Abstract
A 60-year-old man was admitted to our hospital because of massive hemoptysis with acute respiratory failure. Since six months ago, he noticed gradual worsening of hemoptysis and was transferred to our hospital. Chest computed tomography showed a nodular lesion with cavitation in the left upper lobe and surrounding ground-glass opacification. Initially, a hemostatic agent was administered, but we eventually performed bronchial artery embolization (BAE) by ourselves due to persistent hemoptysis. After achieving good hemostasis with BAE bronchoscopy was performed, which gave a diagnosis of pulmonary actinomycosis on histopathologic examination of the transbronchial biopsy specimen without the need for lung resection.
Keywords: Recurrent hemoptysis, transbronchial lung biopsy, pulmonary actinomycosis, bronchial artery embolization (BAE)
Introduction
Actinomycosis is a rare chronic disease caused by Actinomyces species anaerobic Gram-positive bacteria that normally colonize the human oral cavity and gastrointestinal and genital tracts (1). Pulmonary actinomycosis is a rare and slowly progressing infectious disease with nonspecific symptoms similar to those of other chronic suppurative chest diseases (2), commonly presenting as pulmonary infiltrate or a mass on radiologic examination (3). These nonspecific clinical and radiologic findings make pulmonary actinomycosis difficult to diagnose and often lead to misinterpretation as malignancy, rather than an infective process. In fact, even among experienced physicians, delayed diagnosis or misdiagnosis as tuberculosis, lung abscess, or lung cancer is common (4). The diagnosis of pulmonary actinomycosis therefore requires bacterial evidence and characteristic pathologic findings, although flexible bronchoscopy for this purpose is not always diagnostic.
In previous case reports, pulmonary actinomycosis was occasionally resected as pulmonary neoplasm because of the diagnostic difficulty (5). Some cases of pulmonary actinomycosis occasionally present with massive or recurrent hemoptysis and were treated with bronchial artery embolization (BAE) or thoracotomy in order to control bleeding (3,6). Massive hemoptysis is a life-threatening medical emergency, with a mortality of 50% to 80%, if left untreated (7). Here, we report a case of recurrent hemoptysis caused by pulmonary actinomycosis, which could be diagnosed using transbronchial lung biopsy (TBLB) after BAE.
Case presentation
A 60-year-old man was admitted to our hospital because of recurrent hemoptysis with mild desaturation. He had persistent hemoptysis and concomitant cough. His oral hygiene was poor and so he had full dentures in place. He had a medical history of hypertension and type 2 diabetes mellitus, with poor adherence to medications. He was a current smoker of approximately 10 pack-years. He had no known allergies.
On examination, his height was 167 cm and his weight was 58.6 kg, with a body mass index of 21 kg/m2. Blood pressure was 146/78 mmHg and temperature was 36.7 °C. He had tachycardia (heart rate, 103/min) and tachypnea (respiratory rate, 30/min), which were caused by hypoxia (oxygen saturation of 88% on room air). Respiratory sounds were decreased in the left upper field. Cardiac auscultation showed no abnormalities, and there was no leg edema or skin lesions noted.
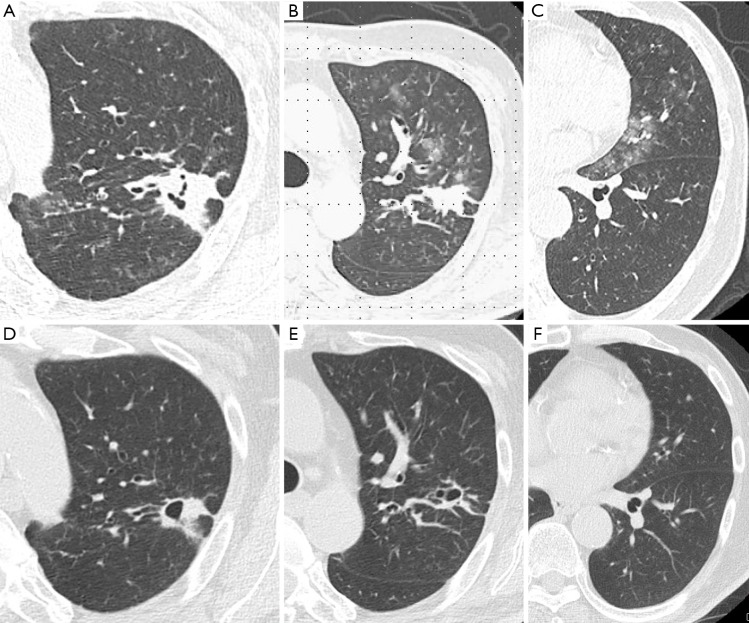
Laboratory findings demonstrated slight anemia and elevated inflammatory markers, such as white blood cell count and C-reactive protein. HbA1c and glucose levels were also high because of poor adherence (Table 1). Anterior-posterior chest radiograph showed slight cardiomegaly, hyperinflation of the lungs, and widespread infiltration with cavitation in the left upper lobe (Figure 1). Chest computed tomography (CT) scan also revealed a patchy nodular shadow with cavitation in the left upper lobe and surrounding ground glass opacities (Figure 2).
Table 1. Laboratory data on admission.
| Hematology | Biochemistry | Serology |
|---|---|---|
| WBC: 12,770/μL | Alb: 2.8 g/dL | CRP: 2.52 mg/dL |
| Neu: 69.1% | T-Bil: 0.3 mg/dL | CEA: 3.3 ng/mL |
| Lymph: 24.4% | AST: 11 IU/L | SCC: 1.6 ng/mL |
| Eos: 1.5% | ALT: 9 IU/L | CYFRA: 2.5 ng/mL |
| RBC: 3.74×104/μL | LDH: 156 IU/L | β-D glucan: 12.2 pg/mL |
| Hb: 11.7 g/dL | CK: 70 IU/L | Aspergillus Ag.: – |
| Hct: 33.7% | BUN: 10.0 mg/dL | ANA (IF): <40 |
| Plt: 30.2×104/μL | Crea: 0.58 mg/dL | ABG analysis (at 1 L oxygen supplement) |
| Coagulation | Na: 137 mEq/L | pH: 7.47 |
| PT-INR: 0.81 | K: 3.9 mEq/L | pCO2: 34.0 mmHg |
| APTT: 25.8 s | Cl: 102 mEq/L | pO2: 68.0 mmHg |
| Fibrinogen: 522 mg/dL | Glu: 181 mg/dL | HCO3: 24.7 mmol/L |
| FDP: 2.0 ìg/mL | HbA1c: 7.9% | Lac: 1.6 mmol/L |
WBC, white blood cell; Neu, neutrophil; Lymph, lymphocyte; Eos, eosinophil; Hb, hemoglobin; Hct, hematocrit; Plt, platelet; PT-INR, prothrombin time-international normalized ratio; APTT, activated partial thromboplastin time; FDP, fibrin degradation products; Alb, albumin; T-Bil, total bilirubin; AST, aspartate transaminase; ALT, alanine transaminase; LDH, lactate dehydrogenase; CK, creatinine phosphokinase; Crea, creatinine; Na, sodium; K, potassium; Cl, chlorine; Glu, glucose; HbA1c, hemoglobin A1c; CRP, C-reactive protein; CEA, carcinoembryonic antigen; SCC, squamous cell carcinoma antigen; CYFRA, cytokeratin 19 fragments; Ag., antigen; ABG, arterial blood gas; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; Lac, lactate.
Figure 1.
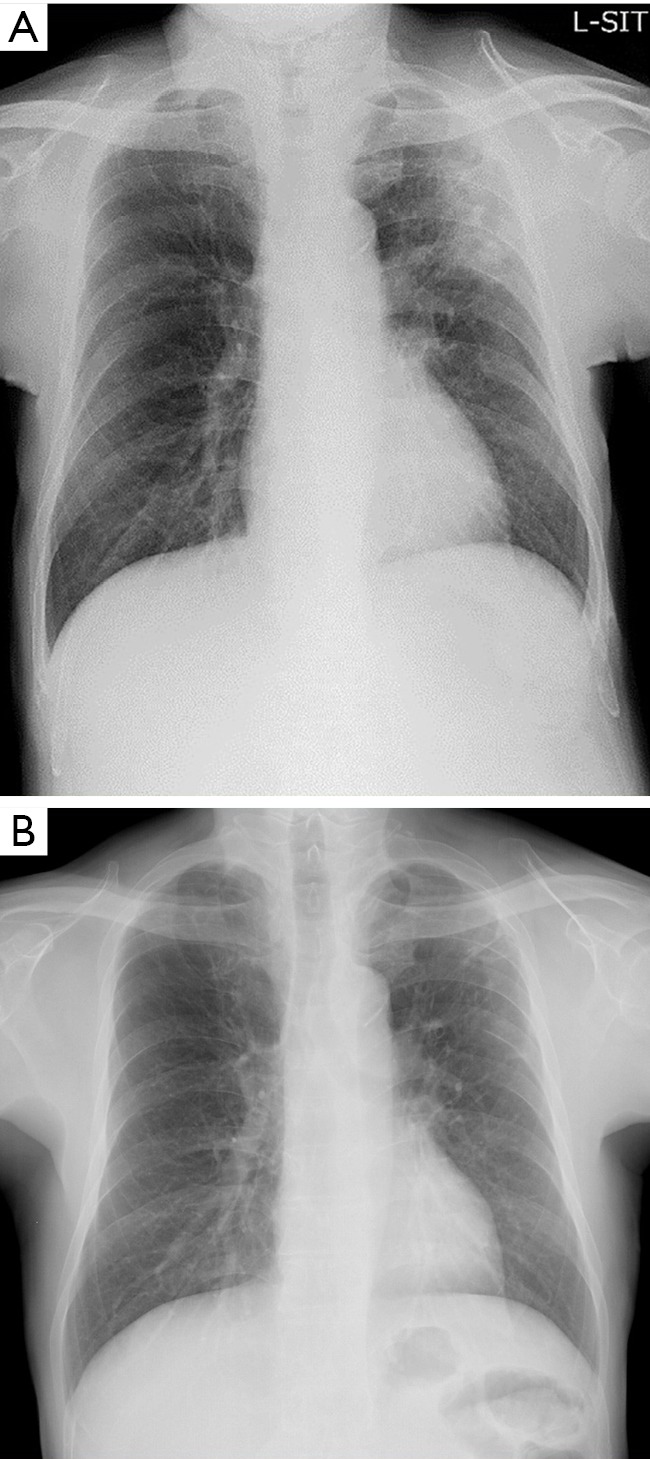
Chest radiograph on admission shows widespread infiltration with cavitation in the left upper lobe (A). Chest radiograph after the standard treatment shows widespread that infiltration with cavitation was improved (B).
Figure 2.
Chest computed tomography scan reveals a patchy infiltration with cavitation (A,B) in left the upper lobe and surrounding ground glass opacities (C) from hemoptysis. After the completion of the standard treatment, CT scan reveals improved patchy infiltration but remained a cavitation in left the upper lobe (D,E). Ground glass opacities (F) from hemoptysis were improved because of disappeared hemoptysis.
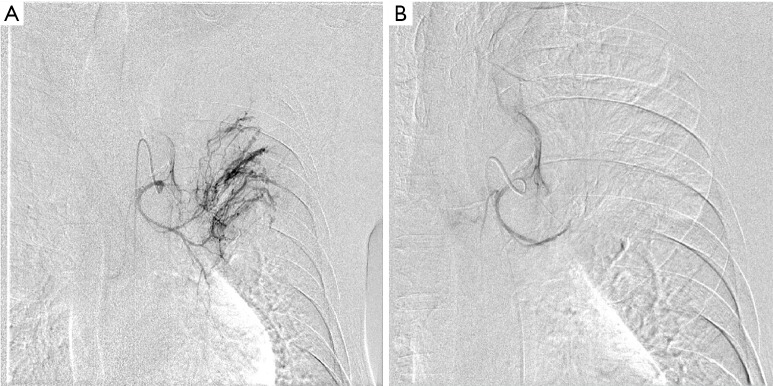
Initially, we treated the patient by giving supplemental oxygen, administering a hemostatic agent, and controlling the elevated blood pressure and hyperglycemia. At first, we suspected pulmonary tuberculosis or mycosis such as chronic pulmonary aspergillosis. However, despite these symptomatic treatments, the hemoptysis persisted and the anemia gradually worsened. Therefore, we performed BAE (Figure 3) by ourselves. The left bronchial artery and 1 intercostal artery were tightly embolized using gelatin sponge particles, the peripheral vascularization and pulmonary artery shunt resolved. After that the patient could be successful control of the hemoptysis.
Figure 3.
Angiography showing the left bronchial artery. (A) The proximal vessel is dilated and tortuous, with peripheral vascularization and presence of pulmonary artery shunt; (B) after bronchial artery embolization with gelatin sponge particles, the peripheral vascularization and pulmonary artery shunt resolved.
We performed 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG-PET/CT) for the purpose of differential diagnosis, but the findings were nonspecific, mostly suspicious for an inflammatory disease because of a mildly elevated SUVmax of 3.51. Because the possibility of lung cancer could not be ruled out, we decided to perform biopsy for definitive diagnosis. Therefore, after the BAE, we attempted to perform bronchoscopy using endobronchial ultrasonography with a guide sheath TBLB on the left upper lobe peripheral lesion.
Culture of the bronchoscopy specimens did not show evidence of Actinomyces spp., Nocardia spp., Aspergillus spp., and Mycobacterium spp. Histopathologic findings of the TBLB specimen revealed no evidence of malignancy, such as lung carcinoma or malignant lymphoma, but there was dense infiltration of neutrophils, plasma cells, and lymphocytes (Figure 4A). Immunostaining showed a Gram-positive filamentous bacterium, which stained positive on Grocott’s staining (Figure 4B and 4C). Based on the clinical course and pathologic findings of the TBLB specimen, we confirmed the cause of recurrent hemoptysis as pulmonary actinomycosis. Thereafter, amoxicillin 2,000 mg/day was started, and his condition gradually improved without recurrence of hemoptysis.
Figure 4.
Histopathologic findings. (A) Hematoxylin and eosin stain (4×) revealed dense infiltration of neutrophils, plasma cells, and lymphocytes. (B) Gram stain (40×) revealed Gram-positive bacteria aggregates of a filamentous bacterium, (C) Grocott’s stain (100×) revealed a filamentous bacterium.
After the completion of the standard treatment against Actinomycosis, infiltrations were improved and there was no recurrent hemoptysis (Figure 1B and Figure 2D,E,F).
Discussion
We encountered a case of recurrent hemoptysis caused by pulmonary actinomycosis, which was diagnosed using TBLB after BAE without the need for lung resection. Actinomycosis is a rare and chronic bacterial infection caused by Actinomyces species, which can colonize cervicofacial, thoracic, abdominal, and pelvic lesions, as well as the skin, brain, and other organs. Pulmonary actinomycosis is the third most common type of actinomycosis, after actinomycosis in the cervicofacial and abdominopelvic locations (1,2). It accounts for 15% of all actinomycosis and is mainly caused by aspiration of oral saprophytes into the respiratory tract (8); therefore, the disease usually shows a lower lobe and peripheral predominance.
The symptoms of pulmonary actinomycosis are nonspecific and similar to those of other forms of chronic suppurative lung disease (2). Previous reports have shown that pulmonary actinomycosis commonly presents with productive cough, fever, and chest pain, but hemoptysis was rare (9). In contrast, Kim et al. recently reported that the most common presenting symptoms were cough (77.7%), hemoptysis (64.9%), and sputum production (61.7%) (10); these results and those of other studies (11,12) are summarized in Table 2. Our case presented with symptoms of chronic lower respiratory tract infection and massive hemoptysis. These results suggest that pulmonary actinomycosis should be suspected in patients with hemoptysis of unknown cause.
Table 2. Clinical manifestations of pulmonary actinomycosis.
| Symptoms and signs | Kim et al. (10), n=94 (%) | Park et al. (11), n=68 (%) | Zhang et al. (12), n=145 (%) |
|---|---|---|---|
| Cough | 73 (77.7) | 62 (91.2) | 127 (87.6) |
| Sputum production | 58 (61.7) | 54 (79.4) | 58 (40.0) |
| Bloody sputum | ND | ND | 53 (36.6) |
| Hemoptysis | 61 (64.9) | 40 (58.8) | 22 (15.2) |
| Massive hemoptysis | ND | 9 (13.2) | ND |
| Chest pain | 12 (12.8) | 16 (23.5) | 34 (23.4) |
| Fever | 17 (18.1) | 16 (23.5) | 34 (23.4) |
| Weight loss | ND | 16 (23.5) | ND |
| Dyspnea | 13 (13.8) | 15 (22.1) | 5 (3.4) |
ND, no data.
The radiologic findings of pulmonary actinomycosis can resemble a broad spectrum of lung pathologies from benign infection to metastatic tumor, which make the diagnosis of this disease difficult, even for experienced physicians (13). In fact, our case showed that chest CT scan revealed a patchy nodular shadow with cavitation in the left upper lobe and surrounding ground glass opacities. Therefore, we suspected pulmonary tuberculosis or mycosis such as chronic pulmonary aspergillosis. However, despite these symptomatic treatments, the hemoptysis persisted and remains a patchy nodular shadow. We considered to be a possibility of differential diagnosis as a malignancy, we also needed to perform 18F-FDG PET/CT. The CT of this patient did not show classic findings of pulmonary actinomycosis, which may be the result of decreased immunity of the patient due to poorly controlled diabetes mellitus. Pulmonary infection is a common cause of morbidity and mortality in immunocompromised patients. Commonly, in immunocompromised patients, the appearance of signs of infection on Chest radiography (CR) may be delayed. For example, in neutropenic fever, CR may appear normal for up to 72 hours, though signs of underlying pneumonia may be apparent on computed tomography (CT) (14). CT has higher sensitivity and specificity than CR and is indicated when there is a strong suspicion of pneumonia with normal or nonspecific CR, especially in immunosuppressed hosts. However a variety of organisms can cause pulmonary infections in immunocompromised patients, and the radiological findings are usually nonspecific. Therefore, it is essential to combine all clinical features and radiological findings. Early accurate diagnosis may potentially reduce the morbidity and mortality associated with pulmonary infections in immunocompromised patients (15,16). Therefore, here, we reviewed the available data on literature regarding the radiologic findings of pulmonary actinomycosis, with reference to this case (Table 3). Commonly, the X-ray presents as a slowly progressive consolidation or mass-like shadow. On CT, pulmonary actinomycosis often presents with dilated bronchiolar shadow or as a characteristic central attenuation area, which comprises ≥1 round or oval low-attenuation area at the center of the shadow (17). Cheon et al. reported that the typical CT findings of pulmonary actinomycosis were chronic segmental airspace consolidations with low-attenuation areas, peripheral enhancement, and adjacent pleural thickening (18). In more than 50% of cases, parenchymal actinomycosis was associated with pleural involvement, such as pleural thickening, effusion, or empyema (10). Multiple small cavities may develop within the parenchymal lesion. Notably, these features are similar to those of other conditions, such as tuberculosis, bacterial or fungal pneumonia, and lung carcinoma (19). For many physicians, the main problem is distinguishing this disease from a neoplasm (17).
Table 3. CT findings of pulmonary actinomycosis.
| Chest CT findings | Kim et al. (10), n=94 | Park et al. (11), n=68 |
|---|---|---|
| Consolidation | 74.5 | 63 (92.6) |
| Central necrosis | ND | 42 (61.8) |
| Cavitation | 23.4 | 13 (19.1) |
| Peripheral subpleural lesion | ND | 44 (64.7) |
| Pleural thickening | ND | 47 (69.1) |
| Focal pleural effusion | 9.6 | 19 (27.9) |
| Mediastinal lymph node enlargement | 29.8 | 27 (39.7) |
| Size of parenchymal lesion, mean (cm, range) | ND | 3.9 (1.5–9.0) |
| Atelectasis | 28.7 | ND |
| Ground-glass opacity (14.9%) | 14.9 | ND |
CT, computed tomography; ND, no data.
Moreover, we also describe for 18F-FDG PET/CT findings of the pulmonary actinomycosis. PET/CT is useful for detecting malignancies, including distant metastases; however, various inflammatory and infectious diseases can also show increased FDG uptake (20,21). Therefore, careful interpretation of PET/CT findings of primary malignant lesions is needed. Only few authors have reported the appearance of pulmonary actinomycosis on 18F-FDG PET/CT (19). In general, PET/CT findings of actinomycosis include intense hypermetabolism that is similar to that of malignancy; in fact, the reported SUVmax of pulmonary actinomycosis was as high as 33.1 (22). For these reasons, pulmonary actinomycosis should be considered a differential diagnosis in cases with intensive uptake on 18F-FDG PET/CT. In this case, the 18F-FDG PET/CT findings were nonspecific, so we had to perform bronchoscopy biopsy in order to confirm the diagnosis.
In cases of suspected pulmonary actinomycosis, confirmation of the diagnosis requires bacterial evidence and characteristic pathologic findings. Actually we were not able to identify causable bacteria in the culture result, we performed TBLB for a diagnosis purpose and comprehensively diagnosed pulmonary actinomycosis from the pathological findings with various findings; such as the bacterial colonies, dense network of gram positive filaments, surrounded by zone of swollen, and radiating and club shaped structures.
Actinomyces is also difficult to culture, because it requires special conditions, such as culture medium or temperature. Gram’s stain of purulent sputum and pathologic examination of infected tissue are usually more sensitive than culture, which remains sterile in more than 50% of cases. Moreover, immunofluorescence techniques are highly specific but have poor sensitivity (2). With regard to flexible bronchoscopy, the formation of a thick inflammatory granulation around the pulmonary actinomycosis has usually made biopsies difficult to perform for an accurate diagnosis. Mabeza et al. showed that among a number of pulmonary actinomycosis cases reported in the preceding 2 decades, more than 50% were diagnosed through surgery and fewer than 20% were diagnosed using bronchoscopy (9). Moreover, the diagnosis requires a combination of several factors, including a positive culture and demonstration of sulphur granules on the infected tissue, correlation with the clinical and radiological features, and response to antibiotic treatment (23).
Mabeza et al. emphasized that the main principle of treatment was the use of high-dose intravenous penicillin for a long duration, specifically at 18–24 million units of penicillin per day for 2–6 weeks, followed by oral penicillin or amoxicillin for 6–12 months (9). In the present case, antibiotic treatment was given for 6 months, which is normally effective in actinomycosis. The hemoptysis however persisted due to inadequate treatment based on an initial diagnosis of bacterial infection.
BAE remains first-line, minimally invasive treatment for hemoptysis in emergency settings, surgically unfit patients, or patients with diffuse or bilateral lung disease. It is a safe and universally accepted procedure to control hemoptysis of varying etiologies, under both emergent and elective settings (24,25). In fact, in some reported cases of pulmonary actinomycosis (10), BAE or thoracotomy was needed to control persistent hemoptysis despite antibiotic treatment. Here, we introduced to the strategy and technique of BAE. At first, we identified signs of possible hemoptysis related artery (HRA) to use previously reported findings (26). These consisted of dilation of the vessels as compared with the normal size for that site, tortuosity of the vessels, direct shunting of the vessels, aneurysmal formation, pleural adhesion with intercostal artery invasion into the lungs and ground glass opacity, suggesting inhaled blood (26). The number and approach sites of the super selective BAE sessions were determined on the basis of the attending endobronchial findings and according to the possible HRAs suggested by the CT findings. We performed via a transfemoral approach with placing a long vascular sheath (25 cm). All possible HRAs identified with CT were super selectively evaluated using arteriography with 4 Fr guiding catheter and 2.8 Fr microcatheter systems with 0.014-inch guide wires during the session. When abnormal findings were observed, such as systemic-pulmonary shunts, the proliferation of the capillary vessels or extravasation of the contrast medium to the lung tissues, they were super selectively embolized using the 2.8 Fr microcatheter system. The embolic materials used in the gelatin sponge particles. We injected embolic materials into the HRA until the proliferation of peripheral blood vessels was disappeared in angiographic appearance.
In this case, the patient had persistent hemoptysis, so we decide to treat with BAE and successfully stopped the bleeding. Thereafter, we were able to confirm the diagnosis using bronchoscopy, and to then start appropriate treatment without the need for lung resection.
Conclusions
Although pulmonary actinomycosis is a rare condition compared to malignancy, early diagnosis using bronchoscopy might enable adequate treatment and avoid unnecessary surgery that is associated with delayed diagnosis.
Acknowledgements
We thank Dr. Teruaki Oka (Kanto Central Hospital) for the valuable comments and suggestions and Dr. Toru Igari (Laboratory Testing Department) for confirming to the diagnosis by the pathological findings.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: A summary of this paper was presented at the 630th Japan Internal Medical Association Kanto Regional Association (February 2017) and received an encouragement prize.
References
- 1.Smego RA, Jr, Foglia G. Actinomycosis. Clin Infect Dis 1998;26:1255-61. 10.1086/516337 [DOI] [PubMed] [Google Scholar]
- 2.Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist 2014;7:183-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu MS, Liu HP, Yeh CH, et al. The role of surgery in hemoptysis caused by thoracic actinomycosis; a forgotten disease. Eur J Cardiothorac Surg 2003;24:694-98. 10.1016/S1010-7940(03)00515-3 [DOI] [PubMed] [Google Scholar]
- 4.Brook I. Actinomycosis: diagnosis and management. South Med J 2008;101:1019-23. 10.1097/SMJ.0b013e3181864c1f [DOI] [PubMed] [Google Scholar]
- 5.Sakon M, Mikami K, Seki H, et al. A case report of pulmonary actinomycosis radiologically mimicking lung metastasis from rectal cancer. Nihon Rinsho Geka Gakkai Zasshi 2008;69:38-43. 10.3919/jjsa.69.38 [DOI] [Google Scholar]
- 6.Hamer DH, Schwab LE, Gray R. Massive hemoptysis from thoracic actinomycosis successfully treated by embolization. Chest 1992;101:1442-43. 10.1378/chest.101.5.1442 [DOI] [PubMed] [Google Scholar]
- 7.Yoon W, Kim JK, Kim YH, et al. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002;22:1395-409. 10.1148/rg.226015180 [DOI] [PubMed] [Google Scholar]
- 8.Colmegna I, Rodriguez-Barradas M, Young EJ, et al. Disseminated Actinomyces meyeri infection resembling lung cancer with brain metastases. Am J Med Sci 2003;326:152-5. 10.1097/00000441-200309000-00010 [DOI] [PubMed] [Google Scholar]
- 9.Mabeza GF, Macfarlane J. Pulmonary actinomycosis. Eur Respir J 2003;21:545-51. 10.1183/09031936.03.00089103 [DOI] [PubMed] [Google Scholar]
- 10.Kim SR, Jung LY, Oh IJ, et al. Pulmonary actinomycosis during the first decade of 21st century: cases of 94 patients. BMC Infect Dis 2013;13:216. 10.1186/1471-2334-13-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JY, Lee T, Lee H, et al. Multivariate analysis of prognostic factors in patients with pulmonary actinomycosis. BMC Infect Dis 2014;14:10. 10.1186/1471-2334-14-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M, Zhang XY, Chen YB. Primary pulmonary actinomycosis: a retrospective analysis of 145 cases in mainland China. Int J Tuberc Lung Dis 2017;21:825-31. 10.5588/ijtld.16.0773 [DOI] [PubMed] [Google Scholar]
- 13.Higashi Y, Nakamura S, Ashizawa N, et al. Pulmonary Actinomycosis Mimicking PulmonaryAspergilloma and a Brief Review of the Literature. Intern Med 2017;56:449-53. 10.2169/internalmedicine.56.7620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pneumonia Franquet E. Semin Roentgenol 2017;52:27-34. 10.1053/j.ro.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 15.Han JY, Lee KN, Lee JK, et al. An overview of thoracic actinomycosis: CT features. Insights Imaging 2013;4:245-52. 10.1007/s13244-012-0205-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peck KR, Kim TJ, Lee MA, et al. Pneumonia in immunocompromised patients: updates in clinical and imaging features. Precis Future Med 2018;2:95-108. 10.23838/pfm.2018.00121 [DOI] [Google Scholar]
- 17.Nagao M, Fukuda A, Matsumura T, et al. Pulmonary actinomycosis mimicking a lung metastasis from esophageal cancer; a case report. BMC Pulm Med 2018;18:39. 10.1186/s12890-018-0602-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheon JE, Im JG, Kim MY, et al. Thoracic actinomycosis: CT findings. Radiology 1998;209:229-33. 10.1148/radiology.209.1.9769836 [DOI] [PubMed] [Google Scholar]
- 19.Heo SH, Shin SS, Kim JW, et al. Imaging of actinomycosis in various organs: a comprehensive review. Radiographics 2014;34:19-33. 10.1148/rg.341135077 [DOI] [PubMed] [Google Scholar]
- 20.Andreani A, Rossi G, Giovannini M, et al. Unexpected positron emission tomography-positive Actinomyces-related mass of the bronchial stump. Can Respir J 2012;19:77-9. 10.1155/2012/502041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu L, Lan L, Feng Y, et al. Pulmonary Actinomycosis imitating lung cancer on 18F-FDG PET/CT: a case report and literature review. Korean J Radiol 2015;16:1262-65. 10.3348/kjr.2015.16.6.1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoekstra CJ, Hoekstra OS, Teengs JP, et al. Thoracic actinomycosis imaging with fluorine-18 fluorodeoxyglucose positron emission tomography. Clin Nucl Med 1999;24:529-30. 10.1097/00003072-199907000-00016 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura S, Kusunose M, Satou A, Senda K, Hasegawa Y, Nishimura K. A case of pulmonary actinomycosis diagnosed by transbronchial lung biopsy. Respir Med Case Rep 2017;21:118-20. 10.1016/j.rmcr.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol 2017;23:307-17. 10.5152/dir.2017.16454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radchenko C, Alraiyes AH, Shojaee S. A systematic approach to the management of massive hemoptysis. J Thorac Dis 2017;9:S1069-86. 10.21037/jtd.2017.06.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa H, Hara M, Ryuge M, et al. Efficacy and safety of super selective bronchial artery coil embolisation for haemoptysis: a single-centre retrospective observational study. BMJ Open 2017;7:e014805. 10.1136/bmjopen-2016-014805 [DOI] [PMC free article] [PubMed] [Google Scholar]