Abstract
The Adolescent Brain Cognitive Development (ABCD) study is designed to be the largest study of brain development and child health in the United States, performing comprehensive assessments of 11,500 children repeatedly for 10 years. An endeavor of this magnitude requires an organized framework of governance and communication that promotes collaborative decision-making and dissemination of information. The ABCD consortium structure, built upon the Matrix Management approach of organizational theory, facilitates the integration of input from all institutions, numerous internal workgroups and committees, federal partners, and external advisory groups to make use of a broad range of expertise to ensure the study’s success.
Keywords: Adolescence, Development, Neuroimaging, Longitudinal, Organizational framework, Governance
1. Introduction
The Adolescent Brain Cognitive Development (ABCD) study is designed to be the largest study of brain development and child health in the United States, performing comprehensive assessments of 11,500 children across the country repeatedly for 10 years. The overarching goal of the study is to integrate structural and functional brain imaging with genetics, neuropsychological, behavioral, and other health assessments to increase our understanding of the numerous facets of brain, cognitive, social, emotional, and physical development during adolescence. As such, the ABCD study requires an interdisciplinary effort involving leading scientists in the fields of child development, psychiatry, neuroscience, genetics, public health, among many others, totaling more than 400 team members and collaborators. The ABCD Coordinating Center, Data Analysis and Informatics Center, 21 data collection sites, and federal collaborators (National Institutes of Health [NIH], Centers for Disease Control and Prevention, National Institute of Justice, National Science Foundation and National Endowment for the Arts) have come together to develop and implement this comprehensive study (see Fig. 1).
Fig. 1.
The ABCD consortium consists of a Coordinating Center (orange), Data Analysis and Informatics Center (green), federal collaborators (grey), and 21 data collection sites comprised of standalone research sites, and coordinated research hubs (connected by dotted lines) in which multiple institutions are members of a single grant award. Sites receiving direct funding are shown in dark blue, and subcontracted sites are shown in light blue. Note: NIDA (National Institute on Drug Abuse); NIAAA (National Institute on Alcohol Abuse and Alcoholism); NCI (National Cancer Institute); NICHD (Eunice Kennedy Shriver Nation Institute of Child Health and Development); NIMH (National Institute of Mental Health); NIMHD (National Institute of Minority Health and Health Disparities); NINDS (Nation Institute of Neurological Disorders and Stroke); OBSSR (NIH Office of Behavioral and Social Sciences Research); ORWH (NIH Office of Research on Women’s Health); CDC (Centers for Disease Control and Prevention); NIJ (National Institute of Justice), NEA (National Endowment for the Arts, and NSF (National Science Foundation) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
An interdisciplinary project of this magnitude requires an organizational framework, governance structure, and communication system for inclusive, sound decision-making within structural and financial limitations. Top-down organizational frameworks are poorly suited for this type of project as they impose constraints on scientific input and stifle innovation (McCall, 1981). Conversely, distributed decision-making in a purely flat structure would be impractical for optimally coordinating the frequent decisions needed for a study this large. The Matrix Management approach of organizational theory emerged in the 1970s as the business world responded to market globalization, intensified competition, accelerated technology development, and other new pressures. This framework uses cross-cutting structures to bridge silos within a hierarchical organizational structure (Stuckenbruck, 1979), thereby increasing integration of diverse perspectives, improving information flow, enhancing creativity, and providing efficient use of resources by reducing redundancy. More recently, this approach has been recommended to improve interdisciplinary research by organizing around research issues rather than disciplines (National Academy of Sciences, 2005). The ABCD Study is an exemplar of such interdisciplinary research in its focus on research questions (e.g., factors that influence adolescent development) crossing disciplines from epidemiology to psychology to brain imaging to genetics. The Matrix Management model provides a framework to address complexity (e.g., 21 sites and a multifaceted protocol), centrality (e.g., a centralized governance body with multi-step decision-making), and formality (e.g., structural and financial requirements from the funding agencies) (Stuckenbruck, 1979), which is well suited to the needs of the ABCD study. This model helps improve efficiency by pooling scientific expertise to optimize a unified protocol across sites while developing and maintaining a centralized informatics infrastructure that serves the consortium. Similar to other successful research consortia such as NIDA’s Clinical Trials Network and the NCAA-DoD Concussion Assessment, Research and Education Consortium, ABCD’s organizational structure is built on these principles to ensure sharing of expertise, inclusive decision-making, and transparent bidirectional communication (Broglio et al., 2017; Tai et al., 2010).
At the heart of the ABCD Study are the Coordinating Center, Data Analysis and Informatics Center, and 21 research sites, which are responsible for executing the study. The Coordinating Center is comprised of Co-Directors Jernigan and Brown, Associate Directors Garavan and Tapert, site quality control monitors, and support for project and communications management and data integration. It is charged with coordinating the scientific and administrative activities of ABCD, providing the organizational framework for management and direction, as well as monitoring site readiness, recruitment progress, quality control metrics, and advancement towards study goals. In addition, they manage the activities of workgroups, committees, and advisory groups established to develop and monitor various aspects of the study, including protocol coordination, policy development, communications, and community engagement. The Data Analysis and Informatics Center is comprised of its Program Director Dale, and expert investigators and programmers in imaging protocol development, image analysis, biostatistics, informatics, and systems analysis. The Data Analysis and Informatics Center is responsible for coordinating the activities of the image acquisition, image analysis, and informatics workgroups as well as ensuring central capture, secure transfer, and storage of many types of data; rigorous quality control and quantitative calibration procedures; centralized image processing and information extraction; development of statistical analysis tools and procedures for the integration of longitudinal information across measures and modalities; and rapid public sharing of data and tools. The Coordinating Center and Data Analysis and Informatics Center work in close collaboration with NIH scientific officials as a part of a centralized Operations Group that meets weekly to ensure that study goals are achieved.
Investigators from all sites, and representatives from each federal partner, the Coordinating Center, and the Data Analysis and Informatics Center comprise the membership of cross-cutting (i.e., horizontal) workgroups as well as the Council of Investigators, which meets via biweekly teleconference to tackle issues that must be considered to make the project a success. Finally, the ABCD consortium has established an advisory structure to provide high-level guidance on scientific directions and clinical risk management through an External Advisory Board and an Observational Study Monitoring Board. Collectively, this framework ensures that appropriate expertise advises the consortium, a variety of perspectives are considered, and the many facets of this complicated study are overseen and addressed in a timely and effective manner.
2. Protocol coordination
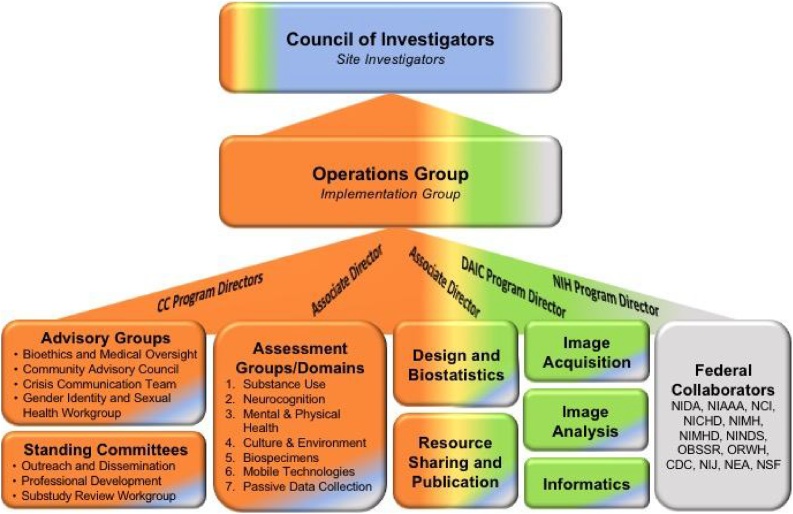
Like similar multi-site neuroimaging collaborations (Brown et al., 2015; Jernigan et al., 2016), the ABCD study was designed to be unified across many sites, all using the same protocol for recruitment, assessment, and imaging. Following the Matrix Management model, workgroups that leverage expertise across institutional boundaries were established to identify, discuss, and recommend protocol elements to be included for the entire consortium and to monitor progress and changes as needed moving forward (see Fig. 2).
Fig. 2.
ABCD committees, advisory groups, and workgroups. The Coordinating Center oversees committees responsible for non-imaging protocol elements and policy and communications activities (orange). The Data Analysis and Informatics Center oversees committees related to informatics and neuroimaging acquisition and analysis (green). The Coordinating Center and Data Analysis and Informatics Center collaboratively oversee groups that involve design, data usage policy, and informatics (blended colors) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
2.1. Design and biostatistics
Led by an Associate Director of the Coordinating Center who is also principal investigator at a research site, the Design and Biostatistics Workgroup oversees all aspects of the study design, including sampling methods, twin cohort (and other forms of relatedness), inclusion and exclusion criteria, and cohort retention. This workgroup, consulting with the Institute for Social Research at the University of Michigan, developed the school-based sampling strategy and school lists for each site, sets appropriate recruitment standards and goals, and monitors recruitment to ensure sampling methods are adhered to and the consortium achieves and maintains desired cohort characteristics. The workgroup ensures that the biostatistical approaches employed to address study aims are optimal for, and take full advantage of, all aspects of the study design. Members of the group include experts in sampling, epidemiology, and biostatistics, and investigators with considerable experience in conducting longitudinal studies from across the consortium and its federal partners.
2.2. Assessment workgroups
The ABCD consortium has divided the non-imaging assessment measures into seven domains, each overseen by a separate assessment workgroup: Substance Use, Neurocognition, Mental & Physical Health, Culture & Environment, Biospecimens, Mobile Technology, and Passive Data Collection. While some overlap exists between domains, having discrete groups focused on each area has allowed experts to fully investigate the best measures for each domain. An Associate Director in the Coordinating Center, who is also principal investigator of a research site, oversees all seven assessment workgroups, serving as a bridge and ensuring procedural consistency and guarding against redundancy or gaps. Each assessment workgroup’s content is detailed in other articles of this special issue.
Leading up to the launch of enrollment for the study, the assessment workgroups were responsible for surveying the field to identify well-validated, age-appropriate measures that address study goals. Individual measures and subsequently the full baseline protocol were piloted, and workgroups reviewed pilot data to optimize, refine, and shorten the protocol. These groups worked with the project informatics support team at the Coordinating and Data Analysis and Informatics Centers to develop appropriate informatics for upload and quality control of assessment results and reporting forms for monitoring data quality and staff training needs.
As the ABCD study progresses, assessment workgroups monitor data quality of the protocol. Workgroups meet regularly to investigate potential inconsistencies, and develop recommendations to address any issues. When indicated, workgroups make recommendations for modifications to assessments or to staff training, indicated by improving technologies, new scientific findings, or demonstrated limitations of existing protocols (e.g., decreasing sensitivity due to a maturing cohort). Errors in protocol administration or data collection discovered by the workgroups are reported to the Data Analysis and Informatics Center, and corrected immediately. Any recommendations for major changes to the protocol or modification for upcoming follow-up waves are reported to the Operations Group via the Associate Director, and then finally approved by the Council of Investigators and the Steering Committee before implementation (see Fig. 4).
Fig. 4.
ABCD process and information flow. Decision making in ABCD incorporates mechanisms and structures that take advantage of expertise across sites and within focused teams. This figure demonstrates how ideas and information are integrated from workgroups, advisory groups, and standing committees through the Operations Group to the Council of Investigators. The workgroups include representation from the Coordinating Center, Data Analysis and Informatics Center, all of the research sites, and federal partners. Note: Orange = Coordinating Center; green = Data Analysis and Informatics Center; blue = data collection sites; grey = federal collaborators (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
As the cohort matures, it is critical to monitor the developmental tailoring of standard measures and developmental milestones (e.g., pubertal changes, social/behavioral functioning) as well as risk and resilience factors that are age, gender, and culture specific. The ABCD consortium will consult with premiere developmental experts for each age to ensure comprehensive coverage, developmental appeal, and appropriateness of all assessment methods as the cohort matures. Additionally, as emerging scientific questions arise, workgroups may be formed to determine how best to incorporate these into the protocol. For example, an additional subgroup was established under the Mental & Physical Health Workgroup to explore how best to incorporate questions about gender identity and sexual health into the protocol as participants mature.
2.3. Imaging protocol
The ABCD study is unique in its goal of conducting brain imaging of a large number of participants across 21 different sites with MRI scanners from 3 different vendors. Ensuring that data are acquired and transferred harmoniously across sites is critical to study success. The Data Analysis and Informatics Center has established Image Acquisition and Image Analysis workgroups with expertise from across the consortium to oversee these efforts. The Image Acquisition Workgroup ensures the quality and consistency of incoming imaging data by overseeing the design of appropriate MRI protocols for structural and functional imaging measures, fMRI stimulus and response monitoring, real-time quality control procedures, calibration within and across scanners and sites, as well as managing issues associated with scanner and software upgrades.
The Image Analysis Workgroup ensures the quality and consistency of derived data. The group is responsible for overseeing the implementation of analysis workflows for structural and functional imaging measures, including cortical and subcortical segmentation, diffusion MRI tractography, and task-related and resting state fMRI. They also oversee software testing and documentation as well as quality control of derived data.
2.4. Informatics
Given the large numbers of measures in the ABCD protocol, it is critically important to have the informatics infrastructure ensure consistent and secure data acquisition, transfer, storage, and sharing. An informatics workgroup, executed by the Data Analysis and Informatics Center, was established with expertise from across the consortium to oversee the implementation of appropriate software solutions and hardware infrastructure for storage and curation of raw and derived data across all domains, including imaging, substance use assessments, neurocognitive measures, questionnaire and interview data, genetic and biosample data, and mobile technologies. This group is responsible for the design and maintenance of the ABCD database and “big data” integration across measures and modalities.
3. Communications and engagement
The ABCD Study has set an ambitious goal of recruiting 11,500 participants over two years and retaining at least 87% of participants over the projected 10-year course of the study. Therefore, engagement with the community is critical to its success. The ABCD Outreach and Dissemination workgroup and each site’s Community Liaison Boards helps establish and maintain these relationships.
The purpose of the Outreach and Dissemination workgroup is to develop and implement a communications strategy that effectively engages ABCD stakeholders and promotes awareness about the study and its potential scientific contributions. This committee, as well as individual ABCD research sites, have engaged stakeholders (e.g., youth and families, educators) from the beginning of study development to ensure that questions of interest to the scientific community are addressed, and needs and expectations of those involved in the study and those that it will ultimately serve are met (for more see Hoffman et al., this issue). This committee develops outreach materials (e.g., website, brochures, recruitment letters, videos), identifies vehicles and influencers to amplify the ABCD message and raise awareness, and works with the Coordinating Center to monitor site outreach, identify gaps, and propose solutions when needed. The Outreach and Dissemination workgroup will also coordinate the dissemination of study findings to stakeholders and constituents as they become available.
Each site is also establishing a Community Liaison Board that receives direct input from stakeholders within the community, including youth and their families, educators, health care providers, other professional organizations, and the general public, and disseminates information and findings from the ABCD study back to the community.
4. Policy development and implementation
Several workgroups were established to develop policies that govern the consortium. It was critical for these groups to capitalize on expertise within the consortium and external to it, to ensure that sensitive issues such as bioethics, data sharing, and crisis communications are appropriately addressed and adopted by consortium membership.
4.1. Bioethics and medical oversight
Given the young age and vulnerability of the cohort, and the delicate balance between the need for frank and accurate information from young participants on the one hand, and the strong incentive to provide supportive and protective recommendations as needed, it is critical for the ABCD consortium to have committees which focus specifically on bioethical concerns. Most ABCD research sites rely on a central Institutional Review Board (cIRB) at the University of California, San Diego for the ethical review and approval of the research protocol, with a few sites obtaining local IRB approval. The Coordinating Center is responsible for coordinating all interactions between relying sites and the cIRB, and providing requisite information to non-relying sites for their IRBs. In addition, the Coordinating Center has established a Bioethics and Medical Oversight advisory group, comprised of experienced clinicians and ethicists from throughout the consortium, which focuses on bioethical concerns, a particularly important responsibility of the Coordinating Center. The Bioethics and Medical Oversight advisory group proposes policies for identifying and responding to information obtained during the course of the study that may indicate significant threats to the participants’ wellbeing (for more see Clark et al., 2017). Anticipated examples of such information include evidence or reports of child abuse and neglect, imminent potential for self-harm or harm to others, serious medical and psychiatric disorders, life-threatening substance use and related problems, imaging incidental findings, and confidentiality limitations. The policies protect the rights of parents and participants to privacy and confidentiality, address the critical need to obtain unbiased information to achieve the study aims, and define responsible protective actions to be taken to address the needs of vulnerable participants.
Each ABCD data collection site has developed site-specific standard operating procedures for the implementation of these policies and procedures. The Bioethics and Medical Oversight group and the Coordinating Center review these site-specific standard operating procedures to ensure the implementation plans comply with these policies. The Coordinating Center has developed site monitoring, reporting and response procedures in accordance with these guidelines, and provides reports of any adverse events and a semiannual report of monitored indices to the ABCD Bioethics and Medical Oversight advisory group and the Observational Study Monitoring Board for review. Over the course of the study, the group provides advice and consultation to the Coordinating Center and research sites regarding the implementation of these policies and procedures, clarification regarding policy interpretation and, when necessary, amendments of policies and procedures to the ABCD Steering Committee.
4.2. Resource sharing and publication
An essential aim of the ABCD Consortium is to produce a valuable data resource for the research community at large. This resource includes raw and curated electronic data from all assessment domains and a biospecimen repository. The Resource Sharing and Publication workgroup develops policies and oversees the sharing of raw and derived data, and associated software and analysis workflows, within and beyond the consortium, including policies and procedures for publication of shared data.
To facilitate an open-science format in a timely fashion, the consortium has adopted an aggressive data sharing schedule, following two timelines. The first, a Fast-Track release, is a rapid, ongoing sharing of “raw” imaging data. The second release includes data from all assessment domains (raw and derived measures). These rigorously curated data, released annually, are cumulative, adding additional curated data from baseline and follow-up visits. All accumulated ABCD data are re-processed for the annual releases, incorporating any improvements in data processing workflows or additions to derived data. Each data release is versioned for reference in publications, and all versions remain available (by request) through the NIMH Data Archive (NDA; https://data-archive.nimh.nih.gov/abcd). The ABCD Coordinating and Data Analysis and Informatics Centers will also provide extensive documentation of protocols and relevant publications and research efforts related to ABCD on its website (abcdstudy.org) so prospective users can learn how to apply for access to ABCD data and interpret findings. Further, ABCD is developing a Data Exploration and Analysis Portal, based on infrastructure created for the Pediatric Imaging, Neurocognition, and Genetics project (Bartsch et al., 2014), to facilitate the use of the ABCD data.
The Resource Sharing and Publication workgroup works closely with NDA and Rutgers University Cell and DNA Repository (RUCDR; http://www.rucdr.org), through which ABCD electronic and biospecimens data are shared, respectively, to maintain compliance with applicable laws, policies, and protocols related to data sharing and publication. For example, while all shared data are de-identified, data use agreements are critical to ensure ethical use of ABCD data. In addition, this workgroup developed a publication policy so that all papers based on ABCD data acknowledge ABCD and NIH support, so that the ABCD scientific impact can be tracked. ABCD monitors requests for access to these data, the number of investigators that download data or obtain biospecimens, and resulting publications made possible by these resources to evaluate the impact of ABCD’s data sharing for the broader scientific community.
4.3. Crisis communications
While the consortium does not anticipate a high incidence of crisis situations, unexpected situations may arise that threaten the integrity or reputation of the study. In those situations, it is vital that the study staff, partners, and spokespersons respond quickly and compassionately to minimize harmful fallout. The Coordinating Center has established a Crisis Communications Team, consisting of representatives from throughout the Consortium as well as external expertise in crisis communications and media, which developed a Crisis Communication Plan to assist the Consortium in preventing incidents from arising and managing them if necessary. The Crisis Communications Team is responsible for anticipating and diffusing potential controversies, and for allaying the concerns of each audience and positioning the consortium to emerge from the incident with its reputation intact. To do this, the crisis communications plan ensures a coordinated initial response, release of information, and consistency of message. The Crisis Communications Team monitors emerging issues, assess the potential for a situation to develop into a communications crisis, identify appropriate communications strategies and actions, brief spokespersons, develop materials to respond to the situation, engage media, advocacy, and community channels as necessary and appropriate, keep partners informed of the situation, and evaluate responses and adjust strategies as needed. Above all, the process ensures bi-directional communication between all parties, i.e., between study sites, the Crisis Communications Team, participants and families, partner organizations, and the public at large, to ensure that all concerns are addressed rapidly and effectively.
5. Monitoring
5.1. Site quality control monitor
While the development and coordination of study protocol is overseen by cross-cutting workgroups, centralized oversight is necessary to ensure that the ABCD protocol is implemented consistently and with fidelity at all ABCD sites. Thus, the Coordinating Center has a site quality control monitor who is responsible for evaluating readiness, performance, and protocol standardization and adherence across all consortium sites. This person assists with the development of study policies and standard operating procedures, and collaborates with the informatics team by providing strategic support and enhancing electronic data collection instruments.
The site quality control monitor travels to all sites to conduct annual on-site visits. Prior to each site being certified to launch enrollment, the site quality control monitor conducted a 3-day site initiation visit that consisted of assessing staff’s core protocol knowledge, monitoring compliance of established administrative guidelines, confirming that required equipment and supplies were in place, determining the accuracy of data collection and data entry, and ensuring the safety and comfort of participants. At each visit, the site quality control monitor observed research lab space and imaging centers, phone screens with parents and baseline visit scheduling; informed consent and the complete youth and parent baseline protocol, reviewed site emergency management procedures, checked subject tracking and post-session data transfer; observed supply shipping, storage, and maintenance of equipment; reviewed regulatory documents and safeguarding of data; underwent the ABCD imaging protocol as multi-site human phantom data for cross-site validation and harmonization; and held a debriefing feedback session with site principal investigators and research staff. The purpose of this comprehensive initial visit was to evaluate the site’s performance and readiness to start assessing participants, identify protocol violations, provide recommendations for needed follow-up, if applicable, and foster collaboration.
Following the site initiation visit, the monitor prepared a detailed report of findings and recommendations for the site PI and staff, and a teleconference was held with study investigators and staff, the Coordinating Center, and Data Analysis and Informatics Center to verify that all site issues were resolved and to discuss any questions or concerns, after which the Coordinating Center and Data Analysis and Informatics Center leadership team determined if the site was ready to launch.
Throughout the study, the quality control monitor visits each site annually to review procedures for the upcoming protocol and ensure against drift in all protocol compliance. The quality control monitor also ensures that standard operations manuals are updated and reflective of ABCD Committees, Advisory groups, and Workgroups decisions. This person organizes and leads a weekly teleconference call with all site coordinators and research assistants, identifies and communicates issues, concerns, and progress from sites, and serves as liaison between assessment workgroups, study sites, the Data Analysis and Informatics Center, and Coordinating Center. Finally, the monitor continuously disseminates and oversees the implementation of policies set forth by governing bodies and works closely with senior investigators and other senior project staff to maintain cross-site fidelity and reliability of the study protocol and compliance with consortium policies for ethical conduct of research across all sites and assessment timelines.
5.2. Recruitment and data quality control monitoring
The Coordinating Center and the Design Workgroup, in collaboration with the Institute for Survey Research, has established a set of enrollment metrics to monitor recruitment progress at each ABCD data collection site. Progress relative to each site’s enrollment target is monitored continuously by site and for the entire consortium. Each site’s targets and progress are examined with regard to sex, risk status (high/low risk), race/ethnicity, and social economic status (education and household income). The four twin sites (University of Minnesota, University of Colorado, Virginia Commonwealth University, and Washington University; see Fig. 1) are monitored for progress towards singleton and twin demographic targets.
The Coordinating Center evaluates the completeness of all required assessments at each data collection site at every visit for all participants/parents, identifying sites or time periods in which protocol adherence deviates. Once identified, plans to address issues are discussed with site staff, and then are monitored closely to confirm resolution.
The Data Analysis and Informatics Center has made these enrollment and data completion metrics available online in real-time as dynamic graphs, allowing each site to view their current data whenever necessary. These metrics are presented and reviewed by the Coordinating Center at the first Council of Investigators meeting each month. These monthly reports include all subjects enrolled through the end of the previous month. From these reports, site principal investigators, as well as NIH staff, are informed of ABCD study progress as a whole and at each site.
This regular review of enrollment and completion metrics is critical for proactive management of the project, and has revealed recruitment challenges, which led to discoveries such as low participant compensation or lack of school engagement that may impact recruitment. To address these challenges, sites worked with the Design and Biostatistics workgroup and the Operations Group to bolster recruitment strategies. Metrics have also revealed recruitment successes, prompting the sharing of productive recruitment strategies in Council of Investigator meetings to assist sites challenged in reaching enrollment targets.
6. Advisory boards
While the ABCD Consortium consists of leading experts in many areas critical to implementing a large longitudinal study, a study of this magnitude must also rely on independent experts who can provide unbiased advice and guidance to ensure the study’s success. To that end, similar to other large studies (Broglio et al.2017; Tai et al., 2010), the ABCD Consortium has an External Advisory Board whose responsibility is to provide scientific guidance on study design and implementation and an Observational Study Monitoring Board who provides recommendations to the NIH about clinical risk management for this vulnerable population.
6.1. External advisory board
The External Advisory Board provides scientific guidance and oversight throughout the study course. This involves review and recommendations about the study protocols before initiation and assessment of progress on a yearly basis and suggestions of course corrections if needed. Board members consult on a variety of issues on an as-needed basis and communicate community interests. The External Advisory Board is composed of national experts in relevant fields and scientific areas critical to the ABCD Study design and implementation (e.g., child and adolescent development, mental health, education research, community outreach, cognitive development, neuroimaging, informatics, data sharing). These experts are unaffiliated with the ABCD Study, and provide unbiased scientific and administrative review of the research, including modifications to the scope of the research program, progress, recruitment and retention, representation of community and scientific views. The External Advisory Board makes recommendations to the Coordinating Center, Data Analysis and Informatics Center, research project sites and the NIH regarding scientific program progress and related activities (see Fig. 3). More information regarding the role of the External Advisory Board are detailed in a following chapter (Charness et al., this issue).
Fig. 3.
ABCD governance structure. The ABCD oversight groups (red) advise the Consortium. The External Advisory Board makes recommendations to the Coordinating Center, Data Analysis and Informatics Center, research project sites, and the federal collaborators regarding scientific program progress and related activities. The Observational Study Monitoring Board provides broad oversight and advice to the NIH administering institute regarding issues of bioethics and safety policies and regulations. The Operations Group integrates information flowing from the consortium workgroups, committees, and advisory groups; develops proposals and reports; and presents these to the Council of Investigators for their information and input. Except when consortium consensus is very high, all proposals and protocols are submitted to the Steering Committee for review and approval. The Operations Group is also responsible to oversee implementation of decisions (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
6.2. Observational study monitoring board
The Observational Study Monitoring Board is an independent advisory board comprised of domain, technical, and ethics experts in areas relevant to a large longitudinal study of youth (e.g., adolescent studies, bioethics, regulations, and legal issues related to consent and confidentiality in studies of adolescents), and reports to the NIDA Director as the organization that administers the ABCD awards (see Fig. 3). The Observational Study Monitoring Board is responsible for: (1) providing broad oversight of bioethics policy with full consideration of national and international level policy and regulations, and (2) advising on issues relevant to bioethics and the safety of the ABCD cohort. The group regularly monitors the data from the observational study, reviews and assesses the performance of its operations to make recommendations to NIH staff with respect to site performance, study progress and participant safety and burden.
The responsibilities of the Observational Study Monitoring Board complement those of the IRBs and the Bioethics and Medical Oversight group. The Observational Study Monitoring Board provides external and unbiased review of ABCD policies regarding protection and medical oversight of the cohort developed by the ABCD Bioethics and Medical Oversight advisory group. The Coordinating Center describes their implementation, and summarizes incident findings on an annual basis, and any follow-up by consortium investigators. Finally, the Observational Study Monitoring Board annually reviews national regulatory and policy changes pertinent to human observational studies with particular attention to youth and brings new policy issues and regulatory requirements relevant to ABCD to the attention of the External Advisory Board, senior leadership of ABCD, and the federal partners.
7. Decision-making processes
The thematic approach of ABCD is to have a variety of different kinds of cross-institution workgroups, advisory groups, and standing committees coordinated by the Coordinating Center and the Data Analysis and Informatics Center (see Fig. 2, Fig. 3). Following the Matrix Management model, leadership from all sites are represented in these various areas. Thus, virtually everyone in the consortium has an opportunity to provide input to the protocol, policies, and communications for the study. Information from these various groups is integrated and discussed by a centralized body, the Operations Group (consisting of the Coordinating Center, Data Analysis and Informatics Center, and NIH leadership) that meets weekly and serves as the implementation team. The Operations Group attempts to combine and reconcile input from workgroups and other sources within the consortium to develop proposed responses to issues that arise. Topics discussed by the Operations Group, including proposals developed there, are presented on a biweekly basis to the Council of Investigators, which comprises all of the investigators across the Consortium (see Fig. 4). At each phase of the decision-making process, the lines of communication are bidirectional. Program Directors and Associate Directors serve as liaisons between the different bodies, to convey information with both a top-down and bottom-up approach. During Council of Investigator meetings, investigators have an opportunity to give feedback regarding the recommendations put forth by various ABCD workgroups. If there are persistent disagreements or concerns about recommendations from workgroups or elsewhere in the consortium, they are discussed by the Operations Group and referred to the ABCD Steering Committee for a vote (see Fig. 3). The Steering Committee consists of representatives from the ABCD Coordinating and Data Analysis and Informatics Centers, NIH staff, rotating membership of site principal investigators whereby every site has representation over the course of the study, and non-voting representation by the chair of External Advisory Board. Unless consortium consensus is very high, any alteration to the core protocol of ABCD requires ratification by the Steering Committee, and all protocol changes are reviewed with External Advisory Board and Observational Study Monitoring Board as relevant.
8. Conclusion
Given the size and scope of the ABCD Study, organized governance and communication workflows are vital for the success of the project. The Matrix Management organizational framework was used to establish cross-institutional workgroups, advisory groups and committees within ABCD to accomplish this goal. The structure provides many reciprocal communication streams that allow all members and their diverse perspectives to be part of the decision-making and implementation process. Annual assessments of investigators, workgroups, advisory boards as well as review of participant feedback and the overall success of the consortium will be evaluated and recommendations incorporated to ensure that this organizational framework meets its intended objectives, ensuring the long-term success of the project.
Conflicts of interest
These authors report no conflict of interest.
Acknowledgements
This work was supported by “ABCD-USA Consortium: Coordinating Center” (1U24 DA041147). National Institute on Drug Abuse (PIs: Jernigan/Brown). We would like to thank all participating faculty, ABCD and NIH staff, schools, and participating families, who have made this project possible.
References
- Bartsch H., Thompson W.K., Jernigan T.L., Dale A.M. A web-portal for interactive data exploration, visualization, and hypothesis testing. Front. Neuroinform. 2014;8:25. doi: 10.3389/fninf.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio S.P., McCrea M., McAllister T., Harezlak J., Katz B., Hack D., Hainline B., CARE Consortium Investigators A national study on the effects of concussion in collegiate athletes and US military service academy members: the NCAA–DoD concussion assessment, research and education (CARE) consortium structure and methods. Sports Med. 2017;47:1437–1451. doi: 10.1007/s40279-017-0707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.A., Brumback T., Tomlinson K., Cummins K., Thompson W.K., Nagel B.J., De Bellis M.D., Hooper S.R., Clark D.B., Chung T., Hasler B.P., Colrain I.M., Baker F.C., Prouty D., Pfefferbaum A., Sullivan E.V., Pohl K.M., Rohlfing T., Nichols B.N., Chu W., Tapert S.F. The national consortium on alcohol and NeuroDevelopment in adolescence (NCANDA): a multisite study of adolescent development and substance use. J. Stud. Alcohol. 2015;76:895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charness The adolescent brain cognitive development study external advisory board. Dev. Cogn. Neurosci. 2018 doi: 10.1016/j.dcn.2017.12.007. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.B., Fisher C.B., Bookheimer S., Brown S.A., Evans J.H., Hopfer C., Hudziak J., Montoya I., Murray M., Pfefferbaum A., Yurgelun-Todd D. Biomedical ethics and clinical oversight in multisite observational neuroimaging studies with children and adolescents: the ABCD experience. Dev. Cogn. Neurosci. 2017 doi: 10.1016/j.dcn.2017.06.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E.A., Howlett K.D., Breslin F., Dowling G.J. Outreach and innovation: communication strategies for the ABCD study. Dev. Cogn. Neurosci. 2018 doi: 10.1016/j.dcn.2018.04.001. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T.L., Brown T.T., Hagler D.J., Jr., Akshoomoff N., Bartsch H., Newman E., Thompson W.K., Bloss C.S., Murray S.S., Schork N., Kennedy D.N., Kuperman J.M., McCabe C., Chung Y., Libiger O., Maddox M., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Sowell E.R., Kenet T., Kaufmann W.E., Mostofsky S., Amaral D.G., Dale A.M. The pediatric imaging, neurocognition, and genetics (PING) data repository. NeuroImage. 2016;124(Pt. B):1149–1154. doi: 10.1016/j.neuroimage.2015.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall M.W., Jr. Center for Creative Leadership; Greensboro, NC: 1981. Leadership and the Professional. Technical Report No. 17. [Google Scholar]
- National Academy of Sciences . Facilitating Interdisciplinary Research. The National Academies Press; Washington, DC: 2005. National academy of engineering, and institute of medicine. [Google Scholar]
- Stuckenbruck L.C. The matrix organization. Proj. Manag. Q. 1979;10:21–33. [Google Scholar]
- Tai B., Straus M.M., Liu D., Sparenborg S., Jackson R., McCarty D. The first decade of the national drug abuse treatment clinical trials network: bridging the gap between research and practice to improve drug abuse treatment. J. Subst. Abuse Treat. 2010;38:S4–S13. doi: 10.1016/j.jsat.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]