Abstract
Key points
To uncover the synaptic profile of Renshaw inhibition on motoneurons, we stimulated thick motor axons and recorded from voluntarily‐activated motor units.
Stimuli generated a direct motor response on the whole muscle and an inhibitory response in active motor units.
We have estimated the profile of Renshaw inhibition indirectly using the response of motor unit discharge rates to the stimulus.
We have put forward a method of extrapolation that may be used to determine genuine synaptic potentials as they develop on motoneurons.
These optimized techniques can be used in research and in clinics to fully appreciate Renshaw cell function in various neurological disorders.
Abstract
Although Renshaw inhibition (RI) has been extensively studied for decades, its precise role in motor control is yet to be discovered. One of the main handicaps is a lack of reliable methods for studying RI in conscious human subjects. We stimulated the lowest electrical threshold motor axons (thickest axons) in the tibial nerve and analysed the stimulus‐correlated changes in discharge of voluntarily recruited low‐threshold single motor units (SMUs) from the soleus muscle. In total, 54 distinct SMUs from 12 subjects were analysed. Stimuli that generated only the direct motor response (M‐only) on surface electromyography induced an inhibitory response in the low‐threshold SMUs. Because the properties of RI had to be estimated indirectly using the background discharge rate of SMUs, its profile varied with the discharge rate of the SMU. The duration of RI was found to be inversely proportional to the discharge rate of SMUs. Using this important finding, we have developed a method of extrapolation for estimating RI as it develops on motoneurons in the spinal cord. The frequency methods indicated that the duration of RI was between 30 and 40 ms depending on the background firing rate of the units, and the extrapolation indicated that RI on silent motoneurons was ∼55 ms. The present study establishes a novel methodology for studying RI in human subjects and hence may serve as a tool for improving our understanding of the involvement of RI in human motor control.
Keywords: Renshaw circuitry, synaptic potentials, direct motor response, human neuronal networks, single motor units
Key points
To uncover the synaptic profile of Renshaw inhibition on motoneurons, we stimulated thick motor axons and recorded from voluntarily‐activated motor units.
Stimuli generated a direct motor response on the whole muscle and an inhibitory response in active motor units.
We have estimated the profile of Renshaw inhibition indirectly using the response of motor unit discharge rates to the stimulus.
We have put forward a method of extrapolation that may be used to determine genuine synaptic potentials as they develop on motoneurons.
These optimized techniques can be used in research and in clinics to fully appreciate Renshaw cell function in various neurological disorders.
Introduction
Recurrent inhibition was one of the first neuronal circuits with feedback in the spinal cord to be identified. It is mediated by inhibitory interneurons of a special type, now referred to as Renshaw cells (RCs) (Eccles et al. 1954). The properties of RC have been extensively investigated over the last 70 years, making them a uniquely well‐defined class of spinal interneurons. RCs are excited by motoneuron (MN) axon collaterals and synapse on MNs. Certainly, one of the most intriguing parts of Renshaw inhibition (RI) is the exertion of its effect back on the same MN pool. This self‐inhibiting recurrent circuit is analogous to negative feedback control of the complex systems known in the field of technology and, perhaps as a result of this resemblance, many researchers have become interested in studying RI. However, despite years of extensive research, the role that RI plays in motor control still remains obscure.
The vast majority of knowledge about RCs and their interaction with MNs was obtained in acute animal experiments, and their limitations (to mention only the influence of anaesthetics on synaptic potentials) may be one of the factors hindering the explanation of the physiological role of RI. In animals, RI has been studied essentially by three methods: (i) morphological study of the recurrent axon collaterals after intracellular staining with HRP (Cullheim & Kellerth, 1978); (ii) in vivo intracellular recordings under anaesthesia, where antidromic RC activation was induced via stimulation of the ventral roots (Hultborn et al. 1971; McCurdy & Hamm, 1994); and (iii) in vitro in spinal cord slices (Lamotte d'Incamps et al. 2012; Bhumbra et al. 2014; Moore et al. 2015). These studies are remarkable with respect to understanding the role of RCs in isolation; however, the use of anaesthetics and a lack of descending inputs limit the use of these findings in terms of understanding the physiological role that RI circuits play under conscious conditions (Fung et al. 1987; Mazzocchio et al. 1994).
On the other hand, human experiments have recently gained attention among those researchers who previously worked with animals (Jankowska & Hammar, 2002; Heckman et al. 2008; Gorassini et al. 2009). Moreover, with respect to the possibility of defining a role for RI, human experiments are more promising because intact MNs can be studied in their physiological environment. Most human studies employed the paired Hoffmann reflex (H‐reflex) technique of Pierrot‐Deseilligny and Bussel (1975). This technique uses graded activation of Ia afferents to induce H‐reflex (Hoffmann, 1910), followed by supramaximal nerve stimulation, seeking to detect the RI response using surface electromyography (SEMG). Using this method, studies on RI in human have been performed, revealing some of the properties of RCs (Rossi et al. 1987; Raynor & Shefner, 1994; Rossi et al. 2003).
Although the double stimulation method was used commonly to investigate RI under different conditions (Rossi et al. 1987; Mazzocchio & Rossi, 2010), a number of variables such as the post‐activation depression (Crone & Nielsen, 1989) limit its reliability (Kudina & Pantseva, 1988). This technique has been criticized as being non‐specific for examining RC systems (Mazzocchio & Rossi, 1989; Rossi & Mazzocchio, 1991; Mazzocchio & Rossi, 1997). Moreover, the double stimulation procedure reveals only the net effect of the RC system on large MN pools of resting muscles, which is somewhat removed from studying the impact of RCs on normally functioning individual MNs. The problems arise from the fact that the double stimulation technique involves stimulating a mixed nerve using two high‐intensity stimuli with an interstimulus interval of 10 ms. Beside activating the spindle primary afferents, such stimulation can also generate tendon organ (Ib afferents) activation, presynaptic inhibition of homonymous spindle primary afferent synapses and post‐activation depression of MNs (Mazzocchio & Rossi, 1989). The success of this technique also depends upon the afterhyperpolarization (AHP) duration of active MNs (Mazzocchio & Rossi, 1989; Rossi & Mazzocchio, 1991). Therefore, a more reliable RI technique must be developed to support, re‐evaluate and understand the background of the diseases.
More reliable information on the RC system and its functions may be obtained in single motor unit (SMU) studies conducted in human subjects. The latency, duration and distribution of RI on MNs are the essential parameters to be investigated. To identify these parameters, isolated stimulation of the motor fibres (M‐only stimulation) was used to investigate antidromic activation of RCs via MN collaterals either in healthy subjects (Kudina & Pantseva, 1988) or in a deafferented patient (Mattei et al. 2003). This method was based on stimulating the thickest axons originating from the largest MNs at the same time as recording stimulus‐evoked responses from the low threshold voluntarily‐activated MNs.
In the present study, we investigated the properties of RI in human experiments using a similar experimental protocol. Our analysis differed from earlier work because we utilized both probability and frequency‐based methods (Türker & Powers, 1999, 2005). Furthermore, we evaluated stimulus‐correlated changes in SMU activity at different levels of background discharge rates. Because we have obtained consistent and reliable data, our approach with respect to investigating RI may become a valuable tool for investigating the normal functions of RCs and their impairment in patients who suffer from various MN disorders. Accordingly, our hypothesis is that a new and reliable method can be developed for use when studying RC function in human MNs in basic and clinical research.
Methods
The experiments were performed in the neurophysiology laboratory of Koç University (Istanbul, Turkey). The Human Ethics Committee of Koç University approved the experimental procedure, which conformed with the Declaration of Helsinki. The study participants comprised 14 subjects (eight males and six females; aged 18–35 years) with no known neuromuscular disorders. Twelve subjects took part in the SMU study, whereas two subjects were recruited for the paired H‐reflex trials. In addition, exclusion criteria included having medication for any type of neuromuscular or psychological problems and ongoing chronic back or leg pain. Before the experiments, subjects provided their written informed consent.
Set‐up
For recording and analysis, Spike2, version 7.20 was used (Cambridge Electronic Design, Cambridge, UK). Recording was performed via a CED 1902 Quad System MKIII amplifier and CED 3601 Power 1401 MKII DAC (Cambridge Electronic Design). A constant current stimulator (model DS7A; Digitimer Ltd, Welwyn Garden Cit, UK) was used for electrical stimulation of the motor axons.
SEMG recording
SEMG recordings were made bipolarly from the soleus and tibialis anterior muscles of the right leg with a 20–10,000 Hz bandpass filter and a 20,000 Hz sampling rate. For soleus muscle, subjects were asked to perform plantar flexion. After determining the lateral portion of the soleus muscle that was the posterolateral part of the leg, routine preparation procedures were used that involved gently rubbing with sandpaper, cleaning with alcohol and applying ultrasound gel. Two standard SEMG electrodes (Ag/AgCl) were placed on the muscle, with one on the muscle belly and the other 4 cm distal to the muscle belly (Tucker & Türker, 2005), and both electrodes were stabilized with tape. For tibialis anterior muscle, subjects were asked to perform dorsiflexion and the same preparation procedure and electrode placement were used.
Intramuscular EMG recording
For SMU recording, we used tip active silver fine‐wire electrodes coated with Teflon (75 μm in core diameter; Medwire, Mt Vernon, NY, USA). Two 25 cm long wires were placed into the 25 G needle and sterilized. The sterile needle was inserted close to the soleus muscle belly between two SEMG electrodes and immediately withdrawn, leaving a pair of fish‐hooked wires inside the soleus muscle. Intramuscular recordings were amplified, bandpass filtered (100–10,000 Hz), sampled at 20,000 Hz and stored for offline analysis. For both surface and intramuscular EMG recordings, a sterile lip‐clip was used as a ground electrode (Türker et al. 1988). Averaging the response of the intramuscular EMG represents the muscle activity in an accurate manner because many units fire continuously most of the time. Therefore, the average response of SMUs involves two or more units that are referred to as the multi‐motor unit (MMU). For better observation of the inhibition, the SMU channel was full‐wave rectified before averaging.
Force recording
The right foot of each subject was stabilized to a force transducer positioned around their sole. This linear strain gauge (Model LC1205‐K020; A&D Co. Ltd, Tokyo, Japan: linear to 196 N) was used to quantify the amount of twitch produced after electrical stimulation. Force signals were amplified 1000 times, filtered with DC‐100 Hz and sampled at 2000 Hz.
Experimental procedure for M‐only technique
The subjects lay comfortably on a bed in the prone position. After placement of the recording electrodes and attaching the right foot to the force transducer, an anode (10 × 12 cm) of the constant current stimulator was placed immediately proximal to the patella and a small cathode (3 × 3 mm) was placed on a slightly lateral part of the popliteal fossa (Ozyurt et al. 2018). The anode and cathode were secured in place using a tight knee pad. The tibial nerve was stimulated with a 1 ms pulse width and a 1–2 s interstimulus interval was delivered randomly. Simultaneously, tibialis anterior SEMG recording was carried out to examine any cross‐talk during tibial nerve stimulation. After the exact stimulation point was determined, subjects were asked to perform three maximum voluntary contractions for 3 s with a 1 min rest between each attempt. When subjects were resting, the M‐response threshold and maximum M‐response (M max) were detected. The M‐response threshold was determined by lowering the stimulus intensity until a minimum detectable M‐response was obtained. The M max, on the other hand, was found by increasing the stimulus intensity above the supramaximal level that produced no further increase in M‐response amplitude. Subjects were then asked to perform plantar flexion to recruit a steadily firing SMU. When the SMU was active and discharging at a predetermined rate using sound and visual feedback, M‐only stimulation was delivered to the tibial nerve. At least two different SMUs discharging at varying rates were obtained from each subject. The stimulus intensities were standardized as percentages of the stimulus strength that induced the M max. The predefined stimulus intensities were applied randomly, and the number of stimuli delivered ranged between 250 and 1000 (mean ± SD: 454 ± 144).
Analysis
SMU potentials were extracted based on their shapes using a template matching Spike2. SMU firing times were recorded and subjected to further analysis by application of output measures: peristimulus time histogram (PSTH), peristimulus frequencygram (PSF) and their cumulative sums (CUSUMs) (Ellaway, 1978). A bin size of 0.1 ms was used in both PSTH and PSF to clearly determine the latency and the duration of inhibition. Averaged SEMG recordings for both muscles and rectified‐averaged intramuscular MMU were obtained in all experiments.
Muscle twitch generated by electrical stimulation of tibial nerve was recorded by a force transducer. Similar to the SEMG recording, force recording was also spike‐triggered averaged. The peak‐to‐peak amplitude of the level between baseline and highest point of the twitch trace was measured.
Waveform template‐based Spike2 was used to recognize and convert SMUs into acceptance pulses. The interval histogram was constructed (Fig. 1 A) to validate the distribution of the interspike intervals of SMUs. After confirming the acceptance pulses using interval histograms, PSTHs and PSFs were constructed. PSF, a discharge rate‐based method, superimposes instantaneous discharge rates around the time of the stimuli (±250 ms in the present study). Because excitatory currents increase and inhibitory currents reduce the discharge rate of action potentials, PSF demonstrates the type of postsynaptic potentials (PSPs) occurring on MNs in terms of discharge rate change (Fig. 1 B). PSTH, a probability‐based stimulus triggered method, superimposes the timing of the accepted pulses pre‐ and poststimulus and detects the probability of the occurrence of units as counts at certain time points referenced to the stimulus (Fig. 1 C). Furthermore, to investigate the gross response of several SMUs detected by the intramuscular electrodes, we full‐wave rectified and averaged the SMU channel that had a number of motor unit potentials (hence, this analysis is called the MMU) (Fig. 1 D). To ensure that the stimulus delivered did not induce an H‐reflex, the averaged SEMG channel was examined online at the M‐response (∼5–20 ms) and the H‐reflex latency (∼30–40 ms) in all experiments (Fig. 1 E).
Figure 1. Analysis of RI from a sample unit.
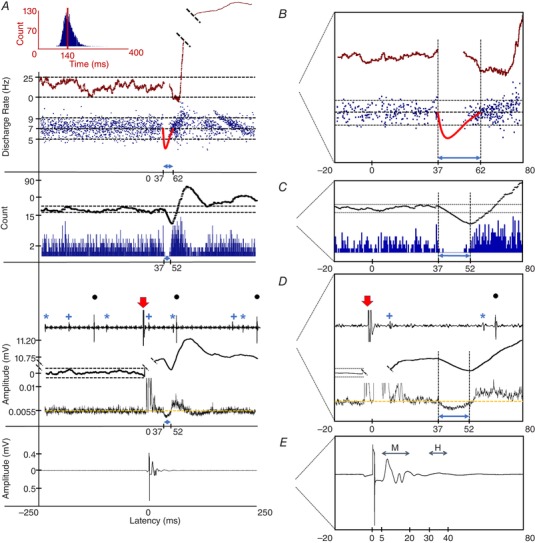
A, interval histogram showing the distribution of the interspike intervals of the unit. The red vertical line shows the mean value (∼140 ms), which gives the mean discharge rate of 7.14 Hz. B, PSF and its CUSUM providing the discharge rate of the unit around the time of the stimulus. The expanded image on the right shows a closer view of the PSF and its CUSUM. Solid red trace represents the imaginary IPSP profile drawn according to PSF CUSUM. Exact amplitude of this IPSP could not be determined because spikes cannot fire during this strong hyperpolarization stage of the IPSP. Therefore, RI amplitudes are only imaginary. C, PSTH and its CUSUM indicate the firing probability of the unit. The horizontal dashed line shows the error box. Right: a closer view of the PSTH and its CUSUM. D, individual units, MMU and MMU CUSUM show the response of all recorded units using the intramuscular EMG. The intramuscular recording was full‐wave rectified and averaged. Yellow line shows the mean background level activity. Black dots above the larger amplitude units are accepted and used in PSF and PSTH, whereas the blue asterisk and plus signs are some other SMUs that are not involved in PSF and PSTH but are involved in the MMU. Right: closer view of the SMUs, MMU and its CUSUM. Red arrows indicate stimulus artefacts. E, averaged‐SEMG recording. Right: closer view of the SEMG recording. The area represented with the indicator ‘M’ is the M‐response region and ‘H’ is the region where H‐reflex would have appeared if the stimulus excited a large number of Ia fibres. The number of stimuli in this unit was 533. [Color figure can be viewed at wileyonlinelibrary.com]
However, as a result of synaptic noise, fluctuations occur in PSTH, PSF and MMU records. Therefore, the significance of inhibitory and excitatory events (IPSP and EPSP, respectively) at poststimulus time was determined using the CUSUM error box method (Türker et al. 1997). To generate the error box for these records, the average prestimulus bin value was subtracted from each of the bin values all along the analysis period. Then, the residual values left in each bin were cumulatively summed to obtain the CUSUM trace (Ellaway 1978). Maximum deviation from the mean at the prestimulus region was taken as the maximal background CUSUM change and was used to build the symmetrical ‘error box’ (Fig. 1 A, horizontal broken lines around CUSUM). The size of this error box was then used to pinpoint significant synaptic activities. Use of error box approach to pinpoint genuine poststimulus events was validated in brain slice experiments (Türker & Powers, 2003).
In CUSUM traces, if the amplitude of the poststimulus deflection was larger than the error box, this was accepted as a significant event. The latency of this event was determined as the first turning time point of the significant deflection. The only exception to this rule is the determination of latency of inhibitions in PSF records. In this case, latency was calculated as the last consistent firing of the unit (time of termination of motor unit firing) (Türker & Powers, 2005). The duration of any significant event, on the other hand, was found by calculating the horizontal distance between the latency and the next turning point in the CUSUM record (Fig. 1 A–D).
Experimental procedure for paired H‐reflex technique
A similar SEMG preparation configuration for soleus muscle as in M‐only technique was used. The anode and cathode were fixed in place using a tight knee pad when the tibial nerve was stimulated with pulses of 1 ms width. The exact location for the cathode that induced H‐reflex only was obtained at the midpoint of the popliteal fossa instead of slightly lateral to the midpoint as in the M‐only technique (Ozyurt et al. 2018). The interval between conditioning (H1) and test stimuli (H') was chosen as 5, 10, 15, 20, 25 and 30 ms (Bussel & Pierrot‐Deseilligny, 1977). H1 was determined as 35% of M max and followed by the supramaximal stimulus that induced H'. Each conditioning test interval protocol was repeated five times and before each protocol H1 was induced to be compared with H' at that particular protocol. Each paired pulse and H1 determination step was separated by at least 10 s to diminish the effect of post‐activation depression (Hultborn et al. 1996). Each protocol was applied in a random order. Lastly, peak‐to‐peak amplitude of the H' was calculated and expressed as percentage of peak‐to‐peak amplitude of H1. After normalization of H' to H1, the results were pooled.
For the analysis of the significance of the changes in latency and duration of RI with changing stimulus intensity and background frequency, we used one‐way ANOVA with Tukey correction for multiple comparisons. Linear correlation was employed to reveal interactions between several variables and duration of RI. P < 0.05 was considered statistically significant.
Results
We recorded 54 SMUs from 12 subjects. For each SMU, the latency and duration of the inhibition were calculated, after confirming the distribution of the discharge rate of the unit using the interval histogram (Fig. 1 A).
For every SMU, the duration and the latency of the inhibition was estimated using three different analysis methods. The mean ± SD duration of RI was found to be 30.8 ± 7.2 ms, 16.1 ± 5.8 ms and 14.4 ± 3.5 ms when estimated using PSF, PSTH and MMU, respectively (Fig. 2 A). Although RI duration between PSTH and MMU was not significant (P = 0.2400), estimation of the duration using PSF was significantly longer than both PSTH (P < 0.0001) and MMU (P < 0.0001).
Figure 2. Overview of the RI properties.
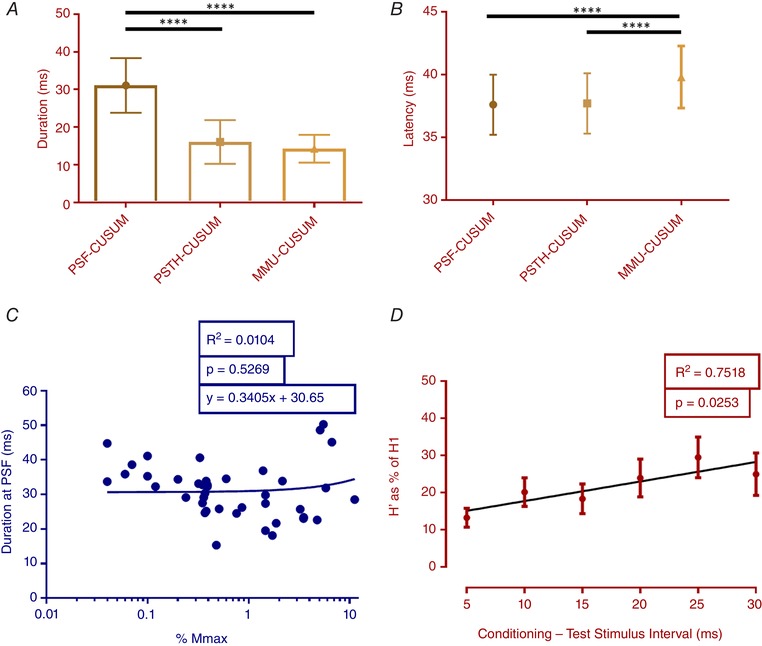
Top: mean duration (A) and latency (B) of all 54 units that were obtained using different analysis methods. Here, the longest duration was obtained at PSF‐CUSUM, whereas the longest latency was recorded in MMU‐CUSUM. Error bars show the SD. **** P < 0.0001. Bottom: effect of the stimulus strength on the duration of inhibition (C) determined using PSF‐CUSUM. Note insignificant regression in (C), which was plotted using a logarithmic abscissa. D, RI by paired H‐reflex protocol was assessed using various conditioning (H1) and test (H') stimulus intervals. Error bars represent the SE. [Color figure can be viewed at wileyonlinelibrary.com]
The RI latency was 37.7 ± 2.3 ms, 37.7 ± 2.4 ms and 39.9 ± 2.3 ms when assessed from PSF, PSTH and MMU, respectively. The inhibition latency in the averaged MMU was significantly longer than that in PSF (P < 0.0001) and PSTH (P < 0.0001), whereas there was no significant difference in latencies recorded using PSF and PSTH (P = 0.9683) (Fig. 2 B). The duration of RI calculated from PSF‐CUSUM showed no correlation with the increasing stimulus intensity (P = 0.3195) (Fig. 2 C).
The paired H‐reflex technique, comprsing the most frequently used protocol for investigating RI, was also employed to evaluate RI in two additional volunteer subjects. An average of 10 experiments from two subjects is presented in Figure 2 D. Use of the paired H‐reflex technique investigating intervals over 30 ms was not practical because the test stimulus should be delivered before the conditioning stimulus arrives at the muscle. Therefore, it is not possible to assess the duration of RI using this method. As the conditioning test interval was increased, a significant rise in H' amplitude was noted (P < 0.05) indicating that the duration of RI was at least 30 ms long. The amplitude of H' was 13.2 ± 2.57% of H1 when the conditioning test interval was 5 ms. The H' increased in amplitude to 29.4 ± 5.48% and to 24.9 ± 5.70% of H1 as the conditioning test interval changed to 25 and 30 ms, respectively.
The latency of RI is longer than H‐reflex
The latency of RI was also compared with the H‐reflex latency using PSTH‐CUSUM. For this reason, the H‐reflex was evoked in seven subjects and the difference between the H‐reflex latency and RI latency was investigated using a paired t test within subjects (Fig. 3). The average H‐reflex latency of SMUs was found to be 35.7 ± 1.2 ms, which is significantly shorter than the RI latency of these subjects, which was 37.4 ± 1.8 ms (P = 0.0074).
Figure 3. The latency difference between the RI and H‐reflex together with their proposed circuitries.
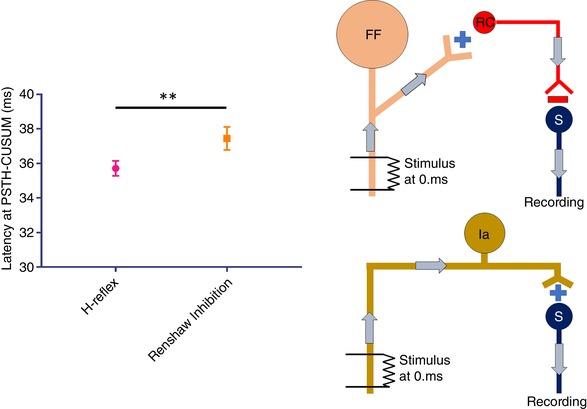
Significantly longer latency of RI compared to H‐reflex is shown on the left, whereas the wiring diagram of RI and H‐reflex is shown on the right. Top right: schematic of the larger MN (FF) stimulation and consecutive RI on smaller MN (S) by RC. Bottom right: primary afferent (Ia) stimulation to evoke H‐reflex. Error bars represent the SD. ** P < 0.01. [Color figure can be viewed at wileyonlinelibrary.com]
The RI duration is shorter on high threshold SMUs
In total, 27 different SMUs were investigated for which the force recruitment threshold could be identified (Fig. 4). A significant negative correlation was found between the duration of RI and the force recruitment thresholds of units (P < 0.001) (Fig. 4 A). However, there was no correlation between the duration and background discharge rate of the units (Fig. 4 B), revealing that the observed change in duration was related to different size of motor units and was not a result of differences in background discharge rates (P = 0.7184). This finding provides evidence indicating that a recurrent IPSP lasts longer in smaller sized MNs compared to larger ones (Fig. 4 C).
Figure 4. The effect of various motor unit sizes on RI duration.
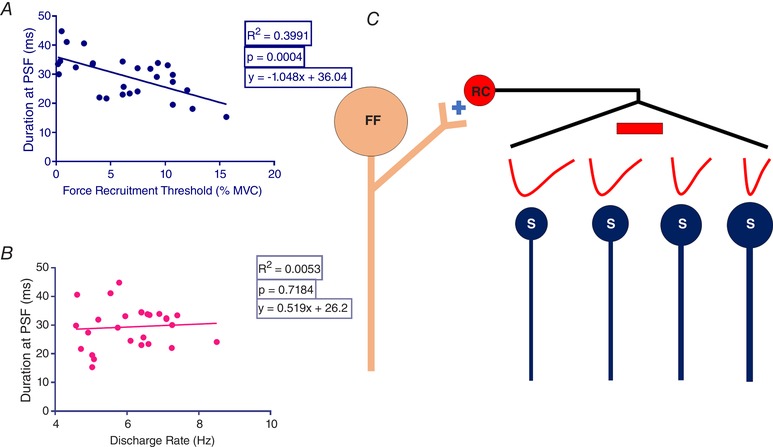
A, correlation between the RI duration and different force recruitment threshold levels of SMUs. B, correlation between the discharge rate and duration of the same SMUs. C, schematized wiring diagram of the RI, evoked by stimulation of the largest MNs (FF), on smaller (S) MNs that are graded in size. Solid red traces represent imaginary IPSP profiles. The amplitude of these traces does not represent the amplitude of the Renshaw IPSP. [Color figure can be viewed at wileyonlinelibrary.com]
Stimulus‐evoked twitch produces long latency synaptic potentials
Because M‐responses, especially larger ones, induce detectable twitches in soleus muscle, which, in turn, can activate tendon organs in homonymous muscle, we examined the relationship between twitch profile and secondary excitation using PSF‐CUSUM (Fig. 5). Electrical stimulation of the thickest motor axons produces the M‐response as the earliest activity and it causes muscle to contract after an electromechanical delay of ∼15 ms. Concomitantly with the muscle contraction, antidromic activity of the thickest motor axons excite RCs via axon collaterals and their inhibitory volley arrives at smaller MNs to reduce their firing rate momentarily.
Figure 5. Hypothesized reflex mechanism behind strong M‐response stimulation of motor axons.
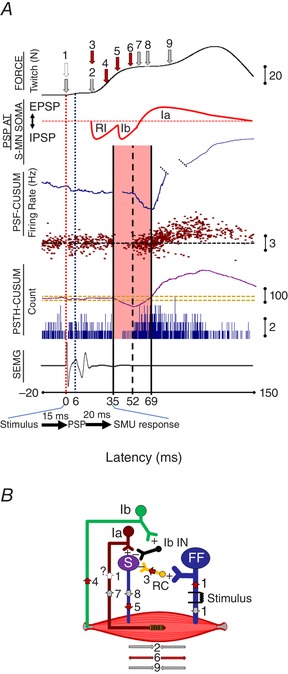
A, a sample recording from stimulation of thick motor axons (FF). From top to bottom: force record, schematic of synaptic potential of a small MN (S) at its soma; PSF and its CUSUM; PSTH and its CUSUM; and SEMG recording. The stimulation (arrow 1) produced biphasic muscle contraction with a relaxation period inbetween. It is also possible that a few Ia fibres were activated during the stimulation, even though we did not observe an H‐reflex. The first twitch (arrow 2) is a result of M‐response induced muscle contraction after an electromechanical delay. Antidromic spike activity arrives at RCs in the spinal cord around the same time period (arrow 3). Because the M‐response induces muscle contraction, tendon organs are activated (arrow 4) and S‐MNs are inhibited via Ib interneurons (Ib‐IN) (arrow 5). Combined recurrent and Ib inhibition cause the muscle to relax (arrow 6). This muscle relaxation may initiate muscle spindle activation (arrow 7), which increases the discharge rate of S‐MN (arrow 8) causing muscle contraction again (arrow 9). Compound PSP at S‐MN soma panel mimics an imaginary recording from the recruited MN. Because we record from the muscle (SEMG, PSTH and PSF) and this hypothetical recording is occurring at the ventral horn of the spinal cord, there is a time delay as a result of conduction along the motor axon. Therefore, any voltage change in S‐MN arrives at the muscle ∼20 ms later. Solid red traces represent imaginary PSP profiles. Although the durations of the IPSPs and the EPSP are determined from the PSF CUSUM findings, the amplitudes of the IPSP traces are only our suggestions. B, hypothesized mechanism underlying these consecutive responses of the muscle and receptors are indicated by corresponding arrows. [Color figure can be viewed at wileyonlinelibrary.com]
A multicomponent inhibition and excitation series was proposed (Fig. 5 A). The hypothesized mechanism behind this complex system is shown schematically in Fig. 5 B. We observed a large twitch in isometric force recordings, especially when the M‐response was also large. The M‐response induces muscle contraction, hence activating tendon organs to send their inhibitory volleys to MNs via Ib afferents. Because RI lasts ∼40 ms, the M‐response induced Ib inhibition may arrive at MNs during the tail of the RI IPSP. These summed inhibitory volleys can relax the contracting muscle and, by doing so, activate stretch sensitive muscle spindles. Spindle activation, after a further electromechanical delay, initiates homonymous muscle contraction via an increased discharge rate of MNs.
The duration of RI changes with the background discharge rate
Background discharge rates of SMUs have been noted to affect the duration of RI (Fig. 6). Because muscle twitch can affect the duration of RI (Fig. 5), we only included the recordings that were free of detectable muscle twitches. Stimulation of 29 units out of 54 did not produce any detectable twitch. For these 29 units, linear regression between inhibition duration and the discharge rate indicated a significant inverse relationship (P < 0.001). A duration of 53.55 ms was calculated by extrapolating the background discharge rate to a silent MN (Fig. 6).
Figure 6. The effect of background firing rate on the duration of RI in single unit recordings.
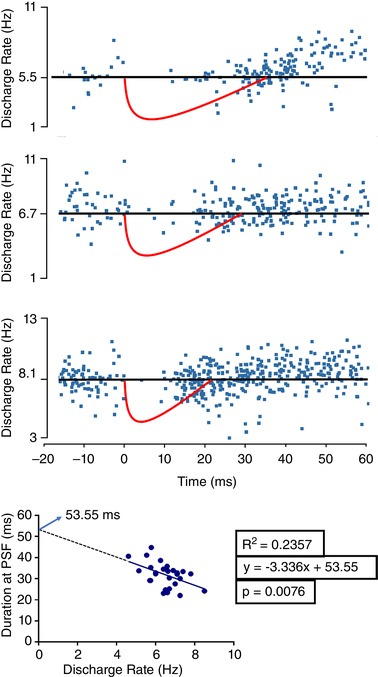
Upper: samples with different background rates. Red shapes are hypothetical IPSP with duration estimated from PSF‐CUSUM. Abscissa represents time with normalized RI latency to 0 ms. Lower: method for extrapolation. [Color figure can be viewed at wileyonlinelibrary.com]
Inhibition was recorded only if the M‐response was present
To ensure that we have stimulated only the motor axons but not afferent fibres, we investigated RI in three different stimulus intensities according to motor threshold (MT): one just below MT (0.9 × MT, 0% M max) and two suprathreshold intensities: one slightly higher than the MT (2% of M max) and one even stronger (10% of M max). The results were evaluated using PSTH‐CUSUM and M‐responses in SEMG recordings (Fig. 7). We found that the stimulus intensity just below MT did not produce an inhibitory response on the SMUs (Fig. 7 A). However, as soon as the stimulus intensity reached, the MT (2% of M max), inhibition appeared (Fig. 7 B) and inhibition increased further at 10% M max (Fig. 7 C). Thus, we confirmed that the observed inhibition was probably a result of antidromic motor axon stimulation.
Figure 7. The effect of the M‐response on the proposed inhibition.
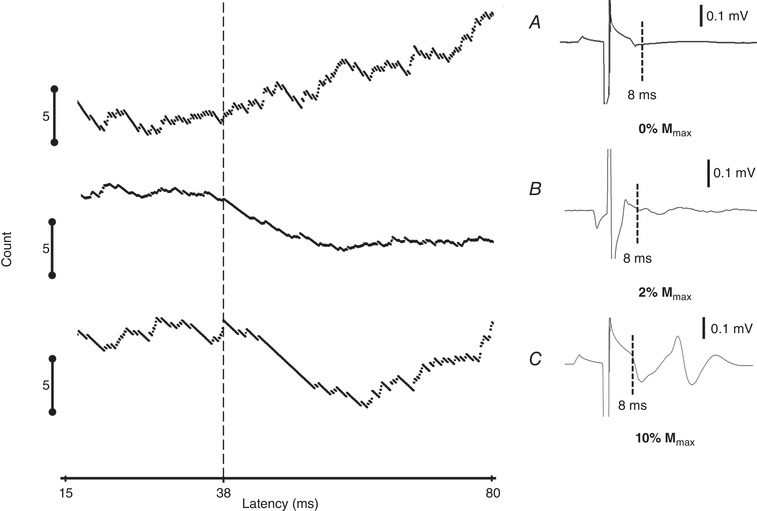
Left: PSTH‐CUSUMs at three different stimulus intensities. Right: averaged SEMG traces. The stimulus intensity just below the M‐response threshold did not result in an M‐response and hence did not produce an inhibition (A), whereas the other two stimulus intensities, 2% (B) and 10% M max (C), resulted in increased inhibition with a latency of ∼38 ms.
The observed inhibition was neither cutaneous, nor reciprocal
To determine whether the observed inhibition could result from stimulation of cutaneous afferents, we stimulated the same sensory dermatome but away from our usual stimulation point using similar stimulus intensities in two subjects. For this, we measured the distance between our usual stimulating point and the upper pelvic bone. Without changing the anode location and stimulus intensity, we moved the cathode proximally to one‐third of that distance in the same dermatome. The data were then analysed using PSTH‐CUSUM (to detect inhibition) and SEMG (to determine the M‐response and H‐reflex). We found that the new stimulation arrangement induced no detectable response (Fig. 8 A).
Figure 8. Possible contaminants for RI.
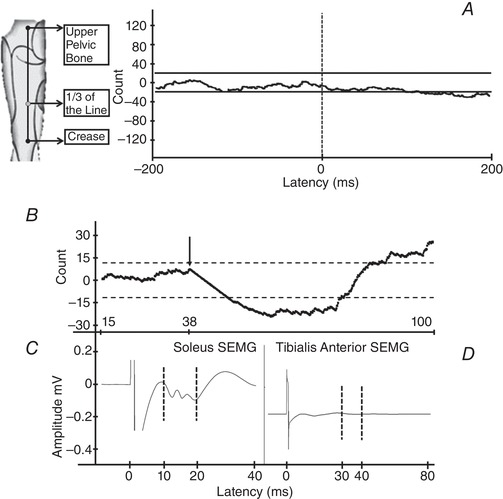
A, the effect of cutaneous stimulation within the same dermatome and the same stimulus intensity (stimulation point indicated on the left). The response of a soleus SMU to the cutaneous stimulation indicates an absence of any cutaneous effect on this SMU. B, PSTH‐CUSUM of a typical RI in a soleus SMU. The black arrow shows the onset of inhibition. C, response of the soleus SEMG to stimuli. The dashed lines show the range of the M‐response. D, no sign of a reflex between dashed lines indicates that there was no stimulus‐induced activity in tibialis anterior muscle.
To test the possibility of activating Ia and Ib afferents, we investigated their possible effect on the antagonist muscle, tibialis anterior. For this, during our usual stimulation of the tibial nerve at the popliteal fossa, we also conducted SEMG recordings from tibialis anterior muscle. We found that there was no noticeable response in averaged tibialis anterior SEMG recording during the period of inhibition in the soleus muscle (Fig. 8 B–D). The lack of stimulus‐induced activity in tibialis anterior muscle may indicate that these afferent fibres were not activated to a level that induced noticeable effects because we found no reciprocal excitation via Ib afferents and no reciprocal inhibition via Ia afferents.
Discussion
Although most of the knowledge concerning the duration and distribution of RCs comes from animal experiments, a reliable tool that can investigate RCs in humans is a necessity for use in research and in clinics. Moreover, with respect to the possibility of a defining role for RI, human experiments are more promising because, here, intact MNs are studied in their physiological environment. To understand the functioning of the RCs in humans, we optimized and improved the precision of a previously used technique that involves electrical stimulation of the largest motor axons in the tibial nerve and recording from the voluntarily‐activated low threshold SMU from the soleus muscle (Kudina & Pantseva, 1988) to determine stimulus‐locked inhibition. Here, we discuss five original findings;
Characteristics of RI using three different methods
Effect of discharge rate on RI duration
Presumed RI duration on silent MN
Distribution of RI to different sized MNs
Reliability of M‐only stimulation
Characteristics of RI using three different methods
We investigated the influence of motor unit discharge rate and stimulus intensity on the latency and duration of RI as estimated using three different methods. We have shown that the stimulus amplitude does not influence the duration of the RI. However, the duration of the RI depends on the background discharge rate.
Latency
The latency of RI did not appear to be connected with any of the controlled variables. The best method for estimating inhibition latency was PSTH‐CUSUM because it deflected down immediately following a reduction in spike counts. The RI latency estimated from PSTH‐CUSUM did not differ from that of the estimation from PSF, although the latency determined from MMU‐CUSUM was longer. This observation can be explained by the fact that PSTH is made up of spikes, each of which only covers a narrow bin width (0.1 ms), whereas the MMU is the rectified averaged raw data from the intramuscular electrode. Effectively, the MMU is a low‐pass filtered version of several motor unit action potentials and hence timing information (similar to the SEMG records) in MMU data are not reliable for latency measurements.
The latency of the RI has been studied to understand its multisynaptic component, which is mostly compared to the H‐reflex. Stimulation of the thickest primary afferents (Ia afferents) produces the H‐reflex monosynaptically. The latency of this reflex upon stimulation from the popliteal fossa varies between 30–35 ms for the soleus muscle depending on the height of the subject (Hoffmann, 1910; Pierrot‐Deseilligny & Mazevet, 2000). We found the H‐reflex latency to be slightly above this value (∼35 ms), which could be a result of our recording region, comprising the distal part of the soleus muscle. The RI latency in our experiments, on the other hand, has been found to be ∼37 ms (2 ms longer than the latency of the H‐reflex), which is in accordance with another single unit study on the soleus muscle (Kudina & Pantseva, 1988). The delay of RI compared to that of H‐reflex is a result of the relatively lower conduction velocity in large motor axons compared to primary afferents. This time delay may also be the result of an additional synapse/s in the RC circuitry and a conduction delay in the RC, which has been found to be between 1.1 and 1.8 ms in animals (Callister & Graham, 2010).
Although it is possible to pinpoint the latency of RI in our experiments, the paired H‐reflex technique could not identify the latency of RI because it relied upon interstimulus intervals that could not be shorter than 5 ms as a result of conduction dispersion in axons. With this limitation, RI latency was reported to be 6 ms longer than the H‐reflex latency by Bussel and Pierrot‐Deseilligny (1977), which falls within the range of spike silence (a gap in PSTH from 2 ms to 17 ms relative to the H‐reflex latency) in our M‐only stimulation method.
Duration
The best method for estimating the duration of the inhibition, on the other hand, was the PSF‐CUSUM because it indicated the end of reduction in discharge rate of the unit very clearly (Türker & Powers, 2005). As a result of the stimulus‐induced synchronization and poor estimation of the tail of the PSPs in PSTH‐CUSUM, the duration measured in PSTH‐CUSUM would be effectively contaminated (Türker & Cheng, 1994; Türker & Powers, 2005).
In our experiments, we found a significantly longer RI duration than that estimated using classical methods (Bussel & Pierrot‐Deseilligny, 1977; Hultborn & Pierrot‐Deseilligny, 1979; Rossi et al. 2003). This is because the conditioning test interval used in the classical method could not be increased over 30 ms in that the test stimulus should be delivered before the conditioning stimulus arrives at the muscle. Therefore, it is not possible to assess the duration of RI using the classical paired H‐reflex method.
Also, this duration was dependent on the background discharge rate of the SMU. The longer lasting duration may originate from differences in the conduction velocity of stimulated motor axons, which might generate dissipated IPSPs on MNs. Also, variability in the RI duration on different sized MNs (Mazzocchio & Rossi, 1997) could be a result of the variable number of inputs arising from RCs or intrinsic properties of larger MNs, such as a shorter AHP duration and a higher firing rate capability (Matthews, 1996). However, the duration determined using the PSF method was still shorter compared to the range of 45–50 ms determined using direct recordings from RCs upon stimulation of ventral roots in animal studies (Renshaw, 1941; Eccles et al. 1954; Ellaway & Murphy, 1981; Callister & Graham, 2010). Nevertheless, the discharge of RCs may occasionally continue for up to 100 ms (Wilson, 1959). This prolonged activity may be a result of the association of slow component receptors, especially NMDA receptors on RCs, in addition to faster nicotinic receptor activity. Slow but longer lasting activation of RCs by NMDA receptors can increase the effective IPSP by increasing the range of temporal summation (Alvarez et al. 2013; Lamotte d'Incamps & Bhumbra, 2017). However, especially in anaesthetized animals, the descending inputs are less effective, which may alter the RI duration as a result of reduced cortical influence (Mazzocchio & Rossi, 2010). This may explain why, in the present study, such long RI durations were not observed.
In addition, because of tendon organ activation that could prolong inhibition duration (Fig. 5), we did not take twitch‐produced intensities into account when evaluating RI duration to eliminate the effect of Ib inhibition.
Discharge rate effect and presumed RI duration
In addition to the receptors (depending on the GABA and/or glycine receptors) underlying the IPSP duration, the state of the MNs in terms of excitability should also be considered for the duration of RI. Larger excitatory drives on MNs make them easily excitable and, more importantly, increase their discharge rate. The MNs that fire at high discharge rates are highly excitable and hence probably respond less to IPSPs. The present study and that of Kudina and Pantseva (1988) showed that, even in the same type of MNs, a faster discharge resulted in a shorter duration of RI. This shortened duration with increasing in discharge rate is even valid for gamma‐MNs. Less pronounced RI on gamma‐MNs as a result of their higher discharge rate up to 80 Hz has been reported (Ellaway & Murphy, 1981). Similarly, it has been shown that Renshaw IPSP durations are shorter in firing MNs than those recorded at rest (Obeidat et al. 2014). This is exactly what was observed in the present study: the higher the discharge rate of the SMU, the shorter the RI duration. Furthermore, because the RI duration is estimated indirectly and is affected by the discharge rate of the unit, we suggest that the correct duration of RI in a MN at rest can be estimated by linear extrapolation from the discharge rate data. A similar linear relationship was proposed by Ellaway and Murphy (1981). We performed this extrapolation in 29 units and estimated the RI duration at ∼55 ms. This is an important step towards understanding synaptic potential profiles in human MNs because they have to be estimated indirectly using SMUs or the entire motor pool in SEMG.
Because the threshold of the largest Ia afferents and thickest motor axons are similar, our M‐only stimulation probably initiates action potentials in a few Ia fibres, although they may not generate a recordable H‐reflex (Fig. 5, dashed arrow 1). Stimulation of Ia afferent fibres can escalate MN excitability and slightly increase the discharge rate of the unit. This may lead to an underestimation of RI. Although we found no correlation between inhibition duration and amount of motor axons stimulated, most of the RI was evoked using low stimulus intensities generating an M‐response of ∼7.6% M max. Therefore, we aimed to minimize the number of stimulated Ia fibres by using low intensity stimulation.
Distribution of RI to different sized MNs
Motor units physiologically differ in size (Burke et al. 1973). Because we used soleus muscle motor units that were recruited at low contraction levels, our units were mostly low‐threshold ones (Gollnick et al. 1974). We were also careful to use low contraction levels to keep the discharge rate regular but low (de Luca et al. 1982; Piotrkiewicz & Türker, 2017). Because the discharge rate was one of the variables that had a significant impact on the indirectly estimated duration of recurrent IPSPs, we wanted to ensure that units fired at their optimum rates. The SMUs that require higher contraction levels to recruit were found to be inhibited by RCs as well, although with a much shorter duration even though these units fired at discharge rates similar to the lower threshold units (Fig. 4). The proposed findings support the results of the studies by Friedman et al. (1981) and Hultborn et al. (1988) who suggested the Renshaw IPSP is larger on smaller MNs. Therefore, we suggest that the lower duration of RI on higher threshold units might be explained by the relatively weaker RC inputs on larger units.
Reliability of the methodology
Most of the knowledge on human RC connections and physiology, as well as their involvement in diseases, was obtained from experiments using the paired H‐reflex technique. Double stimulation of the primary afferent fibres not only activates the RCs through MN collaterals, but also allows the lagging stimulus to pass freely on MNs without any collision of ortho‐ and antidromic activation (Pierrot‐Deseilligny & Bussel, 1975). Although this technique provided valuable information for decades, there are several drawbacks that might directly affect the presumed RI. For example, such a high‐intensity stimulus used in the paired H‐reflex technique probably evokes autogenic Ib inhibition. Because the latencies of both inhibitions are very close to each other, they would have merged together (Delwaide & Oliver, 1988). In addition, primary afferent endings in such frequent stimulation are susceptible to presynaptic inhibition (Hultborn et al. 1996). Those variables and outcomes might lead to misinterpretation of RI when the paired H‐reflex is employed.
M‐only stimulation as applied in the present study, on the other hand, was shown to be highly reliable for investigating the RI with a minimum contamination of other reflex pathways. We showed that the recorded inhibitory effect was neither reciprocal, nor cutaneous inhibition (Fig. 8). In the autogenic inhibition aspect, the voluntary activation of the soleus muscle minimizes the possibility of the Ib inhibition because it is mostly reduced when voluntary contraction takes place (Lafleur et al. 1992). In addition, recorded inhibition in the present study was so much longer than that obtained for Ib inhibition alone (Delwaide & Oliver, 1988). Moreover, to determine whether we have stimulated the Ib fibres that may contaminate RI, we checked if any longer latency responses were obtained in the antagonist muscle. Tendon organ activation or direct Ib afferent stimulation results in excitatory influence on antagonist muscle (i.e. tibialis anterior in the present study) via oligosynaptic pathways (Pierrot‐Deseilligny et al. 1981; Katz et al. 1991). Because we electrically stimulate the mixed nerve and some of the stimulated fibres might be Ib afferents, then we could expect synchronous reciprocal excitation in tibialis anterior muscle. However, we did not observe any excitation several milliseconds later than H‐reflex latency in tibialis anterior muscle (Fig. 8 D). Therefore, it can be proposed that Ib fibres did not effectively contaminate our results.
The extrapolation technique allowed us to deduce the synaptic potential as it develops in a silent MN. This technique provided information about how an IPSP influences a silent MN (Fig. 6). Hence, it may also shed light on why a longer duration of RI was obtained in anaesthetized animals where MNs were mostly silent.
In conclusion, we report an extensive investigation of RI in firing MNs in conscious human volunteers. The duration of RI has been shown to be inversely proportional to the background firing rate of the MNs. The stimulation of afferent fibres with higher intensity produced stronger inhibition. RI was distributed differently onto different sized MNs. The optimized technique, parameters and reported findings can be applied in clinics, particularly for further clarifying the role of RI in MN diseases such as ALS.
Study limitations
The proposed study is only applicable to RI input from larger to smaller MNs. Because the lowest suprathreshold intensity electrical stimulus induces action potentials in the thickest fibres in a mixed nerve, it is possible to stimulate low‐threshold motor axons along with low‐threshold sensory axons (e.g. type Ia and Ib fibres). This means that the results may represent the net effects of all excitatory and inhibitory PSP activity rather than pure RI, especially when large stimulus intensities are used. To minimize these unwanted effects, we used low stimulus intensities and also tested for possible effects of significant contamination by examining their effects in agonist and antagonist muscles (Figs 7 and 8). These cautionary experiments showed that other fibre activity did not effectively contaminate our results. Therefore, we can propose that the dominant mechanism in the net inhibitory effect originates from the Renshaw circuitry.
In addition, although this methodology would be very useful for investigating RI in healthy individuals and in patients with MN loss in an early stage, the application of this technique is limited in cases with severe MN loss where higher stimulus intensities are needed to activate the RC system. This larger intensity stimulation not only induce twitch‐mediated Ib afferent activation, but also activation of Ia spindle primary afferents. Both activations can lead to misinterpretation of RI. Similarly, when studying RI in patients with hyper‐reflexia (e.g. spastic patients), obtaining an M‐only response without an H‐reflex may not be possible, which limits the use of the current method for the investigation of RI.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
M.G.Ö., M.P., H.W.W. and K.S.T. conceived the study. M.G.Ö., B.T. and K.S.T. performed the experiments. M.G.Ö., B.T. and K.S.T. analyzed the raw data. M.G.Ö. wrote the first draft of the manuscript and all authors contributed to final manuscript.
Funding
No funding was received for the present study.
Acknowledgements
We thank the subjects who participated in this project. We thank Koç University School of Medicine and Polish Academy of Sciences for supporting this study.
Biography
Mustafa Görkem Özyurt received his Bachelor's Degree in Bioengineering Department from Ege University. He worked in cellular neuroscience using three‐dimensional tissue culture models to mimic blood–brain barrier in vitro. During his Bachelor's Degree, he visited Magdeburg to investigate the intracellular signalling pathways involved in neuronal differentiation. Subsequently, he moved to Koç University to receive his doctoral training in Renshaw interneurons with electrical stimulation of the motor axons in human and optogenetic stimulation in the mouse. As a PhD Candidate, he is currently studying neuronal circuits and reflex pathways using electrical stimulation of the peripheral nerves, transcranial magnetic stimulation of the motor cortex and the response of the single motor units.

Edited by: Janet Taylor & Richard Carson
Linked articles: This article is highlighted in a Perspectives article by Alvarez. To read this article, visit https://doi.org/10.1113/JP277715.
References
- Alvarez FJ, Benito‐Gonzalez A & Siembab VC. (2013). Principles of interneuron development learned from Renshaw cells and the motoneuron recurrent inhibitory circuit. Ann NY Acad Sci 1279, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumbra GS, Bannatyne BA, Watanabe M, Todd AJ, Maxwell DJ & Beato M. (2014). The recurrent case for the Renshaw cell. J Neurosci 34, 12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P & Zajac FE. (1973). Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234, 723–748.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussel B & Pierrot‐Deseilligny E. (1977). Inhibition of human motoneurones, probably of Renshaw origin, elicited by an orthodromic motor discharge. J Physiol 269, 319–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister RJ & Graham BA. (2010). Early history of glycine receptor biology in mammalian spinal cord circuits. Front Mol Neurosci 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S & Kellerth JO. (1978). A morphological study of the axons and recurrent axon collaterals of cat alpha‐motoneurones supplying different hind‐limb muscles. J Physiol 281, 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C & Nielsen J. (1989). Methodological implications of the post activation depression of the soleus H‐reflex in man. Exp Brain Res 78, 28–32. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP & Xenakis AP. (1982). Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 29, 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ & Oliver E. (1988). Short‐latency autogenic inhibition (IB inhibition) in human spasticity. J Neurol Neurosur Ps 51, 1546–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Fatt P & Koketsu K. (1954). Cholinergic and inhibitory synapses in a pathway from motor‐axon collaterals to motoneurones. J Physiol 126, 524–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH. (1978). Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45, 302–304. [DOI] [PubMed] [Google Scholar]
- Ellaway PH & Murphy PR. (1981). A comparison of the recurrent inhibition of alpha‐ and gamma‐motoneurones in the cat. J Physiol 315, 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WA, Sypert GW, Munson JB & Fleshman JW. (1981). Recurrent inhibition in type‐identified motoneurons. J Neurophysiol 46, 1349–1359. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Pompeiano O & Barnes CD. (1987). Suppression of the recurrent inhibitory pathway in lumbar cord segments during locus coeruleus stimulation in cats. Brain Res 402, 351–354. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Sjödin B, Karlsson J, Jansson E & Saltin B. (1974). Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflügers Archiv 348, 247–255. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Norton JA, Nevett‐Duchcherer J, Roy FD & Yang JF. (2009). Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J Neurophysiol 101, 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C & Schuster J. (2008). Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. (1910). Beitrag zur Kenntnis der menschlichen Reflexe mit besonderer Berucksichtigung der elektrischen Erscheinungen. Arch Anat Physiol 1, 223–246. [Google Scholar]
- Hultborn H, Jankowska E & Lindström S. (1971). Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol 215, 591–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M & Wiese H. (1996). On the mechanism of the post‐activation depression of the H‐reflex in human subjects. Exp Brain Res 108, 450–462. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Katz R & Mackel R. (1988). Distribution of recurrent inhibition within a motor nucleus. II. Amount of recurrent inhibition in motoneurones to fast and slow units. Acta Physiol Scand 134, 363–374. [DOI] [PubMed] [Google Scholar]
- Hultborn H & Pierrot‐Deseilligny E. (1979). Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H‐reflex technique. J Physiol 297, 229–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E & Hammar I. (2002). Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Brain Res Rev 40, 19–28. [DOI] [PubMed] [Google Scholar]
- Katz R, Penicaud A & Rossi A. (1991). Reciprocal Ia inhibition between elbow flexors and extensors in the human. J Physiol 437, 269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudina LP & Pantseva RE. (1988). Recurrent inhibition of firing motoneurones in man. Electroencephalogr Clin Neurophysiol 69, 179–185. [DOI] [PubMed] [Google Scholar]
- Lafleur J, Zytnicki D, Horcholle‐Bossavit G & Jami L. (1992). Depolarization of Ib afferent axons in the cat spinal cord during homonymous muscle contraction. J Physiol 445, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte d'Incamps B & Bhumbra GS. (2017). Segregation of glutamatergic and cholinergic transmission at the mixed motoneuron Renshaw cell synapse. Sci Rep 7, 4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte d'Incamps B, Krejci E & Ascher P. (2012). Mechanisms shaping the slow nicotinic synaptic current at the motoneuron‐renshaw cell synapse. J Neurosci 32, 8413–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei B, Schmied A & Vedel JP. (2003). Recurrent inhibition of wrist extensor motoneurones: a single unit study on a deafferented patient. J Physiol 549, 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB (1996). Relationship of firing intervals of human motor units to the trajectory of post‐spike after‐hyperpolarization and synaptic noise. J Physiol 492, 597–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocchio R & Rossi A. (1989). Recurrent inhibition in human spinal spasticity. Ital J Neurol Sci 10, 337–347. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R & Rossi A. (1997). Involvement of spinal recurrent inhibition in spasticity. Further insight into the regulation of Renshaw cell activity. Brain 120, 991–1003. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R & Rossi A. (2010). Role of Renshaw cells in amyotrophic lateral sclerosis. Muscle Nerve 41, 441–443. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R, Rossi A & Rothwell JC. (1994). Depression of Renshaw recurrent inhibition by activation of corticospinal fibres in human upper and lower limb. J Physiol 481, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy ML & Hamm TM. (1994). Topography of recurrent inhibitory postsynaptic potentials between individual motoneurons in the cat. J Neurophysiol 72, 214–226. [DOI] [PubMed] [Google Scholar]
- Moore NJ, Bhumbra GS & Foster JD. (2015). Synaptic connectivity between renshaw cells and motoneurons in the recurrent inhibitory circuit of the spinal cord. J Neurosci 35, 13673–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeidat AZ, Nardelli P, Powers RK & Cope TC. (2014). Modulation of motoneuron firing by recurrent inhibition in the adult rat in vivo. J Neurophysiol 112, 2302–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyurt MG, Shabsog M, Dursun M & Türker KS. (2018). Optimal location for eliciting the tibial H‐reflex and motor response. Muscle Nerve. 10.1002/mus.26308. [DOI] [PubMed] [Google Scholar]
- Panizza M, Nilsson J, Roth BJ, Rothwell J & Hallett M. (1994). The time constants of motor and sensory peripheral nerve fibers measured with the method of latent addition. Electroencephalogr Clin Neurophysiol 93, 147–154. [DOI] [PubMed] [Google Scholar]
- Pierrot‐Deseilligny E & Bussel B. (1975). Evidence for recurrent inhibition by motoneurons in human subjects. Brain Res 88, 105–108. [DOI] [PubMed] [Google Scholar]
- Pierrot‐Deseilligny E & Mazevet D. (2000). The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin 30, 67–80. [DOI] [PubMed] [Google Scholar]
- Pierrot‐Deseilligny E, Morin C, Bergego C & Tankov N. (1981). Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res 42, 337–350. [DOI] [PubMed] [Google Scholar]
- Piotrkiewicz M & Türker KS. (2017). Onion skin or common drive? Front Cell Neurosci 11, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor EM & Shefner JM. (1994). Recurrent inhibition is decreased in patients with amyotrophic lateral sclerosis. Neurology 44, 2148–2153. [DOI] [PubMed] [Google Scholar]
- Renshaw B. (1941). Influence of discharge of motoneurons upon excitation of neighboring motoneurons. J Neurophysiol 4, 167–183. [Google Scholar]
- Rossi A & Mazzocchio R. (1991). Presence of homonymous recurrent inhibition in motoneurones supplying different lower limb muscles in humans. Exp Brain Res 84, 367–373. [DOI] [PubMed] [Google Scholar]
- Rossi A, Mazzocchio R & Decchi B. (2003). Effect of chemically activated fine muscle afferents on spinal recurrent inhibition in humans. Clin Neurophysiol 114, 279–287. [DOI] [PubMed] [Google Scholar]
- Rossi A, Mazzocchio R & Scarpini C. (1987). Evidence for Renshaw cell‐motoneuron decoupling during tonic vestibular stimulation in man. Exp Neurol 98, 1–12. [DOI] [PubMed] [Google Scholar]
- Tucker KJ & Türker KS. (2005). A new method to estimate signal cancellation in the human maximal M‐wave. J Neurosci Methods 149, 31–41. [DOI] [PubMed] [Google Scholar]
- Türker KS & Cheng HB. (1994). Motor‐unit firing frequency can be used for the estimation of synaptic potentials in human motoneurones. J Neurosci Methods 53, 225–234. [DOI] [PubMed] [Google Scholar]
- Türker KS, Miles TS & Le HT. (1988). The lip‐clip: a simple, low‐impedance ground electrode for use in human electrophysiology. Brain Res Bull 21, 139–141. [DOI] [PubMed] [Google Scholar]
- Türker KS & Powers RK. (1999). Effects of large excitatory and inhibitory inputs on motoneuron discharge rate and probability. J Neurophysiol 82, 829–840. [DOI] [PubMed] [Google Scholar]
- Türker KS & Powers RK. (2003). Estimation of postsynaptic potentials in rat hypoglossal motoneurones: insights for human work. J Physiol 551, 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türker KS & Powers RK. (2005). Black box revisited: a technique for estimating postsynaptic potentials in neurons. Trends Neurosci 28, 379–386. [DOI] [PubMed] [Google Scholar]
- Türker KS, Yang J & Brodin P. (1997). Conditions for excitatory or inhibitory masseteric reflexes elicited by tooth pressure in man. Arch Oral Biol 42, 121–128. [DOI] [PubMed] [Google Scholar]
- Wilson VJ. (1959). Recurrent facilitation of spinal reflexes. J Gen Physiol 42, 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]


