Spasticity following spinal cord injury (SCI) has been clinically defined as an increase in velocity‐dependent tonic stretch reflexes (muscle tone) with exaggerated tendon jerks to passive movement. However, the development of other debilitating symptoms in parallel, such as severe spasms and cutaneous flexor hyperreflexia, may equally contribute to the SCI spasticity syndrome (Bravo‐Esteban et al. 2013; Gomez‐Soriano et al. 2016), by dramatically reducing residual gait function (Bravo‐Esteban et al. 2013) or activities of daily living. Severe SCI spasticity is usually managed with systemic or intrathecal baclofen therapy, but without careful titration this treatment may lead to excessive muscle weakness, complicating the patient's ability to perform essential motor tasks or transfer functions. The development of non‐pharmacological treatment strategies for the SCI spasticity syndrome is therefore a major clinical goal, which may be met by focal modality‐specific sensory stimulation programmes (Gomez‐Soriano et al. 2018) or by the activation of global spinal afferent systems using techniques such as spinal cord stimulation.
The study by Mekhael et al. (2019) published in this issue of the Journal of Physiology shows how short sessions of anodal trans‐spinal direct current stimulation (DCS), applied over the thoracolumbar spinal segments in mice with experimental contusion SCI and repeated daily over 7 days, can reduce stretch hyperreflexia, an effect which is maintained at least up to 4 weeks from the end of stimulation. Importantly, improvement of SCI spasticity was accompanied by better locomotor skills. Furthermore, the team demonstrates that repeated anodal trans‐spinal DCS potentiates spinal reflex inhibition, as shown by an increase in rate‐dependent H reflex depression, and normalises the expression of the Na–K–Cl cotransporter isoform 1 (NKCC1), highlighting one mechanism of action by which spinal stimulation may modulate SCI spasticity. Recently, in a clinical study of hereditary spastic paraplegia, repeated anodal transcutaneous spinal DCS has also been shown to reduce muscle hypertonia up to 2 months after the end of stimulation, although no improvement in gait function was identified (Ardolino et al. 2018).
As the transcutaneous spinal DCS technique is further developed for the control of the SCI spasticity syndrome, it will be necessary to benchmark its clinical effect by using a sensitive objective neurophysiological test of spinal excitability. Our group has shown that cutaneous sensorimotor integration is modified at rest and during controlled movement in subjects with SCI spasticity, characterised by long‐latency hyperreflexia of the tibialis anterior muscle in response to plantar stimulation (Fig. 1 A and B) (Gomez‐Soriano et al. 2016). Although the functional impact of long‐latency cutaneous hyperreflexia on residual muscle strength needs to be further defined (Gomez‐Soriano et al. 2016), our group has shown that focal vibration or transcutaneous electrical nerve stimulation of the plantar pad reduces cutaneous reflex activity (Gomez‐Soriano et al. 2018). Further studies are required to identify whether (1) both proprioceptive and cutaneous afferents are activated with non‐invasive transcutaneous spinal DCS protocols, (2) to determine the relationship between the H reflex and cutaneous hyperreflexia, and, (3) understand which test reflex protocols are sensitive enough to quantify the central effects of these new stimulation paradigms for the control of spasticity.
Figure 1. Measurement of cutaneous lower limb hyperreflexia as a characteristic of the spinal cord injury spasticity syndrome and neuromodulation of long‐latency reflex activity with transcutaneous spinal direct current stimulation.
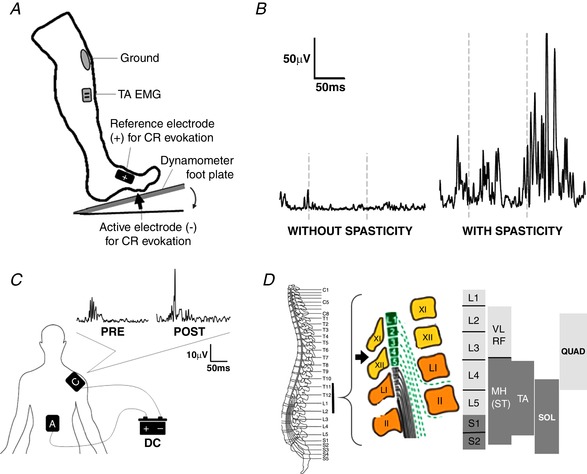
A, cutaneous reflex (CR) test method to quantify hyperreflexia during rest and controlled plantarflexion. Electrical stimulation of the plantar pad is recorded as tibialis anterior (TA) EMG activity (Gomez‐Soriano et al. 2016). B, representative lower limb cutaneous reflex activity recorded from the TA muscle during plantarflexion in subjects with subacute motor incomplete spinal cord injury (SCI) without (left) and with (right) the spasticity syndrome (Gomez‐Soriano et al. 2016). Note the long‐latency cutaneous hyperreflexia in the subject with SCI spasticity syndrome. C, electrode location for anodal transcutaneous spinal direct current stimulation (DCS) in a healthy subject with the anode (A) centred over the 11th thoracic vertebra (T11) and the cathode (C) placed on the right shoulder. Note that one session of T11 anodal transcutaneous spinal DCS increased long‐latency plantar–TA cutaneous reflex activity, similar to a previous study (Hubli et al. 2013). D, schematic diagram of the T11 anodal transcutaneous spinal DCS electrode (black arrow) in relation to the vertebral level, spinal segments and motoneuronal pools innervating key lower limb muscles.
Further characterisation of the effect of transcutaneous spinal DCS parameters on long‐latency cutaneous reflex modulation and clinical spasticity is also required, such as the number of interventions required, stimulation polarity and the spinal level of stimulation. A single session of anodal transcutaneous spinal DCS has in fact been shown to enhance long‐latency cutaneous reflex activity in both healthy subjects (Fig. 1 C) and individuals with motor complete SCI (Hubli et al. 2013), following application at the T11 vertebral level. In contrast, five 20‐min sessions of anodal transcutaneous spinal DCS delivered to the T10–T12 ‘thoracic spinal cord’ lead to long‐term inhibition of muscle hypertonia, albeit for people with hereditary spastic paraplegia (Ardolino et al. 2018). More emphasis should be made to standardise the spinal level of non‐invasive stimulation so that optimal modulation of key lower limb muscles can be achieved (Fig. 1 D). In addition, invasive and non‐invasive high‐frequency stimulation patterns focused at more than one spinal level may also be more effective in modulating spinal reflex excitability, voluntary motor control and clinical measures of spasticity after SCI.
The recent proliferation of clinical studies examining the effect of transcutaneous spinal stimulation protocols to promote residual motor function after SCI will undoubtedly highlight other translational aspects that need to be addressed for the treatment of SCI spasticity syndrome. By understanding the neurophysiological mechanisms underlying the modulatory effect of optimal transcutaneous spinal DCS protocols on muscle hypertonia, hyperreflexia and spasms, attention can then be made to combining non‐invasive spinal stimulation with activity‐based rehabilitation programmes to improve key upper and lower‐limb muscle strength and to facilitate activities of daily living.
Acknowledgements
Funding support was provided for the NeuroTrain project from the Instituto de Salud Carlos III (PI17/00581) and for the Recode project from the Ministerio de Ciencia, Innovación y Universidades (Explora Ciencia/Tecnología 2017, DPI2017‐9111‐EXP).
Edited by: Janet Taylor & Dario Farina
This is an Editor's Choice article from the 15 April 2019 issue.
Linked articles: This Perspectives article highlights an article by Mekhael et al. To read this article, visit https://doi.org/10.1113/JP276952.
References
- Ardolino G, Bocci T, Nigro M, Vergari M, Di Fonzo A, Bonato S, Cogiamanian F, Cortese F, Cova I, Barbieri S & Priori A (2018). Spinal direct current stimulation (tsDCS) in hereditary spastic paraplegias (HSP): a sham‐controlled crossover study. J Spinal Cord Med, 10.1080/10790268.2018.1543926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo‐Esteban E, Taylor J, Abian‐Vicen J, Albu S, Simon‐Martinez C, Torricelli D & Gomez‐Soriano J (2013). Impact of specific symptoms of spasticity on voluntary lower limb muscle function, gait and daily activities during subacute and chronic spinal cord injury. NeuroRehabilitation 33, 531–543. [DOI] [PubMed] [Google Scholar]
- Gomez‐Soriano J, Bravo‐Esteban E, Perez‐Rizo E, Avila‐Martin G, Galan‐Arriero I, Simon‐Martinez C & Taylor J (2016). Abnormal cutaneous flexor reflex activity during controlled isometric plantarflexion in human spinal cord injury spasticity syndrome. Spinal Cord 54, 687–694. [DOI] [PubMed] [Google Scholar]
- Gomez‐Soriano J, Serrano‐Munoz D, Bravo‐Esteban E, Avendano‐Coy J, Avila‐Martin G, Galan‐Arriero I & Taylor J (2018). Afferent stimulation inhibits abnormal cutaneous reflex activity in patients with spinal cord injury spasticity syndrome. NeuroRehabilitation 43, 135–146. [DOI] [PubMed] [Google Scholar]
- Hubli M, Dietz V, Schrafl‐Altermatt M & Bolliger M (2013). Modulation of spinal neuronal excitability by spinal direct currents and locomotion after spinal cord injury. Clin Neurophysiol 124, 1187–1195. [DOI] [PubMed] [Google Scholar]
- Mekhael W, Begum S, Samaddar S, Hassan M, Toruno P, Ahmed M, Gorin A, Maisano M, Ayad M & Ahmed Z (2019). Repeated anodal trans‐spinal direct current stimulation results in long‐term reduction of spasticity in mice with spinal cord injury. J Physiol 597, 2201–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]


