Abstract
Background
To determine the diagnostic performance of qualitative and quantitative shear wave elastography (SWE) and the optimal cutoff values of the quantitative SWE parameters in differentiating malignant from benign breast masses, and to evaluate the association between the quantitative SWE parameters and histological prognostic factors.
Methods
A gray scale ultrasound and SWE were prospectively performed on a total of 244 breast masses (148 benign, and 96 malignant) in 228 consecutive patients before an ultrasound-guided needle biopsy. The qualitative SWE and quantitative SWE parameters (the mean elasticity, maximum elasticity, and elasticity ratio) were measured in each mass. The diagnostic performance of SWE and the optimal cutoff values of the quantitative SWE parameters were obtained. An association analysis of the parameters and histological prognostic factors was performed.
Results
The malignant masses had a more heterogeneous pattern on the qualitative SWE than benign masses (P<0.001). The quantitative SWE parameters of the malignant masses were higher than those of the benign masses (P<0.001); the mean elasticity, maximum elasticity, and elasticity ratio of the benign masses were 19.73 kPa, 23.98 kPa, and 2.78, respectively; and the mean elasticity, maximum elasticity, and elasticity ratio of the malignant masses were 88.13 kPa, 98.48 kPa, and 10.64, respectively. The optimal cutoff value of the mean elasticity was 30 kPa, of the maximum elasticity was 36 kPa, and of the elasticity ratio was 4.5. The maximum elasticity had the highest AUC. Combining the three SWE parameters to differentiate between the malignant and benign masses increased the negative predictive value (NPV), which correctly downgraded 72.73% of BI-RADS category 4A masses to BI-RADS category 3. No statistically significant association was found between the quantitative SWE parameters and the tumor grading, tumor types, axillary lymph node statuses, or molecular subtypes of the breast cancers (P>0.05).
Conclusions
The qualitative and quantitative SWE provided good diagnostic performance in differentiating malignant and benign masses. The maximum elasticity of the quantitative SWE parameters had the best diagnostic performance. Adding the three combined quantitative SWE parameters to the BI-RADS category 4A masses potentially downgraded them to BI-RADS category 3 and avoided unnecessary biopsies. No statistically significant association was found between the quantitative SWE parameters and the histological prognostic factors.
Keywords: Shear wave elastography (SWE), ultrasound, breast cancer
Introduction
Gray scale ultrasound has been widely accepted for use as an effective tool to detect mammographically occult cancers in dense breast tissue and small invasive node-negative breast cancers with high sensitivity, while providing moderate specificity and increasing the biopsy rate (1,2). Generally, the standardized interpretation of breast ultrasound uses the breast imaging reporting and data system (BI-RADS), which was developed by the American College of Radiology (3). In the BI-RADS lexicon, breast masses that are clinically palpable with benign features and masses that are partially well-defined are categorized as 4A (low suspicion for malignancy); a biopsy is recommended in the guidelines in spite of a low positive predictive value (PPV) of malignancy (6%) (4). Recently introduced, shear wave elastography (SWE) is a conjunctive imaging technique that has been employed to assess tissue stiffness by generating an acoustic radiation force from a focused ultrasound beam in order to induce mechanical vibration and create shear waves that are propagated transversely into the tissue. The speed of the shear wave in stiff tissue is faster than in soft tissue (5). The SWE images are displayed in a real-time color overlay box with different colors to indicate the speed of the shear wave (in meters per second, m/sec) or the degree of tissue stiffness (Young modulus; in kilopascal, kPa) in each pixel. The assessment of the stiffness masses can be performed using either a qualitative color map or a quantitative measurement.
Previous studies have revealed that adding qualitative and quantitative SWE to a gray scale ultrasound has the potential to improve diagnostic performance in differentiating benign and malignant breast masses (6-11). Additionally, SWE has the potential to downgrade category 4A masses to category 3 and thus avoid unnecessary biopsies for benign masses (6,12,13). Unfortunately, various cutoff values for the quantitative SWE parameters have been used in the medical literature to differentiate between benign and malignant breast masses.
Furthermore, the breast cancer entity is a heterogeneous disease with several differences in histological prognostic factors, histological grading, tumor types, axillary lymph node statuses, and molecular subtypes. The molecular subtypes of breast cancer have recently been classified into the following four groups, based on the immunohistochemical expression of the hormonal receptors: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, and triple-negative breast cancer (TNBC) subtypes. Poor prognostic disease or recurrent breast cancer patients are usually found to have a high tumor grading, the triple-negative subtype, and/or axillary lymph node metastases. Several studies have identified that gray scale ultrasound features are associated with the histological grading and molecular subtypes of breast cancers (14,15). However, few studies have focused on the use of quantitative SWE parameters as the predictors of prognostic factors.
The purpose of our study was to determine the diagnostic performance of the qualitative and the quantitative SWE (the mean elasticity, maximum elasticity, and elasticity ratio) and the optimal cutoff values in differentiating benign and malignant breast masses, as well as to ascertain the parameter with the highest discriminative performance. Additionally, the study aimed to identify the quantitative SWE parameters which would most probably predict the tumor grading, tumor types, axillary lymph node statuses, and molecular subtypes of breast cancer masses.
Methods
Patients
This prospective study was approved by our hospital’s institutional review board. The study included women older than 18 years who had solid breast masses identified by previous gray scale ultrasounds, and who had an appointment to undergo an ultrasound-guided needle biopsy. Written informed consent to engage in the study was obtained from each participant. Patients who were pregnant or lactating, or had post-treated breast cancer, including those who were still receiving chemotherapy or radiation therapy, were excluded.
From March 2016 to July 2017, 228 consecutive women with 244 lesions were enrolled, with 16 of the patients having 2 masses. The gray scale ultrasounds and SWE measurements were performed using the Aixplorer ultrasound system (Super Sonic Imaging, Aix-en-Provence, France) and a linear array transducer with a frequency range of 7.5–15 MHz. They were administered by 1 of 3 participating radiologists, each of whom had 7–10 years’ experience with breast imaging and 2 years’ experience with SWE. A radiologist performed a gray scale ultrasound at each breast mass and categorized it, based on the BI-RADS lexicon (3). Subsequently, the same radiologist obtained the SWE measurements before performing the ultrasound-guided needle biopsy.
Gray scale ultrasound imaging
The gray scale ultrasound images of the masses were assessed using the BI-RADS lexicon. The final BI-RADS categories were assigned as follows: BI-RADS category 4A, low suspicion of malignancy; category 4B, intermediate suspicion of malignancy; category 4C, moderate suspicion of malignancy; and category 5, highly suggestive of malignancy (3).
SWE imaging
SWE imaging was performed in two perpendicular planes for each mass by applying the probe with minimal pressure and holding it for approximately 10 seconds until the color overlay imaging was complete. The stiffness was displayed as a color map with a range from 0 to 180 kilopascal (kPa); very soft tissue was coded in dark blue, and progressively increasing levels of tissue stiffness were coded as light blue, green, orange, and red. The qualitative features of the masses were characterized by 2 patterns: (I) homogeneous (defined as a homogeneous blue color overlaying the mass and the adjacent breast tissue); and (II) heterogeneous (represented by a heterogeneous color inside the mass and/or stiff rim sign). The criteria for the qualitative features used in our study were the same as those utilized by Feldmann et al. (16) and Zhou et al. (17). Skerl et al. (18) established that a 2-mm region of interest (ROI) provided the best diagnostic performance for all SWE parameters. Therefore, the quantitative SWE measurements in the present study were performed using a round ROI of 2 mm in diameter. The ROI was located at either the area of highest stiffness within the masses or the breast tissue immediately adjacent to the masses (if the adjacent tissue had a higher stiffness measurement than the masses). Thus, some of the malignant breast masses had their highest stiffness in the perilesional area of the color overlay map (17). Additionally, the ROI was positioned such that it avoided any areas of calcification, which would have probably created a stiffness artifact. The procedure was repeated with a second, 2-mm-round, ROI located at the adjacent normal fat tissue. The mean elasticity, maximum elasticity, and elasticity ratio (the ratio of the mean elasticities of the mass and the adjacent normal fat tissue) were automatically calculated by the ultrasound system and displayed on its monitor. The averages of the measurements of the SWE parameters from the two images (i.e., from the two perpendicular planes) were used in the later statistical analyses.
Pathologic examination
All of the 244 breast masses underwent an ultrasound-guided biopsy, which used a 14-gauge needle and a Magnum automatic biopsy gun (Bard Inc., Murray Hill, NJ, USA) with 3–6 tissue cores. Almost all of the 96 malignant masses had a further wide excision (33 masses) and a mastectomy (49 masses). When available, the final malignant pathology was based on the surgical-resection specimens; in the remaining cases, the tissue cores obtained by the ultrasound-guided biopsy were used for the final pathology. Furthermore, immunohistochemistry was done in the malignant masses; this included the estrogen (ER) and progesterone receptors (PR), the detection of overexpression and/or amplification of the HER2 oncogene, and the Ki-67 labeling index, as a means of identifying the tumor subtypes. The intrinsic molecular subtypes of breast cancer were classified as luminal A, luminal B, HER2-enriched, and TNBC. The ER and/or PR positive, HER2 negative, and Ki-67 low (<20%) masses were categorized as luminal A. The ER and/or PR positive, HER2 negative, and Ki-67 high (≥20%) masses were luminal B; ER and/or PR positive, HER2 over-expressed/amplified, and any Ki-67 were also luminal B. The HER2 over-expressed or amplified, ER and PR negative masses were HER-2 enriched. The ER and PR negative and HER2 negative masses were TNBC. The participating breast pathologists each had at least 10 years’ related experience. Of the 96 malignant masses, 75 yielded the axillary lymph node status, which was determined by a sentinel lymph node biopsy or fine needle aspiration, and their histology were recorded.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). The Mann-Whitney U test was used to compare the medians of the continuous data of the two groups. A receiver operator characteristic (ROC) analysis was performed using MedCalc for Windows, version 12.2.0.0 (MedCalc Software BVBA, Mariakerke, Belgium). ROC curves were constructed for the mean and maximum elasticity values, and the median elasticity ratio. The sensitivity, specificity, accuracy, false negative, PPVs, and negative predictive values (NPV) were calculated. The optimal cutoff values of the quantitative SWE parameters were determined using the ROC curves. The Kruskal-Wallis test was used to compare independent groups for categorical variables. Nonparametric tests for trends were used for analyses across ordered groups. P values <0.05 were considered to indicate a significant difference.
Results
Demographic data
The demographic data of the 228 patients with 244 breast masses are summarized in Table 1. The histopathology of the 244 masses revealed that 148 (60.65%) were benign and 96 (39.34%) malignant; of the latter, 83/96 (86.46%) involved an invasive cancer, and the remaining 13/96 (13.54%) a ductal carcinoma in situ (DCIS). The final pathology findings of the 244 masses are presented in Table 2.
Table 1. Demographic data of the 244 breast masses.
| Variables | Benign | Malignant | P value |
|---|---|---|---|
| Age (years) | 48.17±9.68 | 56.17±11.47 | <0.001 |
| Menstruation periods, n (%) | <0.001 | ||
| Premenopausal | 95 (38.93) | 34 (13.93) | |
| Postmenopausal | 53 (21.72) | 62 (25.41) | |
| Size (cm) | 1.25±0.78 | 2.19±2.15 | <0.001 |
| BI-RADS, n (%) | <0.001 | ||
| 4A | 75 (30.73) | 2 (0.82) | |
| 4B | 72 (29.51) | 33 (13.52) | |
| 4C | 1 (0.41) | 25 (10.25) | |
| 5 | 0 (0) | 36 (14.75) | |
Table 2. Final pathology of the breast masses.
| Pathology | Number of masses |
|---|---|
| Malignancy (n=96) | |
| Invasive ductal carcinoma | 71 |
| Invasive lobular carcinoma | 5 |
| Invasive papillary carcinoma | 4 |
| Mucinous carcinoma | 1 |
| Unclassified type cancer | 2 |
| Ductal carcinoma in situ | 13 |
| Benign (n=148) | |
| Fibroadenoma | 57 |
| Benign breast tissue | 28 |
| Sclerosing adenosis | 20 |
| Papilloma | 13 |
| Fibrocystic change | 5 |
| Fibroadenomatoid hyperplasia | 5 |
| Pseudoangiomatous stromal hyperplasia | 4 |
| Inflammation | 3 |
| Hamartoma | 2 |
| Phyllodes | 2 |
| Apocrine cyst | 1 |
| Abscess | 1 |
| Atypical ductal hyperplasia | 1 |
| Columnar cell change | 1 |
| Lobular carcinoma in situ | 1 |
| Old hemorrhage | 1 |
| Radial scar | 1 |
| Stromal fibrosis | 1 |
| Usual ductal hyperplasia | 1 |
Qualitative SWE
A higher proportion of malignant masses had a heterogeneous pattern than benign masses (Figure 1; P<0.001). The diagnostic performance of the qualitative SWE in differentiating the benign and malignant masses is illustrated in Table 3. The heterogeneous pattern had a sensitivity of 73.96%, specificity of 93.24%, PPV of 87.65%, and NPV of 84.66%. The area under the ROC curve (AUC) and 95% confidence interval (CI) were 0.836 (95% CI: 0.778–0.894), as depicted in Figure 2.
Figure 1.
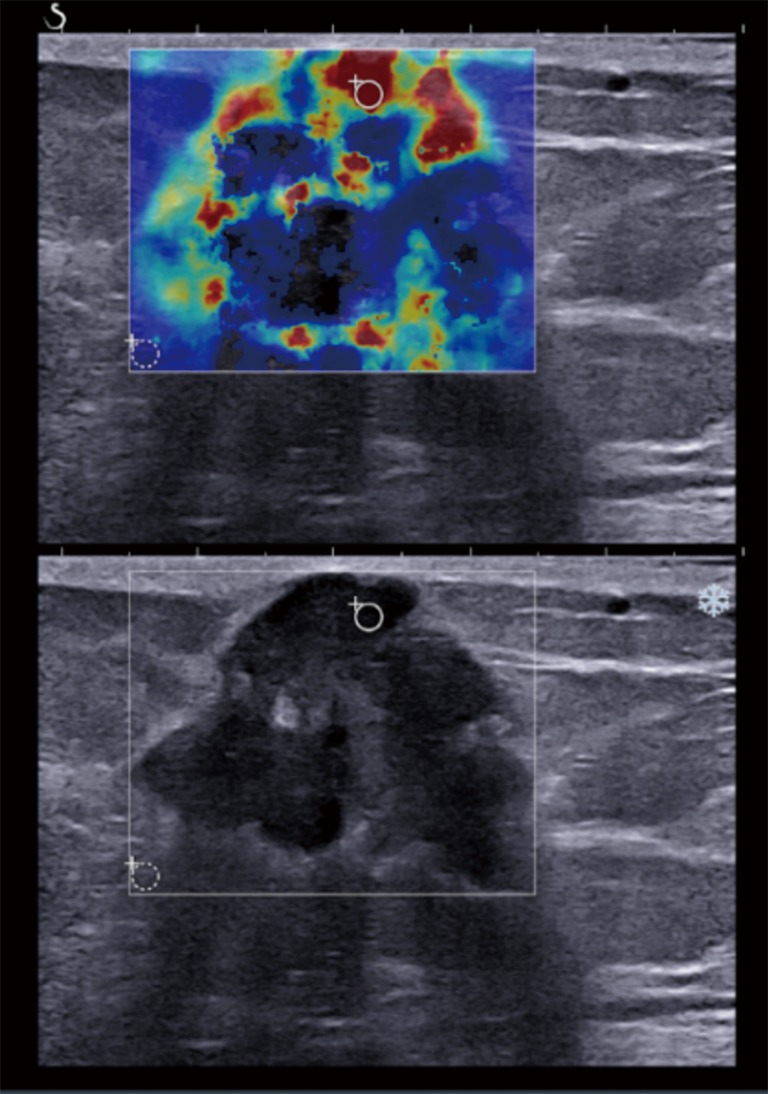
A 56-year-old postmenopausal woman with a pathologically proven grade 3 invasive ductal carcinoma. The gray scale ultrasound feature showed a 3.34 cm, ill-defined, irregular hypoechoic mass, which was assessed as BI-RADS category 5. After applying a color overlay, SWE illustrated heterogeneous pattern with stiff rim sign. The quantitative SWE values were 194 kPa for the mean elasticity, 241 kPa for the maximum elasticity, and 33.80 for the elasticity ratio, all of which were above the corresponding cutoff values. SWE, shear wave elastography.
Table 3. Diagnostic performance of qualitative and quantitative SWE.
| Variables | Heterogeneous pattern | Mean elasticity (cutoff, 30 kPa) | Maximum elasticity (cutoff, 36 kPa) | Elasticity ratio (cutoff, 4.5) | Combination of three parameters |
|---|---|---|---|---|---|
| AUC | 0.836 (0.778–0.894) | 0.903 (0.861–0.946) | 0.905 (0.864–0.946) | 0.860 (0.812–0.907) | 0.813 (0.758–0.868) |
| Sensitivity (%) | 73.96 (64.00–82.35) | 87.50 (79.18–93.37) | 87.50 (79.18–93.37) | 82.29 (73.17–89.33) | 91.67 (84.24–96.33) |
| Specificity (%) | 93.24 (87.93–96.71) | 80.41 (73.09–86.47) | 80.41 (73.09–86.47) | 74.34 (66.50–81.15) | 70.95 (62.92–78.11) |
| Accuracy (%) | 85.66 (80.62–89.80) | 83.20 (77.90–87.66) | 83.20 (77.90–87.66) | 77.46 (71.69–82.54) | 79.10 (73.45–84.02) |
| False-positive rate (%) | 6.76 | 19.59 | 19.59 | 25.66 | 29.05 |
| False-negative rate (%) | 26.04 | 12.50 | 12.50 | 17.71 | 8.33 |
| Positive predictive value (%) | 87.65 (79.41–92.82) | 74.34 (65.45–80.20) | 74.34 (65.45–80.20) | 67.52 (60.88–73.52) | 67.18 (61.24–72.61) |
| Negative predictive value (%) | 84.66 (79.71–88.58) | 90.84 (85.31–94.42) | 90.84 (85.31–94.42) | 86.61 (80.62–90.96) | 92.92 (87.02–96.25) |
Data in round bracket are 95% CI. SWE, shear wave elastography; kPa, kilopascals.
Figure 2.
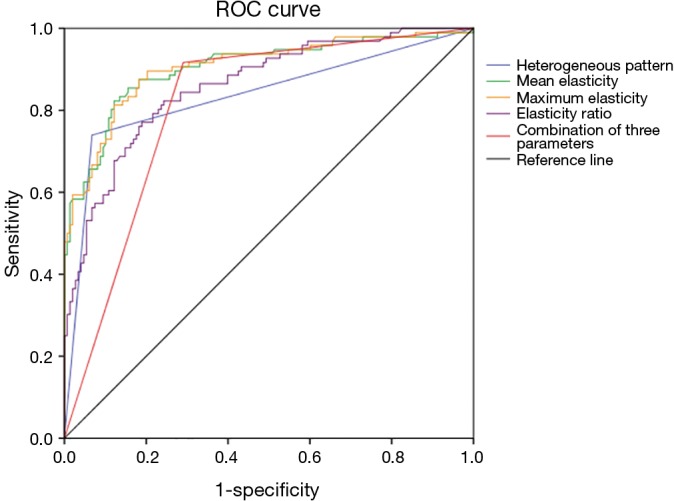
Receiver operating characteristic (ROC) curve of the heterogeneous pattern, mean elasticity, maximum elasticity, elasticity ratio, and the combination of the three SWE parameter values. SWE, shear wave elastography.
Quantitative SWE
The medians of the quantitative SWE parameters of the different variables are at Table 4. The malignant masses had higher values than the benign masses for the three SWE parameters, including the medians of their mean elasticity, maximum elasticity, and elasticity ratio, with statistical significance (P<0.001; Figure 3). Moreover, when the malignant masses were divided into invasive cancer and DCIS, the medians of the SWE parameters of the invasive cancer were statistically significantly higher than those of DCIS (P<0.001). Nevertheless, there were no statistically significant associations between the quantitative SWE parameters and the histological prognostic factors (namely, the tumor grading, tumor types, axillary lymph node statuses, and molecular subtypes) of the breast cancers (P>0.05).
Table 4. Medians of the mean elasticity, maximum elasticity, and elastic ratio, by pathology.
| Variables | Number | Mean elasticity (kPa) | Maximum elasticity (kPa) | Elastic ratio (kPa) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median (min–max) | P value | Median (min–max) | P value | Median (min–max) | P value | ||||
| Pathology (n=244) | <0.001 | <0.001 | <0.001 | ||||||
| Benign | 148 | 19.73 (5.15–104.10) | 23.98 (7.70–113.65) | 2.78 (0.70–17.86) | |||||
| Malignant | 96 | 88.13 (4.25–281.95) | 98.48 (8.15–300.00) | 10.64 (1.57–41.24) | |||||
| Malignant tumor (n=96) | <0.001 | <0.001 | 0.004 | ||||||
| DCIS | 13 | 37.85 (4.25–255.50) | 48.10 (8.15–273.60) | 6.15 (1.69–32.63) | |||||
| Invasive tumors | 83 | 99.45 (8.20–281.95) | 121.25 (12.9–300.0) | 11.38 (1.57–41.24) | |||||
| Invasive tumor | |||||||||
| Grading tumor (n=83) | 0.218 | 0.426 | 0.281 | ||||||
| Grade 1 | 10 | 59.18 (22.80–175.95) | 70.03 (33.3–246.0) | 10.10 (2.56–29.62) | |||||
| Grade 2 | 50 | 108.10 (8.20–274.35) | 125.85 (12.9–300.0 | 13.99 (1.57–41.24) | |||||
| Grade 3 | 23 | 88.10 (14.75–281.95) | 96.05 (18.95–298.95) | 9.99 (2.81–33.75) | |||||
| Invasive tumor types (n=83) | 0.919 | 0.922 | 0.658 | ||||||
| Invasive ductal carcinoma | 71 | 96.65 (8.20–281.95) | 115.45 (12.9–300.0) | 11.78 (1.57–41.24) | |||||
| Invasive lobular carcinoma | 5 | 105.75 (24.05–171.65) | 128.75 (29.2–222.6) | 16.44 (2.66–37.63) | |||||
| Other specified cancers | 7 | 151.90 (31.8–207.55) | 190.25 (35.40–242.15) | 19.25 (3.10–29.23) | |||||
| Axillary lymph node metastases (n=75) | 0.162 | 0.137 | 0.654 | ||||||
| Negative | 51 | 71.35 (8.20–281.95) | 85.00 (12.9–300.0) | 10.29 (1.57–29.27) | |||||
| Positive | 24 | 95.03 (19.55–208.90) | 140.88 (22.90–295.95) | 10.10 (2.35–41.24) | |||||
| Molecular subtypes (n=68) | 0.216 | 0.274 | 0.684 | ||||||
| Luminal A | 25 | 64.60 (8.2–208.9) | 77.05 (12.9–246.0) | 10.44 (1.57–40.33) | |||||
| Luminal B | 36 | 111.93 (12.90–274.35) | 149.68 (18.95–300.00) | 13.37 (2.57–41.24) | |||||
| HER2-enriched | 3 | 71.35 (40.95–192.45) | 85.00 (48.60–222.05) | 13.27 (8.48–14.68) | |||||
| TNBC | 4 | 108.83 (52.00–281.95) | 125.25 (75.75–298.95) | 18.11 (6.18–37.63) | |||||
kPa, kilopascals; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Figure 3.
Box-and-whisker plots of mean elasticity, maximum elasticity, and elasticity ratio of malignant and benign masses. The top and bottom of each box were the 75th and 25th percentiles, respectively; the horizontal line in each box was the median; and the top and bottom of the whiskers were the minimum and maximum, respectively. The mean elasticity, maximum elasticity, and elasticity ratio were significantly higher for malignant than benign masses (all P<0.001).
The optimal cutoff value to differentiate between the benign and malignant masses, while still offering the maximum sensitivity and specificity, was 30 kPa for the mean elasticity, 36 kPa for the maximum elasticity, and 4.5 for the elasticity ratio. The masses with SWE values less than the cutoff values were benign, whereas those masses with SWE values that were equal to, or higher than, the cutoff values were malignant, as shown in Figure 1. Using the combination of the three parameter values to differentiate between the benign and malignant masses means that if the SWE value was equal to, or higher than, the cutoff value of at least one of the three parameters (the mean elasticity, maximum elasticity, or elasticity ratio), the mass was assessed as malignant. When using the SWE cutoff values, the sensitivities were 87.50%, 87.50%, 82.29%, and 91.67%; the specificities were 80.41%, 80.41%, 74.34%, and 70.95%; the accuracies were 83.20%, 83.20%, 77.46%, and 79.10%; the false negatives were 12.50%, 12.50%, 17.71%, and 8.33%; the PPVs were 74.34%, 74.34%, 67.52%, 67.18%; and the NPVs were 90.84%, 90.84%, 86.61%, 92.92% for the mean elasticity, maximum elasticity, elasticity ratio, and the three combined parameters, respectively (Table 3). The area under the ROC curve (AUC) and 95% confidence interval (CI) of the mean elasticity, maximum elasticity, elasticity ratio, and the three combined parameter values were 0.903 (95% CI: 0.861–0.946), 0.905 (95% CI: 0.864–0.946), 0.860 (95% CI: 0.812–0.907), and 0.813 (95% CI: 0.758–0.868), respectively, as demonstrated in Table 3 and Figure 2.
False negative of SWE
The false negative of the qualitative SWE (26.04%) was higher than that of the quantitative SWE in our study. Furthermore, when the optimal cutoff values for the mean elasticity (30 kPa), maximum elasticity (36 kPa), and elasticity ratio (4.5) were used, we found that we had false-negative rates of 12.50%, 12.50%, and 17.71%, respectively.
Additionally, when the combined SWE parameters of mean elasticity, maximum elasticity, and elasticity ratio were used to diagnose malignant breast cancer, it was found that only 8 malignant masses (8.33%) had been misclassified. Those were 4 masses of DCIS (as shown in Figure 4), 1 mass of grade 1 invasive cancer, and 3 masses of grade 2 invasive cancer.
Figure 4.
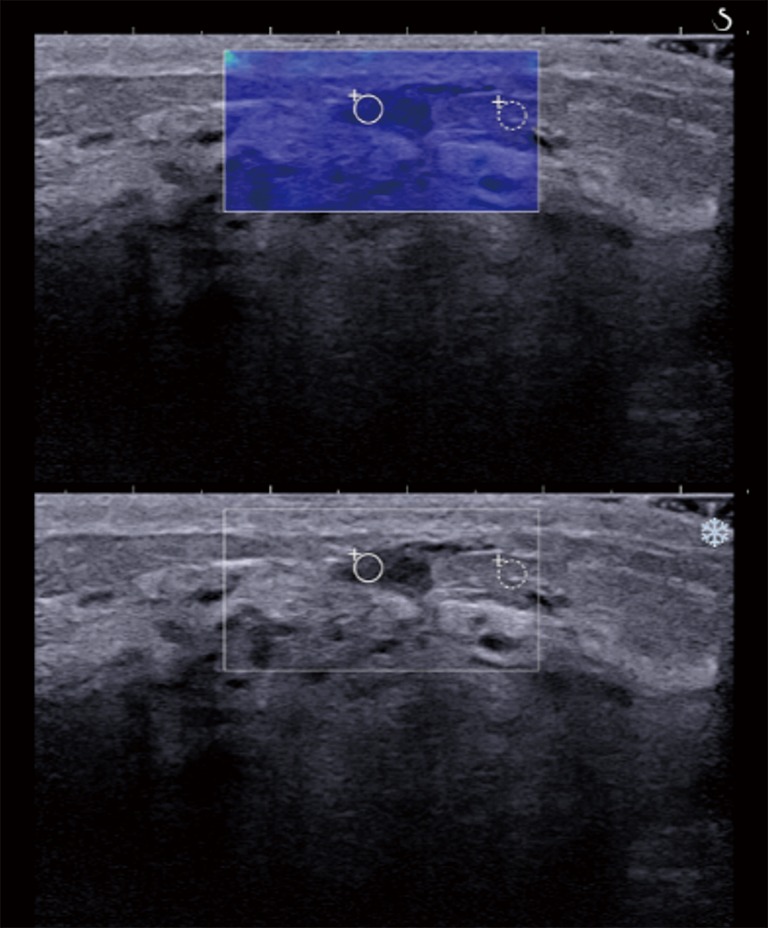
A 50-year-old premenopausal woman with a pathologically proven DCIS. The gray scale ultrasound feature showed a 0.87 cm, ill-defined, oval hypoechoic mass which was assessed as BI-RADS category 4B. After applying a color overlay, SWE showed a homogeneous blue color overlaying the mass. The quantitative SWE measurements were 10.3 kPa for the mean elasticity, 11.7 kPa for the maximum elasticity, and 2.35 for the elasticity ratio, all of which were below the corresponding cutoff values. SWE, shear wave elastography.
False positive of SWE
The qualitative SWE had a false-positive rate of 6.76%. Moreover, the false-positive rates of the quantitative SWE were 19.59%, 19.59%, and 25.66% for the cutoff values of mean elasticity (30 kPa), maximum elasticity (36 kPa), and elasticity ratio (4.5), respectively.
Using the combined SWE parameters of mean elasticity, maximum elasticity, and elasticity ratio to diagnose malignancy, the false-positive rate was 29.05%. The pathology of breast masses that were false positive when using the combined SWE parameters is demonstrated in Table 5.
Table 5. Pathology of false-positive breast masses when using the three combined SWE parameters.
| Pathology | Number of masses |
|---|---|
| Fibroadenoma | 23 |
| Sclerosis adenosis | 7 |
| Papilloma | 2 |
| Inflammation | 2 |
| Dense collagen stroma | 2 |
| Fibrocystic change | 2 |
| Pseudoangiomatous stromal hyperplasia | 1 |
| Atypical ductal hyperplasia | 1 |
| Phyllodes | 1 |
| Old hemorrhage | 1 |
SWE, shear wave elastography.
SWE to downgrade BI-RADS category 4A masses
The malignancy rate for BI-RADS category 4A in our study was 2.6% (2/77). If we used the three combined SWE parameters to downgrade the BI-RADS category 4A masses, 72.73% (56/77) of the masses were correctly downgraded to BI-RADS category 3, thus avoiding benign mass biopsies. No false-negative results occurred in our study when downgrading the masses of BI-RADS category 4A to category 3. Additionally, two malignant masses of BI-RADS category 4A were not downgraded to BI-RADS category 3. One was a DCIS, which had a mean elasticity less than 30 (26.05 kPa) and a maximum elasticity less than 36 (35.3 kPa), but an elasticity ratio greater than 4.5 (5.97). The other was an invasive ductal carcinoma grade 2, which had a higher mean elasticity (109.60 kPa), higher maximum elasticity (122.95 kPa), and higher elasticity ratio (14.82) than the corresponding cutoff values (Figure 5). Finally, the false-positive rate for biopsies for benign category 4A masses was 24.68%.
Figure 5.
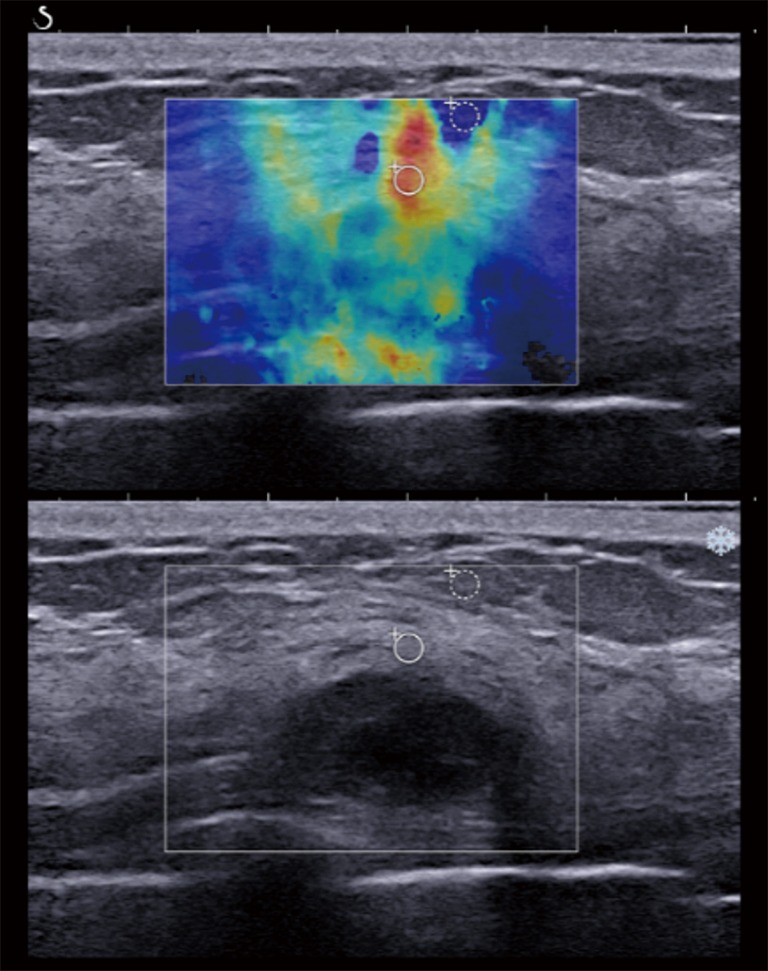
A 48-year-old postmenopausal woman with a pathologically proven grade 2 invasive ductal carcinoma and luminal B subtype. The gray scale ultrasound feature showed a 1.6 cm, partially well-defined, oval hypoechoic mass, which was slightly increased in size compared with a previous US study and was assessed as BI-RADS category 4A. After applying a color overlay, SWE illustrated heterogeneous color pattern. The quantitative SWE values were 109.60 kPa for the mean elasticity, 122.95 kPa for the maximum elasticity, and 14.82 for the elasticity ratio. SWE, shear wave elastography.
Discussion
In our study, a higher proportion of malignant than benign breast masses had a heterogeneous pattern for the qualitative SWE. The heterogeneous pattern in the malignant masses is probably explained by the pathological heterogeneity of malignant lesions that have a dense cellularity with some areas of internal necrosis, including the desmoplastic reaction or tumor infiltrated into the adjacent interstitial tissue (10). The diagnostic performance of the qualitative SWE in our study produced results that were similar to those of other studies. The study by Tozaki et al. (11) divided 100 breast masses (69 malignant and 31 benign) by using 4 color-map patterns (patterns 1 and 2 were benign, similar to the homogeneous pattern in our study, while patterns 3 and 4 were malignant, corresponding to the heterogeneous pattern in our study). The sensitivity was 91.3%, specificity was 80.6%, PPV was 91.3%, NPV was 80.6%, and accuracy was 88%. Likewise, Feldmann et al. (16) reported that 83 breast masses (38 malignant and 45 benign) had an 89% sensitivity, 60% specificity, 65% PPV, and 87% NPV. In the current study, the qualitative SWE had a high specificity and PPV, but a high false-negative rate. As a result, we recommend using the qualitative SWE with upgraded BI-RADS category 3 masses when a suspicious pattern of SWE is found, rather than using it with downgraded BI-RADS category 4A masses.
Our study found that malignant masses had higher values of the three quantitative SWE parameters (mean elasticity, maximum elasticity, and elasticity ratio) than benign masses, with statistical significance (P<0.001). Additionally, there was a statistically significant difference in the three quantitative SWE parameter values of invasive cancer and DCIS, with the former values being higher (P<0.001). The medians of the mean elasticity, maximum elasticity, and elasticity ratio were 19.73 kPa, 23.98 kPa, and 2.78 for benign; 37.85 kPa, 48.10 kPa, and 6.15 for DCIS; and 99.45 kPa, 121.25 kPa, and 11.38 for invasive cancer, respectively. The findings of our study were similar to those of previous studies. Lee et al. (12) reported that malignant masses had a higher value for the maximum elasticity parameter (119.0±52.2 kPa) than did benign masses (41.4±32.1 kPa). Chang et al. (19) found that malignant masses had a higher level of mean elasticity (153.3±58.1 kPa) than benign masses (46.1±42.9 kPa), with statistical significance. In addition, Berg et al. (6) reported that the highest value of the median maximum elasticity was found with invasive cancer (179 kPa), the second-highest with DCIS (133 kPa), and the lowest with benign masses (41 kPa). These results can be explained by the fact that stiffness is correlated more with the degree of tumor cellularity and microvessel density found in malignant masses than in DCIS or benign masses (20,21).
Our study obtained optimal cutoff values for the three quantitative SWE parameters that had maximized sensitivity and specificity. Our cutoff values for the mean elasticity, maximum elasticity, and elasticity ratio were 30 kPa, 36 kPa, and 4.5, respectively. The values were similar to those reported by other studies. For instance, Au et al. (5) found that a total of 123 breast masses (79 benign and 44 malignant) had optimal cutoff values of 42.5 kPa, 46.7 kPa, and 3.56 for mean elasticity, maximum elasticity, and elasticity ratio, respectively. Moreover, Lee et al. (12) used a cutoff value of 30 kPa for the maximum elasticity when downgrading from category 4A to category 3 in screening patients. On the other hand, higher cutoff values than our results have been demonstrated by other researchers. As an example, Youk et al. (10) reported cutoff values of 61.9 kPa, 90.0 kPa, and 4.86 kPa for the mean elasticity, maximum elasticity, and elasticity ratio, respectively. Similarly, a study by Lee et al. (22) showed higher cutoff values for the mean elasticity, maximum elasticity, and elasticity ratio (68.4, 82.3, and 4.39, respectively). The reasons for the various cutoff values for the quantitative SWE parameters reported in the medical literature and our study are probably related to differences in breast thickness, breast composition, ethnicity, and/or the versions of the SWE machines. For instance, studies by Chang et al. (20) and Yoon et al. (23) confirmed that breast thickness is an influencing factor on quantitative SWE results. Additionally, a meta-analysis study showed differences in the diagnostic performance of SWE related to ethnicity; the performance was slightly better for a Caucasian population than an Asian population (9). However, the other influence factors should be extensively investigated in future studies.
Nevertheless, the quantitative SWE parameters in our study showed high diagnostic performance in differentiating between benign and malignant masses (AUC range: 0.860–0.905). The diagnostic performance in our study was similar to those reported by previous studies, even though different cutoff values were used. To illustrate, Youk et al. (24) used higher cutoff values than our study but had similar diagnostic performance results, with an AUC of 0.907, 0.902, and 0.917 for the mean elasticity, maximum elasticity and elasticity ratio, respectively.
In particular, our study revealed that maximum elasticity provided the highest diagnostic performance (AUC =0.905, 95% CI: 0.864–0.946), making it the most discriminative quantitative SWE parameter. This result matched the finding of previous studies (22,25-27): that maximum elasticity is the diagnostic parameter that offers the best performance and the highest AUC. The reason for maximum elasticity being the most discriminative parameter is that the highest stiffness of a mass is usually inside the ROI, regardless of the size of the ROI. In contrast, mean elasticity represents an average value of stiffness, and it is influenced by the size of the ROI, especially given the heterogeneity of malignant masses. As the elasticity ratio is calculated by dividing the mass stiffness by the fat stiffness, any changes in the minimal value of the fat stiffness can result in significant changes in the value of the elasticity ratio (10).
When using the quantitative SWE parameters to differentiate between benign and malignant masses in our study, the range for the false-negative rate was 12.5–17.7%. The rate in our study corresponded with those in other studies (19,23,28). Interestingly, by using the cutoff values from the three combined SWE parameters of mean elasticity, maximum elasticity, and elasticity ratio to diagnose malignant masses, the false-negative rate decreased to 8.3%. When the three combined SWE parameters were used, a half of the false-negative lesions were DCIS. The remaining masses were invasive carcinoma with grade 1 (1 mass) and grade 2 (3 masses; 1 of those had an extensive intraductal component), as well as misclassified invasive cancers of small size (mean diameter, 1.19 cm; range, 0.73–1.87 cm). It is noticeable that DCIS, small tumor, and low-grade tumor were the crucial risk factors for the misclassified masses when using the quantitative SWE. The risk factors of false-negative masses in our results were similar to those reported by previous studies. For instance, Vinnicombe et al. (29) revealed that misclassified malignant masses on the quantitative SWE tend to have a better prognosis than correctly classified malignant masses, namely, small tumor (≤1 cm), low grade invasive tumor, or DCIS.
Our study found that the three combined SWE parameters had a higher NPV and a lower false-negative rate than other SWE parameters. As for the false-negative masses found by using the three combined SWE parameters in our study, all were categorized as BI-RADS 4B or 4C, based on their gray scale ultrasound features. As a result, we recommend using the three combined SWE parameters together with the gray scale ultrasound features for downgraded BI-RADS category 4A to category 3. Our study showed that 72.73% of BI-RADS category 4A could be correctly downgraded to BI-RADS category 3 (and thus avoid unnecessary biopsies), and that the PPV3 (or the positive biopsy rate) of BI-RADS 4A increased from 2.6% to 9.5% when the cutoff values from the three combined SWE parameters were used. In particular, no malignant masses were downgraded from BI-RADS category 4A to category 3 in our study; however, our study only had 2 malignant masses in BI-RADS category 4A. Au et al. (5) reported that one case of invasive ductal carcinoma with mucinous stroma, which had negative features for all of the quantitative SWE parameters, was incorrectly downgraded from BI-RADS category 4A to category 3. Thus, in the cases of downgrades to category 3, a follow-up of lesions should be conducted at regular intervals for at least 2 years to confirm the stability of the masses. Given the fact that there is a considerable likelihood of malignancy in BI-RADS categories 4B (>10% to ≤50%), 4C (>50 to <95%), and 5 (≥95%) (3), we do not suggest using SWE instead of tissue diagnosis to downgrade these highly suspicious masses.
Our study found no statistically significant association between the SWE parameters and the histological prognostic factors (tumor grading, tumor type, axillary lymph node status, and molecular tumor subtype). This result matched that of a study by Youk et al. (30), which reported that there was no association between the molecular tumor subtype and mean elasticity value. Au et al. (31) also demonstrated in a multivariate analysis that there was no correlation between SWE parameters (the mean elasticity and maximum elasticity) and the tumor grading, lymph node status, and molecular tumor subtype. This was in contrast with the findings of a study by Chang et al. (32), which showed higher values of mean elasticity for the aggressive subtypes of breast cancer (high grade tumors, TNBCs, and HER2 positive tumors).
Our study had some limitations. Firstly, the measuring of the quantitative SWE parameters of each mass was performed by only one participating radiologist. Some SWE parameter values would have probably been different if they had been measured by independent radiologists. Nevertheless, previous studies (33,34) have confirmed that quantitative SWE measurements are highly reproducible with good interobserver agreement. A further limitation is that the same radiologist performed the gray scale ultrasound and obtained the SWE without being blinded to the gray scale ultrasound results. Thirdly, determining the BI-RADS category of the gray scale ultrasound features was performed by only one radiologist without seeking consensus. However, previous studies revealed that the BI-RADS lexicon had a good interobserver agreement (4,35). Fourthly, if a benign pathology was confirmed by an ultrasound-guided biopsy, we did not continue with an imaging follow up or undertake a surgical excision. Lastly, all of the malignant masses that had surgery (including wide excision or mastectomy) in our hospital were sent to pathology to obtain an immunohistochemistry profile. However, as our hospital is a tertiary care hospital, some patients were sent for surgical treatment at hospitals closer to their homes. In such cases, we did not collect data relating to the patients’ immunohistochemistry profile.
Conclusions
The qualitative and quantitative SWE parameters provided good diagnostic performance to differentiate benign and malignant breast masses. The optimal cutoff values of the quantitative SWE parameters were 30 kPa, 36 kPa, and 4.5 for the mean elasticity, maximum elasticity, and elasticity ratio, respectively. The maximum elasticity was the most discriminative parameter with the highest AUC. Using the three combined quantitative SWE parameters to downgrade BI-RADS category 4A masses to BI-RADS category 3 can reduce the number of unnecessary biopsies. No statistically significant association was found between the SWE parameters and the histological prognostic factors (tumor grading, tumor types, axillary lymph node status, and molecular tumor subtypes).
Acknowledgements
This research was supported by grant from the Faculty of Medicine, Siriraj Hospital, Mahidol University.
Ethical Statement: The study was approved by our hospital's institutional review board and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening Breast Ultrasound: Past, Present, and Future. AJR Am J Roentgenol 2015;204:234-40. 10.2214/AJR.13.12072 [DOI] [PubMed] [Google Scholar]
- 2.Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, Böhm-Vélez M, Mahoney MC, Evans WP, 3rd, Larsen LH, Morton MJ, Mendelson EB, Farria DM, Cormack JB, Marques HS, Adams A, Yeh NM, Gabrielli G, ACRIN 6666 Investigators Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307:1394-404. 10.1001/jama.2012.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendelson EB, Böhm-Vélez M, Berg WA, Whitman GJ, Feldman MI, Madjar H, Rizzatto G, Baker JA, Zuley M, Stavros AT, Comstock C, Wear VVD. ACR BI-RADS ultrasound. In: D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA. editors. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System. Reston: American College of Radiology, 2013. [Google Scholar]
- 4.Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS Lexicon for US and Mammography: Interobserver Variability and Positive Predictive Value. Radiology 2006;239:385-91. 10.1148/radiol.2392042127 [DOI] [PubMed] [Google Scholar]
- 5.Au FW, Ghai S, Moshonov H, Kahn H, Brennan C, Dua H, Crystal P. Diagnostic performance of quantitative shear wave elastography in the evaluation of solid breast masses: Determination of the most discriminatory parameter. AJR Am J Roentgenol 2014;203:W328-36. 10.2214/AJR.13.11693 [DOI] [PubMed] [Google Scholar]
- 6.Berg WA, Cosgrove DO, Doré CJ, Schäfer FKW, Svensson WE, Hooley RJ, Ohlinger R, Mendelson EB, Balu-Maestro C, Locatelli M, Tourasse C, Cavanaugh BC, Juhan V, Stavros AT, Tardivon A, Gay J, Henry JP, Cohen-Bacrie C; BE1 Investigators. Shear-wave Elastography Improves the Specificity of Breast US: The BE1 Multinational Study of 939 Masses. Radiology 2012;262:435-49. 10.1148/radiol.11110640 [DOI] [PubMed] [Google Scholar]
- 7.Goddi A, Bonardi M, Alessi S. Breast elastography: A literature review. J Ultrasound 2012;15:192-8. 10.1016/j.jus.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, Jordan L, Baker L, Thompson A. Quantitative shear wave ultrasound elastography: Initial experience in solid breast masses. Breast Cancer Res 2010;12:R104. 10.1186/bcr2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue Y, Yao S, Li X, Zhang H. Benign and malignant breast lesions identification through the values derived from shear wave elastography: evidence for the meta-analysis. Oncotarget 2017;8:89173-81. 10.18632/oncotarget.21124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youk JH, Gweon HM, Son EJ. Shear-wave elastography in breast ultrasonography: the state of the art. Ultrasonography 2017;36:300-9. 10.14366/usg.17024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tozaki M, Fukuma E. Pattern classification of ShearWave TM Elastography images for differential diagnosis between benign and malignant solid breast masses. Acta radiol 2011;52:1069-75. 10.1258/ar.2011.110276 [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Chang JM, Kim WH, Bae MS, Seo M, Koo HR, Chu AJ, Gweon HM, Cho N, Moon WK. Added value of shear-wave elastography for evaluation of breast masses detected with screening US imaging. Radiology 2014;273:61-9. 10.1148/radiol.14132443 [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Chung J, Choi HY, Choi SH, Ryu EB, Ko KH, Koo HR, Park JS, Yi A, Youk JH, Son EJ, Chu AJ, Chang JM, Cho N, Jang MJ, Kook SH, Cha ES, Moon WK. Evaluation of Screening US-detected Breast Masses by Combined Use of Elastography and Color Doppler US with B-Mode US in Women with Dense Breasts: A Multicenter Prospective Study. Radiology 2017;285:660-9. 10.1148/radiol.2017162424 [DOI] [PubMed] [Google Scholar]
- 14.Suvannarerg V, Tangcharoensathien W, Thiravit S, Tanasoontrarat W, Muangsomboon K, Korparaphong P. The association between mammographic and ultrasound features and histologic grade in invasive ductal carcinoma of the breast. Siriraj Med J 2018;70:152-8. [Google Scholar]
- 15.Costantini M, Belli P, Bufi E, Asunis AM, Ferra E, Bitti GT. Association between sonographic appearances of breast cancers and their histopathologic features and biomarkers. J Clin Ultrasound 2016;44:26-33. 10.1002/jcu.22312 [DOI] [PubMed] [Google Scholar]
- 16.Feldmann A, Langlois C, Dewailly M, Martinez EF, Boulanger L, Kerdraon O, Faye N. Shear Wave Elastography (SWE): An Analysis of Breast Lesion Characterization in 83 Breast Lesions. Ultrasound Med Biol 2015;41:2594-604. 10.1016/j.ultrasmedbio.2015.05.019 [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Zhan W, Chang C, Zhang X, Jia Y, Dong Y, Zhou C, Sun J, Grant EG. Breast Lesions: Evaluation with Shear Wave Elastography, with Special Emphasis on the “Stiff Rim” Sign. Radiology 2014;272:63-72. 10.1148/radiol.14130818 [DOI] [PubMed] [Google Scholar]
- 18.Skerl K, Vinnicombe S, Giannotti E, Thomson K, Evans A. Influence of region of interest size and ultrasound lesion size on the performance of 2D shear wave elastography (SWE) in solid breast masses. Clin Radiol 2015;70:1421-7. 10.1016/j.crad.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 19.Chang JM, Moon WK, Cho N, Yi A, Koo HR, Han W, Noh DY, Moon HG, Kim SJ. Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat 2011;129:89-97. 10.1007/s10549-011-1627-7 [DOI] [PubMed] [Google Scholar]
- 20.Chang JM, Won JK, Lee KB, Park IA, Yi A, Moon WK. Comparison of Shear-Wave and Strain Ultrasound Elastography in the Differentiation of Benign and Malignant Breast Lesions. AJR Am J Roentgenol 2013;201:W347-56. 10.2214/AJR.12.10416 [DOI] [PubMed] [Google Scholar]
- 21.Jugé L, Doan BT, Seguin J, Albuquerque M, Larrat B, Mignet N, Chabot GG, Scherman D, Paradis V, Vilgrain V, Van Beers BE, Sinkus R. Colon Tumor Growth and Antivascular Treatment in Mice: Complementary Assessment with MR Elastography and Diffusion-weighted MR Imaging. Radiology 2012;264:436-44. 10.1148/radiol.12111548 [DOI] [PubMed] [Google Scholar]
- 22.Lee EJ, Jung HK, Ko KH, Lee JT, Yoon JH. Diagnostic performances of shear wave elastography: which parameter to use in differential diagnosis of solid breast masses? Eur Radiol 2013;23:1803-11. 10.1007/s00330-013-2782-5 [DOI] [PubMed] [Google Scholar]
- 23.Yoon JH, Jung HK, Lee JT, Ko KH. Shear-wave elastography in the diagnosis of solid breast masses: what leads to false-negative or false-positive results? Eur Radiol 2013;23:2432-40. 10.1007/s00330-013-2854-6 [DOI] [PubMed] [Google Scholar]
- 24.Youk JH, Son EJ, Gweon HM, Kim H, Park YJ, Kim JA. Comparison of strain and shear wave elastography for the differentiation of benign from malignant breast lesions, combined with B-mode ultrasonography: qualitative and quantitative assessments. Ultrasound Med Biol 2014;40:2336-44. 10.1016/j.ultrasmedbio.2014.05.020 [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Chang JM, Kim WH, Bae MS, Cho N, Yi A, Koo HR, Kim SJ, Kim JY, Moon WK. Differentiation of benign from malignant solid breast masses: comparison of two-dimensional and three-dimensional shear-wave elastography. Eur Radiol 2013;23:1015-26. 10.1007/s00330-012-2686-9 [DOI] [PubMed] [Google Scholar]
- 26.Xue Y, Yao S, Li X, Zhang H. Value of shear wave elastography in discriminating malignant and benign breast lesions. Medicine (Baltimore) 2017;96:e7412. 10.1097/MD.0000000000007412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng WL, Rahmat K, Fadzli F, Rozalli FI, Mohd-Shah MN, Chandran PA, Westerhout CJ, Vijayananthan A, Abdul Aziz YF. Shearwave Elastography Increases Diagnostic Accuracy in Characterization of Breast Lesions. Medicine (Baltimore) 2016;95:e3146. 10.1097/MD.0000000000003146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans A, Whelehan P, Thomson K, Brauer K, Jordan L, Purdie C, McLean D, Baker L, Vinnicombe S, Thompson A. Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br J Cancer 2012;107:224-9. 10.1038/bjc.2012.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinnicombe SJ, Whelehan P, Thomson K, McLean D, Purdie CA, Jordan LB, Hubbard S, Evans AJ. What are the characteristics of breast cancers misclassified as benign by quantitative ultrasound shear wave elastography? Eur Radiol 2014;24:921-6. 10.1007/s00330-013-3079-4 [DOI] [PubMed] [Google Scholar]
- 30.Youk JH, Gweon HM, Son EJ, Kim JA, Jeong J. Shear-wave elastography of invasive breast cancer: correlation between quantitative mean elasticity value and immunohistochemical profile. Breast Cancer Res Treat 2013;138:119-26. 10.1007/s10549-013-2407-3 [DOI] [PubMed] [Google Scholar]
- 31.Au FW, Ghai S, Lu FI, Moshonov H, Crystal P. Quantitative shear wave elastography: correlation with prognostic histologic features and immunohistochemical biomarkers of breast cancer. Acad Radiol 2015;22:269-77. 10.1016/j.acra.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 32.Chang JM, Park IA, Lee SH, Kim WH, Bae MS, Koo HR, Yi A, Kim SJ, Cho N, Moon WK. Stiffness of tumours measured by shear-wave elastography correlated with subtypes of breast cancer. Eur Radiol 2013;23:2450-8. 10.1007/s00330-013-2866-2 [DOI] [PubMed] [Google Scholar]
- 33.Hong S, Woo OH, Shin HS, Hwang SY, Cho KR, Seo BK. Reproducibility and diagnostic performance of shear wave elastography in evaluating breast solid mass. Clin Imaging 2017;44:42-5. 10.1016/j.clinimag.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 34.Cosgrove DO, Berg WA, Doré CJ, Skyba DM, Henry JP, Gay J, Cohen-Bacrie C; BE1 Study Group. Shear wave elastography for breast masses is highly reproducible. Eur Radiol 2012;22:1023-32. 10.1007/s00330-011-2340-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HJ, Kim EK, Kim MJ, Youk JH, Lee JY, Kang DR, Oh KK. Observer variability of Breast Imaging Reporting and Data System (BI-RADS) for breast ultrasound. Eur J Radiol 2008;65:293-8. 10.1016/j.ejrad.2007.04.008 [DOI] [PubMed] [Google Scholar]



