Abstract
Background
This study aims to assess the effects of probiotic supplementation on the maternal metabolism and the risk of development of gestational diabetes mellitus (GDM) in the pregnant women by a meta-analysis of relevant randomized controlled trials (RCTs).
Methods
The medical literature was searched from PubMed, Web of Science and the Cochrane Library since inception to October 2017. Two investigators independently performed the data extraction and quality assessment. The mean differences (MD) or standardized mean differences (SMD) or relative risk (RR) with 95% confidence intervals (CIs) were calculated with the random-effects model.
Results
From 648 citations, a total of ten RCTs published in 13 articles with 1,139 participants met the inclusion criteria. The meta-analysis showed that probiotics supplementation effectively reduced the fasting blood glucose (FBG) levels (MD −0.11 mmol/L, P=0.0003), serum insulin levels (MD −2.06 µU/mL, P<0.00001), insulin resistance (HOMA-IR) (MD −0.38, P<0.00001). The study found a significant effect of probiotics on decreasing the risk of GDM [risk ratio (RR) 0.52, P=0.003) in early pregnancy. Additionally, there were statistically significant reductions in the total cholesterol and triglycerides levels after probiotic interventions (SMD −0.56, P=0.03; SMD −0.66, P=0.04), respectively.
Conclusions
Our study shows that the probiotic use was associated with improved glucose and lipid metabolism in the pregnant women, and might also contribute to the reduced risk of GDM.
Keywords: Pregnancy, gut microbiota, probiotics, glycometabolism, gestational diabetes mellitus (GDM), lipid profile
Introduction
The pregnancy is characterized by a series of physiologic, metabolic and intestinal microbial changes (1,2). A prospective study conducted by Koren et al. (2) on 91 pregnant women showed that the composition and structure of gut microbiota dramatically change during pregnancy and these changes continue to occur from the first trimester to the third trimester. They concluded that the impact of intestinal microbiota during pregnancy is similar to metabolic syndrome. However, similar results were not observed in all studies. During pregnancy, an imbalance in the intestinal flora resembles metabolic dysfunction with increase in the inflammation, energy content, and decreased insulin sensitivity (3). Increased insulin resistance can raise glucose and free fatty acid (FAA) concentrations, and can also affect fat oxidation in the late gestation. In obese pregnant women, the dysbiosis of gut microbiota can lead to the development of metabolic disorders and gestational diabetes mellitus (GDM) (4). Thus, the prevention and possibly treatment of dysbiosis are essential to decrease the risk of morbidity and mortality in the mother and newborn (5).
In the past decade, the use of probiotics has emerged as a principal approach to maintain the balance of the human gut microbiota (6). Some reports have shown that the probiotic supplementation can improve insulin sensitivity and cholesterol metabolism in the hosts (7,8). The Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) have defined probiotics as live microorganisms, which confer a health benefit on the host under sufficient amounts (9). Probiotics are widely being studied for their beneficial effects on the treatment of various diseases, such as the obesity, type 2 diabetes mellitus (DM-2) and non-alcoholic fatty liver disease (NAFLD) (10). Furthermore, probiotics that alter the bacterial flora in the gut may help infants to thrive or even prevent the development of diseases like asthma and diabetes (11). Thus far, several randomized controlled trials (RCTs) have reported that the probiotics have a beneficial effect on the prevention and treatment of maternal metabolic outcomes (12-16). Therefore, we systematically analyzed the medical literature for all published RCTs to assess the role of probiotics in affecting the metabolic profiles in the pregnant women.
Methods
Literature search
An electronic literature search was conducted on PubMed-MEDLINE, Cochrane Library and Web of Science for relevant RCTs since inception up to October 2017. The search terms included pregnancy-related terms (that is “pregnan*” or “gestation*” or “matern*” or “gestational diabetes mellitus,” or “gestational diabetes”), plus probiotic-related term (that is “probiotics” or “culturelle” or “bacteria*” or “Bifidobacteria” or “Lactobailli” or “Acidophilus” or “yogurt”), plus metabolism-related terms (that is “glucose” or “insulin” or “HOMA*” or “metabol*” or “intervention”). We also searched their references lists without any language restrictions. Primary outcomes of selected studies were GDM and maternal metabolic changes during pregnancy. Secondary outcomes included maternal pregnancy and offspring birth outcomes. This meta-analysis was prospectively registered in PROSPERO as CRD 42017079900. We undertook the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (17).
Selection criteria
Inclusion criteria were defined as follows: (I) completed RCTs reported by the original research articles; (II) pregnant women >18 years-of-age with or without GDM; (III) studies comparing the effects of probiotics with placebo/control, and (IV) articles on fasting plasma glucose or insulin or homeostasis model assessment of insulin resistance (HOMA-IR) or lipid metabolism or GDM. Exclusion criteria were determined as follows: (I) duplicate publications, editorials, literature reviews, and meta-analyses; (II) nonrandomized trials; (III) studies presented only as abstracts with no subsequent full report of study results or primary data; and (IV) insufficient information for data extraction.
Data extraction and qualitative analysis
Two reviewers independently determined eligible studies, extracted data and assessed the risk of bias of included studies. The following details were extracted and tabulated: study (authors/year), study design, sample size (intervention/control), the period of intervention (category, dose, intervention time point, duration of probiotics), and outcome indicators. The included studies were evaluated for bias by using the Cochrane risk-of-bias (RoB) tool (version 5.0) (18). Each included study was evaluated for the following biases: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participant and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. The reviewers’ judgment was categorized as “Low risk,” “High risk” or “Unclear risk” of bias. The discrepancies were resolved after consultation and discussion with a third investigator.
Statistical analysis
The meta-analysis and statistical analysis were undertaken by using the Stata Software (version 11) and Review Manager (RevMan version 5.3). We summarized dichotomous data as relative risk (RR) with 95% confidence intervals (CI) for GDM in the included study. The metabolic indices of selected studies were maternal fasting blood glucose (FBG), insulin, HOMA-IR, quantitative insulin-sensitivity check index (QUICKI), maternal weight change, and morbidity of GDM and lipid metabolism. Secondary outcomes included maternal outcomes (gestation weeks and gestational weight gain), infant birth outcomes (birth weight, birth length, and head circumference) and rate of adverse pregnancy outcome (cesarean delivery, premature baby and macrosomia). The mean difference (MD) was used for the continuous data outcome indicators which measured with the same methods. Otherwise, the standardized mean difference (SMD) was used to combine the trials. We extracted the means and standard deviations (SDs) for post-intervention values or change scores. When the heterogeneity existed, the random-effects model was applied; otherwise, the fixed-effects model was chosen.
Heterogeneity was assessed in each meta-analysis using the T2, I2 and χ2 statistics, with the value >50% indicative of statistical heterogeneity or there was a low P value (P<0.05) in the χ2 test for heterogeneity (19). Subgroup analyses based on glycemic status (FBG, insulin, and HOMA-IR) for age, body mass index (BMI), GDM, dose and species of probiotic, intervention time point and the duration of treatment. Sensitivity analyses were performed by excluding individual trial from the meta-analysis, and the effect size was recalculated to assess the effects of probiotic vs. placebo on metabolisms with pregnancy. The publication bias was analyzed with the method from Egger and colleagues with P<0.10 considered as significant (20).
Results
Description of selected trials and study characteristics
Our initial search in the electronic databases included 648 citations (300 from PubMed/MEDLINE, 255 from Web of Science, and 93 from Cochrane Library). After the screening of titles and abstracts, the full text of 24 articles was assessed. Finally, 13 articles (including 10 RCTs) involving 1,139 participants were included in the meta-analysis, as shown in Figure 1.
Figure 1.
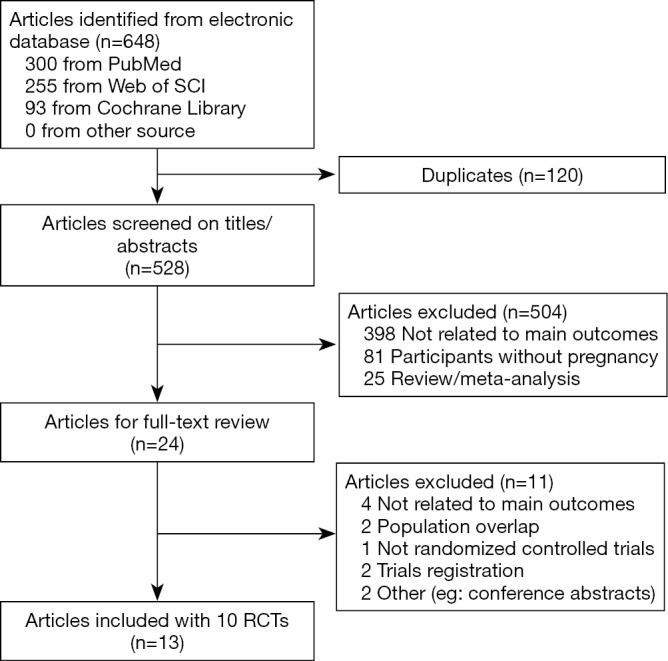
Study flow diagram. RCT, randomized controlled trial.
The characteristics of the studies are shown in Table 1. The age range was 18–40 years. Pre-pregnancy BMI ranged from 26–39.9 kg/m2. Out of the 10 RCTs, five trials included pregnant women with GDM (22-26). All participants were randomly assigned to daily probiotic supplements or placebo, and the daily consumption of probiotics varied from 107 colony-forming units (CFU)/g to 1010 CFU/g. In regards to the composition of probiotics, three studies (21,23,24) used a single species probiotics (Lactobacillus spp.), whereas other studies (12-15,22,25-28) used combination strains (Lactobacillus spp., Bifidobacterium spp. and others). The timing of intervention was the first trimester in four studies (12-14,21-23) and it was the third trimester in the remaining six trials (15,16,24-28). The duration of the intervention was 4–24 weeks. The primary outcome indicators for various interventions included FBG, fasting insulin, HOMA-IR, quantitative insulin sensitivity check index (QUICKI), lipid profiles, maternal weight change, and GDM. The results of the quality assessment had a low risk of bias, as shown in Figure 2.
Table 1. Characteristics of the 10 RCTs (13 articles) included in the analysis.
| Study author/year | Participants sample size (intervention/control) | Study design | Periods of intervention/control | Outcomes |
|---|---|---|---|---|
| Laitinen 2009 (12), Luoto 2010 (13), Hoppu 2014 (14) |
126 (66/60) | Parallel RCT, Double blind for probiotics/placebo, single blind for dietary intervention | Supplement probiotic or placebo from early pregnancy until the end of exclusive breastfeeding. Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12, 1010 colonyforming units/d | ↓ Fasting plasma glucose; ↓ HOMA-IR; ↓ Glycated HbA1c; ↑ Insulin; ↓ QUICKI; ↓ Incidence of GDM; ↔ gestational weight gain; ↔ Total cholesterol; ↔ HDL cholesterol; ↔ LDL cholesterol; ↔ Triglycerides |
| Asemi 2013 (15), Asemi 2012 (16) |
70 (37/33) | Parallel RCT, double-blinded | Supplement probiotic or placebo with 200 g per day of conventional or probiotic yogurts for 9 weeks; Lactobacilli and bifidobacteria, a total of min 1×107 CFU/g | ↔ Fasting plasma glucose; ↓ HOMA-IR; ↓ serum insulin; ↔ Total cholesterol; ↔ HDL cholesterol; ↔ LDL cholesterol; ↔ Triglycerides |
| Lindsay 2014 (21) | 138 (63/75) | Parallel RCT, A single-center, double-blinded | Supplementation probiotic or placebo from 24 to 28 weeks of gestation (4 weeks); Lactobacillus salivarius UCC118, a target (100 mg) dose of 109 CFU | ↔ Fasting plasma glucose; ↔ HOMA-IR; ↔ Insulin; ↔ C-peptide; ↔ Incidence of GDM; ↔ Total cholesterol; ↔ HDL cholesterol; ↔ LDL cholesterol; ↔ Triglycerides; ↔ C-reactive protein; ↔ gestational weight gain |
| Jamilian 2016 (22) | 60 (30/30) | Parallel RCT, double-blinded | Supplementation probiotic or placebo for 12 weeks among pregnant women in the first half of pregnancy; each probiotic capsule contained L. acidophilus (2×109 CFU/g), L. casei (2×109 CFU/g) and B. bifidum (2×109 CFU/g) | ↔ Fasting plasma glucose; ↓ HOMA-IR; ↓ serum insulin ↓ HOMA-B; ↑ QUICKI; ↔ Total cholesterol; ↔ HDL cholesterol; ↔ LDL cholesterol; ↓ Triglycerides; ↓ C-reactive protein |
| Kristin 2017 (23) | 397 (195/202) | Parallel RCT, double-blinded | Supplementation probiotic/placebo from early pregnancy until 6 months postpartum; Lactobacillus rhamnosus HN001, a dose per capsule of 6x109 CFU/g | ↓ Fasting plasma glucose; ↓ incidence of GDM |
| Lindsay 2015 (24) | 100 (48/52) | Parallel RCT, double-blinded | Supplementation probiotic or placebo capsules for 6-week; each capsule contained L. salivarius UCC118 (1×109 CFU/g) | ↔ Fasting plasma glucose; ↔ Insulin; ↔ HOMA-IR; ↔ C-peptide; ↓ Total cholesterol; ↔ CRP; ↔ Triglyceride; ↓ LDL cholesterol; ↔ HDL cholesterol; ↔ gestational weight gain |
| Dolatkhah 2015 (25) | 56 (29/27) | Parallel RCT, double-blinded | Supplementation probiotic capsule with dietary advice or placebo capsule with dietary advice for 8 weeks; each probiotic capsule contained L. acidophilus LA-5, Bifidobacterium BB-12, S. thermophilus STY-31 and L. delbrueckii subsp. Bulgaricus LBY-27 (>4×109 CFU/g) | ↓ Fasting plasma glucose; ↓ HOMA-IR; ↔ QUICKI; ↓ gestational weight gain |
| Jafarnejad 2016 (26) | 72 (37/35) | Parallel RCT, double-blinded | Supplementation probiotic or placebo capsules for 8 weeks. Each probiotic capsule contained VSL#3 (S. thermophilus, B. breve, B. longum, B. infantis, L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. Bulgaricus, 15×109 CFU/g) | ↔ Fasting plasma glucose; ↓ Insulin; ↓ HOMA-IR; ↔ HbA1c; ↓ Interleukin-6; ↓ Tumor Necrosis Factor-alpha; ↓ hs-CRP; ↔ gestational weight gain |
| Karamali 2016 (27) | 60 (30/30) | Parallel RCT, double-blinded | Supplementation probiotic or placebo capsules for 6 weeks; each probiotic capsule contained L. acidophilus (2×109 CFU/g), L. casei (2×109 CFU/g) and B. bifidum (2×109 CFU/g) | ↓ Fasting plasma glucose; ↓ Insulin; ↓ HOMA-IR; ↓ QUICKI; ↔ Total cholesterol; ↔ HDL cholesterol; ↔ LDL cholesterol; ↓ VLDL cholesterol; ↓ Triglycerides; ↔ gestational weight gain |
| Badehnoosh 2018 (28) | 60 (30/30) | Parallel RCT, double-blinded | Supplementation probiotic or placebo capsules for 6 weeks; each probiotic capsule contained Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum (2×109 CFU/g each) | ↓ Fasting plasma glucose; ↓ hs-CRP; ↔ gestational weight gain |
BMI, body mass index; CFU, colony-forming units; GDM, gestational diabetes mellitus; HDL-cholesterol, high density lipoprotein-cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-cholesterol, low density lipoprotein-cholesterol; hs-CRP, hypersensitive C-reactive protein; QUICKI, quantitative insulin sensitivity check index; RCT, randomized controlled trial; VLDL cholesterol, very low-density lipoprotein-cholesterol
Figure 2.
Assessment of the risk of bias of the included studies.
Main outcomes
FBG
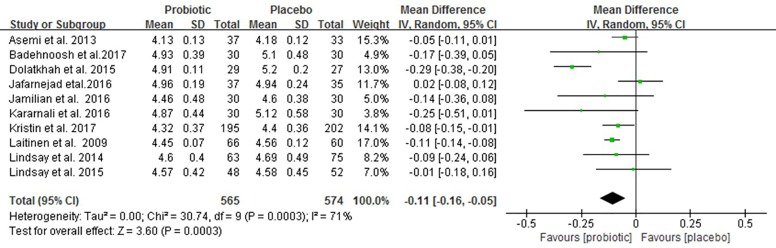
Figure 3 shows the forest plot of the pooled effect of probiotics supplementation on FBG. All studies reported changes in FBG involving 1,139 pregnant women. Compared with placebo/control group, our meta-analysis indicates a significant reduction in FBG (MD –0.11 mmol/L; 95% CI: −0.16 to −0.05; P=0.0003) with high heterogeneity (I2=71%, P<0.001) after probiotic interventions.
Figure 3.
Effect of probiotic supplementation on fasting blood glucose (mmol/L) in pregnant women.
Sensitivity analysis showed that the exclusion of one trial with higher heterogeneity revealed significance in the overall effect as well (MD −0.08 mmol/L, P<0.0001) (25), in Table S1. Subgroup analyses showed that FBG was not improved in the subgroup of pregnant women with GDM, whereas FBG significantly reduced in the subgroup of those without GDM (−0.09 mmol/L; 95% CI: −0.12 to −0.07; P<0.00001). Early pregnancy intervention resulted in a significant reduction in FBG (−0.10 mmol/L; 95% CI: −0.12 to −0.07; P<0.00001). However, there was a decreasing trend in FBG when the interventions were carried in third trimester (−0.12 mmol/L; 95% CI: −0.23 to 0.0; P=0.06). Meta-analysis of trials with multiple probiotics showed a meaningful reduction in FBG (−0.11 mmol/L; 95% CI: −0.18 to −0.04; P=0.001). However, similar results were not found for a single probiotic species. Furthermore, a subgroup analysis with a daily dose of probiotics consumed ≥6×109 CFU found a significant improvement in FBG. Additionally, subgroup analyses for age, BMI and duration of interventions were no longer significant differences (Table 2).
Table S1. Sensitivity analysis of RCTS.
| Groups | Trials | WMD (95% CI) | P value | I2 (%) | Pheterogeneity |
|---|---|---|---|---|---|
| Fasting blood glucose (mmol/L) | |||||
| Excluded heterogeneous studies | 9 | −0.08 (−0.11 to −0.045) | <0.0001 | 25 | 0.22 |
| Insulin (mU/L) | |||||
| Excluded heterogeneous studies | 6 | −1.92 (−2.81 to −1.04) | <0.0001 | 31 | 0.2 |
| HOMA-IR | |||||
| Excluded heterogeneous studies | 7 | −0.30 (−0.43 to −0.18) | <0.0001 | 46 | 0.08 |
| QUICKI | |||||
| Excluded heterogeneous studies | 3 | 0.02 (0.01 to 0.03) | <0.0001 | 0 | 0.67 |
| Weight change (kg) | |||||
| Excluded heterogeneous studies | 3 | −0.17 (−0.33 to −0.01) | 0.03 | 0 | 0.4 |
| Total cholesterol | |||||
| Excluded heterogeneous studies | 5 | −0.21 (−0.39 to −0.04) | 0.02 | 0 | 0.67 |
| Triglycerides | |||||
| Excluded heterogeneous studies | 4 | −0.08 (−0.30 to 0.14) | 0.49 | 28 | 0..24 |
| LDL-cholesterol | |||||
| Excluded heterogeneous studies | 5 | −0.13 (−0.30 to 0.05) | 0.15 | 0 | 0.87 |
HOMA-IR, homeostasis model assessment of insulin resistance; LDL-cholesterol, low density lipoprotein-cholesterol; QUICKI, quantitative insulin sensitivity check index; RCT, randomized controlled trial; WMD, weight mean difference; CI, confidence interval; I2, I-squared.
Table 2. Results of sensitivity and subgroup analysis of included RCTs in meta-analysis of probiotics and glycemic outcomes.
| Subgroup analysis | Weight mean difference (95% confidence interval) | ||
|---|---|---|---|
| Fasting blood glucose (mmol/L) | Insulin (mU/L) | HOMA-IR | |
| Pregnant women of age (≥30 years) | −0.08 (−0.13 to −0.03), P=0.003, n=6 | −2.73 (−4.70 to −0.76), P=0.007, n=5 | −0.55 (−0.87 to −0.22), P=0.001, n=5 |
| Pregnant women of age (<30 years) | −0.16 (−0.32 to −0.01), P=0.04, n=4 | −1.59 (−2.56 to −0.62), P=0.001, n=3 | −0.29 (−0.59 to 0.01), P=0.06, n=3 |
| Pregnant women of BMI (≥28 kg/m2) | −0.16 (−0.29 to −0.04), P=0.008, n=5 | −1.34 (−2.64 to −0.04), P=0.04, n=4 | −0.37 (−0.70 to −0.05), P=0.02, n=4 |
| Pregnant women of BMI (<28 kg/m2) | −0.08 (−0.13 to −0.02), P=0.009, n=4 | −3.77 (−6.61 to −0.94), P=0.009, n=3 | −0.77 (−1.31 to −0.22), P=0.006, n=3 |
| Pregnant women without GDM | −0.09 (−0.12 to −0.07), P<0.00001, n=5 | −1.89 (−2.39 to −1.38), P<0.00001, n=4 | −0.29 (−0.47 to −0.11), P=0.002, n=4 |
| Pregnant women with GDM | −0.08 (−0.29 to 0.03), P=0.10, n=5 | −3.01 (−5.73 to −0.28), P=0.03, n=4 | −0.69 (−1.24 to −0.14), P=0.01, n=4 |
| Early pregnancy intervention | −0.10 (−0.12 to −0.07), P<0.00001, n=4 | −1.87 (−3.14 to −0.60), P=0.004, n=3 | −0.41 (−0.67 to −0.16), P=0.001, n=3 |
| Late pregnancy intervention | −0.12 (−0.23 to 0.00), P=0.06, n=6 | −2.16 (−3.34 to −0.99), P=0.0003, n=5 | −0.37 (−0.58 to −0.17), P=0.0004, n=5 |
| Intervention duration ≥ 8weeks | −0.11 (−0.18 to 0.03), P=0.004, n=6 | −2.16 (−3.19 to −1.12), P<0.00001, n=5 | −0.35 (−0.52 to −0.19), P<0.00001, n=5 |
| Intervention duration <8 weeks | −0.10 (−0.19 to −0.01), P=0.03, n=4 | −2.09 (−4.75 to 0.58), P=0.12, n=3 | −0.55 (−1.20 to 0.11), P=0.10, n=3 |
| Daily dose of probiotics ≥6×109 CFU | −0.09 (−0.14 to −0.04), P=0.0008, n=6 | −4.25 (−6.85 to −1.66), P=0.001, n=4 | −0.89 (−1.60 to −0.17), P=0.01, n=4 |
| Daily dose of probiotics <6×109 CFU | −0.12 (−0.26 to 0.02), P=0.11, n=4 | −1.32 (−2.02 to −0.61), P=0.0003, n=4 | −0.29 (−0.34 to −0.24), P<0.0001, n=4 |
| More than 1 species of probiotics | −0.11 (−0.18 to −0.04), P=0.001, n=7 | −2.41 (−3.49 to −1.33), P<0.001, n=6 | −0.42 (−0.61 to −0.23), P<0.001, n=6 |
| Single species of probiotics | −0.08 (−0.18 to 0.02), P=0.12, n=3 | −0.97 (−2.561 to 0.62), P=0.23, n=2 | −0.24 (−0.61 to 0.13), P=0.21, n=2 |
HOMA-IR, homeostasis model assessment of insulin resistance; RCT, randomized controlled trial; BMI, body mass index; GDM, gestational diabetes mellitus; CFU, colony-forming units.
Serum insulin (µU/mL) and HOMA-IR
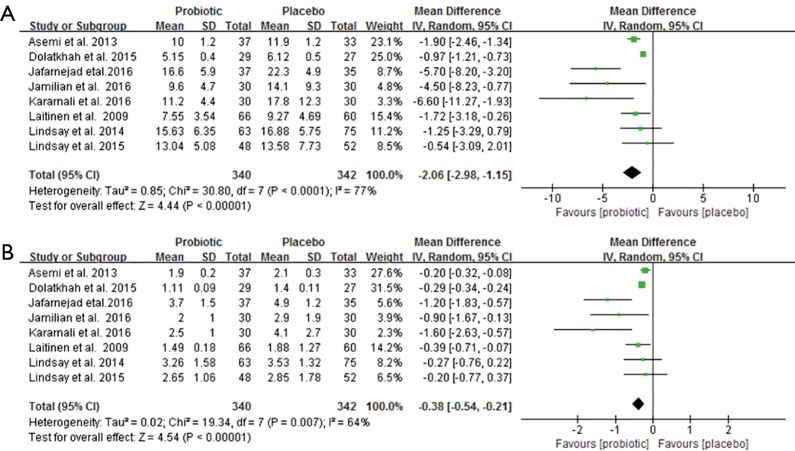
Figure 4 demonstrates forest plots of the pooled effects of probiotics supplementation on serum insulin and HOMA-IR (12,15,21-23,25-27). For serum insulin and HOMA-IR, the pooled MD were (−2.06 µU/mL, 95% CI: −2.98 to −1.15, P<0.00001; −0.38 µU/mL, 95% CI: −0.54 to −0.21, P<0.00001), respectively. However, the significant evidence of inter-study heterogeneity were observed for the meta-analysis in insulin levels and HOMA-IR (I2=77%, P<0.0001 and I2=64%, P=0.007, respectively).
Figure 4.
Effect of probiotics supplementation on (A) serum insulin (µU/mL) and (B) HOMA-IR in pregnant women. HOMA-IR, homeostasis model assessment of insulin resistance.
The sensitivity analysis showed that the overall results of serum insulin changes were influenced mainly by two trials (25,26), and HOMA-IR was influenced by only one trial (25) (Table S1). Subgroup analysis with multiple probiotics found a significant reduction in serum insulin levels and HOMA-IR (−2.41 µU/mL, 95% CI: −3.49 to −1.33, P<0.001; −0.42 µU/mL, 95% CI: −0.61 to −0.23, P<0.001). Additionally, subgroup analysis with duration of interventions ≥8 weeks showed a significant effect on serum insulin levels and HOMA-IR. Although there were no statistical differences of age, BMI, GDM and intervention time points for insulin levels and HOMA-IR, there was high heterogeneity in these subgroups (Table 2).
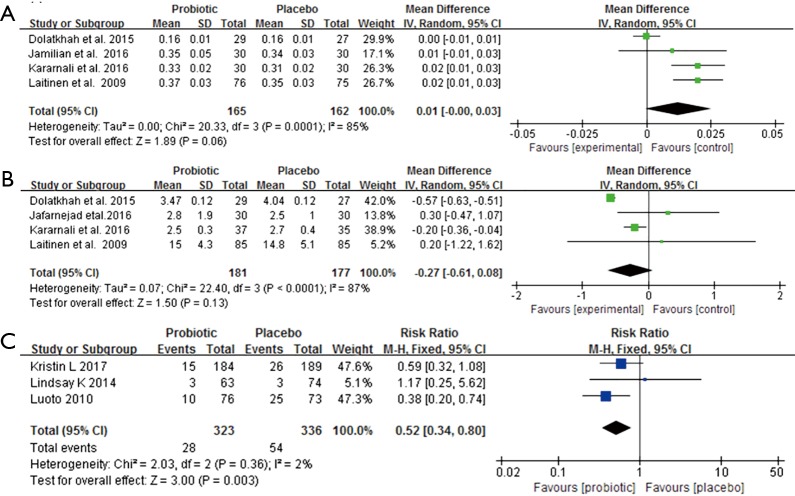
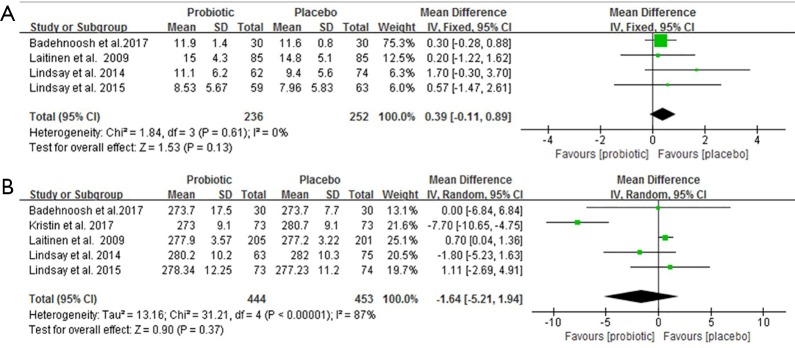
QUICKI, Maternal weight change, and GDM morbidity
Four clinical trials involving 327 pregnant women were included to investigate the impact of probiotics supplementation on QUICKI (12,25-27). The overall results of the meta-analysis showed that probiotics interventions were not significantly related with QUICKI (MD 0.01, 95% CI: 0.00 to 0.03, P=0.06) when compared to placebo/control groups in pregnant women (Figure 5A). Nevertheless, the higher levels of statistical heterogeneity were observed for the meta-analysis of QUICKI (I2=85%, P=0.0001). When a trial (25) was removed, the results suggested a significant change in QUICKI, and the heterogeneity of the study results on QUICKI became insignificant (MD 0.02, 95% CI: 0.01 to 0.03, P<0.0001; I2=0%, P=0.67) (Table S1).
Figure 5.
Effect of probiotics supplementation on (A) QUICKI, (B) maternal weight change and (C) rate of GDM. GDM, gestational diabetes mellitus.
Figure 5B shows a forest plot of the pooled effect of probiotics supplementation on maternal weight change. In these four studies, no significant change was observed in maternal weight (MD −0.27 kg, 95% CI: −0.61 to 0.08, P=0.13) (12,25-27). Significant evidence of inter-study heterogeneity was observed across studies (I2=87%, P<0.0001). After excluding one trial (25), the results showed lower heterogeneity and statistical difference (MD = −0.17kg, P=0.03; I2=0%, P=0.40) (Table S1).
For GDM morbidity, the overall results of the meta-analysis indicated that probiotic supplementation significantly reduced the risk of GDM when compared to placebo during early pregnancy (RR 0.52, 95% CI: 0.34 to 0.80, P=0.003; I2=2.0%, P=0.36) (Figure 5C).
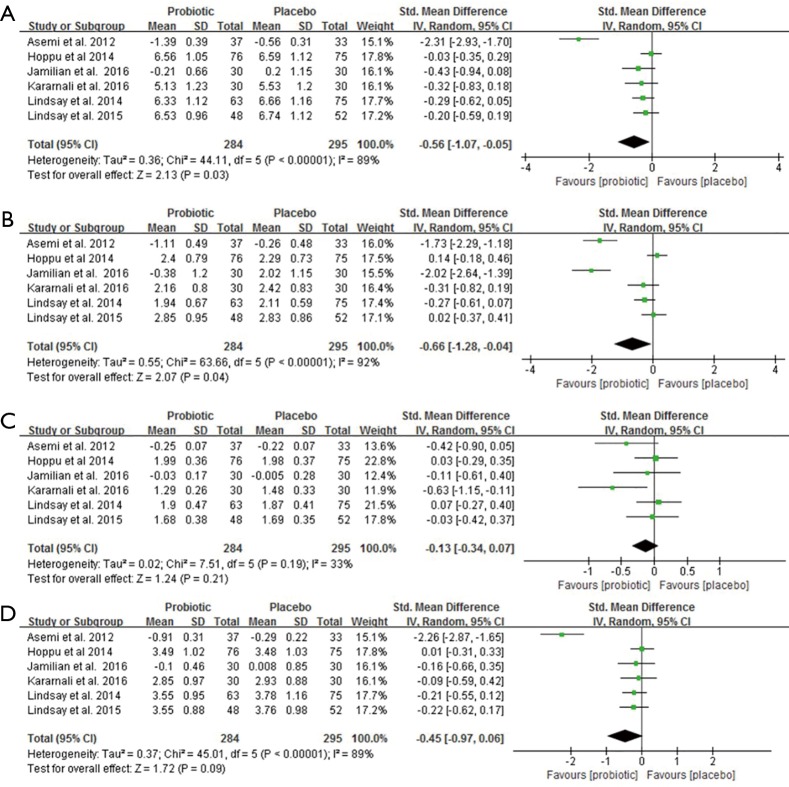
Lipid profiles
Data from six trials involving 579 pregnant women reported the effects of probiotic supplements on total cholesterol, triglycerides, HDL-C and LDL-C levels (14,16,21,22,24,27). There were statistically significant reductions in total cholesterol and triglycerides after probiotic supplementation (SMD −0.56, 95% CI: −1.07 to −0.05, P=0.03; SMD −0.66, 95% CI: −1.28 to −0.04, P=0.04) (Figure 6A,B). However, there was no significant changes in HDL-C and LDL-C levels (SMD −0.13, 95% CI: −0.34 to 0.07, P=0.21; SMD −0.45, 95% CI: −0.97 to 0.06, P=0.09, respectively) (Figure 6C,D). Significant heterogeneity was present in these trials in the overall analysis (all I2>50%, P<0.05), except for HDL-C (I2=33%, P=0.19). In the sensitivity analysis, all the re-pooled results show no significance when removing a single study at one time (Table S1).
Figure 6.
The effect of probiotics on (A) total cholesterol, (B) triglycerides, (C) HDL-C and (D) LDL-C.
Secondary outcomes
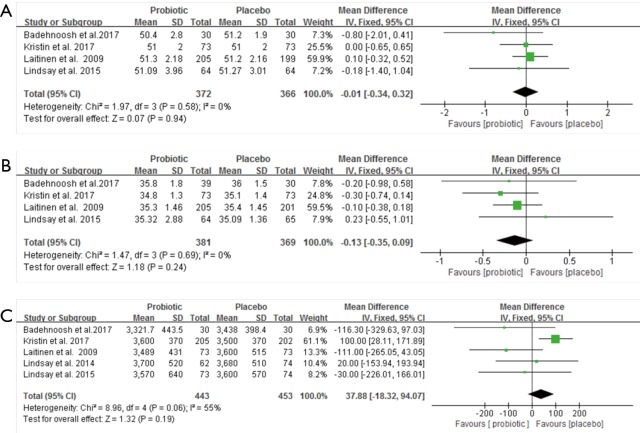
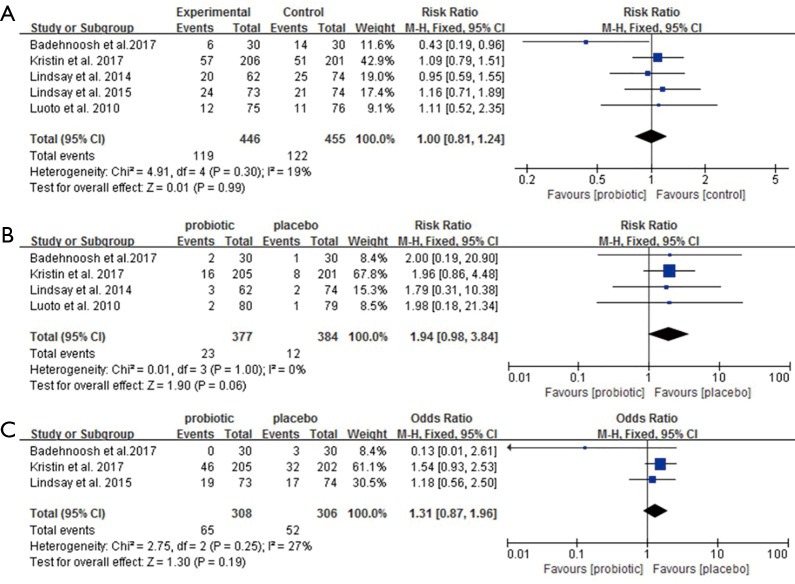
The meta-analysis found that the use of probiotics in pregnancy did not affect the gestational weight gain and days of gestation at the time of birth, the secondary outcome (Figure S1). There were no differences in mean infant birth weight, length and head circumference between the intervention and control group (Figure S2). However, the rate of premature delivery showed a decreasing trend in the probiotic group (Figure S3).
Figure S1.
The effect of probiotics on the outcome of pregnant women. (A) Weight gain over pregnancy (kg); (B) gestation days at the time of birth.
Figure S2.
Effects of probiotics on infant outcomes. (A) Birth length (cm); (B) neonatal head circumference (cm); (C) birth weight (g).
Figure S3.
Effect of probiotics on adverse pregnancy outcomes. (A) Rate of cesarean section; (B) rate of premature delivery; (C) rate of macrosomia.
Publication bias
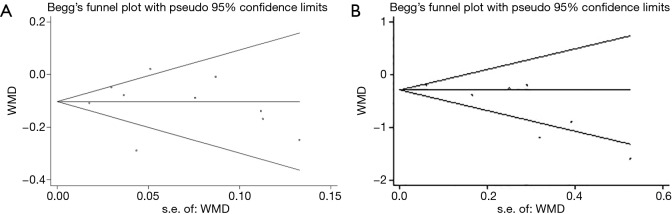
The Egger’s publication bias plot (Figure S4) indicated that no publication bias existed in the studies. This finding was again confirmed with the use of Begg’s test for maternal metabolic outcomes including FBG, serum insulin, HOMA-IR and lipid profiles (all P>0.05).
Figure S4.
Funnel plot with pseudo 95% confidence limits for fasting plasma glucose in pregnant women.
Discussion
The results of our meta-analysis revealed that the probiotic supplementation in pregnant women is effective in decreasing FBG, insulin, and HOMA-IR. These favorable factors may contribute in reduction of the risk of GDM. The use of probiotics is promising approach to prevent and potentially manage the metabolic derangements in GDM.
Probiotics interventions have been inconsistently associated with significant reductions in FBG, insulin, and HOMA-IR in the pregnant women. These discrepant findings could be the result of different dosages and types of probiotic bacteria used for the patient population. According to the FAO/WHO, optimum probiotic use can bring health benefits to the host. In general, >106–108 CFU/g, or >108–1010 CFU/d of viable cells are regarded as sufficient and efficient (29). In our meta-analysis, the probiotic doses were more than 109 CFU/g, and small doses were 1×107 CFU/g. Asemi et al. (15) demonstrated that the probiotic yogurt containing two strains of Lactobacilli and Bifidobacteria with a minimum concentration of 1×107 CFU/g was helpful in maintaining serum insulin concentrations in healthy pregnant women. However, Lindsay et al. (24) showed that the supplementation with a probiotic capsule containing 109 CFU/g of L. salivarius UCC118 had no impact on glycemic controls in women with GDM, and the supplementation with 100 mg of L. salivarius UCC118 109 CFU dose for 4 weeks had no beneficial influence on maternal metabolic profiles in obese pregnant women (21). Furthermore, our subgroup analysis showed that the multiple species probiotics were helpful for glucose metabolism as compared to single species probiotic. We also observed that the subgroup with ≥8 weeks of intervention time had a favorable glucose metabolism as compared to the subgroup with <8 weeks of intervention. The findings of the present meta-analysis suggest that the combination of probiotic species and longer duration of interventions are more effective in improving glucose metabolism.
Pregnant women have increased triglyceride and decreased HDL concentrations, especially in patients with GDM (30). Women with GDM maintain a stable state of the plasma FFA concentrations while promoting insulin secretion in the human body (1). The results from the analysis of six RCTs demonstrated that the probiotic capsule intake could significantly decrease total cholesterol and triglycerides levels in pregnant women. However, some other clinical studies have produced conflicting results (14). A randomized study by Hoppu et al. (14) reported that the dietary counseling with probiotics or placebo in the first trimester of pregnancy showed no difference on the lipid levels in the third trimester. Therefore, the results of the present meta-analysis are encouraging, and further interventional studies are necessary to confirm the results.
The GDM is one of the most common complications in pregnant women (31). Unfortunately, the results of probiotics administration on the risk of GDM have produced contradictory results. Recent a meta-analysis found that probiotics supplementation during pregnancy had beneficial effects on glucose metabolism among pregnant women (32). We found that the risk of GDM in the study from New Zealand (13) was weaker than that of from Finland (23). It could be a possibility that the combined use of the two probiotics in the Finnish study was more effective than HN001 Lactobacillus rhamnosus alone in the New Zealand study (23). Above all, these results may indicate that the intervention period is also a crucial factor, and the treatment of single species probiotics was ineffective for the prevention of GDM. Additionally, the different diagnostic threshold may be a contributor for the differences in the results (13,21,23). Therefore, larger well-designed RCTs are needed to confirm potentially beneficial relationships between probiotics and GDM risk.
The impact of probiotics on metabolic improvement may be due to effect on the gut flora (33,34). However, the detailed mechanism for the relation still remains unclear. Short chain fatty acids (SCFAs) which are the main product of the fermentation of intestinal flora are significantly changed in the intestinal lumen and peripheral blood during pregnancy (35). Furthermore, SCFAs can be recognized by G protein-coupled receptors (GPCRs) such as free fatty acid receptor-2 (FFA2), which is a unique receptor that senses metabolites produced by the gut microbiota (35). Recently, Fuller et al. (36) showed a potential synergy between the gut microbiota, SCFAs, and FFA2 in the regulation of gestational glucose homeostasis. It was suggested that SCFAs play an important role in the metabolism of pregnant women. Another possible mechanism could be the protective action of probiotics on the gut microbiota-SCFAs-glucagon-like peptide-1 (GLP-1) axis in the metabolic processes. Probiotic intake has also been shown to influence the gut flora (i.e., decrease Firmicutes and increase Bacteriodetes and Bifidobacteria), and the variations were associated with increased the levels of butyrate, which promoted the release of hormones like GLP-1 (36,37). Another possible mechanism is a probiotic-gut flora-butyrate-inflammatory pathway. It is known that the anti-inflammatory effects of probiotics (Lactobacillus and Bifidobacterium) may contribute to the treatment of low-grade inflammation (38). For example, probiotics improve insulin resistance in DM-2, and this function is associated with the gut microbiota, butyrate production and the inflammatory response (39,40). In addition, supplementation of probiotics decreases the levels of the inflammatory markers including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) and increase intestinal GLP-1 levels reducing glucotoxicity and increasing increase insulin sensitivity in pregnant women (15,16,26). Therefore, the alterations the composition of gut microbiota can improve the metabolism with pregnant women.
Our meta-analysis had some strengths. This meta-analysis addressed the clinical question, and provided comprehensive and quantitative analyses by quality assessment. Our meta-analysis also considered the types, dosages, time point and duration of probiotic intervention in the pregnant women. Nevertheless, there were some limitations to the present analysis worth mentioning. First, the bias of the included studies such as a single-blinded design and diet consultation management could affect the final results (12-14). Second, there was no justification of sample size or reporting the evaluation of the successfulness of blinding in the included study. Third, studies with shorter intervention time might lead to the discrepancy for the overall results of the meta-analysis, since the subgroup analysis of the studies with the duration of intervention (<8 weeks) did not indicate a meaningful reduction for the metabolic parameters.
Conclusions
Probiotic interventions are associated with improved glucose and lipid metabolism in the pregnant women and may contribute to reducing the risk of GDM. The use of specific probiotics supplementation may be a promising prevention and therapeutic strategy for GDM.
Acknowledgements
Funding: This work was funded by the National Natural Science Foundation of China (No. 81570739).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Xiang AH, Peters RK, Trigo E, et al. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes. Diabetes 1999;48:848-54. 10.2337/diabetes.48.4.848 [DOI] [PubMed] [Google Scholar]
- 2.Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012;150:470-80. 10.1016/j.cell.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiGiulio DB, Callahan BJ, McMurdie PJ, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci USA 2015;112:11060-5. 10.1073/pnas.1502875112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Arango LF, Barrett HL, McIntyre HD, et al. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes 2016;65:2214-23. 10.2337/db16-0278 [DOI] [PubMed] [Google Scholar]
- 5.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 2012;8:639-49. 10.1038/nrendo.2012.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy H, Harris J, Lyon E, et al. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeostasis and immunopathology. Nutrients 2013; 5:1869-912. 10.3390/nu5061869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev 2018;31:35-51. 10.1017/S095442241700018X [DOI] [PubMed] [Google Scholar]
- 8.Guo Z, Liu XM, Zhang QX, et al. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis 2011;21:844-50. 10.1016/j.numecd.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 9.Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iqbal MZ, Qadir MI, Hussain T, et al. Probiotics and their beneficial effects against various diseases. Pak J Pharm Sci 2014; 27:405-15. [PubMed] [Google Scholar]
- 11.DeWeerdt S. How baby's first microbes could be crucial to future health. Nature 2018;555:S18-9. 10.1038/d41586-018-02480-6 [DOI] [PubMed] [Google Scholar]
- 12.Laitinen K, Poussa T, Isolauri E, et al. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr 2009;101:1679-87. 10.1017/S0007114508111461 [DOI] [PubMed] [Google Scholar]
- 13.Luoto R, Laitinen K, Nermes M, et al. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr 2010;103:1792-9. 10.1017/S0007114509993898 [DOI] [PubMed] [Google Scholar]
- 14.Hoppu U, Isolauri E, Koskinen P, et al. Maternal dietary counseling reduces total and LDL cholesterol postpartum. Nutrition 2014;30:159-64. 10.1016/j.nut.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 15.Asemi Z, Samimi M, Tabassi Z, et al. The effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. Eur J Clin Nutr 2013;67:71-4. 10.1038/ejcn.2012.189 [DOI] [PubMed] [Google Scholar]
- 16.Asemi Z, Samimi M, Tabasi Z, et al. Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. J Matern Fetal Neonatal Med 2012;25:1552-6. 10.3109/14767058.2011.640372 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Green SE, Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: Wiley-Blackwell, 2008. [Google Scholar]
- 19.Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97-111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsay KL, Kennelly M, Culliton M, et al. Probotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). Am J Clin Nutr 2014;99:1432-9. 10.3945/ajcn.113.079723 [DOI] [PubMed] [Google Scholar]
- 22.Jamilian M, Bahmani F, Vahedpoor Z, et al. Effects of Probiotic Supplementation on Metabolic Status in Pregnant Women: a Randomized, Double-blind, Placebo-Controlled Trial. Arch Iran Med 2016;19:687-2. [PubMed] [Google Scholar]
- 23.Wickens KL, Barthow CA, Murphy R, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br J Nutr 2017;117:804-13. 10.1017/S0007114517000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay KL, Brennan L, Kennelly MA, et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am J Obstet Gynecol 2015;212:496.e1-11. 10.1016/j.ajog.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 25.Dolatkhah N, Hajifaraji M, Abbasalizadeh F, et al. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J Health Popul Nutr 2015;33:25. 10.1186/s41043-015-0034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jafarnejad S, Saremi S, Jafarnejad F, et al. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J Nutr Metab 2016;2016:5190846. 10.1155/2016/5190846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karamali M, Dadkhah F, Sadrkhanlou M, et al. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab 2016;42:234-41. 10.1016/j.diabet.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 28.Badehnoosh B, Karamali M, Jamilian M, et al. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J Matern Fetal Neonatal Med 2018;31:1128-36. 10.1080/14767058.2017.1310193 [DOI] [PubMed] [Google Scholar]
- 29.Homayoni Rad A, Mehrabany EV, Alipoor B, et al. Do probiotics act more efficiently in foods than in supplements? Nutrition 2012;28:733-6. 10.1016/j.nut.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 30.Gelaye B, Larrabure-Torrealva GT, Qiu C, et al. Fasting lipid and lipoproteins concentrations in pregnant women with a history of migraine. Headache 2015;55:646-57. 10.1111/head.12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davey RX. Gestational diabetes mellitus: a review from 2004. Curr Diabetes Rev 2005;1:203-13. 10.2174/1573399054022776 [DOI] [PubMed] [Google Scholar]
- 32.Zheng J, Feng Q, Zheng S, et al. The effects of probiotics supplementation on metabolic health in pregnant women: An evidence based meta-analysis. PLoS One 2018;13:e0197771. 10.1371/journal.pone.0197771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delzenne NM, Neyrinck AM, et al. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 2011;7:639-46. 10.1038/nrendo.2011.126 [DOI] [PubMed] [Google Scholar]
- 34.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010;328:228-31. 10.1126/science.1179721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Layden BT, Angueira AR, Brodsky M, et al. Short chain fatty acids and their receptors: new metabolic targets. Transl Res 2013;161:131-40. 10.1016/j.trsl.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 36.Fuller M, Priyadarshini M, Gibbons SM, et al. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am J Physiol Endocrinol Metab 2015;309:E840-51. 10.1152/ajpendo.00171.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yadav H, Lee JH, Lloyd J, et al. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem 2013;288:25088-97. 10.1074/jbc.M113.452516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLoughlin RF, Berthon BS, Jensen ME, et al. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr 2017;106:930-45. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Xu Q, Jiang T, et al. A comparative study of the antidiabetic effects exerted by live and dead multi-strain probiotics in the type 2 diabetes model of mice. Food Funct 2016;7:4851-60. 10.1039/C6FO01147K [DOI] [PubMed] [Google Scholar]
- 40.Mohamadshahi M, Veissi M, Haidari F, et al. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 2014;4:83-8. [DOI] [PMC free article] [PubMed] [Google Scholar]