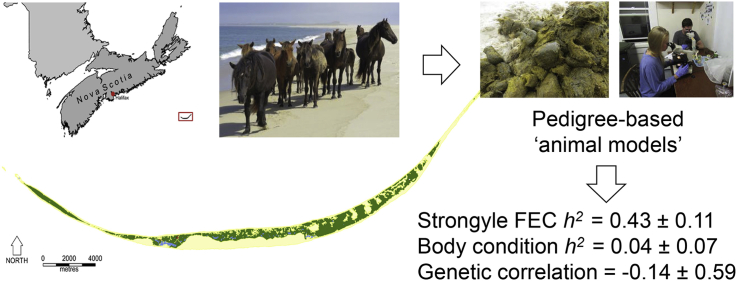
Abstract
Variability in host resistance or tolerance to parasites is nearly ubiquitous, and is of key significance in understanding the evolutionary processes shaping host-parasite interactions. While ample research has been conducted on the genetics of parasite burden in livestock, relatively little has been done in free-living populations. Here, we investigate the sources of (co)variation in strongyle nematode faecal egg count (FEC) and body condition in Sable Island horses, a feral population in which parasite burden has previously been shown to negatively correlate with body condition. We used the quantitative genetic “animal model” to understand the sources of (co)variation in these traits, and tested for impacts of an important spatial gradient in habitat quality on the parameter estimates. Although FEC is significantly heritable (h2 = 0.43 ± 0.11), there was no evidence for significant additive genetic variation in body condition (h2 = 0.04 ± 0.07), and therefore there was also no significant genetic covariance between the two traits. The negative phenotypic covariance between these traits therefore does not derive principally from additive genetic effects. We also found that both FEC and body condition increase from east to west across the island, which indicates that the longitudinal environmental gradient is not responsible for the negative phenotypic association observed between these traits. There was also little evidence to suggest that quantitative genetic parameters were biased when an individual's location along the island's environmental gradient was not incorporated into the analysis. This research provides new and important insights into the genetic basis and adaptive potential of parasite resistance in free-living animals, and highlights the importance of environmental heterogeneity in modulating host-parasite interactions in wild vertebrate systems.
Keywords: Sable island, Parasites, Animal model, Heritability, Genetic correlation, Spatial effects
Graphical abstract
Highlights
-
•
Strongyle faecal egg count and body condition are negatively correlated in Sable Island horses.
-
•
Strongyle faecal egg count is heritable (h2 = 0.43 ± 0.11) but body condition is not (h2 = 0.04 ± 0.07).
-
•
The phenotypic correlation is not primarily genetically derived.
-
•
The island's environmental gradient is not the source of the phenotypic correlation.
1. Introduction
Parasites can have wide-ranging and influential impacts upon the dynamics and evolution of host populations (Tompkins and Begon, 1999; Watson, 2013). This is the case with gastrointestinal nematodes, common and abundant parasites which affect a wide range of host species, both wild and domestic (Hoberg et al., 2001). Within domestic species, genetic variation in parasite burden has been the subject of considerable study. This has primarily been to assess the potential for selective breeding, increasingly of interest due to widespread anthelmintic resistance (Kaplan and Vidyashankar, 2012; Stear and Murray, 1994). However, subclinical disease caused by gastrointestinal nematodes is also ubiquitous in wild populations (Hoberg et al., 2001). These infections can have a number of detrimental effects on individuals, including reductions in body condition due to reduced feed intake and alterations to digestion and metabolism (Fox, 1997; Stien et al., 2002), increased stress hormone levels (Mougeot et al., 2010; Pedersen and Greives, 2008), and consequently reduced survival and fecundity (Coltman et al., 1999; Gulland et al., 1993; Gunn and Irvine, 2003; Murray et al., 1997; Stien et al., 2002). Therefore, these parasites can represent a significant selection pressure for free-living populations, and establishing the genetic basis to variation in parasite burden is vital for establishing the evolutionary consequences. Furthermore, in areas of evolutionary theory, genetic variation in resistance to parasites holds significant implications. For example, an additive genetic basis to parasite resistance is a key assumption in models of host-parasite co-evolution (Sorci et al., 1997), sexual selection (Hamilton et al., 1990), and life-history evolution (Møller, 1997).
Studies examining variation in parasite infection intensity have revealed that it varies significantly between individuals and is often highly aggregated among population subgroups or over time (Shaw et al., 1998). Some of the intrinsic and extrinsic factors influencing this variation, including sex, age, reproductive status, and habitat quality have been well established (Fox, 1992; Poulin, 1996; Wilson et al., 2002; Wood et al., 2013). However, there has been limited work examining the role of genetics in underpinning individual variation in infection intensity under natural conditions and genetic covariation with other traits (but see Brown et al., 2013; Coltman et al., 2001; Hayward et al., 2014; Smith et al., 1999; Wenzel et al., 2015), despite its importance for understanding the evolution of host responses to parasitism. This is likely due to the challenges of implementing the appropriate analyses in wild systems, and the complex interplay of processes affecting covariation among phenotypic traits. For example, (co)variation may be maintained by trade-offs (Cotter et al., 2004; Stearns, 1989). In the case of parasite burden, individuals must balance the costs of greater resistance (preventing infection) and/or tolerance (reducing the impact of infection induced damage), such as increased metabolic activity, reduced nutrient availability, and the potential for immunopathology (Colditz, 2008; Lochmiller and Deerenberg, 2000), against the cost of being parasitized. Therefore, genes underlying variation in parasite-related traits may also be associated with variation in other fitness-related traits, such as body condition, leading to a genetic correlation. Where this is the case, selection on both traits may maintain variation. Therefore, studies examining the heritability of parasite burden in wild populations, as well as investigating the causes of phenotypic correlations between parasite burden and fitness-related traits, such as body condition, will be very important for providing a comprehensive understanding of the evolution of parasite resistance or tolerance in the wild (Lynch and Walsh, 1998).
In comparison to studies of domestic animals, studying the evolutionary effects of parasites under field conditions holds significant challenges, for example in gathering relatedness and phenotypic data for large numbers of wild individuals. The quantitative genetic “animal model” revolutionised such studies by making it possible to utilise all the information contained in complex natural pedigrees and to control for a variety of environmental factors when decomposing phenotypic variation into additive genetic and other components (Kruuk, 2004). Nevertheless, challenges remain in avoiding confounding factors and accounting for the bias that can be induced when space use is heterogeneous across individuals and partially overlaps with kinship. Recently, particular focus has been on the ways that spatial effects can confound (co)variance estimates when relatives use space similarly. However, studies to date that have incorporated spatial effects into quantitative genetic analyses have yet to reach a consensus. Some have illustrated significant impacts on estimates (Stopher et al., 2012; Van Der Jeugd and McCleery, 2002), whilst others suggest this impact may be smaller than previously thought (Germain et al., 2016; Regan et al., 2017). This lack of clarity on the influence of spatial effects may suggest they are population-specific. Further investigation is warranted to determine their role and to provide more reliable estimates for quantitative genetic parameters in wild populations.
Here, we use data from the long-term individual-based study of Sable Island horses to understand whether genetic differences between females are associated with variation in the burden of gastrointestinal nematodes and body condition. We also investigate the negative correlation between nematode burden and body condition that has been previously shown for females in this population (Debeffe et al., 2016) to try to understand whether this negative phenotypic correlation is driven by a negative genetic correlation between these traits. When conducting these analyses, we also use data on individual space use to better understand the environmental determinants of variation in parasite burden and body condition, and to ensure that any similarities between relatives in their space use were accounted for when estimating quantitative genetic parameters.
2. Materials and methods
2.1. Study site and population
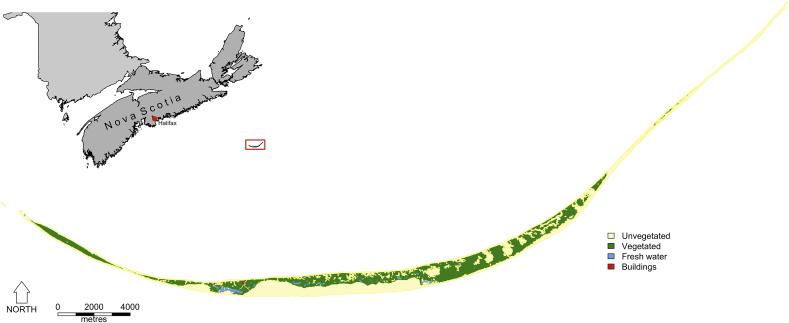
Sable Island is situated in the Atlantic Ocean, 275 km southeast of Halifax in Nova Scotia, Canada (43°55′N; −60°00′W) (Fig. 1). The island, a 49 km long emergent sandbar, has a breadth of 1.25 km at its widest point, with an overall area of approximately 32 km2. The island's north and south sides comprise of parallel sandy beaches, while the centre consists of a mature dune ecosystem. At the eastern and western tips of the island are expanses of sand running for 2–6 km. A habitat gradient occurs across the island with greater availability of freshwater, and important forage species, in the west compared to the east (Contasti et al., 2012). Permanent freshwater pools occur in the west and centre of the island, while at the eastern end the horses obtain water from temporary pools when available, or by digging down to the water table when these disappear (Rozen-Rechels et al., 2015). Vegetation covers approximately one third of the island, and consists primarily of marram grass, beach pea, sandwort and various heathland species.
Fig. 1.
Map of Sable Island, Nova Scotia, Canada, showing its position relative to the Canadian mainland and the predominant land cover types.
Horses were first introduced to the island in the mid-1700s, and have since been permanently free-ranging (Christie, 1995). Occasional further introduction and removal of individuals has occurred since, but, after government protection in 1960, the population has lived independently of human interference. The horses are the only terrestrial mammals present on the island and so live entirely free from predation and inter-specific competition, excluding the potential space competition with the large breeding population of grey seals. The mating system of feral horses is characterised by female-defence polygyny, in which males protect groups of females with which they monopolize breeding opportunities (Linklater et al., 1999). The social structure of Sable Island horses consists of bands of breeding individuals (Stallion, unrelated mares, offspring and occasional subordinate males) and bachelor groups of unmated males. Both male and female offspring disperse from their natal bands (Welsh, 1975), but dispersal between nonadjacent subdivisions of the island appears uncommon (Lucas et al., 2009), potentially resulting in relatives being clustered in space to some degree.
2.2. Data collection
Systematic yearly ground censuses of the horse population began in 2008, following a pilot study in 2007. These occur each summer between July and September, the mid-late breeding season for the horses. The island is divided into seven sections, with at least one section censused daily, allowing the whole island to be covered in a week. Adjacent sections of the island are not censused on consecutive days to avoid collecting larger amounts of data for individuals whose home ranges overlap section boundaries. Thus, we do not consistently cover the island in an east-west or west-east direction. Over the course of the field season, the island is therefore covered multiple times, ensuring all horses are recorded and multiple measurements are taken for each individual. During each census, horses are approached on foot and the position of bands or lone individuals are recorded to the nearest five metres using GPS. Researchers take multiple photographs of each horse and note individual sex, coat colour, age and any distinguishing features to allow individual identification. They also record band membership and female reproductive status (presence or absence of foal). We determine horse age using records for each individual born after the start of research in 2007. Any individuals born before the start of research are grouped and assumed to belong to a different age category. Therefore, we treat age as a factor with values ranging from 0 to 10, where 10 corresponds to all individuals born in 2006 and earlier.
2.3. Faecal egg count
Sable Island horses are parasitised by a range of gastrointestinal nematodes, and strongyle species are the primary parasites within mature individuals (Debeffe et al., 2016). We therefore restricted our analysis to large (strongylids) and small (cyathastomins) strongyle species. We used faecal egg counts (FEC) to study between-individual variation in strongyle burden. FEC is a common measure of strongyle burden, reflecting the abundance of parasites following host attrition at the larval and adult stage, which is relatively consistent over time within individuals (Debeffe et al., 2016; Scheuerle et al., 2016).
We collected faeces when freshly passed, either when opportunistically presented, or by observing individuals until defecation. We stored samples in tied-up nitrile gloves and, where possible, immediately in a cooler containing ice packs. Following transport to the laboratory, we stored all samples in a cooler or fridge until FECs were taken on the same day. Previous analysis has shown that time of collection and storage conditions do not influence FEC (Debeffe et al., 2016), therefore these factors were not included in any analyses. Strongyle species cannot be identified by egg morphology (Campbell et al., 1995), therefore counts reflect an aggregate of all species. We used a modified McMaster protocol to count strongyle eggs in the collected faeces. In a paper cup, we homogenised four grams of faeces with 26 mL of Sheather's sugar solution using a tongue depressor. We then filtered this mixture through a cheesecloth-lined funnel into a separate cup, mixed the solution again, and loaded it into two chambers of a McMaster slide (Chalex Corp., USA). We then allowed two minutes for the eggs to float to the surface before counting each slide using a compound microscope. To scale this up to eggs per gram (EPG) of faeces, we multiplied the total by 25. We do not consider foals (age <1 year) because, given that horse strongyle species take several months to produce eggs following initial infection (Lyons et al., 2011), FECs are primarily zero.
2.4. Body condition measurements
We measured female body condition from photographs using a five-point scale, ranging from emaciated to obese, developed by Carroll and Huntington (1988). The score provides a proxy for the subcutaneous fat levels on the spine, hips and ribs. Scores were only assigned when multiple clear photographs were available, and the scorer was blind to previous measurements. When separate body regions differed in score, half points were awarded (see Debeffe et al., 2016 for details). Because we only have faecal egg counts from females between 2014 and 2016, we also restricted analyses of body condition to data obtained in these years.
2.5. Pedigree information
We used a pedigree constructed from observational data, where maternal links were inferred from observations of foal suckling behaviour and paternity was inferred as the stallion of the band a mare belonged to in the year prior to foaling. The pedigree features 1012 individuals with 693 maternal links and 574 paternal links, where a link refers to an inferred parentage. When the pedigree was pruned to contain only links informative for the analysis of FEC, there were 200 maternities (123 unique mothers), 161 paternities (84 unique fathers), 36 full siblings, and 208 half siblings. In the case of body condition there were 204 maternities (124 unique mothers), 164 paternities (87 unique fathers), 36 full siblings, and 211 half siblings. The use of social information to assign pedigree relationships may influence quantitative genetic parameter estimates; however, studies suggest that results from analyses using social pedigrees are likely to be relatively robust (Charmantier and Réale, 2005; Firth et al., 2015). We do not yet know the degree of error in Sable Island horse paternity assignments, though research on other feral horse populations suggests that dominant stallions may sire between 50% (Gray et al., 2012) and 85% (Kaseda and Khalil, 1996) of offspring. Therefore, it is possible that the use of a genetic pedigree, if available, would be associated with a small change in parameter estimates.
2.6. Statistical analyses
To ensure that residuals from analyses of FEC approximated a normal distribution we log transformed FEC (measured as eggs per gram of faeces) using ln(FEC+25) in all analyses. Though it is clear that a body condition index cannot be distributed normally in reality, visual inspection of model residuals showed a reasonable approximation to a Gaussian distribution. We therefore assumed Gaussian errors for body condition as well.
We estimated genetic and environmental (co)variance components by fitting univariate and bivariate animal models using ASReml-R (Butler et al., 2007) in R version 3.4.1 (R Development Core Team, 2008). In univariate models we included random effects to partition the phenotypic variance (VP) into additive genetic (VA), permanent environmental (VPE), and residual variances (VR). In bivariate animal models we also estimated the additive genetic (COVA), permanent environmental (COVPE), and residual (COVR) covariances between FEC and body condition. Debeffe et al. (2016), using a subset of the data used here, showed that FEC decreased with age and was higher in lactating females. As a result, age and reproductive status were included as fixed effects in both the univariate and bivariate animal models. Female age was included as a ten-level factor and reproductive status as a two-level factor (with foal or without foal). Only females aged three years or over reproduced, with the proportion of females producing a foal remaining relatively consistent from age three to eight, before declining (see Fig. S1). Year (three level factor) and Julian date of measurement (covariate) were also included as fixed effects to account for temporal variation (see Figs. S2–S6 for plots of the raw data across all considered fixed effects).
We assessed the significance of the additive genetic effects using likelihood ratio tests, assuming the test statistic was distributed as a 50:50 mix of χ2 distributions with zero and one degrees of freedom (Self and Liang, 1987). Similarly, we used likelihood ratio tests to assess the significance of covariance terms, comparing a model where the covariance was estimated with a model where the covariance was fixed at zero. In this case we used a χ2 distribution with one degree of freedom. We calculated heritability (h2) as VA/VP, where VP excludes fixed effect variances, and the genetic correlation (rG) as:
2.7. Spatial effects
Sable Island is a particularly tractable system in which to account for location effects as the long, thin nature of the island means it can effectively be modelled as a one-dimensional system running from west to east. We used the median longitude value of each individual's annual sightings as an estimate of the centre of each individual's range (median within-summer band movements have been previously estimated as 2.06 km [Manning et al., 2015]), and scaled these estimates to have a mean of zero and standard deviation of one prior to analysis. We included location as a covariate and compared models including and excluding location to understand whether incorporating spatial effects influenced quantitative genetic parameters.
3. Results
Our dataset consisted of 930 faecal egg counts from 257 females (range of number of observations per individual = 1–11, median = 3), and 2479 measures of body condition (range of number of observations per individual = 1–21, median = 10) from 260 females, all aged at least one year. FEC ranged from 0 to 9575 eggs per gram (mean = 1587.12, SD = 1314.47), whilst body condition scores ranged from 0.5 to 4 (mean = 2.48, SD = 0.59). In 576 cases (214 females) a horse's FEC and body condition were measured on the same day.
3.1. Heritability estimates
We found evidence for a significant additive genetic effect on FEC (VA = 0.39 ± 0.11, χ2(0,1) = 15.56, P < 0.001; Table 1), equating to an estimated heritability of 0.43 (±0.11). The permanent environment effect accounted for a much smaller, and non-significant, portion of the total variance (VPE = 0.12 ± 0.09; χ2(0,1) = 2.08, P = 0.07). In contrast, for female body condition we found evidence for significant permanent environment effects (VPE = 0.12 ± 0.02; χ2(0,1) = 28.10, P < 0.001), but no evidence for significant additive genetic effects (VA = 0.01 ± 0.02, χ2(0,1) = 0.34, P = 0.28) (see Table 2).
Table 1.
Univariate analysis of body condition and FEC. Variance components for strongyle faecal egg count (log-transformed) and body condition in female Sable Island horses. Results shown from analyses where location was and was not included as a fixed effect. Phenotypic variance is shown after fixed effects were accounted for and is equal to the sum of additive genetic variance (VA), permanent environmental variance (VPE) and residual variance (VR). Narrow sense heritability (h2) was calculated as VA/VP, p2 is equal to VPE/VP and r2 is VR/VP. Standard errors are shown in parentheses. Significance of additive genetic and permanent environment components were calculated using likelihood ratio tests. *P < 0.05, **P < 0.01, ***P < 0.001.
| Trait | Location included | VP | VA | VPE | VR | h2 | pe2 | r2 |
|---|---|---|---|---|---|---|---|---|
| FEC (ln EPG+25) | No | 0.931 (0.066) | 0.445 (0.116)*** | 0.089 (0.086) | 0.396 (0.022) | 0.478 (0.105) | 0.096 (0.095) | 0.426 (0.034) |
| FEC (ln EPG+25) | Yes | 0.897 (0.063) | 0.385 (0.112)*** | 0.117 (0.087) | 0.395 (0.022) | 0.429 (0.108) | 0.130 (0.098) | 0.441 (0.034) |
| Body condition | No | 0.318 (0.015) | 0.017 (0.023) | 0.118 (0.025)*** | 0.183 (0.006) | 0.054 (0.072) | 0.371 (0.074) | 0.575 (0.028) |
| Body condition | Yes | 0.310 (0.015) | 0.011 (0.021) | 0.116 (0.024)*** | 0.183 (0.006) | 0.037 (0.068) | 0.373 (0.071) | 0.590 (0.028) |
Table 2.
Bivariate analysis of body condition and FEC. Phenotypic covariances (COVP), additive genetic covariances (COVA), permanent environmental covariances (COVPE) and residual covariances (COVR) are shown from models with and without location included as a fixed effect. Phenotypic covariances and correlations come from models with individual identity (not associated with the pedigree) as the only random effect. Correlations are also provided and standard errors are shown in parentheses.
| Location included | COVP | COVA | COVPE | COVR | rP | rA | rPE | rR |
|---|---|---|---|---|---|---|---|---|
| Yes | −0.113 (0.030) | −0.076 (0.060) | −0.040 (0.054) | −0.067 (0.016) | −0.414 (0.092) | −0.886 (1.087) | −0.939 (3.203) | −0.226 (0.050) |
| Noa | −0.094 (0.031) | −0.007 (0.049) | −0.056 (0.047) | −0.068 (0.016) | −0.326 (0.095) | −1.000 (6.947) | −0.842 (1.249) | −0.229 (0.050) |
Model did not converge normally and thus estimates should be treated with some caution.
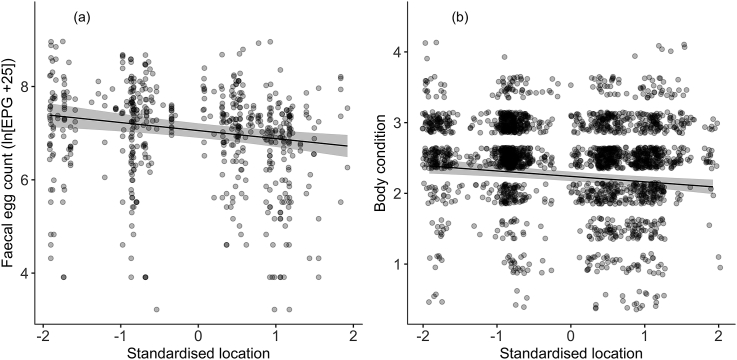
An individual's median annual location was a significant predictor of both FEC and body condition (FEC: βlocation = −0.17 (±0.05) P < 0.001; body condition: βlocation = −0.07 (±0.02) P < 0.001), indicating that values of both traits decreased from west to east (Fig. 2). Incorporating location as a fixed effect was associated with very little change in estimated variance components for either trait (Table 1). The estimated heritability of FEC decreased from 0.48 (±0.11) to 0.43 (±0.11), whilst, the estimated heritability of body condition decreased from 0.05 (±0.07) to 0.04 (±0.07) when location was included. Similarly, the permanent environment components showed little change, increasing slightly from 0.1 (±0.1) to 0.13 (±0.1) in the case of FEC and, remaining at 0.37 (±0.07) in the case of body condition.
Fig. 2.
Predicted relationship between an individual's annual location and a) faecal egg count (measured as the natural logarithm of eggs per gram (EPG) + 25) and b) body condition. Location is scaled to a mean of 0 and standard deviation of 1, therefore 0 represents the centre of the island with −2 at the far west and 2 at the far east. The fitted line comes from the full univariate animal model in each case. In both cases, overlap between points is represented by darker point colour. In 2b. points have been jittered along the y axis to ease visualisation.
3.2. Covariation between FEC and condition
As in Debeffe et al. (2016), we found a significant negative phenotypic correlation between body condition and FEC (rP = −0.41 ± 0.09, χ2(df=1) = 16.03, P < 0.001). As expected, in the absence of genetic variation for condition, we found no significant genetic covariance between body condition and FEC (rA = −0.89 ± 1.09, χ2(df=1) = 1.61, P = 0.20). We also found no evidence for a significant permanent environmental covariance (rPE = −0.94 ± 3.20, χ2(df=1) = 0.58, P = 0.45), as expected given that VPE for FEC was not significantly different from zero.
4. Discussion
In this study, we assessed the basis of (co)variation in FEC and body condition in females of an unmanaged ungulate population. Previous work on this population demonstrated that these traits vary significantly between individuals and are negatively correlated at the phenotypic level (Debeffe et al., 2016). Here, we have further shown that (i) intensity of infection is significantly heritable, but body condition is not, (ii) that this lack of significant heritability for body condition precludes any significant genetic correlation between the two traits and iii) that the negative phenotypic correlation is primarily driven by unknown environmental factors not captured by the island's west-east environmental gradient.
We estimated a heritability of 0.43 for strongyle FEC, when location effects were accounted for. There have been relatively few studies examining the heritability of parasite burden in wild or naturalised mammal populations and, to our knowledge, we provide only the second estimate of heritability for this trait in equids, and the first in an unmanaged horse population. Our estimate is significantly higher than those found by Kornaś et al. (2015) in pure-bred Arabian horses. Kornas et al. showed an increase in heritability with age; however, both the estimate for young horses (0.04 ± 0.02) and older ones (0.21 ± 0.04) was markedly lower than found in this study. Similarly, our heritability estimate is markedly higher than estimates of strongyle FEC from studies of St. Kilda Soay sheep, another feral ungulate population. Coltman et al. (2001) estimated a heritability of between 0.11 and 0.14 for FEC in female Soay sheep, whilst Beraldi et al. (2007) found no evidence for genetic variation in adult strongyle FEC in that population.
Research has illustrated that heritabilities tend to be higher when environmental conditions are favourable (Charmantier and Garant, 2005), and therefore we would expect lower heritability estimates for traits measured in populations such as Sable Island horses where food, water, and shelter are often scarce. Nevertheless, our heritability estimate for FEC falls within the range of 0.3–0.4 that is commonly expected for FEC in domestic species (Sonstegard and Gasbarre, 2001). However, comparing h2 for FEC obtained in different studies is complicated by differences in FEC measurement technique and precision, which influence residual variance and hence h2, as well as models used to generate estimates (Wilson, 2008). For instance, we must acknowledge that our heritability estimated may be upwardly biased by our inability to account for maternal effects. Due to a lack of data, models including a maternal effect term would not converge, and thus we were unable to estimate the additive genetic variance after accounting for maternal effects. Indeed, there is some evidence that maternal characteristics, such as maternal age (Hayward et al., 2010), affect offspring parasite burdens and other quantitative genetic studies of FEC in wild animals have shown that maternal effects can account for a significant portion of the phenotypic variance and their inclusion can be associated with a decrease in the size of the additive genetic component (Coltman et al., 2001). However, models excluding yearlings (the group in which any maternal effects are expected to be greatest) provided similar estimates to models in which they were included (results not shown).
In contrast to FEC, we found that body condition was not significantly heritable. This was a result of effectively no additive genetic variation, rather than due to higher environmental variance. Nevertheless, the large proportion of variance attributed to the permanent environment term indicates that non-(additive) genetic differences between individuals lead to significant between-individual variation in body condition. This variation is likely to derive from many sources. For example, diet or weather conditions during early development (Lindström, 1999), the degree of coinfection (Jolles et al., 2008), variation in gut microbiota (Hayes et al., 2010; Vrieze et al., 2010), stress (Ould and Welch, 1980), or foraging behaviour (Hutchings et al., 2003) may all have long-term influences on body condition. As such, being able to model individual body condition trajectories may shed more light on the heritability of body condition score in this population. However, unfortunately, data limitations mean we are currently unable to do so.
There are a number of potential causes for the lack of significant additive genetic variation in body condition that we have shown here. First, traits closely linked with fitness are expected to have lower heritability as strong selection erodes variance (Merilä and Sheldon, 1999). Condition is often used as a proxy for fitness (Barnett et al., 2015) and, as a component of individual quality in Sable Island horses, condition has been shown to link with reproductive success (Debeffe et al., 2017). Therefore, it is possible that strong selection imposed by the harsh conditions on Sable Island has eroded the additive genetic variance for body condition in this population. However, our result contrasts to other studies of wild populations that have found significant additive genetic variation in body condition (e.g. Gosler and Harper, 2000; Merilä et al., 2001; Jensen et al., 2003), and where the additive genetic variation in condition has actually been found to be higher in low quality environments (Merilä et al., 1999). Second, although body condition scores, such as the one we have used here, have been shown to correlate with fat content in horses (Gentry et al., 2004; Henneke et al., 1983), there has been substantial debate surrounding the reliability of indirect measures of body condition (Green, 2001; Schulte-Hostedde et al., 2005). Therefore, it is possible that analyses using more direct measures of body composition, for example, by modelling weight conditional on skeletal size, would give different results. We must also acknowledge that although our heritability estimate for body condition was not significantly different to zero, this may arise because we were unable to estimate a small additive genetic component with the accuracy necessary to reach statistical significance. Nonetheless, such a result would still support our conclusion that the heritability of body condition is very low.
As expected, due to the lack of significant additive genetic variance in body condition and of significant permanent environmental variance in FEC, we found no significant genetic or permanent environmental correlation between body condition and strongyle FEC. All correlations were negative indicating that individuals with high FEC tended to have lower body condition, but our result suggests that the negative phenotypic correlation reported by (Debeffe et al., 2016), and that we have also reported here, is largely driven by environmental factors because we found no evidence for a significant genetic correlation. Although we acknowledge that our ability to accurately partition genetic and environmental sources of covariation was limited. We are also not yet able to assess the direction of the causality when it comes to the environmental covariance between FEC and body condition. Indeed, the association between body condition and FEC is likely to be bidirectional (Koski and Scott, 2001). For example, depending on their energy reserves, for which condition is a proxy, hosts may regulate how they invest in resistance, relative to self-maintenance (Houston et al., 2007; Warburton et al., 2016). Therefore, individuals in good condition may invest more into reducing infection intensity than those with low reserves. Similarly, high FEC reduces body condition, which in turn reduces the ability of the host to fight parasites, thus increasing FEC and further reducing condition (Beldomenico et al., 2008). To distinguish between these would require experimental manipulations, such as food supplementation or parasite treatment.
Negative correlations between gastrointestinal infection intensity and condition have been shown in other wild populations including red deer (Irvine et al., 2006) and reindeer (Stien et al., 2002). However, to our knowledge, no one has investigated the genetic or environmental basis of this correlation. There has been a study examining the covariance between FEC and body size (both body mass and hindleg length) in Soay sheep in which they found that parasite resistance was positively genetically correlated with body size (Coltman et al., 2001). This suggested that there was no trade-off between parasite resistance and this morphometric trait, and instead that individuals with lower FEC tended to reach larger body sizes. Similarly, another study in the Soay sheep system found no evidence for a trade-off between parasite tolerance and body weight (Hayward et al., 2014). Further studies investigating the basis of phenotypic correlations between measures of parasite resistance/tolerance and fitness-related traits, such as body condition and body size, will be necessary to provide a complete understanding of the evolution of traits in natural populations. As more data become available for the Sable Island horse population we will be able to better disentangle the determinants of phenotypic correlations, such as that between FEC and body condition.
By incorporating information on individual horse locations, we have shown that both FEC and body condition tend to decrease from west to east across the island. Thus, the environmental gradient across the island generates a positive, rather than a negative, covariance between the two traits. The western end of Sable Island provides better access to water and forage species relative to the east, and population density in these areas is higher (Contasti et al., 2012). Therefore, the location effects on body condition may be caused by differences in food availability or energy expended in efforts to obtain water (Contasti et al., 2012; Rozen-Rechels et al., 2015). The environmental gradient across the island is also likely to explain the association between individual location and FEC. The higher density of faeces in western areas and the fact that the developmental success of nematodes is higher when soil moisture is high (Van Der Wal et al., 2000) may mean that there is a higher larval density and increased infection pressure in the west compared to the east. The fact that inbreeding is also higher in the west (Lucas et al., 2009) and that reduced heterozygosity may increase susceptibility to parasites (Coltman et al., 1999) might also contribute to the location trend in FEC. Overall, our results further suggest that the Sable Island horse population is significantly influenced by spatial heterogeneity in the environment and that the strong environmental gradient is likely to influence the dynamics of host-parasite interactions.
Location effects on feral horse parasite burden, driven by variation in salinity, have been found in other populations (Rubenstein and Hohmann, 1989), but only a small number of studies have considered location effects when conducting quantitative genetic analyses (e.g. Van Der Jeugd and McCleery, 2002; Stopher et al., 2012, Regan et al., 2017; Germain et al., 2016). In agreement with recent studies (Regan et al., 2017; Germain et al., 2016), we found that including location effects had little impact on estimates of additive genetic variation. Bias is expected when the environment is heterogeneous and there is natal philopatry because under these conditions relatives are likely to experience similar environmental conditions (Regan et al., 2017). As discussed above, the environment on Sable Island is highly heterogeneous, but given that both males and females disperse it is likely that there is little natal philopatry in this population (Welsh, 1975). Therefore, it is perhaps unsurprising that we found very little change in our (co)variance estimates when we accounted for individual location. Nonetheless, the results from previous quantitative genetic analyses where information on individual space use has been incorporated have been very inconsistent. As a result, further studies like ours will be necessary to better understand the bias that may be induced when spatial effects cannot or are not accounted for.
Our results provide insight into the potential for future evolutionary change in the Sable Island horse population. Additive genetic variation is required for traits to respond to selection, and therefore our results suggest that faecal egg count, but not body condition, will be able to evolve under selection in this population. The population-level impacts of nematodes have been attributed to their impacts on body condition (e.g. Irvine et al., 2006). Therefore, despite genetic variation in FEC, if the population is limited in its ability to evolve higher condition, this may prevent adaptation to reduce the impacts of these subclinical infections. However, it is also possible that the key impacts of strongyle nematodes in this population act through other mechanisms, for example, reduced reproductive ability or susceptibility to other diseases. Independently of the consideration of parasites, the lack of ‘evolvability’ for body condition may mean that if environmental conditions deteriorate on the island, for example due to climate change, this may present a challenge to population persistence.
In this study, we considered the variation in, and covariation between, FEC and body condition in a horse population living independently of human interference. Our results show the presence of significant additive genetic variation in resistance, which is an important assumption in models of host-parasite co-evolution (Sorci et al., 1997), sexual selection (Hamilton et al., 1990), and life-history evolution (Møller, 1997). This result is also of interest for those involved in selective breeding, because it suggests that in the absence of anthelminthic drugs, a significant degree of variation occurs for resistance in horses. Furthermore, our findings show that body condition, a proxy for fitness, has low genetic variation within this population. This has potential implications for the evolution and survival of this isolated population. This work provides a number of avenues for future research. For example, to better understand the evolutionary potential of parasite resistance or tolerance in wild populations, further studies examining the strength of selection on parasite burden and the trade-offs between measures of parasite burden and a wider range of other traits will be necessary. Furthermore, genome wide association (GWAS) studies could be used to provide insights into the genetics underlying the variation found.
Declarations of interest
None.
Funding
The Sable Island Horse Project is/has been funded by the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grant No. 371535-2009 to P.D.M.), the Canada Foundation for Innovation (Leaders Opportunity Grant No. 25046 to P.D.M.), and a Royal Society International Exchange grant (J.P. and P.D.M.), and the L. David Dubé and Heather Ryan Veterinary Health and Research Fund. J.P. received support from the University of Calgary NSERC-CREATE Host-Parasite Interactions Training Program and the Leverhulme Trust. S.G. received support from the University of Exeter M.Sc. program in Conservation and Biodiversity.
Acknowledgements
We want to thank the many students, research assistants, and volunteers, that have helped to collect the data used here by being part of the Sable Island Horse Project. We also thank Fisheries and Oceans Canada (DFO), Canada Coast Guard, the Bedford Institute of Oceanography (DFO Science), Environment and Climate Change Canada, Parks Canada Agency, Maritime Air Charters Limited (Sable Aviation), and Sable Island Station (Meteorological Service of Canada) for their in-kind and logistical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.03.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Barnett C.A., Suzuki T.N., Sakaluk S.K., Thompson C.F. Mass-based condition measures and their relationship with fitness: in what condition is condition? J. Zool. 2015;296:1–5. doi: 10.1111/jzo.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldomenico P.M., Telfer S., Gebert S., Lukomski L., Bennett M., Begon M. Poor condition and infection: a vicious circle in natural populations. Proc. R. Soc. B Biol. Sci. 2008;275:1753–1759. doi: 10.1098/rspb.2008.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraldi D., McRae A.F., Gratten J., Pilkington J.G., Slate J., Visscher P.M., Pemberton J.M. Quantitative trait loci (QTL) mapping of resistance to strongyles and coccidia in the free-living Soay sheep (Ovis aries) Int. J. Parasitol. 2007;37:121–129. doi: 10.1016/j.ijpara.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Brown E.A., Pilkington J.G., Nussey D.H., Watt K.A., Hayward A.D., Tucker R., Graham A.L., Paterson S., Beraldi D., Pemberton J.M., Slate J. Detecting genes for variation in parasite burden and immunological traits in a wild population: testing the candidate gene approach. Mol. Ecol. 2013;22:757–773. doi: 10.1111/j.1365-294X.2012.05757.x. [DOI] [PubMed] [Google Scholar]
- Butler D.G., Cullis B.R., Gilmour A.R., Gogel B.J. Queensland Department of Primary Industries and Fisheries; Brisbane, Australia: 2007. Analysis of Mixed Models for S Language Environments: ASReml-R Reference Manual, Release 2. [Google Scholar]
- Campbell A.J.D., Gasser R.B., Chilton N.B. Differences in a ribosomal DNA sequence of Strongylus species allows identification of single eggs. Int. J. Parasitol. 1995;25:359–365. doi: 10.1016/0020-7519(94)00116-6. [DOI] [PubMed] [Google Scholar]
- Carroll C.L., Huntington P.J. Body condition scoring and weight estimation of horses. Equine Vet. J. 1988;20:41–45. doi: 10.1111/j.2042-3306.1988.tb01451.x. [DOI] [PubMed] [Google Scholar]
- Charmantier A., Garant D. Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B Biol. Sci. 2005;272:1415–1425. doi: 10.1098/rspb.2005.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmantier A., Réale D. How do misassigned paternities affect the estimation of heritability in the wild? Mol. Ecol. 2005;14:2839–2850. doi: 10.1111/j.1365-294X.2005.02619.x. [DOI] [PubMed] [Google Scholar]
- Christie B. Pottersfield Press; Porters Lake, Nova Scotia: 1995. The Horses of Sable Island. [Google Scholar]
- Colditz I.G. Six costs of immunity to gastrointestinal nematode infections. Parasite Immunol. 2008;30:63–70. doi: 10.1111/j.1365-3024.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- Coltman D.W., Pilkington J., Kruuk L.E.B., Wilson K., Pemberton J.M. Positive genetic correlation between parasite resistance and body size in a free-living ungulate population. Evolution. 2001;55:2116–2125. doi: 10.1111/j.0014-3820.2001.tb01326.x. [DOI] [PubMed] [Google Scholar]
- Coltman D.W., Pilkington J.G., Smith J.A., Pemberton J.M. Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution. 1999;53:1259–1267. doi: 10.1111/j.1558-5646.1999.tb04538.x. [DOI] [PubMed] [Google Scholar]
- Contasti A.L., Tissier E.J., Johnstone J.F., McLoughlin P.D. Explaining spatial heterogeneity in population dynamics and genetics from spatial variation in resources for a large herbivore. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0047858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter S.C., Kruuk L.E.B., Wilson K. Costs of resistance: genetic correlations and potential trade-offs in an insect immune system. J. Evol. Biol. 2004;17:421–429. doi: 10.1046/j.1420-9101.2003.00655.x. [DOI] [PubMed] [Google Scholar]
- Debeffe L., McLoughlin P.D., Medill S.A., Stewart K., Andres D., Shury T., Wagner B., Jenkins E., Gilleard J.S., Poissant J. Negative covariance between parasite load and body condition in a population of feral horses. Parasitology. 2016;143:983–997. doi: 10.1017/S0031182016000408. [DOI] [PubMed] [Google Scholar]
- Debeffe L., Poissant J., McLoughlin P.D. Individual quality and age but not environmental or social conditions modulate costs of reproduction in a capital breeder. Ecol. Evol. 2017;7:5580–5591. doi: 10.1002/ece3.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J.A., Hadfield J.D., Santure A.W., Slate J., Sheldon B.C. The influence of nonrandom extra-pair paternity on heritability estimates derived from wild pedigrees. Evolution. 2015;69:1336–1344. doi: 10.1111/evo.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. Epidemiology of nematode infections of Soay sheep (Ovis aries L.) on St Kilda. Parasitology. 1992;105:481–492. doi: 10.1017/s0031182000074667. [DOI] [PubMed] [Google Scholar]
- Fox M.T. Pathophysiology of infection with gastrointestinal nematodes in domestic ruminants: recent developments. Vet. Parasitol. 1997;72:285–308. doi: 10.1016/s0304-4017(97)00102-7. [DOI] [PubMed] [Google Scholar]
- Gentry L.R., Thompson D.L., Gentry G.T., Del Vecchio R.P., Davis K.A., Del Vecchio P.M. The relationship between body condition score and ultrasonic fat measurements in mares of high versus low body condition. J. Equine Vet. Sci. 2004;24:198–203. [Google Scholar]
- Germain R.R., Wolak M.E., Arcese P., Losdat S., Reid J.M. Direct and indirect genetic and fine-scale location effects on breeding date in song sparrows. J. Anim. Ecol. 2016;85:1613–1624. doi: 10.1111/1365-2656.12575. [DOI] [PubMed] [Google Scholar]
- Gosler G., Harper D.G.C. Assessing the heritability of body condition in birds: a challenge exemplified by the great tit Parus major L. (Aves) Biol. J. Linn. Soc. 2000;71:103–117. [Google Scholar]
- Gray M.E., Cameron E.Z., Peacock M.M., Thain D.S., Kirchoff V.S. Are low infidelity rates in feral horses due to infanticide? Behav. Ecol. Sociobiol. 2012;66:529–537. [Google Scholar]
- Green A.J. Mass/length residuals: measures of body condition or generators of spurious results? Ecology. 2001;82:1473–1483. [Google Scholar]
- Gulland F.M.D., Albon S.D., Pemberton J.M., Moorcroft P.R., Clutton-Brock T.H. Parasite-associated polymorphism in a cyclic ungulate population. Proc. Biol. Sci. 1993;254:7–13. doi: 10.1098/rspb.1993.0119. [DOI] [PubMed] [Google Scholar]
- Gunn A., Irvine R.J. Subclinical parasitism and ruminant foraging strategies: a review. Wildl. Soc. Bull. 2003;31:117–126. [Google Scholar]
- Hamilton W.D., Axelrod R., Tanese R. Sexual reproduction as an adaptation to resist parasites (a review) Proc. Natl. Acad. Sci. 1990;87:3566–3573. doi: 10.1073/pnas.87.9.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K.S., Bancroft A.J., Goldrick M., Portsmouth C., Roberts I.S., Grencis R.K. Exploitation of the intestinal microflora by the parasitic nematode trichuris muris. Science. 2010;328:1391–1394. doi: 10.1126/science.1187703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A.D., Garnier R., Watt K.A., Pilkington J.G., Grenfell B.T., Matthews J.B., Pemberton J.M., Nussey D.H., Graham A.L. Heritable, heterogeneous, and costly resistance of sheep against nematodes and potential feedbacks to epidemiological dynamics. Am. Nat. 2014;184:S58–S76. doi: 10.1086/676929. [DOI] [PubMed] [Google Scholar]
- Hayward A.D., Pilkington J.G., Pemberton J.M., Kruuk L.E.B. Maternal effects and early-life performance are associated with parasite resistance across life in free-living Soay sheep. Parasitology. 2010;137:1261–1273. doi: 10.1017/S0031182010000193. [DOI] [PubMed] [Google Scholar]
- Henneke D.R., Potter G.D., Kreider J.L., Yeates B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983;15:371–372. doi: 10.1111/j.2042-3306.1983.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Hoberg E.P., Kocan A.A., Rickard L.G. Gastrointestinal strongyles in wild ruminants. In: Samual W.M., Pybus M.J., Kocan A.A., editors. Parasitic Diseases of Wild Mammals. Iowa State University Press; Ames, Iowa, USA: 2001. [Google Scholar]
- Houston A.I., McNamara J.M., Barta Z., Klasing K.C. The effect of energy reserves and food availability on optimal immune defence. Proc. R. Soc. B Biol. Sci. 2007;274:2835–2842. doi: 10.1098/rspb.2007.0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings M.R., Athanasiadou S., Kyriazakis I., J. Gordon I. Can animals use foraging behaviour to combat parasites? Proc. Nutr. Soc. 2003;62:361–370. doi: 10.1079/pns2003243. [DOI] [PubMed] [Google Scholar]
- Irvine R.J., Corbishley H., Pilkington J.G., Albon S.D. Low-level parasitic worm burdens may reduce body condition in free-ranging red deer (Cervus elaphus) Parasitology. 2006;133:465–475. doi: 10.1017/S0031182006000606. [DOI] [PubMed] [Google Scholar]
- Jensen H., Sæther B.E., Ringsby T.H., Tufto J., Griffith S.C., Ellegren H. Sexual variation in heritability and genetic correlations of morphological traits in house sparrow (Passer domesticus) J. Evol. Biol. 2003;16:1296–1307. doi: 10.1046/j.1420-9101.2003.00614.x. [DOI] [PubMed] [Google Scholar]
- Jolles A.E., Ezenwa V.O., Etienne R.S., Turner W.C., Olff H. Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology. 2008;89:2239–2250. doi: 10.1890/07-0995.1. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Vidyashankar A.N. An inconvenient truth: global worming and anthelmintic resistance. Vet. Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Kaseda Y., Khalil A.M. Harem size reproductive success of stallions in Misaki feral horses. Appl. Anim. Behav. Sci. 1996;47:163–173. [Google Scholar]
- Kornaś S., Sallé G., Skalska M., David I., Ricard A., Cabaret J. Estimation of genetic parameters for resistance to gastro-intestinal nematodes in pure blood Arabian horses. Int. J. Parasitol. 2015;45:237–242. doi: 10.1016/j.ijpara.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Koski K.G., Scott M.E. Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral. Annu. Rev. Nutr. 2001;21:297–321. doi: 10.1146/annurev.nutr.21.1.297. [DOI] [PubMed] [Google Scholar]
- Kruuk L.E.B. Estimating genetic parameters in natural populations using the “animal model”. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:873–890. doi: 10.1098/rstb.2003.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. [DOI] [PubMed] [Google Scholar]
- Linklater W.L., Cameron E.Z., Minot E.O., Stafford K.J. Stallion harassment and the mating system of horses. Anim. Behav. 1999;58:295–306. doi: 10.1006/anbe.1999.1155. [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L., Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Lucas Z.L., McLoughlin P.D., Coltman D.W., Barber C. Multiscale analysis reveals restricted gene flow and a linear gradient in heterozygosity for an island population of feral horses. Can. J. Zool. 2009;87:310–316. [Google Scholar]
- Lynch M., Walsh B. Sinauer; Sunderland: 1998. Genetics and Analysis of Quantitative Traits. [Google Scholar]
- Lyons E.T., Kuzmina T.A., Tolliver S.C., Collins S.S. Observations on development of natural infection and species composition of small strongyles in young equids in Kentucky. Parasitol. Res. 2011;109:1529–1535. doi: 10.1007/s00436-011-2460-y. [DOI] [PubMed] [Google Scholar]
- Manning J.A., Medill S.A., Mcloughlin P.D. Climate fluctuations interact with local demography and resources to predict spatially dynamic adult sex ratios in a megaherbivore. Oikos. 2015;124:1132–1141. [Google Scholar]
- Merilä J., Kruuk L.E.B., Sheldon B.C. Cryptic evolution in a wild bird population. Nature. 2001;412:76–79. doi: 10.1038/35083580. [DOI] [PubMed] [Google Scholar]
- Merilä J., Przyblo R., Sheldon B. Genetic variation and natural selection on blue tit body condition in different environments. Genet. Res. 1999;73:165–176. [Google Scholar]
- Merilä J., Sheldon B.C. Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity. 1999;83:103–109. doi: 10.1046/j.1365-2540.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- Møller A. Parasitism and the evolution of host life history. In: Clayton D., Moore J., editors. Host-parasite Evolution: General Principles and Avian Models. Oxford University Press; Oxford, United Kingdom: 1997. [Google Scholar]
- Mougeot F., Martínez-Padilla J., Bortolotti G.R., Webster L.M.I., Piertney S.B. Physiological stress links parasites to carotenoid-based colour signals. J. Evol. Biol. 2010;23:643–650. doi: 10.1111/j.1420-9101.2009.01926.x. [DOI] [PubMed] [Google Scholar]
- Murray D.L., Cary J.R., Keith L.B., Cary J.R., Keith L.B. Interactive effects of sublethal nematodes and nutritional status on snowshoe hare vulnerability to predation. J. Anim. Ecol. 1997;66:250–264. [Google Scholar]
- Ould P., Welch H.E. The effect of stress on the parasitism of mallard ducklings by Echinuria uncinata (Nematoda: Spirurida) Can. J. Zool. 1980;58:228–234. [Google Scholar]
- Pedersen A.B., Greives T.J. The interaction of parasites and resources cause crashes in a wild mouse population. J. Anim. Ecol. 2008;77:370–377. doi: 10.1111/j.1365-2656.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- Poulin R. Helminth growth in vertebrate hosts: does host sex matter? Int. J. Parasitol. 1996;26:1311–1315. doi: 10.1016/s0020-7519(96)00108-7. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2008. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Regan C.E., Pilkington J.G., Bérénos C., Pemberton J.M., Smiseth P.T., Wilson A.J. Accounting for female space sharing in St. Kilda Soay sheep (Ovis aries) results in little change in heritability estimates. J. Evol. Biol. 2017;30:96–111. doi: 10.1111/jeb.12990. [DOI] [PubMed] [Google Scholar]
- Rozen-Rechels D., van Beest F.M., Richard E., Uzal A., Medill S.A., Mcloughlin P.D. Density-dependent, central-place foraging in a grazing herbivore: competition and tradeoffs in time allocation near water. Oikos. 2015;124:1142–1150. [Google Scholar]
- Rubenstein D.I., Hohmann M.E. Parasites and social behavior of island feral horses. Oikos. 1989;55:312–320. [Google Scholar]
- Scheuerle M.C., Stear M.J., Honeder A., Becher A.M., Pfister K. Repeatability of strongyle egg counts in naturally infected horses. Vet. Parasitol. 2016;228:103–107. doi: 10.1016/j.vetpar.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Schulte-Hostedde A.I., Zinner B., Millar J.S., Hickling G.J. Restitution of mass-size residuals: validating body condition indices. Ecology. 2005;86:155–163. [Google Scholar]
- Self S.G., Liang K.-Y. Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandard conditions. J. Am. Stat. Assoc. 1987;82:605–610. [Google Scholar]
- Shaw D.J., Grenfell B.T., Dobson A.P. Patterns of macroparasite aggregation in wildlife host populations. Parasitology. 1998;117:597–608. doi: 10.1017/s0031182098003448. [DOI] [PubMed] [Google Scholar]
- Smith J.A., Wilson K., Pilkington J.G., Pemberton J.M. Heritable variation in resistance to gastro-intestinal nematodes in an unmanaged mammal population. Proc. R. Soc. B Biol. Sci. 1999;266:1283–1290. doi: 10.1098/rspb.1999.0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonstegard T.S., Gasbarre L.C. Genomic tools to improve parasite resistance. Vet. Parasitol. 2001;101:387–403. doi: 10.1016/s0304-4017(01)00563-5. [DOI] [PubMed] [Google Scholar]
- Sorci G., Møller A.P., Boulinier T. Genetics of host-parasite interactions. Trends Ecol. Evol. 1997;12:196–200. doi: 10.1016/s0169-5347(97)01056-2. [DOI] [PubMed] [Google Scholar]
- Stear M.J., Murray M. Genetic resistance to parasitic disease: particularly of resistance in ruminants to gastrointestinal nematodes. Vet. Parasitol. 1994;54:161–176. doi: 10.1016/0304-4017(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Trade-offs in life-history evolution. Funct. Ecol. 1989;3:259–268. [Google Scholar]
- Stien A., Irvine R.J., Ropstad E., Halvorsen O., Langvatn R., Albon S.D. The impact of gastrointestinal nematodes on wild reindeer: experimental and cross-sectional studies. J. Anim. Ecol. 2002;71:937–945. [Google Scholar]
- Stopher K.V., Walling C.A., Morris A., Guinness F.E., Clutton-Brock T.H., Pemberton J.M., Nussey D.H. Shared spatial effects on quantitative genetic parameters: accounting for spatial autocorrelation and home range overlap reduces estimates of heritability in wild red deer. Evolution. 2012;66:2411–2426. doi: 10.1111/j.1558-5646.2012.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins D.M., Begon M. Parasites can regulate wildlife populations. Parasitol. Today. 1999;15:311–313. doi: 10.1016/s0169-4758(99)01484-2. [DOI] [PubMed] [Google Scholar]
- Van Der Jeugd H.P., McCleery R. Effects of spatial autocorrelation, natal philopatry and phenotypic plasticity on the heritability of laying date. J. Evol. Biol. 2002;15:380–387. [Google Scholar]
- Van Der Wal R., Irvine J., Stien A., Shepherd N., Albon S.D. Faecal avoidance and the risk of infection by nematodes a natural population of reindeer. Oecologia. 2000;124:19–25. doi: 10.1007/s004420050020. [DOI] [PubMed] [Google Scholar]
- Vrieze A., Holleman F., Zoetendal E.G., De Vos W.M., Hoekstra J.B.L., Nieuwdorp M. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53:606–613. doi: 10.1007/s00125-010-1662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton E.M., Pearl C.A., Vonhof M.J. Relationships between host body condition and immunocompetence, not host sex, best predict parasite burden in a bat-helminth system. Parasitol. Res. 2016;115:2155–2164. doi: 10.1007/s00436-016-4957-x. [DOI] [PubMed] [Google Scholar]
- Watson M.J. What drives population-level effects of parasites? Meta-analysis meets life-history. Int. J. Parasitol. Parasites Wildl. 2013;2:190–196. doi: 10.1016/j.ijppaw.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh D. Dalhousie University; 1975. Population, Behavioural and Grazing Ecology of the Horses of Sable Island. [Google Scholar]
- Wenzel M.A., James M.C., Douglas A., Piertney S.B. Genome-wide association and genome partitioning reveal novel genomic regions underlying variation in gastrointestinal nematode burden in a wild bird. Mol. Ecol. 2015;24:4175–4192. doi: 10.1111/mec.13313. [DOI] [PubMed] [Google Scholar]
- Wilson A.J. Why h2 does not always equal VA/VP? J. Evol. Biol. 2008;21:647–650. doi: 10.1111/j.1420-9101.2008.01500.x. [DOI] [PubMed] [Google Scholar]
- Wilson K., Bjørnstad O.N., Dobson A., Merler S., Poglayen G., Randolph S., Read A., Skorpin A. Heterogeneities in macroparasite infections: patterns and processes. In: Hudson P., Rizzoli A., Grenfell B., Heesterbeek H., Dobson A., editors. The Ecology of Wildlife Diseases. Oxford University Press; Oxford, United Kingdom: 2002. [Google Scholar]
- Wood E.L., Matthews J.B., Stephenson S., Slote M., Nussey D.H. Variation in fecal egg counts in horses managed for conservation purposes: individual egg shedding consistency, age effects and seasonal variation. Parasitology. 2013;140:115–128. doi: 10.1017/S003118201200128X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.