Abstract
Background
Surgical site infection (SSI) has a high incidence in diabetic surgical patients. Preoperative antibiotic prophylaxis followed by an intraoperative re-dose was a common strategy in diabetic prolonged procedures. However, there were lacking studies on the relative benefits of this strategy on SSI. Our study aimed to clarify the effect of intraoperative re-dose of prophylactic antibiotics on SSI in diabetic patients.
Methods
A total of 1,840 diabetic patients with prolonged surgeries were included and Cefazolin was the only type of antibiotic prophylaxis. We assessed the relationship between intraoperative re-dose of cefazolin and 30-day incidence of SSI using a retrospective cohort study method.
Results
There were 361 diabetic cases with preoperative antibiotics only and 1,479 cases with pre- plus intraoperative antibiotics, in which 60 subjects suffered from SSI. Pre- plus intraoperative prophylaxis group had a lower rate of SSI in the overall and subgroup analyses when compared with preoperative only group. Operation location, combined with hypertension, poor blood glucose control, high WBC count and ASA score >2 were significantly associated with an increased risk of SSI for diabetic surgical patients (all P<0.05). Intraoperative re-dose of prophylactic antibiotics was statistically related to a lower incidence rate of SSI than preoperative prophylaxis alone (crude RR =0.47; 95% CI, 0.27–0.82; P<0.01), while the association remained significant even after adjusting the potential confounders (adjusted RR =0.51; 95% CI, 0.29–0.90; P=0.02).
Conclusions
For diabetic patients, intraoperative re-dose of prophylactic antibiotics may be an independent protective factor for the prevention of SSI. A specific perioperative antibiotics injection strategy should be encouraged for diabetic patients with prolonged surgeries to minimize the possibility of SSI.
Keywords: Surgical site infection (SSI), antibiotics, diabetes
Introduction
Surgery carries the risks of infection and other complications. These risks can be increased in those with pre-existing and co-morbid conditions, such as diabetes. Diabetes has been reported to have negative effects on surgical and postoperative infection (1), which is thought to be due to diabetic patient’s immunocompromised state, decreased wound healing capacity and poor microvascularization (2). Surgical site infection (SSI), a kind of complication after surgery, can lead to significant morbidity, increased financial burden and potentially mortality (3). It is believed that the identification of patient-related risk factors and their reversal in some cases can lead to a reduction in SSI (4). Quite a number of studies have analyzed the characteristics of patients to identify the risk factors for SSI; however, the results are controversial. And studies specifically addressing SSI problem for diabetic surgical patients are lacking in the literature.
In practice, surgeons often use perioperative antibiotics to reduce the occurrence of SSI and intraoperative antibiotics seems to be considered as an effective method to prevent SSI after surgeries (5). However, the optimal perioperative antibiotic regimen for the prevention of SSI remains unclear and lack of validation (6). In a previous retrospective study, Ko et al. examined the effects of prophylactic antibiotic use in a diabetic population, and found no effects on the reduction of SSI risk for diabetic surgical patients who were given prophylactic antibiotics (7). Although preoperative antibiotic prophylaxis is regarded as one of the most effective methods to reduce the possibility of SSI in many types of surgical procedures, the efficacy of preoperative antibiotics has been reported to diminish with procedure’s duration of time (8). An additional shot of antibiotics is recommended to counteract this reduced efficacy during long procedures. Kasatpibal et al. have proved that failure to re-dose prophylactic antibiotics effectively during long operations could increase the risk of SSI (9), but there are very few published data on the relative benefits of intraoperative re-dose of antibiotics on SSI prevention compared with single dose therapy before operation, particularly as a practical strategy for diabetic surgical patients.
Given these outstanding questions, using a well-defined surgery data of 1,840 diabetic patients as a cohort, we aimed to find the possible risk factors for SSI and further evaluate potential benefits of the intraoperative re-dose of prophylactic antibiotics on reducing the occurrence of SSI in diabetic patients.
Methods
Study participants
We conducted a retrospective cohort study of diabetic patients (all type II) aged over 18 years undergoing operations between January 2016 and December 2017 at the First Hospital of China Medical University. Eligible types of surgery included cardiac operation, orthopedic joint replacement, vascular surgery, neurosurgery, and thoracic and abdominal/pelvic surgical procedures. Furtherly, surgical incision was divided into four types according to the possibility of contamination during surgery (10).
To ensure that the surgical prophylaxis regimen was homogeneous, the drug was limited to the most commonly administered cefazolin. The first dose was given within 60 minutes of incision, and the re-dose of the drug was indicated four hours after initiation of preoperative dose (using the same dose) (11). In addition, to avoid the effect of excessive blood loss on the application of antibiotics, intraoperative blood loss of all the participants was within 600 mL. The exclusion criteria included therapeutic antibiotics given around surgery, patients undergoing re-operations or multiple surgical procedures, the operation time shorter than 4 hours, no records of preoperative antibiotic prophylaxis, antibiotics other than cefazolin used for prophylaxis and patients who were not followed up for up to 30 days since the day of surgery.
This study was approved by the First Hospital of China Medical University Institutional Review Board (IRB#2018-0139), who gave this study the consent to collect the patients’ surgical records and other available medical data by offering patient identification information.
Data collection and definition
Patients that fulfilled our inclusion and exclusion criteria were extracted from the electronic medical record systems. Demographic and clinical characteristics, including age, gender, weight and height, smoking status, operation location, duration of surgery, hypertension history, blood glucose control, American Society of Anesthesiologists (ASA) score, wound class, intraoperative blood loss and blood supply were collected. Above all, perioperative antibiotics usage records including the number of injections and time span between individual injections were carefully examined. All needed laboratory results including white blood cell (WBC) count and hemoglobin level were extracted from the Laboratory Information System of the hospital. Patients were followed up through outpatients and SSI status 30 days after surgery was then recorded. Patients were also followed up by telephone, if necessary.
Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters (normal BMI <24 kg/m2). Diabetes was defined as fasting serum glucose (FPG) ≥7 mmol/L (126 mg/dL) and/or being on treatment for diabetes. Hypertension was defined as having a systolic blood pressure (SBP) ≥140 mmHg and/or having a diastolic blood pressure (DBP) ≥90 mmHg and/or being under antihypertensive treatment.
Outcome definition
The main outcome of the current study was the incidence of SSI 30 days after surgery, which was determined by doctors in charge of the patients. Following the definition specified by Centers for Disease Control (CDC), SSI was defined as a description of symptoms like redness, warmth, fever, tenderness, swelling, and/or drainage around the surgical site within 30 days after surgery (12).
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics Software 19.0 (IBM, Armonk, NY, USA). Data were presented as means with SD for continuous variables and percentages for categorical variables. Student’s t test was employed to compare continuous variables and Chi-square test was used to compare categorical variables. Univariate and multivariate logistic regression models were subsequently applied to estimate risk ratios (RRs) and corresponding 95% confidence intervals (95% CIs). Crude RRs and adjusted RRs were presented. The forward stepwise strategy was applied to screen variables for the multivariate logistic regression model. All statistical tests were two tailed and P<0.05 was considered statistically significant.
Results
During a 2-year period, 1,840 eligible diabetic surgical cases were recorded in the First Hospital of China Medical University’s surgery database. Among them, 361 diabetic patients received preoperative cefazolin only and rest 1,479 cases had records for both pre- and intraoperative cefazolin injections. Baseline characteristics of the cohort are presented in Table 1. In collected diabetic surgical patients, a total of 60 cases (20 ones with preoperative prophylaxis versus 40 ones with pre + intraoperative prophylaxis), which accounted for 3.3% of all participants, developed SSI. Preoperative prophylaxis alone group had statistically higher incidence rate of SSI (5.5%) than pre- plus intraoperative group (2.7%) (Figure 1).
Table 1. Basic clinical characteristics of preoperative prophylaxis alone group and pre- plus intraoperative prophylaxis group.
| Variables | Full cohort (N=1,840) | Preoperative only (N=361) | Pre + intraoperative (N=1,479) | P |
|---|---|---|---|---|
| Age, years | 54.24±12.81 | 52.02±14.24 | 54.78±12.38 | <0.01 |
| <60 | 1,147 (62.3%) | 244 (67.6%) | 903 (61.1%) | 0.02 |
| ≥60 | 693 (37.7%) | 117 (32.4%) | 576 (38.9%) | |
| Gender | ||||
| Female | 778 (42.3%) | 161 (44.6%) | 617 (41.7%) | 0.32 |
| Male | 1,062 (57.7%) | 200 (55.4%) | 862 (58.3%) | |
| BMI (kg/m2) | 23.89±3.52 | 24.22±3.77 | 23.81±3.46 | 0.06 |
| <24 | 998 (54.2%) | 187 (51.8%) | 811 (54.8%) | 0.30 |
| ≥24 | 842 (45.8%) | 174 (48.2%) | 668 (45.2%) | |
| Smoking status | ||||
| No | 1,169 (65.5%) | 228 (63.2%) | 941 (63.6%) | 0.58 |
| Yes | 671 (36.5%) | 133 (36.8%) | 538 (36.4%) | |
| Operation location | ||||
| Head and neck | 583 (31.7%) | 160 (44.3%) | 423 (28.6%) | <0.001 |
| Chest | 365 (19.8%) | 85 (23.5%) | 280 (18.9%) | |
| Abdomen | 729 (39.6%) | 102 (28.3%) | 627 (42.4%) | |
| Limbs and spine | 163 (8.9%) | 14 (3.9%) | 149 (10.1%) | |
| Duration of surgery (min) | 352.72±127.34 | 323.06±129.88 | 359.97±125.70 | <0.001 |
| <360 | 1,148 (62.4%) | 257 (71.2%) | 891 (60.2%) | <0.001 |
| ≥360 | 692 (37.6%) | 104 (28.8%) | 588 (39.8%) | |
| Hypertension | ||||
| No | 1,241 (67.4%) | 239 (66.2%) | 1,002 (67.7%) | 0.83 |
| Yes | 599 (32.6%) | 122 (33.8%) | 477 (32.3%) | |
| Blood glucose control | ||||
| <6.1 mmol/L | 1,421 (77.2%) | 261 (72.3%) | 1,160 (78.4%) | 0.01 |
| ≥6.1 mmol/L | 419 (22.8%) | 100 (27.7%) | 319 (21.6%) | |
| Hemoglobin (g/L) | 133.81±26.92 | 135.66±28.87 | 133.35±26.41 | 0.15 |
| WBC (×109/L) | 7.45±3.46 | 8.17±4.44 | 7.28±3.16 | <0.001 |
| ≤10 | 1,590 (86.4%) | 293 (81.2%) | 1,297 (87.7%) | <0.01 |
| >10 | 250 (13.6%) | 68 (18.8%) | 182 (12.3%) | |
| ASA score | ||||
| ≤2 | 1,763 (95.8%) | 342 (94.7%) | 1,421 (96.1%) | 0.25 |
| >2 | 77 (4.2%) | 19 (5.3%) | 58 (3.9%) | |
| Wound class | ||||
| I–II | 320 (17.4%) | 140 (38.8%) | 180 (12.2%) | <0.001 |
| III–IV | 1,520 (82.6%) | 221 (61.2%) | 1,299 (87.8%) | |
| Intraoperative blood loss | ||||
| No | 1,178 (64.0%) | 261 (72.3%) | 917 (62.0%) | <0.001 |
| Yes | 662 (36.0%) | 100 (27.7%) | 562 (38.0%) | |
| Blood supply | ||||
| No | 1,355 (73.6%) | 292 (80.9%) | 1,063 (71.9%) | <0.001 |
| Yes | 485 (26.4%) | 69 (19.1%) | 416 (28.1%) | |
| SSI | ||||
| No | 1,780 (96.7%) | 341 (94.5%) | 1,439 (97.3%) | <0.01 |
| Yes | 60 (3.3%) | 20 (5.5%) | 40 (2.7%) | |
BMI, body mass index; WBC, white blood cell; ASA, American Society of Anesthesiologists; SSI, surgical site infection.
Figure 1.
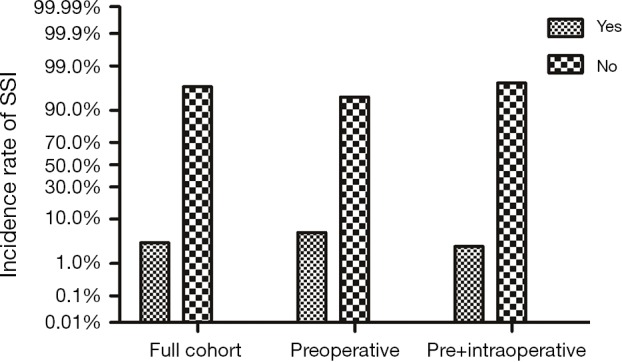
The rate of surgical site infection (SSI) was 3.3% for full cohort, 5.5% for preoperative prophylaxis group and 2.7% for pre- plus intraoperative prophylaxis group.
Furthermore, we compared the incidence of SSI between preoperative only and pre- plus intraoperative prophylaxis group stratified by demographic characteristics (Table 2), we found that pre- plus intraoperative prophylaxis group had a significantly lower incidence rate of SSI in aged ≥60 years, male and high BMI subgroups when compared with that in preoperative only group.
Table 2. Subgroup analyses for the incidence of surgical site infection between different prophylactic antibiotic regimens.
| Variables | Preoperative only, n (%) | Pre + intraoperative, n (%) | χ2 | P |
|---|---|---|---|---|
| Age, years | ||||
| <60 | 10 (4.1%) | 28 (3.1%) | 0.60 | 0.44 |
| ≥60 | 10 (8.5%) | 12 (2.1%) | – | <0.01a |
| Gender | ||||
| Female | 3 (1.9%) | 13 (2.1%) | – | 1.00a |
| Male | 17 (8.5%) | 27 (3.1%) | 11.77 | <0.01 |
| BMI (kg/m2) | ||||
| <24 | 6 (3.2%) | 20 (2.5%) | – | 0.61a |
| ≥24 | 14 (8.0%) | 20 (3.0%) | 9.09 | <0.01 |
| Smoking status | ||||
| No | 12 (5.3%) | 26 (2.8%) | 3.65 | 0.06 |
| Yes | 8 (6.0%) | 14 (2.6%) | – | 0.06 |
a, Fisher’s exact test. BMI, body mass index.
Univariate and multivariate logistic regression models were subsequently applied to examine potential influencing factors on SSI (Table 3). Operation location, combined with hypertension, poor blood glucose control, high WBC count and ASA score >2 were significantly associated with an increased risk of SSI for diabetic surgical patients (all P<0.05). After adjusting the possible confounders, operation location and ASA score >2 were independent risk factors of SSI. Moreover, pre- and intraoperative prophylaxis was correlated with a lower risk of SSI (crude RR =0.47; 95% CI, 0.27–0.82; P<0.01). Furthermore, the protective effects of pre- and intraoperative prophylaxis strategy were still statistically significant even after the adjustment for gender, combined with hypertension, WBC count and ASA score (adjusted RR =0.51; 95% CI, 0.29–0.90; P=0.02) (Table 3).
Table 3. Univariate and multivariate logistic regression analysis of possible influencing factors on surgical site infection.
| Variables | Crude RR (95% CI) | P | Adjusted RR (95% CI) | Pa |
|---|---|---|---|---|
| Age (≥60 vs. <60 years) | 0.96 (0.56–1.63) | 0.87 | – | – |
| Gender (male vs. female) | 2.01 (1.15–3.68) | 0.02 | 0.56 (0.31–1.01) | 0.06 |
| BMI (≥24 vs. <24 kg/m2) | 1.57 (0.94–2.64) | 0.09 | – | – |
| Operation location (dummy variable) | – | – | ||
| Head and neck (reference) | Reference | – | Reference | – |
| Chest | 1.98 (0.81–4.83) | 0.13 | 2.41 (0.96–6.03) | 0.06 |
| Abdomen | 2.83 (1.34–6.00) | 0.01 | 2.96 (1.36–6.43) | <0.01 |
| Limbs and spine | 3.73 (1.46–9.55) | 0.01 | 4.58 (1.71–12.24) | <0.01 |
| Duration of surgery (≥360 vs. <360 min) | 0.89 (0.52–1.53) | 0.67 | – | – |
| Hypertension (yes vs. no) | 1.85 (1.11–3.11) | 0.02 | 1.59 (0.93–2.73) | 0.09 |
| Smoking status (yes vs. no) | 1.01 (0.59–1.72) | 0.97 | – | – |
| Blood glucose control (poor vs. good) | 2.34 (1.38–3.97) | <0.01 | 1.50 (0.85–2.65) | 0.16 |
| WBC (>10 vs. ≤10 ×109/L) | 2.63 (1.47–4.68) | <0.01 | 1.80 (0.96–3.38) | 0.07 |
| ASA score (>2 vs. ≤2) | 5.11 (2.49–10.52) | <0.001 | 3.56 (1.63–7.80) | <0.01 |
| Wound class (III–IV vs. I–II) | 0.62 (0.34–1.13) | 0.12 | – | – |
| Intraoperative blood loss (yes vs. no) | 1.58 (0.94–2.65) | 0.08 | – | – |
| Blood supply (yes vs. no) | 1.53 (0.89–2.62) | 0.13 | – | – |
| Pre + intraoperative vs. preoperative only | 0.47 (0.27–0.82) | <0.01 | 0.43 (0.24–0.78) | <0.01 |
a, adjusted for gender, operation location, hypertension, blood glucose control, WBC, ASA score and antibiotic prophylaxis unless the variable used as an analyzed factor. BMI, body mass index; WBC, white blood cell; ASA, American Society of Anesthesiologists.
Discussion
As far as we know, our study is the first and largest cohort study to assess the potential advantage of the combination of pre- and intraoperative prophylaxis for SSI prediction in diabetic surgical patients. In the present study, our most meaningful discovery was that the receipt of pre- plus intraoperative prophylaxis could be an independent predictor associated with a significant reduction of SSI for diabetic surgical patients.
SSI is a kind of well-known complication of general surgery. Although the overall incidence of SSI is relatively low, it is considered the most common nosocomial infection. SSI negatively influences patients’ outcomes and healthcare costs (13). A complex interaction of patient, procedure, and surgeon related risk factors exists in the etiology of SSI. Diabetes has been regarded as a risk factor for SSI (14). Data has showed that incidence rate of SSI in diabetic patients is much higher than that in non-diabetic patients (15). The poor microcirculation associated with diabetes could result in tissue hypoxia and obstruction of antibiotics delivery, thus facilitating microorganism growth in surgical sites (2). Therefore, the investigation of SSI risk factors in diabetic patients is of great significance for risk stratification and preventive measures. Although several studies have been conducted to determine the risk factors for developing SSI, the results are not uniform, especially for diabetic patients. Optimal antibiotic regimens should be urgently encouraged for diabetic patients to prevent the occurrence of SSI after surgery. Initially, we suggested that diabetic surgical subjects with pre- plus intraoperative antibiotic prophylaxis had a significantly lower risk of SSI than those with preoperative use of antibiotics only in the general as well as subgroup comparisons, especially for those aged over 60 years, male and high BMI patients.
Furthermore, univariate and multivariate logistic regression models were applied to estimate the possible predictors of SSI. Interestingly, our study revealed that operation location, combined with hypertension and poor blood glucose control were risk factors for SSI. Hypertension may impair and delay wound healing due to microangiopathic changes with local tissue ischemia, and reduce tissue concentrations of antibiotics (16). We found that diabetic surgical patients combined with hypertension could significantly increase the site infection risk, which was mirrored elsewhere (17). Strict control of serum glucose and blood pressure may decrease the incidence of SSI after surgery. In addition, our study also suggested that a higher WBC level, which indicated an infection status, was a risk factor markedly affecting SSI. ASA score is more than an index evaluating the preoperative physical status of a patient, but an important predictor of SSI risk (18,19). Multiple analysis of our data showed that ASA score >2 was determined as another independent risk factor which could result in a 3.56-fold higher risk of SSI compared with ASA score ≤2 after adjusting the possible confounders.
In recent years, several studies revealed the preventive effects of preoperative antibiotics on SSI, which mainly focused on the comparative efficacy of single and combined use of prophylactic antibiotics (13,20). A multicenter national cohort study, through comparing postoperative outcomes following administration of two antibiotics versus a single agent for the prevention of SSI, found a significant association between combined prophylaxis and lower incidence of SSI among cardiac surgical patients (12). A previous systematic review and meta-analysis performed by Slobogean et al. suggested that multiple-dose perioperative antibiotic prophylaxis failed to have a superiority over a single preoperative dose in the prevention of SSI during the treatment of closed bone fractures (21). A large multicenter collaborative study from Steinberg et al showed a relationship between antibiotic timing and SSI risk, and confirmed that intraoperative re-dose appeared to reduce SSI risk in operations lasting more than 4 hours, but only when the preoperative dose was given correctly (22). However, there were few researches to discuss the combined effect of pre- and intraoperative prophylactic antibiotics on the risk of SSI in diabetic population. Only one retrospective cross-sectional study with a small sample size (n=144) tried to compare preoperative single dose of Ceftriaxone (n=48) with pre- and post-operative three dose approach (n=96) on the reduction of the occurrence of SSI, and found no reduction in SSI risk for diabetic surgical patients (23). In our study, the protective effects of pre- and intraoperative prophylaxis strategy on SSI of diabetic patients were remarkably demonstrated. Reasons for different results may include study design, sample size, and timing of the re-dose of antibiotic prophylaxis. Findings from our present study can be utilized to optimize SSI reduction strategy. Therefore, clinicians may need to individualize prophylaxis strategy based on diabetic patient-specific factors that influence the risk versus benefit equation. Better recognition of the clinical decision making around perioperative antibiotics could inform future propensity models (24).
Although our cohort was large and robust, there were still a number of limitations. First, the current study was a retrospective study rather than a randomized controlled trial. Thus, there was a lack of information about possible unmeasured confounding factors, such as duration of therapy. As such, further in-depth analysis with a controlled clinical trial would be ideal. Second, potential adverse outcomes following receipt of either preoperative prophylaxis or the combination of pre- and intraoperative prophylaxis were not included in the final analysis, e.g., the incidence of antimicrobial resistance (25) and Clostridium difficile infection (CDI) (12). Third, given the nature of the operation, patients undergoing different types of surgeries can result in a different infection rate (26). Future larger-scale studies based on the subgroup analysis according to the types of surgical procedures will be considered.
In summary, for diabetic patients, pre- plus intraoperative prophylaxis could significantly reduce the postoperative SSI incidence rate compared to preoperative only, especially for those aged ≥60 years, male and high BMI subjects. Operation location and ASA score >2 were independent risk factors of SSI. Meanwhile, the intraoperative re-dose of prophylactic antibiotics in prolonged procedures was related to a significant decrease in postoperative SSI. Future researches are deserved to explore the screening protocols for optimizing and individualizing surgical prophylaxis regimens among diabetic surgical patients.
Acknowledgements
We gratefully acknowledge the help for the statistical analysis from Dr Jing-Mei Jiang from Department of Epidemiology and Biostatistics, Institute of Basic Medicine Sciences, Chinese Academy of Medical Sciences, Beijing, China.
Funding: This study was supported by grants from Modern Surgery and Anaesthesia Safety Management System Construction and Promotion (MSCP) (201402017).
Ethical Statement: This study was approved by the First Hospital of China Medical University Institutional Review Board (IRB#2018-0139), who gave this study the consent to collect the patients’ surgical records and other available medical data by offering patient identification information.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Martin ET, Kaye KS, Knott C, et al. Diabetes and Risk of Surgical Site Infection: A Systematic Review and Meta-analysis. Infect Control Hosp Epidemiol 2016;37:88-99. 10.1017/ice.2015.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satake K, Kanemura T, Matsumoto A, et al. Predisposing factors for surgical site infection of spinal instrumentation surgery for diabetes patients. Eur Spine J 2013;22:1854-8. 10.1007/s00586-013-2783-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards C, Counsell A, Boulton C, et al. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br 2008;90:770-7. 10.1302/0301-620X.90B6.20194 [DOI] [PubMed] [Google Scholar]
- 4.Barie PS. Surgical site infections: epidemiology and prevention. Surg Infect (Larchmt) 2002;3 Suppl 1:S9-21. 10.1089/sur.2002.3.s1-9 [DOI] [PubMed] [Google Scholar]
- 5.Poggio JL. Perioperative strategies to prevent surgical-site infection. Clin Colon Rectal Surg 2013;26:168-73. 10.1055/s-0033-1351133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozgun H, Ertugrul BM, Soyder A, et al. Peri-operative antibiotic prophylaxis: adherence to guidelines and effects of educational intervention. Int J Surg 2010;8:159-63. 10.1016/j.ijsu.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Ko JS, Zwiebel S, Wilson B, et al. Perioperative antibiotic use in diabetic patients: A retrospective review of 670 surgeries. J Plast Reconstr Aesthet Surg 2017;70:1629-34. 10.1016/j.bjps.2017.06.042 [DOI] [PubMed] [Google Scholar]
- 8.de Jonge SW, Gans SL, Atema JJ, et al. Timing of preoperative antibiotic prophylaxis in 54,552 patients and the risk of surgical site infection: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e6903. 10.1097/MD.0000000000006903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasatpibal N, Whitney JD, Dellinger EP, et al. Failure to Redose Antibiotic Prophylaxis in Long Surgery Increases Risk of Surgical Site Infection. Surg Infect (Larchmt) 2017;18:474-84. 10.1089/sur.2016.164 [DOI] [PubMed] [Google Scholar]
- 10.Levy SM, Lally KP, Blakely ML, et al. Surgical wound misclassification: a multicenter evaluation. J Am Coll Surg 2015;220:323-9. 10.1016/j.jamcollsurg.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 11.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013;14:73-156. 10.1089/sur.2013.9999 [DOI] [PubMed] [Google Scholar]
- 12.Branch-Elliman W, Ripollone JE, O'Brien WJ, et al. Risk of surgical site infection, acute kidney injury, and Clostridium difficile infection following antibiotic prophylaxis with vancomycin plus a beta-lactam versus either drug alone: A national propensity-score-adjusted retrospective cohort study. PLoS Med 2017;14:e1002340. 10.1371/journal.pmed.1002340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isik O, Kaya E, Dundar HZ, et al. Surgical Site Infection: Re-assessment of the Risk Factors. Chirurgia (Bucur) 2015;110:457-61. [PubMed] [Google Scholar]
- 14.Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg 2017;224:59-74. 10.1016/j.jamcollsurg.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 15.Ng RR, Myat Oo A, Liu W, et al. Changing glucose control target and risk of surgical site infection in a Southeast Asian population. J Thorac Cardiovasc Surg 2015;149:323-8. 10.1016/j.jtcvs.2014.08.076 [DOI] [PubMed] [Google Scholar]
- 16.Meng F, Cao JMeng X. Risk factors for surgical site infections following spinal surgery. J Clin Neurosci 2015;22:1862-6. 10.1016/j.jocn.2015.03.065 [DOI] [PubMed] [Google Scholar]
- 17.Almustafa MA, Ewen AM, Deakin AH, et al. Risk Factors for Surgical Site Infection Following Lower Limb Arthroplasty: A Retrospective Cohort Analysis of 3932 Lower Limb Arthroplasty Procedures in a High Volume Arthroplasty Unit. J Arthroplasty 2018;33:1861-7. 10.1016/j.arth.2018.01.037 [DOI] [PubMed] [Google Scholar]
- 18.Khan M, Rooh ul M, Zarin M, et al. Influence of ASA score and Charlson Comorbidity Index on the surgical site infection rates. J Coll Physicians Surg Pak 2010;20:506-9. [PubMed] [Google Scholar]
- 19.Kaye KS, Sloane R, Sexton DJ, et al. Risk factors for surgical site infections in older people. J Am Geriatr Soc 2006;54:391-6. 10.1111/j.1532-5415.2005.00651.x [DOI] [PubMed] [Google Scholar]
- 20.Ponce B, Raines BT, Reed RD, et al. Surgical Site Infection After Arthroplasty: Comparative Effectiveness of Prophylactic Antibiotics: Do Surgical Care Improvement Project Guidelines Need to Be Updated? J Bone Joint Surg Am 2014;96:970-7. 10.2106/JBJS.M.00663 [DOI] [PubMed] [Google Scholar]
- 21.Slobogean GP, Kennedy SA, Davidson D, et al. Single- versus multiple-dose antibiotic prophylaxis in the surgical treatment of closed fractures: a meta-analysis. J Orthop Trauma 2008;22:264-9. 10.1097/BOT.0b013e31816b7880 [DOI] [PubMed] [Google Scholar]
- 22.Steinberg JP, Braun BI, Hellinger WC, et al. Timing of antimicrobial prophylaxis and the risk of surgical site infections: results from the Trial to Reduce Antimicrobial Prophylaxis Errors. Ann Surg 2009;250:10-6. 10.1097/SLA.0b013e3181ad5fca [DOI] [PubMed] [Google Scholar]
- 23.Koirala K, Mukhia R, Sharma S, et al. A single-dose antibiotic prophylaxis to prevent surgical site infection in clean-contaminated surgery among diabetic patients. Journal of Patan Academy of Health Sciences 2014;1:8-10. 10.3126/jpahs.v1i2.16637 [DOI] [Google Scholar]
- 24.Charani E, Tarrant C, Moorthy K, et al. Understanding antibiotic decision making in surgery-a qualitative analysis. Clin Microbiol Infect 2017;23:752-60. 10.1016/j.cmi.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 25.Calina D, Docea AO, Rosu L, et al. Antimicrobial resistance development following surgical site infections. Mol Med Rep 2017;15:681-8. 10.3892/mmr.2016.6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saeed MJ, Dubberke ER, Fraser VJ, et al. Procedure-specific surgical site infection incidence varies widely within certain National Healthcare Safety Network surgery groups. Am J Infect Control 2015;43:617-23. 10.1016/j.ajic.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]


