Abstract
Aims
Spontaneous coronary artery dissection (SCAD) was underdiagnosed and poorly understood for decades. It is increasingly recognized as an important cause of myocardial infarction (MI) in women. We aimed to assess the natural history of SCAD, which has not been adequately explored.
Methods and results
We performed a multicentre, prospective, observational study of patients with non-atherosclerotic SCAD presenting acutely from 22 centres in North America. Institutional ethics approval and patient consents were obtained. We recorded baseline demographics, in-hospital characteristics, precipitating/predisposing conditions, angiographic features (assessed by core laboratory), in-hospital major adverse events (MAE), and 30-day major adverse cardiovascular events (MACE). We prospectively enrolled 750 SCAD patients from June 2014 to June 2018. Mean age was 51.8 ± 10.2 years, 88.5% were women (55.0% postmenopausal), 87.7% were Caucasian, and 33.9% had no cardiac risk factors. Emotional stress was reported in 50.3%, and physical stress in 28.9% (9.8% lifting >50 pounds). Predisposing conditions included fibromuscular dysplasia 31.1% (45.2% had no/incomplete screening), systemic inflammatory diseases 4.7%, peripartum 4.5%, and connective tissue disorders 3.6%. Most were treated conservatively (84.3%), but 14.1% underwent percutaneous coronary intervention and 0.7% coronary artery bypass surgery. In-hospital composite MAE was 8.8%; peripartum SCAD patients had higher in-hospital MAE (20.6% vs. 8.2%, P = 0.023). Overall 30-day MACE was 8.8%. Peripartum SCAD and connective tissue disease were independent predictors of 30-day MACE.
Conclusion
Spontaneous coronary artery dissection predominantly affects women and presents with MI. Despite majority of patients being treated conservatively, survival was good. However, significant cardiovascular complications occurred within 30 days. Long-term follow-up and further investigations on management are warranted.
Keywords: Spontaneous coronary artery dissection (SCAD), Myocardial infarction (MI), Women, Fibromuscular dysplasia (FMD), Peripartum, Percutaneous coronary intervention (PCI)
See page 1198 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz048)
Introduction
Spontaneous coronary artery dissection (SCAD) is an underdiagnosed and poorly understood condition that frequently affects young women without cardiovascular (CV) risk factors and can cause myocardial infarction (MI), cardiac arrest, or death.1 Spontaneous coronary artery dissection is defined as a spontaneous, non-traumatic, non-iatrogenic, and non-atherosclerotic separation of the coronary arterial wall by intramural haemorrhage, which can be elicited by intimal tear or spontaneous haemorrhage.1 This creates a false lumen with intramural haematoma (IMH) that compresses the true lumen causing myocardial ischaemia or infarction. Spontaneous coronary artery dissection typically occurs from an underlying predisposing arteriopathy that weakens the wall, with or without precipitating stressors.1,2
Spontaneous coronary artery dissection was previously reported as rare, however, it had been underdiagnosed and the true prevalence is unknown. In a large 2009–14 National Inpatient Sample analysis, ∼1% of ∼750 000 women with MI who underwent coronary angiography were reported to have SCAD.3 However, in contemporary studies with improved SCAD diagnosis, SCAD was reported to cause 24–35% of MI in women age <60 years.4 Among men and women presenting with acute coronary syndrome (ACS) undergoing coronary angiography, SCAD was reported in 1.0–4.0%.2,3 Since angiography does not image arterial walls, intracoronary imaging [optical coherence tomography (OCT) or intravascular ultrasound (IVUS)] may be necessary to confirm SCAD in ambiguous cases.5 New angiographic SCAD classification6 has aided SCAD diagnosis on angiography, which remains the current gold-standard for diagnosis.
Despite recent improvements in diagnosis and recognition of the importance of SCAD, it remains poorly studied and understood. Spontaneous coronary artery dissection publications are mostly limited to case reports and non-prospective series, with absence of randomized trial data. Both the American Heart Association and European Society of Cardiology, SCAD working groups recently published scientific statements summarizing the evidence to date and recommendations for management and screening.2,7 However, there are diverse aetiologies, stressors, and management strategies, with as yet unclear estimates of recurrence and prognosis. Therefore, we designed the Canadian SCAD cohort study, a large, observational, prospective, cohort study, to describe the natural history of SCAD and to provide the justification and foundation for future randomized clinical trials. Here, we report the in-hospital and 30-day outcomes.
Methods
This is a multicentre, prospective, observational study of consecutive patients with non-atherosclerotic SCAD from 20 centres across Canada and two centres in the United States (Supplementary material online, Appendix S1). The study was registered at ClinicalTrials.gov (NCT02188069) and approved by the local research ethics boards of each participating centre. All patients provided written informed consent for participation. We included patients with new SCAD presentation with ACS, and documented SCAD on coronary angiogram confirmed by core laboratory. We excluded patients with atherosclerotic disease in other coronary arterial segments with diameter stenosis ≥50%. The study was managed by the University of British Columbia Cardiology Research group.
Angiographic spontaneous coronary artery dissection diagnosis
All coronary angiograms were reviewed by the independent Cardiovascular Imaging Research core laboratory and classified according to the Saw angiographic SCAD classification.6 In brief, Type 1 SCAD (evident wall stain) depicts classic contrast dye staining of arterial wall with multiple radiolucent lumen, with or without dye hang-up or slow contrast clearing from the lumen. Type 2 SCAD (diffuse stenosis) depicts diffuse (>20 mm) and smooth narrowing that can vary in severity; Type 2A describes presence of normal arterial segments proximal and distal to SCAD; Type 2B describes dissection that extends to distal tip of the artery.5 Type 3 SCAD (mimics atherosclerosis) depicts focal or tubular stenosis that appears similar to atherosclerosis, and typically requires OCT/IVUS to prove presence of IMH and/or intimal dissection. Coronary segments were defined by the Bypass Angioplasty Revascularization Investigation classification.8 Other angiographic characteristics, left ventricular ejection fraction (LVEF), and wall motion abnormality were recorded.
Baseline characteristics
Baseline demographics, past medical history, pregnancy history, hormonal therapy, preceding emotional and physical stressors, CV risk factors, and family history were recorded from patient reviews, hospital records, and patient-completed questionnaires. All patients completed detailed questionnaires on potential predisposing and precipitating stressors, gynaecologic history, clinical symptoms, and family history. Emotional stress was defined as major stress at hospital admission, and categorized as ≥3 severity on a 4-point scale (mild, moderate, high, or severe). The Perceived Stress Scale was also administered. Physical stress was defined as new or unusually intense physical activity within a week of hospitalization. Intense isometric activity was defined as lifting >50 pounds. Active and prior hormonal therapy and other potential precipitating stressors (e.g. intense retching, vomiting, straining with bowel movement, use of recreational drugs, active pregnancy, breastfeeding, labour and delivery) were recorded.
Hospital clinical characteristics
Hospital presentation, electrocardiogram (ECG), laboratory results, need for revascularization [percutaneous coronary intervention (PCI) or coronary artery bypass surgery (CABG)], ventricular arrhythmia (sustained ventricular tachycardia or fibrillation), LVEF, cardiogenic shock, need for haemodynamic support, urgent repeat coronary angiography, and urgent repeat revascularization were recorded.
Predisposing conditions
Pregnancy history (gravidity and parity), current pregnancy or peripartum (3rd trimester or within 12 months of delivery)9 state, presence of fibromuscular dysplasia (FMD), inherited connective tissue disorders (CTDs), systemic inflammatory conditions, and coronary artery spasm history were sought. Multiparity was defined as having given birth ≥4 times and grand multiparity ≥5 times with gestational age ≥24 weeks; grand multigravida defined as pregnancy ≥5 times.10 Fibromuscular dysplasia screen was recommended for the renal, iliac, and cerebrovascular arteries with catheter-based or CT angiography at the discretion of site physicians.10,11 Fibromuscular dysplasia diagnosis was defined according to the American Heart Association criteria of multifocal disease (string-of-bead appearance)12 in ≥1 extracoronary vasculature.
Management of spontaneous coronary artery dissection
Treatment of SCAD was at the discretion of treating physicians. Conservative management was typically recommended if there was no ongoing ischaemia, chest pain, haemodynamic instability, ventricular arrhythmias, or left main (LM) dissection. Percutaneous coronary intervention was usually pursued for ongoing ischaemia, and CABG was reserved for patients with LM or extensive proximal multi-vessel SCAD.13 Percutaneous coronary intervention outcomes were defined as (i) successful if angioplasty/stenting of the dissection accomplished final thrombolysis in myocardial infarction (TIMI) 3 flow with no residual dissection; (ii) partially successful if angioplasty/stenting resulted in residual dissection or stenosis ≤50% of lumen diameter, and with final TIMI 3 or improved flow; and (iii) unsuccessful if angioplasty/stenting concluded with residual dissection or stenosis >50% of lumen diameter, or worsened TIMI flow compared to baseline, or extension of dissection requiring bail-out CABG.
Clinical follow-up and outcomes
Patients were followed routinely during their hospital stay, and after discharge by telephone/office contact at 1, 6, 12 months, and annually thereafter for 3 years. Patients were consented into the study preferably during the acute hospitalization, but may be enrolled within 3 months of the SCAD event if there were logistical challenges (e.g. patients transferred back to referral hospitals, angiograms done at non-enrolling sites). The Seattle Angina Questionnaire was administered at baseline 6, 12, 24, and 36 months. Medications administered on discharge and at each follow-up were recorded. In-hospital major adverse events (MAE) included the composite of all-cause mortality, stroke/transient ischaemic attack (TIA), re-infarction,14 cardiogenic shock (requiring medical or mechanical haemodynamic support), congestive heart failure (CHF), cardiac arrest (severe ventricular arrhythmia requiring defibrillation or antiarrhythmic agents), repeat revascularization (or unplanned revascularization), and cardiac transplantation. High-risk presentation was defined as in-hospital death, cardiac arrest, cardiogenic shock, ejection fraction <35%, or LM dissection. Thirty-day major adverse cardiovascular events (MACE) included the composite of all-cause mortality, stroke/TIA, recurrent MI (including recurrent SCAD), CHF, and revascularization. Recurrent SCAD was defined as de novo recurrent spontaneous dissection with new MI symptoms and enzyme elevation, not involving extension of dissection of the original SCAD lesion.15
Statistical analysis
Patient characteristics were summarized with mean ± standard deviation or median and interquartile range for continuous variables, and with counts and proportions for categorical variables. Event rates and 95% confidence intervals (CIs) are reported for each of in-hospital MAE, post-discharge 30-day MACE, and total 30-day MACE. Univariate and multivariable logistical regression analyses were performed to identify clinical predictors for each outcome. Based on clinical input, 18 predictors were evaluated using univariate models and considered for inclusion in multivariable models. Predictors tested included demographic (age), CV risk factors (hypertension, diabetes, dyslipidaemia, smoking, and CTDs), patient histories (depression, anxiety, prior cerebrovascular accident (CVA), number of pregnancies, post-partum, and use of fertility treatment), predisposing arteriopathies (FMD), and precipitating stressors (emotional, physical, isometric, and hormonal stress). Connective tissue disorder was forced into the multivariable model of in-hospital MAE as it was deemed to be clinically important. Other variables were selected using a forward selection method with a significance level of P-value <0.20. Odds ratios (ORs) with corresponding 95% CIs were reported. The multivariable models for both in-hospital MAE and 30-day MACE are based on the female population only, which comprised 88.5% of the overall SCAD cohort. A two-sided P-value <0.05 was considered to indicate statistical significance. Statistical analyses were performed with SAS version 9.4 (Cary, NC, USA).
Results
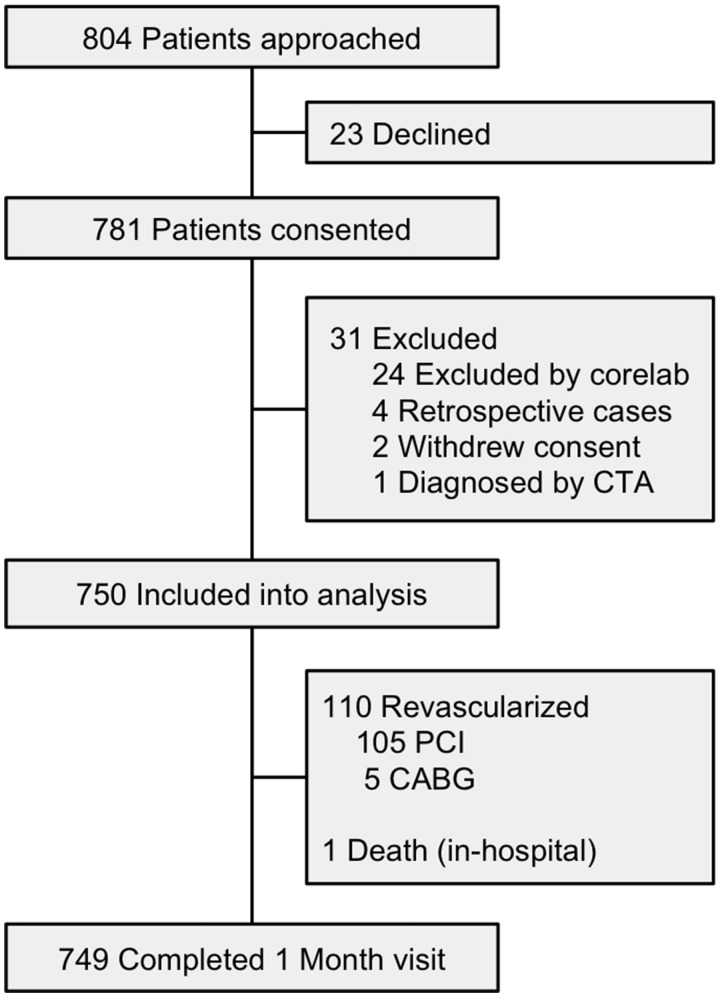
We prospectively enrolled 750 patients from June 2014 to June 2018 from 22 centres presenting with acute SCAD. The study enrolment flowchart is depicted in Figure 1, with complete follow-up at 1 month. Baseline characteristics are summarized in Table 1. Mean age was 51.8 ± 10.2 years (range 24–89 years) (Figure 2), and majority were women (88.5%) and Caucasian (87.7%). At baseline, 33.9% had no CV risk factors, 32.5% had migraines, 19.5% depression, and 19.7% anxiety.
Figure 1.
Canadian spontaneous coronary artery dissection cohort study flow diagram.
Table 1.
Baseline demographics
| Mean ± SD, median (Q1–Q3), or n (%) | N = 750 |
|---|---|
| Age (years) | 51.8 ± 10.2 |
| Sex (female) | 664 (88.5) |
| Weight (kg) | 73.0 (63.0–80.0) |
| Height (cm) | 165 (160–171) |
| Body mass index | 26.4 (23.1–31.2) |
| Race | |
| Caucasian | 658 (87.7) |
| East Asian | 33 (4.4) |
| South Asian | 17 (2.3) |
| African Canadian | 12 (1.6) |
| First nation | 10 (1.3) |
| Other | 20 (2.7) |
| Medical history | |
| Diabetes mellitus | 34 (4.5) |
| Diabetes mellitus on medication | 16 (2.1) |
| Hypertension | 241 (32.1) |
| Dyslipidaemia | 152 (20.3) |
| Current smoker | 87 (11.6) |
| Family history of premature CAD | 285 (38.0) |
| No cardiac risk factors | 254 (33.9) |
| ≥3 cardiac risk factors | 71 (9.5) |
| History of previous revascularization | 13 (1.7) |
| History of previous MI | 63 (8.4) |
| Confirmed cases of previous SCAD | 42 (5.6) |
| History of CVA | 26 (3.5) |
| History of heart failure | 3 (0.4) |
| Relevant clinical history | |
| Tinnitus | 100 (13.3) |
| History of migraines | 244 (32.5) |
| History of depression | 146 (19.5) |
| On medication for depression | 111 (14.8) |
| History of anxiety | 148 (19.7) |
| On medication for anxiety | 88 (11.7) |
| Thyroid dysfunction | 97 (12.9) |
| Hypothyroid | 85 (11.3) |
CAD, coronary artery dissection; CVA, cerebrovascular accident; SD, standard deviation.
Figure 2.
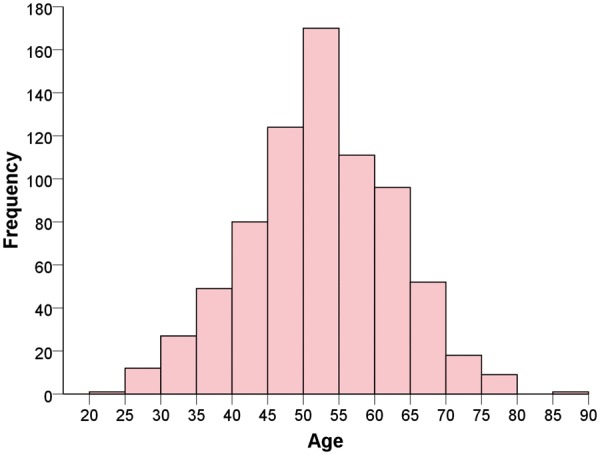
Histogram of age distribution.
Among women with SCAD, 55.0% were post-menopausal (Supplementary material online, Table SA). Only 12.7% had no prior pregnancies; 10.1% had ≥5 prior pregnancies, 9.6% had ≥4 prior births, and 2.6% had ≥5 births. Thirty-four (4.7%) were peripartum (1 in 3rd trimester pregnancy). Nineteen (2.9%) were still breastfeeding during SCAD presentation.
For hospital presentation (Table 2), 29.7% presented with ST-elevation MI, 69.9% non-ST-elevation MI. Ventricular tachycardia/fibrillation occurred in 8.1% (3.9% required cardioversion or defibrillator). Troponin levels were elevated in 97.6%. The main presenting symptom was chest pain (91.5%). Median LVEF was 55% (50–60); 25.6% had LVEF <50% and 3.8% had LVEF <35%. Wall motion abnormality occurred in 82.3%.
Table 2.
Hospital presenting characteristics
| Median (Q1–Q3) or n (%) | N = 750 |
|---|---|
| Acute coronary syndrome | |
| STEMI | 223 (29.7) |
| NSTEMI | 524 (69.9) |
| Unstable angina | 3 (0.4) |
| Presenting main symptom | |
| Chest discomfort | 686 (91.5) |
| Back discomfort | 15 (2.0) |
| Shoulder or arm discomfort | 10 (1.3) |
| Dyspnoea | 7 (0.9) |
| Arrhythmia | 8 (1.1) |
| Other | 24 (3.2) |
| Troponin levels | |
| Elevated troponin | 732 (97.6) |
| Troponin not elevated | 4 (0.5) |
| Troponin value not available | 14 (1.9) |
| ECG changes | |
| Normal ECG | 170 (22.7) |
| Non-specific changes | 81 (10.8) |
| T inversion | 138 (18.4) |
| ST depression | 47 (6.3) |
| ST elevation <1 mm | 85 (11.3) |
| ST elevation >1 mm | 187 (24.9) |
| Q waves | 11 (1.5) |
| LBBB | 5 (0.7) |
| Other | 26 (3.5) |
| Ventricular tachycardia or fibrillation | 61 (8.1) |
| Left ventricular function assessment | |
| Ejection fraction assessed | 737 (98.2) |
| Angiogram | 491 (65.5) |
| Echocardiogram | 243 (32.4) |
| Initial ejection fraction (%) | 55 (50–60) |
| Ejection fraction <50% | 188/734 (25.6) |
| Ejection fraction <35% | 28/734 (3.8) |
| Wall motion abnormality | |
| No abnormality | 114 (15.2) |
| Hypokinesis | 359 (47.9) |
| Akinesis | 215 (28.7) |
| Dyskinesis | 43 (5.7) |
| Not assessed | 19 (2.5) |
ECG, electrocardiogram; LBBB, left bundle branch block; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
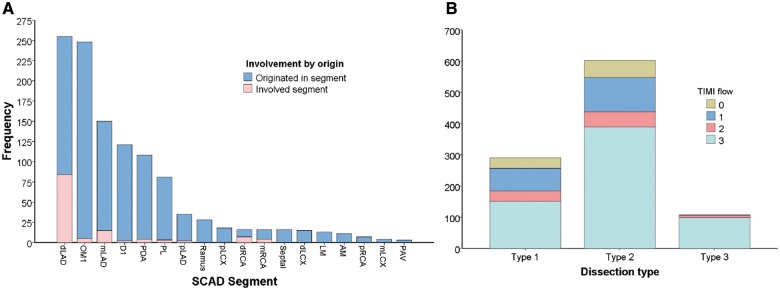
Angiographic characteristics are described in Table 3. Optical coherence tomography was performed in 5.5% and IVUS in 2.1%. Majority of SCAD involved a single coronary artery territory (86.9%). The most common coronary artery dissected was the left anterior descending artery and branches (52.1%) (Figure 3). Among the 1002 dissected arteries, majority had Type 2 angiographic SCAD (60.2%). Type 1 SCAD occurred in 29.0% and Type 3 SCAD in 10.8%. Median angiographic stenosis was 79.0% (65.0–100), and median dissection length was 33.2 mm (22.2–48.9).
Table 3.
Spontaneous coronary artery dissection angiographic characteristics
| N (%), median (Q1–Q3) | N = 750 |
|---|---|
| Radial approach catheterization | 556 (74.1) |
| Femoral approach catheterization | 192 (25.6) |
| OCT-confirmed SCAD | 41 (5.5) |
| IVUS-confirmed SCAD | 16 (2.1) |
| Number of non-contiguous SCAD arteries | |
| 1 | 652 (86.9) |
| 2 | 88 (11.7) |
| 3 | 10 (1.3) |
| Number of affected SCAD segments | |
| 1 | 561 (74.8) |
| 2 | 147 (19.6) |
| 3 | 24 (3.2) |
| 4 | 16 (2.1) |
| 5 | 1 (0.1) |
| 6 | 1 (0.1) |
| ≥2 segments | 189 (25.2) |
| Dissected coronary arteries | |
| LM | 11 (1.5) |
| LAD | 391 (52.1) |
| LCX | 283 (37.7) |
| RCA | 174 (23.2) |
| LM or prox LCX or prox LAD | 57 (7.6) |
| Angiographic SCAD type | N = 1002 dissections |
| 1 | 291 (29.0) |
| 2 | 603 (60.2) |
| 2A | 343 (34.2) |
| 2B | 260 (25.9) |
| 3 | 108 (10.8) |
| Worse TIMI flow | |
| 0 | 89 (8.9) |
| 1 | 185 (18.5) |
| 2 | 89 (8.9) |
| 3 | 639 (63.8) |
| QCA characteristics | |
| Total occlusion (stenosis 100%) | 307 (30.6) |
| Vessel diameter (mm) | 2.4 (2.0–3.0) |
| Segment diameter stenosis (%) | 79.0 (65.0–100) |
| Segment length (mm) | 33.2 (22.2–48.9) |
LAD, left anterior descending; LCX, left circumflex artery; QCA, quantitative coronary analysis; RCA, right coronary artery; TIMI: thrombolysis in myocardial infarction.
Figure 3.
Distribution/frequency of (A) dissected segments and (B) angiographic/TIMI subtypes.
Precipitating stressors and predisposing conditions were frequently observed (Table 4). Overall, 66.4% reported potential precipitating stressors: emotional stressors 50.3% (perceived stress scale ≥20 in 41.2%), physical stressors 28.9%, and heavy isometric activities lifting >50 pounds in 9.8%. Potential predisposing conditions occurred in 49.9%, but 45.2% had no or incomplete screening for FMD (Supplementary material online, Table SB). Fibromuscular dysplasia was most commonly observed, with multifocal changes in 31.1% of our overall cohort [56.7% (233/411) amongst those who had complete FMD screening]. Of the FMD screens performed, 52.4% were CT angiography, 43.6% catheter-based angiography, and 4.0% MR angiography. Cerebral aneurysm was present in 7.1% who underwent cerebrovascular imaging. Other predisposing conditions were less frequent, and 50.1% were deemed idiopathic. Relevant family history included any aneurysm in 13.5%, sudden death 15.1%, any arterial dissection 3.1%, SCAD 2.4%, and FMD 0.8%.
Table 4.
Precipitating stressors and potential predisposing conditions
| N (%) | N = 750 |
|---|---|
| Precipitating stressors | |
| Emotional stress (rated high or severe) | 377 (50.3) |
| Perceived stress scale ≥20 | 288 (41.2) |
| Unusually intense physical stress | 216 (28.9) |
| Isometric stress >50 lb | 74 (9.8) |
| Cocaine/amphetamine use | 2 (0.3) |
| Valsalva-type stress | 90 (12.0) |
| No precipitating factor | 252 (33.6) |
| Predisposing conditions | |
| Fibromuscular dysplasia | 233 (31.1) |
| Systemic inflammatory disease | 35 (4.7) |
| Connective tissue disorder | 27 (3.6) |
| Active hormonal therapy | 75 (10.0) |
| Peripartum | 34 (4.5) |
| Grand multigravida (≥5 pregnancies) | 67 (8.9) |
| Multiparous (≥4 births) | 64 (8.5) |
| Grand multiparity (≥5 births) | 17 (2.3) |
| Idiopathic (none of the above) | 376 (50.1) |
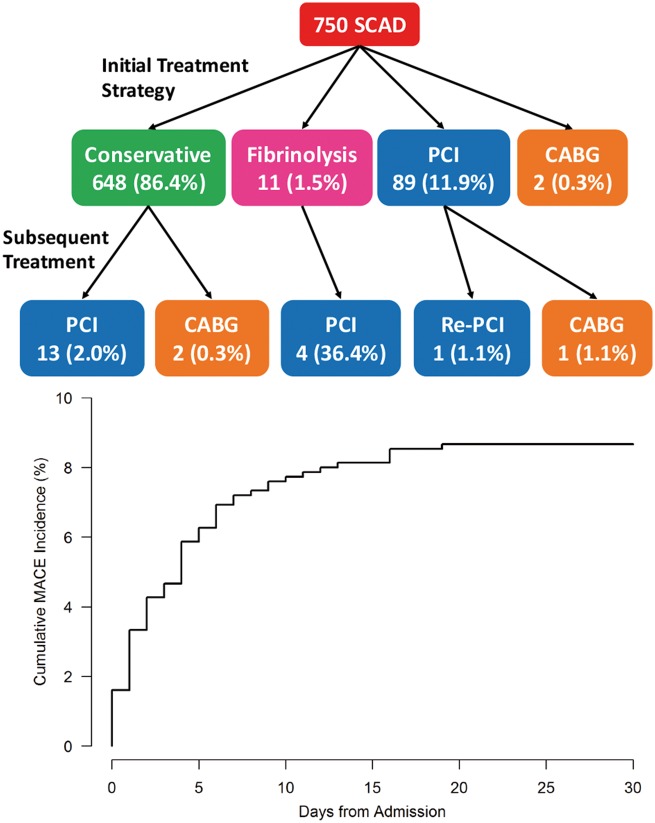
Majority of patients (n = 648, 86.4%) were treated conservatively as the first initial strategy; of these, 13 (2.0%) required subsequent in-hospital PCI, and 2 (0.3%) underwent CABG. Overall, 110 patients (14.7%) underwent revascularization (14.1% PCI, 0.7% CABG) (Table 5 and Take home figure). Eleven (1.5%) underwent fibrinolysis, of which four required subsequent PCI. Of the 106 patients who underwent PCI, 85 cases were planned, 18 unplanned, and three were performed on non-SCAD lesions (SCAD was missed). The rationale for revascularization are listed in Supplementary material online, Table SC, with the most common reasons being ongoing chest pain or ECG ischaemia. Of the 103 PCI cases performed for SCAD, 29.1% was deemed successful, 40.8% partially successful, and 30.1% unsuccessful.
Table 5.
Management strategy and outcomes
| N (%) | N = 750 |
|---|---|
| Treatment strategy | |
| Conservative | 632 (84.3) |
| Fibrinolysis | 11 (1.5) |
| Revascularization (PCI or CABG) | 110 (14.7) |
| PCI | 106 (14.1) |
| CABG | 5 (0.7) |
| PCI performed during admission | N = 106 |
| Planned or ad hoc | 85 (80.2) |
| Unplanned | 18 (17.0) |
| To non-SCAD segment (missed SCAD) | 3 (2.8) |
| SCAD PCI procedures and outcomes | N=103 |
| Wiring only | 15 (14.6) |
| Balloon angioplasty | 21 (20.4) |
| Cutting balloon | 5 (4.9) |
| Stent placement | 67 (65.0) |
| Number of stents implanted | |
| 1 | 21/67 (31.4) |
| 2 | 23/67 (34.1) |
| 3 | 15/67 (22.4) |
| 4 or more | 8/67 (11.9) |
| Final TMI flow | |
| 0 | 16 (15.7) |
| 1 | 6 (5.9) |
| 2 | 13 (12.7) |
| 3 | 67 (65.7) |
| PCI effect on TIMI flow | |
| Improved | 59 (57.6) |
| Unchanged | 40 (38.8) |
| Worse | 4 (3.9) |
| Propagation of SCAD during PCI | 33 (32.0) |
| Overall PCI success | |
| Successful | 30 (29.1) |
| Partial success | 42 (40.8) |
| Unsuccessful | 31 (30.1) |
Take home figure.
Top: treatment flow diagram and bottom: cumulative incidence of 30-day major adverse cardiovascular events.
The median hospital stay was 4 days (IQR 3 days). During hospitalization, MAE occurred in 66 (8.8%) (Table 6). Iatrogenic catheter-induced dissection occurred in 9 cases (1.2%). High-risk presentation occurred in 7.6%. Peripartum SCAD patients were more likely to have high-risk presentation (23.5% vs. 6.8%, P = 0.003) than non-peripartum patients, as well as higher in-hospital MAE, higher troponin elevation, more multivessel SCAD, and iatrogenic dissections (Supplementary material online, Table SD). Post-discharge within 30 days, the composite MACE was 2.7%. The overall MACE within 30 days was 8.8% (Take home figure). Other complications within 30 days included re-admission for chest pain in 2.5% and emergency room visit for cardiac reasons 4.9%.
Table 6.
In-hospital and 30-day cardiovascular events
| n (%) | N = 750 |
|---|---|
| Overall in-hospital MAE | 66 (8.8) (95% CI 6.9–11.1) |
| Death | 1 (0.1) |
| Recurrent MI | 30 (4.0) |
| Extension of SCAD segment | 15 (50.0) |
| Iatrogenic dissection | 9 (30.0) |
| Other | 6 (20.0) |
| Severe ventricular arrhythmia | 29 (3.9) |
| Requiring ICD | 6 (0.8) |
| Haemodynamic instability | 15 (2.0) |
| Use of inotropes | 9 (1.2) |
| IABP | 6 (0.8) |
| LVAD | 2 (0.3) |
| ECMO | 2 (0.3) |
| LV rupture requiring surgery | 1 (0.1) |
| Heart transplant | 0 (0) |
| Unplanned revascularization | 19 (2.5) |
| Stroke/TIA | 6 (0.8) |
| Congestive heart failure | 2 (0.3) |
| Post-discharge 30-day MACE | 19 (2.5) (95% CI 1.5–3.9) |
| Death | 0 (0) |
| Recurrent MI | 16 (2.1) |
| Extension of SCAD segment | 8 (50.0) |
| Iatrogenic dissection | 1 (6.3) |
| New de novo SCAD | 1 (6.3) |
| Other | 6 (37.5) |
| Unplanned revascularization | 1 (0.1) |
| Stroke/TIA | 3 (0.4) |
| Congestive heart failure | 1 (0.1) |
| Total 30-day MACE | 66 (8.8) (95% CI 6.9–11.1) |
| Death | 1 (0.1) |
| Recurrent MI | 46 (6.1) |
| Unplanned revascularization | 20 (2.7) |
| Stroke/TIA | 9 (1.2) |
| Congestive heart failure | 3 (0.4) |
| Other complications within 30 days | |
| Pericarditis | 14 (1.9) |
| New atrial fibrillation | 7 (0.9) |
| Cardiac emergency room visit | 37 (4.9) |
| Admission for chest pain | 19 (2.5) |
ECMO, extracorporeal membrane oxygenation; LV, left ventricle; LVAD, left ventricular assist device.
Medications at hospital discharge and last clinical follow-up are listed in Supplementary material online, Table SE. The vast majority of patients were discharged home on aspirin (93.7%), 67.4% on ADP antagonist, and 84.8% on beta-blocker.
Univariates associated with in-hospital MAE included age, smoking history, ≥5 pregnancies, and peripartum SCAD (Supplementary material online, Table SF, Figure 4). In multivariable analysis, only peripartum SCAD remained significantly associated with in-hospital MAE. Peripartum SCAD was also independent predictor of high-risk presentation (OR 4.56, 95% CI 1.94–10.62; P < 0.001). Univariates associated with 30-day MACE included age, smoking history, CTDs, ≥5 pregnancies, and peripartum SCAD. However, in multivariable analysis, only CTDs and peripartum SCAD remain significantly associated with 30-day MACE.
Figure 4.
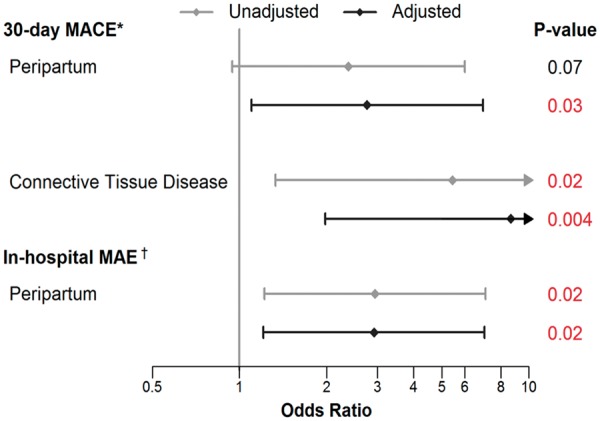
Forest plot for multivariable predictors of in-hospital major adverse events and 30-day major adverse cardiovascular events. aAdjusted model includes peripartum, connective tissue disease, and number of pregnancies. bAdjusted model includes peripartum, connective tissue disease, and history of smoking.
Discussion
This is the first prospective multicentre study of patients presenting acutely with non-atherosclerotic SCAD. It is also the largest and the only study with core laboratory adjudicated SCAD to date. We found that survival to 30 days was good despite the majority being treated conservatively, but 30-day MACE was high (8.8%). Important novel findings include peripartum SCAD and CTD being independent predictors of 30-day MACE.
Our study differs from prior SCAD case series, and extends beyond the single-centre Vancouver experience that we previously reported.10,11,16 Enrolling patients prospectively after acute SCAD ensures that all CV outcomes were methodically collected to ascertain the natural history of this disease. Clinical events were reviewed and verified by source documentations to ensure accuracy. This article provides detailed short-term outcomes in SCAD patients, which were not previously reported and are important to guide management and physician/patient education. Survival to discharge was excellent, with only one in-hospital death, and the remainder survived to 30 days. Despite the majority (86.4%) being treated conservatively as the initial treatment strategy, most patients survived hospitalization without MAE. Of note, in-hospital recurrent MI occurred in 4.0% and unplanned revascularization in 2.5%, highlighting the need for monitoring in-hospital for recurrent ischaemia and potential urgent revascularization for failed conservative therapy. Importantly, high-risk presentations were not infrequent (7.6%). Conservative therapy may be inadequate for these patients, and clinicians should have low thresholds for revascularization and/or supportive haemodynamic therapies.
Within 30 days after discharge, recurrent MI occurred in a further 2.1% of patients, and unplanned revascularization in 0.1%. This highlights the small but residual risks of extension or recurrent dissections post-SCAD, and the need for surveillance of recurrent ischaemia post-discharge. Importantly, repeat presentations with chest pains or other cardiac reasons were frequent (4.9%) within 30 days of SCAD. Although half of emergency room visits did not require admission, these patients should be investigated for recurrent MI. Interestingly, stroke/TIA occurred in 1.2% within 30 days; most were deemed ischaemic, except for one cerebral haemorrhage (no carotid/vertebral dissection). Peripartum SCAD and CTD were independent predictors of 30-day MACE, highlighting the need for clinical vigilance in these cohorts.
Our study also provides valuable insights on the background characteristics of SCAD patients. The mean age of our ‘all-comers’ cohort was 51.8 years, reflecting a young to middle-aged group of women at risk for this condition.16–19 A few smaller studies reported younger mean ages in the forties,20–22 which were likely inaccurate as these were small, retrospective studies, and preferentially included patients who had more severe SCAD (younger peripartum cases) or self-selected (voluntary online registry). The age range of patients in our study was 24–89 years (9.2% were older than 65 years), and 88.5% were women, therefore, SCAD should be in the differential diagnosis of all women presenting with MI, not just young women.
Precipitating stressors were commonly reported (66.4%) in our SCAD cohort, including emotional and/or physical stress. This important observation should be taken into consideration for subsequent lifestyle changes, including referral to cardiac rehabilitation programmes with SCAD-specific recommendations that include restrictions in weight lifting and psychosocial support.23 Predisposing conditions were also common (half our patients), even though only 54.8% had complete FMD screen. We recommended that FMD screening be performed for all patients; however, this was at the discretion of treating physicians and were not routinely done. As such, only 31.1% of the overall cohort screened positive for FMD (56.7% amongst those who had complete FMD screening), which was lower than our previously reported co-prevalence of 63–86%.10,11,16 Furthermore, CTA was the primary mode of FMD screen (lower sensitivity) in this study, as opposed to catheter-angiography in our prior Vancouver series.10,11 We expect more patients to be screened for FMD during remainder of the study, and the incidence of extracoronary FMD should be higher. Other potential predisposing conditions were much less frequent. Patients with peripartum SCAD had higher likelihood of high-risk presentation and in-hospital MAE. They had more LM and proximal artery SCAD, multivessel, and multisegmental SCAD, which culminated in larger MI and worse LVEF. These findings confirm prior Mayo Clinic study on pregnancy-associated SCAD, although they included patients who were pregnant or ≤12 weeks postpartum,24 whereas we defined peripartum as the period from 3rd trimester pregnancy to within 12 months of delivery.
Coronary angiographic core laboratory analysis was central for patient inclusion for our study, and a fundamental strength of this study. This ensured that all enrolled patients had verified angiographic SCAD and were uniformly classified.5,6 Angiographic diagnosis of SCAD can be challenging, both with under-diagnosis and ‘over-calling’. We have observed that angiographers have improved their SCAD diagnostic skills on angiography remarkably over the past few years. Some critics are concerned that the pendulum may have swung too much the other way with ‘over-calling’. Twenty-four cases were excluded by our core laboratory. In ambiguous cases, intracoronary imaging can be invaluable to confirm diagnosis. Of note, iatrogenic catheter-induced dissection can be a drastic complication in patients with SCAD,25 and our reported incidence was 1.3%. Thus, meticulous and cautious angiographic techniques are mandatory when imaging SCAD patients.
From early experience, we learnt that PCI for SCAD patients was fraught with challenges, including poor success rates, extension of dissections, iatrogenic dissections, and need for long stents.10 The decision to revascularize can differ widely amongst clinicians and this is the first study to capture the rationale for revascularization. In our cohort, 86.4% of patients were treated conservatively and the in-hospital event rates were highly favourable. For those that underwent revascularization, the majority had PCI, with 30.1% being unsuccessful. These findings reaffirm current recommendations for conservative therapy as first-line treatment in position statements.2,7 However, patients with high-risk presentation (e.g. peripartum SCAD) should be considered for emergent invasive management if conservative therapy is unlikely to be sufficient.
Limitations
Our study is non-randomized, however, our large, multicentre, prospective enrolment of SCAD patients enabled us to evaluate the natural history and outcomes according to real-world management in an objective manner. We attempted enrolling all consecutive non-atherosclerotic SCAD patients at each site to minimize bias; however, we cannot be certain that all patients were enrolled, particularly those who did not survive to hospital presentation, or diagnosis that was missed on angiography. A small proportion (17.6%, n = 132) of this current cohort was included in prior publication as part of the Vancouver SCAD cohort16; the remainder majority in this current study were new unreported patients. The majority (60.0%) were enrolled acutely in-hospital, 31.3% within a month, and the remainder within 3 months of SCAD presentation. However, we only allowed post-discharge enrolment of patients if all in-hospital and readmission records can be obtained to ensure that all events were collected. The small number of peripartum patients rendered it challenging to make definitive conclusions; nevertheless, peripartum SCAD remained an independent predictor of high-risk presentation and 30-day MACE.
Conclusion
Spontaneous coronary artery dissection predominantly affects young and middle-aged women, and primarily presents with MI. Despite conservative therapy in the majority of patients, acute in-hospital and 30-day survival is good. However, significant CV complications accrued within the first 30 days post-SCAD, including recurrent MI, unplanned revascularization, stroke, and recurrent emergency room visits. Longer-term follow-up of this large prospective cohort, and further investigations on pathophysiology, risk and predictors of recurrence, and management are warranted.
Supplementary Material
Acknowledgements
The authors would like to thank the University of British Columbia Cardiology Research group (Jackie Chow, Andrew Starovoytov, Naomi Uchida, Robyn Tkatch, Brady Robinson, and Ngaire Meadows) for co-ordinating the study, the Cardiovascular Imaging Research team (G.B.J.M., Eunice Yeoh, Craig Kamimura, and Arnold Ryomoto) for angiographic analyses, the BC Center for Improved Cardiovascular Health (K.H., Defen Peng, Yinshan Zhao, and Melissa Pak) for statistical analyses, and the site principal investigators and team for participation and patient enrolment [Vancouver General Hospital (J.S.), Hamilton General Hospital (T.S.), University of Ottawa Heart Institute (D.S.), St. Boniface Hospital (K.M.), Royal Alexandra Hospital (N.B.), Prairie Vascular Research Network (A.L.), Queen Elizabeth Health Centre (H.B.), London Health Sciences Centre (S.L.), Royal University Hospital (C.P.), St Mary’s General Hospital (S.R.), Sunnybrook Health Sciences Centre (M.M.), University of Alberta (R.W.), St. John Regional Hospital (S.L.), Rouge Valley Health System (R.V.), St Paul’s Hospital (E.A.), Foothills Hospital (B.H.), Montreal Heart Institute (R.I.), Cleveland Clinic Foundation (H.G.), University of Michigan (S.G.), St. Michael’s Hospital (C.B.), Centre hospitalier de l’Université de Montréal (A.M.), and McGill University Health Centre (G.M.)].
Funding
This work was supported by Canadian Institutes of Health Research, Abbott Vascular, AstraZeneca, St Jude Medical, and Servier.
Conflict of interest: J.S. has received unrestricted research grant supports (from the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, National Institutes of Health, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, St Jude Medical, Boston Scientific, and Servier), speaker honoraria (AstraZeneca, St Jude Medical, Boston Scientific, and Sunovion), consultancy and advisory board (AstraZeneca, St Jude Medical, Abbott Vascular, and SCAD Alliance), and proctorship honoraria (St Jude Medical and Boston Scientific). D.S. has received unrestricted research grant supports (from the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, National Institutes of Health, Eli-Lilly, Spartan Biosciences, Roche Diagnostics, and Aggredyne), speaker honoraria and advisory board honoraria (AstraZeneca and Bayer). H.L.G. has received research support (from CVR Global), equity/intellectual property (Flexlife Health), and is on advisory board (FMD Society of America). A.L. reports speaking honoraria (Boehringer Ingelheim, Pfizer, BMS Sanofi, Novartis, Servier, and Bayer). S.G. has received research grants from the National Heart, Lung and Blood Institute (HL139672), NIH, Doris Duke Charitable Foundation, and the Frankel Cardiovascular Center at the University of Michigan. She is on advisory boards (FMD Society of America and SCAD Alliance). And all other authors have no conflict of interest.
References
- 1. Saw J, Mancini GB, Humphries KH.. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016;68:297–312. [DOI] [PubMed] [Google Scholar]
- 2. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahmoud AN, Taduru SS, Mentias A, Mahtta D, Barakat AF, Saad M, Elgendy AY, Mojadidi MK, Omer M, Abuzaid A, Agarwal N, Elgendy IY, Anderson RD, Saw J.. Trends of incidence, clinical presentation and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018;11:80–90. [DOI] [PubMed] [Google Scholar]
- 4. Saw J, Aymong E, Mancini J, Sedlak T, Starovoytov A, Ricci D.. Non-atherosclerotic coronary artery disease in young women. Can J Cardiol 2014;30:814–819. [DOI] [PubMed] [Google Scholar]
- 5. Saw J, Mancini GB, Humphries K, Fung A, Boone R, Starovoytov A, Aymong E.. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv 2016;87:E54–E61. [DOI] [PubMed] [Google Scholar]
- 6. Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014;84:1115–1122. [DOI] [PubMed] [Google Scholar]
- 7. Adlam D, Alfonso F, Maas A, Vrints C, Writing Committee.. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 2018;39:3353–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alderman EL, Stadius M.. The angiographic definitions of the bypass angioplasty revascularization investigation. Coron Artery Dis 1992;3:1189–1207. [Google Scholar]
- 9. Vijayaraghavan R, Verma S, Gupta N, Saw J.. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014;130:1915–1920. [DOI] [PubMed] [Google Scholar]
- 10. Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, Robinson S, Vuurmans T, Gao M, Humphries K, Mancini GB.. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645–655. [DOI] [PubMed] [Google Scholar]
- 11. Saw J, Ricci D, Starovoytov A, Fox R, Buller CE.. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6:44–52. [DOI] [PubMed] [Google Scholar]
- 12. Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, Gray WA, Gupta R, Hamburg NM, Katzen BT, Lookstein RA, Lumsden AB, Newburger JW, Rundek T, Sperati CJ, Stanley JC; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Functional Genomics and Translational Biology; American Heart Association Council for High Blood Pressure Research; American Heart Association Council on the Kidney in Cardiovascular Disease; American Heart Association Stroke Council. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American heart association. Circulation 2014;129:1048–1078. [DOI] [PubMed] [Google Scholar]
- 13. Koul AK, Hollander G, Moskovits N, Frankel R, Herrera L, Shani J.. Coronary artery dissection during pregnancy and the postpartum period: two case reports and review of literature. Catheter Cardiovasc Interv 2001;52:88–94. [DOI] [PubMed] [Google Scholar]
- 14. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Gabriel Steg P, Wijns W, Bassand J-P, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon J-L, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; ESC Committee for Practice Guidelines (CPG). Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 15. Main A, Prakash R, Starovoytov A, Sabbaghan A, Aymong E, Mancini GBJ, Saw J.. Characteristics of extension and de novo recurrent spontaneous coronary artery dissection. EuroIntervention 2017;13:e1454–e1459. [DOI] [PubMed] [Google Scholar]
- 16. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, Mancini GBJ.. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017;70:1148–1158. [DOI] [PubMed] [Google Scholar]
- 17. Alfonso F, Paulo M, Lennie V, Dutary J, Bernardo E, Jiménez-Quevedo P, Gonzalo N, Escaned J, Bañuelos C, Pérez-Vizcayno MJ, Hernández R, Macaya C.. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv 2012;5:1062–1070. [DOI] [PubMed] [Google Scholar]
- 18. Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, Latib A, Ferlini M, Trabattoni D, Colombo P, Galli M, Tarantini G, Napodano M, Piccaluga E, Passamonti E, Sganzerla P, Ielasi A, Coccato M, Martinoni A, Musumeci G, Zanini R, Castiglioni B.. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol 2015;116:66–73. [DOI] [PubMed] [Google Scholar]
- 19. Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F, Joerg L, Rickli H.. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv 2017;89:59–68. [DOI] [PubMed] [Google Scholar]
- 20. Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, Holmes DR Jr, Hayes SN, Gulati R.. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777–786. [DOI] [PubMed] [Google Scholar]
- 21. Roura G, Ariza-Solé A, Rodriguez-Caballero IF, Gomez-Lara J, Ferreiro JL, Romaguera R, Teruel L, de Albert M, Gomez-Hospital JA, Cequier A.. Noninvasive follow-up of patients with spontaneous coronary artery dissection with CT angiography. JACC Cardiovasc Imaging 2016;9:896–897. [DOI] [PubMed] [Google Scholar]
- 22. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, Gulati R.. Clinical features, management and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579–588. [DOI] [PubMed] [Google Scholar]
- 23. Chou AY, Prakash R, Rajala J, Birnie T, Isserow S, Taylor CM, Ignaszewski A, Chan S, Starovoytov A, Saw J.. The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol 2016;32:554–560. [DOI] [PubMed] [Google Scholar]
- 24. Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM.. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol 2017;70:426–435. [DOI] [PubMed] [Google Scholar]
- 25. Prakash R, Starovoytov A, Heydari M, Mancini GB, Saw J.. Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. JACC Cardiovasc Interv 2016;9:1851–1853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.