Abstract
Background
Which induction chemotherapy (IC) regimen followed by cisplatin-based concurrent chemoradiotherapy (CCRT) is the best choice among PF (cisplatin and 5-fluorouracil), TP (docetaxel and cisplatin) and TPF (docetaxel, cisplatin, and 5-fluorouracil) remains controversial in locoregionally advanced nasopharyngeal carcinoma (LA-NPC). This Bayesian network meta-analysis investigated the efficacy and toxicity of these three common IC regimens and attempted to find the optimal chemotherapy regimen.
Methods
We searched PubMed, Embase, and the Cochrane Library for randomized controlled trials (RCTs) up to December, 2017. Then, we screened studies for eligibility, extracted data, assessed their quality, tested consistency, and used Bayesian network meta-analysis to combine direct and indirect evidence.
Results
Ten records were identified involving 7 eligible RCTs with 1,570 patients. Results of the Bayesian network meta-analysis shows that TPF [hazard ratios (HRs) 0.68; 95% credibility interval (CrI), 0.42–1.1], TP (HRs 0.70; 95% CrI, 0.22–2.2) and PF (HRs 1.0; 95% CrI, 0.71–1.5) have the probability of 49.61%, 47.45% and 1.57% respectively to be the optimal induction regimen. Docetaxel-based regimens, including TP [risk ratios (RRs) 5.9; 95% CrI, 1.4–26.0) and TPF (RRs 4.5; 95% CrI, 1.1–18.0), significantly increase the incidence of hematological toxicity. As for ≥ grade 3 mucositis, 5-fluorouracil-based regimens, including PF (RRs 2.1; 95% CrI, 0.91–5.8) and TPF (RRs 1.4; 95% CrI, 0.48–4. 6), are higher than TP (RRs 1.1; 95% CrI, 0.30–4.6).
Conclusions
Only considering overall survival (OS), TPF has the highest probability to be the optimal choice in LA-NPC and TP also shows encouraging anti-tumor effects. However, we also noticed that TPF induced worse adverse events, especially in ≥ grade 3 hematological toxicity and oral mucositis.
Keywords: Nasopharyngeal carcinoma (NPC), induction chemotherapy regimen (IC regimen), chemoradiotherapy, network meta-analysis
Introduction
Nasopharyngeal carcinoma (NPC), one of the most common head and neck malignancies, is endemic in China (1). Sixty to seventy percent of patients with NPC are diagnosed as locoregionally advanced (LA) stages (2). The therapeutic effect of radiotherapy alone remains unsatisfactory in LA-NPC, and the 5-year overall survival (OS) rate is only 67–77% (3). Local recurrence and distant metastasis are still main reasons for treatment failure.
The addition of systematic chemotherapy to radiotherapy may improve prognosis in LA-NPC. As reported, Intergroup study 0099 demonstrated that concurrent chemoradiotherapy (CCRT) with adjuvant chemotherapy (3 cycles of cisplatin and 5-fluorouracil regimen) was superior to radiotherapy alone for patients with LA-NPC with respect to progression-free survival (PFS) (P<0.001) and OS (P=0.005) (4). Thus, CCRT followed by adjuvant chemotherapy becomes a standard therapeutic model for LA-NPC. Although cisplatin-based CCRT is widely used in clinical practice, the evidence mainly comes from the era of two-dimensional radiotherapy. In the era of intensity-modulated radiotherapy (IMRT), CCRT needs to be supported by more clinical evidence (5). Meanwhile, it has been reported that adjuvant cisplatin and fluorouracil chemotherapy did not significantly improve failure-free survival after CCRT in LA-NPC [hazard ratios (HRs) 0.74; 95% confidence interval (CI) 0.49–1.10; P=0.13] (6). Hence, whether CCRT with or without adjuvant chemotherapy bring survival benefit is controversial.
In addition, it has been studied whether induction chemotherapy (IC) followed by CCRT can improve survival. In 2016, Ma et al. reported that the addition of IC-CCRT significantly improved PFS (P=0.034), OS (P=0.029), and distance metastasis-free survival (P=0.031), compared with CCRT group. Furthermore, a meta-analysis including 9 randomized clinical trials with 2,215 patients also confirmed that IC-CCRT could significantly improve OS (HRs 0.64; 95% CI, 0.49–0.84, P=0.001) and PFS (HRs 0.68; 95% CI, 0.56–0.81, P<0.001), compared with CCRT alone (7). Moreover, compared with adjuvant chemotherapy after CCRT (CCRT-AC), IC-CCRT still effectively prolonged OS (HRs 0.82; 95% CI, 0.69–0.98, P=0.03) and reduced distant metastasis rate (RRs 0.69; 95% CI, 0.56–0.84, P=0.0002) (8). IC-CCRT, a promising treatment strategy, is a recommended by NCCN guidelines.
Induction regimens, including PF (cisplatin and 5-fluorouracil), TP (docetaxel and cisplatin) and TPF (docetaxel, cisplatin, and 5-fluorouracil), are usually utilized to treat LA-NPC. However, the optimal IC regimens among TPF, TP and PF remains unclear. To solve this essential question, we performed a Bayesian network meta-analysis with a mixed-treatment comparison method to combine direct and indirect evidence while maintaining randomization (9). Our data show that TPF, TP and PF have the probability of 49.61%, 47.45% and 1.57% respectively to be the optimal induction regimen.
Methods
Search strategies and selection
PubMed, Embase, and the Cochrane Library were searched to identify potentially eligible studies up to December, 2017. Medical subject headings (MeSH) terms were as follows: [(‘‘nasopharyngeal neoplasms’’) or (“nasopharynx” and ‘‘neoplasms’’)] and (‘‘induction chemotherapy’’ or “drug therapy”) and (‘‘randomized controlled trial’’).
Literatures were included using the following criteria: (I) participating patients diagnosed as LA-NPC; (II) age 18–70 years old; (III) published randomized controlled trials (RCTs) assessing the efficacy and toxicity between IC plus concurrent chemoradiation and concurrent chemoradiation alone or published RCTs assessed the efficacy and toxicity of different IC regimens followed by concurrent chemoradiation; (IV) IC regimens included TPF, TP, and PF; (V) concurrent chemotherapy regimen was cisplatin alone; (VI) primary endpoint OS was provided. Literatures were excluded by the following criteria: (I) participating patients with early stage disease or metastasis; (II) the main participants were children, adolescents or the old; (III) adjuvant chemotherapy was applied; (IV) concurrent chemotherapy regimen was not cisplatin alone; (V) any review, comment and letter. Literature search and screen were done by two investigators independently. Disagreements were resolved by discussion with a third author.
Data extraction and quality assessment
Data were extracted, including study characteristics, patient characteristics, interventions and outcome data. HRs and corresponding standard errors were estimated from survival curves by Engauge Digitizer 4.1 and calculations spreadsheet according to methods described by Tierney and colleagues (10). If outcome data were indistinct, we attempted to contact author for detailed information. We assessed the risk of bias with Review Manager Software (RevMan 5.3, Cochrane Collaboration, and Oxford, UK), referring to the guidance of Cochrane handbook (5.1.0) (11). We presented network plot, contribution, inconsistency, publication bias with stata12.0 software (Stata Corporation, College station, TX, USA). Data extraction and quality assessment were done by two investigators independently. Disagreements were resolved by discussion with a third author.
Statistical analyses
In network meta-analysis, the available data was not only from direct comparisons of regimen A and regimen B but also from indirect evidences that comparing either A or B to a common comparator C. Network meta-analysis was allowed to analyze all relevant RCTs and overcomes the limitation for lack of direct comparisons (12). In this study, we prespecified OS as the primary outcome. The secondary end points were PFS. The survival endpoint results were expressed as HRs. We used CCRT alone as the baseline regimen to act as the effect measure. Regimens were ranked according to the estimated InHR. The probability of a regimen being superior was exhibited by using the proportion of times a regimen ranked first. As for treatment tolerance, we assessed the completion of CCRT and ≥ grade 3 neutropenia and mucositis by risk ratios (RRs). Gemtc package was used to conduct the network meta-analysis in R software (version 3.4.1) based on Bayesian statistics by JAGS 4.3.0 (13). The median of the posterior distribution as a point estimate was introduced for the treatment effect (14). The each chain overlap well and the smoothed posterior probability densities for the same parameters supporting convergence. When posterior distributions were roughly normally distributed, the credible interval could be interpreted like conventional confidence intervals (9). To assess the feasibility of the model, we used Bayesian deviance information criterion (DIC) statistics to compare different models. The DIC is a Bayesian information criterion that quantifies the information in the model by measuring the efficacy of the model (14). We chose lower value of DIC that indicated better model performance in predicting future values.
Results
Studies and patients
One thousand and six hundred thirty-four records from database searches were identified and detailed search strategies for PubMed, Embase, and the Cochrane Library database were described in supplementary materials. The flow diagram (Figure 1) illustrated that of 31 articles retrieved for detailed review, 10 records were included (all were associated with 7 trials, 1,570 patients). The latest publication of each trial was utilized for network meta-analysis, as cited in the main publications (15-21).
Figure 1.
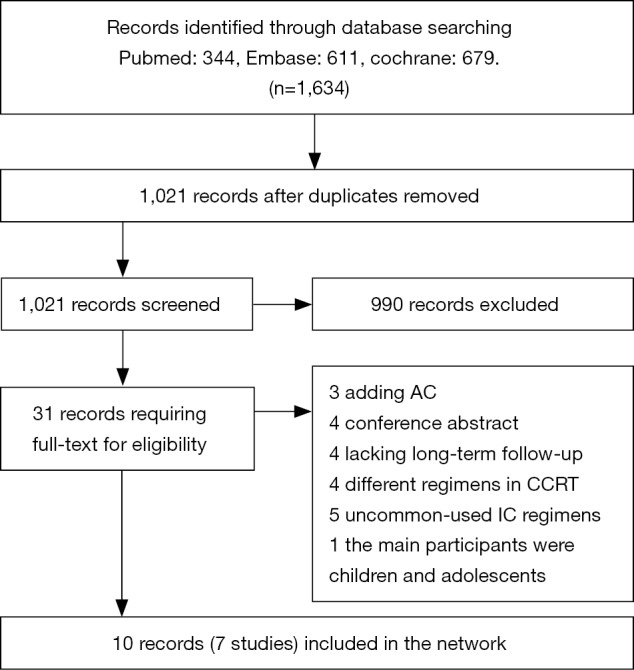
Flow diagram. CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; AC, adjuvant chemotherapy.
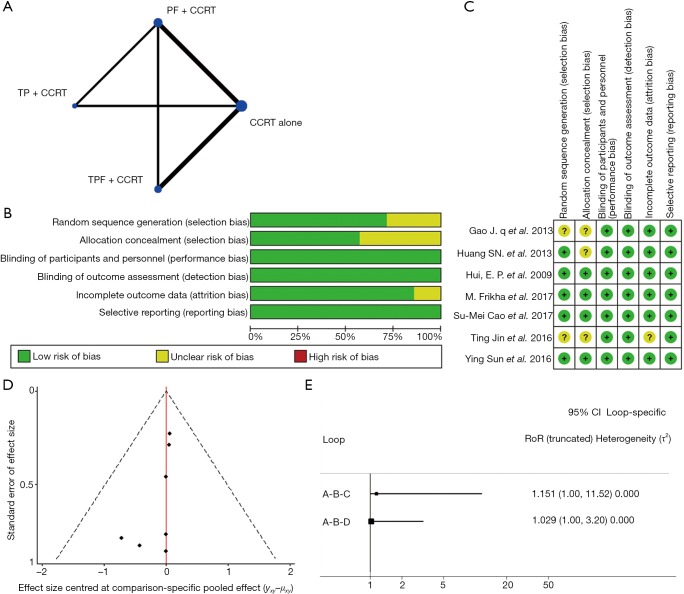
We established a network to compare different IC (Figure 2A). The methodological quality of included all trials was high (Figure 2B). Five trials (5/7) were multicenter and done by cooperative groups. Random sequence generation was adequate in 5 trials, and detailed random sequence generation were not reported in other 2 trials. Allocation concealment was adequate in the 4 trials, and was not reported in remaining 3 trials. We assessed low risk on blinding method, because it was impossible to influence the bias of primary endpoint OS in that death is not susceptible to patient, physician, or outcome assessor. Six trials described the missing data at follow-up in detail and only one trial did not describe (Figure 2C). According to funnel plots, there was no obvious publication bias (Figure 2D). According to evaluation of inconsistency using loop-specific heterogeneity estimates, the indirect evidences were consistent with direct comparisons (Figure 2E).
Figure 2.
The comparisons analyzed within the network, risk of bias of including studies and consistency test. (A) Network plot; (B) risk of bias graph; (C) risk of bias summary; (D) publication bias; (E) evaluation of inconsistency. A: CCRT; B: PF + CCRT; C: TP + CCRT; D: TPF + CCRT. PF, induction chemotherapy regimen of cisplatin, 5-fluorouracil; TP, induction chemotherapy regimen of docetaxel, cisplatin; TPF, induction chemotherapy regimen of docetaxel, cisplatin, 5-fluorouracil; CCRT, concurrent chemoradiotherapy.
The characteristics of the 7 included trials were summarized in the Table 1. More than 99% of including patients were in the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) stages III or IV with Karnofsky performance status scores of at least 70. Although IMRT was not carried out in all clinical trials (some patients were treated with two-dimensional radiotherapy or three-dimensional radiotherapy), treatment associated baseline characteristics were balanced among these four groups. OS was reported in all studies. The survival analysis was based on intention-to-treat principle and adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE 2.0 or CTCAE 3.0). Details of the regimens were showed in Table S1.
Table 1. Characteristics of including studies.
| Study | Inclusion period | median follow-up, m [range] | Multi-center | Study arms | Patients (n) | Median, age, years, [range] | Gender, M:F | Staging criteria | Stage I/II/III/IV |
|---|---|---|---|---|---|---|---|---|---|
| Cao et al. 2017 | 2008.6–2015.2 | 50 [3–94] | Yes | PF + CCRT; CCRT alone | 238; 238 | 44 [19–65]; 42 [21–66] | 173:65; 190:48 | AJCC/UICC 6th edition | 0/1/117/120; 0/0/133/105 |
| Sun et al. 2016 | 2011.3–2013.8 | 45 [39–49] | Yes | TPF + CCRT; CCRT alone | 241; 239 | 42 [36–49]; 44 [39–50] | 193:48; 174:65 | AJCC/UICC 7th edition | 0/0/129/112; 0/0/133/106 |
| Jin et al. 2016 | 2012.4–2014.4 | 36 [24–48] | Yes | TPF + CCRT; PF + CCRT | 138; 138 | 48 [18–68]; 50 [25–69] | 99:39; 98:40 | AJCC/UICC 7th edition | 0/0/86/52; 0/0/94/64 |
| Huang et al. 2013 | 2010.1–2010.6 | 20 [13–29] | No | TP + CCRT; PF + CCRT | 40; 40 | 42 [18–63]; 44 [19–66] | 28:12; 31:9 | AJCC/UICC 6th edition | 0/0/13/27; 0/0/17/23 |
| Hui et al. 2009 | 2002.11–2004.11 | 51 | Yes | TP + CCRT; CCRT alone | 34; 31 | 50 [31–70]; 45 [32–70] | 21:13; 24:7 | AJCC/UICC 5th edition | 0/0/19/15; 0/0/19/12 |
| Frikha et al. 2017 | 2009–2012 | 43.1 | Yes | TPF + CCRT; CCRT alone | 40; 41 | 46; 48 | 28:12; 32:9 | AJCC/UICC 6th edition | NA |
| Gao et al. 2013 | 2008.5–2009.6 | 42† | No | PF + CCRT; CCRT alone | 57; 55 | [18–60] | 43:14; 39:16 | Fuzhou 1992 | 0/0/14/43; 0/0/11/44 |
†, all patients follow-up over 3 years. N, number; M, male; F, female; NA, not available; m, months; AJCC/UICC, The International Union against Cancer/American Joint Committee on Cancer; Fuzhou 1992, the Fuzhou staging system (1992) of nasopharyngeal carcinoma; PF, induction chemotherapy regimen of cisplatin, 5-fluorouracil; TP, induction chemotherapy regimen of docetaxel, cisplatin; TPF, induction chemotherapy regimen of docetaxel, cisplatin, 5-fluorouracil; CCRT, concurrent chemoradiotherapy.
Table S1. Details of chemotherapy regimens.
| Study | IC regimens of experiment arm | IC regimens of control arm | CCRT regimen | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DDP | 5-Fu | Docetaxel | Course | DDP | 5-Fu | Course | DDP | |||
| Cao et al. 2017 | 80 mg/m2; d1 | 800 mg/m2; d1-5 | q3w 2 cycle | 80 mg/m2; q3w 3 cycle | ||||||
| Sun et al. 2016 | 60 mg/m2; d1 | 500 mg/m2; d1-5 | 60 mg/m2; d1 | q3w 3 cycle | 100 mg/m2; q3w 3 cycle | |||||
| Jin et al. 2016 | 75 mg/m2; d1 | 600 mg/m2; d1-4 | 75 mg/m2; d1 | q3w 2 cycle | 100 mg/m2; d1 | 800 mg/m2; d1-5 | q3w 2 cycle | 80 mg/m2; q3w 2 cycle | ||
| Huang et al. 2013 | 80 mg/m2; d1 | 65 mg/m2; d1 | q3w 2 cycle | 40 mg/m2; d1-2 | 500 mg/m2; d1-5 | q3w 2 cycle | 80 mg/m2q3w | |||
| Hui et al. 2009 | 75 mg/m2; d1 | 75 mg/m2; d1 | q3w 2 cycle | 40 mg/m2q1w | ||||||
| Frikha et al. 2017 | 75 mg/m2; d1 | 750 mg/m2; d1-5 | 75 mg/m2; d1 | 40 mg/m2q1w | ||||||
| Gao et al. 2013 | 30 mg/m2; d1-3 | 450 mg/m2; d1-3 | q3w 2 cycle | 40 mg/m2q1w | ||||||
IC, induction chemotherapy; CCRT, concurrent chemoradiotherapy; 5-Fu, 5-fluorouracil; DDP, cisplatin.
Quantitative analysis
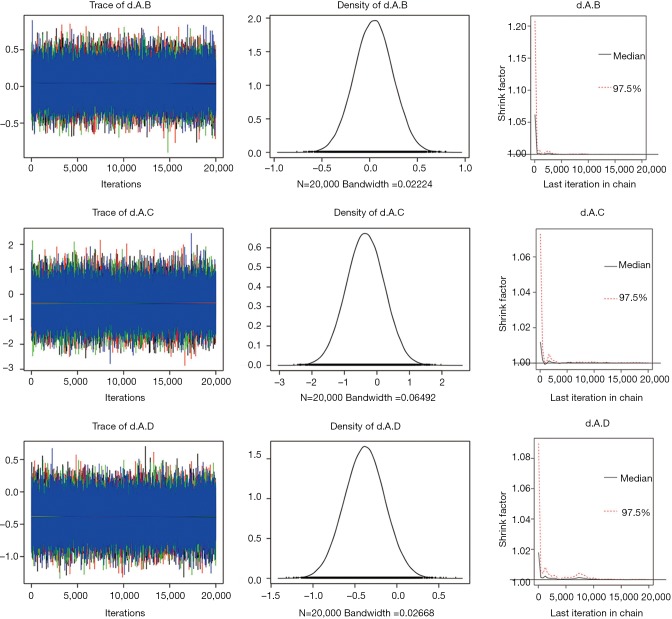
In the analysis of OS, we found that the between-trial heterogeneity within each comparison was negligible (I2=0). Thus, fixed-effects model was used. Models were computed with Markov chain Monte Carlo simulations, using 4 different sets of starting values to fit the model, with Gibbs sampling based on 20,000 iterations after a burn-in phase of 10,000 iterations. The densities nicely overlap, supporting convergence in the posterior distribution (Figure S1).
Figure S1.
The posterior distribution. The each chain overlap well indicating that they converged to the same area and the smoothed posterior probability densities for the same parameters in each chain supporting convergence.
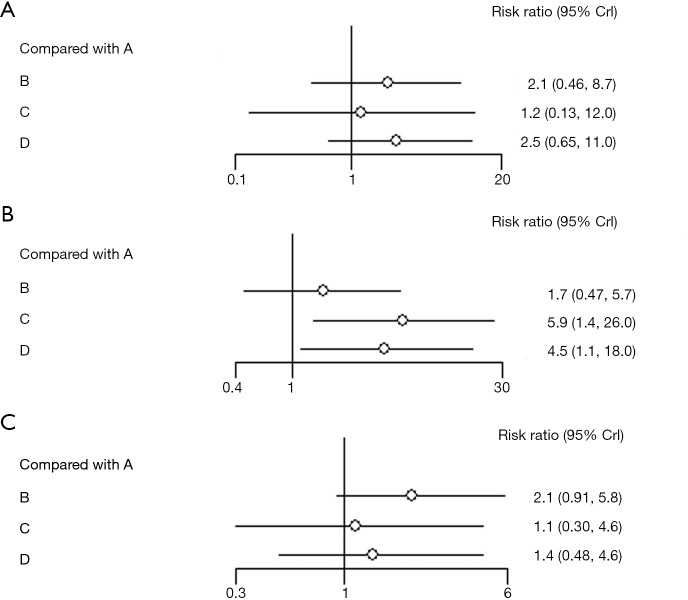
OS was reported in all included clinical trials. Either induction regimen (including TPF, TP and PF) do not reach significant statistical differences in OS, compared with CCRT alone. However, considering the value of HRs, docetaxel-based regimens (including TPF and TP) may bring improved trends in OS, compared with regimen without docetaxel (PF). In detail, TPF shows the highest probability to be the best choice (HRs 0.68; 95% CrI, 0.42–1.1) and TP ranks the second place (HRs 0.70; 95% CrI, 0.22–2.2). HRs value of PF regimen do not show any advantages (HRs 1.0; 95% CrI, 0.71–1.5) (Figure 3A). In terms of each treatment’s probability of being the best regimen, TPF, TP, PF and CCRT alone are 49.61%, 47.45%, 1.57%, and 1.37% respectively (Figure 3B).
Figure 3.
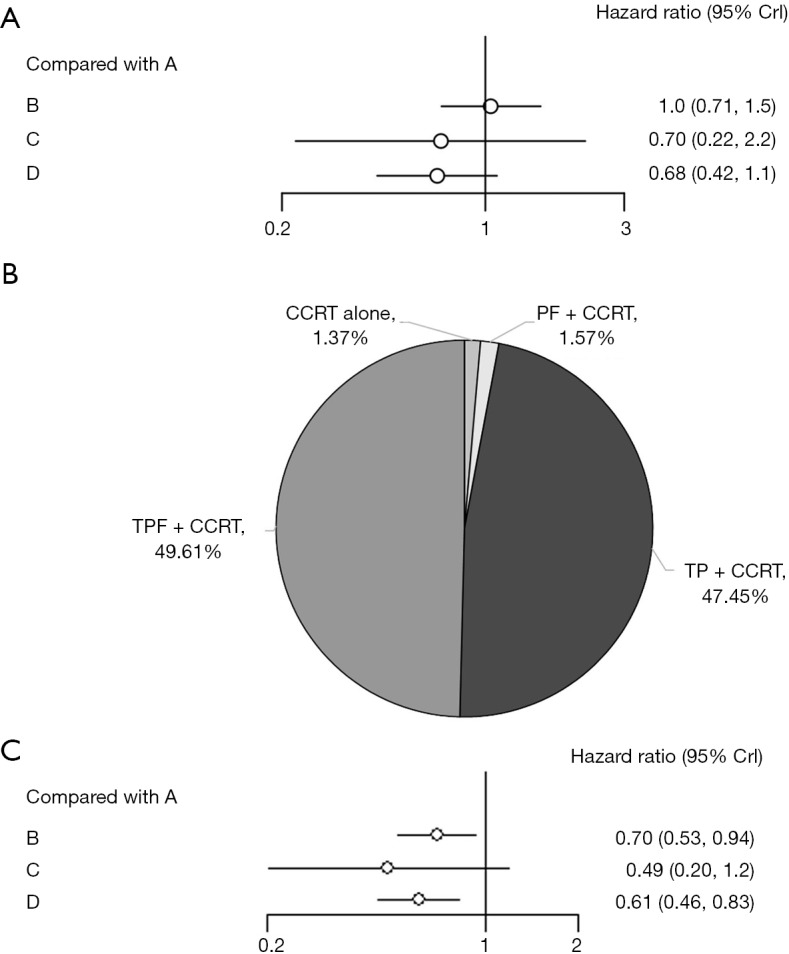
Analysis of efficacy. (A) Forest of overall survival; (B) probability of being the best regimen based on overall survival; (C) forest of progression-free survival. A: CCRT; B: PF + CCRT; C: TP + CCRT; D: TPF + CCRT. PF, induction chemotherapy regimen of cisplatin, 5-fluorouracil; TP, induction chemotherapy regimen of docetaxel, cisplatin; TPF, induction chemotherapy regimen of docetaxel, cisplatin, 5-fluorouracil; CCRT, concurrent chemoradiotherapy.
As for PFS, 6 studies can be used for quantitative analysis. Compared with CCRT alone, adding IC [including TPF (HRs 0.61; 95% CrI, 0.46–0.83) and PF (HRs 0.70; 95% CrI, 0.53–0.94)] significantly improve PFS while TP regimen (HRs 0.49; 95% CrI, 0.20–1.2) does not reach significant statistical differences (Figure 3C).
In addition, we further studied whether IC can affect the delivery of concurrent chemotherapy. Six studies could be used to analyze the effect of IC on completion of CCRT (Figure 4A). The results show that either regimen does not significantly affect the completion of concurrent chemotherapy. However, considering RRs values, 5-fluorouracil-based regimens, including TPF (RRs 2.5; 95% CrI, 0.65–11.0) and PF (RRs 2.1; 95% CrI, 0.46–8.7), may hamper the delivery of concurrent chemotherapy, compared with TP regimen (RRs 1.2; 95% CrI, 0.13–12.0), which may be caused by ≥ grade 3 mucositis. Furthermore, we assessed treatment associated toxicities (mainly including ≥ grade 3 neutropenia and mucositis), because they are the most frequent adverse events accounting for discontinuation of concurrent cisplatin. Six studies were used for quantitative analysis on ≥ grade 3 neutropenia and mucositis. ≥ Grade 3 neutropenia significantly increases in TP (RRs 5.9; 95% CrI, 1.4–26.0) and TPF (RRs 4.5; 95% CrI, 1.1–18.0), compared with CCRT alone. In addition, PF regimen does not show obvious increase in ≥ grade 3 neutropenia (RRs 1.7; 95% CrI, 0.47–5.7) (Figure 4B). Moreover, we observed that IC does not cause significant increase in ≥ grade 3 mucositis. Considering RRs values, 5-fluorouracil-based regimens, including PF (RRs 2.1; 95% CrI, 0.91–5.8) and TPF (RRs 1.4; 95% CrI, 0.48–4.6), show higher ≥ grade 3 mucositis than TP (RRs 1.1; 95% CrI, 0.30–4.6) (Figure 4C).
Figure 4.
Forest of treatment tolerance. (A) The completion of concurrent chemotherapy; (B) ≥ grade 3 neutropenia; (C) ≥ grade 3 mucositis. A: CCRT; B: PF + CCRT; C: TP + CCRT; D: TPF + CCRT. PF, induction chemotherapy regimen of cisplatin, 5-fluorouracil; TP, induction chemotherapy regimen of docetaxel, cisplatin; TPF, induction chemotherapy regimen of docetaxel, cisplatin, 5-fluorouracil; CCRT, concurrent chemoradiotherapy.
Discussion
Previous clinical trials showed that IC could improve prognosis in LA-NPC (22,23). However, it is unclear which IC regimen is optimal among common IC regimens including PF, TP and TPF.
In the network comparisons, we found that adding docetaxel might provide better efficacy than regimen without docetaxel in OS. Only considering OS, TPF shows the highest probability to be the best choice and TP ranks the second place. Previous retrospective studies also found that the treatment efficacy of docetaxel included induction regimens is superior to regimens without docetaxel in patients with LA-NPC (19,24). In addition, compared with the standard regimen PF, IC including docetaxel significantly improved PFS and OS in patients with unresectable squamous-cell carcinoma of the head and neck (25). Our data show that compared with CCRT alone, adding IC, including TPF, TP and PF, shows improved PFS, though TP regimen does not reach significant statistical differences.
According to included studies, the most frequent reasons for discontinuation of treatment plan in these groups were adverse events. The most frequent adverse events leading to discontinuation were hematological toxicity and oral mucositis. In our analysis, we also identified that different IC could affect the delivery of concurrent chemotherapy, which was consistent with the occurrence of oral mucositis. In detail, considering the impact on the delivery of concurrent chemotherapy, three regimens do not significantly affect the completion of concurrent chemotherapy (P>0.05). However, the RRs value from regimens with 5-fluorouracil (PF and TPF) is higher, compared without 5-fluorouracil. That is to say, 5-fluorouracil included regimens are likely to hamper concurrent chemotherapy, possibly because 5-fluorouracil induces worse mucositis. Previous studies also indicated that the administration of 5-fluorouracil often was associated with rates of grade 3–4 oral mucositis >15%, and the addition of radiation therapy might increase the risk of grade 3–4 oral mucositis >30%. Furthermore, among patients with grade 3–4 oral mucositis, 60% of patients had fever, 70% of patients required feeding tubes to maintain adequate nutrition, and 62% of patients required hospitalization, which finally affected the completion of concurrent chemoradiation (26). In addition, we also observed that docetaxel based regimens induce much worse ≥ grade 3 neutropenia. As reported before, patients treated with docetaxel based chemotherapy were more susceptible to the hematological toxicity, and the rate of neutropenia (grade III–IV) related to docetaxel (100 mg/m2) accounted for 75.4% in all severe adverse reactions (27).
There are other IC regimens that had been investigated before. However, they are excluded in this network meta-analysis based on the reasons as follows. For example, compared with CCRT alone, CEP (cisplatin, epirubicin and paclitaxel) (28) and GCP (gemcitabine, carboplatin, and paclitaxel) (29) did not show any significant benefit in response rates or OS and thus are not usually used in clinical practice. As for GP (gemcitabine, cisplatin) (30) and NP (vinorelbine, cisplatin) (31), current published studies did not meet inclusion criteria and thus cannot be combined in our network meta-analysis.
To our knowledge, this is the first network meta-analysis to demonstrate which IC is the optimal choice for LA-NPC. Our data show that TPF significantly improve PFS and may bring improved trends in OS. TP also bring improved trends in OS and PFS. TPF and TP are similar in hematological toxicity. Furthermore, TPF has worse oral mucositis which can impact the delivery of concurrent chemotherapy compared with TP group. In conclusion, TPF has the highest probability to be the optimal choice in LA-NPC and TP also shows encouraging anti-tumor effects, although TPF brings more adverse events
Acknowledgements
Funding: The work was supported by National Natural Sciences Foundation of China (81672386 and 81402494).
Supplementary
Search strategies for PubMed, Embase, and the Cochrane Library database
PubMed

Embase

The Cochrane Library

Stata and R code

Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Mao YP, Xie FY, et al. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother Oncol 2012;104:331-7. [DOI] [PubMed] [Google Scholar]
- 3.Yi JL, Gao L, Huang XD, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: Ten-year experience of a single institution. Int J Radiat Oncol Biol Phys 2006;65:161-8. 10.1016/j.ijrobp.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 4.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. 10.1200/JCO.1998.16.4.1310 [DOI] [PubMed] [Google Scholar]
- 5.He Y, Guo T, Guan H, et al. Concurrent chemoradiotherapy versus radiotherapy alone for locoregionally advanced nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy: a meta-analysis. Cancer Manag Res 2018;10:1419-28. 10.2147/CMAR.S160469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012;13:163-71. 10.1016/S1470-2045(11)70320-5 [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Tian H, Li G, et al. Significant benefits of adding neoadjuvant chemotherapy before concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: A meta-analysis of randomized controlled trials. Oncotarget 2016;7:48375-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.OuYang PY , Xie C, Mao YP, et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol 2013;24:2136-46. 10.1093/annonc/mdt146 [DOI] [PubMed] [Google Scholar]
- 9.Skoetz N, Trelle S, Rancea M, et al. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin's lymphoma: a systematic review and network meta-analysis. Lancet Oncol 2013;14:943-52. 10.1016/S1470-2045(13)70341-3 [DOI] [PubMed] [Google Scholar]
- 10.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie 2011;5:S38. [Google Scholar]
- 12.Garcia-Perdomo HA, Tobias A. Network meta-analysis: mixed and indirect treatment comparisons. a new method to the service of clinical epidemiology and public health. Rev Peru Med Exp Salud Publica 2016;33:149-53. [PubMed] [Google Scholar]
- 13.Liu XP, Meng XY, Yin XH, et al. A network Meta-analysis on survival data achieved by using gemtc package in R software. Chin J Evid Based Cardiovasc Med 2016;7:769-72. [Google Scholar]
- 14.Oravecz Z, Muth C. Fitting growth curve models in the Bayesian framework. Psychon Bull Rev 2018;25:235-55. 10.3758/s13423-017-1281-0 [DOI] [PubMed] [Google Scholar]
- 15.Cao SM, Yang Q, Guo L, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer 2017;75:14-23. 10.1016/j.ejca.2016.12.039 [DOI] [PubMed] [Google Scholar]
- 16.Frikha M, Auperin A, Tao Y, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol 2018;29:731-6. 10.1093/annonc/mdx770 [DOI] [PubMed] [Google Scholar]
- 17.Huang SN, Wang RS, Liang FF, et al. Inductive chemotherapy followed by chemoradiotherapy for locally advanced nasopharyngeal carcinoma. Zhonghua yu fang yi xue hui 2013;20:614-7.
- 18.Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 2009;27:242-9. 10.1200/JCO.2008.18.1545 [DOI] [PubMed] [Google Scholar]
- 19.Jin T, Qin WF, Jiang F, et al. Interim analysis of a prospective randomized non-inferiority trial of cisplatin and fluorouracil induction chemotherapy with or without docetaxel in nasopharyngeal carcinoma. Oncotarget 2016. doi:. 10.18632/oncotarget.10903 [DOI] [Google Scholar]
- 20.Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509-20. 10.1016/S1470-2045(16)30410-7 [DOI] [PubMed] [Google Scholar]
- 21.Gao J, Gao T, Dong z, et al. A prospective and randomized study of induction chemotherapy combined with concurrent chemoradiotherapy in the treatment for nasopharyngeal carcinoma stage T_(3~4)N_ (2~3)M_0. J Chin Oncol 2013;3:161-5. [Google Scholar]
- 22.Tan TH, Soon YY, Cheo T, et al. Neoadjuvant chemotherapy plus concomitant chemoradiation versus concomitant chemoradiation for locoregionally advanced nasopharyngeal carcinoma: A systematic review and meta-analysis of comparative studies. J Clin Oncol 2017;15:e17552 10.1200/JCO.2017.35.15_suppl.e17552 [DOI] [Google Scholar]
- 23.Li WF, Chen L, Sun Y, et al. Induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer 2016;35:94. 10.1186/s40880-016-0157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mi JL, Zhang B, Pan YF, et al. Chemotherapy regimens containing taxanes or fluorouracil in nasopharyngeal carcinoma: Which better? Oral Oncol 2017;74:34-9. 10.1016/j.oraloncology.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 25.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, Fluorouracil, and Docetaxel in Unresectable Head and Neck Cancer. N Engl J Med 2007;357:1695-704. 10.1056/NEJMoa071028 [DOI] [PubMed] [Google Scholar]
- 26.Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100:1995-2025. 10.1002/cncr.20162 [DOI] [PubMed] [Google Scholar]
- 27.Elm’hadi C, Tanz R, Khmamouche MR, et al. Toxicities of docetaxel: original drug versus generics—a comparative study about 81 cases. Springerplus 2016;5:732. 10.1186/s40064-016-2351-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fountzilas G, Ciuleanu E, Kalogera-Fountzila A, et al. Induction chemotherapy (IC) followed by concomitant chemoradiotherapy (CCRT) versus CCRT alone in patients with locally advanced nasopharyngeal carcinoma (LA-NPC) - A randomized phase II study of the Hellenic Cooperative Oncology Group. Eur J Cancer 2009;7:471 10.1016/S1359-6349(09)71595-2 [DOI] [Google Scholar]
- 29.Tan T, Lim WT, Fong KW, et al. Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2015;91:952-60. 10.1016/j.ijrobp.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 30.Yau TK, Lee AW, Wong DH, et al. Treatment of Stage IV(A-B) nasopharyngeal carcinoma by induction-concurrent chemoradiotherapy and accelerated fractionation: impact of chemotherapy schemes. Int J Radiat Oncol Biol Phys 2006;66:1004-10. 10.1016/j.ijrobp.2006.06.016 [DOI] [PubMed] [Google Scholar]
- 31.Han SH, Yu L, Zhang Z, et al. Evaluation of induction chemotherapy with vinorelbine plus cisplatin (NP) or docetaxel plus cisplatin (TP) combined with concurrent chemoradiotherapy for patients with locally advanced nasopharyngeal carcinoma. Zhonghua Zhong Liu Za Zhi 2013;35:623-6. [PubMed] [Google Scholar]