Abstract
Epidemiological data suggest that body mass index and obesity are strong risk factors for depression and anxiety. However, it is difficult to separate cause from effect, as predisposition to obesity may enhance susceptibility to anxiety, or vice versa. Here, we examined the effect of diet and obesity on anxiety-like behaviors in male and female selectively bred obesity-prone and obesity-resistant rats, and outbred Sprague-Dawley rats. We found that when obesity-prone and obesity-resistant rats do not differ in weight or fat mass, measures of anxiety-like behavior in the elevated plus maze and open field are similar between the two groups. However, once weight and fat mass diverge, group differences emerge, with greater anxiety in obesity-prone relative to obesity-resistant rats. This same pattern was observed for males and females. Interestingly, even when obesity-resistant rats were “forced” to gain fat mass comparable to obesity-prone rats (via prolonged access to 60% high-fat diet), anxiety-like behaviors did not differ from lean chow fed controls. In addition, a positive correlation between anxiety-like behaviors and adiposity were observed in male but not in female obesity-prone rats. Finally, diet-induced weight gain in and of itself was not sufficient to increase measures of anxiety in outbred male rats. Together, these data suggest that interactions between susceptibility to obesity and physiological alterations accompanying weight gain may contribute to the development of enhanced anxiety.
Keywords: Genetic susceptibility, Female, DIO, Individual differences, Obesity
1. Introduction
As the global obesity epidemic has progressed, examination of how diet and weight gain influence neural function to impact cognition, motivation, and emotion has come to the forefront of research. Human obesity is often comorbid with anxiety and depression, and recent studies suggest that this may be due in part to effects of diet, weight gain, and accompanying physiological alterations in brain regions that mediate affective processes [1–3]. Consistent with mechanistic, rather than social underpinnings of this association, diet and obesity-induced alterations in gut microbiota can have pro-anxiety and pro-depressant effects in rodents [4–6]. For example, high fat diets can increase the risk of development of psychological disorders such as anxiety and depression [7,8]. Highly palatable diets have been shown to be both anxiolytic as well as anxiogenic depending on a range of factors including the age of the animals and whether or not the animals develop obesity [7,9,39]. In addition, consumption of sugary, fatty foods is sufficient to alter neural systems involved in cognition and anxiety including the hippocampus, nucleus accumbens, prefrontal cortex, and amygdala in ways that are expected to promote poor mental health [10–13].
In humans, weight is a heritable trait [14], although obesity is polygenic and influenced by early life experiences (see Ref. [15] for review). This results in considerable individual differences in susceptibility to weight gain that may interact with experience to influence anxiety and depression [16,17]. Similar variation in susceptibility to weight gain exists in outbred Sprague- Dawley rats, with some readily gaining weight and becoming obese, and others remaining lean, even when given calorie dense diets [18,19]. This naturally occurring variance was amplified through selective breeding, based on propensity or resistance to weight gain, to generate obesity-prone and obesity-resistant rat lines [19]. As in humans, obesity in this model is polygenic and influenced by early life experiences [20,21]. In addition, the obesity-prone phenotype is associated with enhanced striatal function and plasticity [22–25], as well as alterations in hypothalamic and metabolicprocesses (see Ref. [26] for review).
How obesity and palatable, calorie dense diets may affect anxiety-like behaviors in the obesity-prone/obesity-resistant model is poorly understood, and potential differences in females have not been examined. One recent study found mild elevations in anxiety-like behaviors in obesity-prone compared to obesity-resistant male rats prior to diet manipulation [33]. Therefore, in the current study we measured anxiety-like behaviors in both male and female obesity-prone and obesity-resistant rats before and after the development of spontaneous or diet-induced obesity. Additional behavioral studies were conducted in outbred Sprague-Dawley rats. Fasted plasma insulin and leptin levels, as well as weight and body composition were used to evaluate relationships between weight gain, alterations in peripheral hormones, and anxiety-like behaviors.
2. Methods
2.1. Subjects
Selectively bred obesity-prone (OP) and obesity-resistant (OR) rats [19] were bred in house at the University of Michigan Breeding Core using an outbred rotational system within closed populations. Sprague-Dawley (SD) rats were purchased from Envigo (Haslett, MI). For all studies, rats were group or pair housed (according to AAALAC recommendations based on weight and housing size) and were approximately 65–70 days old at the start of the experiment. All rats housed at the University of Michigan (Experiments 1–4) were maintained on a reverse light-dark cycle (12/12; lights off 8 AM) and behavioral tests were conducted between 2 pm and 4 pm. Rats housed at Hope College (Experiment 5) were maintained in 12 h light-dark cycle (lights on 8 AM) with experiments performed between 3 pm and 5 pm. Animal facilities were maintained at 72 °F ( + /- 2 °) and 30–70% humidity and conditions were monitored and recorded daily. All procedures were approved by The University of Michigan or Hope College Committee on the Use and Care of Animals.
2.2. Diet manipulations and measures of obesity
For all diet manipulations, food was available ad libitum and rats were weighed at least 5 times per week. Standard chow diet (Lab Diet 5001: 4.07kcal/g; 13.5% fat, 28.5% protein, 58% carbohydrates; % of caloric content), high-fat diet (HF; Open Source Diets D12492, 5.21kcal/g, 60% fat), or a “junk-food” diet (JF) made in-house were used. The “junk-food” diet was a mash of Ruffles potato chips (40 g), Original Chips Ahoy (130 g), Nesquik (130 g), Jiff creamy peanut butter (130 g), powdered LabDiet 5001 (200 g) and 180 mL of water (19.6% fat, 14% protein, and 58% carbohydrates; 4.5 kcal/g). For all diet manipulations, only one type of food was available at a time, and rats were maintained on the given diet for the duration of the experiment (see also Fig. 1). For studies of effects of high-fat diet, rats were given free access to this food for 4 or 8 weeks. For studies of junk-food, rats were given free access for 4 weeks. Age matched controls were given only chow throughout. Food intake and body weight for male obesity-prone and obesity-resistant rats were monitored throughout the experiment. In addition, nuclear magnetic resonance imaging (NMR; Minispec LF90II, Bruker Optics; conducted by the University of Michigan Animal Phenotyping Core) was used to determine body composition in a subset of subjects.
Fig. 1.
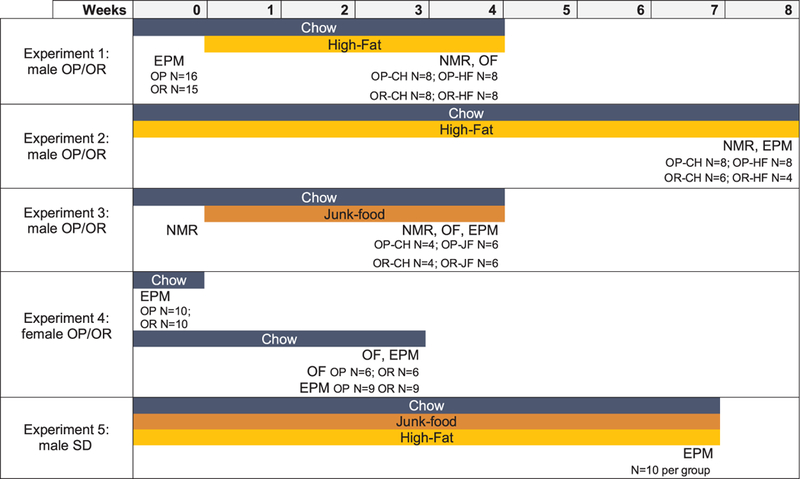
Experiment timelines. Timelines show number of animals used for each experiment and for each measure within an experiment. In addition, the timelines demonstrate which measures were performed for each experiment, and when each measure was performed. EPM=Elevated plus maze; OF=Open Field; CH=Chow; HF=High Fat; JF=Junk-food.
After the completion of all testing, animals were fasted for 16 h and blood samples were collected via tail nick into tubes containing EDTA (1.6 mg/ml, Sarstedt). The nick was covered with antibiotic ointment. Plasma was isolated by centrifugation (1000 × g, 4 °C) and stored (— 20 °C) for analysis. Insulin levels were measured using a 125I- Human insulin tracer (Linco Research, St. Charles, MO), a rat insulin standard (Novo Nordisk, Plainsboro, NJ), a guinea pig anti-rat insulin first antibody (Linco Research), and a sheep anti-guinea pig gamma globulin-PEG second antibody (Michigan Diabetes Research Core). Plasma leptin concentrations were measured using a leptin ELISA kit (#90040, Crystal Chem Inc, Elk Grove Village, IL; Michigan Diabetes Research Core) and conducted according to insert instructions.
2.3. Measures of anxiety-like behaviors
Two well-established measures of anxiety-like behavior were used, open field and elevated plus maze testing [27]. Both tests are based on intrinsic responses of rodents to avoid open areas in which they could be vulnerable to predation, and both tests have predictive validity for detecting anxiolytic and anxiogenic properties of drugs [27]. Open field and elevated plus maze testing occurred in the same testing room under the same lighting conditions. All behavior was video recorded during testing described in detail below, and videos were scored off-line by an observer that was blind to group and testing conditions.
2.3.1. Open field test
Open field testing was done in a 70 × 70 × 31 cm Plexiglas arena placed in the center of a brightly-lit, white room. On the day of testing, rats were brought into the testing room at least 30 min before being placed randomly along an edge of the open field. Behavior was video recorded for 30 min, after which the animal was returned to its home cage. The duration of testing was based on previous studies [28]. The open field was wiped clean with disinfectant prior to testing the next subject. The following behaviors were measured: traverses, defined as number of times the rat went from one side of the box to the opposite side or halfway across one side and returning back to the same side, number of entrances into the center, defined as the number of times all four paws simultaneously entered the center region, as well as time spent in the center, and latency to first center entrance.
2.3.2. Elevated plus maze
The maze consisted of 2 closed arms and 2 open arms; 50 cm long × 10 cm wide × 40 cm tall and 50 cm long × 10 cm wide respectively, with a 10 cm × 10 cm square intersection in the middle that was not within either arm. On the day of testing, rats were brought into the testing room at least 30 min before being randomly placed in the maze and allowed to explore freely for 15 min (Experiments 2 and 4) or 10 min (Experiments 1, 3 and 5). The duration of testing was reduced in Experiments 1, 3 and 5 because rats tended to stop exploring the maze after ~ 10 min, thus this captured the majority of their behavior. The number of entrances to the open and closed arms as well as the time spent in each arm were determined by hand scoring each video. Entry into an arm was defined as the shoulders and both forepaw paws crossing into that arm. Time spent in the central intersection was not counted towards time in any arm.
2.3.3. Locomotor activity
Animals were placed individually into a clean cage (41 × 25.4 × 20.3 cm) with fresh bedding. Locomotor activity was recorded for 45 min using an array of photocell beams to evaluate locomotor behavior (as in Refs. [24,29]). Crossovers were defined as the number of times the animal traveled from one end of the cage to the other, determined by breaking infrared beams at each end of the cage in succession.
2.4. Experiment 1: Differences in anxiety-like behavior in male OP vs. OR rats before and after 4 weeks of high-fat diet consumption
We began by testing the same set of male obesity-prone and obesity- resistant rats both before and after 4 weeks of high-fat diet consumption. Pilot studies indicated that repeated open field or elevated plus maze testing was not feasible, as behavior in both groups changed across repeated testing. Thus, rats were first tested in the elevated plus maze at a time when there were no differences in body weight between OP and OR groups (OP N = 16, OR N = 15; one rat was excluded because it fell off the maze platform). Next, these same rats were divided into chow and high-fat groups, counterbalanced for initial weight and behavioral measures in the elevated plus maze, and allowed free access to their respective diets for 4 weeks (OP-Chow N = 8, OP-4wkHF N = 8, OR-Chow N = 8, OR-4wkHF N = 8). After this manipulation, anxiety-like behavior was evaluated in the open field and body composition was determined.
2.5. Experiment 2: Effect of 8 weeks of high-fat diet consumption on anxiety-like behavior in male OP and OR rats
Although less pronounced than that of obesity-prone rats, increases in adiposity can be induced in obesity-resistant rats through prolonged exposure to high-fat diet. Thus, in order to determine whether anxiety-like behaviors can be enhanced by diet-induced obesity in obesity-resistant rats, a separate set of male OP and OR rats were given access to chow or high-fat diet for 8 weeks (OP-Chow N = 8, OP-8wkHF N = 8, OR-Chow N = 6, OR-8wkHF N = 4). Rats were then tested in the elevated plus maze and body composition was determined.
2.6. Experiment 3: Effects of a junk-food diet on anxiety-like behaviors
Because different dietary sources of calories can produce distinct outcomes in neural function [30], we also determined the effect of 4 weeks of free access to a sugary, fatty, junk-food diet on anxiety-like behavior in male obesity-prone and obesity-resistant rats (OP-Chow N = 4, OP-JF N = 6, OR-Chow N = 4, OR-JF N = 6). In addition, body composition was assessed at baseline and after the 4-week diet manipulation. Behavioral measures in both the elevated plus maze and open field were made only after diet manipulation. In addition, in order to determine whether differences in anxiety-like behavior may be due to reduced overall exploratory behavior in obesity-prone vs. obesity- resistant rats, locomotor activity in a novel environment was also assessed in these same rats.
2.7. Experiment 4: Anxiety-like behaviors in female OP vs. OR rats
Here we assessed anxiety-like behaviors prior to, and after, the spontaneous divergence of weight gain in obesity-prone and obesity- resistant female rats. All animals remained on chow diet throughout the experiment. One set of females was tested in the elevated plus maze at a time when there were no differences in total body weight (OP N = 10, OR N = 10). In addition, two separate groups of rats were tested after spontaneous weight gain in obesity-prone rats in the open field (OR N = 6, OP N = 6) and elevated plus maze (OR N = 9, OP N = 9). Plasma leptin levels were used as an indirect measure of adiposity in these groups. Given that anxiety-like behavior is known to vary across the cycle, estrous cycle phase was monitored daily for a minimum of 10 days in a subset of females tested in the elevated plus maze. This was achieved by observing vaginal epithelial cell cytology and pre-copulatory and copulatory behaviors [31]. In order to avoid additional potential stress on the test day, vaginal lavages were conducted after elevated plus maze in order to confirm estrous phase on the day of testing (OR metestrus and diestrus (M + D) N = 6; OR proestrus and estrus (P + E) N = 3; OP M + D N = 5, OP P + E N = 4).
2.8. Experiment 5: Effects of a junk-food or high-fat diet on anxiety-like behaviors in outbred male rats
Outbred Sprague Dawley male rats were given either a chow (N = 10), junk-food (N = 10), or 60% high-fat diet (N = 10) for 48 days before determining body weight and evaluating anxiety-like behaviors in the elevated plus maze as described above.
2.9. Statistics
Data were analyzed using Prism 6 and Prism 7 (GraphPad, San Diego, CA). Comparison between two groups were made with unpaired or paired two-tailed t-tests, as appropriate. For comparison of three or more groups, one-way and two-way-repeated measures ANOVAs were used, followed by Sidak’s post-hoc multiple comparisons when appropriate. In addition, a priori comparisons of weight gain, fat, and lean mass were made between OP and OR groups within each diet condition. Power analyses were used to determine that group sizes of 4–6 are sufficient to detect effect sizes of 40–50% with a power of 80%.
3. Results
Fig. 1 outlines experimental groups, treatment duration, primary measures, and the number of subjects in each group for each experiment. In addition, average daily food intake for each group within a given experiment is shown in Table 1. Because animals were pair or group housed, food intake data are shown as daily averages based on food intake per cage divided by the number of animals in the cage and indicate general differences between groups and diet conditions. Data are presented as caloric intake, rather than mass, to facilitate comparisons across diets with different caloric density (e.g., high fat and chow). Food intake was comparable across studies including male OP and OR animals, with OP animals consuming more than their OR counterparts (Table 1). Interestingly, food intake was highest in outbred animals (Experiment 5). The reason for this difference is unclear, as outbred animals were age matched to OP/OR rats and housed under similar conditions, albeit at a different institution.
Table 1.
Average daily food intake as kilocalories per day.
| Chow |
High Fat |
Junk-food |
|||||
|---|---|---|---|---|---|---|---|
| OR | OP | OR | OP | OR | OP | ||
| Exp. 1 | average | 121 | 130.9 | 129 | 152.3 | – | – |
| SEM | 3.129 | 3.354 | 3.532 | 9.074 | – | – | |
| Exp. 2 | average | 115.4 | 127.3 | 99.34 | 119.4 | – | – |
| SEM | 2.697 | 1.207 | 0.7095 | 3.293 | – | – | |
| Exp. 3 | average | 117.7 | 126.6.2 | – | – | 159.5 | 179.3 |
| SEM | 2.269 | 8.346 | – | – | 6.488 | 3.041 | |
| outbred males | outbred males | outbred males | |||||
| Exp. 5 | average | 164.4 | 159.3 | 203.9 | |||
| SEM | 2.783 | 4.141 | 4.041 | ||||
3.1. Experiment 1: Anxiety-like behaviors develop with weight gain in obesity-prone male rats
Fig. 2 shows weight and anxiety-like behavior in the same set of male obesity-prone and obesity-resistant rats before and after 4 weeks of chow or high-fat diet consumption. During baseline testing, weight (Fig. 2A) and anxiety-like behavior in the elevated plus maze (Fig. 2B-F) were similar in obesity-prone and obesity-resistant groups. These same rats were then tested in the open field after 4 weeks of free access to chow or high-fat diet (HF). As expected, obesity-prone rats given chow or high-fat diet were significantly heavier than obesity-resistant rats (Fig. 2G: Two-way ANOVA main effect of group: F(1, 12) = 15.33, p < 0.01), had more fat mass (Fig.2H: Two-way ANOVA main effect of group: F(1, 12) = 36.52, p < 0.001), and less lean mass (Fig. 2I: Two-way ANOVA main effect of group: F(1,12) = 22.54, p < 0.001) than obesity-resistant rats. In addition, high fat diet did not produce any increases in weight or fat mass in obesity-resistant rats. Although there were visual trends towards increased fat mass in OP-HF vs OP-Chow groups, these were not statistically significant (Fig. 2H; post-hoc OP-CH vs OP-HF p = 0.2). This is not entirely unexpected given the short duration of diet exposure and pair housing conditions, which slow down weight gain (unpublished observation, CRF). When tested after weight gain, obesity-prone rats showed significantly increased anxiety-like behavior compared to obesity-resistant groups, regardless of diet manipulation. Specifically, obesity-prone rats spent less time in center (Fig. 2J: Two-way ANOVA main effect of group: F(1, 28) = 17.49, p < 0.001), entered the center fewer times (Fig. 2K: Two-way ANOVA main effect of group: F(1, 28) = 17.79, p < 0.001), and made fewer traverses (Fig. 2L: Two-way RM ANOVA main effect of group: F(1, 28) = 16.42, p < 0.001) compared to obesity-resistant rats in chow or high-fat groups. No significant interaction between 4 week high-fat diet and measures of anxiety-like behavior were found. Thus, data show that although anxiety-like behavior is initially comparable between obesity-prone and obesity-resistant rats, differences between these strains emerge with weight gain only in obesity-prone rats (see also discussion).
Fig. 2.
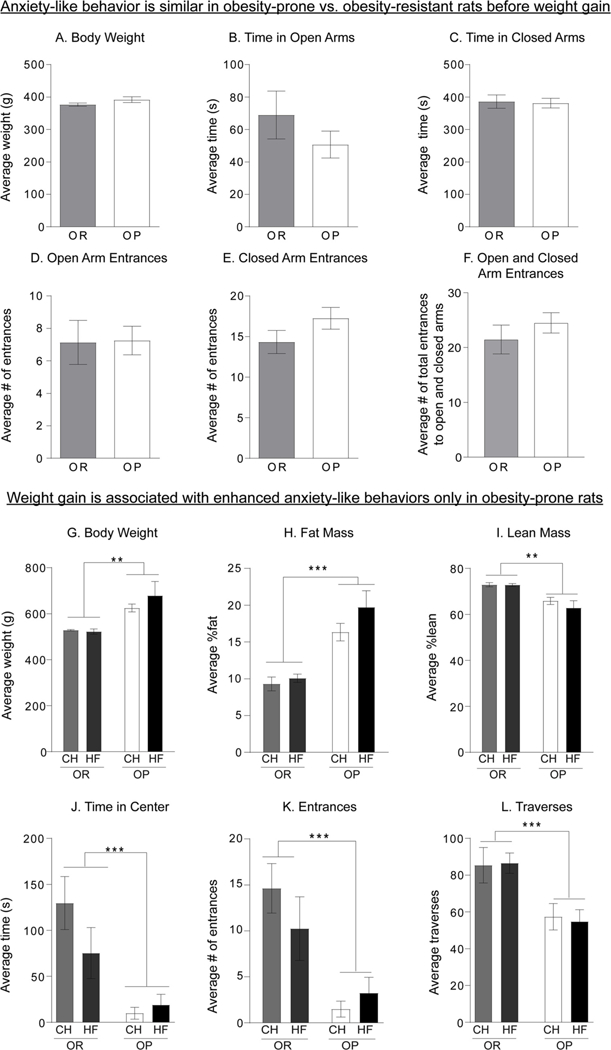
Anxiety-like behaviors emerge with spontaneous weight gain in male obesityprone rats. A) Average weight at the time of elevated plus maze testing is similar between obesity-prone (OP) and obesity-resistant groups (OR). B–F) Measurements of anxietylike behavior in the elevated plus maze were similar in obesity-prone and obesity-resistant groups. G–I) Weight and adiposity in the same obesity-prone and obesity-resistant rats after 4 weeks of high-fat (HF) or regular chow (CH) diet. G–I) Obesity-prone rats were heavier, had more fat mass, and less lean mass than the obesity-resistant rats in both chow and highfat diet fed groups. J–L) Measurements of anxiety- like behaviors in the open field after 4 weeks of high-fat diet. Anxiety-like behavior was enhanced in obesity-prone vs. obesity-resistant rats following spontaneous weight gain chow or high-fat diet. Data are shown as mean ± SEM. * = p < 0.05, *** = p < 0.001.
3.2. Experiment 2: Diet-induced obesity is not sufficient to enhance anxiety-like behavior in obesity-resistant male rats
Fig. 3 shows measures of obesity (A–C) and anxiety-like behavior in the elevated plus maze following 8 weeks of chow or high-fat diet (ORChow, OR-HF, OP-Chow, OP-HF; D-F). As expected, obesity-prone rats were heavier (Fig. 3A: Two-way ANOVA main effect of group: F(1, 22)=88.71, p < 0.0001), had more fat mass (Fig. 3B: main effect of group: F(1, 22)=44.42, p < 0.0001), and less lean mass (Fig. 3C: main effect of group: F(1, 22)=58.88, p < 0.0001) compared to obesity-resistant rats. In addition, while high-fat diet produced significant elevations in all measures of obesity in both obesity-prone and obesityresistant groups (Two-way ANOVA; Fig. 3A: main effect of diet: F(1, 22)=107.9, p < 0.0001; Fig. 3B: main effect of diet: F(1, 22)=84.15, p < 0.0001; Fig. 3C: main effect of diet: F(1, 22)=73.28, p < 0.0001), direct comparisons between groups given high-fat diet, show that obesity-prone rats gained significantly more fat mass than obesity-resistant rats (Fig. 3B: Sidak’s multiple comparison test, p < 0.05). Furthermore, prolonged exposure to high-fat diet also produced significant increases in fat mass in obesity-resistant groups compared to their chow fed counterparts (Fig. 3B: Sidak’s multiple comparison test, p < 0.01). Although the magnitude of increased adiposity was less in obesity-resistant vs. obesity-prone rats given high-fat, fat mass, weight, and lean mass were comparable between obesity-resistant rats given high-fat diet and obesity-prone rats maintained on chow. Thus, 8 weeks of high-fat diet was sufficient to induce moderate obesity in obesityresistant rats that was comparable to spontaneously occurring obesity in the obesity-prone group. Despite this, diet-induced obesity did not alter anxiety-like behavior in the obesity-resistant group (Fig. 3D–F), although greater anxiety-like behavior in obesity-prone vs. resistant groups was still present (Two-way ANOVA; Fig. 3D Time in open arms: main effect of group: F(1, 22)=39.48, p < 0.0001; Fig. 3E Time in closed arms: main effect of group: F(1, 22)=32.2, p < 0.0001; Fig. 3F Open arm entrances: main effect of group: F(1, 22)=45.6, p < 0.0001).
Fig. 3.
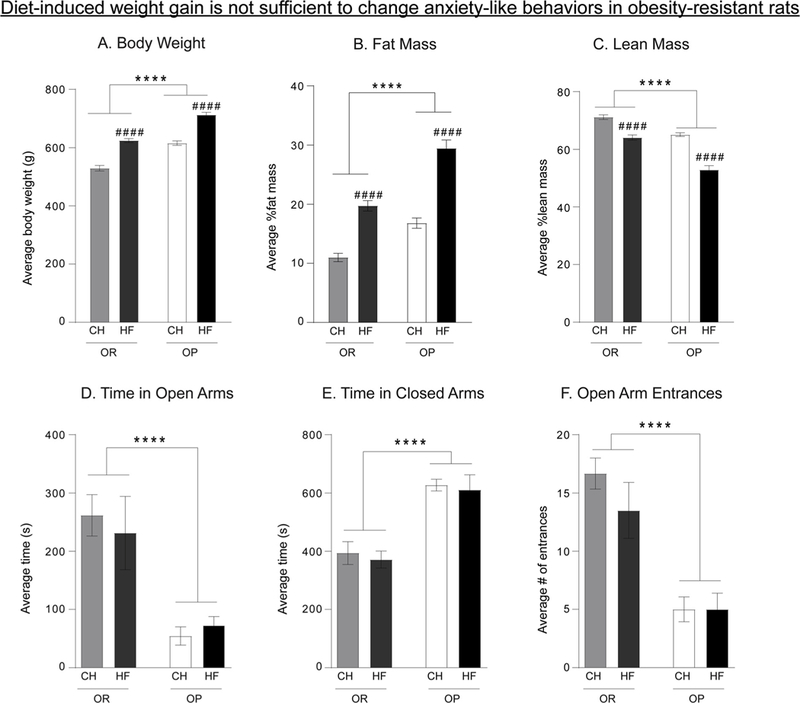
Diet-induced weight gain is not sufficient to enhance anxiety-like behavior in obesity-resistant rats. A–C) Weight, fat mass, and lean mass of obesity-prone and obesity-resistant rats given chow (CH) or high-fat diet for 8 weeks (HF). Eight weeks of high-fat diet was sufficient to induce increases in weight and adiposity, and reductions in lean mass in obesity-resistant rats that are comparable to spontaneous weight gain in obesity-prone rats given chow. In addition, high-fat diet produced additional weight gain, adiposity, and reductions in lean mass in obesity-prone rats compared to their chow-fed counterparts. D–F) Measurements of anxiety-like behavior in the elevated plus maze. Anxiety-like behavior in obesity-resistant rats was unaffected by increases in weight and fat mass, whereas anxiety-like behavior was greater in obesity-prone rats regardless of diet manipulation. Data are shown as mean ± SEM. #### = p < 0.0001 (chow vs. high-fat within group); **** = p < 0.0001 (OR vs. OP).
3.3. Experiment 3: Junk-food diet does not alter anxiety-like behavior in obesity-prone or obesity-resistant rats
Fig. 4 shows measures of adiposity, anxiety-like behavior, and locomotor activity after 4 weeks of free access to chow or junk-food diet. The organization of the graphs here differs from above such that chow and junk-food groups are arranged together on the X axis in order to better highlight differences between obesity-prone and obesity-resistant rats within diet condition. Obesity-prone and obesity-resistant rats given junk-food were significantly heavier than chow groups (Fig. 4A: Two-way ANOVA: main effect of diet F(1,16) = 8.771, p < 0.01), and obesity-prone rats were significantly heavier than their obesity-resistant counterparts (Fig. 4A: Two-way ANOVA: main effect of group, F(1,16) = 11.68, p < 0.01). Furthermore, obesity-prone rats given junk-food were significantly heavier than all other groups (Fig. 4A: Tukey post hoc p < 0.05). Fasted plasma insulin levels were greater in obesity-prone vs. obesity-resistant groups (Fig. 4B: Two-way ANOVA: main effect of group, F(1,16) = 5.868, p = 0.03). Body composition was determined within subjects before and after diet manipulation (Fig. 4C). Fat mass was similar between all groups prior to diet manipulation (Fig. 4C “Pre-JF”). However, 4 weeks of junk-food diet was sufficient to significantly increase fat mass in both obesity-prone and obesity-resistant groups (Fig. 4C: Two-way ANOVA: main effect of diet F(1,16) = 29.52; p < 0.0001), with trends towards greater increases in obesity-prone groups. Consistent with effects observed in the previous two experiments (Figs. 2 and 3), obesity-prone rats again displayed significantly stronger anxiety-like behavior than obesity-resistant rats in the elevated plus maze and open field, with no effect of diet in either group (Fig. 4D open field: Two-way ANOVA: main effect of group F(1,16) = 17.51, p < 0.001; Fig. 4E elevated plus maze: Two-way ANOVA: main effect of group, F(1,16) = 40.09, p < 0.0001; data not shown include: EPM time in open arms, Two-way ANOVA main effect of group: F(1,16) = 8.04, p < 0.05; OF Center Entrances, Two-way ANOVA main effect of group: F(1,16) = 15.6, p < 0.01; and OF Traverses, Two-way ANOVA main effect of group: F(1,16) = 16.28, p < 0.01). In order to determine whether differences in anxiety-like behavior may be due to reductions in overall exploratory behavior in obesity-prone vs. obesity-resistant rats, we assessed locomotor activity in a novel testing environment in this same group of rats (Fig. 4F). No significant differences in locomotor activity were found between any groups, and all rats showed normal habituation (Fig. 4F: Two-way RM ANOVA: no main effect of group p = 0.053, or group x time interaction p = 0.6410; significant main effect of time F(8,120) = 36.81 p < 0.0001). Thus, differences in anxiety-like behavior described above are not likely due to differences in exploratory behavior.
Fig. 4.
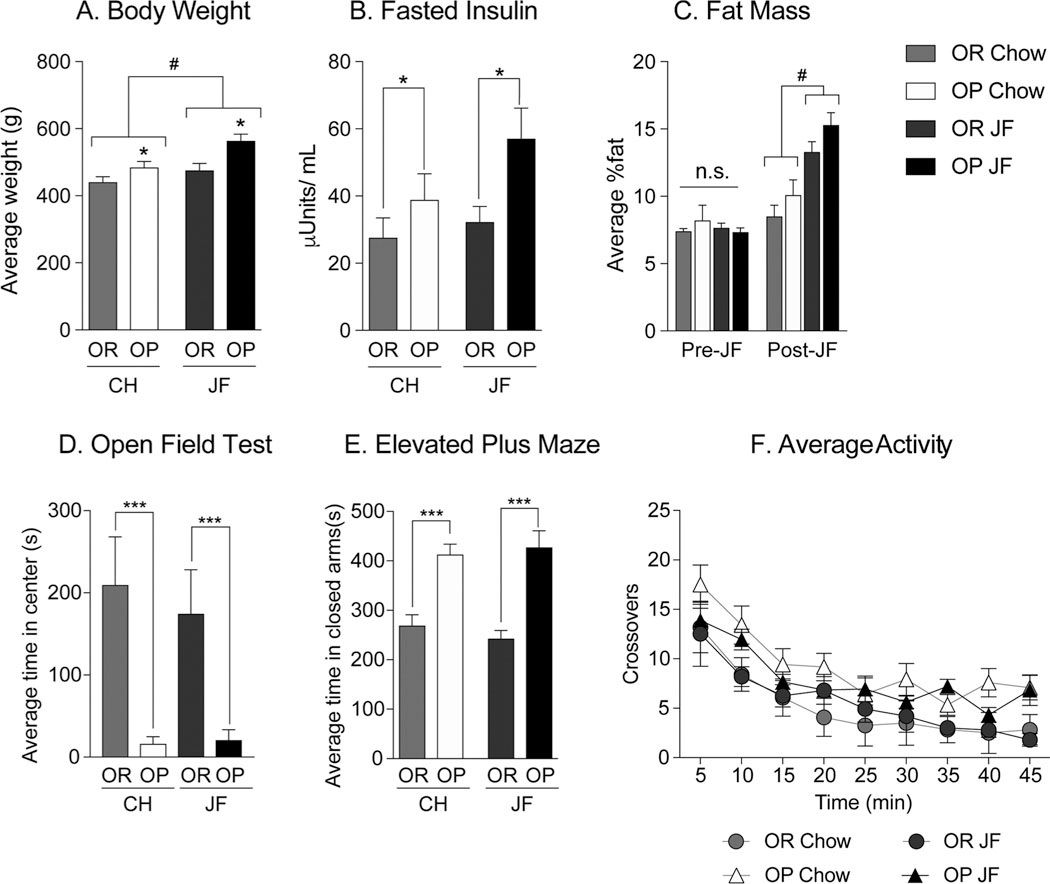
Junk-food diet does not alter anxiety-like behavior in obesity-prone or obesity-resistant rats. A–C) Weight, fasted insulin and fat mass following 4 weeks of free access to chow or junk-food (JF). Junk-food diet resulted in significant increases in weight only in obesity-prone rats, however fat mass was significantly increased by junk-food in obesity-prone and obesity-resistant groups compared to chow. Furthermore, fasted plasma insulin levels were elevated in obesity-prone vs. obesity-resistant groups, regardless of diet. D) Average time spent in the center during open field testing following 4 weeks of chow or junk-food diet. Junk-food did not alter anxiety-like behaviors in either group, but the obesity-prone groups spent significantly less time in the center than obesity-resistant groups. E) Average time spent in closed arms during elevated plus maze testing. Junk-food did not alter anxiety-like behaviors in either group, but the obesity-prone groups spent significantly more time in the closed arm than obesity-resistant groups. F) Average locomotor activity in a novel environment. Locomotor activity was similar between obesityprone and obesity-resistant groups, regardless of diet or strain. Data are shown as mean ± SEM. * =p < 0.05, ***=p < 0.001 (OP vs. OR), #=p < 0.05 (JF vs.Chow).
3.4. Experiment 4: Anxiety-like behavior in female obesity-prone rats is greater compared to obesity-resistant females
Fig. 5 shows measures of adiposity and anxiety-like behavior in female obesity-prone and obesity-resistant rats maintained on chow. Consistent with data in males, no differences in anxiety-like behavior were found when body weight was similar between groups (Fig. 5A-E). However, greater anxiety-like behavior was found in obesity-prone vs. obesity-resistant females that differed in weight (Fig. 5F: Two-tailed unpaired t-test, t(28) = 2.868, p < 0.01). Specifically, in the open field test, obesity-prone females tended to spend less time in the center (Fig. 5H: Two-tailed unpaired t-test, t(8) = 2.181, p = 0.06), and made significantly fewer entries to the center (Fig. 5I: Two-tailed unpaired t-test, t(8) = 2.666, p < 0.05) than obesity-resistant females. No differences in traverses were found between groups (Fig. 5J), thus group differences are not likely attributable to differences in exploratory behavior. Similarly, in the elevated plus maze obesity-prone females spent significantly less time in the open arms (Fig. 5K: Two-tailed unpaired t-test, t(16) = 2.496, p < 0.05), and more time in the closed arms (Fig. 5L: Two-tailed unpaired t-test, t(16) = 2.22, p < 0.05) than obesity-resistant females. Although there were apparent trends, there were no statistical differences in the number of open arm entrances (Fig. 5M: Two-tailed unpaired t-test, t(16) = 1.56, p = 0.14). Despite differences in weight, no differences in plasma leptin concentrations (an indirect measure of adiposity) were observed between obesity-prone and obesity-resistant female rats (Fig. 5G).
Fig. 5.
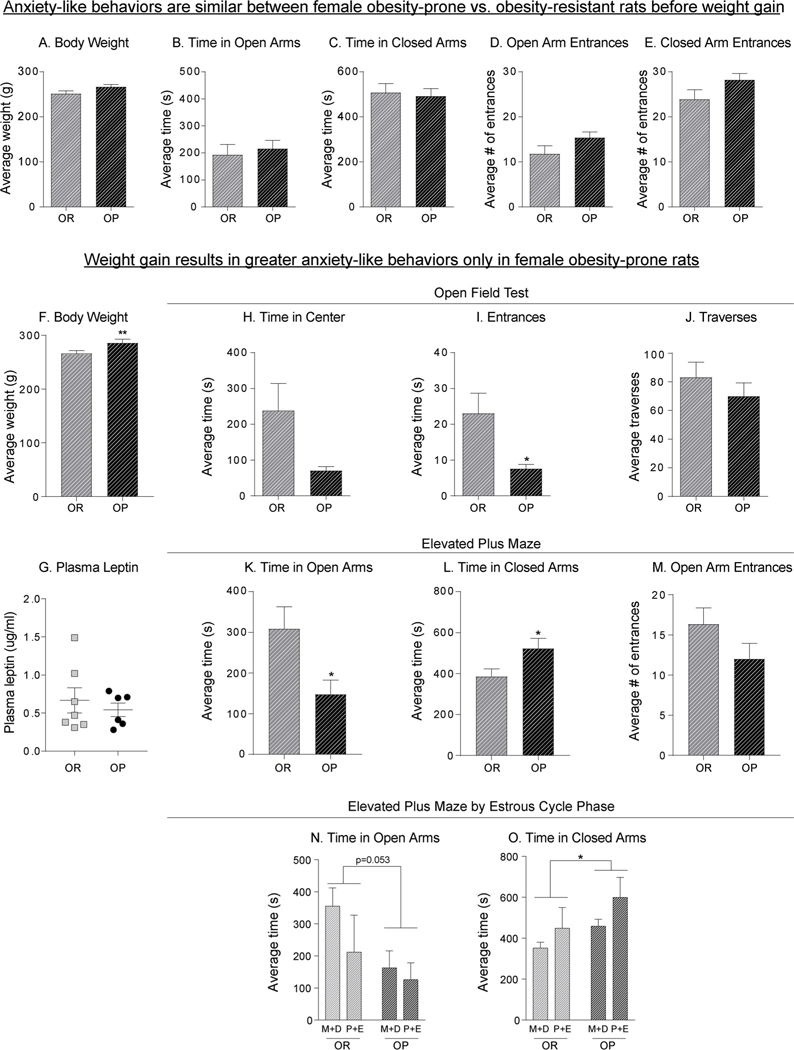
Similar to males, anxiety-like behaviors emerge with spontaneous weight gain in obesity-prone females and anxiety-like behavior varied across the cycle in both groups. A) Average body weight is initially similar between obesity-prone and obesity-resistant female rats. B–E) In the absence of weight differences, anxietylike behavior in the elevated plus maze does not differ between obesity-prone and obesity-resistant females. F) Similar to males, obesity-prone females become heavier than obesity-resistant females as they age. G) Average plasma leptin concentrations did not differ between groups. H–J) Measures of anxiety-like behavior in open field test. Obesity-prone females tend to spend less time in the center, and made significantly fewer entries to the center than obesity-resistant females. K–M) Measures of anxiety-like behavior in elevated plus maze indicate that obesity-prone females display increased anxiety-like behaviors. N–O) Measures of anxiety-like behaviors in elevated plus maze by estrous cycle phase; metestrus and diestrus (M+D), proestrus and estrus (P+E). Obesity-prone females spent less time in the open arms and more time in the closed arms compared to obesity-resistant rats, regardless of estrous cycle phase. Data are shown as mean ± SEM. * =p < 0.05, ** = p < 0.01.
Potential effects of the cycle on behavior in the elevated plus maze were also assessed. Consistent with the data above, obesity-prone females spent less time in open arms (Fig. 5N: Two-way ANOVA: main effect of group, F (1,14) = 4.47, p = 0.05, no group x cycle interaction F (1,14) = 0.644, p = 0.43), more time in the closed arms (Fig. 5O: Two-way ANOVA: main effect of group, F (1,14) = 4.61, p = 0.05; no group x cycle interaction F (1,14) = 0.133, p = 0.72), and showed no differences in open arm entrances (Fig. 5M) compared to obesity-resistant females regardless of estrous cycle phase. Furthermore, there were some apparent trends for anxiety-like behavior to be greater during proestrus/estrus than metestrus/dietestrus in both groups (Two-way ANOVA: time in closed arms, main effect of cycle (F(1,14) = 3.93, p = 0.067). In sum, similar to effects seen in males, differences in anxiety-like behavior were present only following weight gain in female obesity-prone vs. obesity-resistant rats, this group difference did not appear to vary across the cycle, although cycle phase may modulate anxiety-like behavior in both obesity-prone and obesity-resistant strains.
3.5. There is a positive correlation between adiposity and anxiety-like behavior in obesity-prone male, but not female rats
Fig. 6 shows pooled data from studies above using the elevated plus maze (male OP N = 32, female OP N = 10, male OR N = 24, female OR N = 13), and compares the relationship between time spent in the closed arm vs. body weight (Fig. 6A and C) or plasma leptin levels (Fig. 6B and D). These measures were chosen because we obtained leptin levels from both females and males, the largest number of rats were tested in the elevated plus maze, and because circulating leptin levels are proportional to fat mass. Strong positive correlations were found in male obesity-prone rats between time spent in the closed arms and weight (Fig. 6A: r2 = 0.5744; p < 0.0001) as well as time spent in the closed arms and plasma leptin levels (Fig. 6B: r2 = 0.2093; p = 0.0097). No relationship was observed between these factors in obesity-resistant males (Fig. 6A: r2 = 0.00087, p = 0.89; Fig. 6B: r2 = 0.058, p = 0.27). In contrast to male subjects, there was no relationship between weight or plasma leptin levels and time spent in the closed arms in female rats (Fig. 6C: r2 = 0.032, p = 0.59; Fig. 5D: r2 = 0.0001, p = 0.92).
Fig. 6.
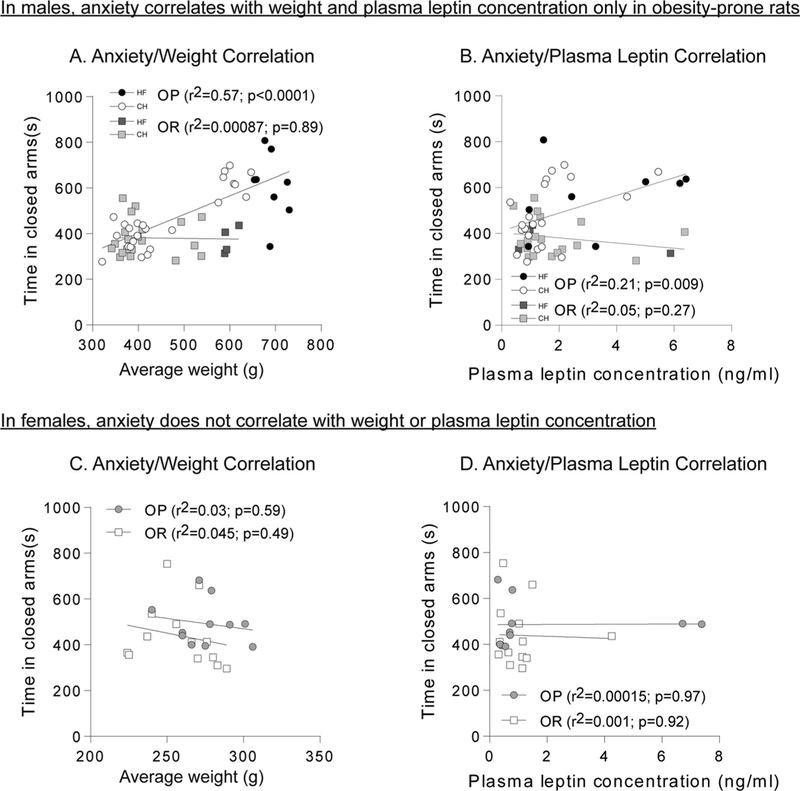
Weight and adiposity are positively correlated with anxiety-like behavior only in male obesity-prone rats. A) Relationship between weight and time spent in closed arms in males. A significant correlation is present in obesity-prone, but not obesity-resistant rats. B) Relationship between plasma leptin concentration and time spent in closed arms in males. A significant correlation is present in obesity-prone, but not obesity-resistant rats. C) Relationship between weight and time spent in closed arms in females. D) Relationship between plasma leptin concentration and time spent in closed arms in females. No significant correlations were found in females.
3.6. Experiment 5: Diet-induced obesity does not alter anxiety-like behavior in outbred male rats
Fig. 7 shows body weight and measures of anxiety-like behavior in the elevated plus maze in separate groups of outbred male rats given prolonged access to chow, junk-food, or high-fat diet. As expected, high-fat fed rats were significantly heavier than chow or junk-food fed groups (Fig. 7A: One-way ANOVA: F(2,27) = 13.52, p < 0.0001; Tukey’s post-test: Chow vs. HF: p < 0.01; JF vs. HF: p < 0.0001). However, no differences were observed between any group for time spent in open arms (Fig. 7B: One-way ANOVA: F (2, 20) = 1.70, p = 0.21), time spent in closed arms (Fig. 7C: One-way ANOVA: F (2, 20) = 0.47, p = 0.63), or number of open arm entrances (Fig. 7D: One-way ANOVA: F(2, 20) = 0.50, p = 0.62).
Fig. 7.
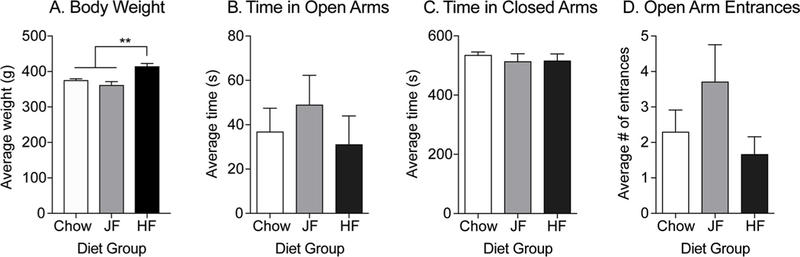
Prolonged junk-food or high-fat diet (48 days) does not alter anxiety-like behavior in outbred male rats. A) Average body weight following 48 days of free access to chow, junk-food (JF) or high-fat (HF) diet. Animals given a high-fat diet were significantly heavier than those fed either chow or junk-food. B–D) Measures of anxiety-like behavior in the elevated plus maze. In outbred males, junk-food or high-fat diet did not alter anxiety-like behavior compared to chow-fed animals. Data are shown as mean ± SEM. ** = p < 0.01.
4. Discussion
Previous studies have demonstrated that anxiety and obesity are often comorbid in both humans [1,2,32] and in rodent models [33–35]. However, it remains difficult to determine whether obesity leads to the development of anxiety, whether basal differences in anxiety may promote obesity, or a combination of the two. Here, we utilized both selectively bred and outbred rats to examine the relationship between pre-existing vs. obesity-induced increases in anxiety-like behavior. Weight gain and fat mass vary from ‘overweight’ to ‘obese’ to ‘obesity accompanied by metabolic dysregulation’. Each of these designations is not one explicit state, but rather is a representation of key stages across a continuum. Based on weight gain, fasted insulin levels, and fat mass, young adult obesity-prone and obesity-resistant rats begin within the normal range of the outbred rodent population [25,36]. As they age, obesity-prone rats diverge from their obesity-resistant counterparts, accumulating more fat and showing mild elevations in fasted plasma insulin levels that do not yet constitute metabolic dysregulation (i.e., overweight; [25,26]). However, when given a high-fat diet, obesity- prone rats rapidly increase their fat mass and become metabolically compromised, though this effect varies by several factors including diet composition and duration of diet consumption (see Ref. [37] for review).
While many studies utilizing high fat diets and/or obesity-prone rats provide access for 6–8 weeks, we observe trends towards differences in adiposity and weight as early as 4 weeks, with some (but not all) cohorts reaching differing statistically in weight and fat mass even at this early timepoint. Many previous studies have utilized single housing, which can increase food intake and decrease activity levels, leading to faster rises in weight. Coincidentally, single housing can also have significant effects on anxiety-like behaviors which led us to maintain our animals in pair or group housing. Obesity-resistant rats can also gain fat mass on a high-fat diet, reaching levels of fat mass that are comparable to that of ‘overweight’ obesity-prone rats given standard lab chow, but that have not yet transitioned to a metabolically compromised state. Thus, this model is useful for examining behavioral differences in obesity-prone vs. -resistant populations prior to and after the development of varying degrees of obesity. We found that in both males and females, increases in anxiety-like behavior emerged with weight gain in obesity-prone but not obesity-resistant rats. These data suggest that interactions between genetic predisposition and alterations accompanying weight gain promote anxiety in some susceptible individuals.
4.1. Relationships between predisposition, weight gain, and anxiety-like behaviors in males
Measures of anxiety-like behavior in the elevated plus maze are similar in obesity-prone and obesity-resistant male rats prior to weight gain (Fig. 2A-F). However, when these same rats were tested again after 4 weeks of high-fat diet or spontaneous weight gain, anxiety-like behavior in the open field was enhanced in obesity-prone vs. obesity- resistant groups (Fig. 2G-L). No statistically significant effect of diet was observed within the obesity-prone rats; however, this appears to be a floor effect with animals on both a chow and high fat diet entering the center very infrequently. It is possible that a different paradigm might be used in the future to tease apart more subtle effects of diet manipulation within this strain. Similar differences in anxiety-like behaviors were found in a separate cohort of rats tested in the elevated plus maze following 8 weeks of high-fat diet consumption, corroborating our initial observation.
In males the degree of anxiety-like behavior correlated with fat mass and fasted plasma leptin levels (Fig. 6A and B). These data appear to be in contrast to a recent study by Vogel et al. [33], which found mild elevations in anxiety-like behaviors in obesity-prone rats prior to any diet manipulation, but no correlation between weight and behavior. This could be due to differences in the lines of rats used, as rats in the current study were offspring of breeders originally obtained from Taconic, whereas rats in the study by Vogel et al. [33] were obtained from Charles River, or to the use of weight gain previously vs. fat mass, plasma insulin, and plasma leptin levels used here. For example, we have found that elevations in peripheral insulin levels precede observable weight differences between obesity-prone and obesity-resistant rats [25,29]. Furthermore, differences in anxiety-like behavior in the Vogel paper were less robust than those observed here. This supports the idea of a continuum in the relationship between anxiety-like behaviors and alterations accompanying weight gain. Consistent with this, Vogel et al. [33] also found greater anxiety-like behaviors in Zucker rats compared to obesity-prone animals, possibly due to greater obesity and associated peripheral dysregulation.
Following our initial 4-week diet manipulation studies, we determined whether moderate obesity was sufficient to enhance anxiety-like behaviors in obesity-resistant rats. To do this, a second set of obesity- resistant and obesity-prone rats were fed a high-fat diet for 8 weeks, leading to significant weight gain in obesity-resistant rats compared to their chow fed counterparts, and increases in fat mass that were comparable to that of obesity-prone rats fed a chow diet (Fig. 3A-C). As was seen previously, compared to obesity-resistant rats given chow, anxiety-like behavior of obesity-prone rats was enhanced (Fig. 3D-F). However, despite having similar fat mass, weight, and fasted leptin levels to chow-fed obesity-prone rats who showed high levels of anxiety-like behavior, anxiety-like behavior remained unaffected in obesity-resistant rats given high-fat diet (Fig. 3D-F). This suggests that weight gain and accompanying alterations, independent of susceptibility, are not sufficient to enhance these measures of anxiety.
We next examined effects of a sugary, fatty junk-food diet on anxiety-like behaviors. Junk-food diet led to significant increases in weight in both strains when compared to chow fed counterparts, and stronger anxiety-like behavior was again observed in obesity-prone groups, regardless of diet (Fig. 4). However, as with the high-fat diet, obesity-resistant rats failed to display any changes in anxiety-like behaviors. This further suggests that diet and weight gain are not sufficient to induce anxiety in obesity-resistant rats, even in the presence of increased adiposity. Given the heterogeneity of effects of different foods on anxiety, we cannot rule out the possibility that a different diet composition may be required to induce anxiety-like behaviors in obesity-resistant rats. For example, diets made from animal fats and trans fats may be more anxiogenic than saturated fats ([38]; for review see Ref. [13]). However, both diets used here are composed largely of animal and soy-based fat, which have been linked to anxiogenic effects ([39] for review). None-the-less, our data do show that increases in fat mass or consumption of fatty, sugary foods in obesity-resistant rats are not in-and-of-themselves sufficient to enhance anxiety.
The role of obesogenic diets and obesity in the development of anxiety-like behaviors is rather complex. It has been recently demonstrated that chronic consumption of high-fat diets increases anxiety- and depressive-like behaviors, heightens the HPA axis and is responsible for biochemical changes in brain reward circuitry [8]. However, highly-palatable diets have different effects on the development of anxiety-like behaviors, depending on the type of diet, length of administration, age that it was fed and whether the rats became obese. For example, some studies have shown that feeding highly-palatable diets to recently weaned rats and during the pre-pubertal periods can be anxiolytic [9]. In addition, another study showed that limited sucrose intake has stress-relieving properties [39]. In contrast, adult rats fed a high-fat diet and that developed an obesity phenotype showed increase anxiety-like behaviors [7]. Hence, the anxiolytic or anxiogenic effects of highly-palatable foods will depend on the administration of the diet, age of the animal, additional stressors, individual susceptibility to obesity and quality of prior experiences to the behavioral test.
In our experiments, increases in anxiety-like behavior in obesity- prone rats were generally similar whether induced by spontaneous weight gain, high-fat, or junk-food diet. This may be due to floor effects, in which case the lack of an effect of diet may be due to the sensitivity of the measure. However, it is important to note that while no differences in these behaviors were seen on average, there was a positive correlation between anxiety-like behaviors and plasma leptin levels as well as fat mass in obesity-prone males (Fig. 6A and B). This suggests that there is not simply an all-or-none relationship between obesity and anxiety, but rather that anxiety-like behavior may scale with physiological alterations that accompany obesity, at least in males (see Section 4.2 for discussion of results of female studies). Of course, we cannot rule out an age effect in the current study, something that should be addressed in future studies.
We also examined the relationship between obesity and anxiety-like behaviors in outbred male Sprague-Dawley rats given chow, junk-food or high-fat diet for 48 days. The inability of the JF diet to induce significant weight gain may be attributable to the similarities in caloric content between the JF diet and a standard chow diet (4.5kcal/g and 4.07 kcal/g respectively), or a result of insufficient heterogeneity in susceptibility to weight gain in this particular cohort. No significant effects on anxiety-like behavior were observed between groups, although high-fat fed animals displayed significant increases in weight (Fig. 7). This could suggest that differences observed in obesity-prone animals may be a result of not only weight gain, but also selective- breeding which is expected to amplify phenotypic differences. It is also interesting to note that similarities exist in anxiety-like behaviors between obesity-prone and outbred rats. The expected response of animals in our behavioral tests, and that observed in both outbred and OP rats, is to limit time spent in open, unprotected spaces. Obesity-resistant rats do not appear to follow this trend. Thus, it is possible that obesity- resistant rats are not as good at perceiving external cues that might be potentially harmful. In addition, we have observed that the obesity- resistant rats are not as good at engaging and perceiving food-associated cues [22]. Hence, obesity-resistant rats may be lacking internal mechanisms to detect and engage with external cues that might be useful for their survival. Alternatively, the absence of an effect in outbred rats may simply have been due to insufficient heterogeneity in weight gain due to a relatively small outbred sample size (N = 10/ group). Additionally, we cannot rule out the possibility that running outbred animals during their light phase may have dampened overall responses, masking more subtle behaviors. Thus, larger scale studies of outbred populations are needed before a firm conclusion can be drawn regarding the generalizability of the effects observed in obesity-prone rats.
4.2. Relationships between predisposition, weight gain, and anxiety-like behaviors in females
To our knowledge, interactions between obesity and anxiety-like behaviors have not previously been examined in female selectively bred obesity-prone vs. obesity-resistant rats, even though females are at a higher risk for developing obesity and anxiety disorders (see Ref. [40] for review). Similar to males, obesity-prone female rats also develop anxiety-like behaviors alongside weight gain compared to female obesity-resistant rats (Fig. 5). Thus, the overall pattern of behavioral data in females was similar to that of males. However, we did not find any correlation between weight, or leptin levels and anxiety-like behavior in females, while there were correlations between these measures and anxiety-like behavior in males. The absence of these correlations in females could simply be due to a lack of sensitivity in these measures, however if this were the case, this should have equally affected data from males. Alternatively, these data could indicate that the mechanisms underlying the interaction between weight gain and enhanced anxiety differ between males and females. For example, neuroinflammation increases with obesity, and contributes to anxiety (see Ref. [41] for review) and males and females differ in their central and peripheral responses to inflammation. For example, in males, accumulation of fat in abdominal and visceral depots correlates with a deleterious metabolic profile that results in an inflammatory response. Whereas accumulation of subcutaneous fat in the gluteofemoral areas in females is correlated with a beneficial metabolic profile and a decrease in inflammatory responses (see Ref. [42] for review). Thus, the absence of correlations between overall fat mass and anxiety-like behavior in females may be an artifact of the general NMR measure used, which does not distinguish between different fat depots.
Although only a few studies have been conducted, data to date suggest that ovarian hormones can influence the expression of anxiety [43]. Here, we observed trends for greater anxiety-like behavior during proestrus/estrus compared to metestrus/diestrus that were similar in obesity-prone and obesity-resistant groups (Fig. 5N and O). This is consistent with previous reports showing that anxiety-like behaviors increase in the proestrus and estrus phases under high light conditions similar to those used in the current study [44], although the opposite relationship has been found when females are tested under low-light conditions [43–46]. However, regardless of any effect of the cycle on anxiety-like behavior, the data here show that behavioral differences between obesity-prone and obesity-resistant females cannot be explained by differences in behavior across the cycle.
4.3. What may be driving increases in anxiety-like behaviors?
While it is challenging to determine which of the many changes cooccurring with weight gain may mediate the observed increases in anxiety, positive correlations between plasma leptin levels and anxiety-like behaviors in obesity-prone, but not obesity-resistant rats may provide clues to potential mechanisms, at least in males. Leptin is involved in the regulation of corticotropin releasing factor (CRF). Some have reported that increases in leptin lead to increases in CRF [47,48]. Furthermore, elevated levels of CRF can increase anxiety-like behaviors in rodent models (See Ref. [49] for review) and alterations in CRF systems have been reported in some anxiety disorders [50–52]. A better understanding of potential changes in CRF signaling and peripheral signals associated with obesity and adiposity in the OP/OR model could be important in understanding the link between genetic susceptibility, obesity-development, and anxiety-like behaviors. Of course, many different hormones and neuromodulators are altered by obesity, thus it’s unlikely that changes in leptin alone may account for behavioral differences. For example, insulin resistance either via knockout of the insulin receptor [53] or via obesity-induced insulin resistance and inflammation [54] can also increase anxiety-like behaviors. Thus, future studies should address the potential role of insulin resistance, metabolic dysregulation, and inflammation in the effects reported here. Of course, it should also be noted that additional studies are necessary to tease apart potential contributions of aging vs increased adiposity to anxiety-like phenotypes that have been observed.
5. Summary
Our data demonstrate interactions between susceptibility to obesity and anxiety-like behaviors in males and females. In multiple cohorts, baseline measures of anxiety-like behavior did not differ between obesity-prone and obesity-resistant rats, but differences emerged even with mild increases in adiposity in obesity-prone groups. Increases in fat mass were not sufficient to induce anxiety-like behaviors in either obesity-resistant or outbred rats. While we have put forth several plausible mechanisms by which obesity development may lead to anxiety-like behaviors, it is important to note that additional studies are needed to determine causal relationships, and that effects found in obesity-prone rats may be due to shared neurobiological factors or to dissociable neurobiological factors that are both influenced by a third factor. It remains to be determined whether selective breeding for an obesity-prone phenotype has co-selected for anxiety-like traits, although a lack of anxiety prior to obesity development suggests that at the least, these traits are related either directly or by a common, as yet unidentified, factor. These data suggest that susceptibility to obesity in combination with physiological alterations accompanying weight gain lead to enhancements in anxiety-like behaviors.
Acknowledgements
This work was supported by a Brain and Behavior Research Foundation NARSAD Young Investigator Award, NIH, NIDDK, USAR01DK115526 and R01-DK106188 to CRF, University of Michigan Rackham Merit Fellowship, R01DK106188–02-S1 and F99 NS108549– 01 to YAC, the Biology of Drug Abuse training grant fellowship T32DA007268 awarded to PJV. Studies also utilized the Chemistry Core of the Michigan Diabetes Research and Training Center funded by DK020572 awarded by NIDDK
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Daumit GL, Clark JM, Steinwachs DM, Graham CM, Lehman A, Ford DE, Prevalence and correlates of obesity in a community sample of individuals with severe and persistent mental illness, J. Nerv. Ment. Dis. 191 (12) (2003) 799–805, 10.1097/01.nmd.0000100923.20188.2d. [DOI] [PubMed] [Google Scholar]
- [2].Dickerson FB, Brown CH, Kreyenbuhl JA, Fang L, Goldberg RW, Wohlheiter K, Dixon LB, Obesity among individuals with serious mental illness, Acta Psychiatr. Scand. 113 (4) (2006) 306–313, 10.1111/jM600-0447.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- [3].Luppino FS, De Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, Zitman FG, Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies, March 1, Arch. Gen. Psychiatry Am. Med. Assoc. (2010), 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- [4].Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. , Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve, Proc. Natl. Acad. Sci. U. S. A. 108 (38) (2011) 16050–16055, 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mackos AR, Eubank TD, Parry NMA, Bailey MT, Probiotic lactobacillus reuteri attenuates the stressor-enhanced severity of citrobacter rodentium infection, Infect. Immun. 81 (9) (2013) 3253–3263, 10.1128/IAI.00278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ohland CL, Kish L, Bell H, Thiesen A, Hotte N, Pankiv E, Madsen KL, Effects of lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome, Psychoneuroendocrinology 38 (9) (2013) 1738–1747, 10.1016/j.psyneuen.2013.02.008. [DOI] [PubMed] [Google Scholar]
- [7].Souza CG, Moreira JD, Siqueira IR, Pereira AG, Rieger DK, Souza DO, Perry MLS, Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior, Life Sci. 81 (3) (2007) 198–203, 10.1016/J.LFS.2007.05.001. [DOI] [PubMed] [Google Scholar]
- [8].Sharma S, Fulton S, Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry, Int. J. Obes. (Lond.) (2012), 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- [9].de Lima Marcolin M, Benitz A, de ND, Arcego DM, Noschang C, Krolow R, Dalmaz C, Effects of early life interventions and palatable diet on anxiety and on oxidative stress in young rats, Physiol. Behav. 106 (4) (2012) 491–498, 10.1016/j.physbeh.2012.03.025. [DOI] [PubMed] [Google Scholar]
- [10].Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG, After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/ acetylcholine imbalance, Physiol. Behav. 94 (3) (2008) 309–315, 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, et al. , Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function, Proc. Natl. Acad. Sci. U. S. A. 112 (51) (2015) 201511593, 10.1073/pnas.1511593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morganstern I, Ye Z, Liang S, Fagan S, Leibowitz SF, Involvement of cholinergic mechanisms in the behavioral effects of dietary fat consumption, Brain Res. 1470 (2012) 24–34, 10.1016/j.brainres.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Murphy M, Mercer JG, Diet-regulated anxiety, Int. J. Endocrinol. (Hindawi Publishing Corporation; ) (2013), 10.1155/2013/701967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wardle J, Carnell S, Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment, Am. J. Clin. Nutr. 87 (2) (2009) 398–404, 10.1007/s12160-009-9116-5. [DOI] [PubMed] [Google Scholar]
- [15].Albuquerque D, Stice E, Rodríguez-López R, Manco L, Nóbrega C, Current review of genetics of human obesity: from molecular mechanisms to an evolutionary perspective, Mol. Genet. Genom. (2015), 10.1007/s00438-015-1015-9. [DOI] [PubMed] [Google Scholar]
- [16].Zender R, Olshansky E, Women’s mental health: depression and anxiety, Nurs. Clin. North Am. (2009), 10.1016/j.cnur.2009.06.002. [DOI] [PubMed] [Google Scholar]
- [17].Zhao G, Ford ES, Dhingra S, Li C, Strine TW, Mokdad AH, Depression and anxiety among US adults: associations with body mass index, Int. J. Obes. 33 (2) (2009) 257–266, 10.1038/ijo.2008.268. [DOI] [PubMed] [Google Scholar]
- [18].Alonso-Caraballo Y, Jorgensen ET, Brown TE, Ferrario CR, Functional and structural plasticity contributing to obesity: roles for sex, diet, and individual susceptibility, Curr. Opin. Behav. Sci. 23 (2018) 160–170, 10.1016/j.cobeha.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Levin BE, Dunn-Meynell A, Balkan B, Keesey RE, Selective breeding for diet- induced obesity and resistance in Sprague-Dawley rats, Am. J. Physiol. 273 (1997) R725–R730. [DOI] [PubMed] [Google Scholar]
- [20].Gorski JN, Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance, Ajp Regul. Integr. Comp. Physiol. 291 (3) (2006) R768–R778, 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- [21].Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, et al. , Long-term characterization of the diet-induced obese and diet- resistant rat model: a polygenetic rat model mimicking the human obesity syndrome, J. Endocrinol. 206 (3) (2010) 287–296, 10.1677/JOE-10-0004. [DOI] [PubMed] [Google Scholar]
- [22].Derman RC, Ferrario CR, Enhanced incentive motivation in obesity-prone rats is mediated by NAc core CP-AMPARs, Neuropharmacology 131 (2017) 326–336, 10.1016/j.neuropharm.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Naneix F, Tantot F, Glangetas C, Kaufling J, Janthakhin Y, Boitard C, et al. , Impact of early consumption of high-fat diet on the mesolimbic dopaminergic system, Eneuro 4 (3) (2017), 10.1523/ENEURO.0120-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Oginsky MF, Maust JD, Corthell JT, Ferrario CR, Enhanced cocaine-induced locomotor sensitization and intrinsic excitability of NAc medium spiny neurons in adult but not in adolescent rats susceptible to diet-induced obesity, Psychopharmacology 233 (5) (2016) 773–784, 10.1007/s00213-015-4157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vollbrecht PJ, Nobile CW, Chadderdon AM, Jutkiewicz EM, Ferrario CR, Pre-existing differences in motivation for food and sensitivity to cocaine-induced locomotion in obesity-prone rats, Physiol. Behav. 152 (Pt. A) (2015) 151–160, 10.1016/j.physbeh.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Levin BE, Why some of us get fat and what we can do about it, J. Physiol. (Lond.) 583 (Pt. 2) (2007) 425–430, 10.1113/jphysiol.2007.135434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cryan JF, Sweeney FF, The age of anxiety: role of animal models of anxiolytic action in drug discovery, Br. J. Pharmacol. 164 (4) (2011) 1129–1161, 10.1111/j.1476-5381.2011.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Walsh RN, Cummins RA, The open-field test: a critical review, Psychol. Bull. 83 (3) (1976) 482–504, 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- [29].Vollbrecht PJ, Mabrouk OS, Nelson AD, Kennedy RT, Ferrario CR, Pre-existing differences and diet-induced alterations in striatal dopamine systems of obesity-prone rats, Obesity 24 (3) (2016) 670–677, 10.1002/oby.21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Murphy T, Dias GP, Thuret S, Effects of diet on brain plasticity in animal and human studies: mind the gap, Neural Plast. (2014), 10.1155/2014/563160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Marcondes FK, Bianchi FJ, Tanno AP, Determination of the estrous cycle phases of rats: some helpful considerations, Braz. J. Biol. (2002), 10.1590/S1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- [32].Gariepy G, Nitka D, Schmitz N, The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis, Int. J. Obes. 34 (3) (2010) 407–419, 10.1038/ijo.2009.252. [DOI] [PubMed] [Google Scholar]
- [33].Vogel H, Kraemer M, Rabasa C, Askevik K, Adan RAH, Dickson SL, Genetic predisposition to obesity affects behavioral traits including food reward and anxiety-like behavior in rats, Behav. Brain Res. 328 (2018) 95–104, 10.1016/j.bbr.2017.02.037. [DOI] [PubMed] [Google Scholar]
- [34].Hryhorczuk C, Sharma S, Fulton SE, Metabolic disturbances connecting obesity and depression, Front. Neurosci. 7 (2013) 177, 10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sivanathan S, Thavartnam K, Arif S, Elegino T, McGowan PO, Chronic high fat feeding increases anxiety-like behaviour and reduces transcript abundance of glucocorticoid signalling genes in the hippocampus of female rats, Behav. Brain Res. (2015), 10.1016/j.bbr.2015.02.036. [DOI] [PubMed] [Google Scholar]
- [36].Lillie LE, Temple NJ, Florence LZ, Reference values for young normal Sprague- Dawley rats: weight gain, hematology and clinical chemistry, Hum. Exp. Toxicol. 15 (8) (1996) 612–616, 10.1177/096032719601500802. [DOI] [PubMed] [Google Scholar]
- [37].Giles ED, Jackman MR, MacLean PS, Modeling diet-induced obesity with obesity-prone rats: implications for studies in females, Front. Nutr. 3 (2016) 50, 10.3389/fnut.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mizunoya W, Iwamoto Y, Shirouchi B, Sato M, Komiya Y, Razin FR, Ikeuchi Y, et al. , Dietary fat influences the expression of contractile and metabolic genes in rat skeletal muscle, PLoS One 8 (11) (2013), 10.1371/journal.pone.0080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ulrich-Lai YM, Fulton S, Wilson M, Petrovich G, Rinaman L, Stress exposure, food intake and emotional state, Stress (Amsterdam, Netherlands) 18 (4) (2015) 381–399, 10.3109/10253890.2015.1062981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lopresti AL, Drummond PD, Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment, Prog. Neuropsychopharmacol. Biol. Psychiatry (2013), 10.1016/j.pnpbp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- [41].Guillemot-Legris O, Muccioli GG, Obesity-induced neuroinflammation: beyond the hypothalamus, Trends Neurosci. (2017), 10.1016/j.tins.2017.02.005. [DOI] [PubMed] [Google Scholar]
- [42].Leeners B, Geary N, Tobler PN, Asarian L, Ovarian hormones and obesity, Hum. Reprod. Update 23 (3) (2017) 300–321, 10.1093/humupd/dmw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Donner NC, Lowry CA, Sex differences in anxiety and emotional behavior, Pflügers Arch.—Eur. J. Physiol. 465 (5) (2013) 601–626, 10.1007/s00424-013-1271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mora S, Dussaubat N, Diaz-Veliz G, Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats, Psychoneuroendocrinology 21 (7) (1996) 609–620, 10.1016/S0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- [45].Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC, Estrous cycle influences the response of female rats in the elevated plus-maze test, Physiol. Behav. 74 (4–5) (2001) 435–440, 10.1016/S0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- [46].ter Horst JP, de Kloet ER, Schachinger H, Oitzl MS, Relevance of stress and female sex hormones for emotion and cognition, Cell. Mol. Neurobiol. 32 (5) (2012) 725–735, 10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Costa A, Poma A, Martignoni E, Nappi G, Ur E, Grossman A, Stimulation of corticotrophin-releasing hormone release by the obese (ob) gene product, leptin, from hypothalamic explants, NeuroReport 8 (5) (1997) 1131–1134, 10.1097/00001756-199703240-00014. [DOI] [PubMed] [Google Scholar]
- [48].Yamagata S, Kageyama K, Akimoto K, Watanuki Y, Suda T, Daimon M, Regulation of corticotropin-releasing factor and urocortin 2/3 mRNA by leptin in hypothalamic N39 cells, Peptides 50 (2013) 1–7, 10.1016/j.peptides.2013.09.010. [DOI] [PubMed] [Google Scholar]
- [49].Koob GF, Corticotropin-releasing factor, norepinephrine, and stress, Biol. Psychiatry 46 (9) (1999) 1167–1180, 10.1016/j.bbagen.2009.07.018. [DOI] [PubMed] [Google Scholar]
- [50].Bangasser DA, Kawasumi Y, Cognitive disruptions in stress-related psychiatric disorders: a role for corticotropin releasing factor (CRF), Horm. Behav. 76 (2015) 125–135, 10.1016/j.yhbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, et al. , Overexpressing corticotropin-releasing factor in the primate amygdala increases anxious temperament and alters its neural circuit, Biol. Psychiatry 80 (5) (2016) 345–355, 10.1016/j.biopsych.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Weber H, Richter J, Straube B, Lueken U, Domschke K, Schartner C, et al. , Allelic variation in CRHR1 predisposes to panic disorder: evidence for biased fear processing, Mol. Psychiatry 21 (6) (2016) 813–822, 10.1038/mp.2015.125. [DOI] [PubMed] [Google Scholar]
- [53].Kleinridders A, Cai W, Cappellucci L, Ghazarian A, Collins WR, Vienberg SG, et al. , Insulin resistance in brain alters dopamine turnover and causes behavioral disorders, Proc. Natl. Acad. Sci. U. S. A. 112 (11) (2015) 3463–3468, 10.1073/pnas.1500877112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Soto M, Herzog C, Pacheco JA, Fujisaka S, Bullock K, Clish CB, Kahn CR, Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism, Mol. Psychiatry 1 (2018), 10.1038/s41380-018-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


