Abstract
Pluripotent stem cells (PSCs) are highly proliferative cells characterized by robust metabolic demands to power rapid division. For many years considered a passive component or “passenger” of cell-fate determination, cell metabolism is now starting to take center stage as a driver of cell fate outcomes. This review provides an update and analysis of our current understanding of PSC metabolism and its role in self-renewal, differentiation, and somatic cell reprogramming to pluripotency. Moreover, we present evidence on the active roles metabolism plays in shaping the epigenome to influence patterns of gene expression that may model key features of early embryonic development.
Keywords: metabolism, mitochondria, stem cells, pluripotency, reprogramming, differentiation, cell fate, epigenetics
Introduction
Most investigators view metabolism, which encompasses the synthesis and utilization of macromolecules and energy, as a passive supporter of self-renewal and rapid proliferation in pluripotent stem cells (PSCs)2 and their differentiated, slower dividing progeny. However, exciting recent studies are changing this perception by showing an active role for metabolism in PSC fate determination.
Metabolism in PSCs and somatic cells
Glycolysis and oxidative phosphorylation
Mammalian cells generate ATP by varying the ratios of glycolysis and oxidative phosphorylation (OXPHOS). Human and mouse PSCs produce energy mainly by the enzymatic conversion of glucose to lactate (1), which contrasts with resting somatic body cells that favor OXPHOS (Fig. 1) (2–5). This difference persists for PSCs made from blastocysts or by somatic cell reprogramming, independent of oxygen availability (6). We interpret this energetic difference as a possible programmed feature of cell state rather than a consequence of relative hypoxia in earliest embryo development. Despite a markedly lower ATP yield per glucose molecule, elevated glycolysis in PSCs, also seen in rapidly dividing cancer cells (Warburg effect), has several key advantages. Increased glycolytic flux fuels the biosynthesis of nucleotides, lipids, and reducing equivalents to support rapid cell replication. Maintaining embryonic integrity, or genomic stability during embryonic development, requires reducing genomic and mitochondrial DNA damage and protein and lipid oxidation from reactive oxygen species (ROS) produced by OXPHOS. A premium for embryonic integrity is also manifest as heightened apoptosis sensitivity in early embryos, germ cells, and PSCs (2, 7, 8). Favoring glycolysis reduces ROS, which, along with augmented antioxidant mechanisms, supports PSC self-renewal until differentiation is triggered with required OXPHOS and ROS elevations (9–11).
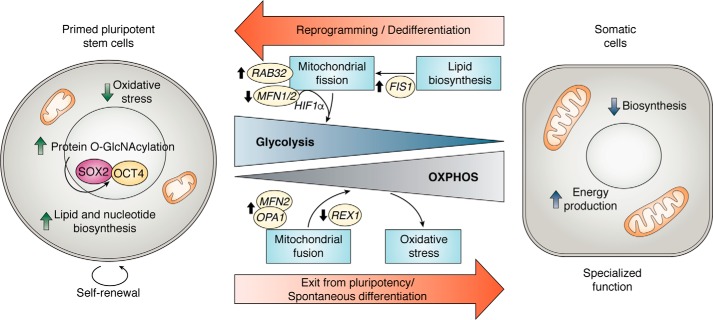
Figure 1.
Metabolic transitions between pluripotency and differentiation. The primed pluripotent state is characterized by elevated glycolysis, which associates with a fragmented mitochondrial network. Glycolytic metabolism fuels rapid proliferation and self-renewal while maintaining molecular integrity from decreased oxidative stress. PSC metabolism appears to favor a relatively high level of protein O-GlcNAcylated motifs, particularly on SOX2 and OCT4, that are quickly erased upon differentiation. These modifications could also regulate other transcription factors and epigenome remodelers upon pluripotency exit. During PSC-derived generation of somatic cells, such as cardiomyocytes, oxidative metabolism is up-regulated by mitochondrial dynamics via REX1 repression and MFN2 and OPA1 expression. This supports efficient mitochondrial energy production at the expense of macromolecular biosynthesis. The consequence of increased OXPHOS is increased ROS production and oxidative stress, which may serve regulatory roles in differentiation. Conversely, the reprogramming of somatic cells toward pluripotency depends on an early glycolytic shift, and mitochondrial morphology remodeling facilitated by RAB32 kinase and lipid biosynthesis induced FIS1 stabilization. The down-regulation of proteins driving MFN1/2 can promote a glycolytic shift through HIF1α stabilization.
Glutamine
Glutamine supports PSC survival and expansion by increasing antioxidant GSH (4) and fueling mitochondrial energy metabolism (12–14). Glutaminolysis generates tricarboxylic acid (TCA) cycle substrates (12) and supports the mitochondrial inner membrane potential (ΔΨ) required for PSC survival (15). A paradoxically high ΔΨ in PSCs compared with PSC-differentiated progeny cells (3, 16, 17), despite low OXPHOS in PSCs, may arise from the hydrolysis of glycolytic ATP by complex V ATP synthase. PSCs show low expression of inhibitory factor 1, which prevents the ATP synthase from running as an ATP hydrolase (2). Because low ΔΨ facilitates the induction of apoptotic pathways, enhanced apoptotic sensitivity of PSCs compared with PSC-differentiated progeny is surprising with comparatively heightened ΔΨ in PSCs and requires further study.
Lipids
Similar to rapidly dividing cancer cells and antigen-activated lymphocytes, robust lipid biosynthesis occurs in PSCs to replenish membranes and organelles (16). Lipid production also supports somatic cell reprogramming to pluripotency (18, 19). Carbons for lipid building are siphoned from glycolysis and glutaminolysis biochemical pathways (12, 14). Indeed, lipid anabolism promotes PSC identity, notably by regulating mitochondrial dynamics (see below).
Regulation of PSC metabolism
Core pluripotency transcription factors
In embryonic stem cells (ESCs), core pluripotency transcription factors (CPTFs), such as OCT4, interact with the promoters of genes encoding glycolytic enzymes hexokinase 2 and pyruvate kinase M2 (PKM2) to drive transcription and augment glycolytic flux (5, 20, 21). Metabolic coordination by CPTFs indicates an essential role for metabolism in pluripotency.
Regulators of mitochondrial carbon routing
Uncoupling protein 2 (UCP2) is a mitochondrial inner membrane protein expressed in PSCs that limits OXPHOS and ROS production (2). Aberrant re-expression of UCP2 in many types of cancer also inhibits OXPHOS to bias ATP production toward aerobic glycolysis or “Warburg” metabolism (22). UCP2 functions as a carbon substrate transporter. In PSCs, UCP2 expels carbon 4 (C4) intermediate metabolites, including oxaloacetate, malate, and l-aspartate from the TCA cycle and mitochondrial matrix, thereby reducing electron-donating substrates for OXPHOS and ATP production (22). In cancer cells, UCP2 conversely promotes the incorporation of carbons from glutaminolysis into the TCA cycle. Recent studies also show that glutamine supports the TCA cycle more robustly in PSCs than in differentiated cardiomyocyte progeny cells (12). Combined, these studies provide a model for PSCs in which UCP2 routes glucose-derived carbons into cytosolic biosynthetic pathways and glutamine-derived carbons into the TCA cycle to maintain ΔΨ and low OXPHOS levels. This model is supported by a study that shows glucose provides metabolites only for the first steps (Ac-CoA, citrate, and cis-aconitate) of the TCA cycle, whereas glutamine contributes metabolites to later TCA cycle steps as an anapleurotic fuel (Fig. 2) (12).
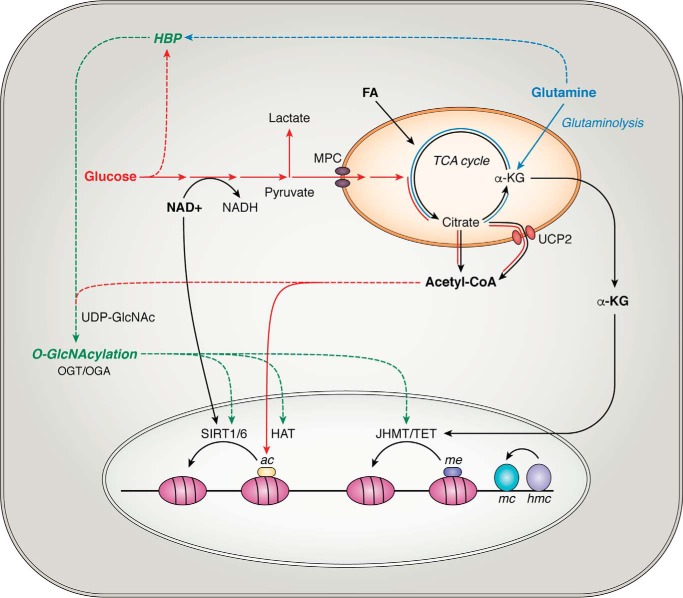
Figure 2.
Influence of metabolism on the epigenome in the maintenance of pluripotency. Glycolytic flux regulates the NAD+/NADH ratio, which controls the activities of sirtuin (SIRT) histone deacetylases. TCA cycle intermediates exported from the mitochondria include citrate and α-KG. Conversion of cytosolic citrate to Ac-CoA provides a donor for HAT-mediated histone acetylation. α-KG is a cofactor for histone and DNA demethylation reactions by JHDM and TET enzymes, respectively. By-products of glucose and glutamine catabolism supply reactants for O-GlcNAc modifications of epigenetic remodeling proteins through the HBP. Highlighted in red, blue, and green are the metabolic pathways associated with pluripotency, their contribution to TCA cycle metabolite production, and their subsequent influence on the epigenome (solid lines). Another potential mechanism for how metabolism can shape transcriptional patterns could be by fueling HBP-derived O-GlcNAcylation of proteins driving epigenome remodeling (dashed line).
Another potential carbon routing regulator in PSCs comes from cancer metabolism studies. Cancer cells show robust glucose import and augmented glycolysis to feed biosynthetic reactions. This activity blocks pyruvate oxidation to CO2 in the mitochondria by repression of the mitochondrial pyruvate carrier (MPC) genes, MPC1/2 (23). Pyruvate exclusion from mitochondrial oxidation may be a more general stem cell strategy beyond cancer cells that requires further study. Supporting this idea, repression of MPC levels occurs in intestinal and hair-follicle adult stem cells, whereas MPC levels increase with differentiation of intestinal crypt stem-like cells (24, 25).
Mitochondrial network
PSCs show punctate mitochondria with immature inner membrane cristae and evidence of reduced functionality with low OXPHOS (2, 4, 5) and ROS production (14, 26). A granular mitochondrial morphology contrasts with elongated interlacing mitochondrial networks in somatic cells and helps to sustain CPTF expression and prevent expression of differentiation genes (27). Conversely, the REX1 pluripotency-associated transcription factor (TF) causes Ser-616 phosphorylation and activation of the mitochondrial fission regulator DRP1 by CDK1/cyclin B (27). Also, repression of mitochondrial fusion proteins MFN1/2 during somatic cell reprogramming is linked to reduced p53 expression and increased proliferation (26). Together, these studies connect mitochondrial network dynamics with pluripotency and proliferation in PSCs.
Mitochondrial dynamics regulators may influence PSC metabolic flux. A granular mitochondrial morphology supports fatty acid (FA) biosynthesis and promotes glycolytic gene expression (14). Studies show that mitochondrial fission with an immature ultrastructure, rather than function of respiratory chain complexes, supports a glycolytic preference (2, 4, 5). In immortalized fibroblasts, mitochondrial dysfunction and a shift to glycolysis occurs with mitochondrial fission factor overexpression (28). Additionally, MFN1/2 depletion can augment the expression and stabilization of the glycolytic master up-regulator, hypoxia-inducible factor 1α (HIF1α) (26). These data suggest that network regulators influence both the cell cycle and metabolism in pluripotency. The potential for mitochondrial network morphology to affect the expression of cell fate and metabolism genes requires further investigation. New insights from recent studies on metabolic control of chromatin structure and gene expression (detailed later) provide a potential mechanism for this connection.
Metabolism in pluripotent cell-fate transitions
Metabolic events during iPSC generation
Reprogramming somatic body cells to induced pluripotent stem cells (iPSCs) is a model for cell-fate transitions. iPSC production provides insight for how metabolism governs pluripotency and self-renewal or differentiation into highly specialized and functional cell types. Stimulating glycolytic flux by modulating pathway regulators or effectors promotes iPSC reprogramming efficiency, whereas impeding glycolysis has the opposite effect (21, 29, 30). Transcriptome and proteome analyses during reprogramming reveal metabolic roles in dedifferentiation. Changes in the expression of metabolic genes that shift OXPHOS to glycolysis precede the induction of pluripotency and self-renewal genes (21, 31–34). An early reprogramming hyper-energetic state, partly mediated by estrogen-related nuclear receptors, shows elevated OXPHOS and glycolysis, with increases in mitochondrial ATP production proteins and antioxidant enzymes (32, 35, 36). An early burst in OXPHOS increases ROS generation and leads to an increase in nuclear factor (erythroid-derived 2)-like 2 (NRF2) activity, which promotes a subsequent glycolytic shift through HIF activation (36). Together, these studies show a progression from a hyper-energetic state to glycolysis during the conversion to pluripotency.
Hypoxia-related pathways in PSC fate transitions
Inducing glycolysis and reducing OXPHOS by modulating p53 and HIFs can influence somatic cell dedifferentiation. p53 inactivation (37–40) and HIF stabilization in low O2 tension promote reprogramming efficiency (34, 41) and reversible pluripotency re-entry during early differentiation (42). Early in reprogramming, HIF1α and HIF2α are stabilized in normoxia and are notably required for metabolic shift by facilitating the expression of glycolysis-enforcing genes such as the pyruvate dehydrogenase kinase 3 (34). However, enforced HIF2 stabilization is deleterious during the last steps of iPSC generation by inducing tumor necrosis factor–related apoptosis inducing ligand (TRAIL) (34).
Conversely, HIFs and hypoxia-related pathways are also effectors in driving early differentiation depending on environmental context. For instance, hypoxia promotes PSC differentiation into definitive endoderm and retinal or lung progenitors (43, 44). In the context of neurogenesis, low O2 tension and HIFs propel a neural fate at the expense of other germ lineages in early differentiation of hPSCs. At later stages of neural specification from neural progenitor cells (NPCs), hypoxia promotes glial rather than neuronal fate by an increase in regulating the activity of Lin28 (45). A synergistic combination of HIF1α and Notch signaling promotes hiPSC-derived NPC differentiation into astrocytes through DNA demethylation of the glial fibrillary acidic protein–encoding gene (46). Overall, by promoting glycolysis and changing epigenome modifications associated with cell identity, HIF1α influences cell fate toward either pluripotency or differentiation depending on the environmental context. O2 tension is an environmental driver that modifies metabolism to enable epigenome remodeling and changes in gene expression to influence cell fate.
Lipid metabolism and mitochondrial dynamics in somatic cell reprogramming
Compared with differentiated cells, iPSCs undergo rewiring of energetic and biosynthetic programs as illustrated by the reduction of mitochondrial oxidative stress pathways and rapid lipogenesis (19). For instance, somatic cell reprogramming efficiency is promoted by an increase in key lipogenic enzymes, such as Ac-CoA carboxylase and FA synthase (18, 19).
The de novo FA biosynthesis controls reprogramming and pluripotency through shifts in mitochondrial dynamics. Equilibrium toward mitochondrial fission is driven by lipogenic enzyme (ACC1) inhibition of mitochondrial fission factor (FIS1), ubiquitin–proteasome degradation, and the lipid generation (14). Mitochondrial dynamics is further supported by reduction of MFN1/2 and an increase of the mitochondrial protein kinase A–anchoring protein RAB32 involved in mitochondrial fission synchronization (26, 47). Genetic or pharmacological perturbation of MFN1/2 endorses HIF1α stabilization through ROS production and promotes iPSC generation by indirectly favoring a metabolic transition from OXPHOS to glycolysis (26, 48). These findings hint at an interconnected mechanism triangulating lipid metabolism, mitochondrial fission, and a metabolic shift to glycolysis that favors the PSC state (Fig. 1).
Metabolism in pluripotency exit and early differentiation
Metabolic remodeling during PSC differentiation
Spontaneous nondirected hPSC differentiation shows an opposite metabolic shift compared with somatic cell reprogramming. A gradual increase in lactate production associates with an increase in O2 consumption, mitochondrial biogenesis, and ROS accumulation (2, 11, 49). This progressive metabolic remodeling is partly mediated by UCP2 repression, which enables complete mitochondrial oxidation of pyruvate leading to an increase in OXPHOS (2). An increase in ROS is a direct consequence of increased OXPHOS and is enforced by repression of major antioxidant defenses (11, 49), suggesting a role for oxidative stress in PSC differentiation. Support for this idea comes from mouse ESC (mESC) cardiac differentiation studies that show ROS augmentation through activation of mitogen-activated protein kinase (MAPK) signaling pathways (9, 10, 50). OXPHOS and ROS are also driven by proteins orchestrating mitochondrial dynamics through REX1 repression (27) and the requirement for MFN2 and OPA1 expression during cardiomyocyte differentiation from mESCs (51).
Metabolites supporting pluripotency exit
Although PSCs and cancer cells have a shared metabolic hallmark, aerobic glycolysis or the Warburg effect, a unique mechanism for how this metabolic shift supports pluripotency exit has recently been proposed. A critical point of divergence is that PSCs strategically utilize glycolysis to produce both lactate and cytosolic Ac-CoA by siphoning glucose-derived citrate from the TCA cycle (Fig. 2). Inhibition or promotion of glycolytic Ac-CoA enhances or delays differentiation, respectively, suggesting that Ac-CoA has a key role during the glycolytic switch in pluripotency exit (52). In PSCs, glycolytic Ac-CoA production is through ATP citrate lyase enzyme activity and may consequently fuel histone acetylation. However, in the 1st h of spontaneous differentiation, repression of this enzyme may assist exit-associated epigenome remodeling by reducing substrate for histone acetylases (52).
Metabolic shift kinetics linked with cell fate
In vitro modeling of embryonic tri-lineage differentiation provides insight for metabolic events during early lineage commitment. Substantially diminished glycolytic flux does not occur for all lineages. Sustained high aerobic glycolytic flux is necessary for early ectoderm lineage commitment and expression of ectoderm-specific differentiation genes (53). Conversely, a metabolic shift toward OXPHOS has recently been observed in early mesoderm and endoderm lineage cells and is consistent with an elevated OXPHOS requirement for PSC generation of cardiomyocytes (54). The utilization of other carbon sources and pathway fluxes beyond glucose in early fate outcome remains unexplored.
Hexosamine biosynthesis and fate transitions
The hexosamine biosynthesis pathway (HBP) is a nutrient-sensing pathway that depends on glucose, glutamine, and cytosolic Ac-CoA levels (Fig. 2). HBP branches from glycolysis to produce UDP-GlcNAc as an end product. UDP-GlcNAc is a substrate of O-GlcNAc transferase (OGT) for post-translational protein modification by O-GlcNAcylation with addition of O-linked β-d-GlcNAc (O-GlcNAc). The removal of O-GlcNAc by O-GlcNAcase (OGA) counteracts OGT activity, and this dynamic cycling of protein GlcNAcylation regulates numerous cell processes such as cell cycle, signaling pathways, transcription, and organelle and chromatin dynamics (55).
During development and general differentiation processes, GlcNAcylation is implicated in the regulation of pluripotency and gene silencing (56–62). Manipulation of O-GlcNAcylation to elevate O-GlcNAc motifs delays mESC differentiation and subsequent generation of cardiomyocytes (56–61). Conversely, a decrease in O-GlcNAcylation disrupts self-renewal and reprogramming (57) but promotes differentiation in neuronal cells (63–65). Moreover, CPTFs such as OCT4 and SOX2 harbor an O-GlcNAcylation motif that is quickly erased upon differentiation (57). Together, these studies suggest that a high rate of O-GlcNAcylation is involved in the maintenance and acquisition of pluripotency, whereas its reduction is linked to pluripotency exit and differentiation. Elevated glucose, glutamine, and Ac-CoA production in PSCs could support enhanced HBP flux to provide substrate for OGT (Fig. 3). CPTF promoter occupancy at the OGA-encoding gene (66, 67) implies a role for protein O-GlcNAcylation dynamics in pluripotency. This suggestion is supported by in vivo studies showing roles for OGT and OGA during embryogenesis (68–70). Remarkably, high-level O-GlcNAcylation was linked with perturbation in glucose homeostasis and neural tube defects in the fetus of diabetic mice (71). Thus, HBP constitutes a direct link between metabolism and protein O-GlcNAcylation, with a suggested role in regulating pluripotency dynamics and cell-fate acquisition by regulating TFs and the epigenome remodeling machinery, but this idea requires further study.
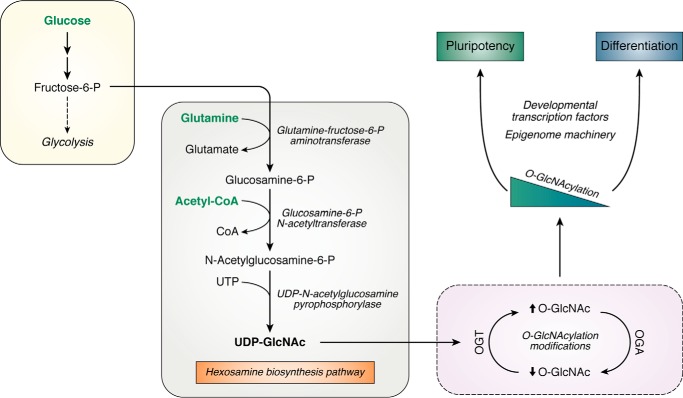
Figure 3.
Nutrient-sensing HBP regulates pluripotency and differentiation. A branch of glycolysis fuels the HBP to produce UDP-GlcNAc. Pathway flux depends on levels of macromolecular metabolism, including carbohydrate, lipid, nucleotide, and amino acid biosynthesis. UDP-GlcNAc is a key intermediate for post-translational modification of Ser and Thr residues. The catalytic activity of OGT and OGA determines the level of protein O-GlcNAcylation. This modification influences pluripotency and differentiation gene networks via transcriptional and epigenetic machinery.
Metabolism modulates epigenetic remodeling
Metabolism has key roles for inducing, maintaining, and exiting pluripotency. It is remarkable that major metabolic pathways can influence the activity of multiple epigenome-modifying enzymes, providing an exciting potential connection to cell-fate plasticity.
TCA cycle–derived metabolites
hPSCs produce cytosolic Ac-CoA from glycolysis, but rapidly lose this activity in early differentiation, which parallels reduced histone acetylation and expression of pluripotency markers coinciding with the expression of early differentiation genes (52). The simultaneous occurrence of these temporal events suggests that a metabolic shift could drive changes in gene expression patterns and cell fate by modulating histone acetylation. A complementary question could be whether metabolism also affects the dynamics of epigenome methylation. One possible connection could be via the TCA cycle metabolites α-ketoglutarate (α-KG) and succinate, respective cofactor and inhibitor for epigenome demethylase remodeling enzymes. α-KG, made from either glucose or glutamine, is a cofactor for Jumonji C-domain containing histone demethylases (JHDM) and ten-eleven–translocase (TET) DNA demethylases that sustain the naïve pluripotent state in mESCs (72). Yet, in the context of early differentiation, increased α-KG levels enhance ectoderm lineage commitment (73), suggesting that metabolism influences chromatin dynamics contextually in pluripotency maintenance or exit.
O-GlcNAcylation
HBP is another potential link between metabolism and epigenome remodeling (Fig. 2). OGT influences DNA methylation and histone modifications by regulating the stabilization, localization, or substrate specificity for specific sets of genes. OGT modifies the epigenome machinery, including TET family enzymes and polycomb group proteins, which are transcriptional repressors that regulate gene expression patterns during embryonic development and differentiation (59). In ESCs, by physically interacting with these proteins, OGT is directed toward the chromatin to influence histone methylation, including H3K4 tri-methylation (H3K4me3), DNA methylation through TET1 interactions, and recruitment of TFs to specific target genes (56–61). Enriched GlcNAc motifs in the catalytic subunit of polycomb complex PRC1 represses neural differentiation genes, whereas this motif is absent on stemness-associated genes, including cell cycle genes (74). Moreover, O-GlcNAcylation may be implicated in neural and neuronal cell-fate determination through epigenetic modulation by the association of OGT with TET3, resulting in recruitment of NeuroD1 to brain-specific target genes (64). O-GlcNAcylation modifications appear to influence the determination of specific neuronal identity. During mESC differentiation, OGA co-localizes with the histone acetyltransferase p300 on the neuropeptide-encoding gene orexin in orexin neurons, whereas OGT co-localizes with histone deacetylase sirtuin 1 (SIRT1) in nonorexin-expressing cells (65). Because of the direct link between HBP and O-GlcNAcylation, we propose that metabolic fluctuations induced by specific microenvironmental differentiation drivers, such as hypoxia, could participate in establishing distinct epigenome patterns associated with cell fate and function acquisitions and maintenance.
S-Adenosylmethionine (SAM)
Methyltransferases for histones and DNA depend on the levels of SAM and threonine, which are required for pluripotency. Extracellular threonine in mESCs or methionine in hPSCs are the only amino acids critical for pluripotency and used for SAM production (75, 76). PSCs use internal regulatory systems to maintain intracellular SAM levels that involve p53 and p38 cell cycle and apoptosis regulators (8, 76). mESCs or hPSCs grown in reduced threonine/methionine show spontaneous differentiation and slowed growth (82, 83). Threonine depletion decreases SAM production and influences H3K4me3 levels, suggesting a possible mechanism for how threonine and SAM levels regulate pluripotent cell fate (75).
Ascorbate
Another regulator of histone modifications is ascorbate, the principal form of vitamin C at physiological pH. Ascorbate is an epigenomic regulator of DNA methylation and a modulator of iPSC reprogramming efficiency (77, 78). It is also a cofactor for TET enzymes that catalyze the oxidation of 5-methylcytosine to 5-hydroxymethylcytosine. By contributing to the demethylation of both DNA and histones, ascorbate appears to augment the erasure of epigenetic memory, and in this manner, it enhances stem cell reprogramming (77). Increased ascorbate enhances iPSC generation, which parallels TET1 deficiency in improving reprogramming efficiency. Conversely, TET1 overexpression impairs reprogramming (76). Although TET1 in the absence of ascorbate remains functional, the addition of ascorbate is critical for the epithelial-to-mesenchymal transition during reprogramming to pluripotency (79).
NAD
Cellular NAD level is directly impacted by the metabolic state, such as glycolytic flux, and regulates the activity of NAD+-dependent histone deacetylase sirtuins (Fig. 2), including SIRT1, which is induced in hPSCs (80). SIRT1 controls cell fate by regulating p53-dependent homeobox protein transcription factor NANOG (homeobox protein transcription factor) expression and transcriptional repression of differentiation genes in hPSCs (8, 80, 81). During hESC differentiation, SIRT1 is repressed, leading to acetylation and reactivation of multiple differentiation genes, including PAX6, TBX3, and DLL (80). Further investigations are required to understand how distinct metabolic shifts during cell-fate conversions influence the NAD+/NADH ratio and, subsequently, how NAD levels impact histone acetylation remodeling events specifically through sirtuin activities.
Metabolism in naïve and primed pluripotency
The pre-implantation blastocyst retains a developmentally unrestricted cell population that differs from its post-implantation counterpart (82). Although the inner cell mass of pre- and post-implantation blastocysts yields ESCs for growth in vitro (83, 84), pre-implantation ESCs exist in a naïve or ground pluripotent state, whereas post-implantation ESCs exist in a primed state. Naïve PSCs introduced in the pre-implantation blastocyst can contribute to a chimeric mouse (85), whereas primed PSCs cannot, discerning their distinct developmental potential. Another distinguishing characteristic between these two PSC states is how metabolism fuels the requirements for self-renewal and proliferation. Pre-implantation blastocyst-derived naïve PSCs utilize glycolysis and OXPHOS and then transition to glycolysis dependence in the primed state, despite having functionally mature mitochondria (86). The coordination of TFs Zic3 and Esrrb regulates bivalent metabolism observed in naïve PSCs (87). Naïve PSCs also have a greater glycolytic output compared with primed PSCs (88). Yet, different patterns of glycolysis and OXPHOS could play additional roles beyond supporting higher proliferation in naïve ESCs (89, 90). For instance, an open question is whether dissimilar metabolic profiles between primed and naïve PSCs is implicated in distinct transcriptional patterns and chromatin organization, ultimately influencing respective developmental potentials. This issue will most likely be a relevant topic in the coming years for the field, as illustrated by the studies conveyed below.
Metabolism and the naïve PSC epigenome
Naïve and primed PSCs show structural changes in chromatin structure, including histone and DNA modifications, that have functional consequences in regard to developmental potential through TF expression and suppression of lineage-restrictive genes (91). Although naïve hESCs with properties similar to mESCs have been claimed (85, 89, 90, 92), these cells show some differences in pluripotency markers and functions that probably require more optimized culture and nutrient conditions to improve consistency between species. There is increasing evidence for interactions between the metabolome and epigenome to sustain a global hypo-methylated profile associated with naïve PSCs (93). Mechanistically, glucose- and glutamine-derived TCA cycle metabolite α-KG, acting as a cofactor for JHMD and TET proteins (Fig. 2), sustains the naïve state in mESCs by global hypo-methylation (72). Additionally, as mentioned previously, HBP couples macromolecular metabolism to O-GlcNAc modifications in proteins, including epigenetic modifiers (Fig. 3) (94). Cycling of these post-translational modifications by OGT and OGA are required for naïve and primed state induction, maintenance, and differentiation (56, 95). OGT interacts with TET1 enzymes preferentially at transcription start sites, thereby influencing gene expression (58). This may implicate OGT in globally regulating the DNA hypo-methylation profile in naïve compared with primed PSCs. As such, the O-GlcNAcylation cycle could have a role in the observed differences of X-chromosome inactivation in primed state female ESCs (97). Thus, a potential link between OGT and X-chromosome inactivation may have functional relevance in the context of naïve and primed PSCs (94).
Moreover, naïve to primed epigenetic transitions are partly mediated by nicotinamide N-methyltransferase (NNMT), which decreases H3K27me3. By consuming SAM and up-regulating Wnt signaling, NNMT prevents histone methylation in the naïve state (98). In addition to the role of ascorbate in reprogramming (84, 85), a combination of ascorbate and retinol has been shown to enhance naïve state acquisition by increasing TET expression and catalysis (99). In contrast, high ratios of l-proline to ascorbate prevents naïve state maintenance, although the mechanism(s) by which l-proline induces epigenetic changes has yet to be elucidated (96). Collectively, these studies suggest a role for metabolism in influencing naïve versus primed pluripotency dynamics by facilitating chromatin remodeling through multiple specific metabolites.
Conclusions
Accumulating evidence illustrates the influence of metabolism on differentiation plasticity by regulating the epigenetic machinery. Our current understanding provides a foundation for further investigations into links between environmental stimuli and subsequent metabolic adaptations that orchestrate epigenome remodeling events, which ultimately drive cell fate. A key open question is what recruits epigenome remodelers, co-factors, and substrates to specific loci to influence patterns of pluripotent and somatic cell gene expression. Here, we suggest that HBP may have a role by acting at crucial nodes connecting metabolism and epigenome remodeling. Answers to fundamental questions raised here will provide a deeper basis for generating safe and accessible PSC-derived cells for use in regenerative medicine, disease modeling, and drug screening applications in the future.
This work was supported by National Institutes of Health Grants CA90571, GM073981, CA18589, and GM114188 and Air Force Office of Scientific Research Grant FA9550-15-1-0406 (to M. A. T.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- PSC
- pluripotent stem cell
- i/h/PSC
- induced/human pluripotent stem cell
- OXPHOS
- oxidative phosphorylation
- TCA
- tricarboxylic acid
- ΔΨ
- mitochondrial inner membrane potential
- CPTF
- core pluripotency transcription factor
- TF
- transcription factor
- UCP2
- uncoupling protein 2
- HBP
- hexosamine biosynthesis pathway
- UDP-GlcNAc
- UDP N-acetylglucosamine
- OGT
- O-GlcNAc transferase
- O-GlcNAc
- O-linked β-d-N-acetylglucosamine. OGA, O-GlcNAcase
- SIRT1
- sirtuin 1
- NPC
- neural progenitor cell
- FA
- fatty acid
- JHDM
- Jumonji C-domain containing histone demethylase
- TET
- ten-eleven-translocase
- α-KG
- α-ketoglutarate
- ESC
- embryonic stem cell
- mESC
- mouse ESC
- NNMT
- nicotinamide N-methyltransferase
- SAM
- S-adenosylmethionine
- Ac-CoA
- acetyl-CoA
- HIF
- hypoxia-inducible factor.
References
- 1. Houghton F. D., Thompson J. G., Kennedy C. J., and Leese H. J. (1996) Oxygen consumption and energy metabolism of the early mouse embryo. Mol. Reprod. Dev. 44, 476–485 [DOI] [PubMed] [Google Scholar]
- 2. Zhang J., Khvorostov I., Hong J. S., Oktay Y., Vergnes L., Nuebel E., Wahjudi P. N., Setoguchi K., Wang G., Do A., Jung H. J., McCaffery J. M., Kurland I. J., Reue K., Lee W. N., et al. (2011) UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 30, 4860–4873 10.1038/emboj.2011.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung S., Dzeja P. P., Faustino R. S., Perez-Terzic C., Behfar A., and Terzic A. (2007) Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 4, Suppl. 1, S60–67 10.1038/ncpcardio0766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prigione A., Fauler B., Lurz R., Lehrach H., and Adjaye J. (2010) The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 28, 721–733 10.1002/stem.404 [DOI] [PubMed] [Google Scholar]
- 5. Varum S., Rodrigues A. S., Moura M. B., Momcilovic O., Easley C. A. 4th., Ramalho-Santos J., Van Houten B., and Schatten G. (2011) Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE 6, e20914 10.1371/journal.pone.0020914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fischer B., and Bavister B. D. (1993) Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J. Reprod. Fertil. 99, 673–679 10.1530/jrf.0.0990673 [DOI] [PubMed] [Google Scholar]
- 7. Madden D. T., Davila-Kruger D., Melov S., and Bredesen D. E. (2011) Human embryonic stem cells express elevated levels of multiple pro-apoptotic BCL-2 family members. PLoS ONE 6, e28530 10.1371/journal.pone.0028530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Setoguchi K., TeSlaa T., Koehler C. M., and Teitell M. A. (2016) P53 regulates rapid apoptosis in human pluripotent stem cells. J. Mol. Biol. 428, 1465–1475 10.1016/j.jmb.2015.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crespo F. L., Sobrado V. R., Gomez L., Cervera A. M., and McCreath K. J. (2010) Mitochondrial reactive oxygen species mediate cardiomyocyte formation from embryonic stem cells in high glucose. Stem Cells 28, 1132–1142 10.1002/stem.441 [DOI] [PubMed] [Google Scholar]
- 10. Xiao Q., Luo Z., Pepe A. E., Margariti A., Zeng L., and Xu Q. (2009) Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am. J. Physiol. Cell Physiol. 296, C711–C723 10.1152/ajpcell.00442.2008 [DOI] [PubMed] [Google Scholar]
- 11. Cho Y. M., Kwon S., Pak Y. K., Seol H. W., Choi Y. M., Park D. J., Park K. S., and Lee H. K. (2006) Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 348, 1472–1478 10.1016/j.bbrc.2006.08.020 [DOI] [PubMed] [Google Scholar]
- 12. Tohyama S., Fujita J., Hishiki T., Matsuura T., Hattori F., Ohno R., Kanazawa H., Seki T., Nakajima K., Kishino Y., Okada M., Hirano A., Kuroda T., Yasuda S., Sato Y., et al. (2016) Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab. 23, 663–674 10.1016/j.cmet.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 13. Zhang H., Badur M. G., Divakaruni A. S., Parker S. J., Jäger C., Hiller K., Murphy A. N., and Metallo C. M. (2016) Distinct metabolic states can support self-renewal and lipogenesis in human pluripotent stem cells under different culture conditions. Cell Rep. 16, 1536–1547 10.1016/j.celrep.2016.06.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L., Zhang T., Wang L., Cai Y., Zhong X., He X., Hu L., Tian S., Wu M., Hui L., Zhang H., and Gao P. (2017) Fatty acid synthesis is critical for stem cell pluripotency via promoting mitochondrial fission. EMBO J. 36, 1330–1347 10.15252/embj.201695417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Green D. R., and Kroemer G. (2004) The pathophysiology of mitochondrial cell death. Science 305, 626–629 10.1126/science.1099320 [DOI] [PubMed] [Google Scholar]
- 16. Armstrong L., Tilgner K., Saretzki G., Atkinson S. P., Stojkovic M., Moreno R., Przyborski S., and Lako M. (2010) Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 28, 661–673 10.1002/stem.307 [DOI] [PubMed] [Google Scholar]
- 17. Schieke S. M., Ma M., Cao L., McCoy J. P. Jr., Liu C., Hensel N. F., Barrett A. J., Boehm M., and Finkel T. (2008) Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J. Biol. Chem. 283, 28506–28512 10.1074/jbc.M802763200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu Y., Chen K., Liu X., Huang L., Zhao D., Li L., Gao M., Pei D., Wang C., and Liu X. (2016) Srebp-1 interacts with c-Myc to enhance somatic cell reprogramming. Stem Cells 34, 83–92 10.1002/stem.2209 [DOI] [PubMed] [Google Scholar]
- 19. Vazquez-Martin A., Corominas-Faja B., Cufi S., Vellon L., Oliveras-Ferraros C., Menendez O. J., Joven J., Lupu R., and Menendez J. A. (2013) The mitochondrial H(+)-ATP synthase and the lipogenic switch: new core components of metabolic reprogramming in induced pluripotent stem (iPS) cells. Cell Cycle 12, 207–218 10.4161/cc.23352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim H., Jang H., Kim T. W., Kang B. H., Lee S. E., Jeon Y. K., Chung D. H., Choi J., Shin J., Cho E. J., and Youn H. D. (2015) Core pluripotency factors directly regulate metabolism in embryonic stem cell to maintain pluripotency. Stem Cells 33, 2699–2711 10.1002/stem.2073 [DOI] [PubMed] [Google Scholar]
- 21. Folmes C. D., Nelson T. J., Martinez-Fernandez A., Arrell D. K., Lindor J. Z., Dzeja P. P., Ikeda Y., Perez-Terzic C., and Terzic A. (2011) Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 14, 264–271 10.1016/j.cmet.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vozza A., Parisi G., De Leonardis F., Lasorsa F. M., Castegna A., Amorese D., Marmo R., Calcagnile V. M., Palmieri L., Ricquier D., Paradies E., Scarcia P., Palmieri F., Bouillaud F., and Fiermonte G. (2014) UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. U.S.A. 111, 960–965 10.1073/pnas.1317400111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schell J. C., Olson K. A., Jiang L., Hawkins A. J., Van Vranken J. G., Xie J., Egnatchik R. A., Earl E. G., DeBerardinis R. J., and Rutter J. (2014) A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol. Cell 56, 400–413 10.1016/j.molcel.2014.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flores A., Schell J., Krall A. S., Jelinek D., Miranda M., Grigorian M., Braas D., White A. C., Zhou J. L., Graham N. A., Graeber T., Seth P., Evseenko D., Coller H. A., Rutter J., et al. (2017) Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 19, 1017–1026 10.1038/ncb3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schell J. C., Wisidagama D. R., Bensard C., Zhao H., Wei P., Tanner J., Flores A., Mohlman J., Sorensen L. K., Earl C. S., Olson K. A., Miao R., Waller T. C., Delker D., Kanth P., et al. (2017) Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat. Cell Biol. 19, 1027–1036 10.1038/ncb3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Son M. J., Kwon Y., Son M. Y., Seol B., Choi H. S., Ryu S. W., Choi C., and Cho Y. S. (2015) Mitofusins deficiency elicits mitochondrial metabolic reprogramming to pluripotency. Cell Death Differ. 22, 1957–1969 10.1038/cdd.2015.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Son M. Y., Choi H., Han Y. M., and Cho Y. S. (2013) Unveiling the critical role of REX1 in the regulation of human stem cell pluripotency. Stem Cells 31, 2374–2387 10.1002/stem.1509 [DOI] [PubMed] [Google Scholar]
- 28. Guido C., Whitaker-Menezes D., Lin Z., Pestell R. G., Howell A., Zimmers T. A., Casimiro M. C., Aquila S., Ando' S., Martinez-Outschoorn U. E., Sotgia F., and Lisanti M. P. (2012) Mitochondrial fission induces glycolytic reprogramming in cancer-associated myofibroblasts, driving stromal lactate production, and early tumor growth. Oncotarget 3, 798–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panopoulos A. D., Yanes O., Ruiz S., Kida Y. S., Diep D., Tautenhahn R., Herrerías A., Batchelder E. M., Plongthongkum N., Lutz M., Berggren W. T., Zhang K., Evans R. M., Siuzdak G., and Izpisua Belmonte J. C. (2012) The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 22, 168–177 10.1038/cr.2011.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J., Nuebel E., Daley G. Q., Koehler C. M., and Teitell M. A. (2012) Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 11, 589–595 10.1016/j.stem.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cacchiarelli D., Trapnell C., Ziller M. J., Soumillon M., Cesana M., Karnik R., Donaghey J., Smith Z. D., Ratanasirintrawoot S., Zhang X., Ho Sui S. J., Wu Z., Akopian V., Gifford C. A., Doench J., et al. (2015) Integrative analyses of human reprogramming reveal dynamic nature of induced pluripotency. Cell 162, 412–424 10.1016/j.cell.2015.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hansson J., Rafiee M. R., Reiland S., Polo J. M., Gehring J., Okawa S., Huber W., Hochedlinger K., and Krijgsveld J. (2012) Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell Rep. 2, 1579–1592 10.1016/j.celrep.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prigione A., Rohwer N., Hoffmann S., Mlody B., Drews K., Bukowiecki R., Blümlein K., Wanker E. E., Ralser M., Cramer T., and Adjaye J. (2014) HIF1α modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1–3 and PKM2. Stem Cells 32, 364–376 10.1002/stem.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mathieu J., Zhou W., Xing Y., Sperber H., Ferreccio A., Agoston Z., Kuppusamy K. T., Moon R. T., and Ruohola-Baker H. (2014) Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell 14, 592–605 10.1016/j.stem.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kida Y. S., Kawamura T., Wei Z., Sogo T., Jacinto S., Shigeno A., Kushige H., Yoshihara E., Liddle C., Ecker J. R., Yu R. T., Atkins A. R., Downes M., and Evans R. M. (2015) ERRs mediate a metabolic switch required for somatic cell reprogramming to pluripotency. Cell Stem Cell 16, 547–555 10.1016/j.stem.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hawkins K. E., Joy S., Delhove J. M., Kotiadis V. N., Fernandez E., Fitzpatrick L. M., Whiteford J. R., King P. J., Bolanos J. P., Duchen M. R., Waddington S. N., and McKay T. R. (2016) NRF2 orchestrates the metabolic shift during induced pluripotent stem cell reprogramming. Cell Rep. 14, 1883–1891 10.1016/j.celrep.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marión R. M., Strati K., Li H., Murga M., Blanco R., Ortega S., Fernandez-Capetillo O., Serrano M., and Blasco M. A. (2009) A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460, 1149–1153 10.1038/nature08287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kawamura T., Suzuki J., Wang Y. V., Menendez S., Morera L. B., Raya A., Wahl G. M., and Izpisúa Belmonte J. C. (2009) Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460, 1140–1144 10.1038/nature08311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hong H., Takahashi K., Ichisaka T., Aoi T., Kanagawa O., Nakagawa M., Okita K., and Yamanaka S. (2009) Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature 460, 1132–1135 10.1038/nature08235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Utikal J., Polo J. M., Stadtfeld M., Maherali N., Kulalert W., Walsh R. M., Khalil A., Rheinwald J. G., and Hochedlinger K. (2009) Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 460, 1145–1148 10.1038/nature08285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshida Y., Takahashi K., Okita K., Ichisaka T., and Yamanaka S. (2009) Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5, 237–241 10.1016/j.stem.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 42. Mathieu J., Zhang Z., Nelson A., Lamba D. A., Reh T. A., Ware C., and Ruohola-Baker H. (2013) Hypoxia induces re-entry of committed cells into pluripotency. Stem Cells 31, 1737–1748 10.1002/stem.1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bae D., Mondragon-Teran P., Hernandez D., Ruban L., Mason C., Bhattacharya S. S., and Veraitch F. S. (2012) Hypoxia enhances the generation of retinal progenitor cells from human induced pluripotent and embryonic stem cells. Stem Cells Dev. 21, 1344–1355 10.1089/scd.2011.0225 [DOI] [PubMed] [Google Scholar]
- 44. Pimton P., Lecht S., Stabler C. T., Johannes G., Schulman E. S., and Lelkes P. I. (2015) Hypoxia enhances differentiation of mouse embryonic stem cells into definitive endoderm and distal lung cells. Stem Cells Dev. 24, 663–676 10.1089/scd.2014.0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xie Y., Zhang J., Lin Y., Gaeta X., Meng X., Wisidagama D. R., Cinkornpumin J., Koehler C. M., Malone C. S., Teitell M. A., and Lowry W. E. (2014) Defining the role of oxygen tension in human neural progenitor fate. Stem Cell Rep. 3, 743–757 10.1016/j.stemcr.2014.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yasui T., Uezono N., Nakashima H., Noguchi H., Matsuda T., Noda-Andoh T., Okano H., and Nakashima K. (2017) Hypoxia epigenetically confers astrocytic differentiation potential on human pluripotent cell-derived neural precursor cells. Stem Cell Rep. 8, 1743–1756 10.1016/j.stemcr.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pei Y., Yue L., Zhang W., Wang Y., Wen B., Zhong L., Xiang J., Li J., Zhang S., Wang H., Mu H., Wei Q., and Han J. (2015) Improvement in Mouse iPSC induction by Rab32 reveals the importance of lipid metabolism during reprogramming. Sci. Rep. 5, 16539 10.1038/srep16539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Son M. J., Jeong B. R., Kwon Y., and Cho Y. S. (2013) Interference with the mitochondrial bioenergetics fuels reprogramming to pluripotency via facilitation of the glycolytic transition. Int. J. Biochem. Cell Biol. 45, 2512–2518 10.1016/j.biocel.2013.07.023 [DOI] [PubMed] [Google Scholar]
- 49. Saretzki G., Walter T., Atkinson S., Passos J. F., Bareth B., Keith W. N., Stewart R., Hoare S., Stojkovic M., Armstrong L., von Zglinicki T., and Lako M. (2008) Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells 26, 455–464 10.1634/stemcells.2007-0628 [DOI] [PubMed] [Google Scholar]
- 50. Schmelter M., Ateghang B., Helmig S., Wartenberg M., and Sauer H. (2006) Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J. 20, 1182–1184 10.1096/fj.05-4723fje [DOI] [PubMed] [Google Scholar]
- 51. Kasahara A., Cipolat S., Chen Y., Dorn G. W. 2nd., and Scorrano L. (2013) Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science 342, 734–737 10.1126/science.1241359 [DOI] [PubMed] [Google Scholar]
- 52. Moussaieff A., Rouleau M., Kitsberg D., Cohen M., Levy G., Barasch D., Nemirovski A., Shen-Orr S., Laevsky I., Amit M., Bomze D., Elena-Herrmann B., Scherf T., Nissim-Rafinia M., Kempa S., et al. (2015) Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 21, 392–402 10.1016/j.cmet.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 53. Cliff T. S., Wu T., Boward B. R., Yin A., Yin H., Glushka J. N., Prestegaard J. H., and Dalton S. (2017) MYC Controls human pluripotent stem cell fate decisions through regulation of metabolic flux. Cell Stem Cell 21, 502–516.e9 10.1016/j.stem.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Teslaa T., and Teitell M. A. (2014) Pluripotent stem cell energy metabolism: an update. EMBO J. 34, 138–153 10.15252/embj.201490446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang X., and Qian K. (2017) Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 18, 452–465 10.1038/nrm.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Speakman C. M., Domke T. C., Wongpaiboonwattana W., Sanders K., Mudaliar M., van Aalten D. M., Barton G. J., and Stavridis M. P. (2014) Elevated O-GlcNAc levels activate epigenetically repressed genes and delay mouse ESC differentiation without affecting naive to primed cell transition. Stem Cells 32, 2605–2615 10.1002/stem.1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jang H., Kim T. W., Yoon S., Choi S. Y., Kang T. W., Kim S. Y., Kwon Y. W., Cho E. J., and Youn H. D. (2012) O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell 11, 62–74 10.1016/j.stem.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 58. Vella P., Scelfo A., Jammula S., Chiacchiera F., Williams K., Cuomo A., Roberto A., Christensen J., Bonaldi T., Helin K., and Pasini D. (2013) Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol. Cell 49, 645–656 10.1016/j.molcel.2012.12.019 [DOI] [PubMed] [Google Scholar]
- 59. Hardivillé S., and Hart G. W. (2016) Nutrient regulation of gene expression by O-GlcNAcylation of chromatin. Curr. Opin. Chem. Biol. 33, 88–94 10.1016/j.cbpa.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shi F. T., Kim H., Lu W., He Q., Liu D., Goodell M. A., Wan M., and Songyang Z. (2013) Ten-eleven translocation 1 (Tet1) is regulated by O-linked N-acetylglucosamine transferase (Ogt) for target gene repression in mouse embryonic stem cells. J. Biol. Chem. 288, 20776–20784 10.1074/jbc.M113.460386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Constable S., Lim J. M., Vaidyanathan K., and Wells L. (2017) O-GlcNAc transferase regulates transcriptional activity of human Oct4. Glycobiology 27, 927–937 10.1093/glycob/cwx055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van den Berg D. L., Snoek T., Mullin N. P., Yates A., Bezstarosti K., Demmers J., Chambers I., and Poot R. A. (2010) An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell 6, 369–381 10.1016/j.stem.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Andres L. M., Blong I. W., Evans A. C., Rumachik N. G., Yamaguchi T., Pham N. D., Thompson P., Kohler J. J., and Bertozzi C. R. (2017) Chemical modulation of protein O-GlcNAcylation via OGT inhibition promotes human neural cell differentiation. ACS Chem. Biol. 12, 2030–2039 10.1021/acschembio.7b00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kizuka Y., Kitazume S., Okahara K., Villagra A., Sotomayor E. M., and Taniguchi N. (2014) Epigenetic regulation of a brain-specific glycosyltransferase N-acetylglucosaminyltransferase-IX (GnT-IX) by specific chromatin modifiers. J. Biol. Chem. 289, 11253–11261 10.1074/jbc.M114.554311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hayakawa K., Hirosawa M., Tabei Y., Arai D., Tanaka S., Murakami N., Yagi S., and Shiota K. (2013) Epigenetic switching by the metabolism-sensing factors in the generation of orexin neurons from mouse embryonic stem cells. J. Biol. Chem. 288, 17099–17110 10.1074/jbc.M113.455899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Boyer L. A., Lee T. I., Cole M. F., Johnstone S. E., Levine S. S., Zucker J. P., Guenther M. G., Kumar R. M., Murray H. L., Jenner R. G., Gifford D. K., Melton D. A., Jaenisch R., and Young R. A. (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 10.1016/j.cell.2005.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee T. I., Jenner R. G., Boyer L. A., Guenther M. G., Levine S. S., Kumar R. M., Chevalier B., Johnstone S. E., Cole M. F., Isono K., Koseki H., Fuchikami T., Abe K., Murray H. L., Zucker J. P., et al. (2006) Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301–313 10.1016/j.cell.2006.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shafi R., Iyer S. P., Ellies L. G., O'Donnell N., Marek K. W., Chui D., Hart G. W., and Marth J. D. (2000) The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc. Natl. Acad. Sci. U.S.A. 97, 5735–5739 10.1073/pnas.100471497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang Y. R., Song M., Lee H., Jeon Y., Choi E. J., Jang H. J., Moon H. Y., Byun H. Y., Kim E. K., Kim D. H., Lee M. N., Koh A., Ghim J., Choi J. H., Lee-Kwon W., et al. (2012) O-GlcNAcase is essential for embryonic development and maintenance of genomic stability. Aging Cell 11, 439–448 10.1111/j.1474-9726.2012.00801.x [DOI] [PubMed] [Google Scholar]
- 70. Olivier-Van Stichelen S., Wang P., Comly M., Love D. C., and Hanover J. A. (2017) Nutrient-driven O-linked N-acetylglucosamine (O-GlcNAc) cycling impacts neurodevelopmental timing and metabolism. J. Biol. Chem. 292, 6076–6085 10.1074/jbc.M116.774042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim G., Cao L., Reece E. A., and Zhao Z. (2017) Impact of protein O-GlcNAcylation on neural tube malformation in diabetic embryopathy. Sci. Rep. 7, 11107 10.1038/s41598-017-11655-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Carey B. W., Finley L. W., Cross J. R., Allis C. D., and Thompson C. B. (2015) Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 10.1038/nature13981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. TeSlaa T., Chaikovsky A. C., Lipchina I., Escobar S. L., Hochedlinger K., Huang J., Graeber T. G., Braas D., and Teitell M. A. (2016) α-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 24, 485–493 10.1016/j.cmet.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Maury J. J., El Farran C. A., Ng D., Loh Y. H., Bi X., Bardor M., and Choo A. B. (2015) RING1B O-GlcNAcylation regulates gene targeting of polycomb repressive complex 1 in human embryonic stem cells. Stem Cell Res. 15, 182–189 10.1016/j.scr.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 75. Shyh-Chang N., Locasale J. W., Lyssiotis C. A., Zheng Y., Teo R. Y., Ratanasirintrawoot S., Zhang J., Onder T., Unternaehrer J. J., Zhu H., Asara J. M., Daley G. Q., and Cantley L. C. (2013) Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339, 222–226 10.1126/science.1226603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shiraki N., Shiraki Y., Tsuyama T., Obata F., Miura M., Nagae G., Aburatani H., Kume K., Endo F., and Kume S. (2014) Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 19, 780–794 10.1016/j.cmet.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 77. Hore T. A., von Meyenn F., Ravichandran M., Bachman M., Ficz G., Oxley D., Santos F., Balasubramanian S., Jurkowski T. P., and Reik W. (2016) Retinol and ascorbate drive erasure of epigenetic memory and enhance reprogramming to naive pluripotency by complementary mechanisms. Proc. Natl. Acad. Sci. U.S.A. 113, 12202–12207 10.1073/pnas.1608679113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Esteban M. A., Wang T., Qin B., Yang J., Qin D., Cai J., Li W., Weng Z., Chen J., Ni S., Chen K., Li Y., Liu X., Xu J., Zhang S., et al. (2010) Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71–79 10.1016/j.stem.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 79. Chen J., Guo L., Zhang L., Wu H., Yang J., Liu H., Wang X., Hu X., Gu T., Zhou Z., Liu J., Liu J., Wu H., Mao S. Q., Mo K., et al. (2013) Vitamin C modulates TET1 function during somatic cell reprogramming. Nat. Genet. 45, 1504–1509 10.1038/ng.2807 [DOI] [PubMed] [Google Scholar]
- 80. Calvanese V., Lara E., Suárez-Alvarez B., Abu Dawud R., Vázquez-Chantada M., Martínez-Chantar M. L., Embade N., López-Nieva P., Horrillo A., Hmadcha A., Soria B., Piazzolla D., Herranz D., Serrano M., Mato J. M., et al. (2010) Sirtuin 1 regulation of developmental genes during differentiation of stem cells. Proc. Natl. Acad. Sci. U.S.A. 107, 13736–13741 10.1073/pnas.1001399107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pawlowski M., Ortmann D., Bertero A., Tavares J. M., Pedersen R. A., Vallier L., and Kotter M. R. (2017) Inducible and deterministic forward programming of human pluripotent stem cells into neurons, skeletal myocytes, and oligodendrocytes. Stem Cell Rep. 8, 803–812 10.1016/j.stemcr.2017.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tesar P. J., Chenoweth J. G., Brook F. A., Davies T. J., Evans E. P., Mack D. L., Gardner R. L., and McKay R. D. (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 10.1038/nature05972 [DOI] [PubMed] [Google Scholar]
- 83. Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., and Marshall V. S. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 [DOI] [PubMed] [Google Scholar]
- 84. Evans M. J., and Kaufman M. H. (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 10.1038/292154a0 [DOI] [PubMed] [Google Scholar]
- 85. Gafni O., Weinberger L., Mansour A. A., Manor Y. S., Chomsky E., Ben-Yosef D., Kalma Y., Viukov S., Maza I., Zviran A., Rais Y., Shipony Z., Mukamel Z., Krupalnik V., Zerbib M., et al. (2013) Derivation of novel human ground state naive pluripotent stem cells. Nature 504, 282–286 10.1038/nature12745 [DOI] [PubMed] [Google Scholar]
- 86. Zhou W., Choi M., Margineantu D., Margaretha L., Hesson J., Cavanaugh C., Blau C. A., Horwitz M. S., Hockenbery D., Ware C., and Ruohola-Baker H. (2012) HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 31, 2103–2116 10.1038/emboj.2012.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sone M., Morone N., Nakamura T., Tanaka A., Okita K., Woltjen K., Nakagawa M., Heuser J. E., Yamada Y., Yamanaka S., and Yamamoto T. (2017) Hybrid cellular metabolism coordinated by Zic3 and Esrrb synergistically enhances induction of naive pluripotency. Cell Metab. 25, 1103–1117.e6 10.1016/j.cmet.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 88. Gu W., Gaeta X., Sahakyan A., Chan A. B., Hong C. S., Kim R., Braas D., Plath K., Lowry W. E., and Christofk H. R. (2016) Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell 19, 476–490 10.1016/j.stem.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ware C. B., Nelson A. M., Mecham B., Hesson J., Zhou W., Jonlin E. C., Jimenez-Caliani A. J., Deng X., Cavanaugh C., Cook S., Tesar P. J., Okada J., Margaretha L., Sperber H., Choi M., et al. (2014) Derivation of naive human embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 111, 4484–4489 10.1073/pnas.1319738111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hanna J., Cheng A. W., Saha K., Kim J., Lengner C. J., Soldner F., Cassady J. P., Muffat J., Carey B. W., and Jaenisch R. (2010) Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. U.S.A. 107, 9222–9227 10.1073/pnas.1004584107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bernstein B. E., Mikkelsen T. S., Xie X., Kamal M., Huebert D. J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., Jaenisch R., Wagschal A., Feil R., Schreiber S. L., and Lander E. S. (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 10.1016/j.cell.2006.02.041 [DOI] [PubMed] [Google Scholar]
- 92. Takashima Y., Guo G., Loos R., Nichols J., Ficz G., Krueger F., Oxley D., Santos F., Clarke J., Mansfield W., Reik W., Bertone P., and Smith A. (2015) Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 162, 452–453 10.1016/j.cell.2015.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Leitch H. G., McEwen K. R., Turp A., Encheva V., Carroll T., Grabole N., Mansfield W., Nashun B., Knezovich J. G., Smith A., Surani M. A., and Hajkova P. (2013) Naive pluripotency is associated with global DNA hypomethylation. Nat. Struct. Mol. Biol. 20, 311–316 10.1038/nsmb.2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hanover J. A., Krause M. W., and Love D. C. (2012) Linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 13, 312–321 10.1038/nrm3334 [DOI] [PubMed] [Google Scholar]
- 95. Miura T., and Nishihara S. (2016) O-GlcNAc is required for the survival of primed pluripotent stem cells and their reversion to the naïve state. Biochem. Biophys. Res. Commun. 480, 655–661 10.1016/j.bbrc.2016.10.111 [DOI] [PubMed] [Google Scholar]
- 96. D'Aniello C., Habibi E., Cermola F., Paris D., Russo F., Fiorenzano A., Di Napoli G., Melck D. J., Cobellis G., Angelini C., Fico A., Blelloch R., Motta A., Stunnenberg H. G., De Cesare D., et al. (2017) Vitamin C and l-proline antagonistic effects capture alternative states in the pluripotency continuum. Stem Cell Rep. 8, 1–10 10.1016/j.stemcr.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shen Y., Matsuno Y., Fouse S. D., Rao N., Root S., Xu R., Pellegrini M., Riggs A. D., and Fan G. (2008) X-inactivation in female human embryonic stem cells is in a nonrandom pattern and prone to epigenetic alterations. Proc. Natl. Acad. Sci. U.S.A. 105, 4709–4714 10.1073/pnas.0712018105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sperber H., Mathieu J., Wang Y., Ferreccio A., Hesson J., Xu Z., Fischer K. A., Devi A., Detraux D., Gu H., Battle S. L., Showalter M., Valensisi C., Bielas J. H., Ericson N. G., et al. (2015) The metabolome regulates the epigenetic landscape during naïve to primed human embryonic stem cell transition. Nat. Cell Biol. 17, 1523–1535 10.1038/ncb3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hore T. A., von Meyenn F., Ravichandran M., Bachman M., Ficz G., Oxley D., Santos F., Balasubramanian S., Jurkowski T. P., and Reik W. (2016) Retinol and ascorbate drive erasure of epigenetic memory and enhance reprogramming to naïve pluripotency by complementary mechanisms. Proc. Natl. Acad. Sci. U.S.A. 113, 12202–12207 10.1073/pnas.1608679113 [DOI] [PMC free article] [PubMed] [Google Scholar]