ABSTRACT
Background
Early protein intake may program later body composition and height growth, perhaps mediated by insulin-like growth factor I (IGF-I). In infancy, higher protein intake is consistently associated with higher IGF-I concentrations and more rapid growth, but associations of protein intake after infancy with later growth and IGF-I are less clear.
Objectives
Our objective was to examine associations of protein intake in early childhood (median 3.2 y) with height, IGF-I, and measures of adiposity and lean mass in mid-childhood (median 7.7 y) and early adolescence (median 13.0 y), and with changes in these outcomes over time. We hypothesized that early childhood protein intake programs later growth.
Methods
We studied 1165 children in the Boston-area Project Viva cohort. Mothers reported children's diet using food-frequency questionnaires. We stratified by child sex and examined associations of early childhood protein intake with mid-childhood and early adolescent BMI z score, skinfold thicknesses, dual-energy X-ray absorptiometry (DXA) fat mass, DXA lean mass, height z score, and IGF-I concentration. We adjusted linear regression models for race/ethnicity, family sociodemographics, parental and birth anthropometrics, breastfeeding status, physical activity, and fast food intake.
Results
Mean protein intake in early childhood was 58.3 g/d. There were no associations of protein intake in early childhood with any of the mid-childhood outcomes. Among boys, however, each 10-g increase in early childhood total protein intake was associated with several markers of early adolescent size, namely BMI z score (0.12 higher; 95% CI: 0.01, 0.23), DXA lean mass index (1.34% higher; 95% CI: −0.07%, 2.78%), and circulating IGF-I (5.67% higher; 95% CI: 0.30%, 11.3%). There were no associations with fat mass and no associations with any adolescent outcomes among girls.
Conclusions
Early childhood protein intake may contribute to programming lean mass and IGF-I around the time of puberty in boys, but not to adiposity development. This study was registered at clinicaltrials.gov as NCT02820402.
Keywords: early childhood protein intake, body composition, height growth, IGF-I, cohort, Project Viva
Introduction
Protein intake in early life is an important driver of early growth. The “early protein hypothesis” attributes accelerated weight gain in the first 2 y of life to higher protein intake, with possible mediation by insulin-like growth factor I (IGF-I) (1). IGF-I is very sensitive to changes in nitrogen balance (2), and secretion may be regulated by certain classes of amino acids both directly and via insulin-dependent pathways (3). Infant formula has a higher protein-to-fat ratio than does breast milk (4), and multiple observational studies have found that nonbreastfed infants have higher circulating IGF-I concentrations in early infancy (5–11) and subsequent indicators of more rapid growth, particularly linear growth (11). In addition, in a randomized trial comparing healthy European infants fed high- and low-protein infant formulas, infants receiving formula with higher protein content had higher IGF-I, weight, and BMI at 6 mo than infants fed the lower-protein formula (8, 12), and follow-up at 6 y in one of these trials found that the high-protein formula group had higher BMI and risk of obesity (1). Whether effects of protein on IGF-I concentrations are transient or result in long-term programming of the IGF axis remains unclear.
The “growth acceleration hypothesis” proposed by Singhal and Lucas (13) postulates that an accelerated rate of weight, height, and adiposity gain during critical periods, including childhood, may program adverse metabolic characteristics and risk of cardiovascular disease. This hypothesis provides a proposed pathway underlying the observed associations of early nutrition with growth and future risk of chronic disease.
Few studies have examined associations of protein intake after infancy with growth markers in later childhood and adolescence. Some studies have found that higher protein intake in early childhood is associated with increased BMI in mid-childhood (14–19) but did not distinguish between gains in fat and lean mass, although some have found associations with direct measures of adiposity (14, 19, 20). In the few studies that have examined early childhood protein intake in relation to subsequent height at early to mid-childhood time points, 1 found an association (17) but others did not (16, 18). These longitudinal studies did not examine IGF-I at the time of mid-childhood growth measurements; the studies that have looked at IGF-I concentrations in relation to childhood protein intake have been cross-sectional and indicate a positive correlation between protein intake and concurrent IGF-I in children (21, 22). Several studies found apparent differences in associations of protein intake with later growth between boys and girls (15, 19, 23, 24), although others did not observe protein–sex interactions (17, 20).
Thus, relations among diet, hormones, and early growth are complex and not fully understood. In addition, previous studies have examined associations of protein intake with later adiposity, lean mass, linear growth, and IGF-I in separate analyses, which does not allow consideration of their complex interplay, and few have followed up past mid-childhood. Our objective in this study was to examine the extent to which protein intake in early childhood (∼3 y) was associated with body composition (including both adiposity and lean mass), height, and IGF-I concentrations in mid-childhood (∼8 y) and early adolescence (∼13 y), and with changes in these outcomes from mid-childhood to early adolescence. Based on sex-specific effects reported in previous studies, we stratified our analyses by child sex.
Methods
Subjects
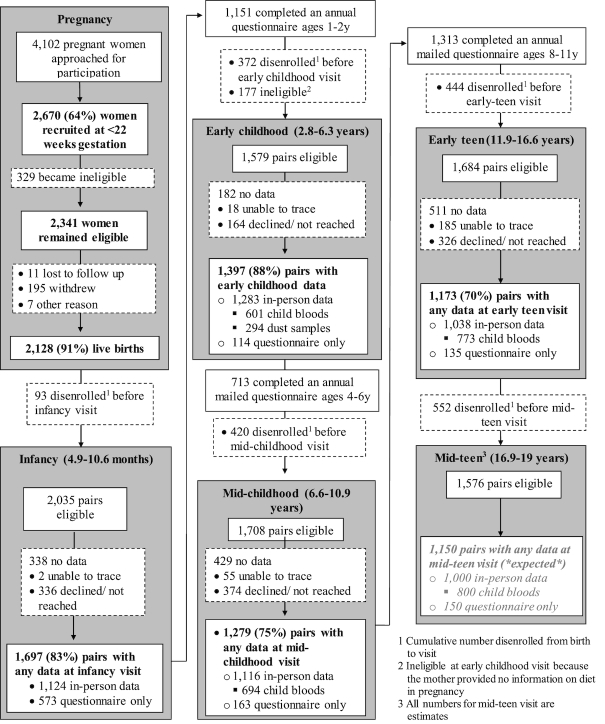
We studied children enrolled in Project Viva (NCT02820402), a prospective cohort study of mother–child pairs examining associations of prenatal, perinatal, and early-life exposures with pregnancy and child health outcomes. Project Viva recruited mothers in 1999–2002 at their initial obstetric appointment from 8 offices of Atrius Health, a large multispecialty group practice in eastern Massachusetts. Mothers enrolled their children after delivery, and Project Viva collected data on the children in infancy, early and mid-childhood, and early adolescence (“early teen” visit). We have previously described detailed recruitment and retention procedures (25) and show the flow of participant involvement in Project Viva in Figure 1. For this study, we used dietary data collected at the early childhood visit (median age 3.2 y, range 2.8–6.3 y), outcome data collected at the mid-childhood (median age 7.7 y, range 6.6–10.9 y) and early teen (median age 13.0 y, range 11.9–16.6 y) visits, and data on potential covariates from the pregnancy, delivery, early childhood, mid-childhood, and early teen visits.
FIGURE 1.
Flow of participant involvement in Project Viva from recruitment through the mid-teen visit. Flow from recruitment through mid-childhood reprinted from Oken et al., Cohort Profile: Project Viva, International Journal of Epidemiology, 2015;44(1):37–48 by permission of Oxford University Press.
Of the 2128 Project Viva children, we included 1165 with available data from the early childhood visit as well as data from either the mid-childhood or early teen visits in this analysis. There were no differences in the proportions of boys and girls or in protein intake between included and excluded participants. The included subset had higher proportions of white children (66% compared with 61%), children who were fully breastfed to 6 mo (29% compared with 20%), children from households with income >$70,000/y at enrollment (63% compared with 52%), and a slightly higher gestational age at delivery (39.5 compared with 39.3 wk) than the excluded group. Institutional Review Boards of participating institutions approved the study protocols, and mothers or other parents/guardians provided written informed consent for the participation of their children.
Measurements
Exposure: protein intake
We derived data on child food and nutrient intake from semiquantitative food-frequency questionnaires (FFQs) validated for use in preschool-age children (26) and completed by the mothers at the early childhood visit. The FFQ assessed the child's diet during the past month, and we estimated usual intake of total, animal, and plant protein using the Harvard nutrient composition database, which includes food composition values from the USDA (27) and is supplemented by other sources. Protein intake was calculated by multiplying the amount of protein in the specific portion size of each food by the consumption frequency of each food and summing across all food items. We adjusted individual nutrient estimates for total energy intake using the nutrient residual method (28–30). Adjustment for total energy intake has been shown to reduce the impact of measurement error inherent in use of FFQs for protein intake specifically (31). We also used a similar approach to generate a measure of protein intake independent of current body weight. The Estimated Average Requirement (EAR), or median nutrient requirement for a given life stage and sex, is based on individual body weight (grams per kilogram per day), and we wanted to account for the weight-based requirements in determining individual protein intakes. However, we felt that it was important to avoid using a ratio incorporating weight in our models given the associations of body weight with many of the outcomes under study. Therefore, we regressed protein intake (in grams per day adjusted for total energy intake) on body weight measured at the early childhood visit. We added the mean protein intake to each residual to allow for comparability with absolute intake and used these residuals for each individual in subsequent exposure–outcome models.
Outcomes: adiposity measures, height, and IGF-I concentrations in mid-childhood
We collected data on height, weight, and several measures of body composition, as well as blood samples, at in-person mid-childhood and early teen visits. Trained research assistants (RAs) measured standing height to the nearest 0.1 cm using a stadiometer (Shorr Board, Weight and Measure LLC). We recorded weight to the nearest 0.1 kg using a Tanita scale (model TBF-300A; Tanita Corporation of America, Inc.) and calculated BMI as kg/m2. We calculated age- and sex-specific z scores for BMI and height using US national reference data (32). RAs measured subscapular (SS) and triceps (TR) skinfold thicknesses to the nearest 0.1 cm using Holtain calipers (Holtain Ltd.), and we calculated the sum of the 2 skinfolds (SS + TR) as a measure of overall adiposity. Finally, RAs performed whole body dual-energy X-ray absorptiometry (DXA) scans (Hologic model Discovery A) from which we obtained measures of total fat mass index and total body lean mass index in kg/m2.
Phlebotomists trained in pediatric blood collection obtained venous blood from participants at the mid-childhood and early teen visits. We obtained fasting samples from 93% of participants in mid-childhood and 88% in early adolescence, and IGF-I and IGF binding protein-3 (IGFBP-3) were analyzed in both fasting and nonfasting samples. Whole blood samples were refrigerated for <24 h, then spun and separated into aliquots for storage in liquid nitrogen (33). We measured IGF-I and IGFBP-3 (ELISA, Alpco Diagnostics, for mid-childhood IGF-I and ELISA, R&D Systems, for early teen IGF-I and mid-childhood and early teen IGFBP-3) in plasma. Day-to-day variability for the assay was <10%.
We calculated the change in each outcome from mid-childhood to early adolescence by subtracting the measure taken in mid-childhood from the measure taken in early adolescence. When calculating the change in IGF-I between mid-childhood and early adolescence, we standardized the measurements at each time point using internal z scores to account for differences in the IGF-I assay between the 2 time points.
Covariates
At the initial prenatal visit (median 9.9 weeks of gestation), mothers reported their education level, height, household income, prepregnancy weight, and the height and weight of the infant's biological father. We calculated maternal and paternal BMI from these reports of height and weight. RAs visited mother–infant pairs after delivery at the 2 primary delivery hospitals and measured recumbent length using a stadiometer (Shorr Board, Weight and Measure LLC). We obtained data on infant sex, delivery date, and birth weight from hospital medical records and calculated sex-specific birth weight for gestational age z scores using US national reference data (32). Mothers self-reported breastfeeding status when the child was 6 mo old and we categorized them as fully breastfeeding (feeding their infant breast milk and no infant formula) or not fully breastfeeding at 6 mo. We asked mothers to indicate the category that best described their child's race/ethnicity during the early childhood interview. At the mid-childhood and early teen visits, mothers reported their child's physical activity via questionnaire. Mothers reported their children's frequency of fast food consumption at the mid-childhood visit, whereas the adolescents reported this for themselves at the early teen visit. We assessed pubertal status at the mid-childhood and early teen visits by maternal report of the child's development using the Pubertal Development Scale (34, 35). All data collection instruments used in Project Viva are publicly available at https://www.hms.harvard.edu/viva/.
Statistical analysis
We examined levels of exposure (early childhood protein intake) and outcomes (adiposity measured by DXA fat mass index, height z score, and circulating IGF-I concentrations measured at the mid-childhood and early teen visits) according to categories of child, parental, and household characteristics. We calculated means ± SDs for normally distributed variables (early childhood protein intake and early adolescent height z score) and geometric means and medians (IQRs) for nonnormally distributed variables (early adolescent DXA fat mass index and IGF-I). We compared differences across categories of each covariate using generalized linear models (SAS PROC GENMOD) and Type 3 analysis to generate P values for global differences across categories of each covariate. We compared means for normally distributed variables and geometric means for nonnormally distributed variables.
We analyzed associations of early childhood total, animal, and plant protein intake with later outcomes using multivariable linear regression models. Model 1 adjusted for household income at maternal enrollment, maternal education level, and child race/ethnicity, age at diet assessment, and age at outcome measurement (except in the case of z score outcomes, which already account for age). Model 2 adjusted for all covariates in Model 1 and also included maternal and paternal BMI and the child's birth weight for gestational age z score (for adiposity outcomes) or maternal and paternal height and the child's birth length (for height and IGF-I outcomes). Model 3 adjusted for all variables in Model 2 and also included breastfeeding status at 6 mo for all outcomes. For the models predicting BMI z score, adiposity (SS + TR skinfolds and DXA fat mass index), and lean mass, we also adjusted for habitual fast food intake and physical activity assessed at the time of outcome measurement. Finally, we examined fully adjusted models predicting IGF-I concentrations in mid-childhood and early adolescence with additional adjustment for IGFBP-3 measured at the same time point, to obtain an estimate for free IGF-I in addition to total. We considered adjustment for pubertal status at the time of outcome measurement, but ultimately decided not to include this characteristic in our final models because it may be in the pathway between early childhood protein intake and later body composition, height, and IGF-I.
We checked for linearity of associations between early childhood protein intake and outcomes measured in mid-childhood and early adolescence using protein intake categorized into quartiles and deciles, which revealed generally linear relations for all outcomes except height z score in mid-childhood, which we examined with quartiles of protein intake. We present all other results with protein intake analyzed as a continuous variable.
To improve the normality of residuals and linearity of the relation between early childhood protein intake and the outcomes, we ln-transformed the following outcomes for the multivariable regression analyses at the individual time points: SS + TR skinfolds, DXA total fat mass index, DXA total lean mass index, and IGF-I. We present geometric means or medians for these outcomes and regression results as a percentage change in the outcome, calculated as: [% change = (exp(β) − 1) × 100], for a 10-g/d difference in protein intake. Because the changes in these outcomes between mid-childhood and early adolescence were more normally distributed, we kept all measurements in their native units for the analyses of change over time.
Because of differences in growth around the time of puberty between males and females, and because several studies have reported sex-specific associations of protein intake with measures of growth and IGF-I (15, 19, 23, 24, 36), we examined all models stratified by child sex. We also checked for a statistical interaction between sex and protein intake by including an interaction term in our regression models.
We used multiple imputation methods to impute missing data. We generated 50 imputed data sets using chained imputation (37) and combined estimates using Rubin's rules (38). We present results from the imputed analysis throughout the article unless otherwise indicated. All 2128 live births were used to generate the imputed dataset, but in our analyses we included only the 1165 participants who completed some portion of the early childhood visit and ≥1 of the following: 1) a portion of the mid-childhood in-person visit (n = 1054), 2) a mid-childhood blood draw (n = 659), 3) a portion of the early teen in-person visit (n = 963), or an early teen blood draw (n = 717). For the models assessing change in body composition or height from mid-childhood to early adolescence, we included the 854 participants who completed some portion of both the mid-childhood and early teen in-person visits, and for the models assessing change in IGF-I we included the 475 participants who completed both a mid-childhood and early teen blood draw.
We used hypothesis-driven models to look for trends and consistency of results across statistical methods, and we interpreted all results in the context of our prespecified hypothesis. We performed all analyses using SAS version 9.4 (SAS Institute).
Results
Estimated early childhood protein intake was high in this cohort; the overall mean ± SD protein intake in the 1165 children was 58.3 ± 8.9 g/d, or 3.77 ± 0.68 g · kg–1 · d–1. The EAR for protein is 0.87 g · kg–1 · d–1 for children ages 1–3 y and 0.76 g · kg–1 · d–1 for children ages 4–8 y. One hundred percent of the children in our sample had an estimated intake above the EAR. Absolute intakes were similar in boys and girls (58.2 ± 8.20 and 58.4 ± 8.48 g/d, respectively); girls had a slightly higher intake for their body weight (3.86 ± 0.75 g · kg–1 · d–1 compared with 3.68 ± 0.69 g · kg–1 · d–1 in boys).
Table 1 displays amounts of early childhood protein intake and adiposity measured by DXA fat mass index, height z score, and circulating IGF-I concentrations measured at early teen visits according to categories of child, parental, and household characteristics. Protein intake was slightly higher in children who were white or of other race/ethnicity than in black children and in children from higher-income households and those whose mothers had a higher level of education (P < 0.05 for all). As expected, girls had higher DXA fat mass index and IGF-I concentrations at both follow-up time points, DXA fat mass index increased with increasing maternal prepregnancy BMI, and children with taller parents had higher height z scores (P < 0.0001 for all). Height z scores at both time points and IGF-I concentrations in mid-childhood were all highest in black children and children from lower-income households, and children of mothers who did not have a college degree had higher DXA fat mass index (P < 0.01 for all). Children who were still fully breastfed at 6 mo of age had lower DXA fat mass index in mid-childhood and early adolescence than children who were not (P < 0.0001 for both).
TABLE 1.
Association of characteristics of 1165 Project Viva participants with early childhood protein intake and adiposity, height, and IGF-I in early adolescence1
| Early adolescence outcomes | |||||
|---|---|---|---|---|---|
| Early childhood protein intake (g/d) | DXA fat mass index (kg/m2) | Height z score | IGF-I (ng/mL) | ||
| % | Mean ± SD or median (IQR)2 | ||||
| Overall | — | 58.3 (8.9) | 5.22 (4.01–7.27) | 0.33 ± 1.02 | 194 (154–237) |
| Child characteristics | |||||
| Sex | |||||
| Male | 50 | 58.2 (8.20) | 4.58 (3.67–6.58) | 0.39 ± 1.05 | 175 (135–219) |
| Female | 50 | 58.4 (8.48) | 5.75 (4.49–7.80) | 0.28 ± 0.99 | 214 (180–251) |
| Race/ethnicity | |||||
| White | 66 | 58.6 (7.86) | 5.18 (4.06–7.03) | 0.31 ± 1.00 | 193 (149–236) |
| Black | 14 | 56.5 (9.48) | 5.57 (3.95–8.56) | 0.62 ± 1.06 | 197 (163–244) |
| Other | 20 | 58.7 (9.41) | 5.22 (3.94–7.44) | 0.21 ± 1.06 | 196 (162–237) |
| Gestational age at birth, wk | |||||
| <37 | 6 | 58.0 (8.37) | 4.98 (3.92–7.63) | 0.08 ± 1.17 | 204 (147–246) |
| ≥37 | 94 | 58.3 (8.25) | 5.22 (4.03–7.27) | 0.35 ± 1.01 | 194 (154–237) |
| Breastfeeding status at 6 mo | |||||
| Not fully breastfed | 71 | 58.2 (8.45) | 5.48 (4.12–7.85) | 0.30 ± 1.03 | 193 (155–239) |
| Fully breastfed | 29 | 58.6 (8.23) | 4.85 (3.78–6.08) | 0.41 ± 1.03 | 196 (151–232) |
| Parent/household characteristics | |||||
| Annual household income at enrollment | |||||
| ≤$70,000 | 37 | 57.5 (8.71) | 5.38 (4.06–7.99) | 0.44 ± 1.02 | 197 (164–243) |
| >$70,000 | 63 | 58.7 (84.6) | 5.18 (4.00–7.04) | 0.27 ± 1.03 | 192 (146–234) |
| Maternal prepregnancy BMI, kg/m2 | |||||
| <18.5 | 3 | 55.4 (9.06) | 4.16 (3.59–4.98) | −0.01 ± 1.00 | 194 (167–220) |
| 18.5–24.9 | 62 | 58.5 (8.28) | 4.91 (3.88–6.33) | 0.26 ± 1.05 | 194 (151–239) |
| 25.0–29.9 | 22 | 58.4 (8.61) | 5.79 (4.19–8.56) | 0.40 ± 0.96 | 191 (146–239) |
| ≥30 | 13 | 57.9 (9.21) | 7.29 (5.22–10.9) | 0.60 ± 0.97 | 203 (164–231) |
| Maternal education | |||||
| <4-y college degree | 29 | 57.0 (8.85) | 5.94 (4.15–8.74) | 0.35 ± 1.06 | 199 (162–244) |
| ≥4-y college degree | 71 | 58.8 (8.43) | 5.08 (3.97–6.89) | 0.33 ± 1.01 | 194 (150–236) |
| Maternal height, m | |||||
| ≤1.57 | 10 | 58.5 (9.37) | 5.71 (4.03–8.14) | −0.26 ± 1.06 | 192 (163–229) |
| >1.57–1.68 | 60 | 58.3 (8.15) | 5.17 (4.07–7.03) | 0.19 ± 0.96 | 192 (151–237) |
| >1.68 | 30 | 58.2 (8.55) | 5.23 (3.95–7.90) | 0.78 ± 0.96 | 199 (158–244) |
| Paternal height, m | |||||
| ≤1.68 | 9 | 56.9 (8.75) | 5.02 (4.12–7.61) | −0.14 ± 1.04 | 194 (167–241) |
| >1.68–1.78 | 40 | 58.3 (8.07) | 5.39 (3.97–7.76) | 0.11 ± 0.98 | 189 (149–232) |
| >1.78 | 51 | 58.5 (8.71) | 5.18 (4.06–6.91) | 0.60 ± 0.99 | 197 (156–239) |
1Total number of children included. n = 963 for analysis of early adolescence fat mass and height; n = 717 for analysis of early adolescence IGF-I. DXA, dual-energy X-ray absorptiometry; IGF-I, insulin-like growth factor I.
2Early childhood protein intake and height z score were normally distributed, and means ± SDs are reported here. Early adolescence DXA fat mass index and IGF-I had skewed distributions, and medians (IQRs) are reported here.
Table 2 presents the results of regression models predicting measures of adiposity (sum of SS and TR skinfolds and DXA total fat mass index), DXA lean mass index, and IGF-I in mid-childhood and early adolescence as a function of early childhood protein intake. We also examined the change in these outcomes from mid-childhood to early adolescence (Table 3). Early childhood protein intake was not associated with any of the outcomes in mid-childhood in either unadjusted models (data not shown) or models adjusted for covariates.
TABLE 2.
Associations of total protein intake in early childhood with adiposity, lean mass, and IGF-I in mid-childhood and early adolescence1
| Mid-childhood2 | Early adolescence3 | |||
|---|---|---|---|---|
| Median (IQR) | % difference (95% CI) per 10-g increase in early childhood protein intake4 | Median (IQR) | % difference (95% CI) per 10-g increase in early childhood protein intake4 | |
| SS + TR skinfolds, mm | ||||
| Boys | 15.0 (12.8–19.4) | 0.63 (−3.30, 4.71) | 21.0 (16.5–31.6) | 1.09 (−3.78, 6.21) |
| Girls | 18.2 (14.9–24.2) | 0.72 (−3.09, 4.69) | 26.1 (20.0–36.2) | 0.84 (−2.90, 4.71) |
| DXA total fat mass index, kg/m2 | ||||
| Boys | 3.41 (2.84–4.42) | 1.14 (−2.76, 5.19) | 4.58 (3.67–6.58) | 1.39 (−3.42, 6.44) |
| Girls | 4.31 (3.49–5.61) | 0.76 (−2.59, 4.23) | 5.75 (4.49–7.80) | 1.28 (−2.31, 5.01) |
| DXA lean mass index, kg/m2 | ||||
| Boys | 13.2 (12.4–14.0) | 1.13 (−0.02, 2.30) | 15.0 (13.8–16.3) | 1.34 (−0.07, 2.78) |
| Girls | 12.4 (11.7–13.3) | 0.18 (−0.94, 1.32) | 14.3 (13.2–15.6) | −0.06 (−1.47, 1.37) |
| IGF-I, ng/mL | ||||
| Boys | 224 (175–279) | −2.25 (−7.34, 3.13) | 175 (134–219) | 5.67 (0.30, 11.3) |
| Girls | 263 (212–326) | −0.43 (−5.30, 4.69) | 214 (180–252) | 0.69 (−3.21, 4.75) |
1Median age at early childhood: 3.2 y. Protein intake is adjusted for total energy intake and body weight at the time of diet assessment. SS + TR skinfolds, DXA total fat mass, DXA total lean mass, and IGF-I were log-transformed before analysis, and percentage difference (95% CI) was calculated as (eβ − 1) × 100. DXA, dual-energy X-ray absorptiometry; IGF-I, insulin-like growth factor I; SS, subscapular; TR, triceps.
2 n = 1054 for analyses of SS + TR skinfolds, DXA total fat mass, and DXA total lean mass; n = 659 for analyses of IGF-I.
3 n = 963 for analyses of SS + TR skinfolds, DXA total fat mass, and DXA total lean mass; n = 717 for analyses of IGF-I.
4From multivariable linear regression models adjusted for child race/ethnicity, age at diet assessment, age at measurement, household income, maternal education, breastfeeding status at 6 mo, and: 1) maternal and paternal BMI, birth weight for gestational age z score, fast food intake, and physical activity (SS + TR skinfolds and DXA fat mass index only); 2) fast food intake and physical activity (DXA lean mass index only); 3) maternal and paternal height and birth length (IGF-I only).
TABLE 3.
Associations of total protein intake in early childhood with changes in BMI, height, adiposity, lean mass, and IGF-I from mid-childhood to early adolescence1
| Δ from mid-childhood to early adolescence2 | ||
|---|---|---|
| Mean ± SD | β (95% CI) per 10-g increase in early childhood protein intake3 | |
| BMI z score | ||
| Boys | −0.04 ± 0.63 | 0.01 (−0.07, 0.09) |
| Girls | −0.04 ± 0.62 | 0.01 (−0.07, 0.08) |
| Height z score | ||
| Boys | 0.14 ± 0.58 | 0.07 (−0.01, 0.14) |
| Girls | 0.09 ± 0.54 | 0.05 (−0.01, 0.11) |
| SS + TR skinfolds, mm | ||
| Boys | 8.41 ± 9.37 | −0.10 (−1.26, 1.07) |
| Girls | 8.04 ± 9.02 | −0.25 (−1.26, 0.75) |
| DXA total fat mass index, kg/m2 | ||
| Boys | 1.70 ± 1.85 | −0.11 (−0.35, 0.13) |
| Girls | 1.73 ± 1.81 | −0.02 (−0.22, 0.17) |
| DXA lean mass index, kg/m2 | ||
| Boys | 1.82 ± 1.46 | 0.06 (−0.11, 0.23) |
| Girls | 1.90 ± 1.33 | −0.06 (−0.21, 0.09) |
| IGF-I z score | ||
| Boys | −0.12 ± 1.15 | 0.17 (−0.02, 0.37) |
| Girls | 0.02 ± 1.29 | 0.12 (−0.08, 0.31) |
1Median age at early childhood: 3.2 y. Protein intake is adjusted for total energy intake and body weight at the time of diet assessment. DXA, dual-energy X-ray absorptiometry; IGF-I, insulin-like growth factor I; SS, subscapular; TR, triceps.
2 n = 854 for analyses of change in BMI z score, height z score, SS + TR skinfolds, DXA total fat mass, and DXA total lean mass; n = 475 for change in IGF-I z score.
3From multivariable linear regression models adjusted for child race/ethnicity, age at diet assessment, and age at measurement (except z score outcomes), household income, maternal education, breastfeeding status at 6 mo, and: 1) maternal and paternal BMI, birth weight for gestational age z score, fast food intake, and physical activity (BMI z score, SS + TR skinfolds, and DXA fat mass index only); 2) fast food intake and physical activity (DXA lean mass index only); 3) maternal and paternal height and birth length (height z score and IGF-I only).
Early childhood protein intake was associated with several outcomes in early adolescence among boys, but no statistically significant associations were observed in girls. A 10-g/d increase in total protein intake in early childhood was associated with a 0.12 unit (95% CI: 0.01, 0.23) greater BMI z score in boys (data not shown). There were no associations between early childhood protein intake and either SS + TR skinfolds or DXA fat mass; however, there was a trend towards higher DXA lean mass index (difference: 1.34%; 95% CI: −0.07%, 2.78%, P = 0.06) among boys. Boys with higher early childhood protein intake also had higher total and free IGF-I concentrations in early adolescence: a 10-g/d increase in early childhood total protein intake corresponded to a 5.67% higher total IGF-I (95% CI: 0.30%, 11.3%) (Table 2) and a 6.10% higher free (unbound) IGF-I (95% CI: 1.19%, 11.3%) (data not shown). Early childhood protein intake was not associated with height z score in early adolescence among boys (β: 0.07; 95% CI: −0.04, 0.18). When looking at change in the outcomes from mid-childhood to early adolescence, point estimates suggested a direct association of early childhood protein intake with changes in height, lean mass, or IGF-I in boys. However, the CIs crossed the null and no associations were statistically significant (Table 3). Among girls, there were no associations between early childhood protein intake and any of the outcomes in early adolescence (Table 2), or change in the outcomes between mid-childhood and early adolescence (Table 3). As a secondary analysis we examined all associations in the full sample with adjustment for child sex as well as testing for statistical interaction between protein intake and sex in each model. The interaction terms were not statistically significant for any of the outcomes (P = 0.13 for BMI z score, P = 0.41 for height z score, P = 0.66 for SS + TR skinfolds, P = 0.69 for DXA fat mass index, P = 0.12 for DXA lean mass index, P = 0.18 for total IGF-I, and P = 0.10 for free IGF-I).
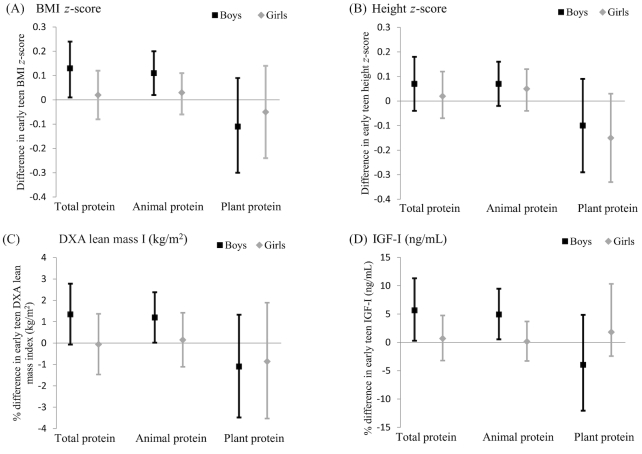
In addition to total protein intake in early childhood, we also examined intake of protein from animal and plant sources separately. As shown in Figure 2, animal protein intake in early childhood was positively associated with BMI z score, DXA lean mass index, and IGF-I in early adolescence among boys. There were no statistically significant associations between plant protein and any of the outcomes (Figure 2).
FIGURE 2.
Associations of total, animal, and plant protein intake in early childhood with BMI z score (A), height z score (B), DXA lean mass index (kg/m2) (C), and IGF-I (ng/mL) (D) in early adolescence among Project Viva boys and girls. Points are βs (BMI z score and height z score) or percentage differences (DXA lean mass index and IGF-I), with 95% CIs represented by error bars. Results were generated from multivariable linear regression models adjusted for child race/ethnicity, child age at outcome measurement (except z score outcomes), child age at diet assessment, household income, maternal education, breastfeeding status at 6 mo, and: 1) maternal and paternal BMI, birth weight for gestational age z score, fast food intake, and physical activity (BMI z score); 2) fast food intake and physical activity (DXA lean mass index); or 3) maternal and paternal height and birth length (height z score and IGF-I). Protein intake is adjusted for total energy intake and body weight at the time of diet assessment. n = 963 for analyses of BMI z score, height z score, and DXA lean mass index; n = 717 for analyses of IGF-I. DXA, dual-energy X-ray absorptiometry; IGF-I, insulin-like growth factor I.
Discussion
In this large sample of generally healthy children from a US cohort, boys with higher protein intake in early childhood had higher BMI z scores, lean mass, and IGF-I in early adolescence, as well as a trend towards greater increases in height z score, lean mass, and IGF-I between mid-childhood and early adolescence. There were no associations between early protein intake and these outcomes in mid-childhood, no associations with measures of adiposity at either time point, and no relations between early childhood protein intake and any of the growth-related outcomes studied in either mid-childhood or early adolescence among girls.
Results of other studies examining childhood protein intake and later growth or size have been inconsistent, possibly owing to differences in the timing of exposure and outcome assessments. In a cohort of German children, Günther et al. (15) found that girls in the highest tertile of habitual protein intake between 12 and 24 mo had a higher BMI z score at onset of the adiposity rebound than girls in the lowest tertile of intake. There was no apparent association in boys, and protein intake was not associated with timing of the adiposity rebound (15). In contrast, Rolland-Cachera et al. (14) found that French children with higher protein intake at 2 y had an earlier adiposity rebound as well as higher subsequent BMI at 8 y. Similarly, in a study of children in Australia, higher intake of protein and meat at 18 mo was associated with a higher BMI at 8 y (18). Among Dutch children in the Generation R cohort, total protein intake at 1 y was associated with higher BMI and specifically fat mass at 6 y, with stronger associations among girls and for animal protein intake (19). In a separate study conducted in Generation R, protein intake at 1 y was associated with greater weight, height, and BMI ≤9 y, with stronger associations for protein from animal sources (17). Finally, protein intake at 2 y was associated with BMI and weight ≤5 y among children in the United Kingdom (16).
In contrast to many of these studies, we did not find associations of protein intake during early childhood with BMI or adiposity in mid-childhood, but we did see an association with higher BMI in early adolescence among boys. This association appeared to be explained by an increase in lean mass rather than fat mass as there were no associations between early childhood protein intake and either SS + TR skinfolds or DXA fat mass. However, there was a trend towards an increase in DXA lean mass index with higher total protein intake, and there was a statistically significant direct association with animal protein intake. Some studies observed stronger associations of protein intake with mid-childhood BMI and fat mass among girls; in contrast, we found associations with BMI, lean mass, and IGF-I only in boys, and the associations were observed only with outcomes measured in early adolescence. This discrepancy may be partly explained by our longer duration of follow-up, extending into adolescence when boys and girls exhibit marked differences in pubertal timing and subsequent growth and body composition. In our study, few participants were pubertal in mid-childhood (17% of boys and 26% of girls), whereas most participants had initiated puberty by early adolescence (91% of boys and 100% of girls). One possible explanation for our finding that early protein intake was associated with body composition and IGF-1 in boys but not girls is that these early adolescent outcomes may be driven primarily by pubertal sex steroids in girls. In boys, who are not as far along in puberty in early adolescence, these outcomes may be more strongly influenced by early life factors such as protein intake.
Günther et al. (20) examined protein intake at multiple infancy and childhood time points in relation to BMI and adiposity at 7 y, and found that 12 mo and 5–6 y were the critical ages at which higher total and animal protein intakes were related to body fat at 7 y. It is possible that we assessed protein intake during a time window that is not important for programming of adiposity, but may be important for programming other growth parameters around the time of puberty. It is also possible, however, that early childhood protein intake tracks with later protein intake, and protein intake closer to the time of puberty is associated with pubertal growth, rather than a programming effect of earlier protein intake.
Our results are consistent with those of Harris et al. (24), who found an association between meat and meat protein intake at 10 y and lean mass at 15 y in German boys but not girls. The authors suggested that higher protein intake may enhance the normal pubertal increases in lean mass among males. We also observed higher concentrations of IGF-I, a key hormone involved in pubertal growth, among boys who had higher protein intake in early childhood. Together these results suggest a possible programming of pubertal increases in lean mass, but not adiposity, by protein intake in early childhood.
Previous work indicates that a higher protein intake in infancy stimulates increases in height, possibly through effects on IGF-I, and that the more rapid linear growth accelerates onset of the adiposity rebound. The adiposity rebound corresponds to the second increase in BMI in early childhood (39) and is followed by a rapid increase in adiposity and BMI (5, 7, 11) and subsequent higher accumulated adiposity in later childhood and into adolescence and adulthood (39, 40). We therefore hypothesized that children with diets higher in protein in early childhood would have greater adiposity at our mid-childhood visit, which should have taken place after the adiposity rebound for most children. Contrary to our hypothesis, there were no associations between protein intake and any of the various measures of weight status and adiposity that we examined (BMI z score, SS + TR skinfolds, or DXA total fat mass index) in mid-childhood. Rolland-Cachera has suggested that there is a distinction between children who have high BMI and adiposity consistently from infancy and those who have a normal or low BMI through early childhood but an early adiposity rebound followed by rapid gain in body fat, and that these 2 pathways to high adiposity in later childhood and adolescence may have different environmental predictors (39). In the Generation R cohort, associations of protein intake at 1 y with fat mass at 6 y were stronger in children with early catch-up growth, suggesting that this group might be more sensitive to higher early protein intake (19). Because our sample likely included a mix of these 2 profiles, we may have failed to detect an association with early protein intake because it is a predictor of adiposity only in children not already on a path to higher body fat in early childhood.
Strengths of our study include a large sample size, reasonably strong measures of diet in early childhood, detailed assessment of several different outcomes measured in both mid-childhood and early adolescence, and consideration of many potential confounders. Many factors are associated with diet and predict height growth and adiposity development, making it important to carefully control for potential confounders when considering associations of child diet with later growth. We accounted for several sociodemographic factors and other strong predictors of child adiposity and height, including parental and birth anthropometrics. Our study also had some limitations, including those associated with dietary assessment using FFQs. FFQ-based estimates of absolute nutrient intakes are unreliable owing to associated measurement error, and the high estimated protein intakes in our cohort suggest that systematic over-reporting was a possibility. However, the absolute protein intake in our study population is comparable to that reported for 2–3-y-olds in the United States based on data from the NHANES 2003–2004 (41). We also addressed potential measurement error by adjusting estimates of protein intake for total energy intake and body weight. FFQs are useful for ranking individuals in relation to others for purposes of comparison and assessment of exposure–outcome relations, which was how they were used in our analyses. In addition, because early childhood protein intake was high in our study population, our results may not be generalizable to other populations with lower protein intakes. Although we did not fully assess child diets after early childhood, we accounted for some of the tracking in dietary habits and healthful lifestyle factors by adjusting our models for fast food intake and physical activity in mid-childhood and early adolescence; accounting for these factors did not substantially change our estimates. Finally, we decided a priori to examine associations separately in boys and girls and found that associations were present among boys but not girls; however, we did not detect a statistically significant interaction between protein intake and sex. We used all available previously collected data from an established cohort study and may not have had sufficient power to detect these interactions owing to limited sample sizes. However, we had decided to conduct a stratified analysis before examining the data, associations were present in boys only, and our analysis was likely underpowered to detect a statistical interaction between protein intake and sex. Therefore, we present all results separately for boys and girls.
In conclusion, we found associations of early childhood total and animal protein intake with BMI, lean mass, and IGF-I in early adolescence in boys, but identified no associations of protein intake with any of the outcomes in mid-childhood, in girls at either time point, or with measures of adiposity at either time point in our unadjusted or adjusted models. Thus, although protein intake in early childhood may be important for stimulating puberty-related growth in boys, our results do not support the hypothesis that protein intake in early childhood programs adiposity development in well-nourished children.
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—KMS, PFJ, AM, and EO: designed the research; KMS: analyzed the data, drafted the manuscript, and had primary responsibility for the final content; PFJ, AM, and EO: provided study oversight; AF: provided scientific expertise in endocrinology and pubertal development; and all authors: provided critical intellectual contributions and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by US NIH grants R01 HD034568 (to EO) and UG3 OD023286 (to EO). EO was supported by grants K24 HD069408 and P30 DK092924.
Abbreviations used: DXA, dual-energy X-ray absorptiometry; EAR, Estimated Average Requirement; FFQ, food-frequency questionnaire; IGF-I, insulin-like growth factor I; IGFBP-3, insulin-like growth factor binding protein 3; RA, research assistant; SS, subscapular; TR, triceps.
References
- 1. Weber M, Grote V, Closa-Monasterolo R, Escribano J, Langhendries J-P, Dain E, Giovannini M, Verduci E, Gruszfeld D, Socha P et al.. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99:1041–51. [DOI] [PubMed] [Google Scholar]
- 2. Setia S, Sridhar MG. Changes in GH/IGF-1 axis in intrauterine growth retardation: consequences of fetal programming?. Horm Metab Res. 2009;41:791–8. [DOI] [PubMed] [Google Scholar]
- 3. Fleddermann M, Demmelmair H, Grote V, Bidlingmaier M, Grimminger P, Bielohuby M, Koletzko B. Role of selected amino acids on plasma IGF-I concentration in infants. Eur J Nutr. 2017;56:613–20. [DOI] [PubMed] [Google Scholar]
- 4. Lonnerdal B. Infant formula and infant nutrition: bioactive proteins of human milk and implications for composition of infant formulas. Am J Clin Nutr. 2014;99:712S–17S. [DOI] [PubMed] [Google Scholar]
- 5. Madsen AL, Larnkjær A, Mølgaard C, Michaelsen KF. IGF-I and IGFBP-3 in healthy 9 month old infants from the SKOT cohort: breastfeeding, diet, and later obesity. Growth Horm IGF Res. 2011;21:199–204. [DOI] [PubMed] [Google Scholar]
- 6. Larnkjaer A, Ingstrup HK, Schack-Nielsen L, Hoppe C, Mølgaard C, Skovgaard IM, Juul A, Michaelsen KF. Early programming of the IGF-I axis: negative association between IGF-I in infancy and late adolescence in a 17-year longitudinal follow-up study of healthy subjects. Growth Horm IGF Res. 2009;19:82–6. [DOI] [PubMed] [Google Scholar]
- 7. Chellakooty M, Juul A, Boisen KA, Damgaard IN, Kai CM, Schmidt IM, Petersen JH, Skakkebaek NE, Main KM. A prospective study of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in 942 healthy infants: associations with birth weight, gender, growth velocity, and breastfeeding. J Clin Endocrinol Metab. 2006;91:820–6. [DOI] [PubMed] [Google Scholar]
- 8. Socha P, Grote V, Gruszfeld D, Janas R, Demmelmair H, Closa-Monasterolo R, Subías JE, Scaglioni S, Verduci E, Dain E et al.. Milk protein intake, the metabolic-endocrine response, and growth in infancy: data from a randomized clinical trial. Am J Clin Nutr. 2011;94:1776S–84S. [DOI] [PubMed] [Google Scholar]
- 9. Savino F, Fissore MF, Grassino EC, Nanni GE, Oggero R, Silvestro L. Ghrelin, leptin and IGF-I levels in breast-fed and formula-fed infants in the first years of life. Acta Paediatr. 2005;94:531–7. [DOI] [PubMed] [Google Scholar]
- 10. Putet G, Labaune J-M, Mace K, Steenhout P, Grathwohl D, Raverot V, Morel Y, Picaud J-C. Effect of dietary protein on plasma insulin-like growth factor-1, growth, and body composition in healthy term infants: a randomised, double-blind, controlled trial (Early Protein and Obesity in Childhood (EPOCH) study). Br J Nutr. 2016;115:271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ong KK, Langkamp M, Ranke MB, Whitehead K, Hughes IA, Acerini CL, Dunger DB. Insulin-like growth factor I concentrations in infancy predict differential gains in body length and adiposity: the Cambridge Baby Growth Study. Am J Clin Nutr. 2009;90:156–61. [DOI] [PubMed] [Google Scholar]
- 12. Closa-Monasterolo R, Ferré N, Luque V, Zaragoza-Jordana M, Grote V, Weber M, Koletzko B, Socha P, Gruszfeld D, Janas R et al.. Sex differences in the endocrine system in response to protein intake early in life. Am J Clin Nutr. 2011;94:1920S–7S. [DOI] [PubMed] [Google Scholar]
- 13. Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis?. Lancet. 2004;363:1642–5. [DOI] [PubMed] [Google Scholar]
- 14. Rolland-Cachera MF, Deheeger M, Akrout M, Bellisle F. Influence of macronutrients on adiposity development: a follow up study of nutrition and growth from 10 months to 8 years of age. Int J Obes Relat Metab Disord. 1995;19:573–8. [PubMed] [Google Scholar]
- 15. Günther ALB, Buyken AE, Kroke A. The influence of habitual protein intake in early childhood on BMI and age at adiposity rebound: results from the DONALD study. Int J Obes. 2006;30:1072–9. [DOI] [PubMed] [Google Scholar]
- 16. Pimpin L, Jebb S, Johnson L, Wardle J, Ambrosini GL. Dietary protein intake is associated with body mass index and weight up to 5 y of age in a prospective cohort of twins. Am J Clin Nutr. 2016;103:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braun KV, Erler NS, Kiefte-de Jong JC, Jaddoe VW, van den Hooven EH, Franco OH, Voortman T. Dietary intake of protein in early childhood is associated with growth trajectories between 1 and 9 years of age. J Nutr. 2016;146:2361–7. [DOI] [PubMed] [Google Scholar]
- 18. Garden FL, Marks GB, Almqvist C, Simpson JM, Webb KL. Infant and early childhood dietary predictors of overweight at age 8 years in the CAPS population. Eur J Clin Nutr. 2011;65:454–62. [DOI] [PubMed] [Google Scholar]
- 19. Voortman T, Braun KVE, Kiefte-de Jong JC, Jaddoe VWV, Franco OH, van den Hooven EH. Protein intake in early childhood and body composition at the age of 6 years: the Generation R study. Int J Obes. 2016;40:1018–25. [DOI] [PubMed] [Google Scholar]
- 20. Günther ALB, Remer T, Kroke A, Buyken AE. Early protein intake and later obesity risk: which protein sources at which time points throughout infancy and childhood are important for body mass index and body fat percentage at 7 y of age?. Am J Clin Nutr. 2007;86:1765–72. [DOI] [PubMed] [Google Scholar]
- 21. Hoppe C, Udam TR, Lauritzen L, Mølgaard C, Juul A, Michaelsen KF. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-y-old Danish children. Am J Clin Nutr. 2004;80:447–52. [DOI] [PubMed] [Google Scholar]
- 22. Rogers IS, Gunnell D, Emmett PM, Glynn LR, Dunger DB, Holly JM. Cross-sectional associations of diet and insulin-like growth factor levels in 7- to 8-year-old children. Cancer Epidemiol Biomarkers Prev. 2005;14:204–12. [PubMed] [Google Scholar]
- 23. Joslowski G, Remer T, Assmann KE, Krupp D, Cheng G, Garnett SP, Kroke A, Wudy SA, Günther ALB, Buyken AE. Animal protein intakes during early life and adolescence differ in their relation to the growth hormone-insulin-like-growth-factor axis in young adulthood. J Nutr. 2013;143:1147–54. [DOI] [PubMed] [Google Scholar]
- 24. Harris C, Buyken A, von Berg A, Berdel D, Lehmann I, Hoffmann B, Koletzko S, Koletzko B, Heinrich J, Standl M. Prospective associations of meat consumption during childhood with measures of body composition during adolescence: results from the GINIplus and LISAplus birth cohorts. Nutr J. 2016;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EM et al.. Cohort profile: Project Viva. Int J Epidemiol. 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blum RE, Wei EK, Rockett HR, Langeliers JD, Leppert J, Gardner JD, Colditz GA. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Matern Child Health J. 1999;3:167–72. [DOI] [PubMed] [Google Scholar]
- 27. US Department of Agriculture Agricultural Research Service. 2001. USDA National Nutrient Database for Standard Reference, Release 14, Beltsville, MD. Nutrient Data Laboratory Home Page, http://www.ars.usda.gov/nutrientdata. [Google Scholar]
- 28. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S.; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 29. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–99. [DOI] [PubMed] [Google Scholar]
- 30. Mackerras D. Energy adjustment: the concepts underlying the debate. J Clin Epidemiol. 1996;49:957–62. [DOI] [PubMed] [Google Scholar]
- 31. Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, Bingham S, Schoeller DA, Schatzkin A, Carroll RJ. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol. 2003;158:14–21.; discussion 22–6. [DOI] [PubMed] [Google Scholar]
- 32. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 33. Parker M, Rifas-Shiman SL, Belfort MB, Taveras EM, Oken E, Mantzoros C, Gillman MW. Gestational glucose tolerance and cord blood leptin levels predict slower weight gain in early infancy. J Pediatr. 2011;158:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–33. [DOI] [PubMed] [Google Scholar]
- 35. Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–5. [DOI] [PubMed] [Google Scholar]
- 36. Wright M, Sotres-Alvarez D, Mendez MA, Adair L. The association of trajectories of protein intake and age-specific protein intakes from 2 to 22 years with BMI in early adulthood. Br J Nutr. 2017;117:750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 38. Rubin D. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: Wiley-Interscience; 2004. [Google Scholar]
- 39. Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes. 2006;30(Suppl 4):S11–17. [DOI] [PubMed] [Google Scholar]
- 40. Rolland-Cachera M, Akrout M, Péneau S. Nutrient intakes in early life and risk of obesity. Int J Environ Res Public Health. 2016;13:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fulgoni VL. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87:1554S–7S. [DOI] [PubMed] [Google Scholar]