ABSTRACT
Background
Mother's own milk (MOM) is protective against gut microbiota alterations associated with necrotizing enterocolitis (NEC) and feeding intolerance among preterm infants. It is unclear whether this benefit is preserved with donor milk (DM) feeding.
Objective
We aimed to compare microbiota development, growth, and feeding tolerance in very-low-birth-weight (VLBW) infants fed an exclusively human milk diet of primarily MOM or DM.
Methods
One hundred and twenty-five VLBW infants born at Texas Children's Hospital were enrolled and grouped into cohorts based on percentage of MOM and DM in enteral feeds. Feeds were fortified with DM-derived fortifier per unit protocol. Weekly stool samples were collected for 6 wk for microbiota analysis [16S ribosomal RNA (rRNA) sequencing]. A research nurse obtained weekly anthropometrics. Clinical outcomes were compared via Wilcoxon's rank-sum test and Fisher's exact test, as well as multivariate analysis.
Results
The DM cohort (n = 43) received on average 14% mothers’ milk compared with 91% for the MOM cohort (n = 74). Diversity of gut microbiota across all time points (n = 546) combined was increased in MOM infants (P < 0.001). By 4 and 6 wk of life, microbiota in MOM infants contained increased abundance of Bifidobacterium (P = 0.02) and Bacteroides (P = 0.04), whereas DM-fed infants had increased abundance of Staphylococcus (P = 0.02). MOM-fed infants experienced a 60% reduction in feeding intolerance (P = 0.03 by multivariate analysis) compared with DM-fed infants. MOM-fed infants had greater weight gain than DM-fed infants.
Conclusions
Compared with DM-fed infants, MOM-fed infants have increased gut microbial community diversity at the phylum and genus levels by 4 and 6 wk of life, as well as better feeding tolerance. MOM-fed infants had superior growth. The incidence of NEC and other gastrointestinal morbidity is low among VLBW infants fed an exclusively human milk diet including DM-derived fortifier. This trial was registered at clinicaltrials.gov as NCT02573779.
Keywords: neonate, premature infant, very low birth weight, microbiota, breast milk, donor milk, feeding intolerance, growth
Introduction
Human milk feeding provides benefits to preterm infants including accelerated gut maturation and decreased infection rates (1). There is evidence that an exclusively human milk–based diet, compared with one that includes bovine milk–based products, decreases rates of necrotizing enterocolitis (NEC) and death (2–5), and is associated with better feeding tolerance in very-low-birth-weight (VLBW) infants born at <1500 g birth weight (BW) (6). Thus, the American Academy of Pediatrics recommends feeding mother's own milk (MOM), supplemented with donor human milk (DM) and fortified as needed for VLBW infants (7).
To provide the benefits of human milk, the use of DM in neonatal intensive care units (NICUs) has increased when MOM is unavailable (8). Numerous studies have favorably compared feeding of MOM to formula (9–11) or DM to formula feeding (12–14). However, relatively few studies have compared MOM to DM (15–17), and they typically include milk supplementation with bovine-based fortifier which theoretically could have effects similar to formula supplementation. There are no randomized controlled trials comparing MOM with DM, because it is unacceptable to not give MOM when available (12).
It has been hypothesized that many beneficial effects of human milk relate to how diet affects gut microbiota and the developing immune system. Evidence suggests an association between gut dysbiosis and disease states such as NEC (18, 19). Specifically, increases in Proteobacteria (e.g., Escherichia coli) and decreases in Firmicutes (specifically Lactobacillus), Actinobacteria (specifically Bifidobacteria), and Bacteroidetes have been implicated in the pathogenesis of NEC (18–20). A longitudinal study by Warner et al. (20) demonstrated dysbiosis in stools from infants before they developed NEC compared with stools from their healthy peers. A meta-analysis by Pammi et al. (21) reported similar findings. Although the association between NEC and altered microbiota is confounded in that infants who develop NEC often experience greater antibiotic exposure before developing the disease, studies indicate that these changes may be important biomarkers for infants at risk of developing NEC and other complications of prematurity.
Trials comparing DM with formula illustrate that the benefit of human milk in preventing NEC and late-onset sepsis is not as clearly identified with DM as with MOM (8, 22). DM may be less beneficial for feeding tolerance and infection prevention due to pasteurization, which removes growth factors, hormones, human milk oligosaccharides, other immunological factors, and (most obviously) beneficial microbes (23–26). Although breast milk appears to promote the development of protective microbiota (27–30), it is unclear if the use of pasteurized DM does the same. Previous studies have raised the concern that DM is associated with poor growth in preterm infants (14). However, Hair et al. (31) found that preterm infants who received MOM, DM, and a DM-derived fortifier (thus avoiding any bovine products) demonstrated superior growth to infants who received a diet of MOM with bovine fortifier or formula.
To our knowledge, this study is the largest to compare intestinal microbiota and clinical outcomes between infants who receive a diet of primarily MOM and those who receive primarily DM, and the first to do so in the setting of an exclusively human milk diet (i.e., one that does not include formula supplementation or fortification with bovine-based fortifier). Our goal was to investigate differences in microbiota development based on type of human milk fed, with the hypothesis that feeding primarily MOM—with its live microbes and higher content of immune factors—is associated with increased microbial diversity and improved short-term outcomes including growth in VLBW infants compared with their peers fed pasteurized DM.
Methods
This prospective cohort study (NCT02573779) included 125 VLBW inborn infants enrolled within the first 72 h of life in the NICU at Texas Children's Hospital—Pavilion for Women. Eligible study participants included premature infants born at <1500 g BW with no barriers to enteral milk feeding. Exclusion criteria included anomalies or birth defects that precluded enteral feeding (e.g., gastroschisis), severe perinatal hypoxia, or <50% projected survival based on the National Institute of Child Health and Human Development Neonatal Research Network Extremely Preterm Birth Outcome Data calculator (32). The study was approved by the institutional review board at Baylor College of Medicine and supported with research nurse staff via the Baylor College of Medicine Clinical Research Center. All study procedures were in accordance with the ethical standards of Baylor College of Medicine. Written parental informed consent was provided for each infant enrolled in the study. Because of the well-established benefits of feeding MOM to VLBW infants, randomization in this study would have been unethical. The sample size was determined based on a primary endpoint of weight gain from birth to 36 wk post-menstrual age (PMA).
Enrolled infants were started on parenteral nutrition while gradually increasing enteral feeds with an exclusively human milk diet per our hospital's standardized feeding protocols for infants <1250 g and 1250–1500 g BW (Supplemental Tables 1 and 2) at the discretion of the attending neonatologist on service. Decisions to decrease or temporarily withhold enteral feeding owing to medical instability were also made per the judgment of the attending neonatologist on service. Infants were preferentially fed MOM when available. When an infant's mother was unavailable, unable (despite attempts to express breast milk with lactation staff support), or unwilling by choice to express adequate milk volume for their infants, pooled DM (pasteurized by Prolacta Bioscience) was provided per hospital protocol. Feeds were fortified with a DM-derived fortifier (Prolacta Bioscience) and calories increased ≤32 kcal/oz (1 oz = 30 mL) with the addition of DM-derived cream (Prolacta Bioscience) for both MOM-fed and DM-fed infants as needed for growth until infants reached 34 wk PMA and 2 kg weight, per protocol. No enrolled subjects received either probiotics or acid-suppression medications because these are not routine practices within our unit.
Study cohorts were determined by overall percentage of enteral feeds consisting of MOM or DM from time of birth to study completion: those who received >50% MOM were grouped into the MOM cohort, and those who received <50% MOM were grouped into the DM cohort. This grouping was accomplished by reviewing the milk bank prep sheet for each infant every day he or she remained in the study and tallying the precise milliliter volume of MOM compared with DM received during the study period. Study participants remained in the study through 36 wk PMA or hospital discharge, whichever came first.
Stool samples, if available, were collected weekly from each enrolled infant for the first 6 wk of life, with the first sample of meconium collected as soon as possible after birth following study enrollment. Bacterial extraction and subsequent sequencing were performed as previously described (33). DNA from stool samples was extracted using PowerSoil DNA isolation kits (Mo Bio Laboratories). Amplification and high-throughput sequencing targeting the V4 region of the 16S ribosomal RNA (rRNA) gene were performed using the NEXTflex 16S V4 Amplicon-Seq Kit 2.0 (BioScientific) and sequences were generated on the Illumina MiSeq platform with a median of 23,492 reads per sample. The forward primer sequence 5′-GACGCTCTTCCGATCTTATGGTAATTGTGTGCCAGCMGCCGCGGTAA-3′ and reverse primer sequence 5′ TGTGCTCTTCCGATCTAGTCAGTCAGCCGGACTACHVGGGTWTCTAAT-3′ from the BioScientific kit were used to amplify the V4 region of the 16S rRNA gene. Sequence data were processed through the LotuS pipeline (34). Briefly, reads were de-multiplexed and paired ends stitched. Quality filtering was performed before operational taxonomic unit (OTU) clustering (at 97% similarity) via the UPARSE algorithm (35). Taxonomic assignment was performed with Ribosomal Database Project 2.10.1 as the classifier and SILVAv123 (36) as the selected database. Organisms potentially classified to the species level were based on individual OTUs of significance. OTUs failing to classify as Bacteria at the kingdom level were removed before further analysis of the data set. Data were rarified using 1015 reads per sample. Bacterial community diversity, evenness, richness, and relative abundance of the OTUs identified in each sample were evaluated using QIIME (37). A mixed-effects linear model with a first-order autoregressive covariance structure was used to compare groups’ OTUs, Shannon Diversity Index (SDI), and organisms’ relative abundance over time while accommodating the repeated measurements on the same patients. Principal coordinates analysis using the Bray-Curtis index as the distance metric was performed using Calypso (38). Comparisons were made across weeks for the DM and MOM groups in parallel.
Primary outcomes included diversity of intestinal microbiota and growth velocities (weight, length, and head circumference). A trained research nurse obtained anthropometrics weekly (weight, length, and head circumference) as previously described (31). Growth velocities were calculated from birth to 36 wk PMA or discharge (whichever came first). Secondary outcomes included feeding tolerance and rates of NEC, spontaneous intestinal perforation (SIP), late-onset sepsis, severe bronchopulmonary dysplasia (BPD), and death. NEC was defined as Stage IIA NEC per Modified Bell's Staging Criteria (39). SIP was defined as intestinal free air on radiograph and/or directly visualized perforation via laparotomy. Late-onset sepsis was defined as positive blood cultures (not deemed contaminated) collected after 72 h of age. BPD was defined as the need for supplemental oxygen >21% for >28 d, and severe BPD was defined as the need for supplemental oxygen >30% or positive pressure ventilation at 36 wk PMA. A preplanned composite outcome analysis of these severe morbidities—all associated with decreased incidence among human milk–fed infants in a prior study by Hair et al. (40)—and death was compared between the cohorts.
Feeding intolerance was assessed by multiple factors: the number of feeds held per total days of enteral feeding during the study period, the number of days required to reach the goal feeding volume (140–160 mL · kg–1 · d–1), days with no enteral feeds (nil per os) after feeding was initiated, and days of total parenteral nutrition. Statistical analysis of clinical outcomes was performed using Wilcoxon's rank-sum test and Fisher's exact test for bivariate analyses as well as multivariable linear and logistic regression analyses that adjusted for BW, duration of antibiotics in the first 14 d of life, as well as 2 baseline variables that were found to be significantly different: ethnicity and use of prophylactic indomethacin. SAS version 9.4 (SAS Institute Inc.) was used for data analysis.
Results
Between September, 2015 and August, 2016, 223 infants <1500 g BW were born at Texas Children's Hospital—Pavilion for Women. Of these, 33 infants were excluded (Supplemental Table 3), 59 could not be enrolled within 72 h, and 6 were not enrolled because their parents declined. Of the 125 enrolled infants, 8 were removed from the study owing to gastrointestinal defects or complications unknown at time of enrollment that precluded enteral feeding (Supplemental Table 4), leaving 117 infants who completed the study. Of the 117 infants who completed the study, there were 90 infants with adequate stools collected for analysis (Supplemental Figure 1). Baseline demographic data are displayed in Table 1.
TABLE 1.
Baseline demographics1
| MOM cohort (n = 74) | DM cohort (n = 43) | P value | |
|---|---|---|---|
| % MOM vs. DM in feeds | 91.2 ± 14.4 | 14.3 ± 15.5 | <0.0001 |
| Gestational age, wk | 28.7 ± 2.0 | 28.4 ± 2.5 | 0.62 |
| Birth weight, g | 1107 ± 242 | 1066 ± 294 | 0.64 |
| Male, n (%) | 39 (52.7) | 25 (55.8) | 0.65 |
| Ethnicity, n (%) | |||
| Caucasian | 24 (32.4) | 14 (32.6) | 0.07 |
| African-American | 27 (36.5) | 13 (30.2) | |
| Hispanic | 15 (20.3) | 15 (34.9) | |
| Asian | 8 (10.8) | 1 (2.3) | |
| SGA at birth (BW <10th percentile), n (%) | 10 (13.5) | 7 (6.3) | 0.68 |
| Received 2 doses of antenatal steroids, n (%) | 62 (83.8) | 36 (83.7) | 0.99 |
| Cesarean delivery, n (%) | 61 (82.4) | 38 (88.4) | 0.39 |
| Apgars—5 min | 7.7 ± 1.2 | 7.0 ± 2.2 | 0.31 |
| Empiric antibiotics ≤48 h after birth, n (%) | 48 (64.9) | 30 (69.8) | 0.59 |
| Days of antibiotics in first 14 d of life | 2.2 ± 2.1 | 2.4 ± 3.0 | 0.78 |
| Received prophylactic indomethacin, n (%) | 11 (14.9) | 17 (39.5) | <0.01 |
| Received surfactant, n (%) | 48 (64.9) | 31 (72.1) | 0.42 |
1Values are means ± SDs (P value from Wilcoxon's rank-sum test) or n (%) (P value from Fisher's exact test). BW, body weight; DM, donor human milk; MOM, mother's own milk; SGA, small for gestational age.
Receipt of MOM differed widely between the 2 cohorts in a bimodal distribution, with infants grouped into the MOM cohort receiving on average 91.2% MOM compared with only 14.3% in the infants grouped to the DM cohort. Eighty-five percent of the enrolled subjects received either >75% or <25% MOM. The 2 cohorts were similar in all other baseline demographics except for higher usage of prophylactic indomethacin in the DM group (P < 0.01). There were no differences in days of antibiotics received in the first 14 d of life between MOM- and DM-fed infants. Stool samples were selected for microbiota analysis from all infants who had samples collected at every time point (n = 90). In total, 546 stool samples from 90 infants—58 in the MOM cohort and 32 in the DM cohort—underwent microbiota analysis.
Microbiota analyses
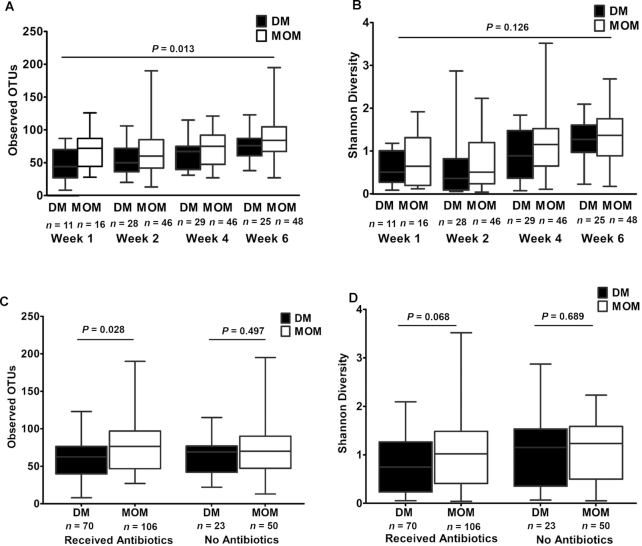
When evaluating the primary outcome of microbial community diversity across all time points measured, MOM infants demonstrated significantly higher diversity in terms of OTU richness (slope = 9.4, SE = 3.6, P = 0.013); however, SDI was not significantly different (slope = 0.22, SE = 0.14, P = 0.126) (Figure 1A, B).
FIGURE 1.
α-Diversity comparisons of gut microbiota from DM and MOM infants by age and antibiotic exposure. Using mixed-effects linear models for data analysis to look across all time points (n = 156 for MOM samples and n = 93 for DM samples), (A) observed OTU richness was significantly higher in the MOM group (slope = 9.4, SE = 3.6, P = 0.013), but (B) the SDI was not significantly different (slope = 0.22, SE = 0.14, P = 0.126). (C) For infants who received empiric antibiotics (≤48 h after birth) observed OTU richness was significantly higher in the MOM group (slope = 10.5, SE = 4.1, P = 0.028), but there was not a significant difference among infants who did not receive antibiotics (slope = 5.1, SE = 7.5, P = 0.497). (D) Among infants who received empiric antibiotics, the SDI tended to be higher for the MOM group (slope = 0.31, SE = 0.15, P = 0.068), but there was not a significant difference for MOM compared with DM among infants who did not receive antibiotics (slope = −0.12, SE = 0.29, P = 0.689). DM, donor human milk; MOM, mother's own milk; OTU, operational taxonomic unit; SDI, Shannon Diversity Index.
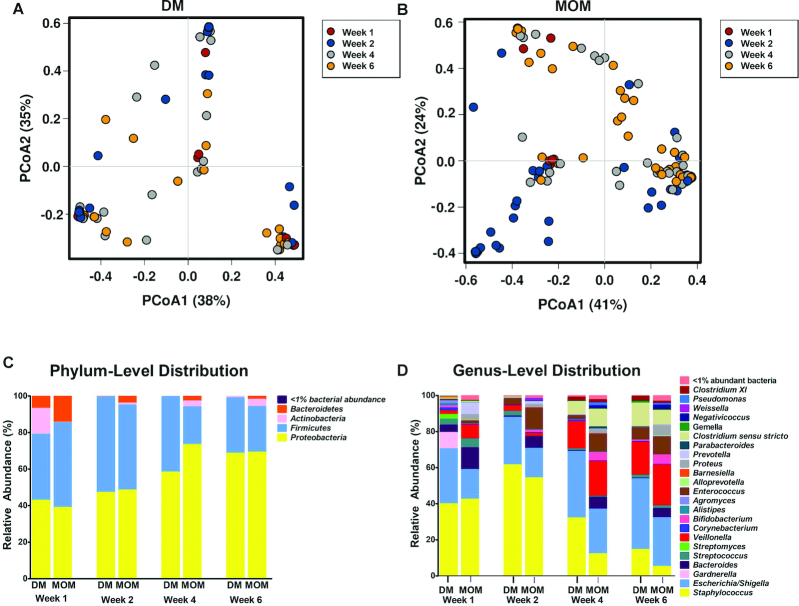
Principal coordinates analysis plots with Bray-Curtis dissimilarity (Figure 2A, B) results revealed a pattern of development with stool samples obtained during the first week of life clustering separately from those obtained during the second week of life, followed by separate clustering of samples obtained in the third or fourth weeks of life, suggesting a developmental pattern among fecal microbial communities in MOM-fed infants (n = 74) (Figure 2B). No obvious clustering was apparent within stool samples obtained from DM-fed infants (n = 43) (Figure 2A).
FIGURE 2.
β-Diversity among gut microbial communities from DM compared with MOM infants over time. (A) Bray-Curtis PCoA ordination of stool samples obtained at 1, 2, 4, and 6 wk of life from DM-fed infants. Samples obtained during the first week of life are shown in red, second week in blue, fourth week in gray, and sixth week in orange. No obvious clustering was apparent within samples obtained from DM-fed infants (n = 43). (B) Bray-Curtis PCoA ordination of samples obtained from MOM-fed infants. Results revealed a pattern of development with stool samples obtained during the first week of life clustering separately from those obtained during the second week of life, followed by separate clustering of samples obtained in the third or fourth weeks of life, suggesting a developmental pattern among fecal microbial communities in MOM-fed infants (n = 74). (C) When comparing longitudinal changes across samples from all study subjects, increasing relative abundance of Proteobacteria was observed. There were no significant differences observed at the phylum level during the first 2 wk of life. By week 4, microbiota from the MOM cohort had significantly higher abundance of Actinobacteria (P = 0.032) and decreased abundance of Firmicutes (P = 0.011). (D) By week 4, microbiota from the MOM cohort had significantly increased abundance of Bacteroides (P = 0.046), Bifidobacterium (P = 0.026), and Enterococcus (P < 0.001) in comparison to the DM cohort. DM infants had significantly higher abundance of Staphylococcus (P = 0.014). DM, donor human milk; MOM, mother's own milk; PCoA, principal coordinates analysis.
As expected and in agreement with earlier studies (41–43), the microbiota in both cohorts underwent dynamic changes over the first 6 wk of life. When comparing longitudinal changes across samples from all study subjects, increasing relative abundance of Proteobacteria was observed (Figure 2C). Between cohorts, there were no significant differences in relative abundance of microbiota at the phylum level in the first 2 wk of life (Figure 2C). However, by week 4, Actinobacteria were more abundant in the MOM cohort (P = 0.03) and Firmicutes were more abundant in the DM cohort (P = 0.01). At the genus level (Figure 2D), by week 4 MOM-fed infants had increased abundance of Bifidobacterium (P = 0.026), in agreement with the previously noted increase in Actinobacteria, as well as a higher abundance of Bacteroides (P = 0.046) and Enterococcus (P < 0.001) in comparison with the DM cohort. Conversely, the previously noted increased abundance of Firmicutes among the DM infants was linked to higher proportions of Staphylococcus (P = 0.014) at the genus level. In addition, with little representation in weeks 1–2, Clostridium and Veillonella appeared in weeks 4–6.
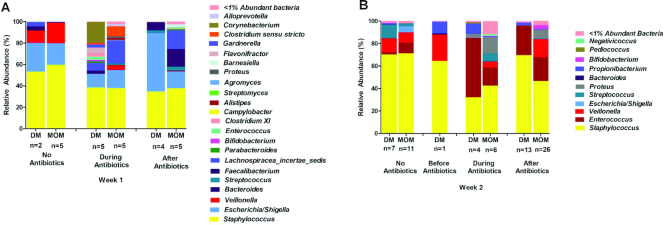
Additional comparisons were made between the cohorts in terms of other variables that are known to affect microbiota development. The impact of early-life antibiotics (≤48 h after birth) on composition of the gut microbiota from DM- and MOM-fed infants at the genus level is shown in Figure 3. The relative abundance of genera during the first week of life, separated by diet and timing by which antibiotic-exposed infants’ stool samples were obtained (before, during, or after exposure to antibiotics), is shown in Figure 3A. No stool samples in week 1 were obtained before the initiation of antibiotics. Sample sizes are small for this group because most premature infants did not have a stool sample of sufficient quantity during the first week of life. The relative abundance of genera during the second week of life, separated by diet and timing by which antibiotic-exposed infants’ stool samples were obtained (before, during, or after exposure to antibiotics), is shown in Figure 3B. One infant receiving DM had a stool sample obtained in week 2 before initiation of antibiotics and this patient's microbiota composition was similar to those obtained in week 2 from the 7 infants receiving DM who never received antibiotics.
FIGURE 3.
Impact of early-life antibiotics on composition of the gut microbiota from DM and MOM infants at the genus level. (A) Relative abundance of genera during the first week of life separated by diet and timing by which antibiotic-exposed infants’ stool samples were obtained (before, during, or after exposure to antibiotics). No stool samples in week 1 were obtained before the initiation of antibiotics. Sample sizes are small for this group because most premature infants did not have a stool sample of sufficient quantity during the first week of life. (B) Relative abundance of genera during the second week of life separated by diet and timing by which antibiotic-exposed infants’ stool samples were obtained (before, during, or after antibiotics). One infant receiving DM had a stool sample obtained in week 2 before initiation of antibiotics, and this patient's microbiota composition was similar to those obtained in week 2 from the 7 infants receiving DM who never received antibiotics. DM, donor human milk; MOM, mother's own milk.
Finally, the microbiota in the first 2 wk of life were evaluated based on mode of delivery, because this is another factor believed to affect gut microbiota development (though data remain inconclusive) (44). α-Diversity of gut microbiota from DM and MOM cohorts by mode of delivery showed no differences in observed OTUs nor SDI between infants delivered via cesarean delivery and those delivered vaginally; however, sample sizes were small (Supplemental Figure 2). The relative abundance of selected genera from DM and MOM cohorts by delivery mode is shown in Supplemental Figure 3A. Both DM- and MOM-fed groups had high rates of cesarean delivery (88% and 82%, respectively), resulting in a very small stool sample size for vaginally delivered infants (especially DM-fed infants: n = 5). The analysis of these data, while interesting, is limited in terms of power to assess interactions.
Clinical outcomes
MOM infants exhibited better growth, as indicated by higher final weight (P < 0.01), length (P = 0.03), and head circumference (P = 0.02) at 36 wk PMA, as well as greater growth velocity (grams per kilogram per day) (P < 0.01) (Table 2). DM infants were significantly more likely to have weight <10th percentile and head circumference <3rd percentile at study conclusion (P = 0.03, P = 0.03, respectively); these findings and other additional growth data, including z scores at 36 wk PMA, can be found in Supplemental Table 5. MOM infants had lower mean incidences of adverse outcomes than DM infants (Table 2). In particular, MOM infants had improved feeding tolerance compared with their DM peers, as indicated by a 60% reduction in held feedings per days fed (0.2 ± 0.4 compared with 0.5 ± 1.0, P = 0.03 adjusted) and fewer days nil per os after initiation of enteral feeds (1.2 ± 2.7 compared with 2.9 ± 5.8, P = 0.04 adjusted). MOM infants also displayed a trend toward requiring fewer days of parenteral nutrition than DM infants (P = 0.09 adjusted).
TABLE 2.
Clinical outcomes of study infants1
| MOM cohort (n = 74) | DM cohort (n = 43) | P value (unadjusted)2 | P value3 (adjusted) | |
|---|---|---|---|---|
| Hospital length of stay, d | 72.5 ± 33.2 | 88.2 ± 62.2 | 0.54 | 0.37 |
| Weight at 36 wk PMA, g | 2188 ± 326 | 2020 ± 320 | 0.01 | <0.01 |
| Length at 36 wk PMA, cm | 43.2 ± 2.2 | 42.5 ± 2.7 | 0.27 | 0.03 |
| HC at 36 wk PMA, cm | 30.7 ± 1.8 | 30.0 ± 1.8 | 0.13 | 0.02 |
| Growth velocity, g · kg–1 · d–1 | 13.6 ± 2.2 | 12.5 ± 1.5 | <0.01 | <0.01 |
| Length velocity, cm/wk | 1.0 ± 0.23 | 1.0 ± 0.22 | 0.64 | 0.95 |
| HC velocity, cm/wk | 0.8 ± 0.19 | 0.8 ± 0.17 | 0.06 | 0.47 |
| Days to final enteral feed volume | 12.4 ± 4.5 | 13.9 ± 7.0 | 0.30 | 0.26 |
| Days of parenteral nutrition | 9.6 ± 5.5 | 12.0 ± 7.8 | 0.17 | 0.09 |
| Number of feeds held per days fed | 0.2 ± 0.4 | 0.5 ± 1.0 | 0.03 | 0.03 |
| Days nil per os >12 h after feeds initiated | 1.2 ± 2.7 | 2.9 ± 5.8 | 0.02 | 0.04 |
| NEC—Stage ≥ IIA,4n (%) | 1 (1.4) | 2 (4.7) | 0.55 | 0.45 |
| SIP, n (%) | 0 | 2 (4.7) | 0.13 | 0.88 |
| BPD,5n (%) | 26 (36.1) | 17 (41.5) | 0.57 | 0.09 |
| Severe BPD,6n (%) | 8 (11.1) | 11 (26.8) | 0.03 | 0.32 |
| Late-onset sepsis, n (%) | 4 (5.4) | 6 (14.0) | 0.17 | 0.36 |
| Death, n (%) | 2 (2.7) | 2 (4.7) | 0.62 | 0.27 |
| NEC, SIP, sepsis, severe BPD, or death, n (%) | 14 (19.0) | 20 (46.9) | <0.01 | 0.02 |
1Values are mean ± SD or n (%). BPD, bronchopulmonary dysplasia; DM, donor human milk; FiO2, fraction of inspired oxygen; HC, head circumference; MOM, mother's own milk; NEC, necrotizing enterocolitis; nil per os, no enteral feeds administered; PMA, post-menstrual age; SIP, spontaneous intestinal perforation.
2 P values from Wilcoxon's rank-sum test for continuous variables and Fisher's exact test for categorical variables.
3Model adjusted for birth weight, ethnicity, receipt of prophylactic indomethacin, and days of antibiotics in first 14 d of life using linear regression for continuous variables and logistic regression for categorical variables.
4Overall NEC (Stage ≥ IIA) among study infants (all exclusively human milk–fed): 2.4% (1.6% surgical NEC).
5FiO2 > 21% for ≥28 d.
6Positive pressure or FiO2 > 30% at 36 wk PMA.
In addition to greater feeding intolerance, DM infants had higher rates of severe BPD (26.8% compared with 11.1%, P = 0.03 unadjusted and P = 0.32 adjusted) and a higher composite outcome of severe morbidity (NEC, SIP, sepsis, severe BPD, or death; P < 0.01 unadjusted and P = 0.02 adjusted). Overall, among both cohorts of infants receiving an exclusively human milk diet there were just 4 cases of serious gastrointestinal morbidity (NEC or bowel perforation), precluding comparative analyses.
Discussion
To our knowledge, this is the first study to directly compare development of gut microbiota in VLBW infants fed primarily MOM with those fed primarily DM in the setting of an exclusively human milk diet (including DM-derived fortifier). Our data highlight several key points. First, the results support our hypothesis that feeding primarily MOM is associated with increased gut microbiota diversity, which has previously been associated with healthier outcomes in VLBW infants such as lower incidence of NEC (18–22). In addition, our data correlate MOM feeding with improved feeding tolerance compared with DM feeding. If MOM could be made available to the vast majority of VLBW infants, even a slight improvement in feeding tolerance could lead to a dramatic reduction in the medical costs related to purchase of alternative formulas (i.e., hydrolyzed and elemental formulas), additional total parenteral nutrition days, and NICU length of stay. The improved growth patterns (weight, length, and head circumference) seen in the MOM cohort are consistent with previously published data demonstrating superior growth with MOM compared with DM (12, 23), although our study is one of the first to demonstrate this difference in the setting of an exclusively human milk diet without bovine fortifier supplementation. A particularly striking observation from our data is the significantly increased percentage of DM infants with head circumference below the third percentile at study conclusion. The fact that DM infants had more than double the incidence of extremely small final head circumferences is potentially concerning, because overall head growth is correlated with increased brain mass, and thus poor head growth is a risk factor for adverse neurodevelopmental outcomes.
There are multiple proposed mechanisms for why infants have improved growth with MOM compared with DM. For one, DM is most often provided by mothers of term infants beyond 1 mo of age, who are likely to produce milk with lower protein content than that of mothers of preterm infants (23). This could perhaps explain why DM infants not only exhibited decreased weight gain, but in particular higher incidence of final head circumference below the third percentile. Another proposed mechanism is that lipase activity is greatly diminished in milk after Holder pasteurization, which may lead to decreased milk fat absorption among infants receiving pasteurized DM (12).
Although our study was insufficiently powered to reveal statistical significance in nearly all of the other reported short-term secondary outcomes, it is striking that the mean rates and incidence for every negative outcome were higher in the DM cohort. Of note, both cohorts of infants fed an exclusively human milk diet exhibited very low rates of NEC—just 2.4% among all enrolled subjects, and only 1.6% for cases of surgical NEC—which prevented us from drawing meaningful conclusions regarding benefits of MOM compared with DM with respect to NEC prevention. In comparison to our low NEC rate, the average rate of NEC for infants <1500 g was 5% in the Vermont Oxford Network for 2017.
Consistent with the clinical findings, and along with the noted increase in microbiota diversity among MOM infants, we found increased representation of genera that contain beneficial bacteria among infants fed MOM. Specifically, infants fed MOM had significantly increased abundance of Bifidobacterium as well as Bacteroides, both of which appear to be protective against morbidities such as NEC (18–21). In addition, decreased proportions of Bacteroides in the gut have been associated with obesity in murine models and with metabolic syndrome in humans, although it is uncertain whether this association represents causation or is merely an effect of a high-fat diet (45–47). DM-fed infants had an increased relative abundance of Firmicutes, which has also been associated with decreased risk of NEC; however, when comparing the cohorts at the genus level, this appears to be due to increased Staphylococcus, a genus that contains pathogenic microbes. Lactobacillus was not detected in significant proportions in either cohort at any of the time points investigated, consistent with multiple studies that suggest lactobacilli are not present in appreciable quantities in preterm infant stool (48–51).
Our data also reveal that antibiotic exposure, mode of delivery, and milk source influence gut microbiota development, confirming prior studies. However, our findings further suggest that the impact of these factors varies based on the type of milk that infants are fed. This finding has potentially important clinical implications among VLBW preterm infants. If MOM could consistently protect the gut microbiota from increased colonization or overgrowth of pathogens, and instead promote the growth of beneficial bacteria, the clinical implications would be enormous.
Unexpectedly, we found that the relative abundance of Proteobacteria increased with age. Previous studies in full-term infants note an initially high abundance of Proteobacteria that decreases with age. However, a similar increase in Proteobacteria has been reported previously in extremely low BW infants (50). Perhaps this finding reflects the unique circumstances affecting this population, such as high rates of early antibiotic exposure, prolonged periods of minimal to no enteral feeding, and nosocomial microbes in the NICU setting, among many other factors.
Strengths of this study include the large sample size relative to prior published studies on preterm gut microbiota, the longitudinal design that permitted multiple microbiota assessments over time, and the specific focus on preterm infants fed an exclusively human milk diet (including DM-derived human milk fortifier), which allowed for a direct comparison of MOM and DM (without confounding effects from bovine formulas or fortifiers). This makes our study distinct from previously published studies that investigated the effects of diet on microbiota or clinical outcomes in VLBW infants, because they typically included infants fed formula or bovine-based human milk fortifier added to MOM or DM.
Owing to the study's observational design, we were unable to account for confounding variables and other potential sources of bias. These include unmeasured variables such as maternal chorioamnionitis and multiple gestation. In addition, the observational design rendered us unable to avoid the discrepancy in the proportion of DM compared with MOM infants who received prophylactic indomethacin in the first few days of life, despite nearly identical mean gestational age and BW between the 2 groups. Given there was a defined institutional protocol that addressed which infants qualified for indomethacin treatment, it is unclear why DM infants received prophylactic treatment more frequently. We cannot exclude the possibility that this difference contributed to some of the microbiota differences, given that pharmacologic agents and gut microbes can interact with and modify one another (52), and adjusting for this difference was the primary reason we analyzed the data via multivariable regression in addition to bivariate analysis. In addition, we were able to categorize stool samples obtained in the first 2 wk of life in relation to exposure to antibiotics; however, the sample sizes were small in the first week of life because of an insufficient amount of stool samples related to infants’ prematurity, which limited the power to detect statistical differences.
Future studies will seek to characterize the MOM microbiome in the context of mother–infant dyads as lactation progresses over the first few weeks of life. Other studies might seek to examine the microbiota from all infants regardless of projected survival (i.e., including infants born at 22-24 weeks gestational age), because these extremely vulnerable neonates might have the most to gain from therapies that help the gut microbiota mature. Finally, future studies may further investigate the role of microbiome-targeting therapies for VLBW infants, especially for those most at risk for alterations in diversity of the microbiota.
Conclusion
MOM- and DM-fed infants have significantly different microbiota at the phylum and genus levels by 4 and 6 wk of life, with increased microbial diversity and prevalence of taxa containing potentially beneficial microbes among MOM-fed infants. Although microbiota development is also affected by factors including antibiotic exposure and mode of delivery, our data suggest that microbiota among infants fed MOM respond differently to these factors compared with infants fed DM. MOM feeding is associated with improved feeding tolerance and may be associated with overall decreased severe morbidity. MOM-fed infants had improved weight gain from birth to 36 wk PMA compared with DM-fed infants. These findings further support efforts to provide robust lactation support to mothers of critically ill infants in the NICU.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the following people for their invaluable assistance with the project: Nancy Hurst, Kristina Tucker, and Mimi Hernandez from Texas Children's Hospital, Houston, TX; Texas Children's Hospital Milk Bank and Texas Children's Hospital Microbiome Center technicians; the Evie Whitlock Foundation, which sponsors research for fellows in neonatal-perinatal medicine; the Baylor College of Medicine Clinical Research Center; and last but not least, the NICU nurses at Texas Children's Hospital—Pavilion for Women.
The authors’ contributions were as follows—SLF, PL, PSG, AOD, JH, AV, MB, RAL, and ABH: designed the research; SLF, PSG, AOD, and ABH: conducted the research; SLF, GAP, JH, AV, MB, RAL, and ABH: analyzed the data; SLF: wrote the first draft of the manuscript; SLF, GAP, JH, MB, RAL, and ABH: wrote the paper; SLF, GAP, JH, RAL, and ABH: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by the Evie Whitlock Foundation Grant for fellows in Neonatal-Perinatal Medicine at Baylor College of Medicine.
There was no honorarium, grant, or other form of payment given to anyone to produce the manuscript.
Supplemental Tables 1–5 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: BPD, bronchopulmonary dysplasia; BW, birth weight; DM, donor human milk; MOM, mother's own milk; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; OTU, operational taxonomic unit; PMA, post-menstrual age; SDI, Shannon Diversity Index; SIP, spontaneous intestinal perforation; VLBW, very-low-birth-weight.
References
- 1. Schanler RJ. Outcomes of human milk-fed premature infants. Semin Perinatol. 2011;35(1):29–33. [DOI] [PubMed] [Google Scholar]
- 2. Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl-Kohlendorfer U, Chan GM, Blanco CL, Abrams S, Cotten CM et al.. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562–7..e1. [DOI] [PubMed] [Google Scholar]
- 3. Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, Dudell G, Rechtman DJ, Lee ML, Lucas A et al.. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163(6):1592–5. [DOI] [PubMed] [Google Scholar]
- 4. Abrams SA, Schanler RJ, Lee ML, Rechtman DJ. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeed Med. 2014;9(6):281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics. 1999;103(6pt 1):1150–7. [DOI] [PubMed] [Google Scholar]
- 6. Sisk PM, Lovelady CA, Gruber KJ, Dillard RG, O'Shea TM. Human milk consumption and full enteral feeding among infants who weigh < 1250 grams. Pediatrics. 2008;121(6):e1528–33. [DOI] [PubMed] [Google Scholar]
- 7. American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–41. [DOI] [PubMed] [Google Scholar]
- 8. Schanler RJ. Mother's own milk, donor human milk, and preterm formulas in the feeding of extremely premature infants. J Pediatr Gastroenterol Nutr. 2007;45(Suppl 3):S175–7. [DOI] [PubMed] [Google Scholar]
- 9. Dewey KG, Heinig MJ, Nommsen-Rivers LA. Differences in morbidity between breast-fed and formula-fed infants. J Pediatr. 1995;126(5):696–702. [DOI] [PubMed] [Google Scholar]
- 10. Le Huërou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23(1):23–36. [DOI] [PubMed] [Google Scholar]
- 11. Duijts L, Ramadhani MK, Moll HA. Breastfeeding protects against infectious diseases during infancy in industrialized countries. A systematic review. Matern Child Nutr. 2009;5(3):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ESPGHAN Committee on Nutrition. Donor human milk for preterm infants: current evidence and research directions. J Pediatr Gastroenterol Nutr. 2013;57(4):535–42. [DOI] [PubMed] [Google Scholar]
- 13. Bertino E, Giuliani F, Baricco M, Di Nicola P, Peila C, Vassia C, Chiale F, Pirra A, Cresi F, Marrtano C et al.. Benefits of donor milk in the feeding of preterm infants. Early Human Dev. 2013;89:S3–S6. [DOI] [PubMed] [Google Scholar]
- 14. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971. [DOI] [PubMed] [Google Scholar]
- 15. Gregory KE, Samuel BS, Houghteling P, Shan G, Ausubel FM, Sadreyev RI, Walker WA. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome. 2016;4(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cong X, Judge M, Xu W, Diallo A, Janton S, Brownell EA, Maas K, Graf J. Influence of feeding type on gut microbiome development in hospitalized preterm infants. Nurs Res. 2017;66(2):123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parra-Lorca A, Gormaz M, Alcantara C, Cernada M, Nunez-Ramiro A, Vento M, Collado MC. Preterm gut microbiome depending on feeding type: significance of donor human milk. Front Microbiol. 2018;9:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3(8):944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mai V, Young CM, Ukhanova M, Warng X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M et al.. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6(6):e20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, Shaikh N, Hoffmann JA, Linneman LA, Hamvas A et al.. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. 2016;387(10031):1928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pammi M, Crope J, Tarr PI, Warner BB, Morrow AL, Mai V, Gregory KE, Kroll JS, McMurtry V, Ferris MJ et al.. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. 2005;116(2):400–6. [DOI] [PubMed] [Google Scholar]
- 23. Montjaux-Régis N, Cristini C, Arnaud C, Glorieux I, Vanpee M, Casper C. Improved growth of preterm infants receiving mother's own raw milk compared with pasteurized donor milk. Acta Paediatr. 2011;100(12):1548–54. [DOI] [PubMed] [Google Scholar]
- 24. García-Lara NR, Vieco DE, De la Cruz-Bértolo J, Lora-Pablos D, Velasco NU, Pallás-Alonso CR. Effect of Holder pasteurization and frozen storage on macronutrients and energy content of breast milk. J Pediatr Gastroenterol Nutr. 2013;57(3):377–82. [DOI] [PubMed] [Google Scholar]
- 25. Marx C, Bridge R, Wolf AK, Rich W, Kim JH, Bode L. Human milk oligosaccharide composition differs between DM and mother's own milk. J Hum Lact. 2014;30(1):54–61. [DOI] [PubMed] [Google Scholar]
- 26. Evans TJ, Ryley HC, Neale LM, Dodge JA, Lewarne VM. Effect of storage and heat on antimicrobial proteins in human milk. Arch Dis Child. 1978;53(3):239–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30(1):61–7. [DOI] [PubMed] [Google Scholar]
- 28. Hanson LA, Korotkova M, Telemo E. Breast-feeding, infant formulas, and the immune system. Ann Allergy Asthma Immunol. 2003;90(6 Suppl 3):59–63. [DOI] [PubMed] [Google Scholar]
- 29. Ward RE, Nirñonuevo M, Mills DA, Lerbrilla CB, German JB. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl Environ Microbiol. 2006;72(6):4497–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Björkström MV, Hall L, Söderlund S, Håkansson EG, Håkansson S, Domellöf M. Intestinal flora in very low-birth weight infants. Acta Paediatr. 2009;98(11):1762–7. [DOI] [PubMed] [Google Scholar]
- 31. Hair A, Hawthorne K, Chetta K, Abrams S. Human milk feeding supports adequate growth in infants < 1250 grams birth weight. BMC Research Notes. 2013;6:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eunice Kennedy Shriver National Institute of Child Health and Human Development. NICHD Neonatal Research Network (NRN): extremely preterm birth outcome data[Internet]. National Institutes of Health; [cited 25 Sep, 2018]. Available from: https://www1.nichd.nih.gov/epbo-calculator/Pages/epbo_case.aspx. [Google Scholar]
- 33. Kourosh A, Luna RA, Balderas M, Nance C, Anagnostou A, Devaraj S, Davis CM. Fecal microbiome signatures are different in food-allergic children compared to siblings and healthy children. Pediatric Allergy Immunol. 2018;29(5):545–54. [DOI] [PubMed] [Google Scholar]
- 34. Hildebrand F, Tadeo R, Voigt AY, Bork P, Raes J. LotuS: an efficient and user-friendly OTU processing pipeline. Microbiome. 2014;2(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–8. [DOI] [PubMed] [Google Scholar]
- 36. Yilmaz P. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014;42:D643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI et al.. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zakrzewski M, Proietti C, Ellis J, Hasan S, Brion MJ, Berger B, Krause L. Calypso: a user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics. 2017;33(5):782–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walsh M, Kliegman R. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hair AB, Peluso AM, Hawthorne KM, Perez J, Smith DP, Khan JY, O'Donnell A, Powers RJ, Lee ML, Abrams SA. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet. Breastfeed Med. 2016;11(2):70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palmer C, Bik EM, DiGriulio DB, Relman DA, Brown PO. Development of the human intestinal microbiota. PLoS Biol. 2007;5(7):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, Stevens HJ, Bennett WE Jr, Shaikh N, Linneman LA et al.. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A. 2014;111(34):12522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou Y, Shan G, Sodergren E, Weinstock G, Walker WA, Gregory KE. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: a case-control study. PLoS One. 2015;10(3):e0118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23(3):314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, O'Toole PW, Cotter PD. The gut microbiota and its relationship to diet and obesity. Gut Microbes. 2012;3(3):186–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and methanogens in anorexic patients. PLoS One. 2009;4:e7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1999;80(3):F167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Magne F, Abély M, Boyer F, Morville P, Pochart P, Suau A. Low species diversity and high interindividual variability in faeces of preterm infants as revealed by sequences of 16S rRNA genes and PCR-temporal temperature gradient gel electrophoresis profiles. FEMS Microbiol Ecol. 2006;57(1):128–38. [DOI] [PubMed] [Google Scholar]
- 50. Drell T, Lutsar I, Stšepetova J, Parm U, Metsvaht T, Ilmoja ML, Simm J, Sepp E. The development of gut microbiota in critically ill extremely low birth weight infants assessed with 16S rRNA gene based sequencing. Gut Microbes. 2014;5(3):304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patel AL, Mutlu EA, Sun Y, Koenig L, Green S, Jakubowicz A, Mryan J, Engen P, Fogg L, Chen AL et al.. Longitudinal survey of microbiota in hospitalized preterm very-low-birth-weight infants. J Pediatr Gastroenterol Nutr. 2016;62(2):292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spanogiannopoulos P, Bess EN, Carmody RN, Turnbaugh PJ. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol. 2016;14(5):273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.