Abstract
Background
With the development of new magnetic resonance imaging (MRI) techniques, an increasing number of articles have been published regarding hepatocellular carcinoma magnetic resonance imaging (HCCMRI) in the past decade. However, few studies have statistically analyzed these published articles. In this study, we aim to systematically evaluate the scientific outcomes of HCCMRI research and explore the research hotspots from 2008 to 2017.
Methods
The included articles regarding HCCMRI research from 2008 to 2017 were downloaded from the Web of Science Core Collection and verified by two experienced radiologists. Excel 2016 was used to analyze the literature data, including the publication years and journals. CiteSpace V was used to perform co-occurrence analyses for authors, countries/regions and institutions and to generate the related collaboration network maps. Reference co-citation analysis (RCA) and burst keyword detection were also performed using CiteSpace V to explore the research hotspots in the past decade.
Results
A total of 835 HCCMRI articles published from 2008 to 2017 were identified. Journal of Magnetic Resonance Imaging published the most articles (79 publications, 9.46%). Extensive cooperating relationship were observed among countries/regions and among authors. South Korea had the most publications (199 publications, 21.82%), followed by the United States of America (USA) (190 publications, 20.83%), Japan (162 publications, 17.76%), and the People’s Republic of China (148 publications, 16.23%). Among the top 10 co-cited authors, Bruix J (398 citations) was ranked first, followed by Llovet JM (235 citations), Kim YK (170 citations) and Forner A (152 citations). According to the RCA, ten major clusters were explored over the last decade; “LI-RADS data system” and “microvascular invasion” (MVI) were the two most recent clusters. Forty-seven burst keywords with the highest citation strength were detected over time. Of these keywords, “microvascular invasion” had the highest strength in the last 3 years. The LI-RADS has been constantly updated with the latest edition released in July 2018. However, the LI-RADS still has limitations in identifying certain categories of lesions by conceptual and non-quantitative probabilistic methods. Plenty of questions still need to be further answered such as the difference of diagnostic efficiency of each major/ancillary imaging features. Preoperative prediction of MVI of HCC is very important to therapeutic decision-making. Some parameters of Gd-EOB-DTPA-enhanced MRI were found to be useful in prediction of MVI, however, with a high specificity but a very low sensitivity. Comprehensive predictive model incorporating both imaging and clinical variables may be the more preferable in prediction of MVI of HCC.
Conclusions
HCCMRI-related publications displayed a gradually increasing trend from 2008 to 2017. The USA has a central position in collaboration with other countries/regions, while South Korea contributed the most in the number of publications. Of the ten major clusters identified in the RCA, the two most recent clusters were “LI-RADS data system” and “microvascular invasion”, indicative of the current HCCMRI research hotspots.
Keywords: Bibliometrics, CiteSpace, co-citation analysis, hepatocellular carcinoma, magnetic resonance imaging
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy in adults and is the most common cause of death in people with cirrhosis. HCC ranks as the second most common cause of cancer death worldwide, and more than 500,000 new patients are diagnosed annually (1-5). Detection, characterization, and identification of appropriate treatment strategies and improvement of HCC prognosis have always been the major concerns in the clinic. Magnetic resonance imaging (MRI) is a powerful tool for evaluating almost all aspects of HCC with sufficient sensitivity and specificity. With the extensive and in-depth application of MRI, the detection, diagnosis and treatment of HCC have been greatly improved.
With the development of new MRI techniques, an increasing number of articles have been published in hepatocellular carcinoma magnetic resonance imaging (HCCMRI) in the past decade (6). However, few studies have statistically analyzed these published articles. Bibliometrics is a useful method to explore the most impactful authors, papers, or countries/regions, construct collaboration networks, and identify research hotspots in particular areas (7-9). With the progression of CiteSpace, bibliometric analysis can be easily performed, and collaboration networks and co-citation networks can even be visualized (10,11).
In this study, we used CiteSpace V to systematically evaluate HCCMRI research from 2008 to 2017. We aimed to visualize the scientific outputs of HCCMRI research with knowledge maps and explore the hotspots of HCCMRI research over time.
Methods
Data search and check
We performed a literature search in the Web of Science Core Collection (WoSCC) on August 29, 2018. To avoid database update bias, we performed all searches within the same day.
The Science Citation Index-Expanded (SCI-E) was selected. The following terms were used to search articles from 2008 to 2017: Topic=(“primary liver carcinoma*” OR “primary hepatic carcinoma*” OR “primary liver cancer*” OR “primary hepatic cancer*” OR “hepatocellular carcinoma*” OR “hcc*” OR “hepatic cell carcinoma*” OR “liver cell carcinoma*” OR “hepatic cell cancer*” OR “liver cell cancer*” OR “hepatocarcinoma*” OR “hepatoma*”) AND Topic=(“nuclear magnetic resonance imaging*” OR “magnetic resonance imaging*” OR “MRI*” OR “NMRI*” OR “MR imaging*” OR “magnetic resonance*” OR “MR” OR “NMR*” OR “nuclear magnetic resonance*”) AND Language= English AND Document type= Article.
Then, raw data were downloaded from WoSCC as plain text files including full records. All downloaded data were independently checked by two radiologists (XP Wang and N Zhang, both with 5 years of abdominal imaging experience) to exclude articles not related to HCCMRI research. In the case of discrepancy between the two reviewers, a consensus was reached with the help of a third independent reviewer (DW Yang). A flowchart of this study is shown in Figure 1.
Figure 1.

A flowchart of the study to retrieve HCCMRI articles from the WoSCC database. HCCMRI, hepatocellular carcinoma magnetic resonance imaging.
Statistical methods
The identified data were then systematically analyzed by Excel 2016 and CiteSpace V (Chaomei Chen, Drexel University, USA). Excel 2016 was used to display the trend in the number of articles published by year and the distribution of the articles by journal. CiteSpace V was used to perform co-occurrence analysis and visualize the collaboration networks of the authors/institutes/countries/keywords. Author co-citation analysis (ACA) and reference co-citation analysis (RCA) were also performed by CiteSpace V, and related knowledge maps were constructed. Burst keyword detection was also performed to investigate the recurrent new keywords. In this study, the 50 most cited or found articles were selected to create the individual network in a 1-year interval. Moreover, log-likelihood ratio (LLR) weighting was used to analyze the contents of each cluster, as recommended by Chen CM (10,11).
Results
Annual publications
A total of 835 papers matched the retrieval criteria and were included for further analysis. The number of articles published annually is shown in Figure 2, which indicates a consistently increasing trend from 37 articles in 2008 to 117 articles in 2017.
Figure 2.

The number of articles published annually in the HCCMRI field from 2008 to 2017. HCCMRI, hepatocellular carcinoma magnetic resonance imaging.
Analysis of journals
A total of 162 scholarly journals have published articles regarding HCCMRI research. The top 10 journals are presented in Table 1. Journal of Magnetic Resonance Imaging [impact factor (IF) 2017=3.612] published the highest number of articles (79 publications, 9.46%), followed by European Journal of Radiology (IF 2017=2.843; 47 publications; 5.63%), Radiology (IF 2017=7.469; 45 publications; 5.39%), and American Journal of Roentgenology (IF 2017 =3.125; 42 publications; 5.03%).
Table 1. The top 10 journals in the HCCMRI field from 2008 to 2017.
| Journal | Country | Number | Percent | IF 2017 |
|---|---|---|---|---|
| Journal of Magnetic Resonance Imaging | United States | 79 | 9.46% | 3.612 |
| European Journal of Radiology | Ireland | 47 | 5.63% | 2.843 |
| Radiology | United States | 45 | 5.39% | 7.469 |
| American Journal of Roentgenology | United States | 42 | 5.03% | 3.125 |
| European Radiology | Germany | 42 | 5.03% | 4.027 |
| Abdominal Imaging | United States | 31 | 3.71% | 2.443 |
| Journal of Computer Assisted Tomography | United States | 24 | 2.87% | 1.292 |
| PLoS One | United States | 22 | 2.63% | 2.766 |
| World Journal of Gastroenterology | People’s R China | 20 | 2.40% | 3.300 |
| Clinical Radiology | England | 18 | 2.16% | 2.282 |
Analysis of countries and institutes
Overall, the 835 articles in HCCMRI research were published by 22 countries/regions. Extensive cooperating relationships were observed among countries/regions (Figure 3). The list of the top 10 countries/regions is presented in Table 2. South Korea had the most publications [199], followed by the United States of America (USA) [190], Japan [162], and the People’s Republic of China [148], accounting for 76.5% of all the articles.
Figure 3.
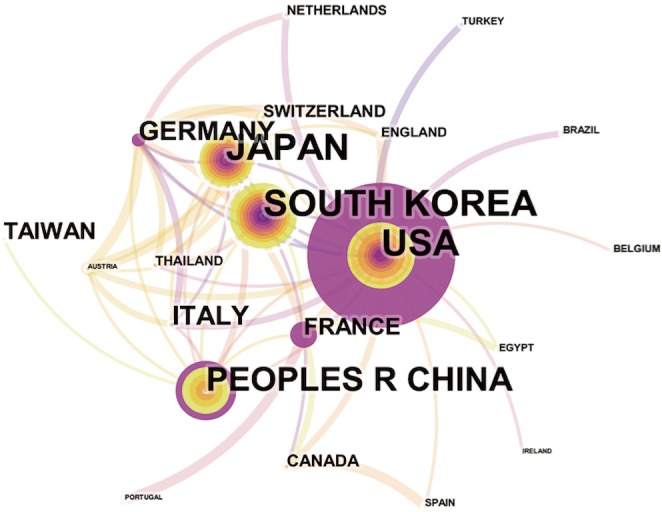
Map of active countries/regions in the HCCMRI field from 2008 to 2017. The font size of each country`s name represents the number of articles in the country/region. Different colors inside the circle represent different time intervals. The purple ring of the circles indicates the core countries/regions. The thickness of the curved connecting line represents the collaborative intensity between countries/regions. HCCMRI, hepatocellular carcinoma magnetic resonance imaging.
Table 2. Ranking of top 10 active countries/regions and institutions in the HCCMRI field from 2008 to 2017.
| Rank | Country/region | Counts (%) | Centrality | Institution | Counts (%) | Centrality |
|---|---|---|---|---|---|---|
| 1 | South Korea | 199 (21.82) | 0.04 | Sungkyunkwan Univ | 57 (8.15) | 0.15 |
| 2 | USA | 190 (20.83) | 1 | Yonsei Univ | 43 (6.15) | 0.17 |
| 3 | Japan | 162 (17.76) | 0.07 | Kinki Univ | 27 (3.86) | 0.4 |
| 4 | China | 148 (16.23) | 0.2 | Fudan Univ | 25 (3.58) | 0.22 |
| 5 | Italy | 54 (5.92) | 0.01 | Seoul Natl Univ | 24 (3.43) | 0.19 |
| 6 | Germany | 50 (5.48) | 0.16 | Seoul Natl Univ Hosp | 24 (3.43) | 0.22 |
| 7 | Taiwan | 24 (2.63) | 0 | Univ Ulsan | 20 (2.86) | 0.06 |
| 8 | France | 24 (2.63) | 0.26 | Korea Univ | 18 (2.58) | 0.03 |
| 9 | Canada | 9 (0.99) | 0.02 | Kyushu Univ | 17 (2.43) | 0 |
| 10 | Switzerland | 8 (0.88) | 0.01 | Sun Yat Sen Univ | 13 (1.86) | 0.08 |
Nearly 128 institutes made contributions to HCCMRI research. Extensive collaborations were observed between institutes (Figure 4). The top 10 institutes (Table 2) published more than 32.1% of all the articles. In this list, the first research echelon was led by Sungkyunkwan University, followed by Yonsei University, Kinki University and Fudan University.
Figure 4.

Map of active institutions in the HCCMRI field from 2008 to 2017. The size of each circle is proportional to the article counts. Different colors inside the circle represent different time intervals. The thickness of the curved connecting line represents the collaborative intensity between institutions. HCCMRI, hepatocellular carcinoma magnetic resonance imaging.
Distribution of authors and co-cited authors
More than 450 authors contributed to HCCMRI research. The collaborative relationships between authors are presented in Figure 5. Of the top 10 contributing authors, Kim SH (40 articles) was ranked first, followed by Kim YK (37 articles), Kim MJ (34 articles) and Lee JM (30 articles) (Table 3).
Figure 5.

Map of active authors in the HCCMRI field from 2008 to 2017. The font size of each author’s name represents the article counts of each author. Different colors inside the circle represent different time intervals. The thickness of the curved connecting line represents the collaborative intensity between authors. HCCMRI, hepatocellular carcinoma magnetic resonance imaging.
Table 3. Ranking of top 10 authors, co-cited authors, and co-cited references in the HCCMRI field from 2008 to 2017.
| Rank | Author | Counts | Co-cited author | Counts | Co-cited reference | Count |
|---|---|---|---|---|---|---|
| 1 | Kim SH | 40 | Bruix J | 398 | Bruix J, 2011, Hepatology, V53, P1020 | 208 |
| 2 | Kim YK | 37 | Llovet JM | 235 | Bruix J, 2005, Hepatology, V42, P1208 | 108 |
| 3 | Kim MJ | 34 | Kim YK | 170 | Kim SH, 2009, Am J Roentgenol, V192, P1675 | 104 |
| 4 | Lee JM | 30 | Forner A | 152 | Ahn SS, 2010, Radiology, V255, P459 | 90 |
| 5 | Han JK | 25 | Kim SH | 149 | Forner A, 2008, Hepatology, V47, P97 | 84 |
| 6 | Kim JH | 25 | Taouli B | 145 | Llovet JM, 2012, J Hepatol, V56, P908 | 82 |
| 7 | Lee WJ | 24 | Huppertz A | 129 | Willatt JM, 2008, Radiology, V247, P311 | 75 |
| 8 | Choi BI | 24 | Vogl TJ | 112 | Nasu K, 2009, Am J Roentgenol, V193, P438 | 65 |
| 9 | Kim KW | 20 | Kudo M | 112 | Kogita S, 2010, Eur Radiol, V20, P2405 | 61 |
| 10 | Choi D | 20 | El-serag HB | 103 | Huppertz A, 2005, Radiology, V234, P468 | 58 |
ACA, which uses authors as the elements of analysis and the pairs of co-cited authors as the variable that reflects the relationship between authors, is usually used in library and information science to explore the core authors in a given field. The top 10 co-cited authors with their citation count according to ACA were identified (Table 3), and the map of the relationships between all cited authors was constructed (Figure 6). Among the top 10 co-cited authors, Bruix J (398 citations) was ranked first, followed by Llovet JM (235 citations), Kim YK (170 citations) and Forner A (152 citations).
Figure 6.
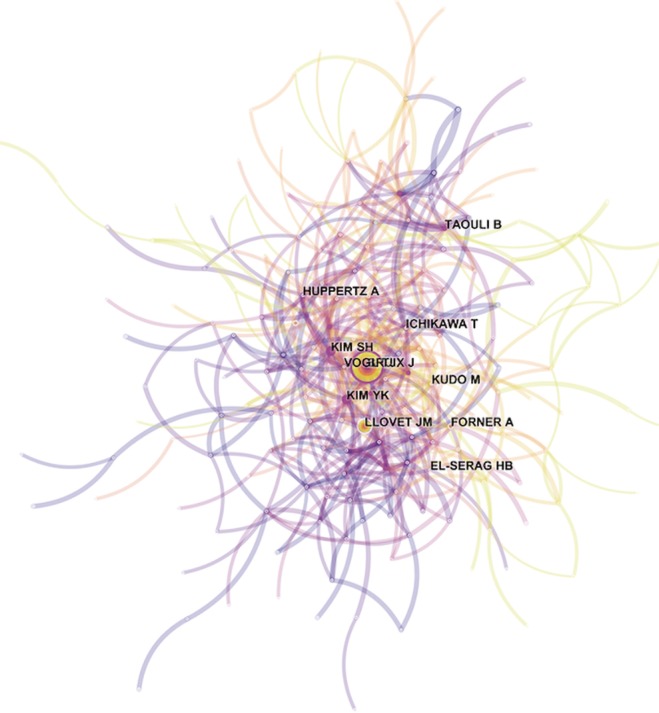
Map of co-cited authors in the HCCMRI field from 2008 to 2017. The thickness of the curved connecting line represents the co-cited intensity between authors. HCCMRI, hepatocellular carcinoma magnetic resonance imaging.
Analysis of reference co-citation
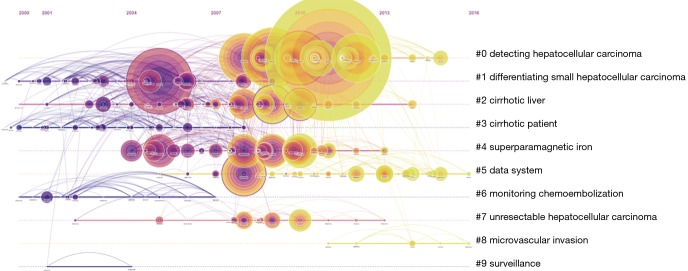
RCA was performed to generalize clusters and construct mapping knowledge domains in cluster (Figure 7) and timeline (Figure 8) views. The top 10 co-cited references with their co-citation counts are presented in Table 3. According to the RCA, the articles regarding HCCMRI research published between 2008 and 2017 can be clustered into 10 major research hotspots, each with different sizes and occurrence periods. The modularity (Q) was 0.6112, and the mean silhouette value was 0.3194. The top 10 clusters, which represent the major research hotspots, were generalized and ordered from the largest to smallest number of co-cited references. The first cluster was ‘‘#0 detecting hepatocellular carcinoma’’, followed by ‘‘#1 differentiating small hepatocellular carcinoma’’. As suggested by the timeline view (Figure 8), a cluster called ‘‘#5 data system’’, which refers to the liver imaging reporting and data system (LI-RADS), and a cluster called ‘‘#8 microvascular invasion’’ appeared recently.
Figure 7.

The cluster view of the knowledge map from the RCA of the HCCMRI field from 2008 to 2017. The convex hulls of different colors represent different clusters; the brighter the convex hull is, the more recently it appears. The number of nodes each convex hull covers is proportional to the number of co-cited references of each cluster. RCA, reference co-citation analysis; HCCMRI, hepatocellular carcinoma magnetic resonance imaging.
Figure 8.
The timeline view of the knowledge map from the RCA of the HCCMRI field from 2008 to 2017. This view clearly presents the differences in the appearance time point and time span of ten clusters. RCA, reference co-citation analysis; HCCMRI, hepatocellular carcinoma magnetic resonance imaging.
Analysis of keywords and burst keywords
According to the keyword co-occurrence analysis, 188 keywords were detected. The keywords with strong citation bursts were explored through CiteSpace V, and 47 keywords with the strongest citation bursts were identified (Figure 9). The appearance time points and periods of the burst keywords varied, as the earliest and highest-strength burst keyword in the past decade was “superparamagnetic iron oxide”; from 2015 to 2017, the keyword with the highest strength was ‘‘microvascular invasion’’.
Figure 9.
The keywords with the strongest citation bursts, with the years of appearance, in the HCCMRI field from 2008 to 2017. The green line represents time period from 2008–2017, while the periods of each burst keyword are plotted by the red line. HCCMRI, hepatocellular carcinoma magnetic resonance imaging.
Discussion
General information
We found a general increasing trend in HCCMRI-related publications from 2008 to 2017, from 37 articles in 2008 to 117 articles in 2017. This trend was consistent with that observed in other research fields (12,13) and can be well explained by the emergence of new and advanced MRI technologies in the last decade, providing more and better tools for studying the popular issues in HCC. Another reason may be that, compared to CT, MRI techniques showed greater advantage in detection, characterization, and following up for HCC.
The top 10 academic journals published 370 articles, accounting for 44.3% of all articles and suggesting that these top 10 journals had strong interest in articles regarding HCCMRI research. These data are helpful for future scholars in choosing journals when submitting HCCMRI-related manuscripts. In addition, most HCCMRI-related articles were published in radiology journals, not hepatology or gastroenterology journals.
Close collaborations were observed between countries/regions. Among the top 10 contributing countries/regions, South Korea published the most articles [199], followed by the USA [190] and Japan [162]. Although the USA published slightly fewer articles than South Korea, the USA is a central collaborator with other countries, as indicated by its highest centrality value. China ranked fourth among the top 10 countries/regions, suggesting its high academic impact in the HCCMRI field. It is well-known that HCC is more common in Asian countries than in European and American countries, which may be one of the reasons why Korea and China become the more productive HCC MRI research publications. Six of the top 10 contributing institutions are from South Korea, followed by China and Japan, each with two institutions. This distribution of institutions was generally consistent with the distribution of the country. Similar to the collaborations between countries, the collaborations between institutions were also significant, especially for institutions in South Korea (Figure 4).
All of the top 10 active authors published at least 20 articles. All top 10 active authors were from South Korea, indicating that South Korean scholars have a leading position in HCCMRI research and have obtained substantial achievements. However, only two of the top 10 active authors are the top 10 co-cited authors, suggesting that highly productive authors do not necessarily have a record of high citation, which is a more effective indicator of an author’s academic impact. Considering the co-cited authors, the top 10 authors with at least 103 co-citations made significant contributions to the HCCMRI field. Bruix J (Barcelona Clinic Liver Cancer (BCLC) Group, Hospital Clínic, University of Barcelona, Spain) (398 co-citations) was ranked first, followed by Llovet JM (BCLC Group, Hospital Clínic, University of Barcelona, Spain) (235 co-citations), Kim YK (Department of Diagnostic Radiology, Medical School, Chonbuk National University Hospital, Republic of Korea) (235 co-citations), Forner A (BCLC Group, Hospital Clínic, University of Barcelona, Spain) (152 co-citations), and Kim SH (Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea) (149 co-citations). Bruix J and Llovet JM are well known for their contribution to developing the guidelines for HCC management (14) and elaborating on the epidemiology of HCC and viral hepatitis (15), respectively, which are important for guiding the direction of HCCMRI research. Both Kim YK (16) and Kim SH (17) investigated the value of gadoxetic acid-enhanced MRI in the preoperative detection and characterization of HCC at the earliest time, although hepatocyte-specific ontrast agent such as Gd-EOB-DTPA (Primovist®, Bayer HealthCare, Germany) and Gd-BOPTA (Multihance®, Bracco Imaging, Italy) were invented in Germany and Italy, respectively.
Research hotspots
RCA, which explores the co-citation relevance between articles and generalizes these data to create major clusters, is usually used to explore research hotspots in a given academic field (18). In our study, 10 major HCCMRI research hotspots in the past decade were generalized and ordered from the largest to smallest number of co-cited references (Figure 7). It is reasonable to assume that the top two hotspots were related to the detection and differentiation of HCC, as the accurate detection and characterization of HCC lesions are the primary purpose of MRI examination during clinical diagnosis and treatment. The timeline view of the knowledge map (Figure 8) indicates that the clusters ‘‘#5 data system’’ and ‘‘#8 microvascular invasion’’ reflect the recent research hotspots, as the phrase “data system” refers to the LI-RADS. This result indicates the current hotspots and highlights future research directions in the HCCMRI field.
The LI-RADS (19,20) is a quality assurance tool created and trademarked by the American College of Radiology in 2011 to standardize the evaluation and reporting, at the earliest occasion, of the risk of nodules being HCC in patients with cirrhosis on the basis of the computed tomography (CT) or MRI features. According to the presence/absence of five major imaging features (observation size, arterial phase hyperenhancement, washout appearance, enhancing capsule, and threshold growth) as well as more than ten ancillary imaging features, a liver nodule detected in patients on CT or MRI with high risk of HCC is categorized as a specific category from LR-1 to LR-5. Observation categorized as LR-1 is defined as definitely benign, while LR-5 entities are defined as 100% HCC. This system enables the radiology community to apply consistent terminologies, reduce errors and variabilities in lesion interpretation, improves communication with referring clinicians, facilitates quality assurance and research, and facilitates therapy decisions such as transplantation or ablative therapy. The LI-RADS has been constantly updated over increasingly short time intervals, with the latest edition released in July 2018. In one study (21), the sensitivity of LI-RADS Category 5 (LR-5) for diagnosing HCC with gadoxetic acid-enhanced MRI was significantly higher than that with gadopentetate dimeglumine-enhanced MRI (73.8% vs. 26.2%, P<0.001). Taking major features according to LI-RADS v2017 into consideration, LR-4 hepatic nodules were upgraded to LR-5. One study (22) found that LI-RADS v2018 with higher sensitivity and accuracy is superior to v2017 for diagnosing HCC. The revisions in v2018 mainly affect the categorization when adopting LR-5 as a predictor of HCC. However, the LI-RADS had many limitations in identifying certain categories of lesions just by conceptual and non-quantitative probabilistic methods. In fact, the LI-RADS was not fully accepted by the European Association for the Study of the Liver Guidelines (EASL) for the management of HCC. Plenty of questions still need to be further answered, such as the difference in diagnostic efficiency of different major/ancillary imaging features, whether some ancillary imaging features can upgrade the categories into LR-5, such as intralesional fat for example, the lack of validation and comparison of two different enhancement modalities (CT and MRI), the absence of a precise distinction of “Targetoid appearance”, the superiority of diagnostic performance of LI-RADS v2018 in comparison to LI-RADS v2017 (22). LI-RADS represents an opportunity for radiologist to improve this system.
Microvascular invasion (MVI) is a pathologic feature strongly associated with aggressive biological behavior and poor prognosis in HCC patients. The diagnostic criteria of MVI in patients with HCC have not been universally accepted; while Sumie et al. (23), Roayaie et al. (24), and the Chinese Society of Pathology (25) have proposed different standard to diagnose MVI. Commonly, MVI refers to the tumor cells invading into the hepatic or portal venous system. Several studies found that MVI was an independent factor to predict the risks of HCC post-operative recurrence and long-term prognosis (26-29). In the past, MVI could not be evaluated without biopsy, which is an invasive procedure with severe complications. However, the identification of MVI before surgery is important to therapeutic decision-making. Recently, with the advancements in MRI techniques, an increasing number of researchers have attempted to evaluate MVI noninvasively by using different MRI techniques. Early studies used morphological indicators to predict the risk of MVI of HCC, such as the smoothness of margin, the completeness of capsule, quantity and size of HCC lesions (30,31). Recently, Gd-EOB-DTPA-enhanced MRI played a pivotal role in predicting MVI. Lee et al. (27) found that a combination of three features (arterial peritumoral enhancement, non-smooth tumor margin, and peritumoral hypointensity on hepatobiliary phase) of gadoxetic acid-enhanced MRI as preoperative biomarkers can be helpful for predicting MVI with a specificity of 99.3%, and two of three imaging features (arterial peritumoral enhancement, peritumoral hypointensity on hepatobiliary phase) were strongly associated with early recurrence after curative HCC resection. Shin et al. (28) also confirmed that peritumoral hypointensity on hepatobiliary phase was an independent risk factor for predicting MVI and postoperative recurrence. However, Gd-EOB-DTPA-enhanced MRI predict MVI with a high specificity but a very low sensitivity, as shown in Lee et al.’s study (27). Xu et al. (29) suggest that it will be a more preferable choice to develop a comprehensive predictive model (nomogram) incorporating additional clinical parameters, such as AFP, PIVKA-II, for improving the sensitivity. Lei et al. (32) developed a nomogram for predicting of MVI of HCC incorporating some imaging and serological parameters, the sensitivities and specificities of the validation cohort were 62% and 81%, respectively. In addition, other MRI techniques also have been used to predict to MVI of HCC. Huang et al. (33) indicated that histogram parameters of contrast-enhanced MR imaging of HCC such as 1th percentiles for portal venous phase images held promise for prediction of MVI of HCC, with area under the ROC curves of 0.76 to 0.88. Min et al. (34) concluded that HCC with intralesional fat detected with MRI had a lower risk for MVI, and a possibly better prognosis. Jhaveri et al. (35) attempt to evaluate blood oxygen level-dependent (BOLD) MRI for prediction of MVI, but they found that BOLD MRI was unable to predict MVI accurately.
It was noted that several studies used the diffusion-weighted imaging (DWI) related MRI techniques for assessment of MVI of HCC, which included the intravoxel incoherent motion (IVIM) (36), diffusion kurtosis imaging (DKI) (37), apparent diffusion imaging (ADC) (38). Although different DWI-related parameters were evaluated as the potential predictors for MVI of HCC, with high diagnostic performances, the reliability and reproducibility of these conclusions remain being questioned. Most reported IVIM studies of biexponential decay model computed the diffusion parameters by using multiple increasingly higher b value from b =0 s/mm2. However, as Wáng (39) found, this decay process may not follow biexponential model, which finally makes the reliability of most IVIM study results questionable. More details could be found in the paper (39). Huang et al. (40) indicated that by excluding the b =0 s/mm2 images for curve fitting and computing the IVIM parameters, partial overlap of liver IVIM parameters that existed when including the b =0 s/mm2 images between healthy subjects and liver fibrosis patients disappeared.
Burst keywords refers to keywords heavily cited by articles over a period of time. Burst keywords are considered another important indicator of research hotspots or emerging trends over time. As seen in Figure 9, the evolution of the burst keywords over the past decade shows the continuing progress in HCCMRI research. In addition, the earliest-appearing and strongest burst keyword in the past decade was “superparamagnetic iron oxide”. Superparamagnetic iron oxide (SPIO), which is specifically taken up by the reticuloendothelial system in the liver or other organs, has been used in diagnostic imaging for various diseases, including lymph node detection, metastasis evaluation, as well as HCC (41,42). SPIO has shown good performance in HCCMRI applications, with many related articles published in the early years (43,44). However, the question remains as to whether SPIO-enhanced MRI is less sensitive and specific than other Gadolinium-enhanced MRI in the detection and characterization of HCC. This question and together with SPIO’s high cost and inconvenient use have led to a gradual reduction of the clinical use and research focus on SPIO. The keyword ‘‘microvascular invasion’’, identified as the strongest burst keyword since 2015 was also found to be one of the latest major clusters in the RCA. As keywords stands for hot research topics and burst keywords represents new research frontier (45,46), this result demonstrates that evaluating the MVI in HCC by MRI techniques is one of the important research hotspots in HCCMRI field currently.
Study limitations
Our study has some limitations. First, the data analyzed in our study originated only from the Web of Science database and did not include data from other important search engines such as PubMed, Embase and Ovid. Thus, the identified articles may not fully represent all HCCMRI research. However, Web of Science, which covers over 12,000 journals and collects most important articles, is currently a premier search platform for information in the sciences, social sciences, and humanities. What is more, data retrieved from the Web of Science, which contains a wealth of information (e.g., author, institution, country, journal), especially for complete references, are very suitable as input data to CiteSpace for ACA and RCA, which could not be performed by the data retrieved from PubMed or Embase database without complete references. This may be, to some extent, a drawback of CiteSpace software when doing a comprehensive bibliometric analysis in a special field. Secondly, the retrieved articles were restricted to those published in English, resulting in some linguistic bias. Nonetheless, English remains the most widely used language for publishing academic articles.
Conclusions
In conclusion, we delineated the overall scientific output of HCCMRI research from 2008 to 2017. “LI-RADS” and “microvascular invasion” are the two most recent research hotspots. Researchers dedicated to HCCMRI research could devote additional attention to these topics in the next few years.
Acknowledgements
Funding: The authors would like to express our enormous appreciation and gratitude to all participants. This work is supported by the Beijing Natural Science Foundation (No. 7184199), WBE Liver Fibrosis Foundation (No. CFHPC2019006).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Choi SH, Lee SS, Park SH, Kim KM, Yu E, Park Y, Shin YM, Lee MG. LI-RADS Classification and Prognosis of Primary Liver Cancers at Gadoxetic Acid-enhanced MRI. Radiology 2019;290:388-97. 10.1148/radiol.2018181290 [DOI] [PubMed] [Google Scholar]
- 2.Jeon SK, Joo I, Lee DH, Lee SM, Kang HJ, Lee KB, Lee JM. Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol 2019;29:373-82. 10.1007/s00330-018-5605-x [DOI] [PubMed] [Google Scholar]
- 3.Hui TCH, Chuah TK, Low HM, Tan CH. Predicting early recurrence of hepatocellular carcinoma with texture analysis of preoperative MRI: a radiomics study. Clin Radiol 2018;73:1056.e11-1056.e16. 10.1016/j.crad.2018.07.109 [DOI] [PubMed] [Google Scholar]
- 4.Lee MW, Lim HK. Management of sub-centimeter recurrent hepatocellular carcinoma after curative treatment: Current status and future. World J Gastroenterol 2018;24:5215-22. 10.3748/wjg.v24.i46.5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver 2016;10:332-9. 10.5009/gnl15257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao Y, Zhang Y, Yin L. Trends in hepatocellular carcinoma research from 2008 to 2017: a bibliometric analysis. PeerJ 2018;6:e5477. 10.7717/peerj.5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guler AT, Waaijer CJ, Palmblad M. Scientific workflows for bibliometrics. Scientometrics 2016;107:385-98. 10.1007/s11192-016-1885-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang G, Wu L. Trend in H2S Biology and Medicine Research-A Bibliometric Analysis. Molecules 2017;22:2087 10.3390/molecules22122087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Hu R, Zhu G. Top 100 cited articles on infection in orthopaedics: A bibliometric analysis. Medicine 2019;98:e14067. 10.1097/MD.0000000000014067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Tec 2006;57:359-77. 10.1002/asi.20317 [DOI] [Google Scholar]
- 11.Chen C, Ibekwe-Sanjuan F, Hou J. The structure and dynamics of cocitation clusters: A multiple-perspective cocitation analysis. J Am Soc Inf Sci Tec 2010;61:1386-409. 10.1002/asi.21309 [DOI] [Google Scholar]
- 12.Shuaib W, Khan MS, Shahid H, Valdes EA, Alweis R. Bibliometric Analysis of the Top 100 Cited Cardiovascular Articles. Am J Cardiol 2015;115:972-81. 10.1016/j.amjcard.2015.01.029 [DOI] [PubMed] [Google Scholar]
- 13.Nafade V, Nash M, Huddart S, Pande T, Gebreselassie N, Lienhardt C, Pai M. A bibliometric analysis of tuberculosis research, 2007-2016. PLos One 2018;13:e0199706. 10.1371/journal.pone.0199706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association For The Study Of The Liver EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 16.Kim YK, Kim CS, Han YM, Kwak HS, Jin GY, Hwang SB, Chung GH, Lee SY, Yu HC. Detection of hepatocellular carcinoma: gadoxetic acid-enhanced 3-dimensional magnetic resonance imaging versus multi-detector row computed tomography. J Comput Assist Tomogr 2009;33:844-50. 10.1097/RCT.0b013e3181a7e3c7 [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Kim S H, Lee J, Kim MJ, Jeon YH, Park Y, Choi D, Lee WJ, Lim HK. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol 2009;192:1675-81. 10.2214/AJR.08.1262 [DOI] [PubMed] [Google Scholar]
- 18.Trujillo CM, Long TM. Document co-citation analysis to enhance transdisciplinary research. Sci Adv 2018;4:e1701130. 10.1126/sciadv.1701130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018;289:816-30. 10.1148/radiol.2018181494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha DI, Jang KM, Kim SH, Kang TW, Song KD. Liver Imaging Reporting and Data System on CT and gadoxetic acid-enhanced MRI with diffusion-weighted imaging. Eur Radiol 2017;27:4394-405. 10.1007/s00330-017-4804-1 [DOI] [PubMed] [Google Scholar]
- 21.Ding Y, Rao SX, Wang WT, Chen CZ, Li RC, Zeng M. Comparison of gadoxetic acid versus gadopentetate dimeglumine for the detection of hepatocellular carcinoma at 1.5 T using the liver imaging reporting and data system (LI-RADS v.2017). Cancer Imaging 2018;18:48. 10.1186/s40644-018-0183-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren AH, Zhao PF, Yang DW, Du JB, Wang ZC, Yang ZH. Diagnostic performance of MR for hepatocellular carcinoma based on LI-RADS v2018, compared with v2017. J Magn Reson Imaging 2019. [Epub ahead of print]. doi: . 10.1002/jmri.26640 [DOI] [PubMed] [Google Scholar]
- 23.Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008;15:1375-82. 10.1245/s10434-008-9846-9 [DOI] [PubMed] [Google Scholar]
- 24.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009;137:850-5. 10.1053/j.gastro.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, Chen J;Guideline Committee. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol 2016;22:9279-87. 10.3748/wjg.v22.i42.9279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 2018;33:347-54. 10.1111/jgh.13843 [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 2017;67:526-34. 10.1016/j.jhep.2017.04.024 [DOI] [PubMed] [Google Scholar]
- 28.Shin SK, Kim YS, Shim YS, Choi SJ, Park SH, Jung DH, Kwon OS, Choi DJ, Kim JH. Peritumoral decreased uptake area of gadoxetic acid enhanced magnetic resonance imaging and tumor recurrence after surgical resection in hepatocellular carcinoma: A STROBE-compliant article. Medicine (Baltimore) 2017;96:e7761. 10.1097/MD.0000000000007761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu XF, Yu JJ, Xing H, Shen F, Yang T. How to better predict microvascular invasion and recurrence of hepatocellular carcinoma. J Hepatol 2017;67:1119-20. 10.1016/j.jhep.2017.06.034 [DOI] [PubMed] [Google Scholar]
- 30.Chandarana H, Robinson E, Hajdu CH, Drozhinin L, Babb JS, Taouli B. Microvascular invasion in hepatocellular carcinoma: is it predictable with pretransplant MRI? AJR Am J Roentgenol 2011;196:1083-9. 10.2214/AJR.10.4720 [DOI] [PubMed] [Google Scholar]
- 31.Renzulli M, Brocchi S, Cucchetti A, Mazzotti F, Mosconi C, Sportoletti C, Brandi G, Pinna AD, Golfierri R. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma?. Radiology 2016;279:432-42. 10.1148/radiol.2015150998 [DOI] [PubMed] [Google Scholar]
- 32.Lei Z, Li J, Wu D, Xia J, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus–Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg 2016;151:356-63. 10.1001/jamasurg.2015.4257 [DOI] [PubMed] [Google Scholar]
- 33.Huang YQ, Liang HY, Yang ZX, Ding Y, Zeng MS, Rao SX. Value of MR histogram analyses for prediction of microvascular invasion of hepatocellular carcinoma. Medicine 2016;95:e4034. 10.1097/MD.0000000000004034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min JH, Kim YK, Lim S, Jeong WK, Choi D, Lee WJ. Prediction of microvascular invasion of hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging: Impact of intra-tumoral fat detected on chemical-shift images. Eur J Radiol 2015;84:1036-43. 10.1016/j.ejrad.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 35.Jhaveri KS, Cleary SP, Fischer S, Haider MA, Pargoankar V, Khalidi K, Moshonov H, Gallinger S. Blood oxygen level‐dependent liver MRI: Can it predict microvascular invasion in HCC?. J Magn Reson Imaging 2013;37:692-9. 10.1002/jmri.23858 [DOI] [PubMed] [Google Scholar]
- 36.Li H, Zhang J, Zheng Z, Guo Y, Chen M, Xie C, Zhang Z, Mei Y, Feng Y, Xu Y. Preoperative histogram analysis of intravoxel incoherent motion (IVIM) for predicting microvascular invasion in patients with single hepatocellular carcinoma. Eur J Radiol 2018;105:65-71. 10.1016/j.ejrad.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 37.Wang WT, Yang L, Yang ZX, Hu XX, Ding Y, Yan X, Fu CX, Grimm R, Zeng MS, Rao SX. Assessment of Microvascular Invasion of Hepatocellular Carcinoma with Diffusion Kurtosis Imaging. Radiology 2018;286:571-80. 10.1148/radiol.2017170515 [DOI] [PubMed] [Google Scholar]
- 38.Zhao J, Li X, Zhang K, Yin X, Meng X, Han L, Zhang X. Prediction of microvascular invasion of hepatocellular carcinoma with preoperative diffusion-weighted imaging: A comparison of mean and minimum apparent diffusion coefficient values. Medicine (Baltimore) 2017;96:e7754. 10.1097/MD.0000000000007754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YX. Living tissue intravoxel incoherent motion (IVIM) diffusion MR analysis without b=0 image: an example for liver fibrosis evaluation. Quant Imaging Med Surg 2019. doi: . 10.21037/qims.2019.01.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H, Che-Nordin N, Wang LF, Xiao BH, Chevallier O, Yun YX, Guo SW, Wáng YX. High performance of intravoxel incoherent motion diffusion MRI in detecting viral hepatitis-b induced liver fibrosis. Ann Transl Med 2019;7:39. 10.21037/atm.2018.12.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurea S, Mainenti P P, Tambasco A, Imbriaco M, Mollica C, Camera L, Liuzzi R, Salvatore M. Diagnostic accuracy of MR imaging to identify and characterize focal liver lesions: comparison between gadolinium and superparamagnetic iron oxide contrast media. Quant Imaging Med Surg 2014;4:181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wáng YX, Idée JM. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant Imaging Med Surg 2017;7:88-122. 10.21037/qims.2017.02.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishie A, Tajima T, Ishigami K, Ushijima Y, Okamoto D, Hirakawa M, Nishihara Y, Taketomi A, Hatakenaka M, Irie H, Yoshimitsu K, Honda H. Detection of hepatocellular carcinoma (HCC) using super paramagnetic iron oxide (SPIO)-enhanced MRI: Added value of diffusion-weighted imaging (DWI). J Magn Reson Imaging 2010;31:373-82. 10.1002/jmri.22059 [DOI] [PubMed] [Google Scholar]
- 44.Kim SH, Lee WJ, Lim HK, Park CK. SPIO-Enhanced MRI Findings of Well-Differentiated Hepatocellular Carcinomas: Correlation with MDCT Findings. Korean J Radiol 2009;10:112-20. 10.3348/kjr.2009.10.2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Ping. Study of international anticancer research trends via co-word and document co-citation visualization analysis. Scientometrics 2015;105:611-22. 10.1007/s11192-015-1689-0 [DOI] [Google Scholar]
- 46.Liang YD, Li Y, Zhao J, Wang XY, Zhu HZ, Chen XH. Study of acupuncture for low back pain in recent 20 years: a bibliometric analysis via CiteSpace. J Pain Res 2017;10:951-64. 10.2147/JPR.S132808 [DOI] [PMC free article] [PubMed] [Google Scholar]