Abstract
Soybean (Glycine max (L.) Merrill) is a globally important crop, providing oil and protein. Diaporthe/Phomopsis complex includes seed-borne pathogens that affect this legume. Non-thermal plasma treatment is a fast, cost-effective and environmental-friendly technology. Soybean seeds were exposed to a quasi-stationary (50 Hz) dielectric barrier discharge plasma operating at atmospheric pressure air. Different carrying gases (O2 and N2) and barrier insulating materials were used. This work was performed to test if the effects of non-thermal plasma treatment applied to healthy and infected seeds persist throughout the entire cycle of plants. To this aim, lipid peroxidation, activity of catalase, superoxide dismutase and guaiacol peroxidase, vegetative growth and agronomic traits were analysed. The results here reported showed that plants grown from infected seeds did not trigger oxidative stress due to the reduction of pathogen incidence in seeds treated with cold plasma. Vegetative growth revealed a similar pattern for plants grown from treated seeds than that found for the healthy control. Infected control, by contrast, showed clear signs of damage. Moreover, plasma treatment itself increased plant growth, promoted a normal and healthy physiological performance and incremented the yield of plants. The implementation of this technology for seeds treatment before sowing could help reducing the use of agrochemicals during the crop cycle.
Keywords: Agricultural science, Biochemistry, Biophysics, Plant biology
1. Introduction
Among the most important crops worldwide, soybean (Glycine max (L.) Merrill) ranks sixth with a production of 347 million of metric tons in 2017–2018 (USDA, 2018). In Argentine, soybean farming has experienced an explosive growth that has placed it as the number one crop, achieving a sown area of 19 million of hectares and a production yield of 57 million metric tons in the last campaign (Terré, 2018). Pathogens and animal pests are known to cause great economical losses all over the world, as they have the potential to reduce crop production severely (Oerke, 2006). Diseases have been threatened soybean yields for the last decades in major world producing countries (Wrather et al., 2010), deriving in financial damage to rural economies and to the economies of the allied industries. More specifically, in our country, losses in soybean yield due to fungal diseases may reach values of 20%, so their adequate management is considered one of the most important technical challenges for improving the productiveness of the crop (Montoya, 2015; Fuentes, 2016; Mesquida, 2018). Diaporthe/Phomopsis (D/P) complex includes some of the most harmful pathogens that affect soybean. Diaporthe caulivora and D. aspalathi are the causal agents of the stem canker (CTS). D. sojae and D. longicolla cause pod and stem blight (TTV) and seed decay (DDS) (Grijalba et al., 2011; Grijalba and del Ridao, 2014; Sánchez et al., 2015; Li et al., 2016; Dissanayake et al., 2017). These fungi may survive unfavourable growth conditions as dormant mycelia within infected seeds, thus both, primary infections (Malvick, 1997; Grijalba and Ridao, 2012) as well as the spreading of the diseases to new areas (Singh and Mathur, 2004; Rossi and Ridao, 2011), result from their dissemination. The causal agents of TTV and DDS may reduce germination, rot the seeds or produce latent infections in seedlings (which may lead to damping-off), while in adult plants they may provoke anticipated death, abortion or poor pod filling, thus, reducing crop yield. The causal agents of the CTS are very destructive and may reduce severely the stand of plants in the field (Rossi and Ridao, 2011; Grijalba and del Ridao, 2016).
Under biotic stress conditions (e.g. infections by pathogenic fungi), a rapid and transient production of huge amounts of ROS (“oxidative burst”) usually occurs and triggers plant antioxidant defences (Wojtaszek, 1997; El-Maarouf-Bouteau and Bailly, 2008), programmed cell death and stomatal changes (Apel and Hirt, 2004; Laloi et al., 2004). Most plant cells possess the ability to detoxify the Reactive Oxygen Species (ROS), that occur in cells as inevitable by-products of normal metabolism or under stressful situations, by protective mechanisms (antioxidant defences) that allow them to maintain the lowest possible levels of ROS inside the cell. In some circumstances, plant defences may be exceeded by accumulation of ROS, leading to cell damage and death. ROS-induced cell death can result from lipid peroxidation, protein oxidation, enzyme inhibition and DNA and RNA damage (Mittler, 2002). Decrease in cell membrane integrity, as a result of oxidative damage, can be measured as the activation of lipid peroxidation through the quantification of malondialdehyde (MDA) formed from the decomposition of lipid peroxidation products (TBARS assay) (Gunes et al., 2007).
According to Bilgin et al. (2010) when facing a biotic stressor, massive defence responses are initiated in plants and this process has always a cost in terms of growth and fitness, as plant must allocate sources from growth to defence, which involves reductions of photosynthetic capacity. Focusing on the interaction fungus-plant, fungi can release specific compounds that may influence stomatal closure or directly interfere with water transport (Chaerle et al., 2004). Literature suggests that leaf temperature inversely correlates with transpiration and stomatal conductance (Jones, 1999). A reduction of stomatal conductance induced by pathogens could reduce photosynthesis, affecting directly on plant growth (Luque et al., 1999; El Omari et al., 2001). A reduction in the photochemical yield of photosystem II (ΦPSII) is a common response of plants to fungal infections (Chou et al., 2000; Swarbrick et al., 2006; Kuckenberg et al., 2009; Prokopová et al., 2010; Muniz et al., 2014; Granum et al., 2015; Tatagiba et al., 2015; Ajigboye et al., 2016; Brugger et al., 2018). In this sense, many authors have successfully addressed the detection of fungal diseases using thermal imaging (Chaerle et al., 2004; Aldea et al., 2006a,b; Stoll et al., 2008; Oerke et al., 2011; Calderón et al., 2015) and monitoring the behaviour of the photosynthetic activity of plants through the measure of the ΦPSII. On the other hand, plants may synthesize specific compounds that can alter the absorption of light impacting on leaves and change the spectrum of reflected, re-emitted, and transmitted light. UV-excited blue–green fluorescence imaging allows the visualization of the accumulation of secondary compounds associated with the response of plants to pathogens (Buschmann and Lichtenthaler, 1998; Cerovic et al., 1999).
The growing world demand for food, together with the paradigms of food security, sustainable agriculture and care for the environment, propose to the food industry (specially to agriculture) the challenge of supplying progressive quantities of food that fulfil the requirements of being economically affordable, safe for humans and clean for the environment. In this regard, there is a worldwide growing interest in pest management strategies with minimum impact on crop yield, human health and the environment. According to Oerke (2006), the use of chemical fungicides for seed treatment and for diseases management in the field is the most common (and sometimes the unique) practice used by farmers to prevent crop losses, but their employment does not mean significant success. Although agrochemicals offer protection for crops and help improving yields, they may constitute potential hazards for human health and for the environment when they are indiscriminate and inadequate used. Some of the chemicals may remain in the environment persistently, bio-accumulate and/or provoke toxicity to the biotic components of the agroecosystems (Ampofo et al., 2009). In addition, the intensive use of fungicides has historically led to the appearance of resistant fungal strains (Hahn, 2014). Integrated Pest Management (IPM) is defined as a package of tools and decision aids for disease management that employs the least disruptive options and reduces the use of pesticides to the lowest practical levels. In an era where food security and sustainable agriculture are a priority, IPM programs are being widely adopted by all pest control disciplines (Jacobsen, 1997; Razdan and Sabitha, 2009). In this scene, non-thermal plasma arises as a novel and promising technology that may be incorporated to IPM programs as it allow reducing seed-borne pathogens while enhancing seed quality in a fast, cost-effective and eco-friendly way (Pérez Pizá et al., 2018).
Non-thermal plasmas (NTP) are (quasi-neutral) partially ionized gases, representing a unique state of matter composed of neutral atoms and molecules, radicals, excited states, ions and electrons (Misra et al., 2016). Non-thermal plasmas have characteristic electron energies of a few eV to 10 eV with ionization degrees that are typically small. These energetic electrons can efficiently generate radicals, charged species, excited states and ultraviolet photons. Most of non-thermal plasmas significantly deviate from thermodynamic equilibrium, with the electron temperature being much higher than the heavy particle (or gas) temperature. NTP sources can produce a chemically rich environment at close to room temperature both at reduced and at ambient pressures, a unique condition that enables the delivery of highly reactive plasma species in a non-destructive and beneficial way to even extremely heat sensitive surfaces as biological tissues (Laroussi, 2005; Adamovich et al., 2017). Among different kind of non-thermal atmospheric-pressure plasma sources, the dielectric barrier discharges (DBD) operate at strongly non-equilibrium conditions and at reasonable high-power levels, allowing a large number of technological applications. The dielectric barrier is responsible for a self-pulsing plasma operation and thus, the formation of a NTP at normal pressure. Because of the capacitive character of the discharge arrangement, alternating or pulsed high voltage is required (Brandenburg, 2017). Depending on their composition and on the structure and composition of the exposed cells, plasmas may disturb biological tissues differently. Several authors showed that non-thermal plasmas are able to inhibit different kind of fungi: food contaminant, clinical and phytopathogenic (Basaran et al., 2008; Sun et al., 2011; Daeschlein et al., 2014; Zhang et al., 2014; Dasan et al., 2016; Bartoš et al., 2017). As non-thermal plasma treatments are known to have multiple mechanisms of action, ranging from intracellular DNA fracture and protein degeneration to oxidation of the outer membrane of fungi (Moisan et al., 2001; Ma et al., 2004), the evolution of microbial resistance is enormously counteracted (Shama and Kong, 2012). On the other hand, studies carried out using different vegetal species have shown that seed treatment by cold plasmas is able to stimulate germination and seedling growth (Jiang et al., 2014; Ling et al., 2016; Jiayun et al., 2014; Stolarik et al., 2015; Ji et al., 2016; Meng et al., 2017; Zhang et al., 2017; Strejckova et al., 2018). Será et al. (2010) verified that the active particles that conform cold plasmas could penetrate through the seed coats and directly interact with the cells inside. These interactions may result in the stimulation of natural signals (e.g. growth factor) (Dobrynin et al., 2009), the alteration of the activities of reserve utilization enzymes (Ling et al., 2016) and the regulation of the demethylation levels of certain genes (Zhang et al., 2017), all of which lead to promote germination and seedling growth. The use of plasma technology does not alter genetic material of seeds (Zivkovic et al., 2004; Jiafeng et al., 2014; Randeniya and Groot, 2015). It is considered not only suitable but also profitable for seed treatment because of its several advantages: 1) provides uniform treatments, 2) does not disturb the function of tissues, 3) does not require chemicals, and 4) no pollutants are produced (Jiang et al., 2014; Ling et al., 2016).
Among the scientific knowledge available actually, there are only a few evidences of the effects of seed treatment with cold plasmas on adult plants grown from those seeds (Jiayun et al., 2014; Jiafeng et al., 2014; Ling et al., 2016; Kriz et al., 2017; Strejckova et al., 2018). Therefore, the main aim of this study was to investigate whether the improvement of soybean seed induced by NTP treatment can persist at different plant stages, focusing on: growth, antioxidant defences, physiological parameters (related to primary and secondary metabolism) and agronomic traits at maturity.
2. Materials and methods
2.1. Plant material
DM 53i53 IPRO soybean seeds (Don Mario Semillas S.A., Buenos Aires, Argentina) were employed in all the experiments carried out in this study. A group of seeds completely free from D/P complex and other fungi, were used as the “healthy control” (HC) and were not treated with plasma. On the other hand, highly infected seeds (with 16 % of D/P complex incidence) were used as the “infected control” (IC).
2.2. Non-thermal plasma treatments
The discharge consisted in a needle-array power electrode and a plate ground electrode covered by a dielectric barrier of either 3 polyester films (Thernofase) or an arrangement of a thin phenolic sheet (Pertinax) and 2 polyester films (Myllar). The gap between the upper surface of the barrier and the tip of the needles was fixed to 10 mm during the experiment. The power supply was a high-voltage sine AC power supply (0–25 kV) operating at 50 Hz. Oxygen and nitrogen were alternatively injected into the discharge active region as carrier gases (measured gas-flow rate of 6 NL min−1). The setup of the experimental prototype is described in detail in Pérez Pizá et al. (2018).
Non-thermal plasma treatments were performed on seeds as previously reported on Pérez Pizá et al. (2018); 500 soybean seeds were placed in the active plasma region on the dielectric barrier and mechanically moved to ensure uniform treatment. During the experiments, seeds were treated with plasma exposure times ranging from 60 to 180 s for each dielectric configuration and carrying gas (Table 1). After treatment, seeds were preserved in sterilized vessels until biological experiments started.
Table 1.
Description of non-thermal plasma treatments.
| Group | Seed | Barrier | Gas | Time (min) | Nomenclature |
|---|---|---|---|---|---|
| Treatments | Healthy | Pertinax-Mylar | N2 | 3 | H + PMN3 |
| O2 | 2 | H + PMO2 | |||
| Thernofase | N2 | 1 | H + TN1 | ||
| O2 | 3 | H + TO3 | |||
| Infected | Pertinax-Mylar | N2 | 3 | I + PMN3 | |
| O2 | 2 | I + PMO2 | |||
| Thernofase | N2 | 1 | I + TN1 | ||
| O2 | 3 | I + TO3 | |||
| Controls | Infected control | IC | |||
| Healthy control | HC | ||||
| Control with fungicide | FC | ||||
2.3. Seed quality
HC, IC and plasma-treated seeds were analysed for germination employing Top of Sand (TS) germination test for soybean (ISTA, 2014). Four replicates of fifty seeds were performed. Germination percentages represent the proportion by number of seeds which have produced normal seedlings under 25 °C, light/dark (12/12 h) within 7 days.
| G (%) = (Number of normal seedling/total number of seeds) × 100 | (1) |
Four replicates of fifty seeds were guided on parallel tests in order to analyse other aspects of germination. The germination percentage was recorded every 48 h for 6 days, following the criterion for germination sensu stricto (Fercha et al., 2016). Total weight and length of seedlings were measured on day 6. Plant material for dry weight was dried at 80 °C for 120 h. Germination rate (GR) and Production rate (PR) were calculated according to Zhang et al. (2017). Vigour index I and II were calculated according to Abdul-Baki and Anderson (1973).
| GR (%) = (Number of germinated seeds in 6 days/total number of seeds) × 100. | (2) |
| PR (%) = Seedling weight (g) on day 6 × GR (%)/seed weight (g). | (3) |
| VI = Seedling length (cm) × GR (%)/100. | (4) |
| VII = Dry weight of seedling (g) × GR (%)/100. | (5) |
The electrical conductivity of seeds leachates was measured as an assessment of the extent of electrolyte leakage from plant tissues which gives an estimation of seed vigour (ISTA, 2014). Four replicates of 50 seed were weighed and soaked in flasks containing 250 mL deionised water. After 24 hours at 25 °C, the measurements were taken employing a portable conductivity meter (Thermo Scientific Orion 10500 105A).
| EC (μS cm−1 g−1) = EC sample − EC water (μS cm−1 g−1)/seed weight (g) | (6) |
2.4. Growing conditions
Seeds were sown and plants grown in sterilized vermiculite in 1-liter plastic pots (1 plant per pot) and maintained in a growth chamber with a 16-h photoperiod and day/night temperatures of 24/25 °C. The photosynthetic photon flux density was 350 μE m−2 s−1. Plants were irrigated every day with half-strength Hoagland solution (Leggett and Frere, 1971). HC, IC and plasma-treated seeds were sown and plants were let to grow until they arrive to the stage V2 – first trifoliate leaf completely developed (Fehr et al., 1971) to analyse lipid peroxidation and antioxidants activities. Another group of plants, grown only from infected-treated seeds, were let to grow until they arrive to the stage V3 – second trifoliate leaf completely developed (Fehr et al., 1971) in order to evaluate their physiological state in relation to D/P diseases, in contrast with the non-treated controls, healthy and infected. With that purpose, thermal and UV-excited blue–green fluorescence imaging were performed and the behaviour of the photosynthetic activity was monitored in the studied plants.
One of the plasma treatments (PMN3) was chosen in order to guide a forth experiment with the aim of measuring biometrical parameters and agronomic traits in plants at the stage R8 – full maturity (Fehr et al., 1971). In this instance, seeds belonging to the infected group were treated with 1 μl g−1 of fungicide Maxim® Evolution (fludioxonil 2.5 g + metalaxil-M 2.0 g + tiabendazole 15 g) before sowing, constituting the fungicide control (FC). The experiment was conducted under greenhouse conditions (temperature max 45.6 °C, min 12.2 °C, mean 27.4 °C). Each pot constituted an experimental unit (EU) and included two plants. Pots were filled with GROWMIX® MULTIPRO™ (peat moss Sphagnum of medium fibre, bark compost, calcite lime, dolomite lime and wetting agents, pH: 4.9, 120 g kg−1 nitrogen, 140 g kg−1 of P2O5, 240 g kg−1 of K2O) mixed with sand in a proportion 10:1 (v/v); 12 mg of triple super phosphate (CaH2PO4) was added to each pot before sowing. The EUs were distributed randomly assuring a density of 16 plants m−2. Sowing was performed on December 1st (2017), after inoculating seeds with 1 μl g−1 of Signum® (Bradyrhizobium sp).
2.5. Lipid peroxidation
Each trifoliate leaf (1 g) was ground with 10 mL of 20% (w/v) trichloroacetic acid (TCA). Homogenates were centrifuged at 3000 g for 20 min and the resulting supernatants were used for malondialdehyde (MDA) determination (Heath and Packer, 1968). Six biological and two technical replicates were performed. The mixture for each assay contained 500 μL of the supernatant, 500 μL of 20% TCA with 0.5% (w/v) thiobarbituric acid (TBA) and 50 μL of 4% butylated hydroxytoluene (BHT) in ethanol. Mixtures were heated at 95 °C for 30 minutes, centrifuged at 3000 g for 10 minutes. Finally, thiobarbituric acid-reactive substances (TBARS) were measured at 532 nm (A1); inespecifics absorbances were determined at 600 nm (A2). TBARS concentrations were obtained through the difference of absorbances (A1-A2) and employing the MDA extinction coefficient (155 mM−1 cm−1). Results were expressed as nmol MDA g tissue−1.
2.6. Antioxidants activities
Each trifoliate leaf was cut in pieces and 0.3 g of tissue were ground and homogenized with 1 mg polyvinylpyrrolidone (PVP) in 3 mL of 50 mM phosphate extraction buffer (pH 7.7) containing 0.5 mM EDTA and 0.5% (v/v) of Triton X-100. Six biological and two technical replicates were performed. The homogenates were centrifuged for 30 minutes at 13000 g and 4 °C. The supernatant fraction was used for the assays. Protein content of the extracts was determined according to the method of Bradford (1976). Catalase activity (CAT) was measured by the decrease in absorbance of H2O2 at 240 nm (Chance et al., 1979). The enzyme assay contained 2 mM H2O2 in 50 mM phosphate buffer (pH 7.0) and 20 μL of enzyme extract in a total volume of 1 mL. Results were expressed in μmol min−1 mg prot−1. For Superoxide dismutase (SOD) activity, the reaction mixture contained 2.2 μM riboflavine, 14.3 mM methionine, 82.5 μL nitro blue tetrazolium (NBT) in 50 mM phosphate buffer (pH 7.7), and 25–200 μL of enzyme extract in a final volume of 3 mL. SOD activity was assayed by measuring the ability of the enzyme extract to inhibit the photochemical reduction of NBT (Becana et al., 1986). Glass test tubes containing the mixture were shaken and placed 30 cm from a light bank of six 15 W fluorescent lamps. The reaction was allowed to run for 10 minutes and then stopped in the darkness; the reduction in NBT was followed by reading absorbance at 560 nm every 2 minutes. One unit of SOD was defined as the enzyme activity which inhibited the photo-reduction of NBT to blue formazan by 50%, and SOD activity of the extracts was expressed as U mg protein-1. Guaiacol peroxidase (GPOX) activity was determined according to Balestrasse et al. (2006), by the oxidation of guaiacol measured at 470 nm (ε: 26,6 mM−1 cm−1) and it was expressed in μmol min−1 mg prot−1. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.2), 2 mM H2O2, 10 mM guaiacol and 150 μL of enzyme extract.
2.7. Evaluation of vegetative growth
2.7.1. Biometric parameters
Plant material for dry weight was dried at 80 °C for 120 h. Fresh and dry weights were registered employing an analytical balance (0.001 g). Root and stem lengths were measure employing a measurement tape. The area of each trifoliate leaf was determined employing the non-destructive millimetre graph paper method (Pandey and Singh, 2011).
2.7.2. Determination of photosynthetic activity
The photosynthetic activity was evaluated as quantum yield of photosystem II (ΦPSII = F′M-FS/F′M; Genty et al. (1989)) using a PAM-101 chlorophyll fluorometer (Walz, Effeltrich, Germany). Three measurements were taken upon the central leaflet of the second trifoliate leaf. Sample size was 6 for every treatment, except for IC with n = 5. To measure fluorescence at the stationary state (FS), white light was used as actinic light 350 μE m−2 s−1 (equivalent to the light intensity in the growth chamber). For each maximum fluorescence in the light (F′M) measurement, a single saturating pulse was 13000 μE m−2 s−1.
2.7.3. Chlorophyll content
Method 1: Total chlorophyll content was measured using a CL-01 Chlorophyll Content Meter (Hansatech Instruments, Norfolk, UK). Four measurements per leaflet were taken upon the second trifoliate leaf and then averaged.
Method 2: Leaves (0.5 g of fresh weight) were homogenized with 96% ethanol (1: 30 w/v). Extracts were heated in a boiling bath until complete bleaching. The absorbance was measured in the supernatant at 665, 649, and 654 nm as described by Wintermans and de Mots (1965).
2.7.4. Thermal imaging
Thermal images of leaves were taken in the growth chamber with a FLIR A305sc infrared camera (FLIR System, Wilsonville, OR, USA) according to Pérez-Bueno et al. (2016). Average temperatures were determined on the second trifoliate leaf, considering three leaf areas, using the software FLIR Research and Development software version 3.4. Average temperatures of each plasma treatment and the HC were obtained from eight plants. Five plants were used to obtain the average temperature of the IC. Images correspond to standard experiments.
2.7.5. Multicolour fluorescence imaging
The activity of the secondary metabolism was analysed in terms of multicolour fluorescence excited by UV light at 360 nm using an Open FluorCam FC 800-O (Photon Systems Instruments, Brno, Czechia). Blue (F440) and green (F520) fluorescence images of the adaxial side of leaves were recorded according to Pérez-Bueno et al. (2015). Image processing was performed employing the FluorCam software version 7.1.0.3 (Photon Systems Instruments).
For each fluorescence parameter and F440/F520 ratio, average values were calculated on the same three leaf areas of the second trifoliate leaf. Six plants were analysed for each plasma treatment and the HC, and four plants for the IC. Images correspond to standard experiments.
2.8. Biometric parameters and agronomic traits at maturity
Root length and height of plants were measure employing a measurement tape. A Vernier caliper was employed for measuring the diameter of stems. Plant material for dry weight was dried at 80 °C for 120 h. Fresh and dry weights were registered employing an analytical balance (0.001 g). The total number of pods and seeds were determined by counting the total pods/seeds harvested from each EU. Material for dry weight of seeds was dried at 80 °C for 120 h. Dry weight of seeds was determined by weighing the total seeds produced per EU. Dry weight of 1000 seeds was calculated from the preceding values and 13.5 g were added to each dry weight in order to obtain the total weight of 1000 seeds with a moisture content of 13.5%.
2.9. Statistical analysis
All data presented correspond to the mean value ± standard error (SE) of the correspondent number of replicates. Analyses were performed using the statistic software package RCommander version 3.1.2 (2014). The variance (p < 0.05) of data was analysed by one-way analysis of variance (ANOVA), after testing for the assumption of the normal distribution. Dennett's test were performed for many-to-one comparisons and Tukey's tests for comparing all the possible pairs of means (p < 0.05).
3. Results and discussion
3.1. Effect of non-thermal plasma treatment on seed quality
The recovery of soybean seed quality following cold plasma treatments (PMN3, PMO2, TN1 and TO3) was evidenced in our previous study (Pérez Pizá et al., 2018), where reductions of about 49–81% from the initial percentage of D/P incidence were achieved. Significant stimulatory effects on germination were also demonstrated, suggesting that plasma treatments helped seeds to germinate by reducing pathogen presence in tissues. In the present study, we evaluated healthy seeds in order to investigate the effects of plasma per se on seeds.
As Fig. 1 shows, germination was enhanced by plasma treatments. Germination (Fig. 1A) of healthy treated seeds showed increments of 5–7 % compared with the HC. The behaviour of this parameter indicated that plasma was capable of stimulating development of a major number of normal seedlings, not only starting from infected (Pérez Pizá et al., 2018) but also from healthy seeds. The PR (Fig. 1B) incremented 10–23 % when treating healthy seeds. These results demonstrated not only that treated seeds germinated properly but also that seedling growth was enhanced by plasma treatment per se, regardless the incidence of pathogens (Pérez Pizá et al., 2018). These observations are in agreement with many authors (Jiafeng et al., 2014; Ji et al., 2016; Li et al., 2017; Meng et al., 2017; Hosseini et al., 2018; Strejckova et al., 2018) who demonstrated that the exposure of healthy seeds to cold plasma can considerably improve the germination rate and seedling growth of a variety of species.
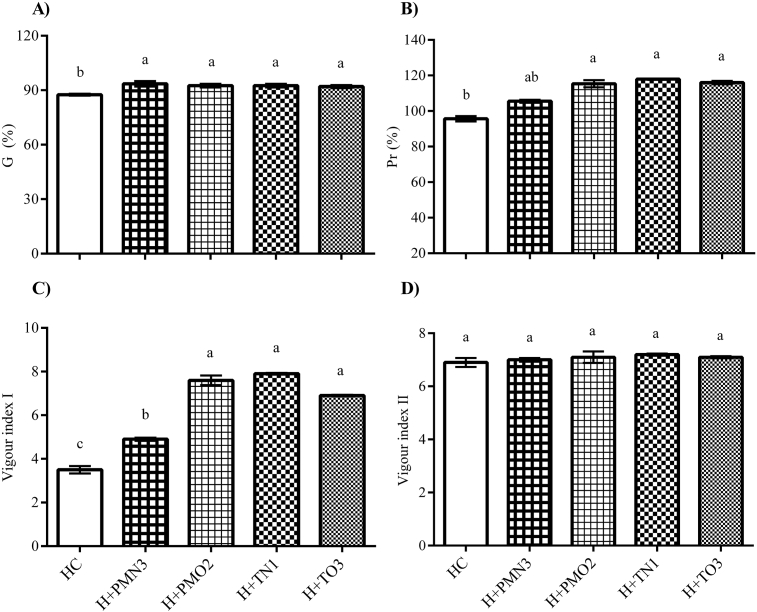
Fig. 1.
Effect of cold plasma on the quality of healthy seeds. Error bars indicate standard error (n = 4). Different lowercase letters denote statistical differences between groups (Tukey's test, P < 0.05).
Vigour Index I (Fig. 1C) was stimulated by plasma treatments. The VI I of treated seeds incremented 1.4–2.25 times compared with the HC. These increases correlated positively with seedling length and fresh weight, which is in agreement with the results documented by Ling et al. (2014) and Zhang et al. (2017) regarding soybean. According to Dobrynin et al. (2009) and Ji et al. (2016), the interaction between plasma and vegetal cells may result in the stimulation of certain natural signals, hormones and enzymes activities, which might explain the changes in germination and early seedling growth observed in our experiment.
Vigour Index II (Fig. 1D) showed no differences between treatments and controls, due to the absence of modifications in total dry weight of seedlings. Dry weight is often used as a quantitative measure of growth and it provides the amount of dry matter, thus, it includes everything but the water (Ördög and Molnár, 2011). In this sense, our results suggested that the stimulation of seedling growth (evidenced by VI I and PR) responded exclusively to changes in cell water content. Considering that at 6 days old seedlings were in a stage of growth in which they still depended on the reserves stored in cotyledons, we understand that increments in dry weight would not occur until seedlings obtained photosynthetic independence.
The electrical conductivities of seed leachates are showed on Table 2. Healthy seeds treated with plasma showed a lower electrical conductivity (18–27 %) than the HC. The same pattern was followed by infected seeds treated with plasma, with decreases of about 12–25 % respect to IC. Considering that the base of EC test is the relation between the organizational level of cellular membranes and the quantity of leachates released to the soaking solution (Wain-Tassi et al., 2012), the amounts of leachates released by seeds indicate the speed of restoring cell membrane integrity (Carvalho et al., 2009). In this sense, the lower electrical conductivities showed by treated seeds (healthy or infected) compared to their correspondent controls, derived from low quantity of leachates released to the soaking solution due to the higher ability of seeds to restore the integrity of cell membranes within 24 h of imbibition. Considering that the changes regarding this parameter did not depend on the healthy-infected condition, we attributed this effect to the plasma treatment itself.
Table 2.
Effect of cold plasma on the electrical conductivity of seeds.
| Group | EC (μS cm−1 g−1) |
|---|---|
| HC | 30,41 ± 1,3c |
| H + PMN3 | 24,44 ± 1,8d |
| H + PMO2 | 25,04 ± 1,6d |
| H + TN1 | 22,21 ± 0,6d |
| H + TO3 | 23,89 ± 1,0d |
| IC | 45,97 ± 1,2a |
| I + PMN3 | 34,27 ± 1,9b |
| I + PMO2 | 39,81 ± 0,8b |
| I + TN1 | 38,33 ± 1,3b |
| I + TO3 | 40,60 ± 1,4b |
Data show mean values of four replicates ± standard error. Different lowercase letters denote statistical differences between the groups (Tukey's test, P < 0.05).
From the preceding results, we confirmed that plasma treatments enhanced seed quality (germination, vigour and health) thereby we infer that the use of this technology may increase the probability of having good performances in the field when employing treated seeds.
3.2. Effect of non-thermal plasma seed treatment on plant growth
Soybean plants grown from plasma treated seeds and from healthy and infected non-treated controls were monitored for 2–3 weeks. Fig. 2 shows the effects of different seed treatments on biometrical parameters of soybean plants of 16 days in the stage V2 (Fehr et al., 1971), which general aspects are shown in Fig. 3.
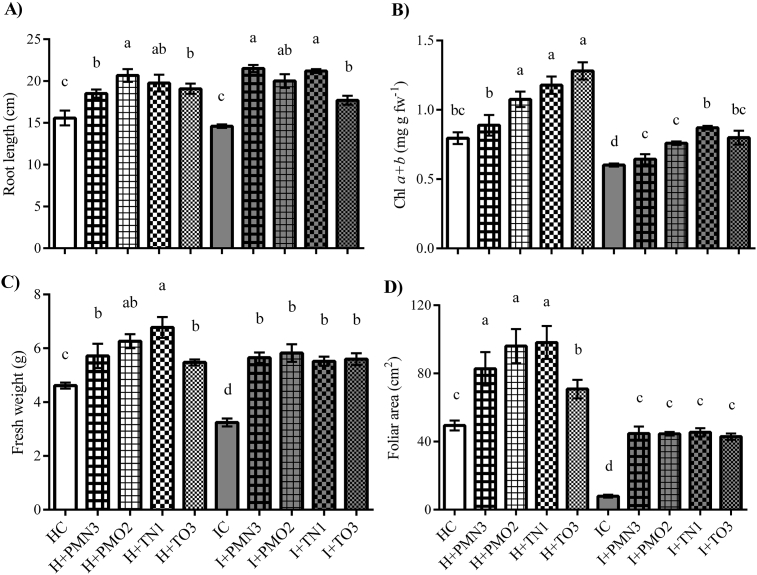
Fig. 2.
Biometrical parameters and foliar chlorophyll content of plants (V2 stage) grown from infected and healthy seeds treated with cold plasma (PMN3, PMO2, TN1 and TO3), as well the controls, healthy and infected. Error bars indicate standard error (n = 8). Different lowercase letters denote statistical differences between the groups (Tukey's test, P < 0.05).
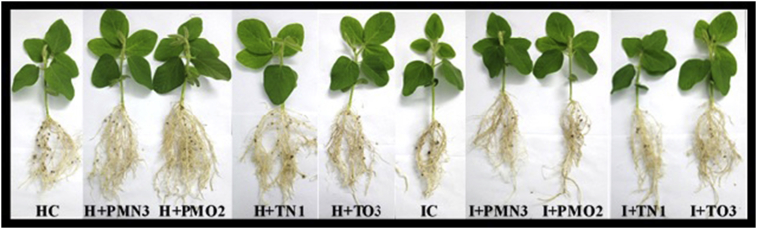
Fig. 3.
Aspect of plants (V2 stage) grown from infected and healthy seeds treated with cold plasma (PMN3, PMO2, TN1 and TO3) as well the negative controls, healthy and diseased.
Fresh weight and root length of plants grown from infected treated seeds (I + PMN3, I + PMO2, I + TN1 and I + TO3) increased significantly in comparison with the IC (70–80% and 20–50%, respectively). On the other hand, plants grown from healthy treated seeds (H + PMN3, H + PMO2, H + TN1 and H + TO3) exhibited significant increases on fresh weight and root length (20–50 % and 20–30%, respectively) compared with the HC. Total leaf area of plants grown from healthy and infected treated seeds was 2 and 5 times higher than the correspondent controls (HC and IC). In general, the improvement effects of plasma treatments when applied to infected seeds are greater in comparison with those obtained when applied to healthy seeds, although the improvement effects of plasma on healthy seeds are quite considerable. In light of the preceding, is possible to affirm that plasma treatments had a dual effect when applied to infected seeds: pathogen removal and growth promotion. The growth promotion effect of seed treatment with non-thermal plasma was described by many authors (Jiang et al., 2014; El-Aragi and Ahmed, 2010; Ji et al., 2016; Ling et al., 2016; Kriz et al., 2017; Kumar et al., 2017; Strejckova et al., 2018), although it has not been proved in advanced stages of soybean growth yet.
On the other hand, focusing in the results obtained for HC and IC plants, the first ones exhibited significantly longer roots (9%), higher fresh weight (41%), and greater leaf area (77%) than the seconds. Taking into account that the only difference between both groups was the presence of D/P fungi in seeds, the observed variations in plants biometry were attributed to fungal pathologies.
3.3. Oxidative stress and antioxidant response of seedlings
In the present study, different parameters were analysed in order to evaluate oxidative stress and antioxidant profile of plants grown plasma-treated seeds. In our previous research (Pérez Pizá et al., 2018), we demonstrated that seeds did not experiment oxidative stress when exposed to different plasma treatments, despite it is well known that plasmas are composed by many different kinds of reactive species (Niemira, 2012; Misra et al., 2016) that may cause damage to the exposed cells. On the other hand, we demonstrated that the presence of D/P complex triggered oxidative damage (and antioxidant responses) on infected seeds and that treated seeds exhibited no biochemical signs of oxidative damage, despite of the fact that plasma-mediated pathogen removal was not absolute (Pérez Pizá et al., 2018).
To give continuity to this research, we analysed the oxidative stress and antioxidant profile of plants grown from infected seeds (I + PMN3, I + PMO2, I + TN1, I + TO3), and included the same plasma treatments applied to healthy seeds (H + PMN3, H + PMO2, H + TN1 and H + TO3), as well the controls, healthy and infected.
Lipid peroxidation of leaves (Table 3) showed that plants grown from infected-treated seeds (I + PMN3, I + PMO2, I + TN1 and I + TO3) presented MDA contents between 27 and 36% lower than the IC. No differences were detected between the different treatments and the HC regarding this parameter, neither for healthy nor for infected seeds. The MDA content of the HC plants were 32% lower than the IC group, revealing that the presence of D/P in seeds provoked oxidative damage to plants as a result of infection. In the light of the above and considering the great decreased in D/P presence reached by plasma treatment (Pérez Pizá et al., 2018), the low MDA content observed in plants grown from treated seeds could be attributed to the absence of infection due to plasma-mediated pathogen removal.
Table 3.
Thiobarbituric acid reactive substances (TBARS) and catalase (CAT), superoxide dismutase (SOD) and guaiacol peroxidase (GPOX) activities of leaves of plants grown from cold plasma treated seeds PMN3, PMO2, TN1 and TO3, as well the controls, healthy and infected.
| Group | TBARS (nmol MDA g fw−1) | CAT (pmol mg protein−1) | GPOX (μmol min−1 mg protein−1) | SOD (U mg protein−1) |
|---|---|---|---|---|
| HC | 6,22 ± 0,38a | 20,46 ± 0,92b | 0,19 ± 0,01b | 97,31 ± 2,01b |
| H + PMN3 | 5,97 ± 0,12a | 15,18 ± 1,28ab | 0,17 ± 0,01ab | 94,54± 7,82b |
| H + PMO2 | 5,74 ± 0,16a | 16,58 ± 1,51ab | 0,12 ± 0,01a | 97,74 ± 6,02b |
| H + TN1 | 6,31 ± 0,20a | 11,85 ± 0,11a | 0,11 ± 0,01a | 90,11 ± 4,55b |
| H + TO3 | 6,54 ± 0,12a | 16,51 ± 1,24ab | 0,12 ± 0,01a | 77,20 ± 1,62a |
| IC | 8,32 ± 0,30b | 26,90 ± 2,02c | 0,25 ± 0,03c | 127,61 ± 5,26c |
| I + PMN3 | 5,87 ± 0,30a | 16,36 ± 1,95ab | 0,20 ± 0,01b | 95,39 ± 4,74b |
| I + PMO2 | 6,71 ± 0,37a | 15,61 ± 1,57ab | 0,19 ± 0,01b | 112,15 ± 3,91bc |
| I + TN1 | 5,92 ± 0,32a | 14,49 ± 1,02ab | 0,20 ± 0,01b | 99,52 ± 5,50b |
| I + TO3 | 5,79 ± 0,31a | 13,20 ± 0,8a | 0,19 ± 0,01b | 99,97 ± 5,21b |
Data show mean values of six replicates ± standard error. Different lowercase letters denote statistical differences between the groups (Tukey's test, P < 0.05).
The antioxidant activities of leaves are shown in Table 3. Regarding infected seeds, all the treatments (I + PMN3, I + PMO2, I + TN1 and I + TO3) exhibited values of CAT activity 40–55% lower than the IC, SOD activity of the IC group almost tripled the values of treatments and GPOX activity showed the same behaviour than the previous, except for treatment I + PMO2 that did not differed from the IC. We observed an exacerbated enzymatic activity in IC in comparison with the HC, suggesting that the D/P fungi could had triggered the antioxidant defences in plants as a response to the oxidative damage caused by them. Plants grown from infected-treated seeds showed levels of antioxidant activities close to the healthy condition, and this was consistent with the low content of MDA. The reduction of D/P incidence in seeds reached by plasma treatment probably explained the absence of oxidative damage and antioxidant response in the leaves of the plants grown from them, giving more support to the results of seed quality documented in our previous work (Pérez Pizá et al., 2018).
Regarding plants grown from healthy seeds, plasma treatments (H + PMN3, H + PMO2, H + TN1 and H + TO3) exhibited, in general, lower antioxidant activity than the HC. CAT activity fall 20–40 % and GPOX and SOD activities declined 10–40 % and 5–20 % (respectively). These results were not consistent with those documented by Zhang et al. (2017) who documented increased activities of SOD, POD (peroxidase) and CAT in soybean seedlings grown from plasma-treated seeds. As Fig. 3 shows, plants grown from treated seeds grew faster than the ones belonging to the HC. Literature evidence affirms that plant cycle involves complexes interactions between ROS, antioxidants and phytohormones (Considine and Foyer, 2014) and that during certain events (e.g. leaf growth), transient quantities of ROS are produced and function like signal molecules of different processes (Morales and Munné-Bosch, 2016). In the light of these knowledges, we suggest that the observed differences between plants grown from treated-seeds and HC plants, in regard with enzyme activities, were associated to differential stages of phenology.
3.4. Effect of non-thermal plasma applied to infected seeds on the physiology of plants grown from them
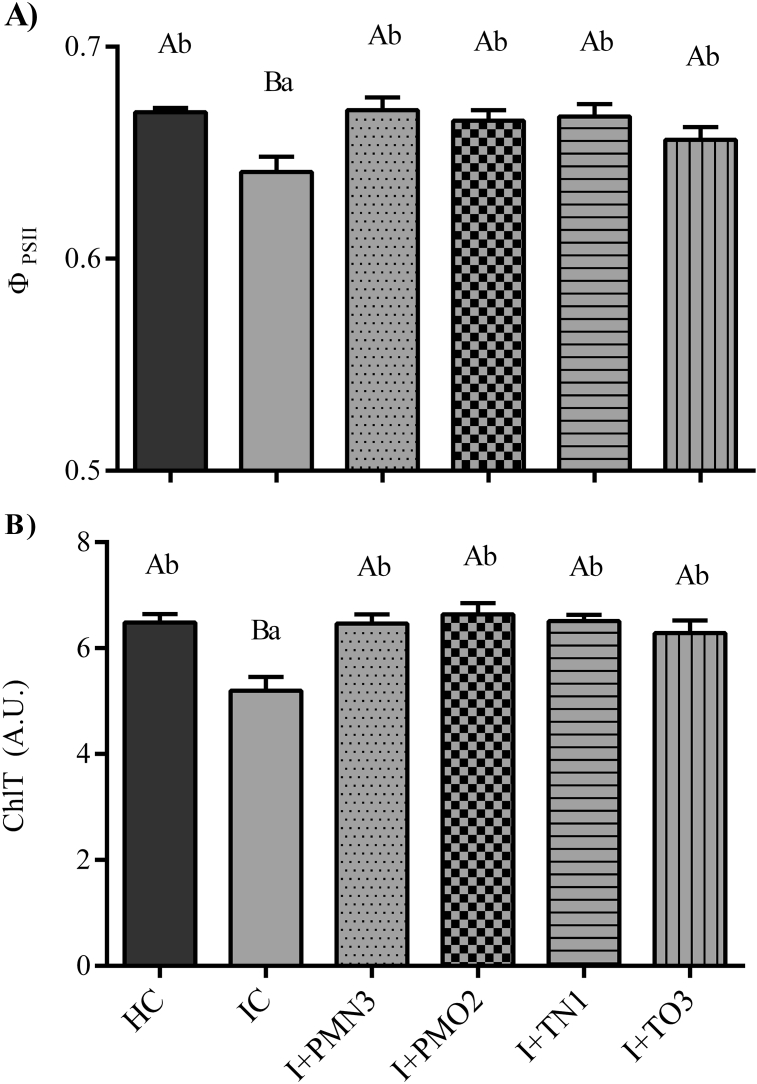
Soybean plants grown from infected seeds treated with plasma and from healthy and infected non-treated controls were analysed at V3 stage with the aim of detecting physiological changes in relation with fungal diseases. Infected control plants reduced significantly their photosynthesis (measured as ΦPSII) respecting to the HC plants (between 2 and 5%), showing a clear impact of the fungal infection on primary metabolism (Fig. 4A). It is worthy to highlight that all plants grown from NTP-treated seeds displayed a photosynthetic performance close to the HC. On the other hand, the total chlorophyll content of leaves (Fig. 4B) of plants grown from NTP-treated seeds presented values similar to HC plants and higher than the IC plants (between 21 and 28%). These results are consistent with a previous work on wheat plants grown from plasma treated seeds (Jiang et al., 2014). It is well known that some fungal infections may induce chlorosis in leaves (Berdugo et al., 2014; Bermúdez-Cardona et al., 2015; Bürling et al., 2011; Leufen et al., 2014; Prokopová et al., 2010) which in turn may affect the photosynthetic efficiency (Berger et al., 2007). Reductions in both photosynthetic activity and chlorophyll content of IC soybean plants respecting to the HC ones correlates negatively with the D/P incidence in seeds (Pérez Pizá et al., 2018), and to our best knowledge, this relation have not been documented up to date in early stages of soybean plant growth.
Fig. 4.
(A) Photosynthetic activity, measured as ΦPSII, (B) total chlorophyll content of leaves of plants (V3 stage) grown from cold plasma treated seeds P + PMN3, P + PMO2, P + TN1 and P + TO3, as well as the controls, HC and IC. Bars indicate standard error. (A) n = 6, except IC with n = 5. (B) n = 8, except IC with n = 6. Different capital letters denote statistical differences between treatment groups and HC; different lowercase letters denote statistical differences between treatment groups and the IC (Dunett's test, P < 0.05).
Leaf temperature of IC plants was 1.9–2.1 °C higher than leaves from HC plants (Fig. 5), suggesting that the presence of D/P complex on seeds of IC plants was the responsible of such increase of temperature in leaves. Moreover, the rise in temperature (pointing to a decrease in stomatal conductance) can be a cause of photosynthesis inhibition found in IC plants. Leaf temperature increments were also found by other authors studying fungal infections (Aldea et al., 2006a,b; Oerke et al., 2006; Stoll et al., 2008; Wang et al., 2012). Remarkably, no differences in leaf temperature were observed between the plants grown from NTP-treated seeds and the HC plants. These results suggest that plasma treatment can counteract the negative effects of D/P complex on leaf temperature and photosynthetic performance.
Fig. 5.
Thermal (T) and F440/F520 images of leaves of plants (V3 stage) grown from cold plasma treated seeds P + PMN3, P + PMO2, P + TN1 and P + TO3, as well the controls, healthy and infected, and their corresponding RGB images.
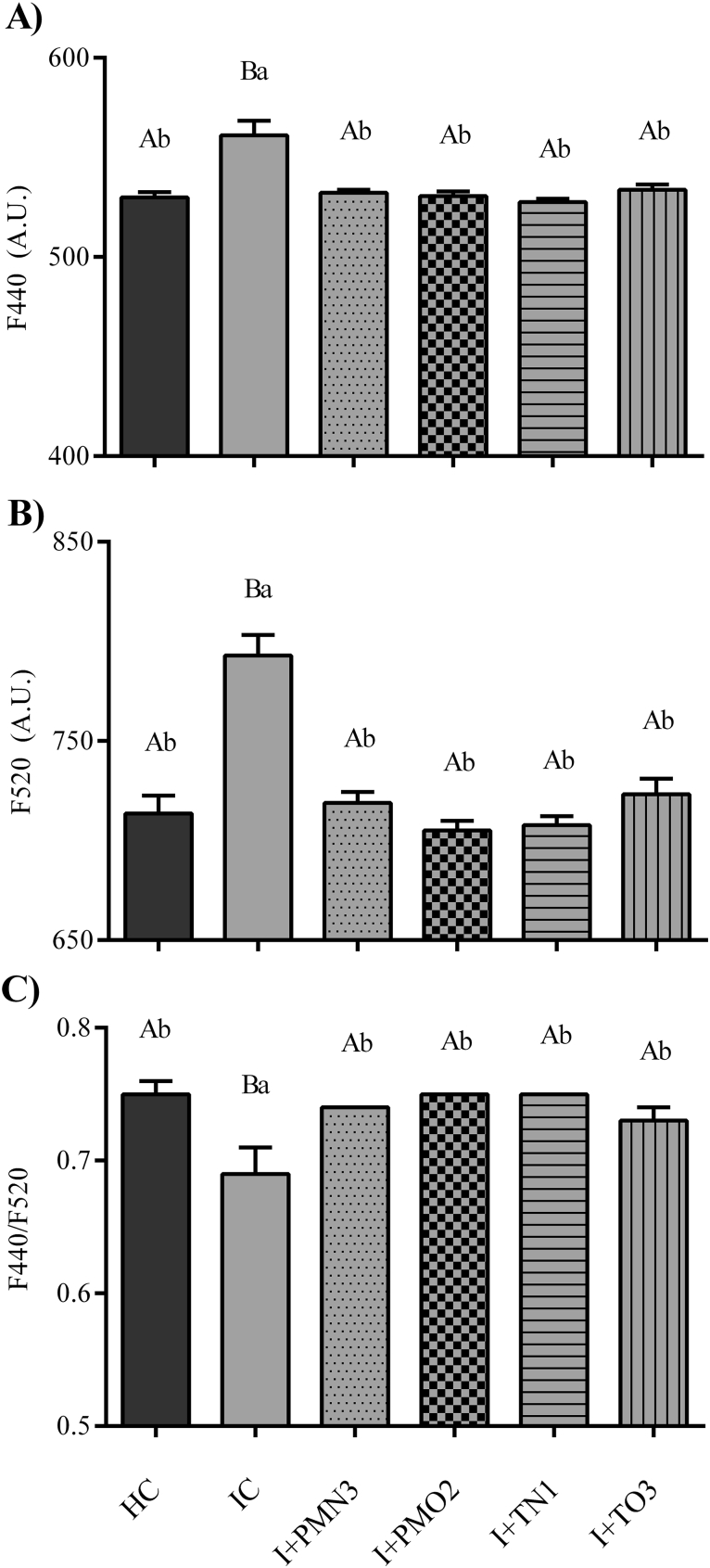
Leaves from plants grown from NTP-treated seeds presented values of blue and green fluorescence lower than the IC plants, and similar to the HC (Fig. 6). A similar increase in blue-green fluorescence of sugar beet plants infected with a powdery mildew has also been demonstrated, and suggested to be explained by a decrease in the chlorophyll content, by an increase of fluorophores or by a combination of both (Leufen et al., 2014). Chlorophylls are able to re-absorb the blue fluorescence, but no the green one (Buschmann and Lichtenthaler, 1998). Thus, the blue-green fluorescence increment detected in leaves of IC plants is only partially caused by the registered decrease in chlorophyll content. It is known that soybean plants biosynthesize phytoalexins as a defence mechanism against the attack of D/P complex (Modolo et al., 2002; Nwachukwu et al., 2013). Such phenolic compounds can account for the increment in blue-green fluorescence (Buschmann and Lichtenthaler, 1998; Cerovic et al., 1999). The fluorescence ratio F440/F520 was lower in leaves of IC plants in comparison with the NTP treatments and the HC plants, which were statistically indistinguishable (Figs. 5 and 6). Bürling et al. (2011) also found that fungal infection diminished this fluorescence ratio due to a decreased chlorophyll content together with a fluorescent secondary metabolites accumulation.
Fig. 6.
(A) Blue fluorescence (F440), (B) green fluorescence (F520) and (C) blue over green fluorescence ratio on leaves of plants (V3 stage) grown from cold plasma treated seeds P + PMN3, P + PMO2, P + TN1 and P + TO3, as well the controls, healthy and infected. Bars indicate standard error. n = 6, except IC with n = 4. Different capital letters denote statistical differences between treatment groups and healthy control; different lowercase letters denote statistical differences between treatment groups and infected control (Dunett's test, P < 0.05).
3.5. Effect of non-thermal plasma seed treatment on the agronomic traits of mature plants
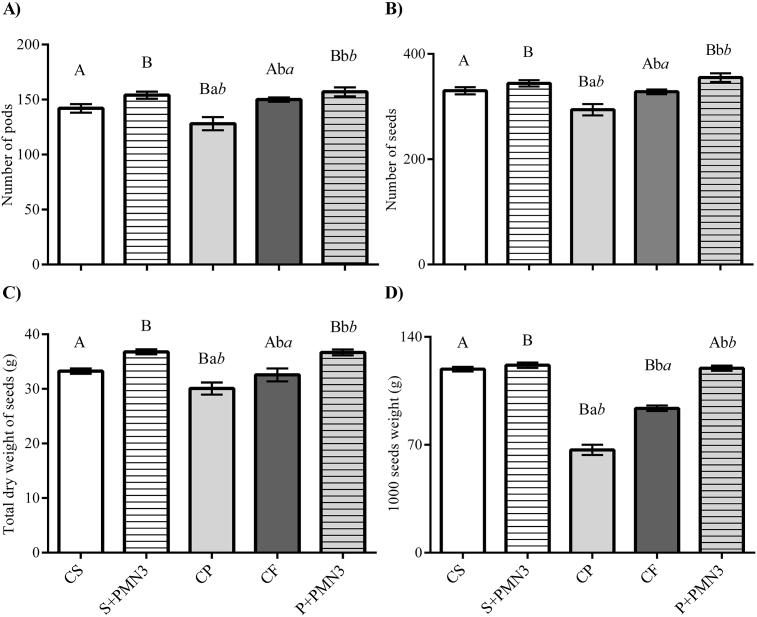
The biometric parameters and agronomic traits of plants at full maturity stage are shown in Table 4 and Fig. 7, respectively. In regard with healthy seeds, plant height, stem diameter and root dry weight of plants from plasma-treated seeds (H + PMN3) showed increases of 3%, 8% and 12% (respectively) compared to HC; the aerial dry weight did not vary significantly. The number of pods and seeds per EU, the total dry weight of seeds and the weight of 1000 seeds of the HC plants were improved by 8%, 4%, 11% and 2%, respectively, by seed treatment with plasma (H + PMN3). These results are consistent with other studies (Meiqiang et al., 2005; El-Aragi and Ahmed, 2010; Abd et al., 2013; Ling et al., 2016; Kumar et al., 2017) that had aborded the effects of non-thermal plasmas on growth and yield of different species (tomato, berseem, alfalfa, peanut, rapeseed and okra), documenting significant improvements in this regard.
Table 4.
Biometrical parameters of plants grown from treated and non-treated seeds, at R8 stage.
| Group | Root dry weight (g) | Aerial dry weight (g) | Height (cm) | Stem diameter (mm) |
|---|---|---|---|---|
| HC | 124,0 ± 0,2A | 70,4 ± 2,0A | 92,1 ± 2,4A | 9,9 ± 0,2A |
| H + PMN3 | 138,8 ± 0,7B | 72,5 ± 1,8A | 94,8 ± 2,0A | 10,7 ± 0,1B |
| IC | 84,5 ± 2,8Bab | 61,1 ± 1,7Bab | 91,4 ± 2,1Aaa | 9,4 ± 0,2Baa |
| FC | 112,2 ± 4,3Bba | 70,0 ± 1,6Aba | 88,3 ± 2,9Aaa | 9,4 ± 0,3Baa |
| I + PMN3 | 149,5 ± 2,5Bbb | 72,1 ± 2,6Aba | 98,5 ± 2,1Bbb | 10,3 ± 0,2Bbb |
Data show mean values of twelve replicates ± standard error. Different lowercase letters denote statistical differences between the different groups and the HC, different uppercase letters mean statistical differences between the different groups and the IC and different lowercase letters in cursive represent statistical differences between the different groups and the FC (Dunett's test, P < 0.05).
Fig. 7.
Yield parameters of plants (R8 stage) grown from cold plasma treated seeds (H + PMN3 and P + PMN3), as well the controls, healthy, infected and treated with fungicide. Bars indicate standard error, n = 12. Notes. Different lowercase letters denote statistical differences between the different groups and the HC, different uppercase letters mean statistical differences between the different groups and the IC and different lowercase letters in cursive represent statistical differences between the different groups and the FC (Dunett's test, P < 0.05).
In reference to infected seeds, cold plasma treatment had positive effects on all the analysed parameters, compared with either IC or with FC. Root and aerial dry weights of plants grown from plasma-treated seeds (I + PMN3) were increased by 77% and 60% compared to IC, and by 33% and 40% in contrast with FC. Stem diameter and height of plants did not differ between IC and FC, but were increased significantly (9% and 10%) by seed treatment with plasma compared to both controls. In contrast with IC, plasma treatment incremented the total number of pods (23%) and seeds (21%) and dry weight (22%) and weight of 1000 seeds (79%). The number of pods and seeds of FC were increased by 5% and 8%, and dry weight and weight of 1000 seeds augmented by 14% and 26%, by seed treatment with plasma. In accordance with these results, non-thermal plasma technology guaranteed the health of plants in the same way the fungicide did. In addition, plasma treatment had differential positive effects on plant growth and on agronomic traits that were independent on pathogen control and could be directly attributable to plasma treatment itself. This suggestion found support on the preceding results in regard to healthy seeds, where the effects of plasma treatment per se on the biometry and yield of mature plants were demonstrated.
4. Conclusions
In this study, we proved that non-thermal plasmas (NTP) applied to soybean healthy seeds enhanced their quality. Germination, production rate, vigour and dry weights of seedlings were markedly increased by all the tested treatments. On the other hand, we demonstrated for the first time that NTPs, applied to soybean seeds with high incidence of D/P, increased plant growth. The photosynthetic performance of plants grown from infected-treated seeds was identical to the healthy ones, suggesting that plasma treatments guaranteed plants a normal and healthy physiological performance as the negative effects of pathogens were avoid, partially because they were greatly removed from seeds and partially due to the effects of plasma per se on seeds. The preceding contributed to obtain optimal plant growth starting from seeds of regular health status which results of great interest for agriculture since plasma technology may help recovering seeds that, otherwise, could not be used for sowing.
Under greenhouse conditions, dry weight of roots, plant height, stem diameter and yield of plants grown from either healthy or infected seeds, were improved by NTP. Considering that plants grown from plasma-treated seeds denoted superiority (in terms of growth and agronomic traits) compared to the ones grown from fungicide-treated seeds, we propose that NTP may be incorporated to the integrated pest management strategies with the certitude that they will not compromise the implantation of crops nor the yields. Nevertheless, we consider that field studies need to be addressed in order to confirm these results.
Concerning to seed-borne D/P complex, we documented here for the first time the oxidative and physiological damage triggered in young soybean plants by these fungi, and the antioxidant responses that this plant-pathogen interaction elicits.
We consider that the implementation of this technology as a routine practice for seeds treatment before sowing could help reducing the use of agrochemicals during the crop cycle, thus contributing to improve the profitability of soybean crops without affecting human health and the environment.
Further studies will investigate the mechanism underlying how cold plasma treatment affects growth and agronomic traits of soybean.
Declarations
Author contribution statement
María C Pérez-Pizá: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Leandro Prevosto, Beatriz Mancinelli: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Pablo E Grijalba, Carla G Zilli: Analyzed and interpreted the data.
Ezequiel Cejas: Performed the experiments.
Karina B Balestrasse: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by grants from: CONICET (PIP 11220120100453), Universidad Tecnológica Nacional (PID 4626), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2015 N°1553), Universidad de Buenos Aires (UBACYT 20020120100145).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
K.B., C.Z. and L.P. are members of CONICET. E.C. thanks CONICET for his doctoral fellowship. M.C.P.P thanks Agencia Nacional de Promoción Científica y Tecnológica for her doctoral fellowship.
We are immensely grateful to María L. Pérez-Bueno, Mónica Pineda and Matilde Barón (Estación Experimental del Zaidín (EEZ), Consejo Superior de Investigaciones Científicas (CSIC), Granada, Spain) who supervised the experiments carried out in their institution and provided insight and expertise that greatly assisted the research. We thank Patricia del Fueyo (Seed Laboratory – Universidad de Buenos Aires, Facultad de Agronomía, Argentine) who provided scientific support and valuable assistance, and to María Marta Caffaro (CONICET – Facultad de Agronomía, Universidad de Buenos Aires, Argentina) for her useful and constructive suggestions during the planning of the greenhouse experiment.
References
- Abd E.D., El-Aragi G.M., Tarrad M.M., Zayed E.M. Effect of non thermal plasma on alfalfa (Medicago sativa L.) forage production. J. Nucl. Technol. Appl. Sci. 2013;1(3):313–320. [Google Scholar]
- Abdul-Baki A.A., Anderson J.D. Vigour determination in soybean seed by multiplication. Crop Sci. 1973;3:630–633. [Google Scholar]
- Adamovich I., Baalrud S.D., Bogaerts A., Bruggeman P.J., Cappelli M., Colombo V., Czarnetzki U., Ebert U., Eden J.G., Favia P., Graves D.B., Hamaguchi S., Hieftje G., Hori M., Kaganovich I.D., Kortshagen U., Kushner M.J., Mason Nigel, Mazouffre S., Thagard S.M., Metelmann H.-R., Mizuno A., Moreau E., Murphy A.B., Niemira B.A., Oehrlein G.S., Petrovic Z.L., Pitchford L.C., Pu Y.-K., Rauf S., Sakai O., Samukawa S., Starikovskaia S., Tennyson J., Terashima K., Turner M.M., Van De Sanden M.C.M., Vardelle A. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D: Appl. Phys. 2017;50(32):323001. [Google Scholar]
- Ajigboye O.O., Bousquet L., Murchie E.H., Ray R.V. Chlorophyll fluorescence parameters allow the rapid detection and differentiation of plant responses in three different wheat pathosystems. Funct. Plant Biol. 2016;43(4):356–369. doi: 10.1071/FP15280. [DOI] [PubMed] [Google Scholar]
- Aldea M., Frank T.D., DeLucia E.H. A method for quantitative analysis of spatially variable physiological processes across leaf surfaces. Photosynth. Res. 2006;90(2):161–172. doi: 10.1007/s11120-006-9119-z. [DOI] [PubMed] [Google Scholar]
- Aldea M., Hamilton J.G., Resti J.P., Zangerl A.R., Berenbaum M.R., Frank T.D., DeLucia E.H. Comparison of photosynthetic damage from arthropod herbivory and pathogen infection in understory hardwood saplings. Oecologia. 2006;149(2):221–232. doi: 10.1007/s00442-006-0444-x. [DOI] [PubMed] [Google Scholar]
- Ampofo J.A., Tetteh W., Bello M. Impact of commonly used agrochemicals on bacterial diversity in cultivated soils. Indian J. Microbiol. 2009;49(3):223–229. doi: 10.1007/s12088-009-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Balestrasse K.B., Gallego S.M., Tomaro M.L. Aluminium stress affects nitrogen fixation and assimilation in soybean (Glycine max L.) Plant Growth Regul. 2006;48(3):271. [Google Scholar]
- Bartoš P., Kříž P., Havelka Z., Bohata A., Olšan P., Špatenka P., Čurn V., Dienstbier M. Plasma technology in food industry: mini-review. Časopis Kvasný průmysl. 2017;63(3):134–138. [Google Scholar]
- Basaran P., Basaran-Akgul N., Oksuz L. Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol. 2008;25(4):626–632. doi: 10.1016/j.fm.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Becana M., Aparicio-Tejo P., Irigoyen J.J., Sanchez-Diaz M. Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol. 1986;82(4):1169–1171. doi: 10.1104/pp.82.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdugo C.A., Zito R., Paulus S., Mahlein A.K. Fusion of sensor data for the detection and differentiation of plant diseases in cucumber. Plant Pathol. 2014;63(6):1344–1356. [Google Scholar]
- Berger S., Sinha A.K., Roitsch T. Plant physiology meets phytopathology: plant primary metabolism and plant–pathogen interactions. J. Exp. Bot. 2007;58(15-16):4019–4026. doi: 10.1093/jxb/erm298. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Cardona M.B., Filho J.A.W., Rodrigues F.Á. Leaf gas exchange and chlorophyll a fluorescence in maize leaves infected with Stenocarpella macrospora. Phytopathology. 2015;105(1):26–34. doi: 10.1094/PHYTO-04-14-0096-R. [DOI] [PubMed] [Google Scholar]
- Bilgin D.D., Zavala J.A., Zhu J.I.N., Clough S.J., Ort D.R., DeLucia E.H. Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ. 2010;33(10):1597–1613. doi: 10.1111/j.1365-3040.2010.02167.x. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandenburg R. Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017;26(5):053001. [Google Scholar]
- Brugger A., Kuska M.T., Mahlein A.K. Impact of compatible and incompatible barley—Blumeria graminis f. sp. hordei interactions on chlorophyll fluorescence parameters. J. Plant Dis. Protect. 2018;125(2):177–186. [Google Scholar]
- Bürling K., Hunsche M., Noga G. Use of blue–green and chlorophyll fluorescence measurements for differentiation between nitrogen deficiency and pathogen infection in winter wheat. J. Plant Physiol. 2011;168(14):1641–1648. doi: 10.1016/j.jplph.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Buschmann C., Lichtenthaler H.K. Principles and characteristics of multi-colour fluorescence imaging of plants. J. Plant Physiol. 1998;152(2-3):297–314. [Google Scholar]
- Calderón R., Navas-Cortés J.A., Zarco-Tejada P.J. Early detection and quantification of Verticillium wilt in olive using hyperspectral and thermal imagery over large areas. Rem. Sens. 2015;7(5):5584–5610. [Google Scholar]
- Carvalho L.F., Sediyama C.S., Reis M.S., Dias D.C.F.S., Moreira M.A. Influence of soaking temperature of soybean seeds in the electrical conductivity test to evaluate physiological quality. Rev. Bras. Sementes. 2009;31:9–17. (in Portuguese, with abstract in English) [Google Scholar]
- Cerovic Z.G., Samson G., Morales F., Tremblay N., Moya I. Ultraviolet-induced fluorescence for plant monitoring: present state and prospects. Agronomie. 1999;19(7):543–578. [Google Scholar]
- Chaerle L., Hagenbeek D., De Bruyne E., Valcke R., Van Der Straeten D. Thermal and chlorophyll-fluorescence imaging distinguish plant-pathogen interactions at an early stage. Plant Cell Physiol. 2004;45(7):887–896. doi: 10.1093/pcp/pch097. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chou H.M., Bundock N., Rolfe S.A., Scholes J.D. Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol. Plant Pathol. 2000;1(2):99–113. doi: 10.1046/j.1364-3703.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- Considine M.J., Foyer C.H. Redox regulation of plant development. Antioxidants Redox Signal. 2014;21(9):1305–1326. doi: 10.1089/ars.2013.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeschlein G., Napp M., von Podewils S., Lutze S., Emmert S., Lange A., Klare I., Haase H., Gümbel D., von Woedtke T., Jünger M. In vitro susceptibility of multidrug resistant skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD) Plasma Process Polym. 2014;11(2):175–183. [Google Scholar]
- Dasan B.G., Mutlu M., Boyaci I.H. Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor. Int. J. Food Microbiol. 2016;216:50–59. doi: 10.1016/j.ijfoodmicro.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Dissanayake A.J., Phillips A.J.L., Hyde K.D., Yan J.Y., Li X.H. The current status of species in Diaporthe. Mycosphere. 2017;8(5):1106–1156. [Google Scholar]
- Dobrynin D., Fridman G., Friedman G., Fridman A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009;11(11):115020. [Google Scholar]
- El Omari B., Fleck I., Aranda X., Moret A. Effect of fungal infection on leaf gas-exchange and chlorophyll fluorescence in Quercus ilex. Ann. For. Sci. 2001;58(2):165–174. [Google Scholar]
- El-Aragi G.M., Ahmed G.A.N. Plasma Science, 2010 Abstracts IEEE International Conference on. IEEE; 2010, June. A preliminary study on the effects of non-thermal plasma technology on growth plant of berseem (Egyptian clover) crop. 1–1. [Google Scholar]
- El-Maarouf-Bouteau H., Bailly C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008;3(3):175–182. doi: 10.4161/psb.3.3.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr W.R., Caviness C.E., Burmood D.T., Pennington J.S. Stage of development descriptions for soybeans, Glycine Max (L.) Merrill 1. Crop Sci. 1971;11(6):929–931. [Google Scholar]
- Fercha A., Capriotti A.L., Caruso G., Cavaliere C., Stampachiacchiere S., Zenezini Chiozzi R., Laganà A. Shotgun proteomic analysis of soybean embryonic axes during germination under salt stress. Proteomics. 2016;16(10):1537–1546. doi: 10.1002/pmic.201500283. [DOI] [PubMed] [Google Scholar]
- Fuentes E. 2016. En defensa de los rindes. Soja: la importancia de acorralar a las enfermedades y evitar pérdidas.https://www.clarin.com/rural/soja-importancia-acorralar-enfermedades-perdidas_0_S1kuld9ll Available from: [Spanish] [Google Scholar]
- Genty B., Briantais J.M., Baker N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989;990(1):87–92. [Google Scholar]
- Granum E., Pérez-Bueno M.L., Calderón C.E., Ramos C., de Vicente A., Cazorla F.M., Barón M. Metabolic responses of avocado plants to stress induced by Rosellinia necatrix analysed by fluorescence and thermal imaging. Eur. J. Plant Pathol. 2015;142(3):625–632. [Google Scholar]
- Grijalba P.E., Ridao A.D.C., Guillin E. Caracterización taxonómica y análisis de la variabilidad del agente causal del cancro del tallo de la soja en Buenos Aires (2005/2007) Rev. Invest. Agropecu. 2011;37(3):290–297. [Google Scholar]
- Grijalba P., del Ridao A.C. Tasa de crecimiento y patogenicidad de aislamientos de Diaporthe phaseolorum var. caulivora. Phyton. 2014;83(2):325–332. [Google Scholar]
- Grijalba P., del Ridao A.C. Growth rate and pathogenicity of isolates of Diaporthe phaseolorum var. caulivora. Phyton. Int. J. Exp. Bot. 2016;83(2):325–332. [Google Scholar]
- Grijalba P., Ridao A.D.C. Survival of Diaporthe phaseolorum var. caulivora (causal agent of soybean stem canker) artificially inoculated in different crop residues. Trop. Plant Pathol. 2012;37(4):271–274. [Google Scholar]
- Gunes A., Inal A., Alpaslan M., Eraslan F., Bagci E.G., Cicek N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007;164(6):728–736. doi: 10.1016/j.jplph.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hahn M. The rising threat of fungicide resistance in plant pathogenic fungi: botrytis as a case study. J. Chem. Biol. 2014;7(4):133–141. doi: 10.1007/s12154-014-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hosseini S.I., Mohsenimehr S., Hadian J., Ghorbanpour M., Shokri B. Physico-chemical induced modification of seed germination and early development in artichoke (Cynara scolymus L.) using low energy plasma technology. Phys. Plasmas. 2018;25(1):013525. [Google Scholar]
- ISTA . Vol. 2014. International Seed Testing Association; 2014. (The Germination Test). (Chapter 5) [Google Scholar]
- Jacobsen B.J. Role of plant pathology in integrated pest management. Annu. Rev. Phytopathol. 1997;35:373–391. doi: 10.1146/annurev.phyto.35.1.373. [DOI] [PubMed] [Google Scholar]
- Ji S.H., Choi K.H., Pengkit A., Im J.S., Kim J.S., Kim Y.H., Park G. Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch. Biochem. Biophys. 2016;605:117–128. doi: 10.1016/j.abb.2016.02.028. [DOI] [PubMed] [Google Scholar]
- Jiafeng J., Xin H., Ling L.I., Jiangang L., Hanliang S., Qilai X., Renhong Y., Yuanhua D. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci. Technol. 2014;16(1):54. doi: 10.1038/srep05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Lu Y., Li J., Li L., He X., Shao H., Dong Y. Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (bacterial wilt) PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiayun T., Rui H., Xiaoli Z., Ruoting Z., Weiwen C., Size Y. Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci. Technol. 2014;16(3):260. [Google Scholar]
- Jones H.G. Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant Cell Environ. 1999;22(9):1043–1055. [Google Scholar]
- Kriz P., Olsan P., Havelka Z., Bartos P., Bohata A., Strejckova M., Curn V., Spatenka P. Optimization of Electrical and Electronic Equipment (OPTIM) & 2017 Intl Aegean Conference on Electrical Machines and Power Electronics (ACEMP), 2017 International Conference on (pp. 1045–1050) IEEE; 2017 May. Enhancement of the yield of rape seeds by plasma discharge and biological protection: Field experiments. [Google Scholar]
- Kuckenberg J., Tartachnyk I., Noga G. Temporal and spatial changes of chlorophyll fluorescence as a basis for early and precise detection of leaf rust and powdery mildew infections in wheat leaves. Precis. Agric. 2009;10(1):34–44. [Google Scholar]
- Kumar R., Thakur A.K., Vikram A., Vaid A., Rane R. Effect of plasma treatment on growth and yield of okra [Abelmoschus esculentus (L.) under field conditions. Int. J. Bio Resour. Stress Manage. 2017;8(5) [Google Scholar]
- Laloi C., Apel K., Danon A. Reactive oxygen signalling: the latest news. Curr. Opin. Plant Biol. 2004;7(3):323–328. doi: 10.1016/j.pbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Laroussi M. Low temperature plasma-based sterilization: overview and state of the art. Plasma Process. Polym. 2005;2(5):391–400. [Google Scholar]
- Leggett J.E., Frere M.H. Growth and nutrient uptake by soybean plants in nutrient solutions of graded concentrations. Plant Physiol. 1971;48(4):457–460. doi: 10.1104/pp.48.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leufen G., Noga G., Hunsche M. Fluorescence indices for the proximal sensing of powdery mildew, nitrogen supply and water deficit in sugar beet leaves. Agriculture. 2014;4(2):58–78. [Google Scholar]
- Li S., Song Q., Martins A.M., Cregan P. Draft genome sequence of Diaporthe aspalathi isolate MS-SSC91, a fungus causing stem canker in soybean. Genom. Data. 2016;7:262–263. doi: 10.1016/j.gdata.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang T., Meng Y., Qu G., Sun Q., Liang D., Hu S. Air atmospheric dielectric barrier discharge plasma induced germination and growth enhancement of wheat seed. Plasma Chem. Plasma Process. 2017;37(6):1621–1634. [Google Scholar]
- Ling L.I., Jiangang L., Minchong S., Jinfeng H., Hanliang S., Yuanhua D., Jiafeng J. Improving seed germination and peanut yields by cold plasma treatment. Plasma Sci. Technol. 2016;18(10):1027. [Google Scholar]
- Ling L., Jiafeng J., Jiangang L., Minchong S., Xin H., Hanliang S., Yuanhua D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014;4:5859. doi: 10.1038/srep05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque J., Cohen M., Savé R., Biel C., Álvarez I.F. Effects of three fungal pathogens on water relations, chlorophyll fluorescence and growth of Quercus suber L. Ann. For. Sci. 1999;56(1):19–26. [Google Scholar]
- Ma F., Cholewa E.W.A., Mohamed T., Peterson C.A., Gijzen M. Cracks in the palisade cuticle of soybean seed coats correlate with their permeability to water. Ann. Bot. 2004;94(2):213–228. doi: 10.1093/aob/mch133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvick D.K. Department of Crop Sciences, University of Illinois; 1997. Pod and Stem Blight, Stem Canker and Phomopsis Seed Decay of soybeans. Report on Plant Diseases. RPD No. 509. [Google Scholar]
- Meiqiang Y., Mingjing H., Buzhou M., Tengcai M. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci. Technol. 2005;7(6):3143. [Google Scholar]
- Meng Y., Qu G., Wang T., Sun Q., Liang D., Hu S. Enhancement of germination and seedling growth of wheat seed using dielectric barrier discharge plasma with various gas sources. Plasma Chem. Plasma Process. 2017;37(4):1105–1119. [Google Scholar]
- Mesquida F. 2018. Soja: las enfermedades de fin de ciclo pueden afectar hasta un 30% del rinde.http://www.infocampo.com.ar/soja-las-enfermedades-de-fin-de-ciclo-pueden-afectar-hasta-un-30-del-rinde/ Available from: [Spanish] [Google Scholar]
- Misra N.N., Schlüter O., Cullen P.J., editors. Cold Plasma in Food and Agriculture: Fundamentals and Applications. Academic Press; 2016. pp. 1–16. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Modolo L.V., Cunha F.Q., Braga M.R., Salgado I. Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorum f. sp. meridionalis elicitor. Plant Physiol. 2002;130(3):1288–1297. doi: 10.1104/pp.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan M., Barbeau J., Moreau S., Pelletier J., Tabrizian M., Yahia L.H. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001;226(1-2):1–21. doi: 10.1016/s0378-5173(01)00752-9. [DOI] [PubMed] [Google Scholar]
- Montoya M.R. 2015. Cómo evitar pérdidas por enfermedades en la producción sojera.https://inta.gob.ar/noticias/como-evitar-perdidas-por-enfermedades-en-la-produccion-sojera.html Available online: [Spanish] [Google Scholar]
- Morales M., Munné-Bosch S. Oxidative stress: a master regulator of plant trade-offs? Trends Plant Sci. 2016;21(12):996–999. doi: 10.1016/j.tplants.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Muniz C.R., Freire F.C.O., Viana F.M.P., Cardoso J.E., Sousa C.A.F., Guedes M.I.F., van der Schoor R., Jalink H. Monitoring cashew seedlings during interactions with the fungus Lasiodiplodia theobromae using chlorophyll fluorescence imaging. Photosynthetica. 2014;52(4):529–537. [Google Scholar]
- Niemira B.A. Cold plasma decontamination of foods. Ann. Rev. Food Sci. Technol. 2012;3:125–142. doi: 10.1146/annurev-food-022811-101132. [DOI] [PubMed] [Google Scholar]
- Nwachukwu I.D., Luciano F.B., Udenigwe C.C. The inducible soybean glyceollin phytoalexins with multifunctional health-promoting properties. Food Res. Int. 2013;54(1):1208–1216. [Google Scholar]
- Oerke E.C. Crop losses to pests. J. Agric. Sci. 2006;144:31–43. [Google Scholar]
- Oerke E.C., Fröhling P., Steiner U. Thermographic assessment of scab disease on apple leaves. Precis. Agric. 2011;12(5):699–715. [Google Scholar]
- Oerke E.C., Steiner U., Dehne H.W., Lindenthal M. Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. J. Exp. Bot. 2006;57(9):2121–2132. doi: 10.1093/jxb/erj170. [DOI] [PubMed] [Google Scholar]
- Ördög V., Molnár Z. Plant Physiology. Debreceni University, Nyugat-Magyarországi University and Pannon University; 2011. Overview on plant growth and development; pp. 64–70. [Google Scholar]
- Pandey S.K., Singh H. A simple, cost-effective method for leaf area estimation. J. Bot. 2011;2011 [Google Scholar]
- Pérez Pizá M.C., Prevosto L., Zilli C., Cejas E., Kelly H., Balestrasse K. Effects of non-thermal plasmas on seed-borne Diaporthe/Phomopsis complex and germination parameters of soybean seeds. Innov. Food Sci. Emerg. Technol. 2018;49:82–91. [Google Scholar]
- Pérez-Bueno M.L., Pineda M., Cabeza F.M., Barón M. Multicolor fluorescence imaging as a candidate for disease detection in plant phenotyping. Front. Plant Sci. 2016;7:1790. doi: 10.3389/fpls.2016.01790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Bueno M.L., Pineda M., Díaz-Casado E., Barón M. Spatial and temporal dynamics of primary and secondary metabolism in Phaseolus vulgaris challenged by Pseudomonas syringae. Physiol. Plant. 2015;153(1):161–174. doi: 10.1111/ppl.12237. [DOI] [PubMed] [Google Scholar]
- Prokopová J., Špundová M., Sedlářová M., Husičková A., Novotný R., Doležal K., Naus J., Lebeda A. Photosynthetic responses of lettuce to downy mildew infection and cytokinin treatment. Plant Physiol. Biochem. 2010;48(8):716–723. doi: 10.1016/j.plaphy.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Randeniya L.K., de Groot G.J. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: a review. Plasma Process. Polym. 2015;12(7):608–623. [Google Scholar]
- Razdan V., Sabitha M. Integrated disease management: concepts and practices. In: Peshin R., Dhawan A.K., editors. Integrated Pest Management: Innovation-Development Process. Springer; Dordrecht: 2009. [Google Scholar]
- Rossi R.L., Ridao A. Main variant of Diaporthe/Phomopsis complex (d. p. var. caulivora) propagated by soybean in the south centre of the province of Buenos Aires. Anál. Semillas. 2011;5(20):80–91. [Google Scholar]
- Sánchez M.C., Ridao A.D.C., Colavita M.L. Diaporthe caulivora: agente causal de cancro del tallo predominante en cultivos de soja del sudeste bonaerense. Fave. Sección Cienc. Agrar. 2015;14(2) 0–0. [Google Scholar]
- Sera B., Spatenka P., Sery M., Vrchotová N., Hruskova I. Influence of plasma treatment on wheat and oat germination and early growth. IEEE Trans. Plasma Sci. 2010;38(10):2963–2968. [Google Scholar]
- Shama G., Kong M.G. Plasma for Bio-Decontamination, Medicine and Food Security. Springer; Dordrecht: 2012. Prospects for treating foods with cold atmospheric gas plasmas; pp. 433–443. [Google Scholar]
- Singh D., Mathur S.B. CRC Press; Washington DC: 2004. Histopathology of Seed-Borne Infections. [Google Scholar]
- Stolárik T., Henselová M., Martinka M., Novák O., Zahoranová A., Černák M. Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L.) Plasma Chem. Plasma Process. 2015;35(4):659–676. [Google Scholar]
- Stoll M., Schultz H.R., Berkelmann-Loehnertz B. Exploring the sensitivity of thermal imaging for Plasmopara viticola pathogen detection in grapevines under different water status. Funct. Plant Biol. 2008;35(4):281–288. doi: 10.1071/FP07204. [DOI] [PubMed] [Google Scholar]
- Strejckova M., Bohata A., Olsan P., Havelka Z., Kriz P., Beran P., Bartos P., Curn V., Spatenka P. Enhancement of the Yield of Crops by Plasma and Using of Entomopathogenic and Mycoparasitic Fungi: From Laboratory to Large-Field Experiments. J. Biomaterials Tissue Eng. 2018;8(6):829–836. [Google Scholar]
- Sun P., Sun Y., Wu H., Zhu W., Lopez J.L., Liu W., Zhang J., Li R., Fang J. Atmospheric pressure cold plasma as an antifungal therapy. Appl. Phys. Lett. 2011;98(2):021501. [Google Scholar]
- Swarbrick P.J., Schulze-Lefert P.A.U.L., Scholes J.D. Metabolic consequences of susceptibility and resistance (race-specific and broad-spectrum) in barley leaves challenged with powdery mildew. Plant Cell Environ. 2006;29(6):1061–1076. doi: 10.1111/j.1365-3040.2005.01472.x. [DOI] [PubMed] [Google Scholar]
- Tatagiba S.D., DaMatta F.M., Rodrigues F.Á. Leaf gas exchange and chlorophyll a fluorescence imaging of rice leaves infected with Monographella albescens. Phytopathology. 2015;105(2):180–188. doi: 10.1094/PHYTO-04-14-0097-R. [DOI] [PubMed] [Google Scholar]
- Terré E. 2018. Balance de oferta y demanda de soja en Argentina.https://www.bcr.com.ar/Pages/Publicaciones/informativosemanal_noticias.aspx?pIDNoticia=958 Available from: (Accessed 26 January 2018). [Spanish] [Google Scholar]
- USDA, FAS . Foreign Agricultural Service; Washington DC, USA: 2018. Oilseeds: World Markets and Trade World Production, Markets, and Trade Reports.https://www.fas.usda.gov/commodities/soybeans Available from: [Spanish] [Google Scholar]
- Wain-Tassi A.L., Santos J.F.D., Panizzi R.D.C., Vieira R.D. Seed-borne pathogens and electrical conductivity of soybean seeds. Sci. Agric. 2012;69(1):19–25. [Google Scholar]
- Wang M., Ling N., Dong X., Zhu Y., Shen Q., Guo S. Thermographic visualization of leaf response in cucumber plants infected with the soil-borne pathogen Fusarium oxysporum f. sp. cucumerinum. Plant Physiol. Biochem. 2012;61:153–161. doi: 10.1016/j.plaphy.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Wintermans J.F.G.M., De Mots A.S. Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. Biochim. Biophys. Acta. 1965;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Wojtaszek P. Oxidative burst: an early plant response to pathogen infection. Biochem. J. 1997;322(3):681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrather A., Shannon G., Balardin R., Carregal L., Escobar R., Gupta G.K., Ma Z., Morel W., Ploper D., Tenuta A. Effect of diseases on soybean yield in the top eight producing countries in 2006. Plant Health Prog. 2010;10:1094. [Google Scholar]
- Zhang J.J., Jo J.O., Huynh D.L., Mongre R.K., Ghosh M., Singh A.K., Jeong D.K. Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes. Sci. Rep. 2017;7:41917. doi: 10.1038/srep41917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liu D., Zhou R., Song Y., Sun Y., Zhang Q., Niu J., Fan H., Yang S.Z. Atmospheric cold plasma jet for plant disease treatment. Appl. Phys. Lett. 2014;104(4):043702. [Google Scholar]
- Zivkovic S., Puac N., Giba Z., Grubisic D., Petrovic Z.L. The stimulatory effect of non-equilibrium (low temperature) air plasma pretreatment on light-induced germination of Paulownia tomentosa seeds. Seed Sci. Technol. 2004;32:693–701. [Google Scholar]