Abstract
Aims
Takotsubo syndrome (TTS) is characterized by acute left ventricular dysfunction often triggered by emotional or physical stress. Severe activation of the sympathetic nervous system with catecholamine release caused by a dysfunctional limbic system has been proposed as a potential mechanism. We hypothesize that brain regions responsible for autonomic integration and/or limbic processing might be involved in the development of TTS. Here, we investigated alterations in resting state functional connectivity in TTS patients compared with healthy controls.
Methods and results
Using brain functional magnetic resonance imaging (fMRI), resting state functional connectivity has been assessed in 15 subjects with TTS and 39 healthy controls. Network-based statistical analyses were conducted to identify subnetworks with altered resting state functional connectivity. Sympathetic and parasympathetic networks have been constructed in addition to the default mode network and whole-brain network. We found parasympathetic- and sympathetic-associated subnetworks both showing reduced resting state functional connectivity in TTS patients compared with controls. Important brain regions constituting parasympathetic- and sympathetic-associated subnetworks included the amygdala, hippocampus, and insula as well as cingulate, parietal, temporal, and cerebellar regions. Additionally, the default mode network as well as limbic regions in the whole-brain analysis demonstrated reduced resting state functional connectivity in TTS, including the hippocampus, parahippocampal, and medial prefrontal regions.
Conclusion
For the first time, we demonstrate hypoconnectivity of central brain regions associated with autonomic functions and regulation of the limbic system in patients with TTS. These findings suggest that autonomic-limbic integration might play an important role in the pathophysiology and contribute to the understanding of TTS.
Keywords: Brain–heart connection, Takotsubo syndrome, Autonomic-limbic integration, Parasympathetic, Sympathetic, Resting state fMRI
If I were to start over today, I would choose a career in neuroscience, because the next 40 or 50 years are going to be all about the nervous system, just as the last half century has been about the circulation.
Eugene Braunwald, MD, MACC (Escaping Death and Prolonging Lives [Part 2], Circulation Research)
Introduction
The connection between the brain and the heart has been acknowledged since the infancy of medicine. Already in 1846, Burrow emphasized the neurocardiac connection in an article published in the British Foreign and Medical Review. While there is strong evidence for the involvement of the brain–heart axis in Takotsubo syndrome (TTS),1–4 the pathomechanism is still poorly understood.5–8 An overstimulation of the sympathetic nervous system due to stressful stimuli is hypothesized as the underlying cause of TTS, however its impact in TTS has not yet been clearly elucidated.9–12 There is compelling evidence that structures associated with the limbic system, particularly the hypothalamus and amygdala, are also credited as mediating the stress response.13,14 Recently, we have demonstrated that structural anatomical brain differences exist between TTS patients and healthy controls.4 These include the limbic network which comprises the insula, amygdala, cingulate cortex, and hippocampus, all which might contribute to the emotional processing and the autonomic nervous system.4 The present study aimed to identify resting state functional connectivity patterns in TTS patients at the whole-brain level and in particular subnetworks constituting brain regions of the central autonomic network and the default mode network.
Methods
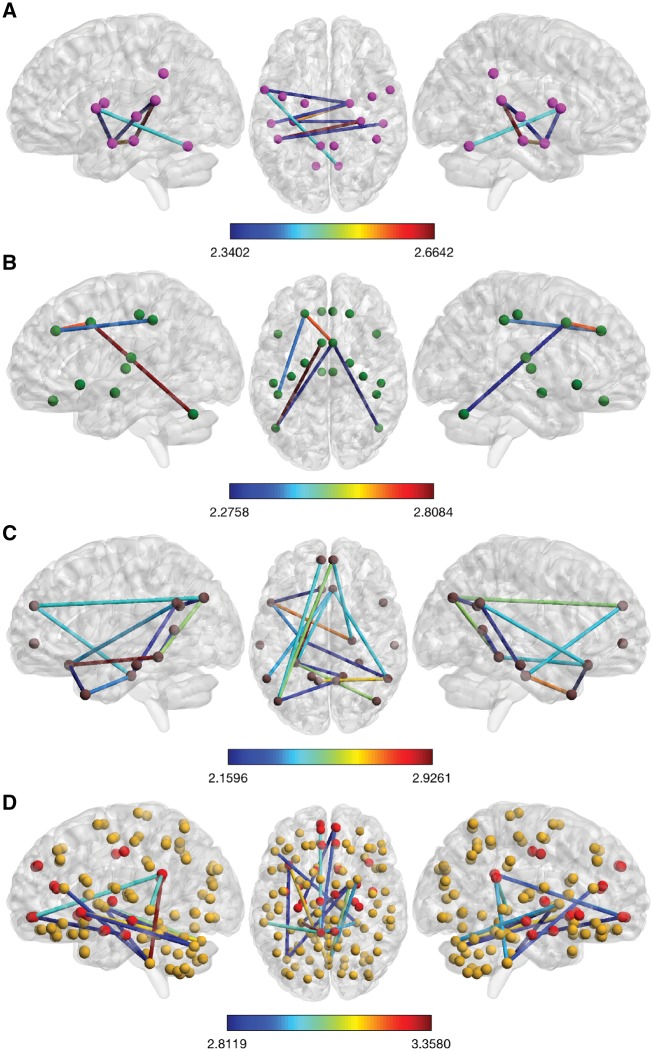
Fifty-four subjects were included in the present analysis. TTS patients were enrolled from the InterTAK Registry, established at the University Hospital Zurich.15 TTS patient inclusion criteria have already been published elsewhere.2 Fifteen TTS patients and 39 age- and gender-matched healthy controls from the IHAB database16 were included. Both groups did not differ regarding age, handedness, Mini-Mental State Examination or Hospital Anxiety and Depression Scale score (all P > 0.05; see Supplementary material online, Table S1). Data were acquired between July 2013 and July 2014 and the median time between the TTS event and the acquisition of the MRI scans was 378 days (inter-quartile range: 116–564 days). Further details about the study participants can be found in the Supplementary material online. Approval of local ethics committee (‘Kantonale Ethikkommission Zürich’) was obtained and the study was conducted in accordance with the declaration of Helsinki. For statistical analyses of the pre-processed MRI data, we defined four different sets of brain regions (network nodes) relevant to the hypotheses stated, that is, a sympathetic network, parasympathetic network, default mode network, and a whole-brain analysis (i.e. nodes covering all brain regions), among which network-based statistics identified subnetworks of altered resting state functional connectivity between groups. A detailed description of the network construction, computation, and the statistical analysis approach of resting state functional connectivity data are described in the Supplementary material online, and elsewhere.17 In brief, resting state functional connectivity, that is, the extent to which two brain regions are simultaneously activated and therefore communicating with each other, of the four differently constructed networks of interest were compared between the TTS patients and healthy controls. Each of the four networks of interest were analysed at two different thresholds. Figure 1A–D represents the subnetworks established at the more conservative threshold, whereas the more liberally thresholded subnetworks are presented in the Supplementary material online. These subnetworks depict connections between brain regions (nodes) in which the two groups differ in resting state functional connectivity.
Figure 1.
Takotsubo syndrome-related hypoconnectivity among central nervous brain structures controlling para- and sympathetic functions as well as regions associated with the default mode network and limbic system. Shown are the more conservatively thresholded subnetworks with reduced resting state functional connectivity in Takotsubo syndrome patients compared with healthy control women. Colour bars represent the set t-value of the connections between which the two groups differ in connectivity strength. The more liberally thresholded subnetworks and detailed information of the nodes and connections involved are shown in the Supplementary materials online. (A) At the higher set threshold (t = 2.30), the Takotsubo syndrome-related parasympathetic-associated hypoconnected subnetwork is composed of seven edges distributed over eight nodes (P = 0.003, FWE-corrected, 5000 permutations). A detailed description of the nodes constituting the subnetworks can be found in Supplementary material online, Table S3. Among these nodes are the right amygdala, left and right hippocampus, left and right middle and superior temporal gyrus, left primary motor cortex and the left supramarginal/angular gyrus, and the left cerebellum. (B) At the higher set threshold (t = 2.25), the Takotsubo syndrome-related sympathetic-associated hypoconnected subnetwork is composed of five edges distributed over six nodes (P = 0.044, FWE-corrected, 5000 permutations). A detailed description of the nodes constituting the subnetworks can be found in Supplementary material online, Table S4. Among these nodes are the left and right amygdala, left and right middle cingulate gyrus, left dorsolateral prefrontal cortex, left anterior insular cortex, left and right cerebellum, and left and right supramarginal gyrus and superior parietal lobule. (C) At the higher set threshold (t = 2.15), the Takotsubo syndrome-related hypoconnected default mode network is composed of 15 edges distributed over 15 nodes (P = 0.033, FWE-corrected, 5000 permutations). A detailed description of the nodes constituting the subnetworks is depicted in Supplementary material online, Table S5. Among these nodes are the left and right hippocampus, left parahippocampal gyrus, left and right dorsal and ventral medial prefrontal cortex, left posterior cingulate cortex, left temporal pole, left and right inferior parietal lobule, and the left and right temporoparietal junction. (D) At the higher set threshold (t = 2.80), the Takotsubo syndrome-related hypoconnected subnetwork is composed of 13 edges distributed over 13 nodes (P = 0.023, FWE-corrected, 5000 permutations). A detailed description of the nodes constituting the subnetworks is depicted in Supplementary material online, Table S6. Among these nodes are the left anterior insular cortex, left posterior cingulate cortex, left and right medial orbitofrontal cortex, left middle temporal gyrus, right pallidum, and the cerebellum.
Results
In general, we found a decreased connectivity strength (hypoconnectivity) in all analysed networks of interest, that is, the parasympathetic- and sympathetic-associated networks as well as in the default mode network and on the whole-brain level. Reduced functional connectivity reflects a decreased or altered communication between brain regions constituting a subnetwork. The specific brain regions with decreased functional connectivity in TTS patients are detailed below.
Demographic and behavioural measures
Demographic and behavioural measures and stressors, risk factors, and TTS-related symptoms can be found in Supplementary material online, Tables S1 and S2, respectively. Statistical analyses revealed no significant differences between TTS patients and controls (see Supplementary material online, Table S1).
Parasympathetic-associated subnetwork
At the higher set threshold (t = 2.30), the TTS-related parasympathetic-associated hypoconnected subnetwork was composed of seven edges distributed over eight nodes (P = 0.003, family wise error (FWE)-corrected, 5000 permutations). The nodes involved were the right amygdala, left and right hippocampus, left and right middle and superior temporal gyrus, left primary motor cortex, left supramarginal/angular gyrus, and the left cerebellum (Figure 1A and Supplementary material online, Figure S1 and Table S3).
Sympathetic-associated subnetwork
At the higher set threshold (t = 2.25), the TTS-related sympathetic-associated hypoconnected subnetwork was composed of five edges distributed over six nodes (P = 0.044, FWE-corrected, 5000 permutations). The nodes involved were the left and right middle cingulate gyrus, left dorsolateral prefrontal cortex, left and right cerebellum, and the left superior parietal lobule/supramarginal gyrus (Figure 1B and Supplementary material online, Figure S2 and Table S4).
Default mode network
At the higher set threshold (t = 2.15), the TTS-related hypoconnected default mode network was composed of 15 edges distributed over 15 nodes (P = 0.033, FWE-corrected, 5000 permutations) including the left and right hippocampus, left parahippocampal gyrus, left and right dorsal and ventral medial prefrontal cortex, left and right posterior cingulate cortex, left temporal pole, left and right posterior inferior parietal lobule, and the left and right temporoparietal junction (Figure 1C and Supplementary material online, Figure S3 and Table S5).
Whole-brain network
We also conducted a whole-brain network analysis using a network composed of 125 nodes. The differentially thresholded subnetworks with reduced resting state functional connectivity in TTS patients compared with healthy control women are presented in Figure 1D and Supplementary material online, Figure S4 and Table S6. At the higher set threshold (t = 2.80), the TTS-related hypoconnected subnetwork was composed of 13 edges distributed over 13 nodes (P = 0.023, FWE-corrected, 5000 permutations), including the left anterior insular cortex, left posterior cingulate cortex, left and right medial orbitofrontal cortex, left middle temporal gyrus, right pallidum, and the cerebellum (Figure 1D).
Discussion
The present study demonstrates that patients with TTS have altered functional connectivity patterns during resting state in comparison to healthy age- and gender-matched controls. Interestingly, we found that brain regions of both the central autonomic network and the default mode network are less functionally connected in patients with TTS compared with controls even months to years after their TTS episode. Our network analysis regarding the central autonomic network, which exerts control over both parts of the autonomic nervous system, revealed a decreased resting state functional connectivity in the parasympathetic as well as the sympathetic subnetworks compared with controls. The parasympathetic hypoconnected subnetwork consisted of the right amygdala, left and right hippocampus, left and right middle and superior temporal gyrus, left primary motor cortex, left supramarginal/angular gyrus, and the left cerebellum. The sympathetic hypoconnected subnetwork included the left and right amygdala, left and right middle cingulate gyrus, left dorsolateral prefrontal cortex, left and right cerebellum, and the left superior parietal lobule/supramarginal gyrus. In addition to these parasympathetic and sympathetic hypoconnected subnetworks, resting state functional connectivity was also reduced in TTS patients within the default mode network. This subnetwork involved the left and right hippocampus, left parahippocampal gyrus, bilateral dorsal and ventral medial prefrontal cortex, left and right posterior cingulate cortex, left temporal pole, bilateral posterior inferior parietal lobule, and the left and right temporoparietal juction. The whole-brain network analysis confirmed the specificity of limbic/autonomic nervous system-related central brain structures showing reduced resting state functional connectivity and hence being important in TTS.
Activation of the sympathetic nervous system is well known to be involved in cardiovascular disease.18 The amygdala is one of the key brain structures that exerts control over the response of the sympathetic nervous system to stress. The study by Tawakol et al. found that an increase in amygdala activity was associated with an increased risk of cardiovascular disease.19 Thus, the amygdala is a major brain structure mediating the interactions between stress and cardiovascular diseases. Neurological disorders such as seizures, intracranial bleeding, and migraines are twice as common in TTS as they are in acute coronary syndromes.2 Additionally, acute episodes of TTS are often triggered by emotional or physical stressors.7,20 Taken together, these findings support the concept that the autonomic nervous system is involved in the pathophysiology of TTS.
Key nodes identified in this study, like the amygdala, hippocampus, and cingulate gyrus, are structures of the limbic system that control emotions, motivation, learning, and memory. The amygdala and cingulate gyrus are also involved in the central control of the autonomic nervous system and the regulation of cardiac function.21 Additionally, the cingulate gyrus is an important structure in depression and other mood disorders that are common among TTS patients.22,23 Interestingly, we observed decreased connectivity pattern in TTS patients in similar brain regions where we previously identified also structural alterations.4,17
Of note, we also demonstrated reduced resting state functional connectivity among nodes constituting the default mode network (Supplementary material online, Table S5). Each component of the default mode network is associated with different psychological functions. The decreased resting state functional connectivity in the default mode network suggests that TTS patients might have a weakened ability to make certain self-relevant decisions. In the presence of a TTS triggering event, the impaired default mode network subsystems may lead to a more pessimistic evaluation of the present and future self. This in turn could cause elevated levels of stress that adversely affect the limbic network and central brain structures that influence the autonomic nervous system.
We acknowledge the following limitations: It remains unknown whether resting state functional connectivity changes observed in TTS patients were present before the onset of the disease or secondary to the acute event; therefore, cause or effect cannot be determined, but a clear association exists. Second, only females TTS patients were included in the present study. However, only a minority of TTS patients are male (10%) and recruitment of a significant number of male patients is quite challenging.
Conclusions
In conclusion, the present study establishes subnetworks of resting state functional connectivity alterations in TTS patients compared with healthy controls. Most of the structures included in these subnetworks are important in emotional processing and autonomic regulation. While there are likely many causes of TTS given the heterogenic nature of the syndrome, the present findings suggest that alterations in the function of certain parts of the central nervous system may result in the onset of a TTS event in response to a stressful trigger.
The underlying pathophysiology of TTS is probably not only restricted to the cardiovascular system alone but also due to the interaction between the brain and the heart. Therefore, our findings might represent a neurological substrate involved in TTS and thereby reinforcing the current concept of the involvement of the brain–heart interaction.
Supplementary Material
Acknowledgements
We thank all TTS patients and healthy controls for their study participation. We also thank H. Baetschmann for providing MATLAB scripts supporting the network-based statistic (NBS) tool.
Funding
This work was supported by ‘Filling the gap’ from the University of Zurich to J.-R.G. and by the University Research Priority Program ‘Dynamics of Healthy Aging’ of the University of Zurich to L.J. C.T. and his research has been supported by a donation of His Highness Sheikh Khalifa bin Hamad bin Abdullah bin Jassim bin Mohammed al Thani to the Foundation of Cardiovascular Research Zurich, Switzerland.
Conflict of interest: The authors have nothing to disclose with the present work. C.T. received research grants from Abbott Vascular and Biosenors not related to the present work. T.F.L. reports research/educational grants from Abbott/St. Jude, AstraZeneca, Bayer Health Care, Biotronik, Boehringer Ingelheim, Daichi-Sankyo, Menarini, Novartis and Servier.
References
- 1. Samuels MA. The brain-heart connection. Circulation 2007;116:77–84. [DOI] [PubMed] [Google Scholar]
- 2. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss H-P, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck K-H, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF.. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 3. Ghadri JR, Sarcon A, Diekmann J, Bataiosu DR, Cammann VL, Jurisic S, Napp LC, Jaguszewski M, Scherff F, Brugger P, Jäncke L, Seifert B, Bax JJ, Ruschitzka F, Luscher TF, Templin C, InterTAK Co-investigators. Happy heart syndrome: role of positive emotional stress in takotsubo syndrome. Eur Heart J 2016;37:2823–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hiestand T, Hänggi J, Klein C, Topka MS, Jaguszewski M, Ghadri JR, Luscher TF, Jäncke L, Templin C.. Takotsubo syndrome associated with structural brain alterations of the limbic system. J Am Coll Cardiol 2018;71:809–811. [DOI] [PubMed] [Google Scholar]
- 5. Templin C, Napp LC, Ghadri JR.. Takotsubo syndrome: underdiagnosed, underestimated, but understood? J Am Coll Cardiol 2016;67:1937–1940. [DOI] [PubMed] [Google Scholar]
- 6. Ghadri JR, Ruschitzka F, Luscher TF, Templin C.. Takotsubo cardiomyopathy: still much more to learn. Heart 2014;100:1804–1812. [DOI] [PubMed] [Google Scholar]
- 7. Kato K, Lyon AR, Ghadri JR, Templin C.. Takotsubo syndrome: aetiology, presentation and treatment. Heart 2017;103:1461–1469. [DOI] [PubMed] [Google Scholar]
- 8. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, S YH, Migliore F, Horowitz JD, Shimokawa H, Luscher TF, Templin C.. International expert consensus document on Takotsubo syndrome (part i): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC.. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–548. [DOI] [PubMed] [Google Scholar]
- 10. Paur H, Wright PT, Sikkel MB, Tranter MH, Mansfield C, O'Gara P, Stuckey DJ, Nikolaev VO, Diakonov I, Pannell L, Gong H, Sun H, Peters NS, Petrou M, Zheng Z, Gorelik J, Lyon AR, Harding SE.. High levels of circulating epinephrine trigger apical cardiodepression in a beta2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation 2012;126:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borchert T, Hubscher D, Guessoum CI, Lam TD, Ghadri JR, Schellinger IN, Tiburcy M, Liaw NY, Li Y, Haas J, Sossalla S, Huber MA, Cyganek L, Jacobshagen C, Dressel R, Raaz U, Nikolaev VO, Guan K, Thiele H, Meder B, Wollnik B, Zimmermann WH, Luscher TF, Hasenfuss G, Templin C, Streckfuss BK.. Catecholamine-dependent beta-adrenergic signaling in a pluripotent stem cell model of Takotsubo cardiomyopathy. J Am Coll Cardiol 2017;70:975–991. [DOI] [PubMed] [Google Scholar]
- 12. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, S YH, Migliore F, Horowitz JD, Shimokawa H, Luscher TF, Templin C.. International expert consensus document on Takotsubo syndrome (part ii): diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith SM, Vale WW.. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 2006;8:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Bockstaele EJ, Colago EE, Valentino RJ.. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol 2006;10:743–757. [DOI] [PubMed] [Google Scholar]
- 15. Ghadri JR, Cammann VL, Templin C.. The international Takotsubo registry: rationale, design, objectives, and first results. Heart Failure Clinics 2016;12:597–603. [DOI] [PubMed] [Google Scholar]
- 16. Zollig J, Merillat S, Eschen A, Rocke C, Martin M, Jäncke L.. Plasticity and imaging research in healthy aging: core ideas and profile of the International Normal Aging and Plasticity Imaging Center (INAPIC). Gerontology 2011;57:190–192. [DOI] [PubMed] [Google Scholar]
- 17. Klein C, Hiestand T, Ghadri JR, Templin C, Jäncke L, Hänggi J.. Takotsubo syndrome – predictable from brain imaging data. Sci Rep 2017;7:5434.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 2010;90:513–557. [DOI] [PubMed] [Google Scholar]
- 19. Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK.. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlossbauer SA, Ghadri JR, Templin C.. Praxis (Bern 1994) 2016;105:1185–1192. [DOI] [PubMed] [Google Scholar]
- 21. Luu P, Posner MI.. Anterior cingulate cortex regulation of sympathetic activity. Brain 2003;126:2119–2120. [DOI] [PubMed] [Google Scholar]
- 22. Hadland KA, Rushworth MF, Gaffan D, Passingham RE.. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia 2003;41:919–931. [DOI] [PubMed] [Google Scholar]
- 23. Drevets WC, Savitz J, Trimble M.. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 2008;13:663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.