Abstract
Key points
Physical activity is associated with reduced mortality rates for survivors of colorectal cancer.
Acute high intensity interval exercise (HIIE) reduced colon cancer cell number in vitro and promoted increases in inflammatory cytokines immediately following exercise.
This acute suppression of colon cancer cell number was transient and not observed at 120 minutes post-acute HIIE.
The acute effects of exercise may constitute an important mechanism by which exercise can influence colorectal cancer outcomes.
Abstract
Physical activity is associated with significant reductions in colorectal cancer mortality. However, the mechanisms by which exercise mediates this anti‐oncogenic effect are not clear. In the present study, colorectal cancer survivors completed acute (n = 10) or chronic (n = 10) exercise regimes. An acute high intensity interval exercise session (HIIE; 4 × 4 min at 85–95% peak heart rate) was completed with serum samples collected at baseline, as well as 0 and 120 min post‐exercise. For the ‘chronic’ intervention, resting serum was sampled before and after 4 weeks (12 sessions) of HIIE. The effect of serum on colon cancer cell growth was evaluated by incubating cells (CaCo‐2 and LoVo) for up to 72 h and assessing cell number. Serum obtained immediately following HIIE, but not 120 min post‐HIIE, significantly reduced colon cancer cell number. Significant increases in serum interleukin‐6 (P = 0.023), interleukin‐8 (P = 0.036) and tumour necrosis factor‐α (P = 0.003) were found immediately following acute HIIE. At rest, short‐term HIIE training did not promote any changes in cellular growth or cytokine concentrations. The acute effects of HIIE and the cytokine flux may be important mediators of reducing colon cancer cell progression. Repetitive exposure to these acute effects may contribute to the relationship between exercise and improved colorectal cancer survival.
Keywords: Colorectal cancer, exercise, exercise‐oncology, physical activity, cytokine, biomarker
Key points
Physical activity is associated with reduced mortality rates for survivors of colorectal cancer.
Acute high intensity interval exercise (HIIE) reduced colon cancer cell number in vitro and promoted increases in inflammatory cytokines immediately following exercise.
This acute suppression of colon cancer cell number was transient and not observed at 120 minutes post-acute HIIE.
The acute effects of exercise may constitute an important mechanism by which exercise can influence colorectal cancer outcomes.
Introduction
Increased physical activity has been associated with significant reductions in mortality for survivors of colorectal cancer (Meyerhardt et al. 2006; Friedenreich et al. 2016). The relationship may partly be explained by exercise‐induced changes in systemic levels of various mechanistic host pathways (e.g. oxidative stress, metabolic hormones, sex hormones), to create a less carcinogenic environment (Betof et al. 2013; Friedenreich et al. 2016).
The direct effect of exercise‐induced changes in serum can be examined by incubation with cancer cells in vitro, allowing the correlation of systemic changes following exercise with changes in cancer cell growth. Exercise training has been shown to significantly reduce breast and prostate cancer cell growth following exercise training, concomitant with reductions in insulin‐like growth factor (IGF)1 and insulin (Ngo et al. 2002; Barnard et al. 2003; Barnard et al. 2006; Sridhar et al. 2015). However, recent data have challenged this notion of chronic adaptations to exercise being the only systemic mechanisms by which exercise may benefit cancer survivors. Dethlefsen et al. (2016) showed that, although long‐term exercise training did not alter breast cancer cell growth, acute exercise suppressed growth and was associated with elevation of several cytokines, including interleukin (IL)‐6 and IL‐8, and tumour necrosis factor‐α (TNF‐α). These findings present an important distinction between the roles of acute and chronic exercise on cancer cell growth, comprising a relationship that is yet to be explored in colorectal cancer survivors. Conceptually, it is important to highlight that the beneficial effects of exercise reflect the cumulative effect of regular alterations in systemic factors in response to acute bouts of exercise. Additionally, transient changes expressed acutely following exercise (e.g. catecholamines, immune responses, cytokines/myokines) have been suggested to have an equally important suppressive effect on cancer cell growth (Dethlefsen et al. 2017). Although acute benefits of exercise on cancer cell growth have been demonstrated (Dethlefsen et al. 2016; Dethlefsen et al. 2017), the time course of these growth‐suppressive effects has not been determined. Exploring and contrasting these responses between acute exercise and exercise training can inform how targeted exercise interventions may improve long‐term outcomes for colorectal cancer survivors.
High intensity interval exercise (HIIE) may be a particularly strong stimulus for promoting changes in colon cancer cell growth. Previous findings have demonstrated a strong relationship between the effects of systemic changes in cytokines following exercise (Dethlefsen et al. 2016; Idorn & Hojman, 2016; Pedersen et al. 2016), with an increased cytokine response (particularly IL‐6) following higher vs. lower intensity exercise (Cullen et al. 2016). Additionally, higher intensity exercise may promote more favourable changes in metabolic variables such as IGF1, insulin and glucose as a result of the increased volume of metabolically active skeletal muscle recruited during high intensity exercise (Edgett et al. 2013; Egan & Zierath Juleen, 2013). The primary aim of this pilot study was to evaluate the effect of exercise serum on colon cancer cell growth, following acute and short‐term HIIE training in colorectal cancer survivors. It was hypothesized that serological changes after both acute and chronic HIIE would lead to reductions in growth in colon cancer cells.
Methods
Participants
Male colorectal cancer survivors were recruited for the present study. Additionally, participants must have completed their last radiation therapy, chemotherapy or surgical treatment ≥ 1 month previously; be aged ≥40 years old (mean ages: acute trial = 66.9 ± 8.4 years; short‐term training = 64.9 ± 6.0 years); not be receiving insulin or any insulin sensitizing agents; and be free of any conditions that prevent safe completion of the exercise demands of the study. Participants were required to obtain physician consent for participation in the programme, and were individually screened via a medical history form and interview with the investigators to determine eligibility. Full details of participant recruitment have been reported elsewhere (Devin et al. 2016). The present study was approved by the Human Research Ethics Committee of The University of Queensland and informed consent was obtained from all participants. No participants died before the experiments were concluded.
Experimental models of exercise
Participants completed either an acute session of HIIE or short‐term HIIE training prescription. The HIIE 38‐min session commenced with a 10‐min warm up at 50–70% peak heart rate (HRpeak) before 4 × 4 minute bouts of cycling at 85–95% HRpeak were completed. Three minutes of active recovery separated each exercise bout. The short‐term training program involved repeating the HIIE protocol three times a week for 4 weeks. Further details of the safety and feasibility of this intervention are reported elsewhere (Devin et al. 2016).
Serum collection and analysis
During the acute exercise sessions, venous blood was sampled at baseline (fasted), immediately post‐exercise (0 min) and 120 min post‐exercise. Following fasting/resting blood sampling, participants consumed a light liquid meal replacement of 0.5 g kg−1 of Sustagen Sport® (Nestle Australia, Sydney, NSW, Australia) mixed with 300 mL of water; they then rested for 30 min before exercising. For the training intervention, fasting blood was sampled between 3 and 7 days before (pre‐intervention testing) and following the intervention (post‐intervention testing) at approximately the same time of day. At each time point, blood (20 mL) was collected from an antecubital vein. Samples were allowed to clot at room temperature (∼30 min), were centrifuged at 900 g for 10 min, frozen at –80°C and then stored for later analysis. Assays using serum samples were partially blinded by allocating samples with sequential number codes prior to analysis.
Culture of colon cancer cell lines
The effects of exercise were investigated in two human colon cancer cell lines (CaCo‐2 and LoVo) purchased from Cell Bank Australia (Westmead, NSW, Australia). These two colon cancer cell lines were chosen for their different genetic features in critical genes related to colorectal cancer: CaCo‐2 are TP53 mutant, whereas LoVo cells are KRAS mutant and wild‐type for the other gene (Ahmed et al. 2013). Cells were cultured in a mycoplasma‐free tissue culture environment in Eagle's minimum essential medium (CaCo‐2) or RPMI‐1640 medium (LoVo), supplemented with 10% fetal bovine serum (FBS), 1% glutamine and 1% penicillin–streptomycin. Cells were incubated at 37°C in 5% CO2 and were routinely passaged at ∼80% confluence.
Cell number assay
Cell number was assessed using the alamarBlue® assay (Thermo Fisher Scientific, Waltham, MA, USA). Cells were counted using an automated cell counter (TC20 Automated Cell Counter; Bio‐Rad, Hercules, CA, USA), after which four replicate wells of CaCo‐2 cells were seeded at ∼2 × 103 cells well−1 and LoVo cells at 1 × 104 cells well−1 in black, clear‐bottom 96‐well plates (Corning Incorporated, Corning, NY, USA). Cells were not incubated in the outer ring of wells (middle 60 wells only) to minimize the influence of evaporation on differences in fluorescence across the plate (Walzl et al. 2012) and quadruplicates were arranged in a two‐by‐two arrangement to minimize the effect of row‐ or column‐specific variability. Serum samples from each individual were used on the same plate to negate inter‐plate variability for comparisons within individuals. Cells were seeded in 100 μL of normal culture medium with 10% FBS for 24 h to allow for attachment, then aspirated and replaced with 100 μL of medium containing 10% serum from individual participants instead of FBS. Separate plates were then incubated for 24, 48 or 72 h. Fluorescence was then measured using a microplate reader (FLUOstar Optima; BMG Labtech, Ortenberg, Germany) at an excitation of 540 nm and emission of 590 nm. The median of four replicates was used for analysis, with background fluorescence being subtracted from the value for each well and then normalised to the fluorescence of control cells grown in 10% FBS instead of participant serum. The intra‐assay coefficient of variation for this assay based on four replicates was 4.3% for CaCo‐2 and 4.6% for LoVo cells.
Cell death assay
Levels of phosphatidylserine externalization were determined using binding of Annexin‐V, a common feature of apoptotic cells. Briefly, CaCo‐2 and LoVo cells were seeded in 60‐mm dishes at a density of 7.5 × 104 cells and 2 × 105, respectively, in 4 mL of their respective growth media. Cells were allowed to attach for 24 h prior to media replacement with 10% patient serum for 72 h. After harvesting via trypsinization, cells were resuspended in 50 μL of Annexin‐V binding buffer (10 mm Hepes, 140 mm NaCl and 2.5 mm CaCl2) and placed on ice for 5 min. Cells were then stained with 5 μL ml−1 Annexin‐V Alexa Fluor® 488 (Thermo Fisher Scientific) in a further 50 μL of buffer and incubated for 15 min in the dark. To distinguish necrotic cells, propidium iodide (Sigma Aldrich, St Louis, MO, USA) was added at a concentration of 1 μg mL−1 in 400 μL of buffer before being analysed on a C6 Accuri flow cytometer (BD Biosciences, San Jose, CA, USA). For an apoptotic positive control, cells were incubated with 1 μm staurosporine for 68 h. Flow cytometry data analysis was conducted using FlowJo™ software (Flow Jo LLC, Ashland, OR, USA). Following doublet discrimination, population gating based on single‐colour and healthy controls was performed to determine healthy (Annexin‐V negative, PI negative), early apoptotic (Annexin‐V positive, PI negative) or late apoptotic/necrotic (Annexin‐V positive or negative, PI positive) cells.
Systemic marker analyses
Systemic concentrations of IL‐6, IL‐8 and TNF‐α were measured using a high‐sensitivity magnetic bead‐based multiplex assay (R&D Systems, Minneapolis, MN, USA) and a Magpix® system (Merck Millipore, Billerica, MA, USA). IGF1 was analysed using an enzyme‐linked immunosorbent assay (Quantikine; R&D Systems). Insulin and glucose were measured using a Cobas e 411 analyser (Roche Diagnostics, Mannheim, Germany) and a Randox RX Daytona+ analyser (Randox Laboratories Limited, Crumlin, UK), respectively. The coefficients of variation for IL‐6, IL‐8, TNF‐α, IGF1, insulin and glucose were 3.1%, 1.8%, 2.9%, 2.1%, 1.1% and 0.8%, respectively.
Statistical analysis
Data were analysed unblinded using SPSS, version 23.0 (IBM Corp., Armonk, NY, USA). For experiments with multiple factors, outcomes were assessed using linear mixed modelling analysis. Combinations of sampling time point and incubation time (24, 48 or 72 h) were treated as fixed factors, with participants treated as a random factor with individual intercepts. Normality of the resulting model residuals were assessed using the Shapiro–Wilk test and via inspection of histogram and quintile–quintile plots. Data found to be positively skewed (insulin, IGF1) were analysed with a generalized linear mixed model specifying a gamma distribution and log‐link as described previously (Azuero et al. 2010). Changes in levels of apoptosis were determined using independent samples t tests. Paired samples t tests and the Wilcoxon signed rank test were used for analysis of systemic markers pre‐ and post‐training. Bonferroni adjustments were made to account for multiple pairwise comparisons within each analysis. Effect sizes (ES) were calculated using the Cohen's d statistic to describe the mean difference in relative cell number (Hopkins, 2003). An alpha level of 0.05 was used for statistical significance.
Results
Effect of acute and short‐term HIIE on colon cancer cell number
Details of the participants in the acute (n = 10) and short‐term training (n = 10) exercise experiments are presented in Table 1. Compared to baseline serum, incubation with serum obtained immediately after HIIE exercise cessation significantly reduced CaCo‐2 cell number after 24 h (ES = –1.3, P = 0.002), 48 h (ES = –1.7, P < 0.001) and 72 h (ES = –1.1, P = 0.035) (Fig. 1). Incubation of LoVo cells with serum collected immediately post‐exercise also reduced cell number at 24 h (ES = –1.2), 48 h (ES = –0.8) and 72 h (ES = –1.1), with significant decreases relative to baseline at 24 h (P = 0.001) and 72 h (P = 0.032). There was no significant difference (P ≥ 0.05) between sera collected at baseline and 120 min post‐exercise across the incubation times in either cell line.
Table 1.
Baseline participant characteristics
| Acute Exposure | Short‐term Exposure | |
|---|---|---|
| Mean SD | Mean SD | |
| n | 10 | 10 |
| Age (years) | 66.9 ± 8.4 | 64.9 ± 6.0 |
| Body mass (kg) | 89.6 ± 15.9 | 96.3 ± 10.9 |
| Body mass index (kg m−2) | 27.7 ± 3.6 | 30.2 ± 3.5 |
| (mL kg−1 min−1) | 28.6 ± 7.3 | 23.2 ± 3.9 |
| Cancer history | ||
| Colon cancer, n (%) | 8 (80) | 8 (80) |
| Rectal cancer, n (%) | 2 (20) | 2 (20) |
| Time since diagnosis (years) | 3.9 ± 0.9 | 3.6 ± 1.1 |
| Time since treatment (years) | 3.4 ± 0.8 | 3.1 ± 1.1 |
| Cancer stage, n (%) | ||
| I | 1 (10) | 2 (20) |
| II A | 1 (10) | 0 (0) |
| III A | 3 (30) | 1 (10) |
| III B | 2 (20) | 1 (10) |
| III C | 0 (0) | 2 (20) |
| IV | 1 (10) | 1 (10) |
| Unknown | 2 (20) | 3 (30) |
| Cancer treatment, n (%) | ||
| Surgery | 3 (30) | 3 (30) |
| Surgery and chemotherapy | 7 (70) | 6 (60) |
| Surgery and radiation | 0 (0) | 1 (10) |
| Exercise intensity | ||
| Heart rate (% HRpeak): HIIE | 84.1 ± 1.8 | 84.6 ± 3.5 |
| Power output (% PPO): HIIE | 64.2 ± 4.6 | 94.2 ± 9.3 |
Continuous variables are presented as mean ± SD; Nominal variables are presented as n (%)
HIIE, high intensity interval exercise; HRpeak, peak heart rate; PPO, peak power output; , peak oxygen consumption
Figure 1. Cell number before or after acute high intensity interval exercise or short‐term training.
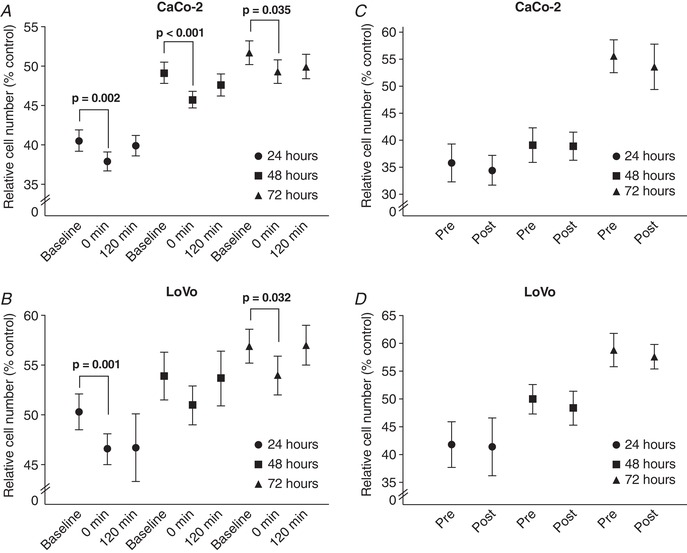
Cell number following incubation of (A) CaCo‐2 and (B) LoVo cells with serum from either baseline (pre‐exercise), 0 or 120 min post‐high intensity interval exercise, or (C) CaCo‐2 and (D) LoVo cells with serum collected at rest (fasting) either pre‐ or post‐exposure to short‐term high intensity interval exercise training (baseline and post‐intervention). Cells were incubated for 24, 48 or 72 h. Data presented as the mean and 95% CI
Incubation with serum collected at rest (3–7 days) following the 4‐week HIIE training resulted in no significant differences for either CaCo‐2 (P = 0.223) or LoVo (P = 0.375) cells compared to treatment with serum from the pre‐intervention control conditions. Consideration of cancer site (colon and rectal) had no influence (P ≥ 0.05) on the in vitro colon cancer cell number assays.
Effect of exercise serum on colon cancer cell apoptosis
A reduction in cell number may be a result of increased apoptosis, as reported previously with exercise serum treatment (Leung et al. 2004). Therefore, serum incubation experiments were also performed to determine the effect of exercise serum on colon cancer cell apoptosis. Compared to cells incubated with serum collected prior to acute HIIE, there were no significant differences in the proportion of apoptotic (Annexin‐V positive, PI negative) CaCo‐2 (‐0.04%, 95% CI = 0.24–0.17%; P = 0.702) or LoVo cells (0.64%, 95% CI = 0.92–2.20%; P = 0.395) following 72 h of incubation with serum collected immediately post‐HIIE. There were no significant differences (P ≥ 0.05) in the proportion of healthy cells or any other measures of cell death from this assay.
Changes in serological factors
Immediately following acute HIIE, significant increases were observed for TNF‐α (+0.7 pg mL−1, +15.2%, P = 0.003), IL‐6 (+0.30 pg mL−1 +44.8%, P = 0.023), IL‐8 (+2.3 pg mL−1 +24.7%, P = 0.036) and insulin (+3.1 pmol L−1, +38.8%, P = 0.023). The concentration of TNF‐α, IL‐6 and IL‐8 returned to baseline levels at 120 min post‐exercise (P ≥ 0.05), with insulin being significantly lower than baseline at 120 min (–2.1 pmol L−1, P = 0.001). No significant differences in TNF‐α (P = 0.765), IL‐6 (P = 0.338), IL‐8 (P = 0.074), IGF1 (P = 0.725), insulin (P = 0.976) or glucose (P = 0.138) were observed between resting serum collected at baseline and after 4 weeks of HIIE.
Discussion
By combining exercise with in vitro cell function assays, the present study indicates that systemic changes following acute HIIE supress the growth of colon cancer cells. Acute HIIE promoted transient increases in systemic cytokine concentrations (IL‐6, Il‐8 and TNF‐α) immediately following exercise, which abated by 2 h post‐exercise in concordance with the changes in cell number. Despite probably inducing repetitive upregulation of these acute growth suppressive effects of HIIE immediately following each session, short‐term HIIE training was not associated with changes in cell number or common metabolic factors related to the risk of colorectal cancer when measured at rest. These data provide support for the benefits of transient systemic changes following HIIE; repeated acute benefits resulting from regular exercise may favour a systemic profile less conducive to colon cancer cell growth.
To the best of our knowledge, this is the first study to demonstrate that the acute serological changes observed immediately following HIIE are associated with a reduction in colon cancer cell growth. The distribution of apoptotic cells in either cell line following incubation with pre‐exercise serum or serum immediately‐post HIIE was not significantly different, suggesting that the observed reductions in overall cell number are probably mediated via reductions in cellular proliferation rather than increased rates of apoptosis. Rundqvist et al. (2013) also showed that reductions in prostate cancer cell viability following post‐acute exercise serum incubation were a result of the inhibition of proliferation, with no changes in levels of apoptosis. Dethlefsen et al. (2016) reported a reduction in breast cancer cell viability following incubation with serum obtained immediately after a 2‐h exercise session (resistance and high intensity aerobic exercise). Similar to the present study, significant increases in TNF‐α, IL‐6 and IL‐8 were reported immediately post‐exercise, with increases in epinephrine and norepinephrine also being observed (Dethlefsen et al. 2016). The present study extends these findings to show that the transient suppression of cancer cell growth had subsided by 2 h post‐exercise, coinciding with the return of TNF‐α, Il‐6 and IL‐8 to baseline levels at this time point. These findings tend to suggest that the transient increases in cytokine concentrations immediately following HIIE in colorectal cancer survivors may be important mechanisms contributing to the observed growth suppression effect in colon cancer cells.
Recent data on tumour progression have provided evidence showing that the exercise‐induced acute cytokine response is an important mechanism underlying the anti‐carcinogenic effects of exercise (Idorn & Hojman, 2016; Pedersen et al. 2016). Pedersen et al. (2016) showed that exercise‐mediated increases in natural killer (NK) cell mobilization (catecholamine‐mediated) and redistribution (IL‐6‐mediated) substantially reduced the incidence of tumour following exercise in a murine model. However, in both the present study and that Dethlefsen et al. (2016), the absence of NK cells in culture following acute exercise serum‐replacement suggests that reductions in tumour cell viability according to this catecholamine‐NK cell‐cytokine mechanism must reflect the downstream cellular consequences of effector cytokine release (such as TNF‐α) following exercise rather than direct NK cell‐mediated apoptosis (Warren & Smyth, 1999; Wang et al. 2012; Idorn & Hojman, 2016; Shimasaki et al. 2016).
Furthermore, the hypothesized role of inflammatory cytokines is somewhat perplexing given that chronic inflammation (including increases in C‐reactive protein, IL‐6 and TNF‐α) is a hallmark of cancer and is associated with an increased risk of incidence (Dethlefsen et al. 2017). This presents an interesting juxtaposition between the proposed anti‐carcinogenic effects of IL‐6, IL‐8 and TNF‐α following acute exercise and the role of chronic elevations in these markers in cancer development. The precise mechanisms by which these factors may influence cellular growth in vivo currently remain unknown. Future research is needed to confirm the relative temporal changes in these cytokines and establish whether a causal relationship exists with the anti‐oncogenic properties of acute exercise, as well to investigate the role of additional immune cells in the response to exercise (Dethlefsen et al. 2017). It remains possible that these markers may not influence cellular progression in vivo but may be markers of the release of other effector mediators following this catecholamine–NK cell–cytokine sequence that directly influence cellular outcomes.
Surprisingly, yet in agreement with previous reports (Dethlefsen et al. 2016), short‐term HIIE training did not promote similar growth reductions to acute HIIE. Several factors may explain this finding. Previous reported reductions in prostate (Barnard et al. 2003; Leung et al. 2004) and breast (Barnard et al. 2006) cancer cellular growth following an intervention (exercise and/or dietary modification) were associated with significant decreases in IGF1 and insulin, as well as increases in IGF binding protein 1. IGF binding protein 1 can sequester IGF1, preventing initiation of the tumour promoting intracellular signalling, and is associated with improved insulin resistance (Maddux et al. 2006; Pollak, 2008). The lack of change in IGF1 or insulin observed in the present study may therefore explain the absence of changes in response to incubation with serum following short‐term HIIE training. At baseline, participants in this cohort had notably low levels of IGF1 relative to age‐referenced normative values (Andreassen et al. 2009). This may be partly explained by the time since diagnosis and the treatment of participants included in the present study (Table 1). IGF1 and insulin have been shown to promote tumour progression (Giovannucci, 2001; LeRoith & Roberts, 2003; Pollak, 2008) and systemic overexpression of these factors has been associated with an increased risk of colorectal cancer (Jenab et al. 2007; Chi et al. 2013). Therefore, the absence of these risk factors at baseline in the present cohort may have supressed the potential for improvements following exercise training.
Acute excursions in factors following exercise (e.g. cytokines) and chronic changes in biomarkers (e.g. metabolic markers) at rest are two separate mechanisms that may contribute to improvements in cancer prognosis with increased levels of physical activity (Dethlefsen et al. 2016; Friedenreich et al. 2016; Dethlefsen et al. 2017). Suppressed cell growth following acute HIIE warrants further investigations that aim to assess the translatability into clinically meaningful improvements for colorectal cancer survivors and that also evaluate the mechanisms specific to the biology of in vivo tumours by which this may occur. Additionally, whether the acute HIIE response differs between sedentary and trained individuals remains to be determined, although it may provide further insight into how long‐term exercise programmes may improve colorectal cancer outcomes. Further research is also warranted to investigate the effects of time since diagnosis and the effects of treatment on colon cancer cell growth.
Finally, given the transient nature of the changes in cell number and cytokines following exercise, which had abated at 2 h post‐exercise, the importance of engagement in and adherence to regular physical activity cannot be overstated. It is improbable that a single bout of exercise would produce a sufficient volume of circulating factors in such a short post‐exercise duration to contribute to improvements in prognosis. However, as part of a long‐term exercise programme, the repetitive induction of acute changes in cytokines and the eventual accumulation of chronic changes in other risk factors for colorectal cancer (e.g. IGF axis) may explain the relationship between exercise and improvements in cancer prognosis (Dethlefsen et al. 2017).
Conclusions and implications
The present study demonstrates that the serological changes associated with acute HIIE transiently reduce colon cancer cell number. There are several limitations worthy of comment. Because this was a pilot study, the findings are limited by the small sample size, which should be considered when drawing conclusions. Despite the small sample size, post hoc power analysis based on cell viability effect sizes following incubation for 24, 48 or 72 h with serum immediately following acute HIIE achieved a power of between 87.1 and 99.7% for CaCo‐2 and between 61.6 and 92.0% for LoVo cells. The encouraging within‐group changes provides support for the design of larger, well‐powered trials implementing this type of analysis to investigate differences between prescriptions of exercise to more adequately assess these outcomes. Second, the assay used in the present trial does not account for the in vivo environment in which tumour cells survive, which limits conclusions regarding whether the observed systemic effects were sufficiently large to directly influence tumour cells in vivo. Although the present trial has demonstrated distinct systemic cytokine (TNF‐α, IL‐6 and IL‐8) responses between acute and chronic exercise, it is possible that other contributory mechanisms may underlie the observed reductions in colon cancer cell growth. Given the pleiotropic effects of exercise, which influences a multitude of systems, we cannot conclude that the observed changes in colon cancer cell growth were the result of any one series of factors (Hawley et al. 2014). Finally, the lack of a non‐exercising control is a limitation that should be considered when drawing conclusions from these results.
Notwithstanding these limitations, the acute serological changes following exercise in the present trial and others (Dethlefsen et al. 2016; Pedersen et al. 2016) tend to suggest that the exercise‐induced cytokine changes may constitute an important mechanism contributing to the changes observed in colon cancer cell growth. Therefore, even without inducing chronic changes in various systemic factors associated with colorectal cancer risk, the repetitive induction of the exercise‐induced cytokine flux associated with HIIE training may translate into a more favourable systemic profile. Given the apparent importance of the transient serological responses to acute exercise, this may be an important mechanism contributing to the relationships observed between physical exercise and cancer mortality.
Additional Information
Competing interests
The authors declare that they have no competing interests.
Author contributions
All authors contributed to the conception and design of the work; acquisition, analysis or interpretation of the data; drafting or revising the work critically for important intellectual content. All authors have approved the final version of the manuscript submitted for publication and agree to be accountable for its content.
Funding
This study was funded by The University of Queensland HABS/MABS Collaboration Seeding Grant, Sports Medicine Australia Research Foundation and The University of Queensland Graduate School International Travel Award.
Biography
James Devin is an early career researcher who received his PhD from the University of Queensland. James also completed a Bachelor of Exercise and Sports Science, majoring in Clinical Exercise Physiology, and concurrently works as a Clinical Exercise Physiologist. His research interests focus on exercise oncology for colorectal cancer patients and survivors, aiming to better understand the mechanistic link between exercise and colorectal cancer progression. His work ultimately aims to contribute to the growing body of evidence for how exercise can be used to improve health outcomes for people affected by colorectal cancer.

Edited by: Scott Powers & Karyn Hamilton
This is an Editor's Choice article from the 15 April 2019 issue.
References
- Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknaes M, Hektoen M, Lind GE & Lothe RA (2013). Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2, e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen M, Nielsen K, Raymond I, Kristensen LØ & Faber J (2009). Characteristics and reference ranges of insulin‐like growth factor‐i measured with a commercially available immunoassay in 724 healthy adult Caucasians. Scand J Clin Lab Invest 69, 880–885. [DOI] [PubMed] [Google Scholar]
- Azuero A, Pisu M, McNees P, Burkhardt J, Benz R & Meneses K (2010). An application of longitudinal analysis with skewed outcomes. Nurs Res 59, 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard RJ, Gonzalez JH, Liva ME & Ngo TH (2006). Effects of a low‐fat, high‐fiber diet and exercise program on breast cancer risk factors in vivo and tumor cell growth and apoptosis in vitro. Nutr Cancer 55, 28–34. [DOI] [PubMed] [Google Scholar]
- Barnard RJ, Ngo TH, Leung PS, Aronson WJ & Golding LA (2003). A low‐fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate 56, 201–6. [DOI] [PubMed] [Google Scholar]
- Betof AS, Dewhirst MW & Jones LW (2013). Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun 30, S75–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi F, Wu R, Zeng YC, Xing R & Liu Y (2013). Circulation insulin‐like growth factor peptides and colorectal cancer risk: an updated systematic review and meta‐analysis.Mol Biol Rep 40, 3583–3590. [DOI] [PubMed] [Google Scholar]
- Cullen T, Thomas AW, Webb R & Hughes MG (2016). Interleukin‐6 and associated cytokine responses to an acute bout of high‐intensity interval exercise: the effect of exercise intensity and volume. Appl Physiol Nutr Metab 41, 803–808. [DOI] [PubMed] [Google Scholar]
- Dethlefsen C, Lillelund C, Midtgaard J, Andersen C, Pedersen BK, Christensen JF & Hojman P (2016) Exercise regulates breast cancer cell viability: systemic training adaptations versus acute exercise responses. Breast Cancer Res Treat 159, 469–479. [DOI] [PubMed] [Google Scholar]
- Dethlefsen C, Pedersen KS & Hojman P (2017). Every exercise bout matters: linking systemic exercise responses to breast cancer control. Breast Cancer Res Treat 162, 399–408. [DOI] [PubMed] [Google Scholar]
- Devin JL, Sax AT, Hughes GI, Jenkins DG, Aitken JF, Chambers SK, Dunn JC, Bolam KA & Skinner TL (2016). The influence of high‐intensity compared with moderate‐intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised controlled trial. J Cancer Surviv 10, 467–479. [DOI] [PubMed] [Google Scholar]
- Edgett BA, Foster WS, Hankinson PB, Simpson CA, Little JP, Graham RB & Gurd BJ (2013). Dissociation of increases in PGC‐1alpha and its regulators from exercise intensity and muscle activation following acute exercise. PLoS ONE 8, e71623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B & Zierath Juleen R (2013). Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17, 162–184. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Neilson HK, Farris MS & Courneya KS (2016). Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res 22, 4766–4775. [DOI] [PubMed] [Google Scholar]
- Giovannucci E (2001). Insulin, insulin‐like growth factors and colon cancer: A review of the evidence. J Nutr 131, 3109S–3120S. [DOI] [PubMed] [Google Scholar]
- Hawley J, Hargreaves M, Joyner M & Zierath J (2014). Integrative biology of exercise. Cell 159, 738–749. [DOI] [PubMed] [Google Scholar]
- Hopkins WG (2003). Sportscience website: a spreadsheet for analysis of straightforward controlled trials. Aukland, New Zealand: Sportscience. Available from: sportsci.org/jour/03/wghtrials.htm [Accessed Septermber 22, 2016].
- Idorn M & Hojman P (2016). Exercise‐Dependent regulation of NK cells in cancer protection. Trends Mol Med 22, 565–577. [DOI] [PubMed] [Google Scholar]
- Jenab M, Riboli E, Cleveland RJ, Norat T, Rinaldi S, Nieters A Biessy C, Tjønneland A, Olsen A, Overvad K, Grønbaek H, Clavel‐Chapelon F, Boutron‐Ruault MC, Linseisen J, Boeing H, Pischon T, Trichopoulos D, Oikonomou E, Trichopoulou A, Panico S, Vineis P, Berrino F, Tumino R, Masala G, Peters PH, van Gils CH, Bueno‐de‐Mesquita HB, Ocké MC, Lund E, Mendez MA, Tormo MJ, Barricarte A, Martínez‐García C, Dorronsoro M, Quirós JR, Hallmans G, Palmqvist R, Berglund G, Manjer J, Key T, Allen NE, Bingham S, Khaw KT, Cust A & Kaaks R (2007). Serum C‐peptide, IGFBP‐1 and IGFBP‐2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 121, 368–376. [DOI] [PubMed] [Google Scholar]
- LeRoith D & Roberts CT (2003). The insulin‐like growth factor system and cancer. Cancer Lett 195, 127–137. [DOI] [PubMed] [Google Scholar]
- Leung P‐S, Aronson WJ, Ngo TH, Golding LA & Barnard RJ (2004). Exercise alters the IGF axis in vivo and increases p53 protein in prostate tumor cells in vitro. J Appl Physiol 96, 450–454. [DOI] [PubMed] [Google Scholar]
- Maddux BA, Chan A, De Filippis EA, Mandarino LJ & Goldfine ID (2006). IGF‐binding protein‐1 levels are related to insulin‐mediated glucose disposal and are a potential serum marker of insulin resistance. Diabetes Care 29, 1535–1537. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA & Fuchs CS (2006) Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24, 3527–3534. [DOI] [PubMed] [Google Scholar]
- Ngo T, Barnard RJ, Tymchuk C, Cohen P & Aronson W (2002). Effect of diet and exercise on serum insulin, IGF‐I, and IGFBP‐1 levels and growth of LNCaP cells in vitro (United States). Cancer Causes Control 13, 929–935. [DOI] [PubMed] [Google Scholar]
- Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, Nielsen J, Gehl J, Pedersen BK, Thor Straten P & Hojman P (2016) Voluntary running suppresses tumor growth through epinephrine‐ and IL‐6‐dependent NK cell mobilization and redistribution. Cell Metab 23, 554–562. [DOI] [PubMed] [Google Scholar]
- Pollak M (2008). Insulin and insulin‐like growth factor signalling in neoplasia. Nat Rev Cancer 8, 915–928. [DOI] [PubMed] [Google Scholar]
- Rundqvist H, Augsten M, Stromberg A, Rullman E, Mijwel S, Kharaziha P, Panaretakis T, Gustafsson T & Östman A (2013). Effect of acute exercise on prostate cancer cell growth. PLoS ONE 8, e67579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki N, Coustan‐Smith E, Kamiya T & Campana D (2016). Expanded and armed natural killer cells for cancer treatment. Cytotherapy 18, 1422–1434. [DOI] [PubMed] [Google Scholar]
- Sridhar R, Bond V Jr., Dunmore‐Griffith J, Cousins VM, Zhang R & Millis RM (2015). Relationship between aerobic fitness, the serum IGF‐1 profiles of healthy young adult african american males, and growth of prostate cancer cells. Am J Mens Health 11, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzl A, Kramer N, Mazza G, Rosner M, Falkenhagen D, Hengstschläger M, Schwanzer‐pfeiffer D & Dolznig H (2012). A simple and cost efficient method to avoid unequal evaporation in cellular screening assays, which restores cellular metabolic activity. Int J Appl Sci Technol 2, 9. [Google Scholar]
- Wang R, Jaw JJ, Stutzman NC, Zou Z & Sun PD (2012). Natural killer cell‐produced IFN‐γ and TNF‐α induce target cell cytolysis through up‐regulation of ICAM‐1. J Leukoc Biol 91, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren HS & Smyth MJ (1999). NK cells and apoptosis. Immunol Cell Biol 77, 64–75. [DOI] [PubMed] [Google Scholar]


