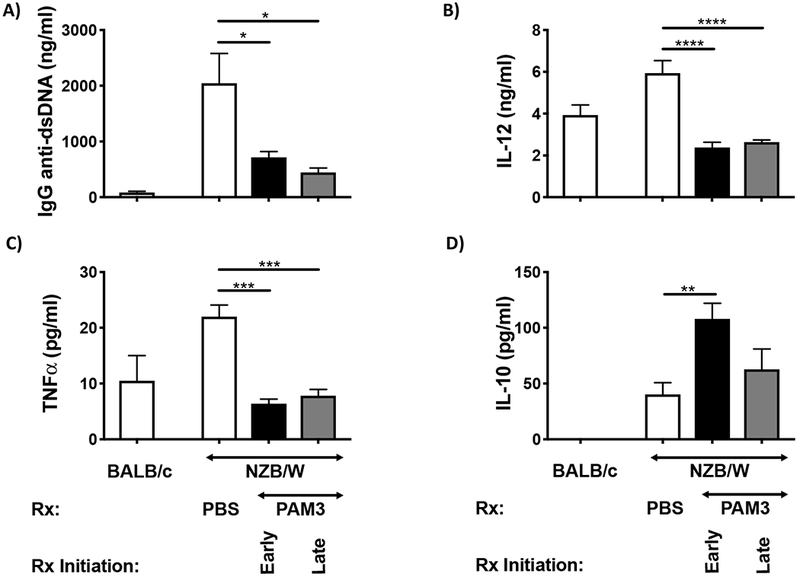
Effect of PAM3 treatment on the immune manifestations of lupus.
Mice were treated as described in Fig 4. Serum collected at 6 months was analyzed for (A) IgG anti-dsDNA and (B) IL-12. Spleen cells were cultured ex vivo without further stimulation for 24 hr and culture supernatants tested for (C) TNFα and (D) IL-10 levels by ELISA (N = 5/group of lupus mice and 2 for BALB/c controls).
** p<.01; *** p<.001; **** p<.0001 PAM3 vs PBS treated NZB/W mice.
3.6. Clinical effect of PAM3 treatment.
The effect of PAM3 treatment initiated either before or after the onset of proteinuria was monitored in two independent experiments. PAM3 reduced proteinuria in both experiments and both groups (p < 0.005 in the early and <0.01 in the late treatment groups, Fig 6A). It also normalized the BUN:creatinine ratio that reached abnormally high levels as glomerulonephritis developed in control NZB/W mice (Fig 6B).
Figure 6:
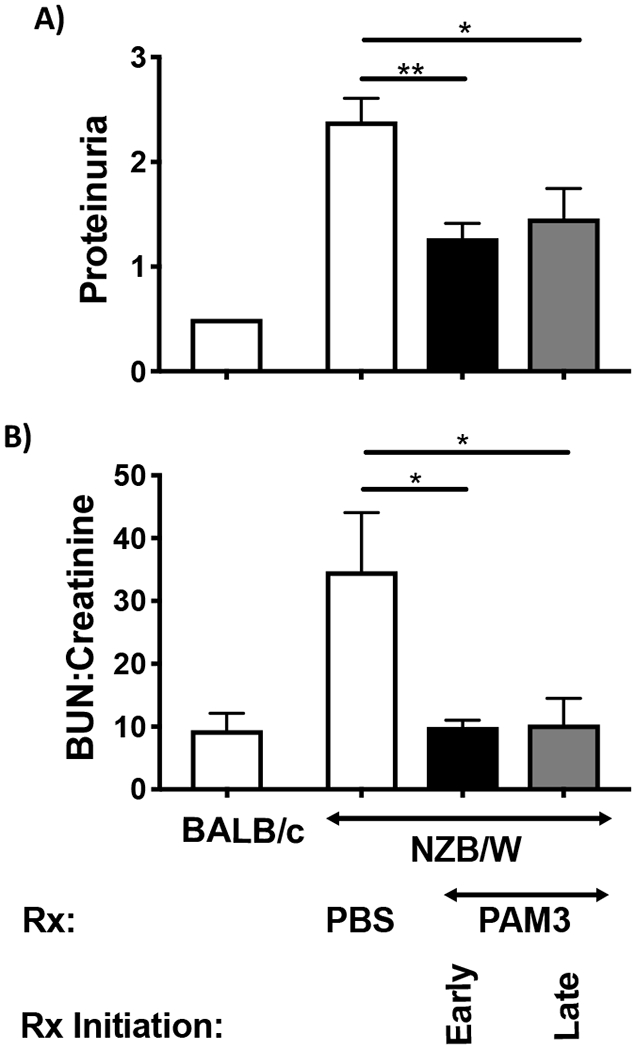
Effect of PAM3 treatment on lupus nephritis.
NZB/W mice were treated with PAM3 or PBS as described in Fig 4. A) Proteinuria was measured at 6 months (N=11 for each PAM3 treated group and 21 for PBS treated mice). B) The serum BUN:creatinine ratio was measured at 6 months of age (N=5 for NZB/W, N=3 BALB/c).
* p<0.05; ** p<0.01 PAM3 vs PBS treated NZB/W mice
The natural history of disease was examined by monitoring kidney histology and animal survival. Immunohistochemical staining of kidneys from control NZB/W mice showed that number of CD206+ M2 macrophages remained low as disease progressed (Fig 7 A,B). By comparison, the number of M2 macrophages in the kidneys of PAM3 treated mice increased over time consistent with the normalization of M1:M2 ratio observed in Fig 4. Histological analysis of these kidneys confirmed that glomerular and tubular infiltration by inflammatory cells was delayed by PAM3 treatment (Fig 7 C-G). Of greatest import, PAM3 treatment significantly prolonged survival, with >60% of mice whose treatment starting at 7 wk of age surviving through 11 months of age vs 5% in the control group (p=.0037, Figure 8). Late initiation of therapy was less effective at slowing disease progression.
Figure 7:

Effect of PAM3 treatment on kidney pathology and the accumulation of M2 macrophages.
NZB/W mice were treated as described in Fig 6. A,B) The number of M2 macrophages present in their kidneys was monitored over time by immunohistochemical staining of PBS and PAM3 treated mice. C) The severity of glomerulonephritis was evaluated histologically. N=9 for PBS and 5 for PAM3 treated animals. D-G) Representative PAS stained kidney sections from 28 week old NZB/W mice treated with PBS (D, E) or PAM3 (F, G). Note the increased interstitial inflammation (arrows) in panel D and hyaline deposits (--), cellular crescent formation (—) and karyorrhexis (arrow) in panel E vs the slight increase in endocapillary proliferation in panel G. Panels D, F were 100× and panels E, G were 800× magnification.
Figure 8:

Effect of PAM3 on survival
Survival was monitored through 45 weeks of age in mice treated with PAM3 starting at 7 or 13 weeks of age (N=6/group) or PBS treated controls (N=21). ** p=.0037 when compared to PBS group, Log rank test.
4. Discussion
This work establishes that the TLR 2/1 agonist PAM3 induces monocytes from lupus patients to preferentially differentiate into M2-like macrophages capable of suppressing autoimmune disease. The therapeutic potential of PAM3 was examined in the NZB/W model of lupus. Weekly treatment with PAM3 normalized the M2:M1 ratio and shifted the cytokine profile towards the production of suppressive rather than inflammatory factors. Serum levels of anti-dsDNA autoantibodies fell, glomerulonephritis was delayed, and survival was prolonged by PAM3 therapy.
The conventional treatment of SLE relies on a combination of immunosuppressive drugs, steroids, and agents that deplete/inhibit autoreactive B cells [3,36,37]. Novel treatments designed to inhibit interferon or lymphocyte signaling are being evaluated in phase III clinical trials [37]. The over-abundance of pro-inflammatory M1-like macrophages present in SLE patients provides an additional target for therapeutic intervention [11,12, 38–40]. In the MRL/lpr model, depleting macrophages by knocking out CSF-1, its receptor, or by systemic administration the selective CSF-1R inhibitor GW2580 reduced disease severity [12,41–44]. Proteinuria and glomerular damage (but not autoAb levels) were also reduced when monocyte infiltration was inhibited by treating MRL/lpr or NZB/W mice with agents that antagonized macrophage differentiation/proliferation [44,45].
The dominance of M1-like macrophages in lupus patients is likely driven by the increased level of pro-inflammatory cytokines including IL-12, TNFα, IFNγ and type I IFNs present in that disease. These support the generation of M1-like macrophages via pathways involving JAK/Stat-1, NFKB and interferon regulatory factors [46–48]. Chemokines that promote monocyte infiltration (e.g. MCP-1, CX3CL1) and macrophage differentiation/proliferation are also elevated in mice and patients with lupus and may contribute to this process [12,41].
While murine studies show that reducing overall macrophage abundance slows disease progression, Li et al showed that increasing the frequency of M2 macrophages accomplished the same goal [13]. That group adoptively transferred M2 macrophages into lupus prone mice and documented a significant reduction in disease severity [13]. Unfortunately, adoptive M2 macrophage transfer is not a practical clinical strategy. We therefore examined whether treatment with PAM3, a TLR agonist that induces normal human monocytes to differentiate into M2-like macrophages [14], might be used to normalize the M1:M2 ratio in SLE patients. As shown in Fig 2C, that outcome was obtained in studies of cultured lupus monocytes. In the absence of exogenous stimulation, lupus monocytes preferentially mature into M1-like macrophages (Fig 2D). This may reflect their in vivo exposure to factors that support the generation of inflammatory macrophages, as described above. Just the opposite is observed in cancer patients, whose monocytes respond to suppressive serum factors by preferentially maturing into M2-like macrophages [49, 50].
The potential of PAM3 to increase M2 macrophage frequency represents a novel approach to the treatment of autoimmunity. In contrast to agents that non-specifically deplete all types of macrophages (thereby increasing host susceptibility to bacterial infections) PAM3 selectively supports the generation of immunosuppressive macrophages (Fig 2C).
The patients evaluated in this study were treated with a variety of conventional agents including hydroxychloroquine, prednisone, mycophenolate mofetil (MMF) and azathioprine (AZA) (Table 1). Hydroxychloroquine is an antimalarial that also interferes TLR7 and TLR9 signaling [3,51,52]; prednisone is a glucocorticoid that inhibits T and B cell responses [53]; MMF is an immunosuppressive agent that inhibits lymphocyte proliferation and antibody production [3,54]; while AZA regulates DNA synthesis thereby inhibiting cell proliferation [3]. None of these drugs is known to selectively act on monocytes/macrophages or influence monocyte differentiation.
The relative excess of M1-like macrophages in lupus patients contributes to the increased level of inflammatory cytokines and reduced apoptotic capacity found in that disease [24–27]. When apoptotic cells are not removed by phagocytosis, they leak DAMPS and self-antigens in a process known as secondary necrosis. These can trigger inflammation and stimulate B cells to produce autoantibodies [55]. Treatment with PAM3 reversed these abnormalities (Fig 3A), generating M2-like macrophages with good endocytic activity that preferentially secreted the immunosuppressive cytokine IL-10 rather than the inflammatory cytokines IL-12 and TNFα (Fig 3 C-E). Monocytes from patients with active lupus express elevated levels of the co-stimulatory molecule CD40. This improves their ability to activate B cells (which express the CD40 ligand) and supports autoAb production [6,23]. Current findings confirm that lupus monocytes express high levels of CD40. Treatments that support the differentiation of monocytes into M1-like (IFNγ and R848) but not M2-like (PAM3) macrophages correlated with increased CD40 expression (Fig 2E).
To evaluate the effect of PAM3 in vivo, NZB/W mice were treated weekly starting either before or after abnormalities in glomerular function (proteinuria) arose (at 7 and 13 weeks of age, respectively). PAM3 normalized the M1:M2 ratio, inhibited the progression of proteinuria, reduced the BUN:creatinine ratio and prolonged survival (Figs 4,6,8). Initiating PAM3 treatment at 7 weeks of age maximized its survival benefit. Starting at 13 weeks had less of an effect on the magnitude of proteinuria and survival, indicating that treatment could not reverse pre-existing kidney damage. Histologic evaluation of kidneys from these animals confirmed that glomerular and tubular infiltration were reduced by PAM3 treatment while the frequency of CD206+ M2 macrophages rose (Fig 7). While human lupus is characterized by periods of relative inactivity punctuated by disease flares, glomerulonephritis in NZB/W mice is genetically determined and advances inexorably. Thus, the significant delay in disease progression observed in this murine model may translate into even greater benefit for patients. In that context, treatment with PAM3 significantly altered the immune milieu of NZB/W mice. The production of the pro-inflammatory cytokines IL-12 and TNFα was reduced (Figs 3C, D; 5 B, C). Previous reports showed that neutralizing IL-12 could prevent the development of Th1-mediated autoimmune disease [56]. Similarly, TNFα levels in the sera of lupus patients correlates with disease activity and there are reports that anti-TNF therapy improves disease outcome [29,57] (Fig 3C,5C). IL-10 is an immunosuppressive cytokine produced by M2 macrophage. IL-10 impedes APC activation and alleviates disease in NZB/W mice (although its effect in human lupus remains controversial) [29,58,59]. Human monocytes stimulated with PAM3 and splenocytes from PAM3 treated mice produced IL-10 (Fig 3E, 5D), an effect linked with a reduction in autoAb levels in NZB/W mice (Fig 5A).
The findings in this report establishes that PAM3 supports the preferential generation of M2-like macrophages from lupus monocytes. That activity altered the internal milieu, increasing levels of suppressive versus inflammatory cytokines, reducing autoantibody production, delaying disease progression and significantly prolonging survival. We propose that PAM3 mediated modulation of macrophage differentiation be evaluated for efficacy in human SLE.
5. Conclusions
Current findings support the conclusion that macrophage contribute to the pathogenesis of SLE. Our results demonstrate that the TLR2/1 agonist PAM3 induces monocytes from lupus patients to preferentially differentiate into M2-like macrophages. The same effect is observed when PAM3 is administered to lupus-prone NZB/W mice, culminating in the normalization of the M1:M2 balance. Disease progression is delayed and survival significantly prolonged by weekly PAM3 treatment in this murine model. By regulating macrophage differentiation, PAM3 thus represents a potentially novel approach to the therapy of SLE.
Supplementary Material
Supplementary Figure 1: Comparison of monocyte subpopulations in lupus patients with active vs inactive disease.
Monocytes as a percentage of total PBMC (A) and the relative frequency of each monocyte subpopulation (B) was evaluated in freshly isolated peripheral blood samples. N = 4 patients with active disease (SLEDAI score >6) and N = 21 patients with inactive disease.
Highlights.
PAM3 induces monocytes from SLE patients to mature into M2-like macrophages.
PAM3 corrects the M1:M2 macrophage imbalance in lupus-prone NZB/W mice.
PAM3 reduces autoantibody and inflammatory cytokine production, delays glomerulonephritis and prolongs survival in NZB/W mice
Acknowledgements
The authors thank Debra Tross, Fatima Haidar and Madhivanan Elango for their support of the animal experiments, Luz Blanco for organizing the transfer of patient samples and Engin Kayraklioglu for the analysis of kidney sections. This work was supported by the Intramural Research Program of the NIH, NCI. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van VR, Ruiz-Irastorza G, and Hughes G. Systemic lupus erythematosus. Nat.Rev.Dis.Primers, 2 (2016) 16039. [DOI] [PubMed] [Google Scholar]
- [2].Tsokos GC, Lo MS, Costa RP, and Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat.Rev.Rheumatol, 12 (2016) 716–730. [DOI] [PubMed] [Google Scholar]
- [3].Yildirim-Toruner C and Diamond B. Current and novel therapeutics in the treatment of systemic lupus erythematosus. J.Allergy Clin.Immunol, 127 (2011) 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rottman JB and Willis CR. Mouse models of systemic lupus erythematosus reveal a complex pathogenesis. Vet.Pathol, 47 (2010) 664–676. [DOI] [PubMed] [Google Scholar]
- [5].Perry D, Sang A, Yin Y, Zheng YY, and Morel L. Murine models of systemic lupus erythematosus. J.Biomed.Biotechnol, 2011 (2011) 271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li Y, Lee PY, and Reeves WH. Monocyte and macrophage abnormalities in systemic lupus erythematosus. Arch.Immunol.Ther.Exp (Warsz.), 58 (2010) 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mukherjee R, Kanti BP, Kumar TP, Tripathy R, Kumar DB, and Ravindran B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci.Rep, 5 (2015) 13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Martinez FO and Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime.Rep., 6 (2014) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gordon S and Pluddemann A. Macrophage Clearance of Apoptotic Cells: A Critical Assessment. Front Immunol., 9 (2018) 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, and Barton GM. Tissue-Resident Macrophages Are Locally Programmed for Silent Clearance of Apoptotic Cells. Immunity, 47 (2017) 913–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Funes SC, Rios M, Escobar-Vera J, and Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology, 154 (2018) 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chalmers SA, Chitu V, Ramanujam M, and Putterman C. Therapeutic targeting of macrophages in lupus nephritis. Discov.Med, 20 (2015) 43–49. [PubMed] [Google Scholar]
- [13].Li F, Yang Y, Zhu X, Huang L, and Xu J. Macrophage Polarization Modulates Development of Systemic Lupus Erythematosus. Cell Physiol Biochem, 37 (2015) 1279–1288. [DOI] [PubMed] [Google Scholar]
- [14].Bayik D, Tross D, Haile LA, Verthelyi D, and Klinman DM. Regulation of the maturation of human monocytes into immunosuppressive macrophages. Blood Adv, 1 (2017) 2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang J, Shirota Y, Bayik D, Shirota H, Tross D, Gulley JL, Wood LV, Berzofsky JA, and Klinman DM. Effect of TLR agonists on the differentiation and function of human monocytic myeloid-derived suppressor cells. J.Immunol, 194 (2015) 4215–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hill GS, Delahousse M, Nochy D, and Bariety J. Class IV-S versus class IV-G lupus nephritis: clinical and morphologic differences suggesting different pathogenesis. Kidney Int, 68 (2005) 2288–2297. [DOI] [PubMed] [Google Scholar]
- [17].Gordon S and Taylor PR. Monocyte and macrophage heterogeneity. Nat.Rev.Immunol, 5 (2005) 953–964. [DOI] [PubMed] [Google Scholar]
- [18].Mills CD, Kincaid K, Alt JM, Heilman MJ, and Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J.Immunol, 164 (2000) 6166–6173. [DOI] [PubMed] [Google Scholar]
- [19].Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev.Immunol, 32 (2012) 463–488. [DOI] [PubMed] [Google Scholar]
- [20].Bayik D, Tross D, and Klinman DM. Factors Influencing the Differentiation of Human Monocytic Myeloid-Derived Suppressor Cells Into Inflammatory Macrophages. Front Immunol., 9 (2018) 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu L, Allman WR, Coleman AS, Takeda K, Lin TL, and Akkoyunlu M. Delayed onset of autoreactive antibody production and M2-skewed macrophages contribute to improved survival of TACI deficient MRL-Fas/Lpr mouse. Sci.Rep., 8 (2018) 1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pilling D, Fan T, Huang D, Kaul B, and Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS.One, 4 (2009) e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Katsiari CG, Liossis SN, Souliotis VL, Dimopoulos AM, Manoussakis MN, and Sfikakis PP. Aberrant expression of the costimulatory molecule CD40 ligand on monocytes from patients with systemic lupus erythematosus. Clin.Immunol, 103 (2002) 54–62. [DOI] [PubMed] [Google Scholar]
- [24].Tas SW, Quartier P, Botto M, and Fossati-Jimack L. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Ann.Rheum.Dis, 65 (2006) 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jung JY and Suh CH. Incomplete clearance of apoptotic cells in systemic lupus erythematosus: pathogenic role and potential biomarker. Int.J.Rheum.Dis, 18 (2015) 294–303. [DOI] [PubMed] [Google Scholar]
- [26].Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, and Herrmann M. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum, 46 (2002) 191–201. [DOI] [PubMed] [Google Scholar]
- [27].Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, and Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum, 41 (1998) 1241–1250. [DOI] [PubMed] [Google Scholar]
- [28].Murray PJ and Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat.Rev.Immunol, 11 (2011) 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yap DY and Lai KN. Cytokines and their roles in the pathogenesis of systemic lupus erythematosus: from basics to recent advances. J.Biomed.Biotechnol, 2010 (2010) 365083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, and Liew FY. TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. J.Immunol, 174 (2005) 7558–7563. [DOI] [PubMed] [Google Scholar]
- [31].Tunis MC, Dawod B, Carson KR, Veinotte LL, and Marshall JS. Toll-like receptor 2 activators modulate oral tolerance in mice. Clin.Exp Allergy, 45 (2015) 1690–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ha T, Lu C, Liu L, Hua F, Hu Y, Kelley J, Singh K, Kao RL, Kalbfleisch J, Williams DL, Gao X, and Li C. TLR2 ligands attenuate cardiac dysfunction in polymicrobial sepsis via a phosphoinositide 3-kinase-dependent mechanism. Am.J.Physiol Heart Circ.Physiol, 298 (2010) H984–H991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lambert PH and Dixon FJ. Pathogenesis of the glomerulonephritis of NZB/W mice. J.Exp.Med, 127 (1968) 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators.Inflamm., 2015 (2015) 816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H, Madaio MP, Bottinger EP, and Davidson A. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J.Immunol, 180 (2008) 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liao J, Chang C, Wu H, and Lu Q. Cell-based therapies for systemic lupus erythematosus. Autoimmun.Rev, 14 (2015) 43–48. [DOI] [PubMed] [Google Scholar]
- [37].Touma Z and Gladman DD. Current and future therapies for SLE: obstacles and recommendations for the development of novel treatments. Lupus Sci.Med, 4 (2017) e000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Byrne JC, Ni GJ, Lazzari E, Mahony R, Smith S, Stacey K, Wynne C, and Jefferies CA. Genetics of SLE: functional relevance for monocytes/macrophages in disease. Clin.Dev.Immunol, 2012 (2012) 582352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Laria A, Lurati A, Marrazza M, Mazzocchi D, Re KA, and Scarpellini M. The macrophages in rheumatic diseases. J.Inflamm.Res, 9 (2016) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Orme J and Mohan C. Macrophage subpopulations in systemic lupus erythematosus. Discov.Med, 13 (2012) 151–158. [PubMed] [Google Scholar]
- [41].Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, Schwarting A, and Kelley VR. Circulating CSF-1 promotes monocyte and macrophage phenotypes that enhance lupus nephritis. J.Am.Soc Nephrol, 20 (2009) 2581–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chalmers SA, Wen J, Shum J, Doerner J, Herlitz L, and Putterman C. CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus. Clin.Immunol, 185 (2017) 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lenda DM, Stanley ER, and Kelley VR. Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice. J.Immunol, 173 (2004) 4744–4754. [DOI] [PubMed] [Google Scholar]
- [44].Inoue A, Hasegawa H, Kohno M, Ito MR, Terada M, Imai T, Yoshie O, Nose M, and Fujita S. Antagonist of fractalkine (CX3CL1) delays the initiation and ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum, 52 (2005) 1522–1533. [DOI] [PubMed] [Google Scholar]
- [45].Hasegawa H, Kohno M, Sasaki M, Inoue A, Ito MR, Terada M, Hieshima K, Maruyama H, Miyazaki J, Yoshie O, Nose M, and Fujita S. Antagonist of monocyte chemoattractant protein 1 ameliorates the initiation and progression of lupus nephritis and renal vasculitis in MRL/lpr mice. Arthritis Rheum, 48 (2003) 2555–2566. [DOI] [PubMed] [Google Scholar]
- [46].Wang N, Liang H, and Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol, 5 (2014) 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Midgley A, McLaren Z, Moots RJ, Edwards SW, and Beresford MW. The role of neutrophil apoptosis in juvenile-onset systemic lupus erythematosus. Arthritis Rheum, 60 (2009) 2390–2401. [DOI] [PubMed] [Google Scholar]
- [48].Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, and Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J.Exp Med, 197 (2003) 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hallam S, Escorcio-Correia M, Soper R, Schultheiss A, and Hagemann T. Activated macrophages in the tumour microenvironment-dancing to the tune of TLR and NF-kappaB. J.Pathol, 219 (2009) 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chanmee T, Ontong P, Konno K, and Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers.(Basel), 6 (2014) 1670–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kyburz D, Brentano F, and Gay S. Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling. Nat.Clin.Pract.Rheumatol, 2 (2006) 458–459. [DOI] [PubMed] [Google Scholar]
- [52].Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, Meraviglia P, Capetti A, Biasin M, Trabattoni D, and Clerici M. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood, 118 (2011) 3263–3272. [DOI] [PubMed] [Google Scholar]
- [53].Auphan N, DiDonato JA, Rosette C, Helmberg A, and Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science, 270 (1995) 286–290. [DOI] [PubMed] [Google Scholar]
- [54].Yong PF and D’Cruz DP. Mycophenolate mofetil in the treatment of lupus nephritis. Biologics, 2 (2008) 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Poon IK, Hulett MD, and Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death.Differ, 17 (2010) 381–397. [DOI] [PubMed] [Google Scholar]
- [56].Nakajima A, Hirose S, Yagita H, and Okumura K. Roles of IL-4 and IL-12 inthe development of lupus in NZB/W F1 mice. J.Immunol, 158 (1997) 1466–1472. [PubMed] [Google Scholar]
- [57].Zhu LJ, Yang X, and Yu XQ. Anti-TNF-alpha therapies in systemic lupus erythematosus J.Biomed.Biotechnol, 2010 (2010) 465898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Beebe AM, Cua DJ, and de Waal MR. The role of interleukin-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth Factor Rev, 13 (2002) 403–412. [DOI] [PubMed] [Google Scholar]
- [59].Hou LF, He SJ, Li X, Wan CP, Yang Y, Zhang XH, He PL, Zhou Y, Zhu FH, Yang YF, Li Y, Tang W, and Zuo JP. SM934 treated lupus-prone NZB x NZW F1 mice by enhancing macrophage interleukin-10 production and suppressing pathogenic T cell development. PLoS.One., 7 (2012) e32424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Romero-Diaz J, Isenberg D, and Ramsey-Goldman R. Measures of adult systemic lupus erythematosus: updated version of British Isles Lupus Assessment Group (BILAG 2004), European Consensus Lupus Activity Measurements (ECLAM), Systemic Lupus Activity Measure, Revised (SLAM-R), Systemic Lupus Activity Questionnaire for Population Studies (SLAQ), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI). Arthritis Care Res.(Hoboken.), 63 Suppl 11 (2011) S37–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gladman DD, Ibanez D, and Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J.Rheumatol, 29 (2002) 288–291. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Comparison of monocyte subpopulations in lupus patients with active vs inactive disease.
Monocytes as a percentage of total PBMC (A) and the relative frequency of each monocyte subpopulation (B) was evaluated in freshly isolated peripheral blood samples. N = 4 patients with active disease (SLEDAI score >6) and N = 21 patients with inactive disease.


