Abstract
Background:
N-3 polyunsaturated fatty acid (PUFA) supplementation has been associated with reduced mortality and inflammation in patients with cardiovascular disease. There are limited data on the effects of n-3 PUFA supplementation in patients with peripheral artery disease (PAD).
Materials and methods:
The OMEGA-PAD II trial was a double-blinded, randomized, placebo-controlled trial to assess the effect of 3 mo of high-dose oral n-3 PUFA supplementation on inflammation, endothelial function, and walking ability in patients with PAD.
Results:
Twenty-four patients with claudication received 4.4 g/d of fish oil or placebo for 3 mo. Outcomes measured included high-sensitivity C-reactive protein levels, the omega-3 index, endothelial function as measured via flow-mediated vasodilation, walking impairment questionnaire, and a 6-min walk test. Plasma levels of specialized pro-resolving lipid mediators (SPMs) were measured by liquid-chromatography-tandem mass spectrometry. In patients treated with fish oil, the absolute mean omega-3 index significantly increased from baseline (fish oil: 7.2 ± 1.2%, P < 0.001; placebo: −0.4 ± 0.9%, P = 0.31; between-group P < 0.001). Furthermore, there were significant increases in several pathway markers of SPM biosynthesis, including several mono-hydroxyeicosapentaenoic acids and mono-hydroxydocosahexaenoic acids. We also observed significant increases in the SPM lipoxin A5 (fish oil: 0.57 ± 0.70 pg/mL, P = 0.05; placebo: 0.01 ± 0.38 pg/mL, P = 0.93; between-group P = 0.04) and resolvin E3 (fish oil: 154 ± 171 pg/mL, P = 0.04; placebo: 32 ± 54 pg/mL, P = 0.08; between-group P = 0.04). There were no significant changes in high-sensitivity C-reactive protein, flow-mediated vasodilation, walking impairment questionnaire, or 6-min walk test in the fish oil group.
Conclusions:
Fish oil increases SPMs in plasma of patients with PAD. Further studies are required to determine whether these early changes translate to clinical improvements in patients with PAD.
Keywords: Peripheral artery disease, n-3 polyunsaturated fatty acids, Fish oils, Specialized pro-resolving lipid mediators, Other pharmacotherapy
Introduction
The effects of n-3 polyunsaturated fatty acid (PUFA) supplementation on systemic inflammation1,2 and cardiovascular disease (CVD)3 have been described in many populations. In addition, studies have shown that n-3 PUFA supplementation is associated with improvements in endothelial function,4,5 reduction in platelet aggregation,6 and improved atherosclerotic plaque stability.7 Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the main PUFAs in fish oil and are substrates for enzymes that give rise to specialized proresolving lipid mediators (SPMs), specifically D-series resolvins, E-series resolvins, maresins, and protectins.8 These SPMs have receptor-mediated actions on human leukocytes, platelets, and vascular cells and have been shown to actively promote resolution of vascular inflammation and decrease atherosclerosis and vascular injury in animal models.8–10
Analysis of National Health and Nutrition Examination Survey data suggests that populations consuming a diet high in n-3 PUFAs may have a decreased prevalence of peripheral artery disease (PAD).11 As such, investigators have examined the relationship between n-3 PUFAs and PAD,12 but heterogeneity in dosage and intervention length has likely contributed to inconsistent results. To more rigorously investigate oral n-3 PUFA supplementation in patients with PAD, the OMEGA-PAD I trial was designed.13 This randomized, double-blinded, placebo-controlled trial demonstrated that 1 mo of high-dose n-3 PUFA (4.4 g/d) supplementation can change the metabololipidomic profile in patients with PAD toward increased production of mediators of resolution.14
Based on these results, we designed the OMEGA-PAD II trial with the hypothesis that high-dose n-3 PUFA supplementation over 3 mo would lead to changes in systemic inflammation, endothelial function, SPM profile, and claudication symptoms.
Methods
Trial design and participants
The OMEGA-PAD II trial is a randomized, double-blinded, placebo-controlled trial. The study protocol was based on the previously published OMEGA-PAD I trial13,14 and took place at the San Francisco Veterans Affairs Medical Center (SFVAMC) between 2014 and 2016. Patients aged ≥50 y presenting to the outpatient vascular surgery clinic at the SFVAMC with intermittent claudication (Rutherford I-III) and PAD were recruited to the study. PAD was defined as an ankle-brachial index (ABI) of <0.9, toe pressure <70 mm Hg, or ≥50% stenosis in segments of the aortoiliac, femoral, or tibial arteries on imaging. Patients were excluded from participating if they were taking immunosuppressive medications or steroids, had a severe acute illness (e.g., infection, surgery, critical limb ischemia) within the last 30 d, or had severe hepatic (Child-Pugh ≥ B), renal (creatinine ≥ 2 mg/dL), or nonvascular inflammatory disease.
Participants enrolled in the trial were randomized to one of two groups: fish oil or placebo. Patients were randomized by a block randomization with four subjects per block with a ratio of 1:1 for each block. The randomization was done by research pharmacists who maintained the key until the end of the study.
N-3 PUFA supplementation was achieved with four capsules of ProOmega (Ultimate Omega) taken twice daily (Nordic Naturals, Watsonville, CA), corresponding to a total of 4.4 g/d. Each ProOmega (Ultimate Omega) capsule contains 325 mg of EPA and 225 mg of DHA. The dose of 4 g/d corresponds to the American Heart Association’s recommendations for the treatment of hypertriglyceridemia.15 The placebo group took the same number of capsules containing soybean (Nordic Naturals) that was designed to appear the same as the treatment capsules. Study subjects attended a baseline visit and underwent a comprehensive vascular physiology assessment and then started on the study drug or placebo for 3 mo. After 3 mo of intervention, they returned for a second visit where the assessment was repeated (Supplemental Fig. 1). Compliance with daily supplementation was addressed using a pill count that was performed at the follow-up visit. Dietary information was not collected throughout the trial; however, participants in both groups were encouraged to engage in healthy dietary habits according to national society guidelines.
The primary end point was a change in plasma high-sensitivity C-reactive protein (hsCRP). Several secondary end points were measured to evaluate a wide range of potential effects that n-3 PUFA supplementation may have. These included changes in other biomarkers of inflammation, SPM profile, omega-3 index, brachial artery flow-mediated vasodilation (FMD), and measures of walking ability. SPMs measured included bioactive products and their biosynthetic pathway markers generated from EPA, DHA, and arachidonic acid (AA) (see below).
Institutional review board approval was granted for this study by the Committee on Human Research at the University of California, San Francisco as well as the SFVAMC Research and Development Office, with all participants giving informed written consent. The study was registered with Clinical-Trials.gov (NCT01979874).
Measurements
Demographics, anthropometries, medical history, and hemodynamic measurements
Basic demographic information was provided by the participant through an intake questionnaire. Information on past medical history and medication use was obtained through the SFVAMC electronic medical record, and common atherosclerotic risk factors such as coronary artery disease, hypertension, hyperlipidemia, diabetes mellitus, and smoking history were recorded. ABIs were measured bilaterally using current guidelines and standards.16
Renal, lipid, metabolic, and inflammation measurements
Blood samples were collected in a fasting state and assayed the same day per standard methodology (Beckman Coulter Analyzer, Miami, FL) for measurement of creatinine, estimated glomerular filtration rate, albumin, hemoglobin A1C, and lipids. Plasma was isolated from venous blood and assayed for hsCRP the same day as collection per standard methodology (Beckman Coulter Analyzer). Serum was stored at −80° C until assayed for interleukin-6 (IL-6) and soluble intracellular adhesion molecule-1 (ICAM-1) using commercially available enzyme-linked immunosorbent assay (ELISA) kits per standard protocol (R&D Systems Inc, Minneapolis, MN). The typical coefficients of variation for IL-6 and ICAM-1 are 7.4% and 4.6%, respectively. The lower limits of detection are 0.04 pg/mL and 0.10 ng/mL, respectively.
Omega-3 index
The omega-3 index represents the red blood cell (RBC) content of the two major long-chain n-3 fatty acids (FAs), EPA and DHA, and equates to EPA + DHA as a percent of total RBC FAs.17 The RBCs were isolated from whole venous blood and were assayed for n-3 FAs, n-6 FAs, AA, EPA, and DHA, according to the HS-Omega-3 Index methodology.18 The typical coefficient of variation for the HS-Omega-3 Index using this procedure is 3%. The average omega-3 index in the United States population is 4.5%, with values ranging from 2.7% in the lowest fifth percentile to 8.8% in the highest 95th percentile.19
Mass spectrometry—based lipid mediator metabolomics
Frozen plasma samples were subjected to solid-phase extraction and profiled for bioactive lipid mediators using liquid-chromatography-tandem mass spectrometry (LC-MS/ MS) using methodologies that have been previously described.20 Briefly, three volumes of methanol containing internal deuterium-labeled standards (i.e., d5-RvD2, d5-LXA4, d4-PGE2, d4-LTB4, d8–5-HETE) were added to plasma samples before solid-phase extraction to assess extraction recovery in each chromatographic region. Methyl formate fractions were collected and dried under a steady stream of N2 gas, resuspended in methanol:water (50:50), and analyzed by LC-MS/MS. Identification of mediators was accomplished using specific multiple reaction monitoring transitions and matching of retention time and diagnostic fragmentation spectra as compared to authentic standards. Abundance of lipid mediators was quantified using standard curves constructed with synthetic or authentic standards for each compound.
Brachial artery FMD
Brachial artery FMD was measured according to current guidelines and standards21 and as already described.14,22 FMD in healthy subjects is expected to be above 7%21 and has been reported to range between 0.2 and 19.2%.23
Functional tests
The 6-min walk test was administered according to standard procedures,24 and the distance to claudication and the time to claudication were recorded. Patients completed the walking impairment questionnaire, which is a validated survey that assesses a patient’s perceived walking capacity and limitation due to claudication across three domains: distance, speed, and stair climbing.25
Statistical analysis
Sample size was estimated based on the primary end point (reduction in hsCRP). It was estimated that a hsCRP value of 5.0 ± 5.0 mg/L can be expected in the PAD population13 and that 3 mo of n-3 PUFA supplementation would result in a 30% decrease in hsCRP.26 A sample size of 30 patients per group (60 in total) would have 80% power to conclude that hsCRP reduction is significantly higher in the treatment group.
Statistical analyses were performed using STATA 15 (StataCorp, College Station, TX), and variables were summarized by appropriate descriptive statistics. Baseline demographics and clinical variables were compared between the placebo and fish oil group using Fisher’s exact test for categorical variables and Student’s t-test for continuous variables. Paired Student’s t-tests were used to compare baseline variables with postintervention variables. All analyses were based on intention to treat.
To visualize changes driven by n-3 PUFA supplementation, interaction network pathway analyses of the EPA lipid mediator metabolome were performed between treatment groups using Cytoscape. To accomplish these analyses, a missing value imputation that replaced nondetected values with half the minimum value for each mediator was used. In addition, a log transformation of the data was performed. These pathways graphically illustrate both the magnitude of change from baseline to 3-month follow-up for each treatment and the mean abundance of each mediator in the 3-month follow-up samples.
Results
Twenty-four patients were enrolled in the study (all male), with 11 randomized to the fish oil group and 13 randomized to the placebo group (Supplemental Fig. 2). Recruitment for the trial was slower than expected and was ended by the principal investigator before reaching target enrollment. Two subjects (one per group) did not complete a follow-up visit and dropped out of the trial because of personal reasons. One subject in the placebo group developed headaches of an unknown cause and was removed from the study, reported to the institutional review board, and referred to the neurology service, who did not suspect that the placebo pills were the cause of the headaches. One subject in the fish oil group was hospitalized for a stroke and stopped taking the intervention while in the hospital. No participant in this trial dropped out of the study because of gastrointestinal upset, which is a commonly reported adverse effect of fish oil supplementation. This resulted in an overall dropout rate of 17%. Both groups were balanced after randomization with the exception of lower aspirin use in the fish oil (7/11 versus. 13/13, P = 0.03) (Table 1).
Table 1 —
Baseline characteristics of participants
| Characteristics | Fish oil (n = 11) |
Placebo (n = 13) |
P value* |
|---|---|---|---|
| Age (y) | 69 ± 8 | 73 ± 7 | 0.14 |
| Male sex | 11 (100%) | 13 (100%) | 1.0 |
| Caucasian | 7 (63%) | 10 (77%) | 0.66 |
| BMI (kg/m2) | 29 ± 5 | 28 ± 5 | 0.56 |
| Waist-hip ratio | 0.99 ± 0.05 | 1.02 ± 0.07 | 0.32 |
| History of smoking | 10 (91%) | 13 (100%) | 0.46 |
| Pack y | 43 ± 35 | 31 ± 18 | 0.28 |
| Omega-3 index (%) | 5.5 ± 2.1 | 5.7 ± 1.5 | 0.77 |
| Index ABI | 0.60 ± 0.09 | 0.66 ± 0.09 | 0.11 |
| Rutherford | |||
| Mild claudication | 3 (27%) | 5 (39%) | 0.86 |
| Moderate claudication | 7 (64%) | 6 (46%) | |
| Severe claudication | 1 (9%) | 2 (15%) | |
| History of revascularization | 4 (36%) | 3 (23%) | 0.66 |
| Lower extremity bypass | 2 (50%) | 1 (33%) | 0.86 |
| Lower extremity percutaneous | 2 (50%) | 2 (67%) | 1.0 |
| Comorbidities | |||
| Hypertension | 9 (82%) | 11 (85%) | 1.0 |
| Hyperlipidemia | 8 (73%) | 12 (92%) | 0.30 |
| Type 2 diabetes mellitus | 3 (27%) | 4 (31%) | 1.0 |
| Coronary artery disease | 4 (36%) | 7 (54%) | 0.44 |
| Systolic blood pressure (mm Hg) | 149 ± 20 | 154 ± 22 | 0.52 |
| Diastolic blood pressure (mm Hg) | 78 ± 8 | 82 ± 12 | 0.35 |
| Medications | |||
| Aspirin | 7 (64%) | 13 (100%) | 0.03 |
| ACE-inhibitor | 5 (45%) | 6 (46%) | 1.0 |
| Beta-blocker | 6 (55%) | 7 (54%) | 1.0 |
| Statin | 11 (100%) | 12 (92%) | 1.0 |
| Laboratory studies | |||
| Total cholesterol (mg/dL) | 153 ± 38 | 171 ± 52 | 0.36 |
| LDL (mg/dL) | 87 ± 33 | 79 ± 18 | 0.53 |
| HDL (mg/dL) | 48 ± 11 | 54 ± 21 | 0.45 |
| Triglycerides (mg/dL) | 92 ± 42 | 145 ± 116 | 0.17 |
| Serum Cr, mg/dL | 0.91 ± 0.2 | 1.10 ± 0.3 | 0.08 |
| eGFR (mL/min) | 85 ± 20 | 71 ± 24 | 0.14 |
| Albumin (g/dL) | 4.1 ± 0.3 | 4.2 ± 0.3 | 0.35 |
| HbA1C (%) | 5.9 ± 0.7 | 5.9 ± 0.9 | 0.99 |
| Vitamin D (ng/mL) | 31 ± 10 | 29 ± 14 | 0.75 |
| Inflammation | |||
| hsCRP (mg/L) | 3.4 ± 2.4 | 3.8 ± 6.2 | 0.85 |
| IL-6 (pg/mL) | 1.4 ± 0.8 | 2.5 ± 3.4 | 0.35 |
| ICAM-1 (ng/mL) | 576 ± 204 | 535 ± 141 | 0.57 |
| Brachial artery FMD | |||
| Brachial FMD (%) | 8.0 ± 4.6 | 5.8 ± 4.5 | 0.25 |
| Patient-perceived walking performance | |||
| Walking distance (score, from 0 to 100) | 42 ± 32 | 42 ± 35 | 0.99 |
| Walking speed (score, from 0 to 100) | 38 ± 18 | 41 ± 30 | 0.77 |
| Stairs climbing (score, from 0 to 100) | 40 ± 34 | 49 ± 30 | 0.50 |
| 6-min walk test | |||
| Time to claudication (s) | 113 ± 86 | 165 ± 99 | 0.30 |
| Distance to claudication (meters) | 119 ± 74 | 219 ± 125 | 0.07 |
Values are as “means ± SD” or “n (%).” Boldface P values were below the 0.05 level required for statistical significance.
ABI = ankle-brachial index; ACE = angiotensin-converting enzyme; BMI = body mass index; bpm = beats per minutes; Cr = creatinine; eGFR = estimated glomerular filtrate rate; FMD = flow-mediated vasodilation; HDL = high-density lipoprotein; HbA1c = hemoglobin A1c; hsCRP = high-sensitivity C-reactive protein; ICAM-1 = intercellular adhesion molecule-1; IL-6 = interleukin-6; LDL = low-density lipoprotein.
Calculated using Fisher’s exact test for categorical variables or a two-tailed Student’s t-test for continuous variables.
There was no significant change in the primary outcome of hsCRP levels after intervention in the fish oil or placebo group (Table 2). This was also true for IL-6 and ICAM-1. However, the omega-3 index increased more than two-fold in the fish oil group only (7.2 ± 1.2% increase from baseline, P < 0.001; between group differential P < 0.001).
Table 2 —
Changes in lipid, inflammatory, hemodynamic profile, and vascular function with treatment.
| Measurement | Fish oil (n = 11) |
Placebo (n = 13) |
Difference between groups† | ||
|---|---|---|---|---|---|
| Change compared to baseline | P value* | Change compared to baseline | P value* | ||
| Omega-3 index | |||||
| Omega-3 index (%)‡ | 7.2 ± 1.2 | <0.001 | −0.4 ± 0.9 | 0.31 | <0.001 |
| Lipid profile | |||||
| Total cholesterol (mg/dL) | −8.7 ± 21 | 0.26 | −5.7 ± 14 | 0.20 | 0.71 |
| Triglycerides (mg/dL) | −14 ± 46 | 0.40 | 6 ± 28 | 0.51 | 0.26 |
| HDL (mg/dL) | 2.2 ± 6.2 | 0.31 | −1.5 ± 7.2 | 0.52 | 0.25 |
| LDL (mg/dL) | −8.2 ± 25.1 | 0.35 | −3.3 ± 8.6 | 0.26 | 0.57 |
| Inflammation | |||||
| hsCRP (mg/L) | 0.8 ± 5.5 | 0.67 | −0.7 ± 3.4 | 0.52 | 0.47 |
| IL-6 (pg/mL) | −0.2 ± 0.6 | 0.37 | −0.4 ± 2.4 | 0.64 | 0.87 |
| ICAM-1 (ng/mL) | 71 ± 145 | 0.20 | 16 ± 139 | 0.70 | 0.41 |
| Hemodynamic parameters | |||||
| Systolic blood pressure (mm Hg) | −2.5 ± 12 | 0.50 | −0.5 ± 23 | 0.93 | 0.79 |
| Diastolic blood pressure (mm Hg) | −0.8 ± 8.3 | 0.75 | −1.5 ± 10.8 | 0.62 | 0.86 |
| Index ABI | 0.06 ± 0.18 | 0.34 | 0.05 ± 0.11 | 0.16 | 0.81 |
| Patient-perceived walking Performance | |||||
| Walking distance (score, from 0 to 100) | 11 ± 14 | 0.06 | 1 ± 26 | 0.87 | 0.35 |
| Walking speed (score, from 0 to 100) | 0.3 ± 23.5 | 0.97 | 1.2 ± 31.6 | 0.90 | 0.95 |
| Stairs climbing (score, from 0 to 100) | 4.2 ± 17 | 0.47 | 3.1 ± 18 | 0.58 | 0.89 |
| 6-min walk test | |||||
| Time to claudication (s) | 94 ± 88 | 0.12 | 7 ± 89 | 0.83 | 0.14 |
| Distance to claudication (meters) | 106 ± 92 | 0.06 | −6 ± 99 | 0.86 | 0.06 |
| Brachial artery FMD | |||||
| Brachial FMD (%)‡ | −1.5 ± 3.7 | 0.27 | 2.9 ± 3.6 | 0.03 | 0.02 |
Values are as “means ± SD.” Boldface P values were below the 0.05 level required for statistical significance.
ABI = ankle-brachial index; FMD = flow-mediated vasodilation; HDL = high-density lipoprotein; hsCRP = high-sensitivity C-reactive protein; ICAM-1 = intercellular adhesion molecule-1; IL-6 = interleukin-6; LDL = low-density lipoprotein.
Calculated using a paired Student’s t-test.
Calculated using an unpaired Student’s t-test.
Absolute difference between follow-up and baseline visit.
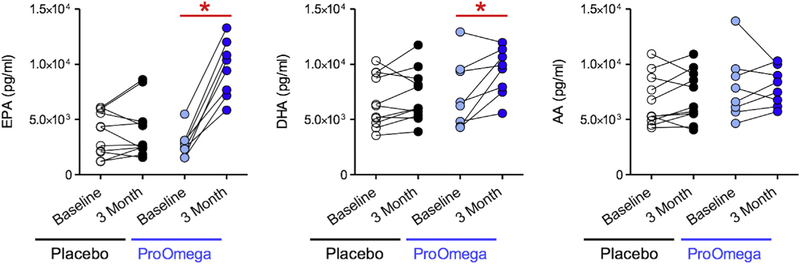
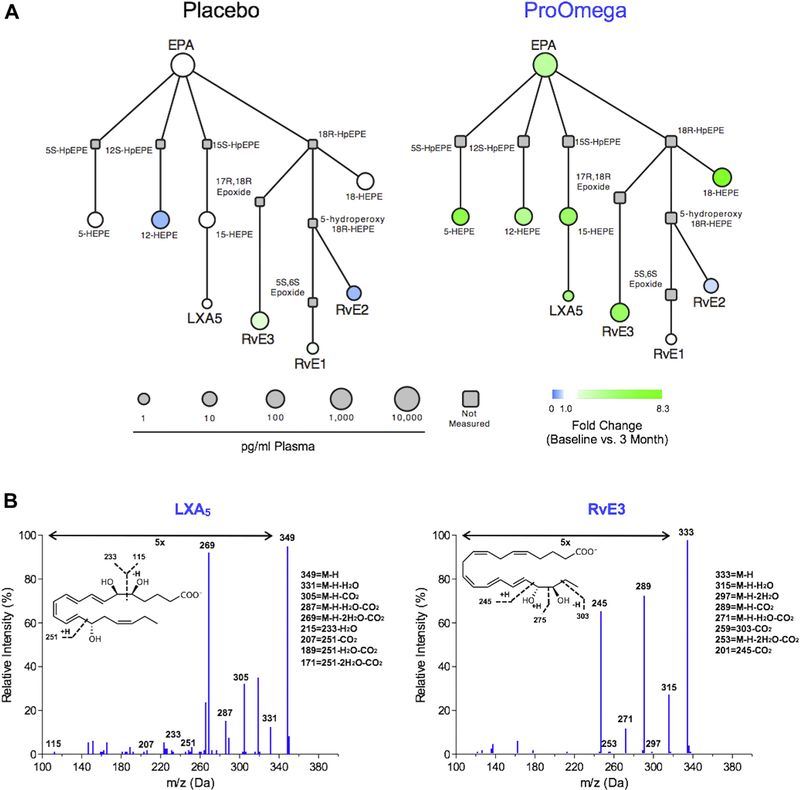
Consistent with increases in the omega-3 index observed in the fish oil group, several downstream omega-3 PUFA products increased in this group as well. Fish oil supplementation resulted in increases in total plasma DHA (2046 ± 2094 pg/mL, P = 0.03) and EPA (6785 ± 2039 pg/mL, P < 0.001) (Fig. 1). The increase in EPA observed in the fish oil group was significantly greater than the change in EPA observed in the placebo group (P < 0.001). Using interaction network pathway analyses to visualize quantitative differences in the EPA metabolome from baseline to follow-up, we observed enrichment in SPM biosynthesis pathways in the fish oil group (Fig. 2). Several downstream EPA products that are SPM biosynthesis pathway markers were identified (Table 3 and Fig. 2). These included 15-hydroxyeicosapentaenoic acid (15-HEPE), a marker of lipoxin A5 (LXA5) biosynthesis, and 18-HEPE, which is a marker of E-series resolvin biosynthesis.8 The levels of 15-HEPE and 18-HEPE increased significantly after 3 mo of fish oil supplementation, whereas there was no change in the placebo group (Table 3 and Fig. 3). Similarly, levels of LXA5 increased in the fish oil group but remained the same in the placebo group. E-series resolvins (i.e., RvE1 and RvE3) increased in the fish oil group, but only changes in RvE3 were statistically significant (Table 3 and Fig. 3).
Fig. 1 —
N-3 polyunsaturated fatty acid (PUFA) supplementation (ProOmega) increases levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the plasma. *Statistical significance as defined by a P value < 0.05. (Color version of figure is available online.)
Fig. 2 —
Interaction network pathway analysis of the eicosapentaenoic acid (EPA) metabolome after n-3 polyunsaturated fatty acid supplementation (ProOmega) or placebo is shown in (A). Representative MS/MS fragmentation spectra used for identification of lipoxin A5 (LXA5) and resolvin E3 (RvE3) are shown in (B). (Color version of figure is available online.)
Table 3 —
Changes in DHA- and EPA-derived lipid mediator profiles with n-3 PUFA supplementation.
| Product | Fish oil (n = 11) |
Placebo (n = 13) |
Difference between groups† | ||||
|---|---|---|---|---|---|---|---|
| Pre [range] | Post [range] | P value* | Pre [range] | Post [range] | P value* | ||
| Plasma EPA | 2783 ± 1244 [1528, 5464] | 9568 ± 2546 [5829, 13,280] | <0.001 | 3773 ± 1979 [1182, 6082] | 4023 ± 2480 [1559, 8612] | 0.58 | <0.001 |
| EPA products | |||||||
| 5-HEPE | 11 ± 11 [2, 37] | 67 ± 42 [31, 139] | 0.003 | 10.0 ± 5.9 [3, 23] | 14 ± 10 [5, 43] | 0.07 | <0.001 |
| 11-HEPE | 3.2 ± 2.7 [1, 10] | 39 ± 32 [17, 116] | 0.02 | 4.7 ± 3.2 [1, 10] | 5.9 ± 3.8 [2, 14] | 0.28 | 0.002 |
| 12-HEPE | 24 ± 21 [3, 63] | 90 ± 64 [9, 201] | 0.02 | 142 ± 165 [6, 371] | 67 ± 42 [14, 170] | 0.17 | 0.04 |
| 15-HEPE | 19 ± 16 [9, 57] | 99 ± 49 [45, 192] | 0.002 | 20 ± 12 [5, 39] | 24 ± 13 [5, 43] | 0.08 | <0.001 |
| 18-HEPE | 23 ± 16 [12, 62] | 195 ± 119 [113, 469] | 0.005 | 36 ± 20 [11, 68] | 41 ± 32 [13, 121] | 0.47 | <0.001 |
| LXA5 | 0.05 ± 0.13 [0, 0.4] | 0.62 ± 0.66 [0, 2] | 0.05 | 0.19 ± 0.31 [0, 1] | 0.20 ± 0.56 [0, 2] | 0.93 | 0.04 |
| RvE1 | 0.32 ± 0.90 [0, 3] | 0.69 ± 0.83 [0, 2] | 0.06 | 0.33 ± 0.88 [0, 3] | 0.83 ± 1.10 [0, 3] | 0.22 | 0.78 |
| RvE2 | 13 ± 14 [0, 32] | 9.7 ± 3.5 [0, 22] | 0.54 | 13 ± 31 [0, 101] | 6.2 ± 6.1 [0, 17] | 0.54 | 0.83 |
| RvE3 | 33 ± 38 [0, 107] | 187 ± 199 [0, 539] | 0.04 | 26 ± 38 [0, 119] | 58 ± 82 [0, 183] | 0.08 | 0.04 |
| Plasma DHA | 7252 ± 3083 [4265, 12,918] | 9298 ± 2166 [5539, 11,951] | 0.03 | 6629 ± 2356 [3542, 10,312] | 7121± 2344 [3878, 11,734] | 0.23 | 0.06 |
| DHA products | |||||||
| 4-HDHA | 3.1 ± 3.0 [1, 10] | 11.0 ± 2.8 [8, 16] | 0.001 | 5.0 ± 2.9 [2, 9] | 5.6 ± 3.9 [2, 15] | 0.48 | <0.001 |
| 7-HDHA | 3.4 ± 2.1 [0, 6] | 4.7 ± 1.7 [3, 7] | 0.07 | 2.0 ± 1.2 [1, 4] | 2.8 ± 2.0 [1, 6] | 0.23 | 0.59 |
| 13-HDHA | 10.2 ± 6.5 [5, 26] | 28.3 ± 9.2 [20, 49] | 0.004 | 11.0 ± 6.8 [2, 26] | 12.1 ± 7.9 [3, 28] | 0.55 | <0.001 |
| 14-HDHA | 11 ± 11 [1, 32] | 44 ± 27 [20, 86] | 0.01 | 9.0 ± 4.7 [2, 17] | 24 ± 24 [3, 77] | 0.04 | 0.12 |
| 17-HDHA | 25 ± 21 [11, 76] | 90 ± 30 [48, 128] | <0.001 | 27 ± 16 [4, 56] | 38 ± 30 [6, 92] | 0.10 | <0.001 |
| 21-HDHA | 9.7 ± 6.8 [4, 23] | 31.7 ± 8.8 [22, 48] | <0.001 | 9.1 ± 4.9 [3, 17] | 12 ± 12 [3, 44] | 0.23 | <0.001 |
| RvD1 | 0.10 ± 0.27 [0, 1] | 0.12 ± 0.35 [0, 1] | 0.87 | 0 ± 0 [0, 0] | 0.16 ± 0.38 [0, 1] | 0.21 | 0.52 |
| RvD2 | 0 ± 0 [0, 0] | 15 ± 30 [0, 87] | 0.19 | 0.9 ± 1.8 [0, 5] | 1.5 ± 1.9 [0, 5] | 0.48 | 0.12 |
| RvD3 | 0.13 ± 0.17 [0, 0.4] | 0.17 ± 0.23 [0, 1] | 0.55 | 0.04 ± 0.07 [0, 0.2] | 0.03 ± 0.07 [0, 0.2] | 0.92 | 0.55 |
| RvD4 | 2.1 ± 0.4 [0, 17] | 0 ± 0 [0, 0] | 0.35 | 7.7 ± 20.5 [0, 68] | 59 ± 191 [0, 636] | 0.34 | 0.39 |
| RvD5 | 0 ± 0 [0, 0] | 0.58 ± 1.09 [0, 3] | 0.18 | 0 ± 0 [0, 0] | 0 ± 0 [0, 0] | NA | 0.09 |
| 17R-RvD1 | 0.01 ± 0.04 [0, 0.1] | 0.11 ± 0.23 [0, 1] | 0.30 | 0.5 ± 1.7 [0, 6] | 0.9 ± 2.6 [0, 9] | 0.23 | 0.43 |
| 17R-RvD3 | 0 ± 0 [0, 0] | 0.03 ± 0.05 [0, 0.1] | 0.11 | 0 ± 0 [0, 0] | 0 ± 0 [0, 0] | NA | 0.05 |
| PD1 | 2.3 ± 5.0 [0, 15] | 5.1 ± 12.6 [0, 36] | 0.34 | 5.6 ± 14.2 [0, 48] | 3.6 ± 5.8 [0, 17] | 0.61 | 0.35 |
| 17R-PD1 | 1.8 ± 5.1 [0, 15] | 0.9 ± 2.5 [0, 7] | 0.35 | 4.6 ± 7.7 [0, 20] | 4.2 ± 9.4 [0, 31] | 0.92 | 0.91 |
| 10S, 17S-diHDHA | 0.02 ± 0.05 [0, 0.2] | 0.31 ± 0.82 [0, 2] | 0.36 | 0.08 ± 0.21 [0, 1] | 2.5 ± 6.2 [0, 21] | 0.22 | 0.35 |
| MaR1 | 0.13 ± 0.20 [0, 0.53] | 0.24 ± 0.23 [0, 0.54] | 0.20 | 0.04 ± 0.09 [0, 0.3] | 0.06 ± 0.13 [0, 0.4] | 0.76 | 0.31 |
| MaR2 | 2.2 ± 2.0 [0, 5.4] | 2.5 ± 2.5 [0, 7] | 0.51 | 0.9 ± 1.6 [0, 5] | 1.3 ± 2.0 [0, 6] | 0.10 | 0.82 |
| 7S,14S-diHDHA | 0.01 ± 0.03 [0, 0.1] | 0.04 ± 0.11 [0, 0.3] | 0.35 | 0.16 ± 0.39 [0, 1] | 0.22 ± 0.35 [0, 1] | 0.45 | 0.75 |
Values are as “means ± SD” in units of pg/mL. Boldface P values were below the 0.05 level required for statistical significance.
7S,14S-diHDHA = 7S,14S-dihydroxydocosahexaenoic acid; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; HDHA = hydroxydocosahexaenoic acid; HEPE = hydroxyeicosapentaenoic acid; LXA5 = lipoxin A5; MaR1 (2) = maresin 1 (2); n-3 PUFA = n-3 polyunsaturated fatty acid; PD1 = protectin D1; RvD1 (2, 3, 4, 5) = resolvin D1 (D2, D3, D4, D5); RvE1 (2, 3) = resolvin E1 (E2, E3).
Calculated using a paired Student’s t-test.
Calculated using an unpaired Student’s t-test.
Fig. 3 —
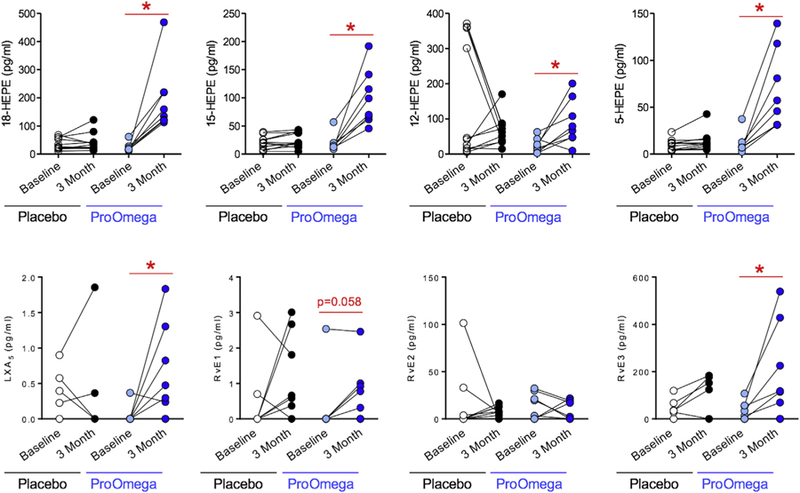
N-3 polyunsaturated fatty acid (PUFA) supplementation (ProOmega) increases levels of monohydroxy intermediates (A) and specialized pro-resolving lipid mediators (B) generated from eicosapentaenoic acid (EPA). *Statistical significance as defined by a P value < 0.05.. HEPE = hydroxyeicosapentaenoic acid; LXA5 = lipoxin A5; RvE1, E2, E3 = resolvin E1, E2, E3. (Color version of figure is available online.
Similar to products of EPA metabolism, several downstream DHA products that serve as SPM biosynthesis pathway markers were identified in the plasma. In the fish oil group, levels of 14-hydroxydocosahexaenoic acid (14-HDHA) and 17-HDHA, which are markers of the maresin and D-series resolvin pathways, respectively, increased significantly after fish oil supplementation (Table 3). We also identified D-series resolvins (RvD1–5), maresins (MaR1, MaR2), and protectins (PD1,17R-PD1) in the plasma (Table 3). However, no significant relationship between their levels and fish oil supplementation was observed. Similarly, we identified several AA products, including lipoxins, leukotrienes, and prostaglandins, but no significant relationships between the fish oil and placebo groups were observed (Supplemental Table 1).
With regard to functional measurements, after intervention, the fish oil group had a greater time to claudication (94 ± 88 s increase from baseline, P = 0.12) and a greater distance to claudication (106 ± 92 m increase from baseline, P = 0.06) after intervention as measured by the 6-min walk test (Table 2). Although these changes were not statistically significant, these trends were not observed in the placebo group. There were no significant changes in perceived walking performance as measured by the walking impairment questionnaire in either group. In addition, there was no significant change in FMD in the fish oil group, although FMD increased in the placebo group, which was significantly different between groups (Table 2).
Discussion
The OMEGA-PAD II trial was designed to investigate the effects of 3 mo of high-dose n-3 PUFA supplementation on inflammation and vascular function in patients with PAD. No difference in hsCRP was observed between the fish oil and placebo group, and no significant differences in functional outcomes were observed. However, the omega-3 index, a predictor of cardiovascular risk,27 increased more than twofold in the fish oil group with no significant changes noted in the placebo group. In addition, there were significant increases in downstream EPA and DHA products, including several SPMs, which have been demonstrated to be potent mediators of inflammation-resolution and have biological roles that could potentially explain several previously reported cardioprotective effects of n-3 PUFAs.8,9 Although the OMEGA-PAD I trial originally identified significant increases in intermediates of SPM biosynthesis (e.g., HDHA and HEPE) after 1 mo of fish oil supplementation, it did not identify significant changes in SPM end products (e.g., resolvins and lipoxins) as reported in the current trial.
Primary end point
PAD is associated with elevated levels of inflammation, and markers of inflammation have been identified as predictors of mortality28 and poor surgical outcomes.29 N-3 PUFA consumption has previously been reported to be associated with lower serum levels of several inflammatory markers.1 Siasos et al. reported reductions in IL-6 and tumor necrosis factor-α after 3 mo of oral n-3 PUFA supplementation.26 However, participants in that study did not have preexisting CVD, PAD, or any known clinical atherosclerosis. Although there are limited data analyzing the effects of oral n-3 PUFA supplementation specifically in patients with PAD, Schiano et al. reported no changes in hsCRP levels after 3 mo of n-3 PUFA treatment.30 The current trial did not detect any significant changes in hsCRP, IL-6, or ICAM-1. These gross measures of systemic inflammation might not adequately measure local inflammation at the vascular level and might not be the best way to assess the effects of n-3 PUFA supplementation on atherogenesis and PAD. It is also possible that the small sample size of this trial did not allow for the detection of changes in inflammatory markers or that 3 mo of n-3 PUFA supplementation is an inadequate time period to see changes in these biomarkers.
Secondary end points
Deficiencies in the levels of EPA and DHA in RBCs, as measured by the omega-3 index, have been implicated with adverse cardiac events.27 Efforts to address deficiencies in the omega-3 index could result in reduced morbidity and mortality, which is particularly applicable to PAD cohorts given their numerous atherosclerotic risk factors and high risk for adverse cardiac events.31 The OMEGA-PAD I trial reported an absolute mean increase of 4% in the omega-3 index after 1 mo of fish oil supplementation,14 whereas this trial reports an absolute mean increase of more than 7% after 3 mo of fish oil supplementation.
The mechanism through which n-3 PUFA supplementation and the omega-3 index could have protective effects has been proposed to be via increasing the production of mediators of resolution of inflammation, specifically SPMs. Recent research demonstrates that the resolution of inflammation is an active process driven by SPMs, which are derived from n-3 and n-6 PUFAs.8 These SPMs are generated via specific biosynthetic pathways and represent distinct classes including lipoxins derived from AA or EPA; the E-series resolvins generated from EPA; and the DHA-derived D-series resolvins, protectins, and maresins.8 SPMs have been shown to have potent proresolution effects in several models of disease, including atherosclerosis.8,10,32 As SPMs orchestrate termination of inflammation and return to tissue homeostasis, they may be protective against atherosclerosis, vascular injury, and PAD.33–36 This trial, despite the small sample size, provides substantial evidence that patients with PAD are capable of utilizing EPA and DHA in endogenous enzymatic pathways that increase the production of SPMs.
Lipoxins and resolvins regulate leukocyte-endothelial interactions,37,38 reduce the formation of reactive oxygen species,39 and regulate the production of prostacyclin40 and nitric oxide.41 Although clinical studies assessing the role of SPMs in PAD are sparse, Ho et al. reported a lower level of 15R-LXA4 in patients with PAD when compared with healthy subjects,33 suggesting that patients with PAD might have a deficit of mediators of resolution. A growing body of evidence suggests that mediators of resolution and n-3 PUFA isolates, such as icosapent ethyl, play a protective role in CVD.42,43 Results of this trial further support that n-3 PUFA supplementation increases plasma levels of vasculoprotective SPMs.
There are several reports of n-3 PUFA supplementation improving endothelial function44; however, there are limited data analyzing the effects of supplementation specifically in patients with PAD. Schiano et al. reported an improvement in FMD in patients with PAD after 3 mo of n-3 PUFA supplementation when compared with their control group.30 The OMEGA-PAD I trial reported an improvement in FMD after 1 mo of n-3 PUFA supplementation, but this difference was not significant when compared with changes observed in the placebo group.14 Results from the OMEGA-PAD II trial report a paradoxical decrease in FMD in the fish oil group and increase in FMD in the placebo group. These results are surprising, and the mechanism underlying them is unclear. Although measurements of FMD were done by trained and experienced staff, there are several factors that can acutely alter the results of FMD measurements including caffeine use, medication use, exercise, anxiety, and stress. Patients were directed to refrain from activities that are known to affect FMD results before measurement, but not all of these factors are controllable and may have contributed to the paradoxical results reported in this study. These results contradict previous evidence and should be further explored using larger sample sizes.
Although not statistically significant, results of this trial suggest that n-3 PUFA supplementation may play a role in increasing time to claudication and walking distance. Previous studies have assessed the effects of n-3 PUFA supplementation on functional outcomes, such as walking distance, and have reported mixed results. Carrero et al. and Madden et al. both examined the effects of n-3 PUFAs in intermittent claudicants and reported improvements in walking distance.45,46 In contrast, a recent meta-analysis representing a total of 425 participants from nine randomized placebo-controlled clinical trials that measured the effects of n-3 PUFAs on claudication symptoms failed to identify significant improvements between treatment and control groups in pain-free walking distance and maximal walking distance.47 However, all of the trials included in this meta-analysis varied in supplementation length, dose, and n-3:n-6 PUFA ratio, which could have contributed to the suboptimal results.
Limitations
This study is limited by a small sample size. Trial recruitment was closed early, and only 40% of the target sample was achieved. The comprehensive vascular physiology assessment that was completed at each study visit amounted to more than 6 h and this time commitment likely impaired study enrollment. In addition, the sample calculation was based on a baseline mean hsCRP of 5.0 mg/L, yet the mean hsCRP of this sample was much lower, and therefore even a sample size of 60 would have been underpowered to detect a difference in the primary end point. The groups were largely balanced after randomization, but differences in aspirin use could have altered the results. In addition, the use of cilostazol was not recorded and may have affected our results. Although not significant, patients in the fish oil group had a higher baseline FMD than the placebo group and could represent falsely elevated baseline FMD, which may have affected the postintervention results. Although recruitment was open to men and women, this cohort reflects the Veterans Affairs population and was made up completely of men, which limits the generalizability of the results.
Conclusion
Although the role of n-3 PUFA supplementation in PAD is poorly understood, there have been limited data that support their beneficial effects in patients with PAD, including the OMEGA-PAD I trial. In this follow-up trial, 3 mo of n-3 PUFA supplementation resulted in significant increases in the omega-3 index and SPMs and their biosynthetic pathway markers in patients with PAD. This provides evidence of the potential role that n-3 PUFA, or SPM, supplementation may play in addressing an inflammation resolution deficit in patients with PAD. Further research utilizing high-dose n-3 PUFA supplementation in larger, more diverse, cohorts is required to confirm and expand the results of this trial.
Supplementary Material
Acknowledgment
The authors would like to acknowledge the generous contribution of Nordic Naturals in providing ProOmega (Ultimate Omega) capsules and placebo for this study. The authors would also like to acknowledge the work of David P. Cheng and Kimberly A. Spaulding in assisting in collection of data throughout the trial.
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), United States, through University of California, San Francisco Clinical and Translational Science Institute Grant Number TL1-TR001871 with additional student research support from the Society for Vascular Surgery Student Research Fellowship Award and the American Heart Association Student Scholarship (J.L.R.). Furthermore, this work was supported by start-up funds from the University of California, San Francisco and the Northern California Institute for Research and Education, by Award Number KL2-RR024130 from the National Center for Research Resources, Award Number K23-HL122446–01 from the National Heart, Lung, and Blood Institute (NHLBI), United States, and a Society for Vascular Surgery Seed Grant and Career Development Award (SMG). The authors acknowledge additional support from the NIH/NHLBI by Award Number R01-HL119508–01 (M.S.C.) and R01-HL106173 (M.S.) as well as the support of a National Research Service Award from the NIH/NHLBI (F32-HL136044; B.E.S.). The authors further acknowledge support of NIH-NIGMS P01-GM095467 (Core B; M.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Northern California Institute for Research and Education, National Center for Research Resources, NIH, Society for Vascular Surgery, or American Heart Association. The funding organizations were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Disclosure
The authors have no conflicts of interest to report.
REFERENCES
- 1.He K, Liu K, Daviglus ML, et al. Associations of dietary longchain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol 2009;103:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohsawa M, Itai K, Onoda T, et al. Dietary intake of n-3 polyunsaturated fatty acids is inversely associated with CRP levels, especially among male smokers. Atherosclerosis 2008;201:184–191. [DOI] [PubMed] [Google Scholar]
- 3.Rizos EC, Ntzani EE, Bika E, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012;308:1024–1033. [DOI] [PubMed] [Google Scholar]
- 4.Khan F, Elherik K, Bolton-Smith C, et al. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovasc Res 2003;59:955–962. [DOI] [PubMed] [Google Scholar]
- 5.Morgan DR, Dixon LJ, Hanratty CG, et al. Effects of dietary omega-3 fatty acid supplementation on endothelium-dependent vasodilation in patients with chronic heart failure. Am J Cardiol 2006;97:547–551. [DOI] [PubMed] [Google Scholar]
- 6.Gao LG, Cao J, Mao QX, et al. Influence of omega-3 polyunsaturated fatty acid-supplementation on platelet aggregation in humans: a meta-analysis of randomized controlled trials. Atherosclerosis 2013;226:328–334. [DOI] [PubMed] [Google Scholar]
- 7.Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 2003;361:477–485. [DOI] [PubMed] [Google Scholar]
- 8.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sansbury BE, Spite M. Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ Res 2016;119:113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu B, Mottola G, Schaller M, et al. Resolution of vascular injury: specialized lipid mediators and their evolving therapeutic implications. Mol Aspects Med 2017;58:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane JS, Magno CP, Lane KT, et al. Nutrition impacts the prevalence of peripheral arterial disease in the United States. J Vasc Surg 2008;48:897–904. [DOI] [PubMed] [Google Scholar]
- 12.Grenon SM, Hughes-Fulford M, Rapp J, et al. Polyunsaturated fatty acids and peripheral artery disease. Vasc Med 2012;17:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenon SM, Owens CD, Alley H, et al. n-3 Polyunsaturated fatty acids supplementation in peripheral artery disease: the OMEGA-PAD trial. Vasc Med 2013;18:263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grenon SM, Owens CD, Nosova EV, et al. Short-Term, highdose fish oil supplementation increases the production of omega-3 fatty acid-derived mediators in patients with peripheral artery disease (the OMEGA-PAD I trial). J Am Heart Assoc 2015;4:e002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kris-Etherton PM, Harris WS, Appel LJ, et al. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol 2003;23:e20–e30. [DOI] [PubMed] [Google Scholar]
- 16.Grenon SM, Gagnon J, Hsiang Y. Video in clinical medicine. Ankle-brachial index for assessment of peripheral arterial disease. N Engl J Med 2009;361:e40. [DOI] [PubMed] [Google Scholar]
- 17.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr 2008;87:1997S–2002S. [DOI] [PubMed] [Google Scholar]
- 18.Farzaneh-Far R, Lin J, Epel ES, et al. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA 2010;303:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris WS, Pottala JV, Varvel SA, et al. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: observations from 160,000 patients. Prostaglandins Leukot Essent Fatty Acids 2013;88:257–263. [DOI] [PubMed] [Google Scholar]
- 20.Dalli J, Colas RA, Walker ME, et al. Lipid mediator metabolomics via LC-MS/MS profiling and analysis. Methods Mol Biol 2018;1730:59–72. [DOI] [PubMed] [Google Scholar]
- 21.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 22.Owens CD, Wake N, Conte MS, et al. In vivo human lower extremity saphenous vein bypass grafts manifest flow mediated vasodilation. J Vasc Surg 2009;50:1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bots ML, Westerink J, Rabelink TJ, et al. Assessment of flowmediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. Eur Heart J 2005;26:363–368. [DOI] [PubMed] [Google Scholar]
- 24.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 25.Nicolai SP, Kruidenier LM, Rouwet EV, et al. The walking impairment questionnaire: an effective tool to assess the effect of treatment in patients with intermittent claudication. J Vasc Surg 2009;50:89–94. [DOI] [PubMed] [Google Scholar]
- 26.Siasos G, Tousoulis D, Oikonomou E, et al. Effects of Omega-3 fatty acids on endothelial function, arterial wall properties, inflammatory and fibrinolytic status in smokers: a cross over study. Int J Cardiol 2013;166:340–346. [DOI] [PubMed] [Google Scholar]
- 27.von Schacky C The Omega-3 Index as a risk factor for cardiovascular diseases. Prostaglandins Other Lipid Mediat 2011;96:94–98. [DOI] [PubMed] [Google Scholar]
- 28.Vidula H, Tian L, Liu K, et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med 2008;148:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owens CD, Ridker PM, Belkin M, et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg 2007;45:2–9. discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiano V, Laurenzano E, Brevetti G, et al. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr 2008;27:241–247. [DOI] [PubMed] [Google Scholar]
- 31.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation 2006;114:688–699. [DOI] [PubMed] [Google Scholar]
- 32.Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and proinflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun 2016;7:12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho KJ, Spite M, Owens CD, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol 2010;177:2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyahara T, Runge S, Chatterjee A, et al. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J 2013;27:2220–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatterjee A, Sharma A, Chen M, et al. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS One 2014;9:e113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chattopadhyay R, Mani AM, Singh NK, et al. Resolvin D1 blocks H2O2-mediated inhibitory crosstalk between SHP2 and PP2A and suppresses endothelial-monocyte interactions. Free Radic Biol Med 2018;117:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009;461:1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norling LV, Dalli J, Flower RJ, et al. Resolvin D1 limits polymorphonuclear leukocytes recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol 2012;32:1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, et al. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost 2007;97:88–98. [PubMed] [Google Scholar]
- 40.Brezinski ME, Gimbrone MA Jr, Nicolaou KC, et al. Lipoxins stimulate prostacyclin generation by human endothelial cells. FEBS Lett 1989;245:167–172. Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. 1989/03/13. [DOI] [PubMed] [Google Scholar]
- 41.Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, et al. 15-epilipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med 2004;200:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2018;380:11–22. [DOI] [PubMed] [Google Scholar]
- 43.Elajami TK, Colas RA, Dalli J, et al. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J 2016;30:2792–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zehr KR, Walker MK. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: a review. Prostaglandins Other Lipid Mediat 2018;134:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrero JJ, Lopez-Huertas E, Salmeron LM, et al. Daily supplementation with (n-3) PUFAs, oleic acid, folic acid, and vitamins B-6 and E increases pain-free walking distance and improves risk factors in men with peripheral vascular disease. J Nutr 2005;135:1393–1399. [DOI] [PubMed] [Google Scholar]
- 46.Madden J, Brunner A, Dastur ND, et al. Fish oil induced increase in walking distance, but not ankle brachial pressure index, in peripheral arterial disease is dependent on both body mass index and inflammatory genotype. Prostaglandins Leukot Essent Fatty Acids 2007;76:331–340. [DOI] [PubMed] [Google Scholar]
- 47.Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev 2013:CD003833. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.