Abstract
Objectives:
To investigate the wear behavior of novel graded glass/zirconia materials and their abrasiveness to the antagonist relative to homogeneous zirconias (polished or glazed) and a glass-ceramic.
Methods:
Graded glass/zirconia specimens were prepared by sintering with concurrent glass-infiltration of pre-sintered zirconia (3Y-TZP) with a polished or as-machined surface. Monolithic zirconia samples were sintered and their surfaces were polished or glazed (as-machined). Glass-ceramic samples were obtained and the surface polished. All specimens were subjected to chewing simulations with a steatite antagonist (r = 3 mm) and a cyclic load of 50 N. Quantitative measurements of wear and roughness were performed on ceramics and antagonists for prescribed number of cycles. Damage sustained in ceramics and antagonists was analyzed by SEM.
Results:
The polished zirconia presented little to no variation in wear depth (2 μm) and roughness (0.06 μm). Graded and glazed zirconia experienced a rapid increase in wear depth while the superficial glass layer was present (until 1000 cycles), but showed little variations afterwards—at 450k cycles ~15 μm for graded and 78 μm for glazed zirconia. The glass-ceramic presented the greatest wear depth (463 μm) and roughness (1.48 μm). Polished zirconia, polished graded zirconia and glazed zirconia yielded significantly lower volumetric wear (~3 mm3) of the antagonist than as-machined graded zirconia and glass-ceramic (~5 mm3).
Significance:
Polished graded zirconia and polished zirconia presented little wear and roughness, as well as yielded reduced antagonist wear. Glassy materials are both more susceptible to wear and more abrasive to the antagonist relative to zirconia.
Keywords: Graded zirconia, glass-infiltration, Y-TZP, glass-ceramic, steatite, sliding wear, volume loss
1. INTRODUCTION
Full-contour zirconia crowns are increasingly used in restorative dentistry, due to their superior fracture resistance relative to porcelain-fused-to-zirconia (PFZ) restorations [1–4]. Although only preliminary clinical results on the lifetime of monolithic zirconia restorations are available [1,2,5–8], there is a general belief that these crowns are likely immune to fracture. Nonetheless, concerns exist regarding (1) the abrasiveness of zirconia restorations towards their antagonists, and (2) the esthetic appearance of such restorations. The high hardness characteristics of zirconia [9,10] makes it a tedious process to achieve a good surface finish on crowns with complex anatomies via hand polishing [11], since a poorly polished zirconia occlusal surface can give rise to increased antagonist wear [12,13]. Extensive in vitro research [14–19] and early results of clinical trials [1,7,8,20,21] have reported that well-polished or glazed zirconia may cause more antagonist wear than natural teeth, yet still within the range of other dental ceramics. In addition, fairly esthetic monolithic zirconia restorations can be produced by using shaded materials, while excellent esthetics may be achieved by means of post-sintering staining and glazing. Both patients and dentists have reported great satisfaction with the esthetic results of glazed monolithic zirconia crowns after up to 3 years [6], which is similar to the satisfaction reported for the esthetics obtained with PFZ restorations [22].
An alternative technique to obtain an esthetically pleasing monolithic zirconia crown is to glass infiltrate its cameo surface [23–25]. This method is different from glazing, which is to merely fuse a glass layer onto the surface of a dense zirconia. In the glass-infiltration process [23], glass penetrates the grain boundaries of the surface zirconia layer driven by capillary pressure. The resulting structure consisted of a thin, outer surface glass layer (~15 μm thick), followed by a graded glass/zirconia layer (~120 μm thick), and a dense homogeneous zirconia interior. The graded layer consisted of ~45 vol.% glass near the interface with the outer surface glass layer. The glass content gradually decreased with depth towards the dense 3Y-TZP core [26]. Superior esthetics can be achieved by using shaded glasses for infiltration and by subsequent staining. Furthermore, this technique allows for relatively easy polishing of zirconia surface at the softer pre-sintered state prior to glass-infiltration.
Previous studies have shown that the glass-infiltrated graded zirconia surface is orders of magnitude more resistant to sliding contact fracture than PFZ, and even more superior to the homogeneous zirconia surface [23,26–28]. In addition, glass-infiltration of the intaglio surface also enhances the load bearing capacity of zirconia [4,26,27,29], while facilitating a strong resin bond [30,31]. Recent studies have further demonstrated the feasibility of glass infiltration of high-translucency zirconias, leading to similar strengthening effects while retaining excellent translucency properties [29,32], suggesting its potential indications for thinner, more translucent, yet stronger restorations for more conservative preparation of teeth.
Despite the aforementioned excellent properties of the graded glass/zirconia material, further evidence is needed to elucidate its wear behavior in relation to other dental ceramics. We postulate that (1) the graded glass/zirconia cameo surface with reduced stiffness is more gentle to the antagonist compared to a homogeneous zirconia surface; (2) compared with glazed zirconia, once the superficial glass layer is worn out, an underlying graded glass/zirconia layer is more favorable due to its relatively low hardness and stiffness; and (3) compared with a glass-ceramic, graded glass/zirconia undergoes less wear due to superior contact damage resistance. Therefore, this study was designed to systematically investigate the wear behavior of ceramic and antagonist, comparing graded glass/zirconia (with polished or as-machined zirconia subsurface) to homogeneous zirconia (polished or glazed) and a glass-ceramic. The null hypothesis was that there these various ceramic materials for monolithic restorations have similar wear behavior.
2. MATERIALS AND METHODS
2.1. Specimen preparation
Five groups (n = 8) were tested in this study: polished zirconia, polished graded zirconia, as-machined graded zirconia, as-machined glazed zirconia, and a glass-ceramic. For the glass-ceramic group, plate-specimens (2 mm thick) were cut from IPS Empress CAD blocks (Ivoclar Vivadent, Schaan, Liechtenstein), and polished sequentially with 15 μm, 6 μm, 3 μm, and 1 μm diamond impregnated pads in an automatic polisher (Buehler, Illinois, USA). For all zirconia groups, disc-shaped specimens (Ø14 mm × 2 mm) were prepared with 5.18 wt.% Y2O3 powder (TZ-3Y-E grade, Tosoh, Tokyo, Japan) by cold isostatic pressing and light sintering, replicating the exact process used in the production of commercial dental zirconia CAD/CAM blocks. The discs were then roughened with 240-grit sandpaper to simulate CAD-CAM surface finish (53 – 63 μm diamond burs). For the polished zirconia group, specimens were sintered at 1450°C for 2 h with a 10°C/min heating and cooling rate, then polished down to 1 μm finish using the same technique described above. For the glazed zirconia group, as-machined samples were sintered as described above, then cleaned in an ultrasonic bath with alcohol, dried, and glazed with Zenostar Glaze for full-contour zirconia (Wieland, Germany) following the manufacturer’s instructions: 575°C preheat temperature, 5 min drying time, 910°C firing temperature, 1 min dwell time, 45°C/min heating rate, and 6 min relief cooling. The preparation of graded zirconia samples followed a method described elsewhere [23]. Briefly, Y-TZP discs were pre-sintered (1350 °C for 1 h), then randomly assigned to polished or as-machined groups. For the polished graded group, the pre-sintered zirconia discs were finished with a 3 μm diamond impregnated pad. Then both groups were cleaned in an ultrasonic bath with alcohol, dried, and received a layer of an in-house prepared glass in the form of powder slurry. Glass-infiltration and densification occurred in a single step at 1450 °C for 2 h.
All specimens were cemented to hydrated glass-fiber reinforced epoxy resin rods (Epoxyglas G10, Acculam, USA) with a resin-based, dual-polymerizing luting material (Multilink Automix, Ivoclar). Hydration was carried out by storing G10 in distilled water at 37°C for 21–30 days. G10 has similar elastic behavior to that of human dentin [33,34]. The elastic behavior of the supporting material and luting cement were found to play a governing role in the fracture resistance of materials under cyclic fatigue [34–37]. The bonding surface of the G10 rods was acid-etched with 5% hydrofluoric acid for 2 min, washed in tap water and air-dried. Then, a primer was applied (Monobond Plus, Ivoclar). The zirconia surface was sandblasted with 50 μm alumina particles at 2 bars and a stand-off distance of 1 cm, ultrasonically cleaned in water for 2 min, then dried and coated with the primer. The glass-ceramic surface was acid etched with 5% hydrofluoric acid for 1 min, ultrasonically cleaned in water for 2 min, dried and coated with the primer. The luting agent was then applied to the surfaces with a mixing tip. The specimen was positioned on the G10 rod and finger pressed. Gross cement excess was removed with a microbrush, then a static load of 1 Kg was applied onto the samples for 2 min for uniform cement layer thickness. Photopolymerization of the luting agent was carried out by exposing the cement/ceramic interface to a LED curing light with an irradiance of 850 mW/cm2 for 40 s, four times from different directions (Ultra Lume LED 5, Ultradent, USA). All cementation procedures were carried out by a single operator. After cementation, the specimens were stored in distilled water at 37°C for 5 to 7 days for continued polymerization and hydration of the cement layer prior to the wear testing.
2.2. Sliding wear test
A 4-chamber Oregon Health Science University (OHSU) oral wear simulator (Proto-tech, USA) was used in this study. The load cell for each chamber was calibrated prior to the commencement of each wear testing regimen using a custom-designed device provided with the equipment. The specimens were subjected to sliding wear using a spherical steatite antagonist. The radius of the steatite sphere is r = 3 mm, which is in the midrange of the molar cuspal radii (2 – 4 mm) [38]. The physical properties of steatite are similar to those of dental porcelains, glass-ceramics, and tooth enamel [39]. The suitability of steatite as an antagonist for the standardization of dental wear studies has been established elsewhere [25]. A 50 N vertical load was applied through the steatite antagonist while it sliding across the specimen surface in an 8 mm horizontal path, followed by the indenter liftoff and returning to the starting point of the sliding path. This simulated chewing motion was repeated for 450k cycles at a frequency of 1 Hz, in water. The rationale for selecting a 50 N load was based on a previous study where the reported average physiological biting force of non-bruxist patients was 50 N [40]. Thus, the test conditions used in this study represent those occurring in the mouth during chewing.
2.3. Wear damage analysis
In order to investigate the progression of wear on the ceramic and antagonist, the cyclic wear testing was interrupted at 10, 50, 100, 500, 1k, 10k, 50k, and 100k cycles; then ended at 450k cycles. In each time point, an impression was taken of the specimen and antagonist using a low viscosity + putty silicone impression material (Elite HD+, Zhermack, Germany). The antagonists were never removed from the wear simulator, and the impression of the specimens was taken from inside the specimen chambers. Thus, the cyclic wear was resumed in the exact same position after every time point. Impressions were poured with epoxy resin (McMaster-Carr, USA) to produce replicas [41] of the ceramic specimens and steatite antagonists. Roughness and wear analyses were all carried out on the epoxy replicas [1,41].
Ceramic wear depth was analyzed using a contact profilometer (SJ-410, Mitutoyo, Tokyo, Japan) in profile mode. The total profile parameter Pt was measured with the following test conditions: cut-off length 0.8 mm, resolution 0.0001 μm (8 μm range), speed 0.5 mm/s, and total length 8 mm. The authors acknowledge that wear volume is a more precise estimation of material loss due to the interaction of opposing surfaces [42–44]. However, the OHSU wear tester performs a horizontal excursion of 8 mm, which is far longer than the horizontal excursion in physiological chewing motions [45]. Thus, the total volume loss of the ceramics tested herein would not be representative of their clinical wear behavior. Nevertheless, a significant correlation has been found between the wear depths observed after OHSU chewing simulation and in vivo clinical wear depth [46]. The roughness on the worn ceramic surface was analyzed by the same contact profilometer, but in roughness mode. The amplitude parameter Ra was measured with the following test conditions: cut-off length 0.25 mm, resolution 0.0001 μm (8 μm range), speed 0.5 mm/s, and total length 1 mm.
The wear damage on the antagonists was comprised of the formation and progressive expansion of a flat wear facet on the bottom most tip of the steatite ball. The antagonist wear was quantified using the geometry equations for “sphere cap height” (Equation 1), to calculate wear depth (D), and “sphere cap volume” (Equation 2) for volume loss (V). The diameter of the sphere cap (wear facet) on the steatite balls was measured on standardized digital photographs using a software for image processing (Photoshop CC).
| (1) |
| (2) |
where D is the wear depth (mm), V is the volume loss (mm3), R is the radii of the sphere (3 mm), r is the measured radii of the wear facet (mm).
Qualitative analysis of the damage sustained in both the ceramic specimens and steatite antagonists following the 450k wear cycles was performed using a scanning electron microscope (SEM – S3500N, Hitachi, Tokyo, Japan). Representative specimens were evaluated for subsurface damage using a sectioning technique [47,48]. The specimens were embedded in clear epoxy resin and sectioned along the direction of sliding contact and slightly away from the center of the wear crater, using a water-cooled low speed diamond saw (Isomet, Buehler, Lake Buff, IL). The cross-sections were polished down to the center of the wear crater with sequence of diamond grinding discs of 15, 9, 6, 3, and 1 μm and analyzed using SEM microscope.
2.4. Statistical analyses
Wear and roughness data passed the tests for normality and equality of variances. Thus, the ceramic and the steatite wear loss data at 450k cycles were separately analyzed using One-Way ANOVA; whereas the ceramic roughness data at baseline and 450k cycles were analyzed using Two-Way ANOVA (Factors: time and ceramic group). All pairwise multiple comparisons were performed by using the Tukey’s post hoc method. The significance level was set at 5% with a power of analyses greater than 80%.
3. RESULTS AND DISCUSSION
3.1. Ceramic wear
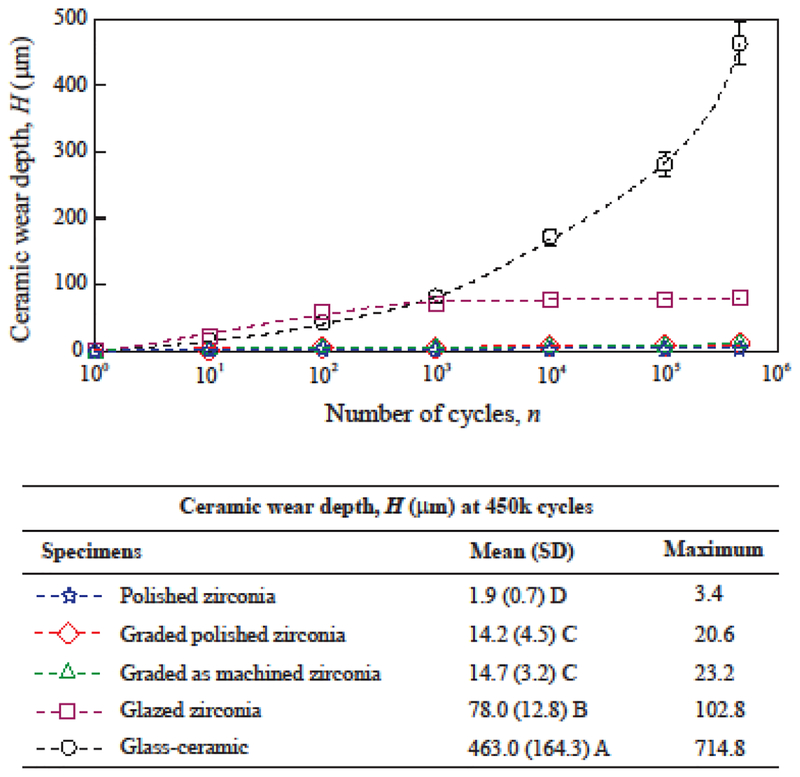
Progressive wear depth for all ceramic groups is shown in Figure 1. The polished zirconia presented an average wear depth of ~2 μm (maximum 3.4 μm) after 450k wear cycles. No wear depth was measurable in the earlier stages of the cyclic wear for the polished zirconia. This group presented the lowest ceramic wear depth, which was 7 to 34 times lower than the other zirconias tested herein. The 450k simulated chewing cycles can be considered equivalent to approximately 2 years in the mouth [45,49]. Two years is also the maximum follow-up reported for the clinical wear behavior of monolithic zirconia in recent studies [1,7,8,20,21]. In three of these papers, the occlusal surface of the zirconia crowns was polished and not glazed, and no quantitative wear of the zirconia was reported [1,7,21]. This is in line with the results of the present study, since the 2 μm wear depth observed herein fall under the uncertainty thresholds of the current techniques used in clinical wear evaluations [1,7,20].
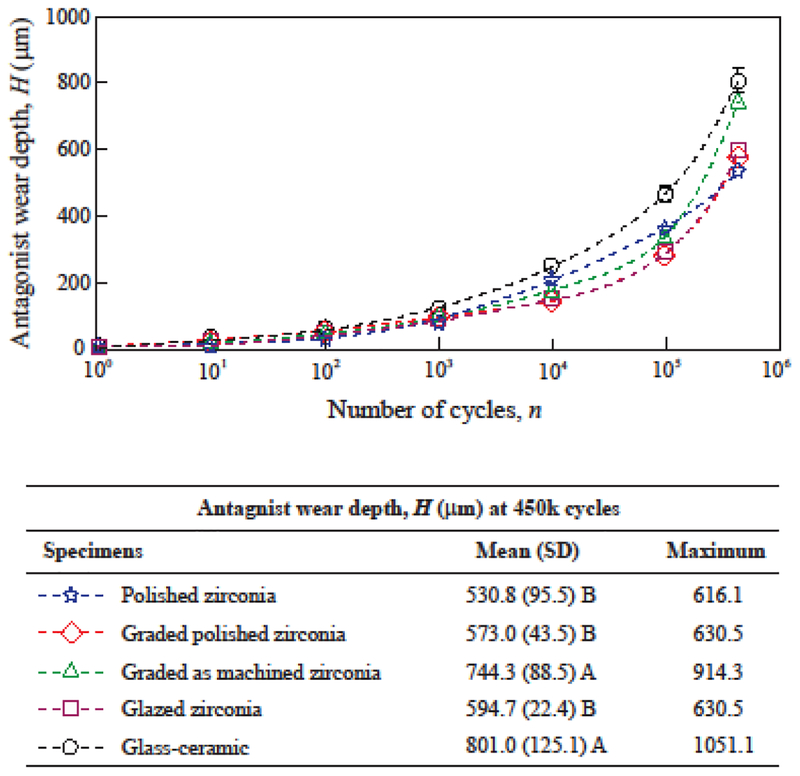
Figure 1:
Progression of ceramic wear depth. Data points indicate average wear depths (μm) for each cycle count, and error-bars indicate standard errors. For graded and glazed zirconia, the surface glass layer was worn out exposing the underlying zirconia surface before 1000 cycles. Means (SD = standard deviations) and maximum measured wear depths after 450k cycles are shown in the table. Distinct letters indicate statistical differences among the materials.
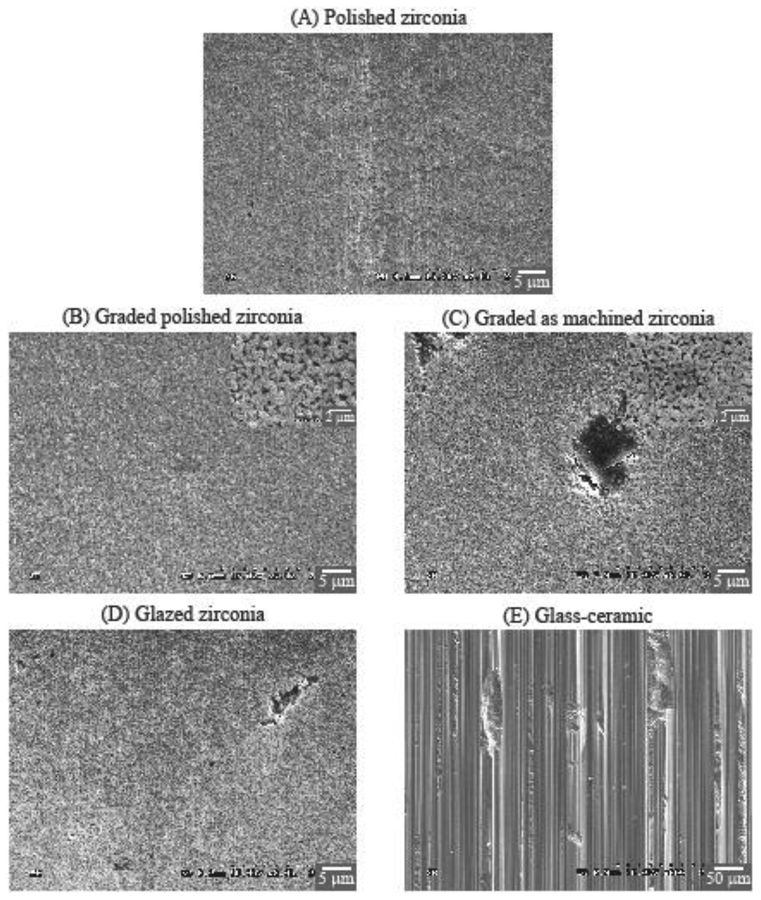
The two graded groups exhibited similar wear behavior in the initial stage, with a rapid increase in wear depth while the surface glass layer was visibly present (until ~1000 cycles). After 1000 cycles, the two graded zirconia showed little progression of mean wear depth (from ~10 μm to ~15 μm). Furthermore, with a similar maximum wear depth (~20 μm) in both polished and as-machined graded zirconias at 450k cycles, our findings suggested that wear occurred mainly in the outer surface residual glass layer of graded zirconias [25,26]. These results are also corroborated by the SEM images (Figure 2), which showed that the wear loss remained within the graded layer for both polished and as-machined graded zirconias (Figs. 2B and 2C), as one may see in the magnified images (×10k, insets of Figs. 2B and 2C). One should also note that steatite is harder and more abrasive than tooth enamel [25], yet caused minimal wear in graded zirconias, resulting in more of a polish effect rather than material loss. In addition, wear on graded zirconia would progressively expose regions with less glass and more zirconia [23,27]. Thus, the restoration would become even more resistant to wear. Based on these findings, the graded glass/zirconia layer is not likely to undergo significant wear in occlusal function.
Figure 2:
SEM images of the center area of the wear crater. To better depict the macro-scale roughness of the glass-ceramic wear crater surface (E), the image was captured using a field-width 10 times wider (×200 magnification) than that for the zirconia groups (A to D, ×2k magnification).
Glazed zirconia also presented a rapid increase in wear depth while the glaze layer was visibly present (until 1000 cycles). After 1000 cycles, little progression of mean wear depth was observed (from 72 μm to 78 μm). In a clinical trial, with 6 months of follow-up, the maximum wear depth for glazed zirconia crowns was 43 (14) μm for 17 patients [50], while after 2 years of follow-up the maximum wear depth was 60 (11) μm [8]. Considering that the underlying zirconia would undergo minimum wear, the wear depth data presented by Stober and collaborators [8,50] is likely associated with the thickness of the glaze layer. The wear depths observed for the glazed zirconia group is in accordance with the observed glaze layer thicknesses and corroborated by the clinical observations [8]. Also, for both groups with underlying as-machined zirconia surface (as-machined graded zirconia -- Fig. 2C, and glazed zirconia -- Fig. 2D), some remaining glass “islands” were observed on the smooth worn surface. These observations further corroborate the minimal zirconia wear, which did not reach the bottom of the machining groves (filled with glass).
Considerably distinct wear behavior was observed for the glass-ceramic in comparison to the zirconias (Fig. 1). A continuous increase in wear depth with cycle count was observed, reaching 463 (164) μm at 450k cycles. The glass-ceramic underwent significantly greater wear than all other groups, which was more than 200 times greater than polished zirconia, 30 times greater than graded zirconia, and 6 times greater than glazed zirconia. The results of this study showed that feldspathic glass, which is present in the constituent of this glass-ceramic, as well as used for infiltration in graded zirconia, and glazing glass is quite susceptible to wear, showing rapid substance loss when exposed to cyclic chewing. The clinical wear behavior of a few feldspathic glass-based ceramics has been investigated in the literature [51,52], with findings indicating increased wear depths over time. The wear depths of feldspathic glass-based ceramics ranged from ~60 μm to more than 300 μm on the occlusal surfaces after two years of clinical service [51,52], depending on the material investigated.
It is well known that complex mechanisms are involved in the wear of restorative materials in an oral cavity: 2-body abrasion, 3-body abrasion, adhesive wear, corrosive wear, and fatigue wear. Thus, as a limitation of this study, the authors acknowledge that the two-body wear test used herein is unable to fully elucidate the wear behavior of the materials tested. Nonetheless, the differences in ceramic wear presented herein are in accordance with previous studies showing that material type, microstructure, and physical properties affect their wear behavior [18,51–55]. Robust materials like zirconia and graded zirconia [23,27,56–60] undergo mild wear due to the slow dislodgment of grains [16,61] when exposed to physiological chewing forces [40]. In contrast, a rapid material loss occurs in feldspathic glass, which is susceptible to the formation and propagation of shallow lateral cracks, resulting in superficial microfractures leading to spalling [62]. Such different wear behaviors are associated with distinct surface characteristics, as seen in Figure 2, resulting in respectively distinct surface roughness.
3.2. Ceramic roughness
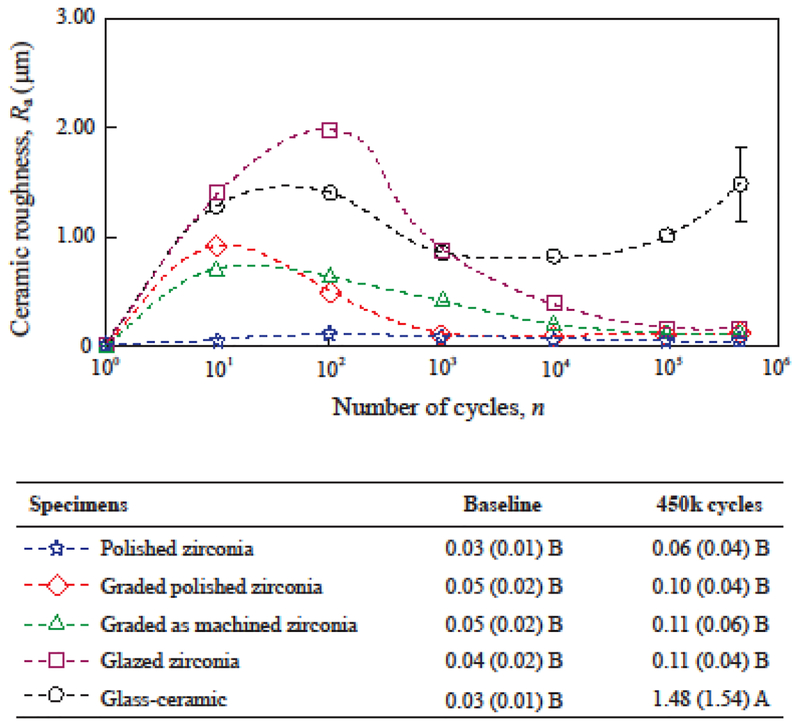
The progression of roughness is shown in Figure 3 for all ceramic groups. No difference in baseline roughness (Ra ≤ 0.05 μm) was found among the groups, regardless of the material and surface finish. Clinical thresholds for roughness have been described in the literature. On one hand, a material incapable of attaining and/or maintaining an Ra value below 0.2 μm in vitro has been linked with increased susceptibility to plaque accumulation and higher risk for caries and periodontal inflammation [63]. These are complications not likely associated with the roughness of occlusal contact surfaces, as those investigated herein. In such areas, the cyclic shear forces applied during chewing prevent the formation and maturation of more complex biofilms. On the other hand, a clinical trial showed that most patients could detect uncomfortably rough dental surfaces when the Ra values were above 0.3 μm [64]. The patient satisfaction and comfort with the restorations is a determining factor for their success and longevity. In the present study, all materials were initially polished to a smooth finish (Ra ≤ 0.05 μm) considerably below the aforementioned thresholds. This condition, however, was not retained throughout the whole course of the study.
Figure 3:
Progression of roughness (Ra, μm) inside the wear crater. Data points indicate the average Ra in each cycle count, and error-bars indicate standard errors. For graded and glazed zirconia, the surface glass layer was worn out exposing the underlying zirconia surface before 1000 cycles. Means (standard deviations) of roughness (Ra) at baseline and after 450k cycles are shown with distinct letters indicating statistical differences among the materials.
The possibility of chewing forces leading to increased roughness over time is associated with surface damage and wear [1,52]. The four zirconia groups presented an increase in Ra values in early cycle counts, but roughness reduced to Ra ≤ 0.10 μm at 450k cycles, which was statistically similar to the baseline (p ≥ 0.697). Representative SEM images (Fig. 2), at the center of the wear crater after 450k cycles corroborate the findings for surface smoothness in all zirconia groups (Fig. 2A–D). Polished zirconia (Fig. 3) presented little to no variation in the average Ra with cycle count. The two graded and the glazed zirconia groups presented higher Ra values while the surface glass layer was present (until 1000 cycles). The polished graded zirconia presented and maintained low roughness after the underlying smooth zirconia surface was exposed. Whereas for as-machined graded and glazed zirconia groups, a reduction in roughness of the exposed zirconia was observed with an increasing cycle count. The simulated chewing using steatite as antagonist had a “polishing” effect on rough zirconia surfaces, yielding Ra values similar to those obtained at baseline and below the clinical acceptability thresholds [63,64]
The glass-ceramic group (Fig. 3) presented increase in Ra values from 0.03 μm at baseline to above 1 μm in the early stages of the wear cycles; the Ra values remained high throughout the test. According to the findings from Dr. Jones and collaborators [64], the early and continuous high Ra values observed for the glass-ceramic would be noticeable and uncomfortable for patients. After 450k cycles, the roughness of the glass-ceramic was significantly greater than that of the other ceramics (Ra = 1.48 μm; p < 0.001), as well as greater than its own baseline (p < 0.001). Severe wear can lead to shallow lateral cracks resulting in superficial microfractures and spalling [62], which were clearly observed in the SEM images of the glass-ceramic (Fig. 2E), explaining the higher roughness quantified for this group. Lateral cracks and superficial microfractures [62] are also likely reasons for the increased roughness observed while the superficial glass layer was still present in graded and glazed zirconia groups. Furthering this, severe wear can also cause deep penetrating partial cone cracks [65] and might lead to the fracture of the restoration initiated from the wear facet at the occlusal surface [66]. In the present study, cracks were not observed on the wear crater surface (Fig. 2) or subsurface (cross-section images not presented herein). Note that two years of simulated chewing is too soon to expect fractures. Clinical studies have shown very little if any early fractures of glass-ceramic crowns [67]. Ideally, a ceramic dental prosthesis should not surpass a mild wear condition in the long-term. Nevertheless, progressive high roughness due to severe wear at the occlusal contact surfaces increases the friction at the interface of the two contacting bodies, reducing the resistance to sliding contact fracture [65,66].
3.3. Antagonist wear
Although the 450k simulated chewing cycles yield ceramic wear depth equivalent to approximately 2 years in the mouth [45,46,49], a distinct reasoning should be considered for the wear of the antagonist in the present study. In the mouth, the extent of the chewing sliding phase is dictated by the anatomy of teeth [45,68]. Assuming a physiologic chewing motion [40,45], a 0.5 mm sliding path can be expected in the first molar region with a projected magnitude of 0.35 mm in the horizontal plane [45]. The OHSU oral wear simulator generates a horizontal excursion of 8 mm. Thus, one may estimate that, in this chewing simulator, one cycle yields antagonist wear orders of magnitude greater than one chewing cycle in the mouth. Provided that each antagonist performed 450k wear cycles in the oral wear simulator, its wear measurements are expected to emulate a few decades of antagonist occlusal wear in the mouth. However, the accuracy of such a prediction could not be validated clinically, since no wear studies have reported such a long follow-up time. Therefore, as a limitation of the present study, the antagonist wear results are not comparable to clinical data due to this extensive discrepancy between the simulated and actual length of horizontal chewing excursions. Nevertheless, it has been reported that the wear of antagonists is affected by the type of restorative material, its microstructure, physical and mechanical properties [18,51–55]. Thus, the differences in antagonist wear among the materials tested herein elucidate some important guidance for their clinical performance.
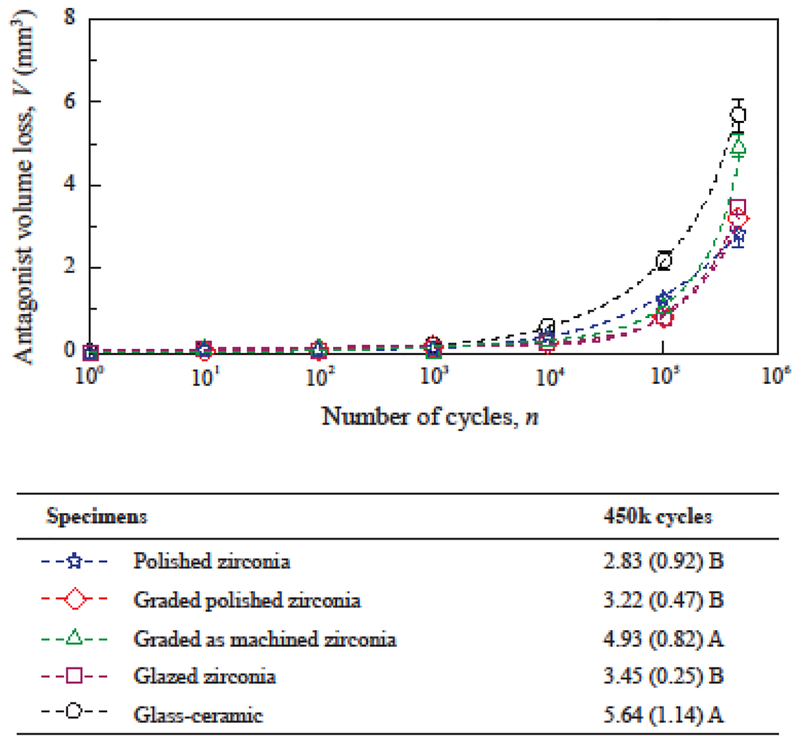
As discussed earlier, the quantification of material volume loss is a more accurate expression of the interaction of opposing surfaces [42–44]. However, wear depth is far more commonly reported in the dental literature than volume loss [1,7,8,52]. From a clinical standpoint, a linear measure of tooth height loss is easier to visualize than volumetric tooth loss. The progression of antagonist volume loss and wear depths are shown in Figures 4 and 5 for all ceramic groups. The results show a continuous increase of antagonist wear with cycle count for all groups when considering either the results expressed in volume loss (Fig. 4) or wear depth (Fig. 5). Similar observations were reported for antagonists opposing ceramic restorations in clinical trials [8,51,52]. As observed in Figure 4, polished zirconia (2.83 mm3), polished graded zirconia (3.22 mm3) and glazed zirconia (3.45 mm3) caused similar volumetric wear on their antagonists (p ≥ 0.55), showing better results than the other groups (p ≤ 0.009). The analysis of wear depth was consistent with the analysis of volume loss.
Figure 4:
Progression of antagonist volume loss. Data points indicate the average volume loss (mm3) for each cycle count, and error-bars indicate standard errors. For graded and glazed zirconia, the surface glass layer was worn out exposing the underlying zirconia surface before 1000 cycles. Means (standard deviations) of antagonist volume loss at 450k cycles are shown along with distinct letters indicating statistical differences among the materials.
Figure 5:
Progression of antagonist wear depth. Data points indicate the average wear depth (mm) for each cycle count, and error-bars indicate standard errors. For graded and glazed zirconia, the surface glass layer was worn out, exposing the underlying zirconia surface before 1000 cycles. Means (SD = standard deviations) and maximum measured wear depths after 450k cycles are shown in the table. Distinct letters indicate statistical differences among the materials.
4. CONCLUSIONS
The null hypothesis was rejected since significant differences in wear behavior were found among the ceramic materials tested. In general, polished zirconia and polished graded glass/zirconia presented the best performance, showing both minimal ceramic surface damage and reduced abrasiveness towards the antagonist. It is known that in clinical occlusal function, monolithic zirconia restorations yield similar or lower wear on their antagonists relative to their glass-ceramic counterparts. Thus, adequate clinical wear behavior can be anticipated for polished graded zirconia crowns.
HIGHLIGHTS.
The wear behavior of polished graded glass/zirconia is similar to polished zirconia
Polished graded glass/zirconia exhibits a smooth worn surface and yields reduced antagonist wear
Glassy materials are more abrasive and prone to wear than polished zirconia
ACKNOWLEDGEMENTS
The authors would also like to thank Dr. Asima Chughtai for helping with the preparation of the specimen replicas. This work was supported by the National Institutes of Health (NIH/NIDCR Grant Nos. R01DE017925, R01DE026279 and R01DE026772).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lohbauer U, Reich S. Antagonist wear of monolithic zirconia crowns after 2 years. Clin Oral Investig 2016; [DOI] [PubMed] [Google Scholar]
- 2.Sulaiman TA, Abdulmajeed AA, Donovan TE, Cooper LF, Walter R. Fracture rate of monolithic zirconia restorations up to 5 years: A dental laboratory survey. J Prosthet Dent 2016; 116:436–439. [DOI] [PubMed] [Google Scholar]
- 3.Sun T, Zhou S, Lai R, Liu R, Ma S, Zhou Z, Longquan S. Load-bearing capacity and the recommended thickness of dental monolithic zirconia single crowns. J Mech Behav Biomed Mater 2014; 35:93–101. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Lee JJ, Srikanth R, Lawn BR. Edge chipping and flexural resistance of monolithic ceramics. Dent Mater 2013; 29:1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batson ER, Cooper LF, Duqum I, Mendonca G. Clinical outcomes of three different crown systems with cad/cam technology. J Prosthet Dent 2014; 112:770–777. [DOI] [PubMed] [Google Scholar]
- 6.Bomicke W, Rammelsberg P, Stober T, Schmitter M. Short-term prospective clinical evaluation of monolithic and partially veneered zirconia single crowns. J Esthet Restor Dent 2016; [DOI] [PubMed] [Google Scholar]
- 7.Mundhe K, Jain V, Pruthi G, Shah N. Clinical study to evaluate the wear of natural enamel antagonist to zirconia and metal ceramic crowns. J Prosthet Dent 2015; 114:358–363. [DOI] [PubMed] [Google Scholar]
- 8.Stober T, Bermejo JL, Schwindling FS, Schmitter M. Clinical assessment of enamel wear caused by monolithic zirconia crowns. J Oral Rehabil 2016; 43:621–629. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Lawn BR. Evaluating dental zirconia. Dent Mater 2018. August 29 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Lawn BR. Novel zirconia materials in dentistry. J Dent Res 2018; 97:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Haj Husain N, Camilleri J, Ozcan M. Effect of polishing instruments and polishing regimens on surface topography and phase transformation of monolithic zirconia: An evaluation with xps and xrd analysis. J Mech Behav Biomed Mater 2016; 64:104–112. [DOI] [PubMed] [Google Scholar]
- 12.Amer R, Kurklu D, Kateeb E, Seghi RR. Three-body wear potential of dental yttrium-stabilized zirconia ceramic after grinding, polishing, and glazing treatments. J Prosthet Dent 2014; 112:1151–1155. [DOI] [PubMed] [Google Scholar]
- 13.Stawarczyk B, Frevert K, Ender A, Roos M, Sener B, Wimmer T. Comparison of four monolithic zirconia materials with conventional ones: Contrast ratio, grain size, four-point flexural strength and two-body wear. J Mech Behav Biomed Mater 2016; 59:128–138. [DOI] [PubMed] [Google Scholar]
- 14.Burgess JO, Janyavula S, Lawson NC, Lucas TJ, Cakir D. Enamel wear opposing polished and aged zirconia. Oper Dent 2014; 39:189–194. [DOI] [PubMed] [Google Scholar]
- 15.Janyavula S, Lawson N, Cakir D, Beck P, Ramp LC, Burgess JO. The wear of polished and glazed zirconia against enamel. J Prosthet Dent 2013; 109:22–29. [DOI] [PubMed] [Google Scholar]
- 16.Kaizer MR, Gierthmuehlen PC, dos Santos MBF, Cava SS, Zhang Y. Speed sintering translucent zirconia for chairside one-visit dental restorations: Optical, mechanical, and wear characteristics. Ceram Int 2017; 43:10999–11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mormann WH, Stawarczyk B, Ender A, Sener B, Attin T, Mehl A. Wear characteristics of current aesthetic dental restorative cad/cam materials: Two-body wear, gloss retention, roughness and martens hardness. J Mech Behav Biomed Mater 2013; 20:113–125. [DOI] [PubMed] [Google Scholar]
- 18.Park JH, Park S, Lee K, Yun KD, Lim HP. Antagonist wear of three cad/cam anatomic contour zirconia ceramics. J Prosthet Dent 2014; 111:20–29. [DOI] [PubMed] [Google Scholar]
- 19.Stawarczyk B, Ozcan M, Schmutz F, Trottmann A, Roos M, Hammerle CH. Two-body wear of monolithic, veneered and glazed zirconia and their corresponding enamel antagonists. Acta Odontol Scand 2013; 71:102–112. [DOI] [PubMed] [Google Scholar]
- 20.Esquivel-Upshaw JF, Kim MJ, Hsu SM, Abdulhameed N, Jenkins R, Neal D, Ren F, Clark AE. Randomized clinical study of wear of enamel antagonists against polished monolithic zirconia crowns. J Dent 2018; 68:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartkamp O, Lohbauer U, Reich S. Antagonist wear by polished zirconia crowns. Int J Comput Dent 2017; 20:263–274. [PubMed] [Google Scholar]
- 22.Nejatidanesh F, Moradpoor H, Savabi O. Clinical outcomes of zirconia-based implant- and tooth-supported single crowns. Clin Oral Investig 2016; 20:169–178. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Kim JW. Graded structures for damage resistant and aesthetic all-ceramic restorations. Dent Mater 2009; 25:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren L, Janal MN, Zhang Y. Sliding contact fatigue of graded zirconia with external esthetic glass. J Dent Res 2011; 90:1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaizer MR, Bano S, Borba M, Garg V, Dos Santos MBF, Zhang Y. Wear behavior of graded glass/zirconia crowns and their antagonists. J Dent Res 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y. Overview: Damage resistance of graded ceramic restorative materials. J Eur Ceram Soc 2012; 32:2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Ma L. Optimization of ceramic strength using elastic gradients. Acta Materialia 2009; 57:2721–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Kim JW. Graded zirconia glass for resistance to veneer fracture. J Dent Res 2010; 89:1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao L, Kaizer MR, Zhao M, Guo B, Song YF, Zhang Y. Graded ultra-translucent zirconia (5y-psz) for strength and functionalities. J Dent Res 2018; 97:1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai H, Kaizer M, Chughtai A, Tong H, Tanaka C, Zhang Y. On the interfacial fracture resistance of resin-bonded zirconia and glass-infiltrated graded zirconia. Dent Mater 2015; 31:1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos N, Kaizer M, Campos T, Zhang Y, Marinho R. Silica-based infiltrations for enhanced zirconia-resin interface toughness. J Dent Res 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaizer MR, Zhang Y. Novel strong graded high-translucency zirconias for broader clinical applications. Dent Mater 2018; 31:e140–e141. [Google Scholar]
- 33.Kelly JR, Rungruanganunt P, Hunter B, Vailati F. Development of a clinically validated bulk failure test for ceramic crowns. J Prosthet Dent 2010; 104:228–238. [DOI] [PubMed] [Google Scholar]
- 34.Kinney JH, Marshall SJ, Marshall GW. The mechanical properties of human dentin: A critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med 2003; 14:13–29. [DOI] [PubMed] [Google Scholar]
- 35.Lawn BR, Deng Y, Lloyd IK, Janal MN, Rekow ED, Thompson VP. Materials design of ceramic-based layer structures for crowns. J Dent Res 2002; 81:433–438. [DOI] [PubMed] [Google Scholar]
- 36.Ma L, Guess PC, Zhang Y. Load-bearing properties of minimal-invasive monolithic lithium disilicate and zirconia occlusal onlays: Finite element and theoretical analyses. Dent Mater 2013; 29:742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Kim JW, Kim JH, Lawn BR. Fatigue damage in ceramic coatings from cyclic contact loading with a tangential component. J Am Ceram Soc 2008; 91:198–202. [Google Scholar]
- 38.Jung YG, Peterson IM, Kim DK, Lawn BR. Lifetime-limiting strength degradation from contact fatigue in dental ceramics. J Dent Res 2000; 79:722–731. [DOI] [PubMed] [Google Scholar]
- 39.Shortall AC, Hu XQ, Marquis PM. Potential countersample materials for in vitro simulation wear testing. Dent Mater 2002; 18:246–254. [DOI] [PubMed] [Google Scholar]
- 40.Gibbs CH, Mahan PE, Lundeen HC, Brehnan K, Walsh EK, Holbrook WB. Occlusal forces during chewing and swallowing as measured by sound-transmission. J Prosthet Dent 1981; 46:443–449. [DOI] [PubMed] [Google Scholar]
- 41.Scherrer SS, Quinn JB, Quinn GD, Wiskott HA. Fractographic ceramic failure analysis using the replica technique. Dent Mater 2007; 23:1397–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Archard JF. Contact and rubbing of flat surfaces. JAppl Phys 1953; 24:981–988. [Google Scholar]
- 43.DeLong R. Intra-oral restorative materials wear: Rethinking the current approaches: How to measure wear. Dent Mater 2006; 22:702–711. [DOI] [PubMed] [Google Scholar]
- 44.Fleming GJP, Reilly E, Dowling AH, Addison O. Data acquisition variability using profilometry to produce accurate mean total volumetric wear and mean maximum wear depth measurements for the ohsu oral wear simulator. Dent Mater 2016; 32:e176–e184. [DOI] [PubMed] [Google Scholar]
- 45.DeLong R, Douglas WH. Development of an artificial oral environment for the testing of dental restoratives: Bi-axial force and movement control. J Dent Res 1983; 62:32–36. [DOI] [PubMed] [Google Scholar]
- 46.Heintze SD, Faouzi M, Rousson V, Ozcan M. Correlation of wear in vivo and six laboratory wear methods. Dent Mater 2012; 28:961–973. [DOI] [PubMed] [Google Scholar]
- 47.El Zhawi H, Kaizer MR, Chughtai A, Moraes RR, Zhang Y. Polymer infiltrated ceramic network structures for resistance to fatigue fracture and wear. Dent Mater 2016; 32:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shembish FA, Tong H, Kaizer M, Janal MN, Thompson VP, Opdam NJ, Zhang Y. Fatigue resistance of cad/cam resin composite molar crowns. Dent Mater 2016; 32:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krejci I, Lutz F. [in-vitro test results of the evaluation of dental restoration systems. Correlation with in-vivo results]. Schweiz Monatsschr Zahnmed 1990; 100:1445–1449. [PubMed] [Google Scholar]
- 50.Stober T, Bermejo JL, Rammelsberg P, Schmitter M. Enamel wear caused by monolithic zirconia crowns after 6 months of clinical use. J Oral Rehabil 2014; 41:314–322. [DOI] [PubMed] [Google Scholar]
- 51.Etman MK, Woolford M, Dunne S. Quantitative measurement of tooth and ceramic wear: In vivo study. Int J Prosthodont 2008; 21:245–252. [PubMed] [Google Scholar]
- 52.Esquivel-Upshaw JF, Rose WF Jr., Barrett AA, Oliveira ER, Yang MC, Clark AE, Anusavice KJ. Three years in vivo wear: Core-ceramic, veneers, and enamel antagonists. Dent Mater 2012; 28:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beuer F, Stimmelmayr M, Gueth JF, Edelhoff D, Naumann M. In vitro performance of full-contour zirconia single crowns. Dent Mater 2012; 28:449–456. [DOI] [PubMed] [Google Scholar]
- 54.Heintze SD, Cavalleri A, Forjanic M, Zellweger G, Rousson V. Wear of ceramic and antagonist--a systematic evaluation of influencing factors in vitro. Dent Mater 2008; 24:433–449. [DOI] [PubMed] [Google Scholar]
- 55.Sabrah AH, Cook NB, Luangruangrong P, Hara AT, Bottino MC. Full-contour y-tzp ceramic surface roughness effect on synthetic hydroxyapatite wear. Dent Mater 2013; 29:666–673. [DOI] [PubMed] [Google Scholar]
- 56.Al-Amleh B, Lyons K, Swain M. Clinical trials in zirconia: A systematic review. J Oral Rehabil 2010; 37:641–652. [DOI] [PubMed] [Google Scholar]
- 57.Denry I, Kelly JR. State of the art of zirconia for dental applications. Dent Mater 2008; 24:299–307. [DOI] [PubMed] [Google Scholar]
- 58.Lawn BR, Pajares A, Zhang Y, Deng Y, Polack MA, Lloyd IK, Rekow ED, Thompson VP. Materials design in the performance of all-ceramic crowns. Biomaterials 2004; 25:2885–2892. [DOI] [PubMed] [Google Scholar]
- 59.Srikanth R, Kosmac T, Della Bona A, Yin L, Zhang Y. Effects of cementation surface modifications on fracture resistance of zirconia. Dent Mater 2015; 31:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tong H, Tanaka CB, Kaizer MR, Zhang Y. Characterization of three commercial y-tzp ceramics produced for their high-translucency, high-strength and high-surface area. Ceram Int 2016; 42:1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Cheng YB, Lathabai S. Erosion of alumina ceramics by air- and water-suspended garnet particles. Wear 2000; 240:40–51. [Google Scholar]
- 62.Zhang Y, Cheng YB, Lathabai S. Influence of microstructure on the erosive wear behaviour of ca -sialon materials. J Eur Ceram Soc 2001; 21:2435–2445. [Google Scholar]
- 63.Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent Mater 1997; 13:258–269. [DOI] [PubMed] [Google Scholar]
- 64.Jones CS, Billington RW, Pearson GJ. The in vivo perception of roughness of restorations. Br Dent J 2004; 196:42–45; discussion 31. [DOI] [PubMed] [Google Scholar]
- 65.Ren L, Zhang Y. Sliding contact fracture of dental ceramics: Principles and validation. Acta Biomater 2014; 10:3243–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pang Z, Chughtai A, Sailer I, Zhang Y. A fractographic study of clinically retrieved zirconia-ceramic and metal-ceramic fixed dental prostheses. Dent Mater 2015; 31:1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heintze SD, Rousson V. Fracture rates of ips empress all-ceramic crowns--a systematic review. Int J Prosthodont 2010; 23:129–133. [PubMed] [Google Scholar]
- 68.Gibbs CH, Lundeen HC, Mahan PE, Fujimoto J. Chewing movements in relation to border movements at the first molar. J Prosthet Dent 1981; 46:308–322. [DOI] [PubMed] [Google Scholar]