Abstract
Background
Transcatheter closure for atrial septal defect (ASD) with inferior-posterior rim deficiency has been scarcely reported with proper identification of the indications and limits. We aimed to assess the safety and feasibility of transcatheter closure of ASDs with deficient rims, paying particular attention to cases with inferior-posterior rim deficiency.
Methods
From January 2008 to January 2013, 241 patients underwent transcatheter ASD closure, including 50 cases (20.7%) with deficient rims, other than the anterior-superior one. Eighteen patients (12 females) presented inferior-posterior rim deficiency. Their median age was 8 (1.4–85) years and their median weight was 24 [9–97] kg. Transcatheter closure was performed in all cases under transesophageal echocardiography (TEE) guidance in children and intracardiac echocardiography (ICE) guidance in adults.
Results
Out of 18 patients with inferior-posterior rim deficiency, only 8 underwent successful immediate transcatheter closure. Four cases failed to be closed. Major complications occurred in 6 patients, including 4 device embolizations, 1 pericardial effusion and 1 complete atrioventricular block that resolved after surgical removal of the device. During a median follow up of 54±13 months, a residual right-to-left shunt was documented in 2 more cases, requiring surgery in one case because of cyanosis. Transcatheter closure was successfully performed in the rest of the 223 patients, including in the 32 cases with deficient rims other than inferior-posterior.
Conclusions
Transcatheter closure of ASDs with inferior-posterior rim deficiency cannot be recommended.
Keywords: Closure, heart septal defect, atrial, congenital heart disease, adults, pediatrics
Introduction
Transcatheter closure has become an accepted alternative to surgical repair for most types of atrial septal defect (ASD) (1,2). The technique is commonly used in patients with a defect inferior to 38 mm in diameter without deficient rims, and allows safe and effective ASD closure in nearly 80% of unselected cases (3). Complex ASDs, which are defined as defects above 38 mm in diameter and/or the presence of deficient rims other than anterior superior, represent approximately 20% of patients with ASD (3,4).
Recently, several studies described transcatheter ASD closure for complex cases, using a variety of modified implantation methods (4-12). However, the authors mostly emphasized that transcatheter closure in complex ASDs was usually feasible in experienced hands, but did not clarify which types of defect were unsuitable for transcatheter closure. In their single center study, Ohno et al. reported that patients with a ratio ASD diameter over weight above 1.5 and deficient rims were not eligible for transcatheter ASD closure (13). However, the authors did not analyze complex transcatheter ASD closure procedures and the operator bias was not controlled. In many cases, patients are directly referred to surgery for ASD closure because of physician’s experience or family preference. Consequently, the types of ASDs to be considered as unsuitable for transcatheter closure remain unclear.
In previous studies, we demonstrated the feasibility of transcatheter closure in complex ASDs under transesophageal echocardiography (TEE) and intracardiac echocardiography (ICE) guidance (4,9). Moreover, all types of complex ASDs referred for transcatheter closure to our institution in the past decade have been systematically collected between 2008 and 2013. Therefore, from this cohort, we aimed to determine which ASD characteristics should contraindicate transcatheter closure.
Methods
Patient population
The study population consisted of 241 consecutive patients referred to our institution for ASD closure over a five-year period (January 1st, 2008 to January 1st, 2013). In this population patients with deficient rims were screened to further analyze cases with inferior-posterior rim deficiency. Patients’ data were collected during the study period. This observational retrospective study was approved by our institutional ethics committee.
The patient population comprises cases with a hemodynamically significant ASD (right ventricle overload and left-to-right shunt with Qp: Qs ratio >1.5), including patients with heart failure and arrhythmia (14,15). In addition, transcatheter closure was proposed for patients with ASDs presenting with a stroke or showing any evidence of paradoxical embolization, regardless the hemodynamic significance. All patients with a patent foramen ovale (PFO) were excluded from the study.
Pre-catheter procedure protocol
All patients had a physical examination, a standard 12-lead electrocardiogram (ECG), and a transthoracic echocardiography (TTE). TEE was only performed prior to the intervention in the adult population. TTE and TEE included multiple views to assess the ASD number, position, diameter, rim adequacy and relation with adjacent cardiac structures. The rim was classified as either adequate (≥5 mm) or inadequate in case of deficiency (<5 mm) around the ASD for the following structures: coronary sinus rim, inferior-posterior rim (towards the inferior vena cava), inferior rim (towards the atrioventricular valves), posterior rim (towards the pulmonary veins) and superior-posterior rim (towards the superior vena cava) (Figure 1) (3). As retro-aortic rim deficiency is well known to not influence the success rate for transcatheter ASD closure (3,4,16), such patients were not considered as complex cases in this study. All complex cases were discussed in our joint cardiac surgical conference.
Figure 1.
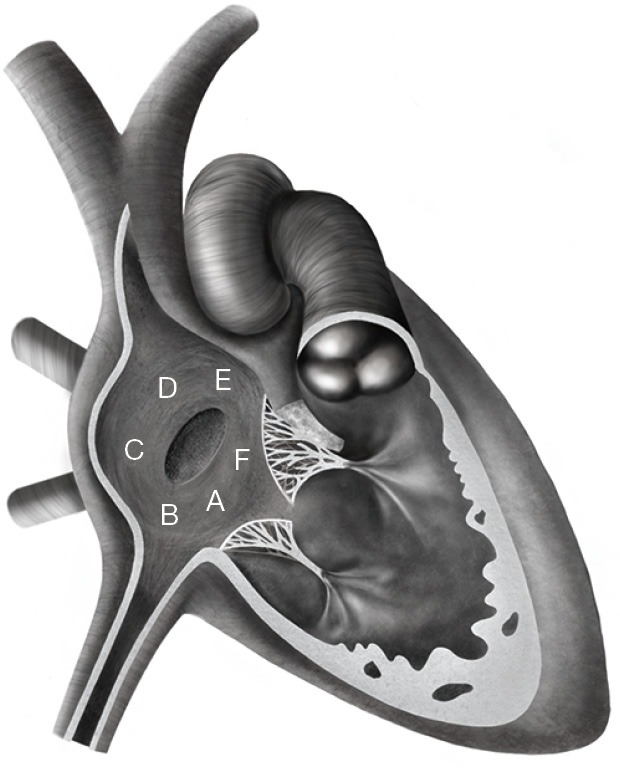
Relation of ASD to surrounding rims and structures. A, coronary sinus; B, inferior-posterior rim (towards the inferior vena cava); C, posterior rim; D, superior-posterior rim (towards the superior vena cava); E, anterior-superior (or retro-aortic) rim; F, inferior rim (towards the tricuspid valve). ASD, atrial septal defect.
Transcatheter ASD closure procedure
We used our previously reported device closure protocol (4). In children, transcatheter closure was performed using venous access through the femoral vein under general anesthesia and TEE guidance. In adults, procedures were performed under local anesthesia and ICE guidance (Acuson Corporation, Siemens, Mountain View, CA, USA), using the 8-French probe through the contralateral femoral vein access (9,17,18). All patients received heparin (100 units/kg IV, maximum: 5,000 units) and antibiotic prophylaxis (cefamandole, 50 mg/kg). All the patients underwent right heart catheterization as a first step of the interventional procedure.
The Amplatzer Septal Occluder (ASO) was used for single or adjacent multiple defects, the Amplatzer Cribriform for distant multiple defects (AGA Medical Corporation, Golden Valley, MN, USA). A Cardia Occluder device (Eagan, MN, USA) was used in 2 cases. For small defects (largest measured diameter on Color-Doppler flow <20 mm by TEE or ICE), the occluding device was 2 to 4 mm larger than the largest measured diameter (19). Balloon sizing of the defect was performed for large (≥20 mm) or complex defects, with the Meditech balloon (Meditech, Boston Scientific, Watertown, MA, USA). When the initial attempt of implantation using standard method failed, a modified implantation technique involving the sizing balloon was employed, as previously described (4).
Patients were considered to have had successful ASD closure if color Doppler flow imaging found no significant residual shunt.
Follow-up
Before discharge, all patients underwent a TTE examination the day after ASD closure, to verify the position of the occluding device, and the absence of thrombus, pericardial effusion, or valvular lesion. Any complications during and after the intervention were recorded.
Patients were subsequently evaluated by clinical examination, ECG and TTE at 1 week, 1 month and 6 months after the procedure. Any complications related to the device implantation were noted at each visit.
Medical treatment
For a 6 month-period after catheter procedure, patients received oral daily anti-platelet therapy (acetylsalicylic acid, 3.5 mg/kg; maximum: 250 mg in adult patients) and, when necessary, antibiotic prophylaxis of endocarditis (20). Patients with pre-existing anticoagulation therapy were maintained under the same medication, without any additional anti-platelet treatment.
Statistical analyses
Data were expressed as frequency or percentage for the nominal variables and as mean ± standard deviation (SD) for continuous variables. When their distribution was not Gaussian, quantitative variables were described with median and extreme values.
Results
Patient population
During the 5-year study period, 241 patients underwent transcatheter closure of an ASD. Fifty patients (21%) had one or several deficient rims, other than the anterior-superior one. Inferior-posterior rim deficiency was found in 18 patients, including 12 females and 6 males, with a median age of 8 (1.4–85) years and median weight of 24 [9–97] kg. Five patients had inferior-posterior rim deficiency associated with superior-posterior (N=3) or inferior (N=2) rim deficiencies. In patients with inferior-posterior rim deficiency, the median ASD diameter was 20 [17–40] mm, among which three patients had an ASD diameter above 38 mm (Table 1). The mean overall follows up was 49±10 months.
Table 1. Characteristics of patients with complex ASD and inferior-posterior rim deficiency.
| Patient | Age (years) | Weight (kg) | Rim deficiency | ASD index size (mm/m2) | ASD size (mm) | Device size (mm) | Imaging guidance | Success | Complications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 85 | 60 | IP | 16 | 26 | 28 | ICE | Yes | None |
| 2 | 1.4 | 9 | IP | 35 | 17 | 18 | TEE | Yes | None |
| 3 | 67 | 97 | IP | 15 | 30 | 32 | ICE | Yes | None |
| 4 | 10 | 37 | IP; SP | 24.4 | 30 | 32 | TEE | Yes | Device embolization |
| 5 | 12 | 24 | IP | 26.5 | 26 | 30 | TEE | Yes | Device embolization |
| 6 | 16 | 33 | IP | 22.5 | 30 | 32 | TEE | No | None |
| 7 | 32 | 50 | IP; SP | 27.2 | 40 | 40 | ICE | Yes | Pericardial effusion |
| 8 | 6 | 20 | IP | 35.7 | 25 | 30 | TEE | Yes | Device embolization |
| 9 | 55 | 51 | IP | 26.5 | 40 | 40 | ICE | Yes | None |
| 10 | 6 | 14 | IP | 33 | 21 | 22 | TEE | Yes | Right-to-left shunt |
| 11 | 3 | 14.5 | IP | 32 | 21 | 22 | TEE | Yes | None |
| 12 | 6 | 24 | IP; SP | 22 | 20 | 22 | TEE | Yes | Device embolization |
| 13 | 3 | 13 | IP | 32 | 18 | 20 | TEE | No | None |
| 14 | 6 | 17.5 | IP; I | 23 | 17 | 18 | TEE | Yes | Right-to-left shunt |
| 15 | 6 | 24 | IP | 28 | 30 | 28 | TEE | No | None |
| 16 | 8 | 20 | IP | 29 | 24 | 26 | TEE | No | None |
| 17 | 17.7 | 95 | IP; I | 20.5 | 39 | 40 | TEE | Yes | None |
| 18 | 1.6 | 9.2 | IP | 51 | 22 | 20 | TEE | Yes | Complete atrioventricular block |
ASD, atrial septal defect; ICE, intracardiac echocardiography; TEE, transesophageal echocardiography; IP, inferior-posterior rim; I, inferior rim; SP, superior-posterior rim.
Transcatheter closure in cases without inferior-posterior rim deficiency
All 223 patients without any inferior-posterior rim deficiency underwent successful ASD transcatheter closure. No major complication occurred in this group: mild pericardial effusion in 3 cases and groin hematoma in 5 cases. In greater detail, all patients with deficient rims other than the inferior-posterior one underwent successful transcatheter ASD closure. Transcatheter closure was performed with ASO in all cases with deficient rims and in most of the other patients. An Amplatzer Cribriform device was used in 9 cases with multiple defects.
Results of transcatheter closure in cases with inferior posterior rim deficiency
In this group of 18 patients, 13 were under 18 years old, 5 weighed less than 15 kg, 5 had more than one deficient rim, 4 had reversible pulmonary arterial hypertension (mean pulmonary arterial pressure >25 mmHg, Qp: Qs ratio >2 and low pulmonary vascular resistance <4 WU·m2), and 6 had an ASD index over 30 mm/m2 (Table 1).
The technical success rate for transcatheter closure during the procedure was 78% (14 out of 18) in patients with inferior-posterior rim deficiency. Four patients could not have their defect closed despite the use of multiple modified implantation methods. The median size of ASO devices was 28 [18–40] mm. A 40 mm ASO was used for the 3 patients with an ASD diameter above 38 mm. The modified sizing balloon assisted technique was used in all cases. Oversizing of ASO, defined as over four millimeters, was present in 2 patients.
As regards early complications in the inferior-posterior rim deficiency group, 4 patients experienced device embolization, in the right ventricle (N=3) or the left atrium (N=1). Embolization in the right ventricle occurred within 2 to 4 hours after catheter procedure and was associated with ventricular ectopics and non-sustained ventricular tachycardia (Figure 2). Embolization in the left atrium was diagnosed 24 hours after catheter procedure during TTE in an asymptomatic patient. Device embolization was well tolerated in all cases. Surgical removal of the device and ASD closure under cardiopulmonary bypass were successfully performed in the 4 patients. The inferior-posterior rim deficiency was confirmed in the 4 cases (Figure 3). One 9 kg-child with inferior-posterior rim deficiency experienced a complete atrioventricular block three hours after implantation of a 20-mm device. The device was surgically removed the following day and the patient recovered normal sinus rhythm. Finally, one adult patient with inferior-posterior rim deficiency and ASD closure with a 40 mm ASO experienced mild pericardial effusion that spontaneously resolved without requiring any intervention or medication.
Figure 2.
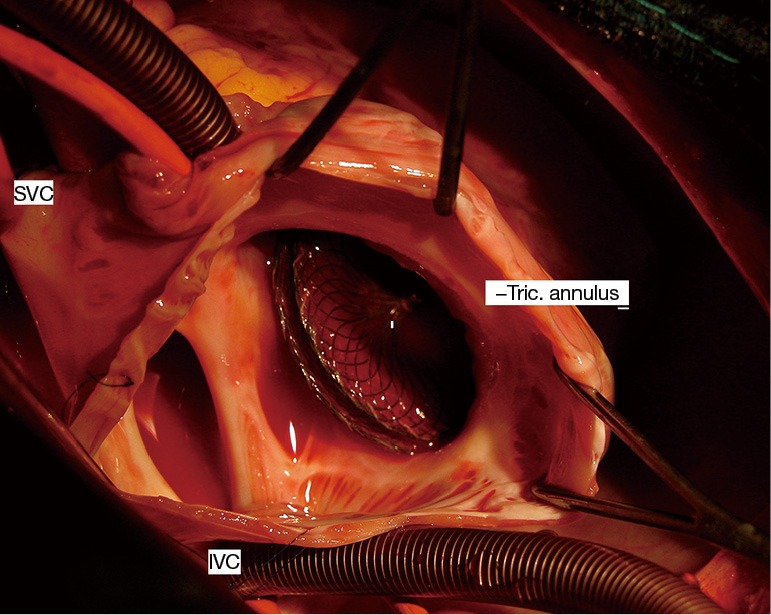
Intra-operative view of Amplatzer device embolization in the right ventricle. IVC, inferior vena cava; SVC, superior vena cava; Tric. annulus, tricuspid annulus.
Figure 3.
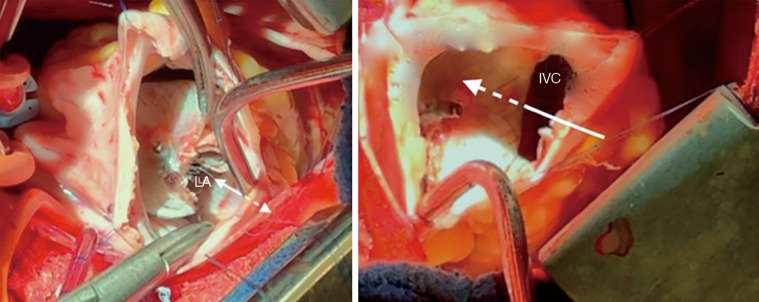
Intra-operative view from the right atrium in the patient who experienced cyanosis during exercise because of a device straddling over the inferior vena cava. In this view from the right atrium we can see that part of the inferior vena cava is not seen, being “redirected” towards the left atrium by the device (arrows). LA, left atrium; IVC, inferior vena cava.
During a mean follow up was 54±13 months in this group of 18 patients with inferior-posterior rim deficiency, right-to-left shunt was diagnosed in 2 cases. In one, cyanosis was documented during cardiopulmonary exercise test performed 3 years after transcatheter procedure. A cardiac CT-scan demonstrated straddling of the inferior vena cava by the device with a residual defect between the inferior border of the device and the inferior vena cava. Blood of the inferior vena cava was then redirected towards the left atrium, causing clinically significant cyanosis during exercise (Figure 4). The patient underwent surgery under right thoracotomy. The position of the device over the inferior vena cava, as well as the residual defect, were confirmed during intraoperative examination. The device was explanted and the residual defect was closed with a pericardial patch. Postoperative course was uncomplicated. In the remaining patient with right-to-left shunt, this was suspected during follow-up echocardiography 3 years after transcatheter closure and confirmed by contrast TTE. Surgery has been decided and is pending. All the other patients are doing well with no residual shunt.
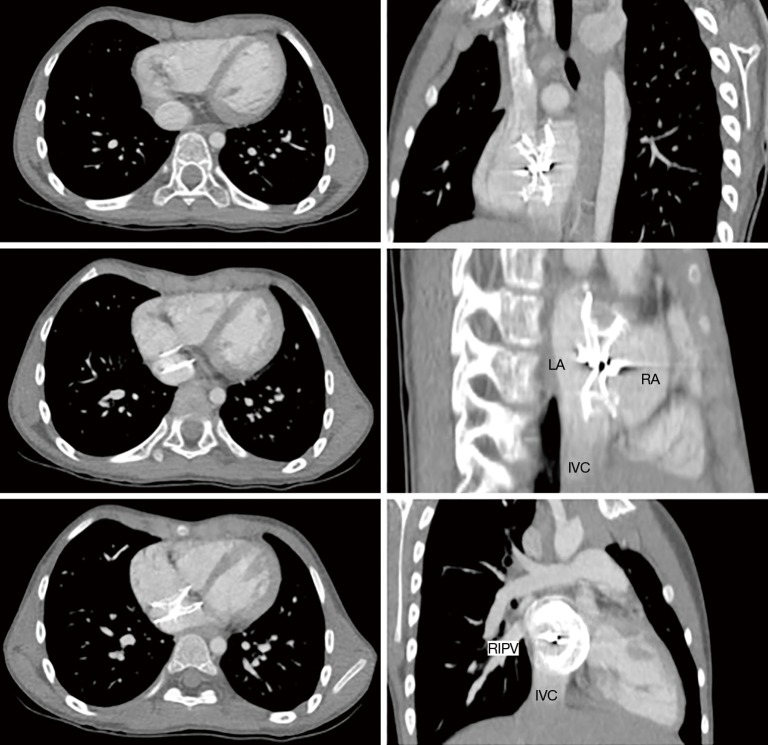
Figure 4.
Cyanosis at exercise after transcatheter ASD closure. CT-scan showing device straddling over the inferior vena cava, with part of cava venous blood redirected to the left atrium. LA, left atrium; RA, right atrium; IVC, inferior vena cava, RIPV, right inferior pulmonary vein; ASD, atrial septal defect.
Discussion
Our “aggressive” strategy to attempt transcatheter closure in all types of ASDs regardless the size of the defect and rim’s deficiency (4,9,21) resulted in a significant number of failures or complications in cases with inferior-posterior rim deficiency. Out of 18 patients with deficient inferior-posterior rim only 50% had eventually their defect successfully closed, whereas we experienced 4 failures, 4 embolizations and 1 complete atrioventricular block necessitating surgical removal after 24 hours. Moreover, right-to-left shunt was diagnosed in 2 additional patients during follow-up, which brings the overall success rate down to only 44%. Interestingly, iatrogenic diversion of the inferior vena cava to the left atrium has also been reported after surgical ASD closure (22). Conversely, all the remaining 223 cases of our study population, with or without rim deficiency, underwent successful transcatheter closure with no major complication.
Many studies reported the safety and feasibility of transcatheter ASD closure in non-complex cases (3,23). In complex cases with deficient rims, few studies have demonstrated the feasibility of transcatheter closure. In those cases, modified implantation methods have been described to optimize implantation of the device (5,6). Although the majority of patients had anterior-superior rim deficiency in the reported cohorts, a recent study pointed out the feasibility of transcatheter closure in patients with posterior rim deficiency, using modified implantation techniques (7). However, transcatheter closure is almost always possible in the latest (24), whereas in the present study our results do not support transcatheter closure in cases with deficiency.
Large ASDs with deficient posterior-inferior rim were previously considered for transcatheter closure in small cohorts. Device closure seems feasible in a limited number of cases (5,24,25). Varma et al. reported deficient inferior rim as a possible explanation for unsuccessful transcatheter closure (26). Mathewson et al. defined the absence of an inferior-posterior rim as a rim <3 mm. As the difference in radius length between right and left ASO atrial disks is 2–3 mm, a rim <3 mm will not allow both disks to hang on both sides of the rim. Although device implantation is possible in such cases, Mathewson et al. suggested that, these defects were at risk for complications, such as pulmonary vein or inferior vena caval obstruction, encroachment onto the anterior leaflet of the mitral valve or embolization (25). Such statements are limited because they rely on the 2-dimensional echocardiographic assessment of three-dimensional structures. Finally, the more recent self-expandable nitinol device Figulla Flex Occluder (Occlutech GmbH, Jena, Germany) with a lower and more flexible left-sided profile may provide better alignment with the atrial septum and decrease the need for modified implantation techniques in cases with deficient rim (27). However, no comparison between the 2 devices has been reported yet for closure of ASD with rim deficiencies.
In their prospective study on more than 1,000 patients referred for ASD closure, Butera et al. reported a 3.3% rate of patients diagnosed with inferior-posterior rim deficiency, and considered it as a contraindication for ASD percutaneous closure (3). By using immediate and late complications after ASD percutaneous closure as a primary endpoint, our retrospective study reinforces this statement. Based on our results, transcatheter closure cannot be recommended in patients with inferior-posterior rim deficiency, whereas this seems suitable in most of other cases. Importantly there is often confusion between the posterior and the inferior-posterior rim. The latest is difficult to diagnose and especially is not well seen with TEE guidance, which was the imaging modality guidance in the majority of our cases. In most cases, inferior-posterior rim is only seen on a short axis TTE sub-costal view, by following the inferior vena cava towards the right atrium. We did not use intracardiac echocardiography in children, but this might be helpful especially when performing the balloon sizing test: if the rim is truly deficient or really floppy after very gentle inflation (e.g., producing no pressure inside the balloon), there will be no waist over the sizing balloon. When inferior-posterior rim is absent, the risk for embolization is major because even if it is initially well positioned the device will tend to slip towards the inferior vena cava. The connection of the IVC into the right atrium is a very large, funnel-shaped and compliant structure that will not prevent slipping of the device. Finally, these types of ASD with inferior-posterior rim deficiency may be classified as inferior vena cava type sinus venosus defects rather than ostium secundum defects (28).
Study limitations
Our results are limited by the retrospective study design, and the small sample size from a single center experience. However, no focus on transcatheter ASD closure in inferior-posterior rim deficiency had been previously reported. Moreover, we did not assess partial or complete inferior-posterior rim deficiency. However, only 3D assessment of the rims would be accurate and this is not possible retrospectively as 3D echocardiography is not yet used in routine practice in all cases.
Conclusions
Transcatheter closure of secundum ASDs is safe and effective, except for patients with inferior-posterior rim deficiency. In the present study, nearly two thirds of the patients with inferior-posterior rim deficiency experienced failure or complications, such as device embolization, complete atrioventricular block, and right-to-left shunt. All patients with other types of rim deficiencies underwent successful transcatheter closure. Based on our experience, patients with ASDs associated with inferior-posterior rim deficiency should rather be considered for surgical closure.
Acknowledgements
We thank Sebastien Mounier, graphic designer, for his work on the Figure 1.
Ethical Statement: This observational retrospective study was approved by our institutional ethics committee (number 2018-IRB-MTP-05-04).
Footnotes
Conflicts of Interest: A Fraisse is consultant and proctor for Abbott (Amplatzer products) and for Occlutech. The other authors have no conflicts of interest to declare.
References
- 1.Du ZD, Hijazi ZM, Kleinman CS, et al. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol 2002;39:1836-44. 10.1016/S0735-1097(02)01862-4 [DOI] [PubMed] [Google Scholar]
- 2.Vida VL, Berggren H, Brawn WJ, et al. Risk of surgery for congenital heart disease in the adult: a multicentered European study. Ann Thorac Surg 2007;83:161-8. 10.1016/j.athoracsur.2006.07.045 [DOI] [PubMed] [Google Scholar]
- 3.Butera G, Romagnoli E, Carminati M, et al. Treatment of isolated secundum atrial septal defects: impact of age and defect morphology in 1,013 consecutive patients. Am Heart J 2008;156:706-12. 10.1016/j.ahj.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 4.Kammache I, Mancini J, Ovaert C, et al. Feasibility of transcatheter closure in unselected patients with secundum atrial septal defect, using Amplatzer devices and a modified sizing balloon technique. Catheter Cardiovasc Interv 2011;78:665-74. 10.1002/ccd.23077 [DOI] [PubMed] [Google Scholar]
- 5.Du ZD, Koenig P, Cao QL, et al. Comparison of transcatheter closure of secundum atrial septal defect using the Amplatzer septal occluder associated with deficient versus sufficient rims. Am J Cardiol 2002;90:865-9. 10.1016/S0002-9149(02)02709-1 [DOI] [PubMed] [Google Scholar]
- 6.Thanopoulos BD, Dardas P, Ninios V, et al. Transcatheter closure of large atrial septal defects with deficient aortic or posterior rims using the "Greek maneuver". A multicenter study. Int J Cardiol 2013;168:3643-6. 10.1016/j.ijcard.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 7.Papa M, Gaspardone A, Fragasso G, et al. Feasibility and safety of transcatheter closure of atrial septal defects with deficient posterior rim. Catheter Cardiovasc Interv 2013;81:1180-7. 10.1002/ccd.24633 [DOI] [PubMed] [Google Scholar]
- 8.Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm Amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv 2005;66:580-4. 10.1002/ccd.20468 [DOI] [PubMed] [Google Scholar]
- 9.Assaidi A, Sumian M, Mauri L, et al. Transcatheter closure of complex atrial septal defects is efficient under intracardiac echocardiographic guidance. Arch Cardiovasc Dis 2014;107:646-53. 10.1016/j.acvd.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 10.Baruteau AE, Petit J, Lambert V, et al. Transcatheter closure of large atrial septal defects: feasibility and safety in a large adult and pediatric population. Circ Cardiovasc Interv 2014;7:837-43. 10.1161/CIRCINTERVENTIONS.113.001254 [DOI] [PubMed] [Google Scholar]
- 11.Romanelli G, Harper RW, Mottram PM. Transcatheter closure of secundum atrial septal defects: results in patients with large and extreme defects. Heart Lung Circ 2014;23:127-31. 10.1016/j.hlc.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 12.Pillai AA, Rangaswamy Balasubramanian V, Selvaraj R, et al. Utility of balloon assisted technique in trans catheter closure of very large (>/=35 mm) atrial septal defects. Cardiovasc Diagn Ther 2014;4:21-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohno N, Chaturvedi R, Lee KJ, et al. Characteristics of secundum atrial septal defects not percutaneously closed. Catheter Cardiovasc Interv 2015;85:234-9. 10.1002/ccd.25700 [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. 10.1093/eurheartj/ehq249 [DOI] [PubMed] [Google Scholar]
- 15.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e143-263. 10.1016/j.jacc.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 16.O’Byrne ML, Glatz AC, Sunderji S, et al. Prevalence of deficient retro-aortic rim and its effects on outcomes in device closure of atrial septal defects. Pediatr Cardiol 2014;35:1181-90. 10.1007/s00246-014-0914-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim NK, Park SJ, Shin JI, et al. Eight-French intracardiac echocardiography - safe and effective guidance for transcatheter closure in atrial septal defects. Circ J 2012;76:2119-23. 10.1253/circj.CJ-11-1286 [DOI] [PubMed] [Google Scholar]
- 18.Alboliras ET, Hijazi ZM. Comparison of costs of intracardiac echocardiography and transesophageal echocardiography in monitoring percutaneous device closure of atrial septal defect in children and adults. Am J Cardiol 2004;94:690-2. 10.1016/j.amjcard.2004.05.048 [DOI] [PubMed] [Google Scholar]
- 19.Hijazi ZM. Device closure of secundum atrial septal defects: To balloon size or not to balloon size. Ann Pediatr Cardiol 2011;4:34-5. [PMC free article] [PubMed] [Google Scholar]
- 20.Amedro P, Soulatges C, Fraisse A. Infective endocarditis after device closure of atrial septal defects: Case report and review of the literature. Catheter Cardiovasc Interv 2017;89:324-34. 10.1002/ccd.26784 [DOI] [PubMed] [Google Scholar]
- 21.Fraisse A, Trivedi KR. Transcatheter closure of atrial septal defects: how large is too large? Cardiovasc Diagn Ther 2014;4:213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain SA, Pinto R, Dalvi B. Iatrogenic diversion of IVC to left atrium after surgical closure of ASD. Ann Pediatr Cardiol 2012;5:72-4. 10.4103/0974-2069.93716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masura J, Gavora P, Formanek A, et al. Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn 1997;42:388-93. [DOI] [PubMed] [Google Scholar]
- 24.Pedra CA, Pedra SR, Esteves CA, et al. Transcatheter closure of secundum atrial septal defects with complex anatomy. J Invasive Cardiol 2004;16:117-22. [PubMed] [Google Scholar]
- 25.Mathewson JW, Bichell D, Rothman A, et al. Absent posteroinferior and anterosuperior atrial septal defect rims: Factors affecting nonsurgical closure of large secundum defects using the Amplatzer occluder. J Am Soc Echocardiogr 2004;17:62-9. 10.1016/j.echo.2003.09.018 [DOI] [PubMed] [Google Scholar]
- 26.Varma C, Benson LN, Silversides C, et al. Outcomes and alternative techniques for device closure of the large secundum atrial septal defect. Catheter Cardiovasc Interv 2004;61:131-9. 10.1002/ccd.10700 [DOI] [PubMed] [Google Scholar]
- 27.Haas NA, Soetemann DB, Ates I, et al. Closure of Secundum Atrial Septal Defects by Using the Occlutech Occluder Devices in More Than 1300 Patients: The IRFACODE Project: A Retrospective Case Series. Catheter Cardiovasc Interv 2016;88:571-81. 10.1002/ccd.26497 [DOI] [PubMed] [Google Scholar]
- 28.Snarr BS, Liu MY, Zuckerberg JC, et al. The Parasternal Short-Axis View Improves Diagnostic Accuracy for Inferior Sinus Venosus Type of Atrial Septal Defects by Transthoracic Echocardiography. J Am Soc Echocardiogr 2017;30:209-15. 10.1016/j.echo.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]