Abstract
Chimeric antigen receptor T-cell (CAR T-cell) therapy has been shown to be clinically effective for managing a variety of hematological cancers. However, CAR T-cell therapy is associated with multiple adverse effects, including neurotoxicity and cytokine release syndrome (CRS). CRS arises from massive cytokine secretion and can be life-threatening, but it is typically managed with an anti-IL-6Ra mAb or glucocorticoid administration. However, these treatments add to a patient's medication burden and address only the CRS symptoms. Therefore, alternative strategies that can prevent CRS and neurotoxicity associated with CAR T-cell treatment are urgently needed. Here, we explored a therapeutic route aimed at preventing CRS rather than limiting its consequences. Using a cytokine-profiling assay, we show that granulocyte–macrophage colony-stimulating factor (GMCSF) is a key CRS-promoting protein. Through a combination of in vitro experiments and gene-editing technology, we further demonstrate that antibody-mediated neutralization or TALEN-mediated genetic inactivation of GMCSF in CAR T-cells drastically decreases available GMCSF and abolishes macrophage-dependent secretion of CRS biomarkers, including monocyte chemoattractant protein 1 (MCP-1), interleukin (IL) 6, and IL-8. Of note, we also found that the genetic inactivation of GMCSF does not impair the antitumor function or proliferative capacity of CAR T-cells in vitro. We conclude that it is possible to prevent CRS by using “all-in-one” GMCSF-knockout CAR T-cells. This approach may eliminate the need for anti-CRS treatment and may improve the overall safety of CAR T-cell therapies for cancer patients.
Keywords: immunotherapy, cancer therapy, gene knockout, gene transfer, T-cell, CAR T-cell therapy, cytokine release syndrome (CRS), granulocyte–macrophage colony-stimulating factor (GMCSF), interleukin signaling
Introduction
CAR T-cell therapy3 has been shown to be clinically effective in the treatment of a variety of cancer types (1–4). However, the widespread use of CAR T-cell therapy is complicated by potential side effects observed in ∼75% of treated patients (5, 6). Almost all patients treated with CD19 CAR T-cells will develop cytokine release syndrome (CRS), manifested by a high-grade fever and hypotension. About 30% of treated patients progress to more severe forms of CRS (grade 3 or 4), characterized by organ failure, neurotoxic effects (7, 8), and eventually death, if not controlled. Recent studies that used cytokine profiling of patients with CRS following CAR T-cell treatment (5, 6) shed light on the cytokines that are differentially expressed in low- versus high-grade CRS. These studies showed a correlation between CRS outcome and elevated MCP-1, IL-6, and other cytokines. In addition, one study using a patient-derived xenograft model of pediatric ALL, and an in vitro co-culture system showed that monocytes were the primary source of IL-6 increase after CAR T-cell treatment. Elevated IL-6 has been reported in other inflammatory diseases (9), and two additional studies showed that IL-1B was critical in mediating CRS in murine models (10, 11).
Clinical studies also demonstrated that blocking IL-6 signaling can mitigate CRS symptoms. Tocilizumab, a mAb that acts as an IL-6 receptor antagonist, was reported to be effective at treating severe CRS (12–14). In addition, the use of corticosteroids has been shown to alleviate the neurotoxic symptoms of CRS in some patients (13, 15). Although these treatments are promising, they failed to prevent CRS-associated death in some cases (16, 17), and treatment with an antibody can cause further toxicities such as infection and hepatic dysfunction (18). Thus, alternative strategies are needed to control CRS symptoms better or even to prevent CRS in patients receiving CAR T-cell therapies. In this study, we demonstrate that GMCSF secretion by tumor-activated CAR T-cells plays a key role in monocyte activation and in the secretion of CRS biomarkers. Armed with this knowledge, we developed a straightforward and drug-free strategy to prevent CRS during CAR T-cell therapy through the development of GMCSF-knockout CAR T-cells.
Results and discussion
Cytokine profiling assay to decipher the contribution of CAR T-cells, tumor cells, and monocytes in secreting CRS mediators
To study the complex interplay between CAR T-cells, tumor cells, and monocytes that generate CRS biomarkers, we developed a transwell co-culture assay with engineered CAR T-cells obtained though targeted insertion of an anti-CD22 CAR cassette under the control of TCR (Figs. S1 and S2) (19, 39). Using multiplex human inflammation cytokine measurements, we observed obvious differences in the cytokines produced by CAR T-cells and monocytes in the context of the transwell assay (Fig. S3). Indeed, CAR T-cells produced TNFα, GMCSF, IL-8, IL-10, soluble CD40L, and CD25, whereas the monocytes produced IL-6, PTX3, and MCP-1 (Fig. S3). No cytokines were detected in the absence of tumor cells or in the absence of T-cells (Fig. S5 and data not shown). Thus, upon tumor engagement, CAR T-cells secrete pro-inflammatory cytokines such as GMCSF and IL-8, which promote the release of the major CRS biomarker IL-6 as well as MCP-1 and IL-8, cytokines that were previously shown to be monocyte-dependent (9). CRS biomarker release did not depend on physical contact between CAR T-cells and monocytes, suggesting that it may be possible to prevent CRS by inhibiting soluble factors involved in macrophage activation.
Neutralization of GMCSF decreases monocyte-dependent inflammatory cytokines
To explore a novel therapeutic strategy for preventing the biogenesis of CRS, we focused on GMCSF because GMCSF is specifically up-regulated in a CAR-dependent manner and because it is known as a pro-inflammatory cytokine. Therefore, GMCSF can be either neutralized or deleted in CAR T-cells even before CRS develops. We observed GMCSF secretion as early as 4 h after co-incubation of CAR T-cells with tumor cells, with no other cytokines detected under the experimental conditions (Fig. S4 and data not shown). GMCSF is also known to increase the proliferation and activation of macrophages and blood monocytes, increasing their pro-inflammatory properties during infection (20, 21). In addition, GMCSF increases macrophages' responsiveness to CSF-1 (macrophage CSF), further enhancing the proliferation of resident macrophages in tissues. Finally, the GMCSF receptor has been shown to be highly expressed by microglia, brain macrophages, and astrocytes, suggesting that these cells can respond to the cytokines. Although GMCSF levels are low in normal healthy individuals, activated lymphocytes produce elevated GMCSF levels under pathologic conditions such as infection (22).
To verify that GMCSF plays a key role in monocyte activation, we first treated human monocytes with recombinant GMCSF protein at various doses and measured IL-6 induction, as a surrogate readout for inflammation. We observed that adding GMCSF alone promoted IL-6 induction starting at 4 h and peaking at 24 h, although no such induction was observed with other recombinant proteins such as TNFα and IL-8 (Fig. S4). Interestingly, neutralizing either GMCSF or GMCSF Ra on monocytes inhibited GMCSF-mediated IL-6 secretion, suggesting that monocyte activation occurs specifically via the GMCSF/GMCSF Ra receptor axis (Fig. S4). We observed no effect on IL-6 induction with the vehicle control or the isotype Ab control. Therefore, our results indicate that GMCSF produced by CAR T-cells promotes CRS–mediator secretion. These findings warrant testing the neutralization Ab against GMCSF as a therapeutic approach.
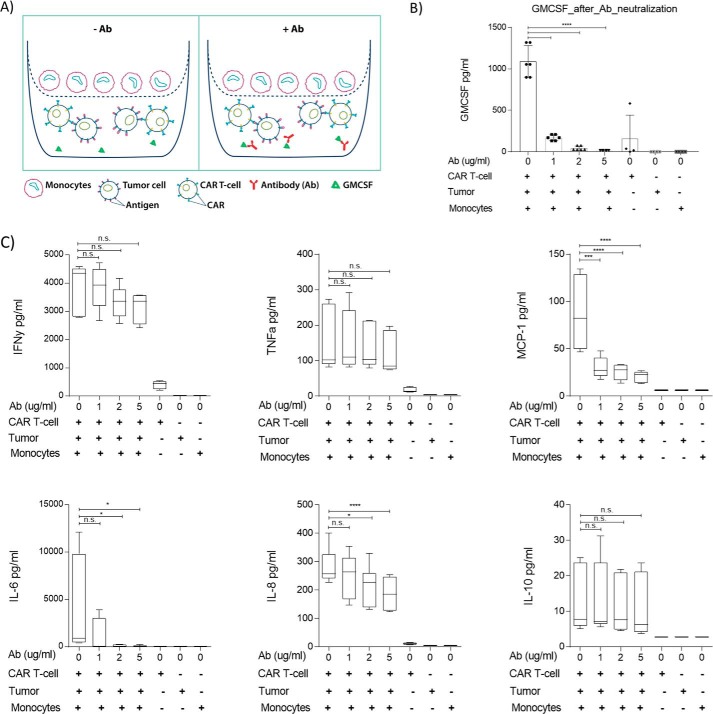
To assess the effect of the GMCSF neutralizing Ab on pro-inflammatory cytokine secretion, we performed a transwell assay followed by a multiplex cytokine assay. We first added different amounts of Ab to the lower chambers of the transwell apparatus to assess its effect on GMCSF secreted by CAR T-cells. As expected, the neutralizing Ab was able to decrease GMCSF secretion at low doses, with barely any GMCSF detected at the higher doses tested (Fig. 1). We observed an average of 1,090 pg/ml of GMCSF released without any antibody treatment, which was reduced to 170, 43, and 16 pg/ml of GMCSF upon treatment with 1, 2, and 5 μg/ml of the Ab, respectively. This suggests that CAR T-cell–induced GMCSF can be completely neutralized by higher antibody doses. No significant Ab-mediated toxicity was observed. After validating that GMCSF was neutralized, we performed multiplex assays to analyze different cytokines. No significant effect of neutralizing Ab on the production of key T-cell cytokines such as IFNγ, TNFα, and IL-10 was observed, even at higher doses. However, a significant reduction in pro-inflammatory cytokines such as IL-6, MCP-1, and IL-8 was observed in an Ab dose-dependent manner (Fig. 1). MCP-1 was found to be significantly lower after treatment with even the lowest tested antibody dose (1 μg/ml). IL-6 and IL-8 were reduced at 1 μg/ml, and a statistically significant reduction was seen at the 2 and 5 μg/ml doses. In addition, the cytokine release was antigen-dependent, as CD22+ KO Raji cells showed no induction of any cytokines, and similar findings were confirmed in Daudi cells, another CD22+ tumor cell line (Fig. S5). These findings suggest that the neutralization of GMCSF can be successfully used to inhibit IL-6 secretion, which is known to cause CRS, and to reduce the production of other pro-inflammatory factors such as MCP-1 and IL-8, cytokines with known roles in immune cell trafficking that have been shown to be elevated in high-grade CRS (23, 24).
Figure 1.
GMCSF antibody neutralization prevents cytokine release by monocytes. A, schematic showing transwell assay system with and without GMCSF antibody. Supernatants were collected after a 16-h incubation and analyzed by ELISA (B) or by Multiplex cytokine FACS-based assays (C). The results are summarized as the average of three independent experiments. One-way ANOVA was used for statistical analysis followed by Dunnett's multiple comparisons test. ****, p < 0.0001; ***, p = 0.0001; *, p < 0.05; n.s., not significant.
GMCSF KO CAR T-cells as a therapeutic approach to prevent CRS
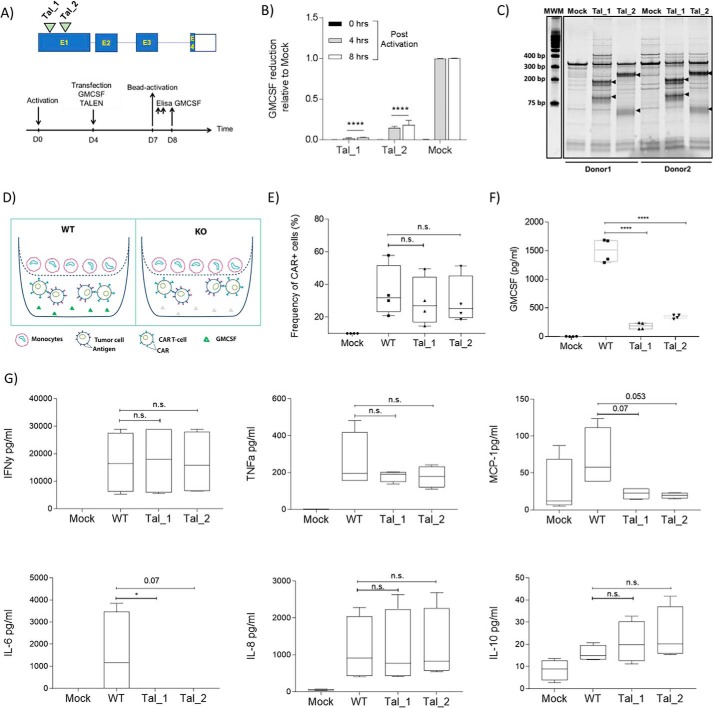
To translate these findings into a relevant next-generation CAR T-cell product, we sought to disrupt the GMCSF gene in CAR T-cells. This strategy could have several advantages over the Ab neutralization approach. First, GMCSF reduction in GMCSF KO CAR T-cells prior to CRS onset may be beneficial for patients' safety, as it will avoid any antibody-mediated side effects and because the GMCSF antibody dosing schedule is not yet fully established. In addition, it would avoid Ab treatment, which adds substantial costs and treatment time in a managed care setting. In contrast, the genetic inactivation of GMCSF could be easily incorporated into a CAR T-cell production scheme, with relatively little cost increase. Therefore, we designed and screened two different TALEN molecules targeting exon1 of the GMCSF gene (Tal_1 and Tal_2) in two independent T-cell donors (Fig. 2, A and B). Both TALEN constructs reduced GMCSF levels significantly (98 and 86%, respectively) compared with mock-transfected cells. Consistently, T7E1 analysis confirmed GMCSF knockout at the genomic level (Fig. 2C).
Figure 2.
Engineered GMCSF KO CAR T-cells thwart cytokine release by monocytes. A, upper panel, schematic showing TALEN target location relative to GMCSF genomic organization; lower panel, experimental design for screening TALEN activity in T-cells. B, GMCSF-specific ELISA was performed on supernatants collected at the indicated time points after activation. The average of two independent donors is plotted. C, T7 endonuclease I (T7E1) assay showing editing of GMCSF at a genomic level with two different TALEN reagents (Tal_1 and Tal_2) performed in two independent donors. DNA was collected 7 days post-transfection of GMCSF TALEN mRNA (2 μg/million cells) into bead-activated T-cells. D, schematic showing transwell assay system performed with WT GMCSF CAR T-cells versus GMCSF KO CAR T-cells. Mock-treated cells were engineered in the absence of the AAV6 carrying the CAR construct. E, expression of CAR on TCR a/b KO cells. After 60 h, the cells were stained with Qbend10 anti-CD34 antibody and analyzed using flow cytometry. No significant differences in CAR frequency were seen in the different groups. F, ELISA confirmation of GMCSF reduction in GMCSF KO CAR T-cells in two independent clones, measured post-16 h of activation. G, multiplex cytokine FACS-based assay showing changes in different cytokines GMCSF KO CAR T-cells, measured post-16 h. D–F, results are displayed as average of four independent donor experiments. B, two-way ANOVA was used for statistical analysis followed by Dunnett's multiple comparisons test. D–F, one-way ANOVA was used for statistical analysis followed by Dunn's multiple comparisons test. ****, p < 0.0001; *, p < 0.05; n.s., not significant.
We then used these two independent TALEN molecules to engineer GMCSF KO CAR T-cells. We opted to use both reagents in parallel to confirm the validity of our approach by independent constructs. Utilizing the anti-CD22 CAR expression cassette targeting the TCR locus described earlier, we successfully engineered GMCSF KO CAR T-cells by co-transfecting TRAC and GMCSF TALEN mRNAs. We observed no differences in CAR expression among different groups (Fig. 2, D and E). However, GMCSF KO resulted in a 90% reduction in GMCSF secretion by CAR T-cells after 16 h of co-incubation with tumor cells (Fig. 2F). To confirm that GMCSF KO did not impair the proliferation and anti-tumor function of CAR T-cells, we then performed a tumor-mediated proliferation assay and a 24-h anti-tumor assay, respectively (Fig. S6). We observed no change in either the proliferation capacity or anti-tumor properties of CAR T-cells after GMCSF KO in four independent donors treated with two different GMCSF TALEN constructs, suggesting that GMCSF KO does not impair the normal functions of CAR T-cells. We also carried out a serial killing assay to challenge GMCSF KO CAR T-cells with daily doses of tumor cells for six consecutive days. This assay showed similar results, with no impaired activity of GMCSF KO CAR T-cells compared with GMCSF wildtype (WT) cells performed at different effector to target (E/T) cell ratios (Fig. S6). Finally, we observed no difference in the expansion of GMCSF KO CD4 CAR T-cells and GMCSF KO CD8 CAR T-cells (Fig. S6).
Because GMCSF KO CAR T-cells proliferate as well as GMCSF WT CAR T-cells and exhibit similar anti-tumor properties, we then subjected these cells to the transwell assay described above. Similar to what was observed in the GMCSF Ab neutralization experiment (Fig. 1), we observed that GMCSF KO CAR T-cells suppressed the secretion of inflammatory cytokines by monocytes. Consistent with our activity tests, GMCSF KO did not impair the production of key CAR T-cell cytokines such as IFNγ. In addition, both TALEN treatments led to a significant reduction in IL-6 and MCP produced by monocytes (Fig. 2G). GMCSF KO also led to a decrease in TNFα, albeit one that was not statistically significant, and no change in IL-8 compared with CAR T-cells with WT GMCSF (Fig. 2G). These data further confirm that GMCSF inhibition in CAR T-cells attenuates the release of several inflammatory cytokines by monocytes and that GMCSF KO can be used to engineer safer but equally effective CAR T-cell–based therapies.
Interestingly, while we were preparing our manuscript, another study (25) showed that GMCSF neutralization via specific antibody treatment was able to reduce CAR T-cell–mediated neurotoxicity. Using Crispr/Cas9-mediated gene-editing approach, Sterner et al. (25) showed that knocking out GMCSF in anti-CD19 CAR T-cells prevented CRS symptoms such as weight loss and encephalopathy in a primary ALL xenograft. In an independent in vivo model, the authors showed improved overall survival in mice with NALM6 xenografts treated with anti-CD19 GMCSF KO CAR T-cells. Therefore, this study demonstrates the therapeutic potential of GMCSF KO in vivo, further corroborating our findings regarding the CAR T-cell–mediated CRS. In addition, Sentman et al. (26) has shown previously in a syngeneic mouse model that NKG2D CAR T-cells generated from germline GMCSF knockout mice were less inflammatory. Therefore, our data on anti-CD22 CAR T-cells along with published data on anti-CD19 and NKG2D CAR T-cells indicate that GMCSF-mediated CRS promotion is unlikely to be an antigen- and/or CAR-dependent phenomenon. Moreover, the role of GMCSF in mediating inflammation is well established in several immune and autoimmune diseases (27). Earlier studies measuring cytokines in patients with inflammatory disorders found elevated GMCSF levels in synovial fluid and blood (28, 29). Other studies reported elevated GMCSF in the cerebrospinal fluid of patients with active multiple sclerosis and showed that GMCSF activates resident microglial cells within the CNS, promotes blood–brain barrier breakdown, and enables inflammation by other immune cells (30–33). As a result, inhibiting GMCSF signaling, either via a mAb against GMCSF (namilumab) (34, 35) or via an antibody against GMCSF receptor (mavrilumumab), has been tried in multiple clinical trials against inflammatory diseases (36, 37). Similarly, our work points toward a strategy that could be used to prevent the side effects of CAR T-cell therapy.
This work is a substantial improvement over current symptomatic treatments for CAR T-cell–initiated CRS. GMCSF KO CAR T-cells would simply prevent CRS in patients receiving CAR T-cell therapy. Currently, CAR T-cell therapy is indicated for cancer patients who have previously received treatment; these individuals have a high disease burden and are in relatively poor health. For these patients, high-grade CRS could be life-threatening. Thus, preventing this side effect rather than treating its symptoms will provide a significant benefit. Furthermore, “all-in-one” GMCSF KO CAR T-cells could be easily generated during the manufacturing process due to the efficiency of TALEN-mediated gene inactivation. For example, we observed ∼90% efficiency in GMSCF KO with the two-tested TALEN; this inactivation is sufficient to reduce monocyte-mediated cytokine release. However, before entering the clinic, it is imperative to evaluate the genomic integrity of these engineered cells. In addition, it will be important to monitor the GMCSF serum levels in preventing CRS in the clinic, particularly in the presence of other GMCSF-responsive cells such as endothelial or hepatic cells. We have shown that the identities of monocyte-specific cytokines observed in our transwell assay in vitro are in agreement with cytokines observed in patients with CRS (5–7). This dataset corroborates the findings of other researchers in identifying monocytes as the main source of CRS mediator release in humans and in mouse models (9–11). However, we cannot exclude that other cells could participate in this phenomenon in vivo. For example, human endothelial and fibroblast cells possess the GMCSF receptor (20, 38) and thus could respond to GMCSF secreted by CAR T-cells. To this point, it is also important to evaluate the effect of GMCSF KO on the anti-tumor properties of CAR T-cells in several antigen model systems. Despite the fact that the T-cells do not possess a functional receptor for GMCSF and thus do not rely on GMCSF for proliferation and anti-tumor activity, it must be determined whether GMCSF KO CAR T-cells are equally potent in in vivo conditions in the presence of other immune cells. One study in mice did show some effect of GMCSF on the anti-tumor properties of mouse CAR T-cells (26).
In conclusion, we describe a strategy to engineer safer “all-in-one” CAR T-cells that confer lesser cytokine-mediated toxicity. Our results demonstrate that GMCSF neutralization, either through a specific antibody treatment or through TALEN-mediated GMCSF KO in the CAR T-cells, is sufficient to reduce inflammatory cytokine release from monocytes in vitro. Interestingly, inhibiting GMCSF, which is able to cross the blood–brain barrier, also mitigated CAR T-cell–mediated neurotoxicity in vivo (25). Thus, our data present a strategy for engineering safer CAR T-cells lacking GMCSF. This strategy could be easily incorporated into the CAR T-cell manufacturing process and may ease the burden both on physicians and patients entering CAR T-cell clinical trials for a variety of cancers.
Experimental procedures
Cells
Cryopreserved human PBMCs were acquired from ALLCELLS (catalog no. PB006F), and human monocytes were acquired from STEMCELL Technologies (catalog no. 70035.1). Both PBMCs and monocytes were cultured in X-vivo-15 media (Lonza, catalog no. BE04-418Q), containing IL-2 (Miltenyi Biotec, catalog no. 130-097-748) and human serum AB (Seralab, catalog no. GEM-100-318). Raji CD22 WT, Raji CD22 KO, and Daudi cells were cultured in RPMI 1640 media supplemented with 10% v/v FBS (Gibco, catalog no. 10437036) and 5% v/v penicillin and streptomycin.
Antibodies and reagents
Human T-activator CD3/CD28 (Life Technologies, Inc., catalog no. 11132D) was used to activate T-cells. CAR T-cells were stained using CD34 antibody QBEND10-APC (R&D Systems, catalog no. FAB7227A). Monocyte phenotyping was performed using antibodies against human CD14, CD11b, and CD16 from Miltenyi Biotec (catalog nos. 130-110-524, 130-110-552, and 130-113-389, respectively). GMCSF neutralization antibody was purchased from R&D Systems (catalog no. MAB215). Human recombinant proteins GMCSF, IL-8, and TNFα were purchased from R&D Systems (catalog nos. 215-GM and 208-IL-010) and PeproTech (catalog no. 50-813-404), respectively. Human ELISA kits for GMCSF, IFNγ, IL-6, and TNFα were obtained from R&D Systems (catalog nos. DGM00, DIF50, H600C, and DTA00D, respectively). LEGENDplex cytokine assays (13-plex), with human inflammation panels 1 and 2, were obtained from the BioLegend (catalog nos. 740118 and 740775, respectively).
Targeted integration of CAR in primary T-cells
The targeted integration at TRAC was performed as follows. PBMC cells were first thawed, washed, resuspended, and cultivated in X-vivo-15 complete media (X-vivo-15, 5% AB serum, 20 ng/ml IL-2). One day later, the cells were activated with the Dynabeads® human T activator CD3/CD28 (25 μl of beads/1E6 CD3 positive cells) and cultivated at a density of 1E6 cells/ml for 3 days in X-vivo complete media at 37 °C in the presence of 5% CO2. The cells were then split in fresh complete media and transduced/transfected the next day according to the following procedure. On the day of transduction–transfection, the cells were first de-beaded by magnetic separation (EasySep), washed twice in Cytoporation buffer T (BTX Harvard Apparatus, Holliston, MA), and resuspended at a final concentration of 28E6 cells/ml in the same solution. The cell suspension was mixed with 5 μg of mRNA encoding TRAC TALEN® arms (see Table 1 for sequences) with or without GMCSF TALEN (see Table 1 for sequences) in a final volume of 200 μl. Transfection was performed using Pulse Agile Technology (BTX Harvard Apparatus). The electroporated cells were immediately transferred to a 12-well plate containing 1 ml of prewarmed X-vivo-15 serum-free media and incubated for 37 °C for 15 min. The cells were then concentrated to 8E6 cells/ml in 250 μl of the same media in the presence of AAV6 particles (multiplicity of infection = 3E5 vg/cells) comprising the donor matrices in 48-well regular-treated plates. After 2 h of culture at 30 °C, 250 μl of Xvivo-15 media supplemented by 10% AB serum and 40 ng/ml IL-2 was added to the cell suspension, and the mix was incubated 24 h in the same culture conditions. One day later, the cells were seeded at 1E6 cells/ml in complete X-vivo-15 media and cultivated at 37 °C in the presence of 5% CO2.
Table 1.
TALEN target sequences used in this study
Left and right binding sites are indicated in uppercase, and spacers are indicated in lowercase.
| TALEN | Sequence targeted |
|---|---|
| TRAC | TTGTCCCACAGATATCCagaaccctgaccctgCCGTGTACCAGCTGAGA |
| GMCSF_Tal_1 | TGTGGCTGCAGAGCCTGctgctcttgggCACTGTGGCCTGCAGCA |
| GMCSF_Tal_2 | TCCTGAACCTGAGTAGAgacactgctgcTGAGATGGTAAGTGAGA |
Transwell assay
Transwell assays were performed using anti-CD22 CAR T-cells (GMCSF WT of KO) from multiple donors, co-cultured with tumor cells (bottom chamber) and human CD14+ monocytes (top chamber), and separated by a polystyrene membrane with a pore size of 0.4 μm. Briefly, 1E5 CAR T-cells and 5E4 tumor cells were incubated with 1E5 monocytes for various time points in the absence or presence of GMCSF antibody at increasing concentrations. The supernatant was collected after 16 h, unless stated otherwise, to measure cytokines using a BioLegend Human Inflammation 13-plex kit or ELISA.
The CD14+ human monocytes used in this assay were acquired from STEMCELL Technologies. Approximately 1 h prior to the experiment, the cells were thawed at 37 °C in a water bath, and after a brief spin at 300 × g for 5 min, the cells were resuspended and counted. For the transwell experiment, the cells were suspended in X-vivo media supplemented with 5% v/v human AB serum, the same media used for CAR T-cells suspension. This quick transition (∼1 h) between thawing and starting the experiment prevented any differentiation of monocytes into any other lineages.
Serial killing assay
To assess the antitumor activity of the engineered CAR T-cells, a serial killing assay was performed according to Ref. 19 using a suspension of 2.5E5 Raji-luc tumor cells mixed with CAR T-cells at variable E/T ratios (5:1, 3.5:1, 2.5:1, and 1:1) in a total volume of 1 ml of Xvivo media supplemented with 5% AB serum.
Statistical analysis
Statistical analysis was performed using Prism 6 (GraphPad Software) using either one-way or two-way ANOVA for comparisons wherever appropriate. p value significance was calculated using post-test Bonferroni or Dunnett's multiple comparisons test.
Author contributions
M. S. conceptualization; M. S., L. P., and J. V. data curation; M. S., L. P., and J. V. formal analysis; M. S. methodology; M. S. writing-original draft; M. S., P. D., L. P., and J. V. writing-review and editing; P. D., L. P., and J. V. supervision; S. D., L. P., and J. V. validation; J. V. investigation.
Supplementary Material
Acknowledgment
We thank Clementine Bonnin for assisting with the graphics included in this report.
The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S6.
- CAR T-cell
- chimeric antigen receptor T-cell
- CRS
- cytokine release syndrome
- GMCSF
- granulocyte macrophage–colony-stimulating factor
- ANOVA
- analysis of variance
- IFNγ
- interferon γ
- TNFα
- tumor necrosis factor α
- IL
- interleukin
- Ab
- antibody
- PBMC
- peripheral blood mononuclear cell
- ALL
- acute lymphoblastic leukemia
- TCR
- T-cell receptor
- AAV6
- adeno-associated virus type 6
- vg
- viral genome.
References
- 1. Neelapu S. S., Locke F. L., Bartlett N. L., Lekakis L. J., Miklos D. B., Jacobson C. A., Braunschweig I., Oluwole O. O., Siddiqi T., Lin Y., Timmerman J. M., Stiff P. J., Friedberg J. W., Flinn I. W., Goy A., et al. (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grupp S. A., Kalos M., Barrett D., Aplenc R., Porter D. L., Rheingold S. R., Teachey D. T., Chew A., Hauck B., Wright J. F., Milone M. C., Levine B. L., and June C. H. (2013) Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maude S. L., Laetsch T. W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M. R., Stefanski H. E., Myers G. D., Qayed M., De Moerloose B., Hiramatsu H., Schlis K., Davis K. L., et al. (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Porter D. L., Levine B. L., Kalos M., Bagg A., and June C. H. (2011) Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teachey D. T., Lacey S. F., Shaw P. A., Melenhorst J. J., Maude S. L., Frey N., Pequignot E., Gonzalez V. E., Chen F., Finklestein J., Barrett D. M., Weiss S. L., Fitzgerald J. C., Berg R. A., Aplenc R., et al. (2016) Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 6, 664–679 10.1158/2159-8290.CD-16-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gust J., Hay K. A., Hanafi L. A., Li D., Myerson D., Gonzalez-Cuyar L. F., Yeung C., Liles W. C., Wurfel M., Lopez J. A., Chen J., Chung D., Harju-Baker S., Özpolat T., Fink K. R., et al. (2017) Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 10.1158/2159-8290.CD-17-0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fitzgerald J. C., Weiss S. L., Maude S. L., Barrett D. M., Lacey S. F., Melenhorst J. J., Shaw P., Berg R. A., June C. H., Porter D. L., Frey N. V., Grupp S. A., and Teachey D. T. (2017) Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit. Care Med. 45, e124–e131 10.1097/CCM.0000000000002053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonifant C. L., Jackson H. J., Brentjens R. J., and Curran K. J. (2016) Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 3, 16011 10.1038/mto.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh N., Hofmann T. J., Gershenson Z., Levine B. L., Grupp S. A., Teachey D. T., and Barrett D. M. (2017) Monocyte lineage–derived IL-6 does not affect chimeric antigen receptor T-cell function. Cytotherapy 19, 867–880 10.1016/j.jcyt.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giavridis T., van der Stegen S. J. C., Eyquem J., Hamieh M., Piersigilli A., and Sadelain M. (2018) CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade letter. Nat. Med. 24, 731–738 10.1038/s41591-018-0041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M., Sanvito F., Ponzoni M., Doglioni C., Cristofori P., Traversari C., Bordignon C., Ciceri F., Ostuni R., Bonini C., et al. (2018) Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 10.1038/s41591-018-0036-4 [DOI] [PubMed] [Google Scholar]
- 12. Frey N. V., Levine B. L., Lacey S. F., Grupp S. A., Maude S. L., Schuster S. J., Shaw P., Hwang W.-T., Wasik M. A., Obstfeld A., Leung M., Shen A., Ericson S. G., Melenhorst J. J., June C. H., et al. (2014) Refractory cytokine release syndrome in recipients of chimeric antigen receptor (CAR) T cells. Blood 124, 2296 [Google Scholar]
- 13. Frey N. (2017) Cytokine release syndrome: who is at risk and how to treat. Best Pract. Res. Clin. Haematol. 30, 336–340 10.1016/j.beha.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 14. Schuster S. J., Svoboda J., Chong E. A., Nasta S. D., Mato A. R., Anak Ö., Brogdon J. L., Pruteanu-Malinici I., Bhoj V., Landsburg D., Wasik M., Levine B. L., Lacey S. F., Melenhorst J. J., Porter D. L., et al. (2017) Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377, 2545–2554 10.1056/NEJMoa1708566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang X. Y., Sun Y., Zhang A., Hu G. L., Cao W., Wang D. H., Zhang B., and Chen H. (2016) Third-generation CD28/4–1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol. BMJ Open 6, e013904 10.1136/bmjopen-2016-013904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee D. W., Gardner R., Porter D. L., Louis C. U., Ahmed N., Jensen M., Grupp S. A., and Mackall C. L. (2014) How I treat current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davila M. L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S. S., Stefanski J., Borquez-Ojeda O., Olszewska M., Qu J., Wasielewska T., He Q., Fink M., Shinglot H., et al. (2014) Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra25 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones G., and Ding C. (2010) Tocilizumab: a review of its safety and efficacy in rheumatoid arthritis. Clin. Med. Insights Arthritis Musculoskeletal Disord. 3, 81–89 10.4137/CMAMD.S4864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valton J., Guyot V., Boldajipour B., Sommer C., Pertel T., Juillerat A., Duclert A., Sasu B. J., Duchateau P., and Poirot L. (2018) A versatile safeguard for chimeric antigen receptor T-cell immunotherapies. Sci. Rep. 8, 8972 10.1038/s41598-018-27264-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Becher B., Tugues S., and Greter M. (2016) GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity 45, 963–973 10.1016/j.immuni.2016.10.026 [DOI] [PubMed] [Google Scholar]
- 21. Chen B. D., Clark C. R., and Chou T. H. (1988) Granulocyte/macrophage colony-stimulating factor stimulates monocyte and tissue macrophage proliferation and enhances their responsiveness to macrophage colony-stimulating factor. Blood 71, 997–1002 [PubMed] [Google Scholar]
- 22. Vogel D. Y., Kooij G., Heijnen P. D., Breur M., Peferoen L. A., van der Valk P., de Vries H. E., Amor S., and Dijkstra C. D. (2015) GM-CSF promotes migration of human monocytes across the blood brain barrier. Eur. J. Immunol. 45, 1808–1819 10.1002/eji.201444960 [DOI] [PubMed] [Google Scholar]
- 23. Russo R. C., Garcia C. C., Teixeira M. M., and Amaral F. A. (2014) The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 10, 593–619 10.1586/1744666X.2014.894886 [DOI] [PubMed] [Google Scholar]
- 24. Deshmane S. L., Kremlev S., Amini S., and Sawaya B. E. (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29, 313–326 10.1089/jir.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterner R. M., Sakemura R., Cox M. J., Yang N., Khadka R. H., Forsman C. L., Hansen M. J., Jin F., Ayasoufi K., Hefazi M., Schick K. J., Walters D. K., Ahmed O., Chappell D., Sahmoud T., et al. (2019) GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood 133, 697–709 10.1182/blood-2018-10-881722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sentman M.-L., Murad J. M., Cook W. J., Wu M.-R., Reder J., Baumeister S. H., Dranoff G., Fanger M. W., and Sentman C. L. (2016) Mechanisms of acute toxicity in NKG2D chimeric antigen receptor T cell–treated mice. J. Immunol. 197, 4674–4685 10.4049/jimmunol.1600769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wicks I. P., and Roberts A. W. (2016) Targeting GM-CSF in inflammatory diseases. Nat. Rev. Rheumatol. 12, 37–48 10.1038/nrrheum.2015.161 [DOI] [PubMed] [Google Scholar]
- 28. Williamson D. J., Begley C. G., Vadas M. A., and Metcalf D. (1988) The detection and initial characterization of colony-stimulating factors in synovial fluid. Clin. Exp. Immunol. 72, 67–73 [PMC free article] [PubMed] [Google Scholar]
- 29. Xu W. D., Firestein G. S., Taetle R., Kaushansky K., and Zvaifler N. J. (1989) Cytokines in chronic inflammatory arthritis. II. Granulocyte–macrophage colony-stimulating factor in rheumatoid synovial effusions. J. Clin. Invest. 83, 876–882 10.1172/JCI113971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fiehn C., Wermann M., Pezzutto A., Hüfner M., and Heilig B. (1992) GM-CSF plasma concentrations in rheumatoid arthritis, systemic lupus erythematosus and spondyloarthropathy. Z. Rheumatol. 51, 121–126 [PubMed] [Google Scholar]
- 31. Okada Y., Wu D., Trynka G., Raj T., Terao C., Ikari K., Kochi Y., Ohmura K., Suzuki A., Yoshida S., Graham R. R., Manoharan A., Ortmann W., Bhangale T., Denny J. C., et al. (2014) Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506, 376–381 10.1038/nature12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ponomarev E. D., Shriver L. P., Maresz K., Pedras-Vasconcelos J., Verthelyi D., and Dittel B. N. (2007) GM-CSF production by autoreactive T cells is required for the activation of Microglial cells and the onset of experimental autoimmune encephalomyelitis. J. Immunol. 178, 39–48 10.4049/jimmunol.178.1.39 [DOI] [PubMed] [Google Scholar]
- 33. King I. L., Dickendesher T. L., and Segal B. M. (2009) Circulating Ly-6C + myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood 113, 3190–3197 10.1182/blood-2008-07-168575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huizinga T. W., Batalov A., Stoilov R., Lloyd E., Wagner T., Saurigny D., Souberbielle B., and Esfandiari E. (2017) Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res. Ther. 19, 53 10.1186/s13075-017-1267-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Papp K. A., Gooderham M., Jenkins R., Vender R., Szepietowski J. C., Wagner T., Hunt B., Souberbielle B., and NEPTUNE investigators. (2018) GM-CSF as a therapeutic target in psoriasis: randomised, controlled investigation using namilumab–a specific, human anti-GM-CSF monoclonal antibody. Br. J. Dermatol. 2018, 10.1111/bjd.17195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burmester G. R., Feist E., Sleeman M. A., Wang B., White B., and Magrini F. (2011) Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann. Rheumat. Dis. 70, 1542–1549 10.1136/ard.2010.146225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamilton J. A. (2015) GM-CSF as a target in inflammatory/autoimmune disease: current evidence and future therapeutic potential. Expert Rev. Clin. Immunol. 11, 457–465 10.1586/1744666X.2015.1024110 [DOI] [PubMed] [Google Scholar]
- 38. Postiglione L., Montagnani S., Riccio A., Ladogana P., Salzano S., Vallefuoco L., and Rossi G. (1998) Expression of GM-CSF receptor and “in vitro” effects of GM-CSF on human fibroblasts. Life Sci. 63, 327–336 10.1016/S0024-3205(98)00281-1 [DOI] [PubMed] [Google Scholar]
- 39. Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S. J., Hamieh M., Cunanan K. M., Odak A., Gönen M., and Sadelain M. (2017) Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 10.1038/nature21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.