Abstract
Purpose
To document the development of disc torsion.
Methods
Consecutive disc photographs obtained at an interval of at least 1 year were reviewed retrospectively in 173 eyes of 173 Korean children. The angle of the vertical disc axis (AVDA) was measured in each fundus photograph with the fovea-disc center axis set at 0°. The associated change in the morphology of the optic disc was assessed by measuring the ratio of the horizontal to vertical disc diameters and the ratio of the maximum parapapillary atrophy width to vertical disc diameter. Eyes were divided into two groups with respect to the development of disc torsion: torsion and non-torsion group. Progressive torsion was defined as a change in AVDA between baseline and follow-up photographs beyond the coefficient of intraobserver repeatab ility. Factors associated with optic disc torsion were evaluated using logistic regression analysis.
Results
Mean subject age and refractive error at the time of baseline fundus examination were 6.8 ± 1.7 (range, 2 to 11) years and 0.2 ± 2.6 (range, −6.0 to +5.5) diopters, respectively. Mean follow-up period was 44.8 ± 21.1 (range, 12 to 103) months. Forty-two eyes (24%) were classified as torsion group who showed changes in AVDA that were greater than the intraobserver measurement variability (4.5°) during the follow-up period. The development of optic disc torsion was associated with greater myopic shift, a decrease in horizontal to vertical disc diameters, and an increase in parapapillary atrophy width to vertical disc diameter.
Conclusions
Progressive optic disc torsion was a common phenomenon in the children included in this study. Torsion occurred as the result of optic disc tilt in an oblique axis in most cases. The findings provide a framework for understanding torsion-related glaucomatous optic nerve damage.
Keywords: Disc tilt, Disc torsion, Optic disk
Myopia, especially high myopia, is associated with various vision-threatening diseases such as myopic macular degeneration [1,2], retinal breaks and detachment [3], and glaucoma [4,5]. Since myopia is growing in prevalence, particularly among younger people [6,7,8,9,10], ocular morbidity related to myopia may be more of problem in future elderly populations, thereby constituting an important clinical and public health problem [11].
It has long been recognized that myopia increases the risk of glaucoma [4,5]. Several hypotheses have been proposed for the association between myopia and glaucoma. Cahane and Bartov [12] suggested that myopic eyes are subject to greater stress due to their axial elongation. Laplace's law states that the wall tension of a sphere is proportional to its radius, such that wall tension in the lamina cribrosa and/or parapapillary sclera may be increased in myopic eyes. Quigley [13] suggested that myopic eyes are anatomically weaker due to scleral stretching. In line with this, Ren et al. [14] reported that the lamina cribrosa and peripapillary sclera were thinner in eyes with longer axial length. However, the definitive pathologic relationship between myopia and glaucomatous optic neuropathy (GON) remains to be determined.
Optic disc torsion has recently become a focus of interest in relation to glaucoma in myopic eyes. Park et al. [15] reported that the direction of optic disc torsion was related to the location of visual field defects. Lee et al. [16] demonstrated that the prevalence and degree of optic disc torsion were significantly greater in the affected eyes of young myopic patients with a unilateral visual field defect than in contralateral normal eyes. These data together suggest that optic disc torsion significantly influences the development of GON in myopic eyes. However, the precise mechanism underlying how optic disc torsion is associated with glaucoma remains unclear. Elucidating this association first requires clarification of what torsion is and how it develops.
Our group proposed that the horizontally oval optic disc (i.e., disc torsion) may represent horizontal disc tilt rather than true rotation of the optic disc based on the lamina cribrosa configuration [17]. However, the true nature of optic disc torsion can be confirmed only by observing the development of a torted disc in a longitudinal study. The purpose of the present study was to document the development of disc torsion in children.
Materials and Methods
This study performed a retrospective analysis of serial optic disc photographs obtained from subjects aged younger than 18 years who were consecutively enrolled from a database of patients first examined for suspected glaucomatous optic disc between October 2004 and October 2014 at Seoul National University Bundang Hospital. This retrospective, observational study was approved by the institutional review board of Seoul National University Bundang Hospital (B-1705/396-110). The requirement for informed consent was waived owing to the retrospective nature of the study. The study followed the tenets of the Declaration of Helsinki.
Each subject underwent ophthalmic examination that included best-corrected visual acuity, cycloplegic refraction, slit-lamp biomicroscopy, and disc photography. Subjects were eligible for inclusion when their medical records included serial color- or red-free fundus photographs with an interval between the first and last photographs of at least 1 year. Eyes with a history of intraocular surgery, a history or evidence of glaucoma, other optic neuropathies or retinal disease, an elevated intraocular pressure (IOP), or a spherical equivalent (SE) of less than −6 diopters were excluded. The medical records of selected subjects were reviewed, and data on sex, age, laterality, and cycloplegic refractive error at the initial and final examinations were collected. When both eyes were eligible for inclusion, one eye was randomly selected.
Fundus photography
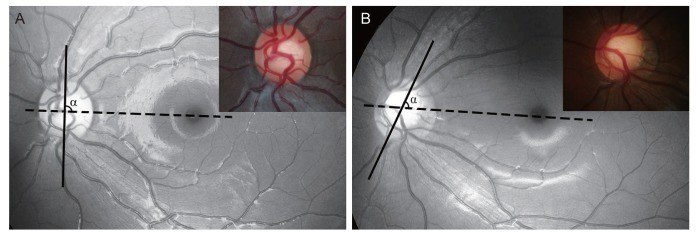
Color- or red-free fundus photographs were acquired using a digital fundus camera (EOS D60, Canon, Utsunomiyashi, Japan or Kowa VX-10a, Kowa, Tokyo, Japan). Serial photographs were assessed on an LCD monitor by an observer (JAK) who was masked to the clinical information of the subjects. The angle of the vertical optic disc axis (AVDA) in each fundus photograph was measured with the fovea-disc enter axis set at 0° using ImageJ ver. 1.52 (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) (Fig. 1A, 1B). Progressive torsion was defined in this study as a change in AVDA between the baseline and follow-up photographs beyond the coefficient of intraobserver repeatability. “Considerable torsion” was deemed to be present when the AVDA change exceeded 15° based on criteria often used in this field [18,19].
Fig. 1. Measuring the vertical disc axis. (A,B) Baseline and follow-up red-free fundus photograph and disc photograph (inset). The angle of the vertical disc axis (solid line) was measured from the reference line connecting the fovea and the disc center (dashed line).
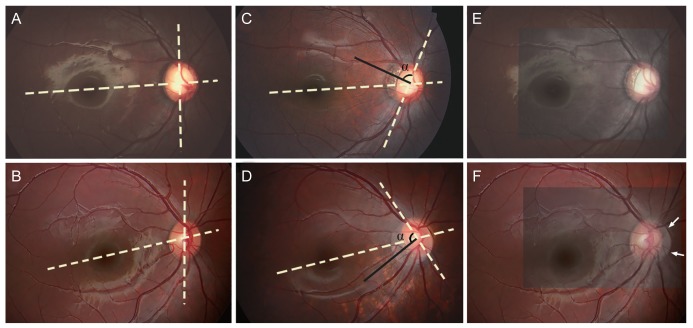
Changes associated with torsion were evaluated by measuring the ratio of horizontal to vertical disc diameters (HVDR) and the ratio of maximum parapapillary atrophy (PPA) width to vertical disc diameter (PVDR) in each photograph. To determine whether torsion can be fully explained by disc tilt, the baseline (Fig. 2A, 2B) and follow-up (Fig. 2C, 2D) photographs were superimposed with blood vessels aligned. Optic disc margin was expected to overlap at least partially when torsion was the result of the optic disc tilt (Fig. 2E). However, when torsion represented the true rotation of the optic disc axis, not even a small segment of the disc margin was expected to overlap (Fig. 2F). This analysis was performed only in eyes with considerable torsion in order to maximize the clarity of the analysis.
Fig. 2. Representative cases showing the development of optic-disc torsion. (A) Baseline fundus photograph of a 4-year-old girl. (B) Baseline fundus photograph of a 3-year-old girl. (C) Superior optic-disc torsion was observed 6 years later. (D) Inferior optic-disc torsion was observed 3 years later. (E) When the baseline and follow-up photographs were superimposed using the blood vessels as references, the nasal disc margin of the two photographs overlapped, indicating that the apparent torsion resulted from disc tilt on an oblique axis. (F) The disc margin of the two photographs did not overlap at any region when the two photographs were superimposed. The current disc margin is vaguely seen (arrows). (C,D) Note that the vertical disc axis (dashed line) and direction of the longest parapapillary atrophy width (black line) are approximately perpendicular.
Data analysis
To evaluate the intraobserver and interobserver reproducibilities of measurements of the optic disc axis and PPA parameters, 30 randomly selected disc photographs were evaluated by two independent observers (JAK and TWK). The coefficient of intraobserver repeatability was defined as 1.96 times the intermeasurement standard deviation. Based on the coefficient of intraobserver repeatability, eyes were classified into two groups: eyes with torsion (torsion group) and eyes without torsion (non-torsion group). Comparisons between these two groups were performed with the chi-square and Student's t tests. Logistic regression analysis was performed to identify factors associated with optic disc torsion. All statistical tests were performed using IBM SPSS Statistics ver. 20.0 (IBM Corp., Chicago, IL, USA). A probability value of p < 0.05 was considered to be indicative of statistical significance.
Results
This study initially included the 398 eyes of 199 subjects who had more than 1 year of follow-up using serial disc photography. Of these, 65 eyes were excluded because of a history or evidence of congenital glaucoma (n = 16), juvenile glaucoma (n = 21), or other optic neuropathy (n = 10), or SE <−6 diopters (n = 18), leaving a final sample of 333 eyes of 173 subjects. When both eyes were eligible for inclusion, one eye was randomly selected.
Subject age and refractive error at the time of initial fundus examination were 6.8 ± 1.7 years (range, 2 to 11 years) and 0.2 ± 2.6 diopters (range, −6.0 to +5.5 diopters), respectively. The follow-up period was 44.8 ± 21.1 months (range, 12 to 103 months). The coefficient of the intraobserver repeatability for measuring AVDA was 4.5°. Based on this value, progressive torsion was defined as an AVDA change exceeding 5°.
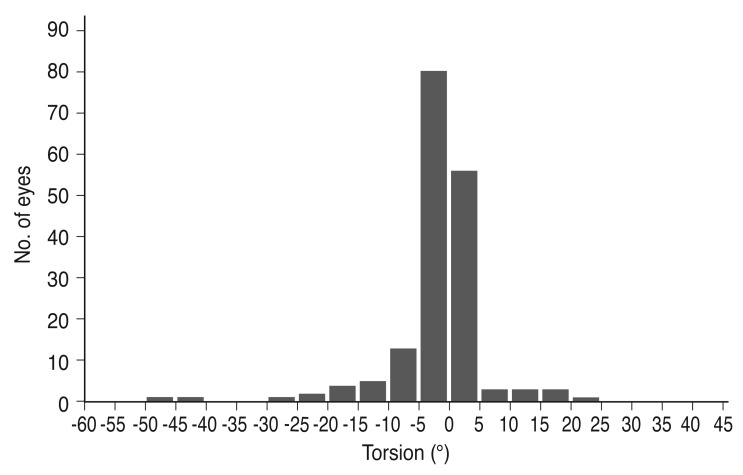
Of the 173 finally included eyes, 42 (24.3%) had progressive torsion and were classified as the torsion group; the remaining 131 eyes were classified as the non-torsion group. Of the 42 eyes in the torsion group, 15 and 27 had superior and inferior torsion, respectively, as defined by clockwise and counterclockwise rotations, respectively, of the vertical diameter axis at a right-eye orientation (Fig. 3). Considerable torsion was found in 21 eyes (12.1%).
Fig. 3. Frequency distribution of the degree of torsion. Note that inferior torsion was more common than superior torsion.
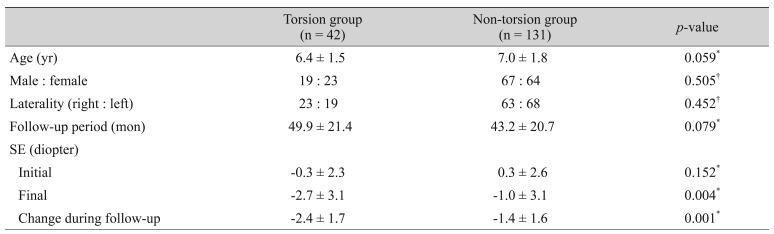
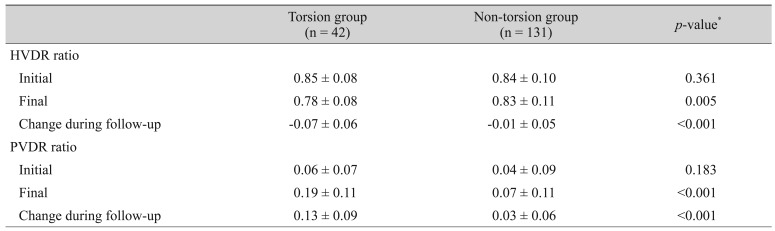
Table 1 compares the baseline characteristics between the torsion and non-torsion groups. No significant difference was found between groups with regard to age, sex, laterality, or follow-up period. The final SE was more myopic in the torsion group than in the non-torsio n group (p = 0.004), and the degree of myopic shift was also significantly greater in the torsion group than in the non-torsion group (−2.4 ± 1.7 vs. −1.4 ± 1.6 diopters, p = 0.001).
Table 1. Demographic and clinical characteristics.
Values are presented as mean ± standard deviation or number.
SE = spherical equivalent.
*Analyzed with the Student t-test; †Analyzed with the chi-square test.
Table 2 compares changes in the optic disc and parapapillary region between the two groups. The final HVDR was smaller in the torsion group than in the non-torsion group (p = 0.005). In addition, the change in HVDR was significantly larger in the torsion group (p < 0.001). PVDR did not differ significantly between the two groups on initial examination (p = 0.183), but the subsequent change in PVDR was significantly larger in the torsion group (p < 0.001).
Table 2. Comparison of the changes in the optic disc and parapapillary region between the two groups.
Values are presented as mean ± standard deviation.
HVDR = ratio of horizontal disc diameter to vertical disc diameter; PVDR = ratio of maximum parapapillary atrophy width to vertical disc diameter.
*Analyzed with the Student's t-test.
The torsion in 19 of the 21 eyes with considerable disc torsion was fully explained by the tilt of the optic disc on an oblique axis. In the remaining eyes, torsion could not be explained solely by tilt, and additional rotation was considered to be present in those eyes (Fig. 2D–2F).
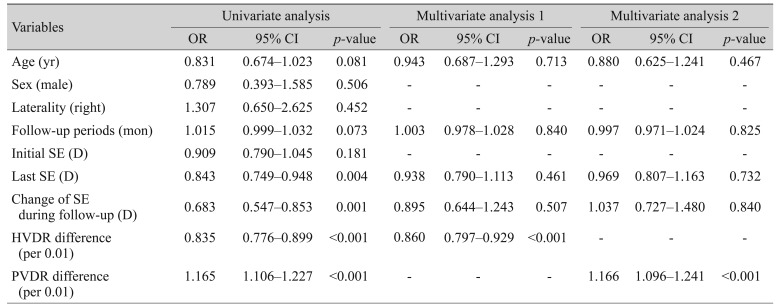
In univariate analysis, torsion was significantly associated with the last measured value of SE (p = 0.004), changes in SEs (myopic shift, p = 0.001), and changes in HVDR and PVDR during follow-up (p < 0.001 and p < 0.001, respectively). Since changes in HVDR and PVDR represent different aspects of optic disc changes associated with myopic shift [20], multivariate analysis was performed in two ways to avoid multicollinearity between them. Changes in HVDR and PVDR during follow-up remained statistically significant in multivariate analysis (p < 0.001) (Table 3).
Table 3. Factors associated with optic nerve head torsion.
OR = odds ratio; CI = confidence interval; SE = spherical equivalent; D = diopters; HVDR = ratio of horizontal disc diameter to vertical disc diameter; PVDR = ratio of maximum parapapillary atrophy width to vertical disc.
Discussion
We identified 42 cases in which progressive optic disc torsion occurred during the study period. Torsion was associated with disc tilt and the development/enlargement of PPA in all eyes. Additional rotation of the optic disc axis was observed in two patients. To the best of our knowledge, this study is the first to document the development of disc torsion using serial fundus photographs.
We previously demonstrated the presence of optic disc tilt in children with incipient myopia [20]. Tilt was accompanied by the development/enlargement of PPA and was associated with myopic shift, suggesting that disc tilt derives from scleral stretching secondary to axial elongation. In the present study, torsion was universally associated with a decrease in HVDR (i.e., disc tilt), and also with the development/enlargement of PPA and myopic shift. These findings suggest that torsion represents tilt on an oblique axis. Axial elongation can occur in various directions [21], and the direction of axial elongation can influence the direction of disc tilt. For instance, when the posterior center of the axis of elongation is located superotemporally, scleral stretching would occur in the superotemporal direction, resulting in PPA in the superotemporal region, with oblique disc tilt being perpendicular to the direction of scleral stretching. The obliquely tilted disc would have an oblique disc axis, and be identified as torsion.
The changes in almost all cases in the torsion group were explainable by oblique disc tilt. However, in two eyes torsion could not be explained by oblique tilt because the nasal disc margin was deviated from the expected disc margin after oblique disc tilt. This finding suggests that true rotation of the disc axis also occurs in some eyes. Although we do not have a clear explanation for such rotation, we speculate that it is attributable to axial elongation in multiple directions. Such axial elongation could result in complex changes in the optic disc, resulting in apparent rotation of the optic disc axis.
Torsion is a common feature in myopic eyes [15], and it has recently become a focus of interest in relation to how it influences the development of GON. In young patients with unilateral myopic glaucoma, greater torsion was found in the affected eye. In particular, it is intriguing that the direction of torsion was related to the location of retinal nerve fiber layer (RNFL) defect: this defect was seen in the superotemporal sector in eyes with superior torsion, and in the inferotemporal sector in eyes with inferior torsion [15]. Our findings may provide a plausible explanation for the relationship between optic disc torsion and the location of glaucomatous damage. The superotemporal and inferotemporal sectors are preferentially involved in glaucoma due to the presence of less support for the axons from connective tissue [22]. In eyes with optic disc tilt, tensile and/or shearing stresses arising from scleral stretching may induce axonal damage. In this process the direction of scleral stretching may result in stress varying by region of the optic nerve head. For instance, in eyes with superior torsion, the change would be largest near the superior disc margin and thereby increase the possibility of axonal damage in the superior optic nerve (and vice versa for eyes with inferior torsion).
An RNFL defect was not detected on follow-up photographs in any of the eyes in the torsion group in this study (data not presented). However, especially in myopic patients aged in their 20s or early 30s, RNFL defect is not rarely seen despite low IOP, particularly among those with disc torsion [23,24]. We speculate that the tensile stress derived from torsion does not immediately lead to development of axonal damage. However, eyes with torsion will be subject to sustained tensile stress, which may eventually damage the axons. An analogy to this finding may be found in the relationship between ocular hypertension and glaucoma. Eyes with high IOP do not necessarily exhibit optic nerve damage (i.e., ocular hypertension) at initial presentation, but some develop glaucomatous damage years later [25].
AVDA was larger than 15° at baseline in 10 of the 173 included eyes. These eyes all had PPA and a tilted disc appearance. We speculate that those discs were torted before the baseline photograph was obtained. Progressive considerable torsion was not observed during the study period in these 10 eyes, and so the incidence of considerable disc torsion may have been underestimated in this study.
The relationship between myopia and glaucoma remains unclear. It is generally acknowledged that myopia is a risk factor for glaucoma. In contrast, recent studies have demonstrated that glaucoma progression does not occur more rapidly in myopic eyes [26,27]. Our data may provide some insight into this puzzle. The tensile stress associated with torsion may increase the susceptibility of axons to glaucomatous damage. Thus, it is possible that eyes subject to torsion will develop glaucoma despite the relatively low IOP-induced stress not being sufficient to induce glaucomatous damage in eyes without torsion. Meanwhile, the tensile stress would remain focused near the superior or inferior pole of the optic disc, depending on the direction of torsion. Thus, once glaucomatous damage occurs in the respective polar area in these eyes, the remaining axons may remain healthy if the glaucomatous insult (i.e., IOP-induced stress) is not sufficient to induce glaucoma by itself. In this situation the rate of disease progression would decrease. This may be a factor contributing to the slow rate of disease progression in myopic patients.
This study was subject to several limitations. First, we did not measure axial length, and myopic shift was used as a surrogate of axial elongation. However, myopic shift is attributed to axial elongation that occurs in childhood [28]. Second, we could not measure the true length or distance values in fundus photographs because we could not correct for the magnification effect due to the actual axial length being unknown. However, all measurements in the study were of angles and ratio parameters that would not be affected by magnification errors. Third, the subjects were glaucoma suspect patients, and so the findings of this study might not be applicable to a general population. Fourth, the subjects were not observed until the end of eye growth. It is possible that the non-torsion group could show changes in the future. Lastly, disc changes were evaluated based on two-dimensional analyses. This was because three-dimensional measurements of the optic nerve head were not feasible in most of the children, who were aged 6.8 ± 1.7 years at the time of baseline examination. Three-dimensional analysis may provide greater insight into optic disc tilt. Nonetheless, our data clearly indicate that optic disc torsion can be an acquired feature arising from scleral stretching.
In conclusion, we have demonstrated the development of optic disc torsion in children. Torsion was mostly explained by optic disc tilt on the oblique axis. The findings provide a framework for understanding torsion-related GON.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Ito-Ohara M, Seko Y, Morita H, et al. Clinical course of newly developed or progressive patchy chorioretinal atrophy in pathological myopia. Ophthalmologica. 1998;212:23–29. doi: 10.1159/000027254. [DOI] [PubMed] [Google Scholar]
- 2.Avila MP, Weiter JJ, Jalkh AE, et al. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984;91:1573–1581. doi: 10.1016/s0161-6420(84)34116-1. [DOI] [PubMed] [Google Scholar]
- 3.Pierro L, Camesasca FI, Mischi M, Brancato R. Peripheral retinal changes and axial myopia. Retina. 1992;12:12–17. doi: 10.1097/00006982-199212010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki Y, Iwase A, Araie M, et al. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study. Ophthalmology. 2006;113:1613–1617. doi: 10.1016/j.ophtha.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–2015. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 6.Kempen JH, Mitchell P, Lee KE, et al. The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. Arch Ophthalmol. 2004;122:495–505. doi: 10.1001/archopht.122.4.495. [DOI] [PubMed] [Google Scholar]
- 7.Guo K, Yang DY, Wang Y, et al. Prevalence of myopia in schoolchildren in Ejina: the Gobi Desert Children Eye Study. Invest Ophthalmol Vis Sci. 2015;56:1769–1774. doi: 10.1167/iovs.14-15737. [DOI] [PubMed] [Google Scholar]
- 8.Williams KM, Verhoeven VJ, Cumberland P, et al. Prevalence of refractive error in Europe: the European Eye Epidemiology (E(3)) Consortium. Eur J Epidemiol. 2015;30:305–315. doi: 10.1007/s10654-015-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena R, Vashist P, Tandon R, et al. Prevalence of myopia and its risk factors in urban school children in Delhi: the North India Myopia Study (NIM Study) PLoS One. 2015;10:e0117349. doi: 10.1371/journal.pone.0117349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster PJ, Jiang Y. Epidemiology of myopia. Eye (Lond) 2014;28:202–208. doi: 10.1038/eye.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seet B, Wong TY, Tan DT, et al. Myopia in Singapore: taking a public health approach. Br J Ophthalmol. 2001;85:521–526. doi: 10.1136/bjo.85.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahane M, Bartov E. Axial length and scleral thickness effect on susceptibility to glaucomatous damage: a theoretical model implementing Laplace's law. Ophthalmic Res. 1992;24:280–284. doi: 10.1159/000267179. [DOI] [PubMed] [Google Scholar]
- 13.Quigley HA. Reappraisal of the mechanisms of glaucomatous optic nerve damage. Eye (Lond) 1987;1:318–322. doi: 10.1038/eye.1987.51. [DOI] [PubMed] [Google Scholar]
- 14.Ren R, Wang N, Li B, et al. Lamina cribrosa and peripapillary sclera histomorphometry in normal and advanced glaucomatous Chinese eyes with various axial length. Invest Ophthalmol Vis Sci. 2009;50:2175–2184. doi: 10.1167/iovs.07-1429. [DOI] [PubMed] [Google Scholar]
- 15.Park HY, Lee K, Park CK. Optic disc torsion direction predicts the location of glaucomatous damage in normal-tension glaucoma patients with myopia. Ophthalmology. 2012;119:1844–1851. doi: 10.1016/j.ophtha.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Lee KS, Lee JR, Kook MS. Optic disc torsion presenting as unilateral glaucomatous-appearing visual field defect in young myopic Korean eyes. Ophthalmology. 2014;121:1013–1019. doi: 10.1016/j.ophtha.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Lee KM, Lee EJ, Kim TW. Lamina cribrosa configuration in tilted optic discs with different tilt axes: a new hypothesis regarding optic disc tilt and torsion. Invest Ophthalmol Vis Sci. 2015;56:2958–2967. doi: 10.1167/iovs.14-15953. [DOI] [PubMed] [Google Scholar]
- 18.Witmer MT, Margo CE, Drucker M. Tilted optic disks. Surv Ophthalmol. 2010;55:403–428. doi: 10.1016/j.survophthal.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 19.How AC, Tan GS, Chan YH, et al. Population prevalence of tilted and torted optic discs among an adult Chinese population in Singapore: the Tanjong Pagar Study. Arch Ophthalmol. 2009;127:894–899. doi: 10.1001/archophthalmol.2009.134. [DOI] [PubMed] [Google Scholar]
- 20.Kim TW, Kim M, Weinreb RN, et al. Optic disc change with incipient myopia of childhood. Ophthalmology. 2012;119:21–26. doi: 10.1016/j.ophtha.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 21.Moriyama M, Ohno-Matsui K, Hayashi K, et al. Topographic analyses of shape of eyes with pathologic myopia by high-resolution three-dimensional magnetic resonance imaging. Ophthalmology. 2011;118:1626–1637. doi: 10.1016/j.ophtha.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Dandona L, Quigley HA, Brown AE, Enger C. Quantitative regional structure of the normal human lamina cribrosa. A racial comparison. Arch Ophthalmol. 1990;108:393–398. doi: 10.1001/archopht.1990.01070050091039. [DOI] [PubMed] [Google Scholar]
- 23.Doshi A, Kreidl KO, Lombardi L, et al. Nonprogressive glaucomatous cupping and visual field abnormalities in young Chinese males. Ophthalmology. 2007;114:472–479. doi: 10.1016/j.ophtha.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Park HY, Lee KI, Lee K, et al. Torsion of the optic nerve head is a prominent feature of normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2014;56:156–163. doi: 10.1167/iovs.13-12327. [DOI] [PubMed] [Google Scholar]
- 25.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 26.Araie M, Shirato S, Yamazaki Y, et al. Risk factors for progression of normal-tension glaucoma under β-blocker monotherapy. Acta Ophthalmol. 2012;90:e337–e343. doi: 10.1111/j.1755-3768.2012.02425.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee JY, Sung KR, Han S, Na JH. Effect of myopia on the progression of primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2015;56:1775–1781. doi: 10.1167/iovs.14-16002. [DOI] [PubMed] [Google Scholar]
- 28.Hyman L, Gwiazda J, Hussein M, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005;123:977–987. doi: 10.1001/archopht.123.7.977. [DOI] [PubMed] [Google Scholar]