Abstract
Angiopoietin-1 (Ang1) and its receptor Tie2 regulate vascular function. Our previous study demonstrated that thymosin beta 4 (Tβ4) ameliorates neurological function of diabetic peripheral neuropathy. Mechanisms underlying the therapeutic effect of Tβ4 on diabetic peripheral neuropathy have not been fully investigated. The present in vivo study investigated whether the Ang1/Tie2 signaling pathway is involved in Tβ4-improved neurovascular remodeling in diabetic peripheral neuropathy. Diabetic BKS. Cg-m+/+Leprdb/J (db/db) mice at age 20 weeks were treated with Tβ4 and neutralizing antibody against mouse Tie2 for 4 consecutive weeks. Neurological functional and neurovascular remodeling were measured. Administration of the neutralizing antibody against Tie2 attenuated the therapeutic effect of Tβ4 on improved diabetic peripheral neuropathy as measured by motor and sensory nerve conduction velocity and thermal hypoesthesia compared to diabetic db/db mice treated with Tβ4 only. Histopathological analysis revealed that the neutralizing antibody against Tie2 abolished Tβ4-increased microvascular density in sciatic nerve and intraepidermal nerve fiber density, which were associated with suppression of Tβ4-upregulated occludin expression and Tβ4-reduced protein levels of nuclear factor-κB (NF-κB) and vascular cell adhesion molecule-1 (VCAM1). Our data provide in vivo evidence that the Ang1/Tie2 pathway contributes to the therapeutic effect of Tβ4 on diabetic peripheral neuropathy.
Keywords: Thymosin β4, anti-Tie2, mouse, diabetic peripheral neuropathy
1. Introduction
Peripheral neuropathy is a major complication of diabetes causing damage to the microvessels and peripheral nerves (Cameron et al., 2001; Yagihashi et al., 2011). Current treatment options for diabetic peripheral neuropathy are limited.
Thymosin β4 (Tβ4), a G-actin-sequestering peptide, has multiple biological functions including amplification of neurorestorative processes after nerve damage by increasing neurovascular remodeling and reducing chronic inflammation (Chopp and Zhang, 2015; Morris et al., 2010; Xiong et al., 2011; Zhang et al., 2016). Our previous studies demonstrated that Tβ4 improves neurological function in type II diabetic db/db mice with diabetic peripheral neuropathy, and this improvement is closely associated with amelioration of sciatic nerve neurovascular function in these mice (Wang et al., 2015a; Wang et al., 2012). The molecular mechanisms underlying the therapeutic effect of Tβ4 on diabetic peripheral neuropathy have not been fully elucidated. To aid in the translation of neurorestorative therapies such as Tβ4 to the clinic for diabetic peripheral neuropathy, it is important to understand the molecular mechanisms that mediate the therapeutic effect of Tβ4.
Angiopoietin-1 (Ang1) promotes vascular stabilization, maturation and anti-inflammatory actions by activating the Tie2 tyrosine kinase receptor (Suri et al., 1996). Ang1-induced Tie2 phosphorylation is an indispensable process for regulation of vascular endothelial cell adhesion and vascular junction integrity (Saharinen et al., 2008). Disruption of Tie2 function in transgenic mice results in embryonic death due to vascular defects (Jones et al., 2001). In addition, the Ang1/Tie2 signaling pathway mediates neurite outgrowth of dorsal root ganglion (DRG) neurons (Kosacka et al., 2005). Our previous studies indicate that diabetic db/db mice exhibit substantial reduction of Ang1 levels in endothelial cells and Schwann cells of the sciatic nerves, which is highly associated with neurovascular dysfunction (Wang et al., 2015a; Wang et al., 2012). More importantly, Tβ4 reverses downregulation of Ang1 expression and promotes neurovascular function in the sciatic nerves of diabetic db/db mice, suggesting that the Ang1/Tie2 signaling pathway may be involved in Tβ4-improved neurovascular function (Wang et al., 2012). However, the cause-effect of the Ang1/Tie2 signaling pathway on the therapeutic effect of Tβ4 in diabetic peripheral neuropathy remains unknown. Using a neutralizing antibody against mouse Tie2 which blocks Tie2 activity (Ohab et al., 2006; Rosa et al., 2010), the present study provides in vivo evidence to show that the Ang1/Tie2 pathway contributes to the therapeutic effect of Tβ4 on diabetic peripheral neuropathy.
2. Materials and Methods
2.1. Animals
All use of animals and procedures were conducted following the national guidelines and the relevant national laws on the protection of animals and were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital (IACUC#1486). Male BKS. Cg-m+/+Lepdb/J (db/db) mice and heterozygote mice (db/m, a non-penetrant genotype) (Jackson Laboratories) at age 20 weeks were be employed, when db/db mice exhibit peripheral neuropathy.
2.2. Tβ4 and anti-Tie2 treatment
Diabetic db/db mice were treated with Tβ4 (30 mg/kg, intraperitoneal injection, i.p. RegeneRx, Inc,) daily for 4 weeks, biotin-conjugated neutralizing antibody against mouse Tie2 (1.2 μg/mouse, R&D systems) or same volume of saline vehicle were administered (i.p.) daily for 4 weeks starting one day before Tβ4 treatment (Ohab et al., 2006; Wang et al., 2015a). The specificity of this neutralizing antibody to block Tie2 activity has been demonstrated (Ohab et al., 2006; Rosa et al., 2010). Mice were randomly assigned to four groups (n=10/group): 1. Non-diabetic db/m mice+vehicle; 2. diabetic db/db mice+vehicle; 3. diabetic db/db mice+ Tβ4 + vehicle; and 4. diabetic db/db mice + Tβ4 + anti-mouse Tie2. After 4 weeks of treatment, all mice were sacrificed. Doses of Tβ4 and anti-Tie2 antibody were selected based on previous studies (Ohab et al., 2006; Wang et al., 2015a; Xiong et al., 2011).
Blood glucose and HbA1c levels were measured by test strips for glucose (Roche Diagnostics) and A1C NOW+ Multi-Test A1C System (Quick Medical), respectively. Body weights, blood glucose, HbA1c and functional tests were performed prior to the treatment, and then before sacrifice. All procedures and analyses were conducted by persons who were blinded to experimental groups.
2.3. Measurement of nerve conduction velocity
Motor and sensory nerve conduction velocities (MCV and SCV) of the sciatic nerve were measured according to published methods (Ii et al., 2005; Wang et al., 2012). Briefly, animals were anesthetized with ketamine/xylazine (i.p., 100/10 mg/kg) and their rectal temperature maintained at 37 ± 1.0°C using a feedback controlled water bath. The sciatic notch and knee were stimulated with electrodes which were connected to a stimulator (Model 2100, A-M Systems). The simultaneous electromyographies were recorded from the dorsum of the foot by two sterilized electrodes with an Amplifier (Model P5, Grass Instruments). Motor and sensory nerve conduction velocities were calculated based on published studies (Ii et al., 2005; Wang et al., 2015b; Wang et al., 2012).
2.4. Evaluation of thermal sensation
Plantar test (Hargreaves Method) was conducted using the Plantar Test and Tail Flick Analgesia Meter (Model 336 TG, IITC Life Science), as previously described (Wang et al., 2015a; Wang et al., 2015b). Briefly, animals were acclimated for 20 min in a chamber resting on a transparent glass surface. The temperature of the floor was set at ~32-33°C (manufacturer’s setup). The radiant heat source (15% intensity which produced a heating rate of ~1.3°C per sec) was placed under the hind paw. The paw-withdrawal latency to the radiant heat stimuli was recorded, with a cut-off time 30 sec. For each mouse, five readings were taken at 15 min intervals, and the mean value was calculated (Wang et al., 2015b).
2.5. Measurement of microvascular function in sciatic nerve
Regional sciatic nerve blood flow was measured by mean of Laser Doppler flowmetry (LDF, PeriFlux PF4, Perimed AB) after 4 weeks of initial treatment (Wang et al., 2015b). Briefly, the mouse was mounted on a Kopf stereotaxic apparatus under anesthesia (ketamine/xylazine, i.p., 100/10 mg/kg, JHP Pharmaceuticals LLC.). The left sciatic nerve in the mid-thigh region was exposed and temperature of mice were kept at 37 ± 1.0°C by a water bath. Relative flow values expressed as perfusion units were measured using the LDF probe placed at the surface of the sciatic nerve, every 5 minutes for a total of 5 recordings. Regional sciatic nerve blood flow values from non-diabetic mice were defined as baseline values and data are expressed as a percentage change from baseline (Wang et al., 2015b).
To assess microvascular perfusion of the sciatic nerve, fluorescein isothiocyanate (FITC)-dextran (0.2 mL of 50 mg/mL, 2×106 molecular weight, Sigma Aldrich) was intravenously injected to the mice (Wang et al., 2015b; Wang et al., 2012). Ten minutes later, the sciatic nerve tissues were rapidly harvested and placed in 2% of paraformaldehyde for 2 hours. The whole-mount sciatic nerves were digitized under a 10x objective by a laser-scanning confocal microscope (Zeiss LSM 510 NLO, Carl Zeiss) (Wang et al., 2015b; Wang et al., 2012). The sciatic nerves were then embedded in optimum cutting temperature (OCT) compound and 20 μm thickness cross section were cut. They were imaged under a 20x microscope objective (Zeiss Axiophot) via a Micro Computer Imaging Device (MCID) system (Imaging Research Inc.). Three sections at 60 μm intervals from each nerve were applied. The vascular density was expressed as the total number of FITC-dextran perfused vessels divided by the total tissue-area (mm2) (Wang et al., 2015b; Wang et al., 2012).
2.6. Immunohistochemistry
The sciatic nerve tissues were post-fixed in 4% paraformaldehyde and then embedded in paraffin (Wang et al., 2012). Footpad skin was fixed in ice-cold Zamboni's fixative for 2 hours and then incubated in 30% sucrose/PBS at 4°C overnight. The skin samples were then frozen in OCT compound. Three longitudinal 6-μm-thick sciatic nerve and 20-μm-thick skin sections from each animal were used (Wang et al., 2015b). For immunostaining, the following primary antibodies were applied: polyclonal rabbit anti-protein gene product 9.5 (PGP9.5, 1:1,000, MILLIPORE)(Liu, 2017), monoclonal mouse anti-CD31 antibody (1:500, BD Biosciences)(Otsuru et al., 2008), polyclonal rabbit anti-vascular cell adhesion molecule-1 (VCAM1, 1:500, Santa Cruz)(Man et al., 2009). Nonspecific binding sites were blocked with PBS with 1% bovine serum albumin goat serum for 1 h at room temperature. The tissues were then incubated with the primary antibodies listed above and with CY3 or FITC-conjugated secondary antibodies. Negative controls by omitting the primary antibody were used to verify that conditions of the immunostaining did not result in a non-specific background, as previously described (Liu et al., 2012). DAPI (4′ 6-Diamidino-2-phenylindole, 1:5000, Thermo Fisher Scientific) was used as a nuclear counterstain.
2.7. Quantitative analysis
To measure intraepidermal nerve fiber (IENF) density, nerve profiles were imaged under a 40x microscope objective (Carl Zeiss AxiostarPlus Microscope) using the MCID system. The number of nerve fibers crossing the dermal-epidermal junction were measured and interepidermal nerve fiber density was presented as number of fibers per millimeter of length of epidermis (Wang et al., 2015b). Representative images of intraepidermal nerve fibers were obtained using a laser-scanning confocal microscope (Zeiss LSM 510 NLO, Carl Zeiss, Germany).
2.8. Western blot assay
Western blot was conducted as in published protocols (Wang et al., 2006). Briefly, equal amounts of protein were separated in 10% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. The following antibodies were used: polyclonal rabbit anti-Ang1 (1:1000, Abcam)(Ma et al., 2017), polyclonal rabbit anti-phospho-Tie2 (p-Tie2, 1:1000, Millipore Sigma)(Ghosh et al., 2016), monoclonal mouse anti-Tie2 (1:1000, Millipore Sigma) (Makinde and Agrawal, 2011), monoclonal mouse anti-phospho-NFκB p65 (p-NFκB, 1:1000, Cell Signaling Technology)(Ciaraldi et al., 2016), polyclonal rabbit anti-vascular cell adhesion molecule-1 (VCAM1, 1:500, Santa Cruz)(Mendoza et al., 2004), polyclonal rabbit-anti-monocyte chemotactic protein-1 (MCP1, 1:1000, Abcam)(Li et al., 2013), monoclonal mouse anti-occludin (1:500, Zymed)(Ni et al., 2017) and rabbit anti-β-actin (1:10000, Abcam)(Miscianinov et al., 2018). β-actin were determined as a loading control. The density of protein bands were Quantified using Scion Image analysis program (Scion Image).
2.9. Statistical analysis
Data were evaluated for normality and presented as mean ± SE. The generalized estimating equation (GEE) Global Test was used to test the effects of Tβ4 and anti-Tie2 treatment on nerve conduction velocity (MCV and SCV) at 4 week post treatment (the primary endpoint). The repeated measure analysis of variance (ANCOVA) was used to study the treatment effect in individual motor and sensory nerve conduction velocities and Plantar test over time. The analysis started testing for group by time interaction, followed by testing the main effect of group and subgroup analyses. The one-way analysis of variance (ANOVA) followed by the Tukey test was used to compare all pairs of groups on immunostaining, biochemistry, Western blot, and MCV, SCV, Plantar test, body weight, blood glucose and A1C at 4 weeks, respectively. Differences were considered to be significant when p<.05. All analyses and sample sizes were pre-specified before experiments were conducted.
3. Results
3.1. Blockage of Tie2 abolishes Tβ4-improved neurological function in diabetic mice with peripheral neuropathy
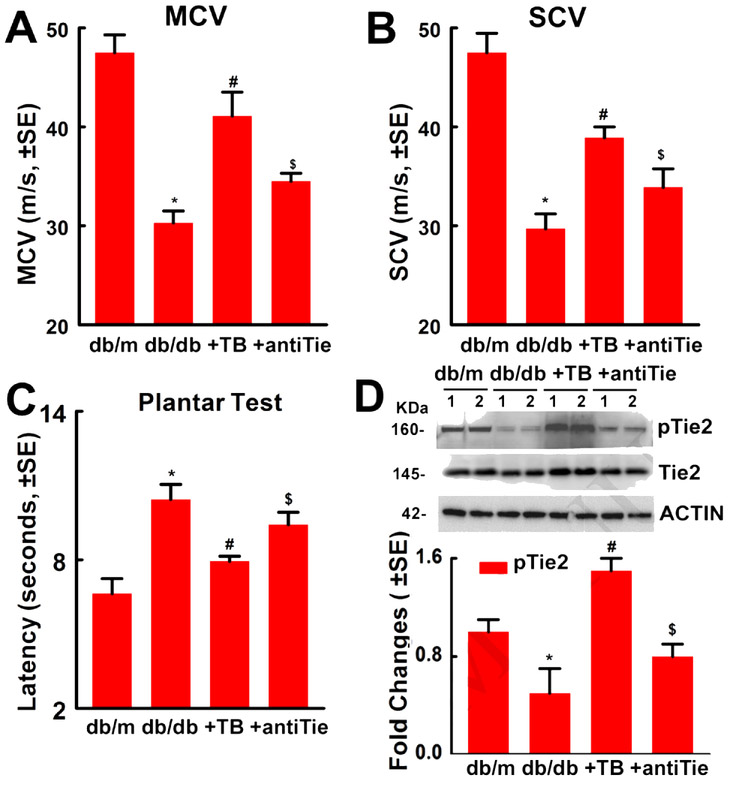
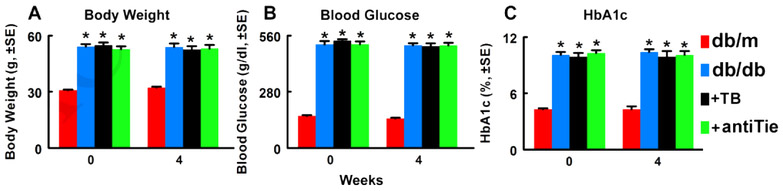
To examine whether blockage of Tie2 affects the therapeutic effect of Tβ4 on diabetic peripheral neuropathy, db/db mice were treated with Tβ4 and the neutralizing antibody against mouse Tie2. Compared to non-diabetic db/m mice, db/db mice exhibited peripheral neuropathy, and the treatment of these db/db mice with Tβ4 (30mg/kg) for 4 weeks significantly improved diabetes reduced sciatic nerve conduction velocity and thermal sensitivity, which are consistent with our previous findings (Wang et al., 2015a; Wang et al., 2012). However, administration of the neutralized antibody against mouse Tie2 to db/db mice abolished the therapeutic effect of Tβ4 on peripheral neuropathy (Fig. 1). Western blot analysis showed that the neutralized antibody significantly reversed Tβ4-increased phosphorylated Tie2 (Fig. 1), indicating inactivation of Tie2 by this antibody. The neutralized antibody did not affect body weight, blood glucose and Hemoglobin A1c (HbA1c) (Fig.2). Thus, these results suggest that Tie2 signaling contributes to therapeutic effect of Tβ4 on diabetic peripheral neuropathy.
Figure 1: Blockage of Tie2 abolishes Tβ4-improved neurological function in diabetic mice with peripheral neuropathy.
Panels A to C show that neurological function outcome measured by motor nerve conduction velocities (MCV, A, F=20.59, P<0.0001), sensory nerve conduction velocities (SCV, B, F=18.11, P<0.0001) and thermal sensitivity (Plantar Test, C, F=9.08, P=0.0007). One-way ANOVA test. n=10/group. Panel D shows Western blot analysis of pTie2 and total Tie2 levels in the sciatic nerve tissues (D, F=90.86, P<0.0001). β-actin was used as a loading control. One-way ANOVA test. n=10/group. *P<0.01, #P<0.01 and $P<0.05 versus the db/db controls and db/db mice treated with Tβ4, respectively, (Tukey’s test). db/m=non-diabetic mice treated with vehicle, db/db=diabetic mice treated with vehicle, TB=diabetic mice treated with Tβ4 and vehicle, and antiTie= diabetic mice treated with Tβ4 and a neutralized antibody against Tie2.
Figure 2: Blockage of Tie2 does not change body weight, blood glucose and HbA1c levels in diabetic mice with peripheral neuropathy.
Panels A to C show body weight (A, F=100.68, P<0.0001), blood glucose (B, F=614.91, P<0.0001) and HbA1c (C, F=189.73, P<0.0001). One-way ANOVA test. n=10/group. *P<0.01 versus the db/m mice (Tukey’s test). db/m=non-diabetic mice treated with vehicle, db/db=diabetic mice treated with vehicle, TB=diabetic mice treated with Tβ4 and vehicle, and antiTie=diabetic mice treated with Tβ4 and the neutralized antibody against Tie2.
3.2. Blockage of Tie2 diminishes Tβ4-improved neurovascular function in diabetic mice with peripheral neuropathy
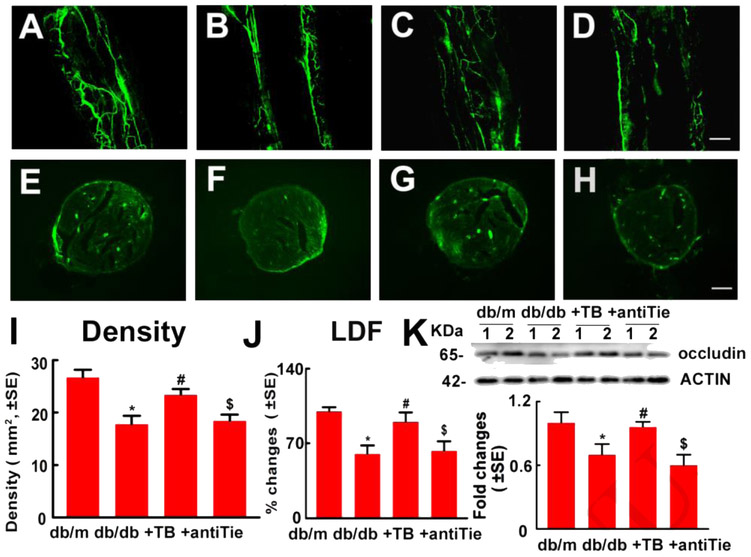
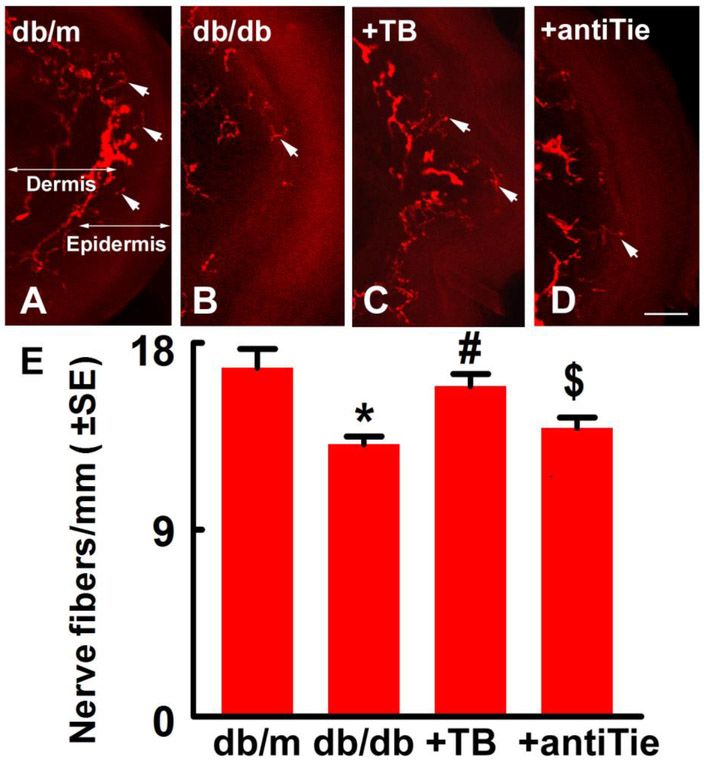
Tβ4 ameliorates diabetes-induced neurovascular dysfunction (Wang et al., 2012). To further verify whether blockage of Tie2 attenuates Tβ4-improved vascular function, LDF and FITC-dextran perfused microvessels in the sciatic nerve were measured. Consistent with previous studies, diabetic db/db mice at aged 24 weeks exhibited vascular dysfunction in the sciatic nerve compared with non-diabetic db/m mice. Tβ4 treatment substantially increased regional blood flow and plasma-perfused vessel density, as well as increased occludin, a tight junction protein expression in diabetic sciatic nerve compared to vehicle-treated diabetic mice. However, administration of anti-Tie2 neutralizing antibody with Tβ4 attenuated Tβ4-improved vascular function in sciatic nerve of db/db mice (Fig.3). These data indicated that Tie2 signaling pathway contributes to Tβ4-ameliorated vascular dysfunction in the sciatic nerve of diabetic mice. To examine the cause-effect of Tie2 signaling on Tβ4-increased distal nerve fibers of diabetic mice, intraepidermal nerve fiber in footpad skin were measured. We observed that diabetic db/db mice exhibited substantial reduction of protein gene product 9.5 (PGP 9.5) positive epidermal nerve fiber (IENF) density in footpad skin compared to non-diabetic mice. Tβ4 significantly increases IENF density compared to diabetic control mice. However, deletion of the Tie2 signaling pathway with anti-Tie2 neutralizing antibody abolished Tβ4-increased of IENF density compared to Tβ4 treated diabetic mice (Fig.4). Therefore, Tie2 signaling mediates Tβ4-improved neurovascular remodeling in diabetic mice.
Figure 3: Blockage of Tie2 diminishes Tβ4-improved microvascular function in sciatic nerves of diabetic mice with peripheral neuropathy.
Representative images of whole-mounted (A to D) and cross sections (E to H) of FITC-dextran perfused blood vessels (green) in the sciatic nerve tissue from non-diabetic db/m mice (A and E), diabetic db/db mice (B and F), db/db mice treated with Tβ4 (C and G) and db/db mice treated with Tβ4 and the neutralized antibody against Tie2 (D and H). Panels I and J show quantification of FITC-dextran perfused functional blood vessel density in the sciatic nerve tissue based on cross sections (I, F=8.10, P=0.0067, n=5/group) and regional blood flow in the sciatic nerve measured by LDF (J, F=3,67, P=0.046, n=10/group). Western blot analysis showed occludin levels in the sciatic nerve tissue (K, F=14.26, P=0.0003, n=10/group). β-actin was used as a loading control. One-way ANOVA test *P<0.05, #P<0.05 and $P<0.05 versus the db/m mice, db/db mice and db/db mice treated with Tβ4, respectively. (Turkey’s test). Bar=100βm. db/m=non-diabetic mice treated with vehicle, db/db=diabetic mice treated with vehicle, TB=diabetic mice treated with Tβ4 and vehicle, and antiTie=diabetic mice treated with Tβ4 and the neutralized antibody against Tie2.
Figure 4: Blockage of Tie2 diminishes Tβ4-increased intraepidermal nerve fibers (IENF) in skin of diabetic mice with peripheral neuropathy.
Panels A to D show representative images of PGP 9.5 immunoreactive IENF (red, arrows) in the footpad skin from db/m mouse (A), db/db mouse (B), db/db mouse treated with Tβ4 (C) and db/db mouse treated with the neutralized antibody against Tie2 with Tβ4 (D). Panel E shows quantitative data (F=5.59, P=0.0062. One-way ANOVA test). n=10/group. *P<0.05, #P<0.05 and $P<0.05 versus the db/m mice, db/db mice and db/db mice treated with Tβ4, respectively (Tukey’s test). Bar=50βm. db/m=non-diabetic mice treated with vehicle, db/db=diabetic mice treated with vehicle, TB=diabetic mice treated with Tβ4 and vehicle, and antiTie=diabetic mice treated with Tβ4 and the neutralized antibody against Tie2.
3.3. Blockage of Tie2 suppresses Tβ4-downregulated proinflammatory mediators in diabetic mice with peripheral neuropathy
Western blot analysis showed that treatment of diabetic db/db mice with Tβ4 increased Ang1 expression in the sciatic nerve tissue compared to diabetic control mice. The neutralized antibody against Tie2 reversed Tβ4-induced Ang1 expression (Fig.5). Inflammatory mediators participate in the pathogenesis of diabetic peripheral neuropathy (Wright, 2011). Tβ4 has anti-inflammatory properties. Thus, we test whether Tie2 signaling is involved in Tβ4-regulated proinflammatory mediators. Western blot analysis of sciatic nerve tissue showed that p-NFkB, VCAM1 and monocyte chemotactic protein-1 (MCP1) were markedly increased in diabetic db/db mice at aged 24 weeks compared with non-diabetic mice. In contrast, their expressions were significantly decreased in diabetic db/db mice treated with Tβ4. However, inhibition of Tie2 activity partially impaired Tβ4-downregulated NFkB activation and VCAM1 expression, but had no effect on MCP1 expression compared to Tβ4 treated diabetic mice (Fig.5). In addition, double immunostaining revealed that VCAM1 immunoreactive cells in the sciatic nerve tissue were CD31 (a marker of endothelial cells) positive (Fig.5). Together, these data demonstrate that the Tβ4 treatment of diabetic neuropathy in db/db mice decreases the activation of NFkB and the expression of VCAM1. The Tie2 signaling pathway mediates Tβ4-decreased activation of NFkB and expression of VCAM1.
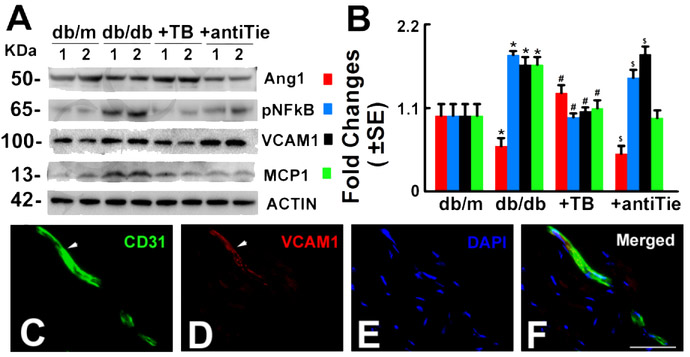
Figure 5: Blockage of Tie2 suppresses Tβ4-downregulated proinflammatory mediators in diabetic mice with peripheral neuropathy.
Panels A and B show Western blot analysis of Ang1 (F=69.31, P<0.0001). p-NFkB (F=475.86, P<0.0001), VCAM1 (F=390.65, P<0.0001) and MCP1 (F=16.05, P=0.0001) levels in the sciatic nerve tissue, β-actin was used as a loading control. n=10/group, One-way ANOVA test. *P<0.01, #P<0.01 and $P<0.05 versus the db/m mice, db/db mice and db/db mice treated with Tβ4, respectively. (Tukey’s Test). db/m=non-diabetic mice treated with vehicle, db/db=diabetic mice treated with vehicle, TB=diabetic mice treated with Tβ4 and vehicle, and antiTie=diabetic mice treated with Tβ4 and the neutralized antibody against Tie2. Double immunofluorescent staining reveals that CD31 positive vessels (C and F green, arrows) immunoreactivity was co-localized with VCAM1 signals (D and F red, arrows). DAPI (E and F, blue) was used as a nuclear counter-staining. Bar=100μm.
4. Discussion
We previously reported that Tβ4 promotes neurovascular function in the sciatic nerve and improves functional outcome of diabetic peripheral neuropathy in diabetic db/db mice (Wang et al., 2015a; Wang et al., 2012). The present study showed that treatment of diabetic mice with the neutralized antibody against Tie2 significantly attenuated Tβ4-improved neurovascular function and neurological outcome in diabetic mice with peripheral neuropathy. These in vivo data indicate that Tie2 signaling contributes to the therapeutic effect of Tβ4 on diabetic peripheral neuropathy.
Preclinical and clinical studies have indicated that microvascular dysfunction is strongly associated with diabetic peripheral neuropathy (Goncalves et al., 2017; Hwang et al., 2016; Stirban, 2014; Tesfaye et al., 2005). Studies from biopsy samples of patients with diabetic peripheral neuropathy show these patients have impaired microvessels (Mohseni et al., 2017; Young et al., 1996). The degree of microvascular impairments correlates with the clinical severity of diabetic peripheral neuropathy (Cameron et al., 2001; Giannini and Dyck, 1995). A treatment strategy targeting both microvascular function and neural regeneration in diabetic neuropathy has been explored (Yagihashi et al., 2011).
The Ang1/Tie2 signaling pathway regulates vascular function by stabilizing and maturating blood vessels. In addition to vascular function, the Ang1/Tie2 pathway affects neuronal function (Kosacka et al., 2005). Ang1 induces neurite outgrowth of DRG neurons (Kosacka et al., 2005). Diabetes disrupts the Ang1/Tie2 signaling pathway by downregulation of Ang1 expression, which is highly associated with vascular dysfunction and diabetic peripheral neuropathy (Chen and Stinnett, 2008; Jin et al., 2008). Elevation of Ang1 levels by administration of a variant of native Ang1 engineered by replacing the N-terminus of Ang1 with cartilage oligomeric matrix protein (COMP-Ang1) increases intraepidermal-nerve fibers and subcutaneous capillary density and reduces diabetic peripheral neuropathy in db/db mice (Jin et al., 2008). In ob/ob mice, COMP-Ang1 enhances sciatic nerve microvessels (Kosacka et al., 2012). This engineered Ang1 also promotes diabetic wound healing and diabetic retinopathy through improvement vascular function and neurovascular normalization in diabetic mice (Cahoon et al., 2015; Cho et al., 2006)
Deletion of Tie2 could block the interaction of Tie2 with Ang1, and simultaneously impair vascular function and nerve regeneration. Reduction of Tie2 activity induces vascular destabilization in diabetic retinopathy (Campochiaro and Peters, 2016). In addition to Ang1, Ang2 binds to receptor Tie2 (Hansen et al., 2010). Our previous study showed substantial reduction and increase of Ang1 and Ang2 levels, respectively, in the sciatic nerve of db/db mouse. Treatment of the db/db mouse with Tβ4 significantly increased Ang1 expression, but decreased Ang2 expression (Wang et al., 2012). Thus, it is likely that the Tie2 neutralized antibody also blocks Ang2.
Endothelial cells and Schwann cells express and secrete Ang1 (Wang et al., 2015a; Wang et al., 2012). Tie2 expression is primarily in the endothelial cells, and also in neurons and Schwann cells of sciatic nerve (Poncet et al., 2003; Valable et al., 2003). High glucose downregulates Ang1 expression in endothelial cells and DRG neurons, which leads to impairment of in vitro angiogenesis and to suppression of neurite outgrowth in DRG neuron, respectively (Wang et al., 2015a; Wang et al., 2012). These findings provide evidence that Ang1 directly acts on endothelial cells and neurons. Tβ4 reverses hyperglycemia-reduced Ang1 and neurovascular impairment in vitro and in vivo. The present study showed that blockage of the Ang1/Tie2 abolished its therapeutic effect on diabetic peripheral neuropathy. We speculate that other Tie 2 blockers, such as the soluble decoy Tie2 receptor (AdTie2Fc) and siRNA against Tie2, will also be effective in blocking the therapeutic effect of Tβ4. Collectively, these data indicate that the Ang1/Tie2 signaling pathway-mediated neurovascular function plays an important role in diabetic peripheral neuropathy and that activation of the Ang1/Tie2 signaling by Tβ4 is critical for Tβ4 therapeutic effect.
Tβ4 has anti-inflammatory properties by inhibiting TNFa-induced NFkB activation (Qiu et al., 2011; Sosne et al., 2007). The present study suggests that activation of Ang1/Tie2 signaling also contributes to the anti-inflammatory effect of Tβ4, as we showed inhibition of Tie2 activity with anti-Tie2 neutralizing antibody abolished Tβ4-suppressed p-NFkB and VCAM1 expression, but not MCP1 expression. Others have shown that activation of the Ang1/Tie2 pathway suppresses inflammation by reducing NFκB activation, and VCAM1 (Kim et al., 2001; Lee et al., 2007; Makinde and Agrawal, 2008). Comp-Ang1 reduces inflammation in diabetic neuropathy by inhibiting the NFkB pathway (Jin et al., 2008). MCP1 (CCL2) is a chemokine and its receptor is CCR2 (Bose and Cho, 2013). Thus, blockage of the Ang1/Tie2 pathway with the antibody against Tie2 may not affect MCP1.
5. Conclusions
In conclusion, based on our previous data and current studies, we demonstrated that inactivation of the Ang1/Tie2 pathway by a neutralizing antibody against Tie2 suppressed Tβ4-improved neurovascular function in diabetic sciatic nerve. Therefore, the Ang1/Tie2 pathway likely mediates Tβ4-induced improvement of neurological function in mice with diabetic peripheral neuropathy.
Highlights.
Blockage of Tie2 abolishes Tβ4-improved neurological function in diabetic mice.
Blockage of Tie2 diminishes Tβ4-improved neurovascular remodeling.
Blockage of Tie2 suppresses Tβ4-downregulated proinflammatory mediators.
Ang1/Tie2 pathway contributes to Tβ4-ameliorated diabetic peripheral neuropathy.
Acknowledgement
The authors thank Julie Landschoot-Ward and Qing-e Lu for their technical assistance.
Funding sources
This work was supported by the National Institutes of Health [NINDS grants RO1 NS075084 (LW) and NIDDK RO1 DK097519 (LW)]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Bose S, Cho J, 2013. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch Pharm Res 36, 1039–1050. [DOI] [PubMed] [Google Scholar]
- Cahoon JM, Rai RR, Carroll LS, Uehara H, Zhang X, O'Neil CL, Medina RJ, Das SK, Muddana SK, Olson PR, Nielson S, Walker K, Flood MM, Messenger WB, Archer BJ, Barabas P, Krizaj D, Gibson CC, Li DY, Koh GY, Gao G, Stitt AW, Ambati BK, 2015. Intravitreal AAV2.COMP-Ang1 Prevents Neurovascular Degeneration in a Murine Model of Diabetic Retinopathy. Diabetes 64, 4247–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NE, Eaton SE, Cotter MA, Tesfaye S, 2001. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 44, 1973–1988. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Peters KG, 2016. Targeting Tie2 for Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Current diabetes reports 16, 126. [DOI] [PubMed] [Google Scholar]
- Chen JX, Stinnett A, 2008. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arteriosclerosis, thrombosis, and vascular biology 28, 1606–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT, Hong HJ, Yoo OJ, Koh GY, 2006. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proceedings of the National Academy of Sciences of the United States of America 103, 4946–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Zhang ZG, 2015. Thymosin beta4 as a restorative/regenerative therapy for neurological injury and neurodegenerative diseases. Expert Opin Biol Ther 15 Suppl 1, S9–12. [DOI] [PubMed] [Google Scholar]
- Ciaraldi TP, Ryan AJ, Mudaliar SR, Henry RR, 2016. Altered Myokine Secretion Is an Intrinsic Property of Skeletal Muscle in Type 2 Diabetes. PloS one 11, e0158209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh CC, David S, Zhang R, Berghelli A, Milam K, Higgins SJ, Hunter J, Mukherjee A, Wei Y, Tran M, Suber F, Kobzik L, Kain KC, Lu S, Santel A, Yano K, Guha P, Dumont DJ, Christiani DC, Parikh SM, 2016. Gene control of tyrosine kinase TIE2 and vascular manifestations of infections. Proceedings of the National Academy of Sciences of the United States of America 113, 2472–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini C, Dyck PJ, 1995. Basement membrane reduplication and pericyte degeneration precede development of diabetic polyneuropathy and are associated with its severity. Annals of neurology 37, 498–504. [DOI] [PubMed] [Google Scholar]
- Goncalves NP, Vaegter CB, Andersen H, Ostergaard L, Calcutt NA, Jensen TS, 2017. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol 13, 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TM, Singh H, Tahir TA, Brindle NP, 2010. Effects of angiopoietins-1 and -2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cellular signalling 22, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Pyun SB, Kwon HK, 2016. Relationship of Vascular Factors on Electrophysiologic Severity of Diabetic Neuropathy. Annals of rehabilitation medicine 40, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii M, Nishimura H, Kusano KF, Qin G, Yoon YS, Wecker A, Asahara T, Losordo DW, 2005. Neuronal nitric oxide synthase mediates statin-induced restoration of vasa nervorum and reversal of diabetic neuropathy. Circulation 112, 93–102. [DOI] [PubMed] [Google Scholar]
- Jin HY, Piao MH, Park JH, Baek HS, Lee S, Kim W, Park SK, Kim CH, Koh GY, Park TS, 2008. Effect of cartilage oligomeric matrix protein angiopoietin-1 on peripheral nerves in db/db diabetic mice. Curr Ther Res Clin Exp 69, 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N, Voskas D, Master Z, Sarao R, Jones J, Dumont DJ, 2001. Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function. EMBO reports 2, 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Moon SO, Park SK, Chae SW, Koh GY, 2001. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circulation research 89, 477–479. [DOI] [PubMed] [Google Scholar]
- Kosacka J, Figiel M, Engele J, Hilbig H, Majewski M, Spanel-Borowski K, 2005. Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell and tissue research 320, 11–19. [DOI] [PubMed] [Google Scholar]
- Kosacka J, Nowicki M, Kloting N, Kern M, Stumvoll M, Bechmann I, Serke H, Bluher M, 2012. COMP-angiopoietin-1 recovers molecular biomarkers of neuropathy and improves vascularisation in sciatic nerve of ob/ob mice. PloS one 7, e32881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim W, Moon SO, Sung MJ, Kim DH, Kang KP, Jang KY, Lee SY, Park BH, Koh GY, Park SK, 2007. Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrol Dial Transplant 22, 396–408. [DOI] [PubMed] [Google Scholar]
- Li S, Du L, Zhang L, Hu Y, Xia W, Wu J, Zhu J, Chen L, Zhu F, Li C, Yang S, 2013. Cathepsin B contributes to autophagy-related 7 (Atg7)-induced nod-like receptor 3 (NLRP3)-dependent proinflammatory response and aggravates lipotoxicity in rat insulinoma cell line. J Biol Chem 288, 30094–30104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, 2017. MicroRNA-146a Mimics Reduce the Peripheral Neuropathy in Type II Diabetic Mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Kassis H, Jia LF, Hozeska-Solgot A, Zhang RL, Chen C, Cui YS, Zhang ZG, 2012. Valproic acid increases white matter repair and neurogenesis after stroke. Neuroscience 220, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Li Z, Shou K, Jian C, Li P, Niu Y, Qi B, Yu A, 2017. Negative pressure wound therapy: Regulating blood flow perfusion and microvessel maturation through microvascular pericytes. International journal of molecular medicine 40, 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinde T, Agrawal DK, 2008. Intra and extravascular transmembrane signalling of angiopoietin-1-Tie2 receptor in health and disease. Journal of cellular and molecular medicine 12, 810–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinde TO, Agrawal DK, 2011. Increased expression of angiopoietins and Tie2 in the lungs of chronic asthmatic mice. American journal of respiratory cell and molecular biology 44, 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S, Tucky B, Bagheri N, Li X, Kochar R, Ransohoff RM, 2009. alpha4 Integrin/FN-CS1 mediated leukocyte adhesion to brain microvascular endothelial cells under flow conditions. Journal of neuroimmunology 210, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza L, Valcarcel M, Carrascal T, Egilegor E, Salado C, Sim BK, Vidal-Vanaclocha F, 2004. Inhibition of cytokine-induced microvascular arrest of tumor cells by recombinant endostatin prevents experimental hepatic melanoma metastasis. Cancer research 64, 304–310. [DOI] [PubMed] [Google Scholar]
- Miscianinov V, Martello A, Rose L, Parish E, Cathcart B, Mitic T, Gray GA, Meloni M, Al Haj Zen A, Caporali A, 2018. MicroRNA-148b Targets the TGF-beta Pathway to Regulate Angiogenesis and Endothelial-to-Mesenchymal Transition during Skin Wound Healing. Molecular therapy : the journal of the American Society of Gene Therapy 26, 1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni S, Badii M, Kylhammar A, Thomsen NOB, Eriksson KF, Malik RA, Rosen I, Dahlin LB, 2017. Longitudinal study of neuropathy, microangiopathy, and autophagy in sural nerve: Implications for diabetic neuropathy. Brain and behavior 7, e00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DC, Chopp M, Zhang L, Lu M, Zhang ZG, 2010. Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience 169, 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Teng T, Li R, Simonyi A, Sun GY, Lee JC, 2017. TNFalpha alters occludin and cerebral endothelial permeability: Role of p38MAPK. PloS one 12, e0170346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST, 2006. A neurovascular niche for neurogenesis after stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y, 2008. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells 26, 223–234. [DOI] [PubMed] [Google Scholar]
- Poncet S, Gasc JM, Janzer RC, Meyer S, Juillerat-Jeanneret L, 2003. Expression of Tie-2 in human peripheral and autonomic nervous system. Neuropathol Appl Neurobiol 29, 361–369. [DOI] [PubMed] [Google Scholar]
- Qiu P, Wheater MK, Qiu Y, Sosne G, 2011. Thymosin beta4 inhibits TNF-alpha-induced NF-kappaB activation, IL-8 expression, and the sensitizing effects by its partners PINCH-1 and ILK. FASEB J 25, 1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AI, Goncalves J, Cortes L, Bernardino L, Malva JO, Agasse F, 2010. The angiogenic factor angiopoietin-1 is a proneurogenic peptide on subventricular zone stem/progenitor cells. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 4573–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K, 2008. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nature cell biology 10, 527–537. [DOI] [PubMed] [Google Scholar]
- Sosne G, Qiu P, Kurpakus-Wheater M, 2007. Thymosin beta 4: A novel corneal wound healing and anti-inflammatory agent. Clinical ophthalmology 1,201–207. [PMC free article] [PubMed] [Google Scholar]
- Stirban A, 2014. Microvascular dysfunction in the context of diabetic neuropathy. Current diabetes reports 14, 541. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD, 1996. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH, Group EPCS, 2005. Vascular risk factors and diabetic neuropathy. The New England journal of medicine 352, 341–350. [DOI] [PubMed] [Google Scholar]
- Valable S, Bellail A, Lesne S, Liot G, Mackenzie ET, Vivien D, Bernaudin M, Petit E, 2003. Angiopoietin-1-induced PI3-kinase activation prevents neuronal apoptosis. FASEB J 17, 443–445. [DOI] [PubMed] [Google Scholar]
- Wang L, Chopp M, Jia L, Lu X, Szalad A, Zhang Y, Zhang R, Zhang ZG, 2015a. Therapeutic Benefit of Extended Thymosin beta4 Treatment Is Independent of Blood Glucose Level in Mice with Diabetic Peripheral Neuropathy. Journal of diabetes research 2015, 173656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chopp M, Szalad A, Jia L, Lu X, Lu M, Zhang L, Zhang Y, Zhang R, Zhang ZG, 2015b. Sildenafil ameliorates long term peripheral neuropathy in type II diabetic mice. PloS one 10, e0118134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chopp M, Szalad A, Liu Z, Lu M, Zhang L, Zhang J, Zhang RL, Morris D, Zhang ZG, 2012. Thymosin beta4 promotes the recovery of peripheral neuropathy in type II diabetic mice. Neurobiology of disease 48, 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M, 2006. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 5996–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N.M.W.a.D.E., 2011. Inflammatory Mediators in Diabetic Neuropathy. Journal of Diabetes & Metabolism S5:004. doi: 10.4172/2155-6156.S5-004 [DOI] [Google Scholar]
- Xiong Y, Mahmood A, Meng Y, Zhang Y, Zhang ZG, Morris DC, Chopp M, 2011. Treatment of traumatic brain injury with thymosin beta in rats. Journal of neurosurgery 114, 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagihashi S, Mizukami H, Sugimoto K, 2011. Mechanism of diabetic neuropathy: Where are we now and where to go? J Diabetes Investig 2, 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ, Bennett JL, Liderth SA, Veves A, Boulton AJ, Douglas JT, 1996. Rheological and microvascular parameters in diabetic peripheral neuropathy. Clin Sci (Lond) 90, 183–187. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang ZG, Li Y, Lu M, Zhang Y, Elias SB, Chopp M, 2016. Thymosin beta4 promotes oligodendrogenesis in the demyelinating central nervous system. Neurobiology of disease 88, 85–95. [DOI] [PubMed] [Google Scholar]