Abstract
Blood flow between the aorta and atrium is a rare but complex pathological condition, also known as aorto-atrial fistula (AAF). The exact incidence of this condition is unknown, as are the major precipitating factors and best treatment options. We carried out a systematic review of the available case report literature reporting AAF. We systematically reviewed literature on AAF formation and closure. Separate Medline (PubMed), EMBASE, and Cochrane database queries were performed. The following MESH headings were used: atrium, ventricle, fistula, cardiac, shunts, aortic, aorto-atrial tunnels and coronary cameral fistula. All papers were considered for analysis irrespective of their quality, or the journal in which they were published. Fistula formation from the ascending aorta to the atria occurred more often in the right atrium compared to the left. Endocarditis was the major cause of AAF formation, whilst congenital causes were responsible for nearly 12%. In a number of cases fistula formation occurred secondary to cardiac surgery, whilst chest traumas were a relatively rare cause of AAF. Correction via an open surgical approach occurred in 73.5% of cases, whilst percutaneous intervention was utilised in 10% of patients. In 74.3% of all studied cases the fistula repair was successful and patients survived the procedures. In 14.7% of the cases patients did not survive. Similar outcomes were observed between percutaneous and surgical interventions. Data from larger populations with AAF is lacking, meaning that specific data regarding incidence and prevalence does currently not exist.
Keywords: Aorta, fistula, atria
Introduction
The presence of blood flow between the aorta and atrium is a rare but complex pathological condition, also known as aorto-atrial fistula (AAF). Systemic symptoms such as heart failure, weakness and oedema may occur as well as more local symptoms including dyspnoea, chest pain, palpitations, and fatigue or coughing.
The exact incidence of AAF is currently unknown and there are many gaps in our knowledge regarding various aspects of AAF, such as diagnosis strategies and management options. The aim of the present study was to systematically review the available case report literature reporting AAF. These data will provide an overview of the demographic characteristics of AAF, the pre-operative imaging techniques used to diagnose AAF, the anatomy and causes of AAF and finally give some information on the therapeutic options for AAF and the respective outcomes. These data should enable surgeons to prevent this condition occurring initially, as well as treating it optimally when it does occur.
Evidence acquisition
We systematically reviewed literature on AAF formation and closure. Separate Medline (PubMed), EMBASE, and Cochrane database queries were performed. The following MESH headings were used: atrium, ventricle, fistula, cardiac, shunts, aortic, aorto-atrial tunnels and coronary cameral fistula. All papers were considered for analysis irrespective of their quality, or the journal in which they were published. Strict criteria and screening of titles and abstracts were used to select relevant papers. All papers and reports on AAF formation and closure were included. Reports not written in English were excluded, as well as reports without a clear description of AAF or AAF closure. No randomized controlled trials or clinical studies were identified. We evaluated the remaining case reports. We identified 132 case reports with a total of 136 patients for our analysis (Figure 1). In each of these reports we extracted the following information: year of publication, number of patients, age, sex, previous cardiac surgery, time between surgery and AAF formation, fistula tract, presumed AAF cause, surgical technique for AAF closure, follow-up and outcome (Table 1).
Figure 1.
Schematic of Study Selection.
Table 1. List of all analyzed articles.
| Reference | Year published | Sex/age (years) | Previous surgery | Time between surgery and AAF | Type of AAF | Cause of AAF | Closure technique | Follow-up/outcome |
|---|---|---|---|---|---|---|---|---|
| RB Hsu | 2000 | M/67 | None | N/A | AscAo-RA | Endocarditis | Surgical | Lived |
| W Dewilde | 2008 | M/51 | AVR + MVR | 4 years | AscAo-LA | Prosthetic valve endocarditis | Conservative medical treatment | Lived |
| D Patsouras | 2002 | M/70 | AVR 2x | 7 years; 1 month | AscAo-LA | Aortic dissection | Conservative medical treatment | Died |
| CS Balestrini | 2013 | M/57 | CABG | 12 years | AscAo-RA | SVG aneurysm | Percutaneous | Lived |
| DA Chung | 2000 | M/52 | Aortic root replacement + CABG | 17 years | AscAo-RA | Aortic dissection | Surgical | Lived |
| F Haddad | 2008 | M/66 | AVR + ascending aorta replacement | 10 days | AscAo-RA | Aortic dissection (Giant cell arthritis) | Surgical | Lived |
| SS Dhawan | 2008 | M/65 | AVR | 10 weeks | AscAo-LA | Prosthetic valve endocarditis | Patient refused surgery | Not described |
| TP Archer | 1997 | M/61 | None | N/A | AscAo-LA | Endocarditis | Patient died before surgery | Died |
| BN Shah | 2012 | F/54 | AVR | 8 years | AscAo-LA | Complication of cardiac surgery | Surgical | Not described |
| K Suzuki | 2006 | F/77 | Aortic arch replacement | 8 years | AscAo-RA | Complication of cardiac surgery | Surgical | Lived |
| S Cheng Siang | 1967 | M/55 | None | N/A | AscAo-LA | Aneurysm | Conservative medical treatment | Died |
| O Candan | 2012 | F/55 | MVR 2x, mitral valve repair | 4 years; 3 years;1 month | AscAo-RA | Prosthetic valve endocarditis | Surgical | Died |
| Y Sakano | 2007 | F/70 | Ascending aorta replacement | 16 years | AscAo-RA | Complication of cardiac surgery | Surgical | Lived |
| S Bouchez | 2012 | M/61 | None | N/A | AscAo-LA | Accidental finding | No procedure performed | Lived |
| K Ananthasubramaniam | 2005 | M/66 | AVR 2x | Not described; 4 years | AscAo-LA | Complication of cardiac surgery | Surgical | Lived |
| PR Menon | 2011 | F/73 | Mitral valve repair + tricuspid valve repair | 1 year | AscAo-RA | Complication of cardiac surgery | Surgical | Lived |
| S Pagni | 2013 | F/69 | CABG | 8 years | AscAo-RA | Aortic dissection | Surgical | Lived |
| R Estévez-Loureiro | 2012 | M/44 | AVR | 9 years | AscAo-LA | Prosthetic valve endocarditis | Percutaneous | Not described |
| J Swampillai | 2012 | F/16 | None | N/A | AscAo-RA | Endocarditis | Surgical | Not described |
| R Dalla Pozza | 2009 | F/72 | Aortic valve repair | <1 week | AscAo-LA + AscAo-RA | Complication of cardiac surgery | Percutaneous | Lived |
| MSL Sey | 2010 | M/20 | Percutaneous closure of ASD | 3 weeks | AscAo-RA | ASD device closure | Surgical | Lived |
| Y Cho | 2005 | F/61 | None | N/A | AscAo-LA | Endocarditis | Surgical | Lived |
| M Sreedharan | 2006 | M/11 | None | N/A | AscAo-RA | Congenital | Percutaneous | Lived |
| N Ozer | 2007 | F/41 | AVR | 13 years | AscAo-RA | Prosthetic valve endocarditis | Patient died before surgery | Died |
| MM Stechert | 2007 | M/65 | AVR | 6 months | AscAo-LA | Prosthetic valve endocarditis | Surgical | Lived |
| C Russo | 2001 | F/70 | Ascending aorta replacement | 6 years | AscAo-RA | Aortic dissection | Surgical | Lived |
| A Melua | 1998 | M/30 | None | N/A | AscAo-RA | Behcet’s disease | Surgical | Lived |
| WM Wilson | 2010 | M/17 | Percutaneous closure of ASD | 3 months | AscAo-LA | ASD device closure | Surgical | Lived |
| A Kalra | 2013 | F/61 | Myectomy for hypertrophic cardiomyopathy + CABG | 2 months | AscAo-RA | Aortic dissection | Surgical | Not described |
| H Matsuhisa | 2004 | M/85 | None | N/A | AscAo-RA | Aortic dissection | Surgical | Lived |
| VR Aligeti | 2012 | M/61 | RFA 2x | Not described | AscAo-RA | Complication of cardiac surgery | Surgical | Lived |
| AF Elwatidy | 2003 | F/3 | None | N/A | DescAo-RA | Congenital | Surgical | Lived |
| A Alozie | 2012 | M/19 | None | N/A | AscAo-RA | Aneurysm | Surgical | Lived |
| MS Topcuoðlu | 1997 | M/20 | None | N/A | AscAo-LA | Congenital | Surgical | Lived |
| M Sehgal | 2002 | M/53 | TIPS procedure, TIPS revision | 4 years 5 months; 3 years 11 months | AscAo-RA | Stent protruding RA | Patient refused surgery | Died |
| AK Sarkar | 2013 | M/5 months | None | N/A | DescAo-LA | Congenital | Patient refused surgery | Not described |
| D Patsouras | 2009 | M/84 | AVR | 18 years | AscAo-LA | Prosthetic valve endocarditis | Conservative medical treatment | Lived |
| MT Barrio-López | 2012 | M/56 | Multiple implantations of endovascular prostheses in IVC | 1 day | AscAo-RA | Stent protruding RA | Surgical | Lived |
| M Chacko | 2005 | M/58 | None | N/A | AscAo-LA | Aneurysm | Surgical | Not described |
| S Moral | 2009 | F/27 | AVR + Aortic root replacement | 3 months | AscAo-RA + AscAo-RV | Prosthetic valve endocarditis | Not described | Not described |
| S Bartus | 2008 | M/53 | Percutaneous closure of congenital ASD | 18 months | AscAo-RA | ASD device closure | Spontaneous closure | Lived |
| JM Hernández-García | 2005 | F/72 | Resection left atrial myxoma 2x | 18 years; 15 years | AscAo-LA | Complication of cardiac surgery | Percutaneous | Lived |
| S Maffè | 2012 | M/69 | AVR | 8 years | AscAo-RA | Prosthetic valve endocarditis | Surgical | Died |
| S Rubin | 2006 | M/30 | None | N/A | AscAo-RA | Trauma | Surgical | Lived |
| DK Millward | 1972 | M/32 | None | N/A | AscAo-RA | Aortic dissection | Patient died before surgery | Died |
| GY Jang | 2005 | F/54 | Percutaneous closure of ASD | 2 months | AscAo-RA | ASD device closure | Surgical | Lived |
| AD Berman | 1987 | F/60 | AVR | 10 years | AscAo-RA | Aortic dissection | Surgical | Lived |
| A Caruso | 2000 | M/41 | AVR | 8 years | AscAo-LA | Aortic dissection | Surgical | Lived |
| B Bell | 2010 | M/65 | PCI + CABG | 25 years; 19 years | AscAo-RA | SVG aneurysm | Percutaneous | Died |
| T Sugimoto | 2006 | M/65 | CABG | 20 years | AscAo-RA | SVG aneurysm | Surgical | Lived |
| R Benham | 1992 | M/21 | None | N/A | AscAo-LA | Endocarditis | Surgical | Lived |
| AJ Page | 1973 | M/60 | None | N/A | AscAo-RA | Aortic dissection | Surgical | Lived |
| JH Kay | 1959 | F/39 | Exploration through left posterolateral thoracotomy due to cardiac mass | 2 months | AscAo-LA | Aortic dissection | Surgical | Lived |
| A Ebringer | 1969 | F/21 | None | N/A | AscAo-LA | Endocarditis | No procedure performed | Died |
| EJ Hickey | 2008 | M/72 | CABG | 8 years | AscAo-RA | SVG aneurysm | Surgical | Lived |
| U Filizcan | 2011 | M/62 | None | N/A | AscAo-RA | RCA aneurysm | Surgical | Lived |
| AC Henze | 1991 | M/48 | AVR | 3 years | AscAo-RA | Aortic dissection | Surgical | Lived |
| JS Oliveira | 1991 | F/37 | None | N/A | AscAo-LA | Aortic dissection | Patient died before surgery | Died |
| A Kalangos | 2000 | M/18 | None | N/A | AscAo-RA | Congenital | Surgical | Not described |
| M/7 | None | N/A | AscAo-RA | Congenital | Surgical | Not described | ||
| C Türkay | 2003 | M/29 | None | N/A | AscAo-RA | Congenital | Surgical | Not described |
| PR James | 2002 | M/34 | AVR | 1 week | AscAo-RA | Prosthetic valve endocarditis | Surgical | Lived |
| PA Crean | 1983 | F/65 | None | N/A | AscAo-RA | Rheumatoid arthritis | Patient died before surgery | Died |
| W Beck | 1964 | M/42 | None | N/A | AscAo-LA | Aneurysm | Surgical | Lived |
| JR Büchler | 1983 | M/53 | CABG | 2 years | AscAo-RA | Aortic dissection | Surgical | Died |
| W Knirsch | 2005 | M/3 | Percutaneous closure of ASD | 4 weeks | AscAo-LA | ASD device closure | Surgical | Lived |
| CS Krishna | 2010 | F/11 | None | N/A | AscAo-RA | Congenital | Surgical | Lived |
| M/24 | None | N/A | AscAo-RA | Congenital | Surgical | Lived | ||
| YC Tsai | 2002 | F/2 | None | N/A | AscAo-RA | Not described | Surgical | Lived |
| AM Esen | 2003 | M/44 | None | N/A | AscAo-LA | Endocarditis | Surgical | Lived |
| H Nakano | 2000 | M/65 | AVR | 15 years | AscAo-RA | Aortic dissection | Surgical | Lived |
| M Dulake | 1964 | M/49 | None | N/A | AscAo-RA | Aortic dissection | Conservative medical treatment | Died |
| AD Timmis | 1985 | M/72 | None | N/A | AscAo-RA | Aortic dissection | Surgical | Died |
| D Vaidiyanathan | 1990 | M/30 | None | N/A | AscAo-RA | Aortic dissection | Conservative medical treatment | Lived |
| P Nicod | 1984 | F/52 | CABG | 9 years | AscAo-RA | Aortic dissection | Surgical | Not described |
| P Nihoyannopoulos | 1987 | M/4 | None | N/A | DescAo-LA | Congenital | Surgical | Lived |
| S Chandra | 2011 | F/12 | None | N/A | AscAo-RA | Congenital | Percutaneous | Lived |
| A Schwartzbard | 1998 | F/75 | AVR | 2 years | AscAo-LA | Prosthetic valve endocarditis | Surgical | Died |
| T Feldman | 2006 | F/76 | MVR | 10 years | AscAo-LA | Complication of cardiac surgery | Percutaneous | Lived |
| DS Chun | 2003 | M/10 | Percutaneous closure of ASD | 3 months | AscAo-RA | ASD device closure | Surgical | Lived |
| PA Grayburn | 2005 | F/41 | Percutaneous closure of ASD | 20 months | AscAo-RA | ASD device closure | Surgical | Lived |
| VS Mahadevan | 2006 | F/17 | Percutaneous closure of ASD | 25 months | AscAo-LA | ASD device closure | Percutaneous | Lived |
| DM Mello | 2005 | F/16 | Percutaneous closure of ASD | 6 months | AscAo-LA | ASD device closure | Surgical | Lived |
| D Danilowicz | 1989 | F/5 days | None | N/A | AscAo-RA | Congenital | Surgical | Lived |
| JW Jukema | 1992 | F/68 | CABG | 8 years | AscAo-RA | SVG aneurysm | Surgical | Lived |
| C Nathaniel | 1996 | M/59 | CABG | 16 years | AscAo-RA | SVG aneurysm | Surgical | Died |
| L Gruberg | 1999 | M/52 | AVR + CABG | 17 years | AscAo-RA | SVG aneurysm | Surgical | Lived |
| W Fares | 2003 | M/73 | CABG, dual chamber pacemaker placement | 21 years; 9 years | AscAo-RA | SVG aneurysm | Percutaneous | Lived |
| SA Photiou | 1981 | M/55 | AVR | 10 months | AscAo-RA | Aneurysm | Surgical | Lived |
| A DeSa'Neto | 1979 | M/17 | None | N/A | AscAo-RA | Trauma | Surgical | Lived |
| F Moraes | 2004 | M/1 | None | N/A | AscAo-RA | Congenital | Surgical | Lived |
| AE Weyman | 1975 | M/24 | None | N/A | AscAo-RA | Aneurysm | Surgical | Lived |
| M Aiba | 2013 | M/71 | CABG, Graft replacements of abdominal aorta, bilateral femoral + popliteal arteries, descending aorta | 20 years; 16 years; 7 years; 3 years | AscAo-RA | SVG aneurysm | Surgical | Lived |
| M Yuce | 2011 | M/70 | CABG + cardioverter-defibrillator implantation | 22 years; 7 years | AscAo-RA | SVG aneurysm | Patient refused surgery | Not described |
| MP Richardson | 1992 | M/74 | CABG | 11 years | AscAo-RA | SVG aneurysm | Surgical | Died |
| H Le Breton | 1998 | M/62 | CABG | 21 years | AscAo-RA | SVG aneurysm | Surgical | Lived |
| ML Williams | 2004 | M/58 | CABG | 12 years | AscAo-LA | SVG aneurysm | Surgical | Lived |
| HD Danenberg | 1995 | F/49 | Percutaneous transjugular stent placement to IVC and left hepatic vein | 14 months | AscAo-RA | Complication of internal jugular vein catheterization | Surgical | Lived |
| SK Aggarwal | 2007 | F/12 | None | N/A | AscAo-RA | Congenital | Surgical | Lived |
| M/33 | None | N/A | AscAo-RA | Aneurysm | Surgical | Lived | ||
| K Nandate | 2016 | M/19 | None | N/A | AscAo-LA | Trauma | Surgical | Lived |
| E Valero | 2016 | F/60 | Ascending aorta reconstruction without AVR | 8 years | AscAo-LA | Endocarditis | Surgical | Lived |
| M Alkouli | 2017 | M/84 | AVR | 3 days | AscAo-RA | Complication of cardiac surgery | Percutaneous | Lived |
| T Ahmad | 2014 | M/71 | None | N/A | AscAo-LA | Complication of cardiac surgery | Surgical | Lived |
| ES John | 2014 | F/21 | None | N/A | AscAo-RA | Endocarditis | Surgical | Lived |
| S Chandra | 2013 | M/20 | None | N/A | AscAo-LA | Endocarditis | Surgical | Lived |
| AM Noyes | 2015 | M/35 | None | N/A | AscAo-LA | Endocarditis | Surgical | Lived |
| M Bashir | 2014 | M/27 | Percutaneous closure of ASD | 6 weeks | AscAo-RA | ASD device closure | Surgical | Lived |
| P Sytnik | 2015 | M/63 | Ascending aorta reconstruction without AVR | 9 days | AscAo-RA | Aortic dissection | Surgical | Lived |
| PA Villablanca | 2014 | F/51 | None | N/A | AscAo-RA | Endocarditis | Patient refused surgery | Lived |
| M Yesin | 2015 | F/41 | MVP + MVR | 4 months | AscAo-LA | Complication of cardiac surgery | Surgical | Lived |
| N Raut | 2016 | M/48 | MVR | 4 years | AscAo-LA | Complication of cardiac surgery | Surgical | Lived |
| Y Agrawal | 2016 | M/57 | None | N/A | AscAo-LA | Endocarditis | Patient died before surgery | Died |
| A Ikeda | 2016 | M/45 | None | N/A | AscAo-RA | Endocarditis | Surgical | Lived |
| F Sabzi | 2015 | F/37 | None | N/A | AscAo-LA + AscAo-RA | Endocarditis | Surgical | Lived |
| L Frey | 2014 | F/45 | None | N/A | AscAo-RA | Endocarditis | Surgical | Lived |
| I Ece | 2015 | F/7 | None | N/A | AscAo-RA | Congenital | Percutaneous | Lived |
| H Matsumoto | 2014 | F/72 | AVR | 3 years | AscAo-RA | Complication of cardiac surgery | Surgical | Lived |
| C Siebers | 2014 | M/62 | AVR | 5 years | AscAo-RA | Prosthetic valve endocarditis | Surgical | Lived |
| AC Aykan | 2013 | M/21 | AVR + MVR | Not described | AscAo-LA | Prosthetic valve endocarditis | Surgical | Lived |
| MY Tsang | 2014 | F/43 | Multiple catheter ablations | Not described | AscAo-RA | Complication of percutaneous catheterization | Surgical | Not described |
| A Gunarathne | 2013 | M/28 | None | N/A | AscAo-RA + AscAo RV | Endocarditis | Surgical | Lived |
| E Sener | 2014 | F/19 | None | N/A | AscAo-RA | Aneurysm | Surgical | Lived |
| R Pancas | 2012 | F/51 | None | N/A | AscAo-RA | Not described | Surgical | Not described |
| V Patel | 2010 | F/72 | 2x AVR | 10 years, several weeks | AscAo-RA + AscAo RV | Complication of cardiac surgery | Patient died before surgery | Died |
| T Takamura | 2009 | F/82 | AVR | 6 months | AscAo-RA | Prosthetic valve endocarditis | Surgical | Lived |
| MM El Yaman | 2007 | M/32 | Coarctation repair, MVR, MVR, AVR+MVR | early childhood, 29 years, 25 years, 15 years | AscAo-LA | Complication of cardiac surgery | Percutaneous | Lived |
| O Badak | 2003 | F/49 | AVR + MVR | 10 months | AscAo-RA | Complication of cardiac surgery | Surgical | Lived |
| M Hachida | 1994 | F/57 | AVR + MVR + Manouguian procedure | 6 years | AscAo-LA | Complication of cardiac surgery | Surgical | Lived |
| F/49 | AVR + MVR + Manouguian procedure | 3 years | AscAo-LA + AscAo-LV | Complication of cardiac surgery | Surgical | Lived | ||
| NE Liddell | 1992 | M/51 | AVR + Aortic root replacement | 8 weeks | AscAo-LA | Complication of cardiac surgery | Surgical | Lived |
| H Chang | 1989 | M/25 | None | N/A | AscAo-RA | Trauma | Surgical | Lived |
| R Hayward | 1988 | F/72 | Ascending aorta reconstruction without AVR | 5 weeks | AscAo-RA | Complication of cardiac surgery | Percutaneous | Lived |
| JL Taylor | 1982 | M/61 | None | N/A | AscAo-RA | Aortic dissection | Surgical | Lived |
| R Berkowitz | 1973 | M/24 | Emergency thoracotomy and suturing of laceration RA appendage and RV | 33 days | AscAo-RA | Trauma | Surgical | Lived |
| JS Ladowski | 1984 | F/56 | Closed mitral commissurotomy | 25 years | AscAo-RA | Complication of percutaneous catheterization | Surgical | Lived |
AAF, aorto-atrial fistula; AscAo, ascending aorta; DescAo, descending aorta; RA, right atrium; LA, left atrium; RV, right ventricle; AVR, aortic valve replacement; MVR, mitral valve replacement; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; SVG, saphenous vain graft; IVC, inferior vena cava; ASD, atrial septal defect; RFA, radiofrequency ablation; TIPS, transjugular intrahepatic portosystemic shunt.
Evidence synthesis
Demographics
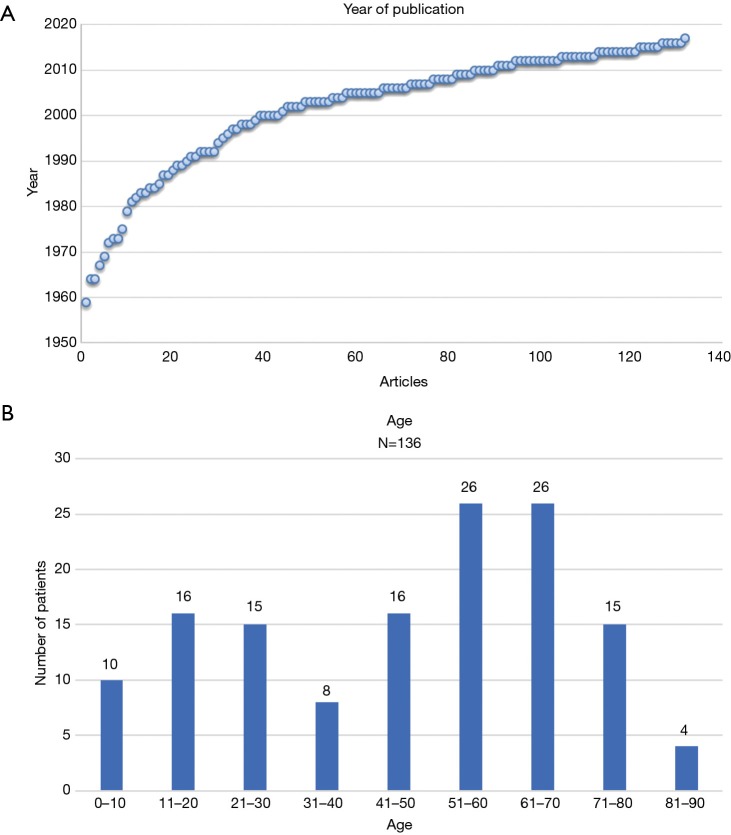
7% of the studied articles were published in the period from 1960 till 1980. Around 70% of the articles were published after 2000 whilst approximately 35% were published after 2010 (Figure 2A). Most case reports on AAF were from the United States of America (39 cases) followed by the United Kingdom (12 cases). Most Asian publications stemmed from India (11 cases), Japan (12 cases) and Turkey (12 cases). From the 136 cases analysed the occurrence of AAF had a male to female ratio of approximately 2:1. The age of the patients in the case reports described ranged between 5 days and 85 years old (median 51 years, average 46 years) (Figure 2B).
Figure 2.
Demographics of Patient Population with AAF. (A) Number of articles published about AAF in time; (B) occurrence of AAF per age group. AAF, aorto-atrial fistula.
Imaging
In 130 out of the 136 cases information was provided regarding use of preoperative imaging techniques. In the majority of the cases where imaging was utilised, echocardiography (83.1%) and angiography (59.6%) were favoured (Tables 2 and 3). Out of the 113 reported cases where echocardiography was used, the use of transthoracic (23.9%) as well as transesophageal (24.8%) or the combination of both (23.0%) seemed to be equally distributed, whilst in 28.3% of cases the technique the authors had used was not specified (Table 3).
Table 2. Pre-operative diagnostic tests, number of cases, all cases (136).
| Pre-operative diagnostic tests (N=136) | Number of cases | Percentage |
|---|---|---|
| Echocardiography only | 47 | 34.6 |
| Echocardiography + Angio | 34 | 25.0 |
| Echocardiography + Angio + CT scan | 20 | 14.7 |
| Echocardiography + Angio + CT-scan + MRI | 1 | 0.7 |
| Echocardiography + Angio + MRI | 2 | 1.5 |
| Echocardiography + CT-scan | 8 | 5.9 |
| Echocardiography + CT-scan + MRI | 1 | 0.7 |
| Angio only | 11 | 8.1 |
| Angio + CT-scan | 3 | 2.2 |
| Angio + MRI | 1 | 0.7 |
| CT-scan only | 2 | 1.5 |
| None described | 6 | 4.4 |
Table 3. Preoperative diagnostic tests, percentages, all cases (136).
| Preoperative diagnostic tests (N=136) | Number of cases | Percentage |
|---|---|---|
| Echocardiography | 113 | 83.1 |
| TTE | 27 | 19.9 (23.9% of 113 cases) |
| TEE | 28 | 20.6 (24.8% of 113 cases) |
| TTE + TEE | 26 | 19.1 (23.0% of 113 cases) |
| Type of echocardiography not specified | 32 | 23.5 (28.3% of 113 cases) |
| Angio | 81 | 59.6 |
| CT-scan | 35 | 25.7 |
| MRI | 5 | 3.7 |
| Not described | 6 | 4.4 |
TTE, transthoracic echocardiography; TEE, transesophageal echocardiography.
Anatomy
Fistula formation from the ascending aorta to the atria occurred more often into the right atrium (86 of the 136 cases) compared to the left atrium (41 of the 136 cases), at a LA to RA ratio of 1:2.1. In two patients, the fistula tract originated from the descending aorta into the left atrium (DescAo-LA) (1,2). Some very rare cases included fistula formation from the descending aorta into the right atrium (DescAo-RA) (3), fistula formation to both the left and right atrium (4,5) and fistula formation between the ascending aorta, right atrium as well as the right ventricle (6-8) and fistula formation between the ascending aorta, left atrium as well as the left ventricle (9) (Figures 3,4 and Table 4).
Figure 3.
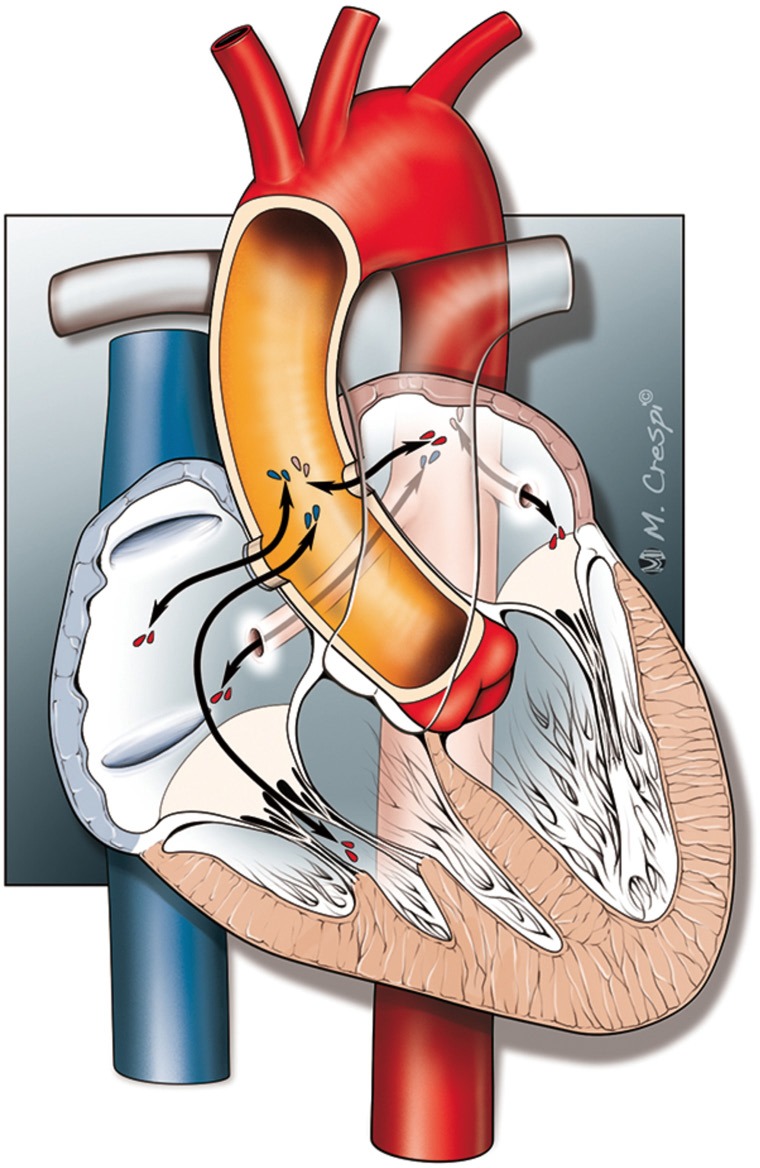
Types of AAF. AAF, aorto-atrial fistula.
Figure 4.
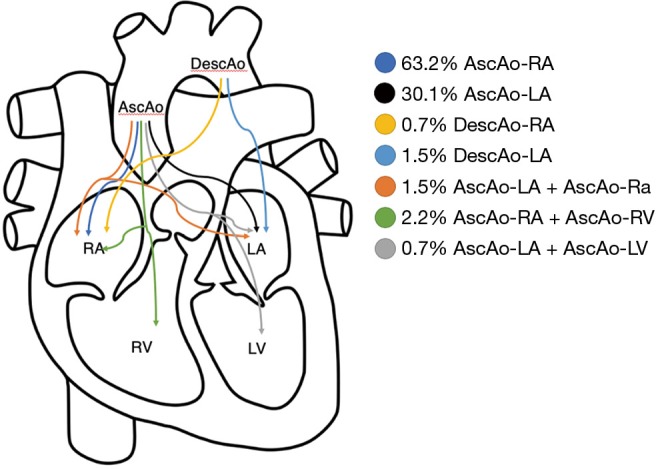
A Schematic outline illustrating the occurrence rates of different types of AAF. AAF, aorto-atrial fistula.
Table 4. Type of AAF, all cases (136).
| Type of AAF (N=136) | Number of cases | Percentage |
|---|---|---|
| AscAo-RA | 86 | 63.2 |
| AscAo-LA | 41 | 30.1 |
| DescAo-RA | 1 | 0.7 |
| DescAo-LA | 2 | 1.5 |
| AscAo-LA + AscAo-RA | 2 | 1.5 |
| AscAo-RA + AscAo-RV | 3 | 2.2 |
| AscAo LA + AscAo LV | 1 | 0.7 |
AAF, aorto-atrial fistula; AscAo, ascending aorta; DescAo, descending aorta; RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle.
Causes
In the majority of cases (22.8%) endocarditis was the cause of AAF formation. In 71% of these cases, a paravalvular abscess was also present. Furthermore, 45.2% of these patients had prior surgery with a prosthetic valve. Aortic aneurysms (22.1%), mainly complicated with dissected aortic walls, were the 2nd most common cause. Congenital causes (11.8%) were also responsible for a number of AAFs. In this group 81.2% of the fistula tracts were from the aorta to the right atrium whilst only 18.8% led into the left atrium. Interestingly, chest traumas were a relatively rare cause of AAF, with this being the cause in just 3.7% of all cases reported.
In 15.4% of the cases fistula formation occurred secondary to cardiac surgery. Saphenous vein graft (SVG) aneurysms after coronary artery bypass surgery were responsible for 9.6% of the AAF formations in the reported cases. Furthermore, in 7.4% of the cases a previous atrial septal defect closure with a device was responsible for AAF (Table 5).
Table 5. Causes of AAF, all cases (136).
| Cause of AAF (N=136) | Number of cases | Percentage | Sub-analysis individual groups |
|---|---|---|---|
| Aneurysm | 30 | 22.1 | N=30 |
| With dissection | 22 | 16.2 | 73% |
| Without dissection | 8 | 5.9 | 27% |
| Endocarditis (total) | 31 | 22.8 | N=31 |
| With abscess formation | 22 | 16.2 | 71% |
| Without abscess formation | 9 | 6.6 | 29% |
| Endocarditis no artificial valves | 17 | 12.5 | 54.8% |
| With abscess formation | 11 | 8.1 | 64.7% |
| Without abscess formation | 6 | 4.4 | 35.3% |
| Prosthetic valve endocarditis | 14 | 10.3 | 45.2% |
| With abscess formation | 11 | 8.1 | 78.6% |
| Without abscess formation | 3 | 2.2 | 21.4% |
| Congenital | 16 | 11.8 | N=16 |
| Congenital ARAF | 13 | 9.6 | 81.2% |
| Congenital ALAF | 3 | 2.2 | 18.8% |
| SVG aneurysm | 13 | 9.6 | – |
| Complication of cardiac surgery | 21 | 15.4 | – |
| ASD device closure | 10 | 7.4 | – |
| Chest trauma | 5 | 3.7 | – |
| Stent protruding RA | 2 | 1.5 | – |
| Complication of percutaneous catheterization | 3 | 2.2 | – |
| RCA aneurysm | 1 | 0.7 | – |
| Behcet’s disease | 1 | 0.7 | – |
| Rheumotoid arthritis | 1 | 0.7 | – |
| Accidental finding | 1 | 0.7 | – |
AAF, aorto-atrial fistula; ARAF, aorto-right atrial fistula; ALAF, aorto-left atrial fistula; SVG, saphenous vein graft; ASD, atrial septal defect; RA, right atrium.
Therapy
As shown in Table 6, AAF was corrected via an open surgical approach in 73.5% of all cases. In 10.3% the fistula was closed via a percutaneous intervention, whilst in 4.4% of cases a conservative medical approach was advocated (e.g., diuretics and blood transfusions), due to the high surgical risk. In one case the patient was scheduled for surgical closure of the fistula, but echocardiography showed a spontaneous closure. In 3.6% of cases the patient refused corrective surgery and in 5.1% of all cases the patient died before surgery could go ahead. In 1.5% of all cases with AAF there was no procedure or medical intervention performed, either due to a very high operational risk or due to the fact that the fistula had no hemodynamic effects. In 0.7% of the cases the treatment of the AAF was not described. In 42% of the cases where a surgical approach was utilized, the fistula was closed with sutures. In 20% of the cases it was opted to close the fistula with a patch, whilst in 4% the tract was ligated. A combined approach of suturing and the use of patches occurred in 5% of cases. The closing technique during corrective surgery was not described in 29% of cases (Table 6). When percutaneous closure of the fistula tract was employed, closure with an Amplatzer device was the treatment of choice (71.4%), followed by coil embolization (14.3%), covered stents (7.15%) and finally balloon closures (7.15%) (Table 6).
Table 6. Treatment of AAF, all cases (136).
| Type of treatment (N=136) | Number of cases | Percentage | Sub-analysis individual groups |
|---|---|---|---|
| Surgical closure | 100 | 73.5 | N=100 |
| Suture closure of fistula | 42 | 30.9 | 42% |
| Patch closure of fistula | 20 | 14.7 | 20% |
| Combination of suture and patch | 5 | 3.7 | 5% |
| Ligation of fistula | 4 | 2.9 | 4% |
| Closing technique not described | 29 | 21.3 | 29% |
| Percutaneous closure | 14 | 10.3 | N=14 |
| Amplatzer device | 10 | 7.4 | 71.4% |
| Coil embolisation | 2 | 1.5 | 14.3% |
| Covered stents | 1 | 0.7 | 7.15% |
| Balloon closure | 1 | 0.7 | 7.15% |
| Conservative medical treatment | 6 | 4.4 | – |
| Patient died before surgery | 7 | 5.1 | – |
| Patient refused surgery | 5 | 3.7 | – |
| No procedure performed | 2 | 1.5 | – |
| Procedure not described | 1 | 0.7 | – |
| Spontaneous closure | 1 | 0.7 | – |
AAF, aorto-atrial fistula.
Outcomes
In 74.3% of all studied cases the fistula repair was successful and patients survived the procedures. In 14.7% of the cases patients did not survive, whilst in 11.0% of the reported cases patient’s outcome was not mentioned. In 83% of all surgical cases the fistula repair was successful and patients survived the procedures. In 85.7% of all the percutaneous fistula corrections, the repair was successful and patients survived the procedures (Table 7).
Table 7. Outcome of AAF, all cases (136).
| Type of treatment (N=136) | Survived | Died | Not described | Number of cases |
|---|---|---|---|---|
| Intervention | ||||
| Surgical closure | 83 (83%) | 7 (7%) | 10 (10%) | 100 |
| Suture closure | 37 (88.1%) | 1 (2.4%) | 4 (9.5%) | 42 |
| Patch closure | 16 (80%) | 2 (10%) | 2 (10%) | 20 |
| Suture and patch closure | 5 (100%) | – | – | 5 |
| Ligation | 4 (100%) | – | – | 4 |
| Closing technique not described | 21 (72.4%) | 4 (13.8%) | 4 (13.8%) | 29 |
| Percutaneous closure | 12 (85.7%) | 1 (7.1%) | 1 (7.1%) | 14 |
| Amplatzer device | 9 (90%) | – | 1 (10%) | 10 |
| Coil embolization | 2 (100%) | – | – | 2 |
| Covered stents | – | 1 (100%) | – | 1 |
| Balloon closure | 1 (100%) | – | – | 1 |
| Procedure not described | – | – | 1 (100%) | 1 |
| Overall treatment success | 95 (82.6%) | 8 (7.0%) | 12 (10.4%) | 115 |
| No intervention | ||||
| Conservative medical treatment | 3 (50%) | 3 (50%) | – | 6 |
| No procedure performed | 1 (50%) | 1 (50%) | – | 2 |
| Patient refused surgery | 1 (20%) | 1 (20%) | 3 (60%) | 5 |
| Spontaneous closure | 1 (100%) | – | – | 1 |
| Patient died before surgery | – | 7 (100%) | – | 7 |
| Overall | 101 (74.3%) | 20 (14.7%) | 15 (11.0%) | 136 |
AAF, aorto-atrial fistula.
Discussion
We systematically reviewed the literature for reports on AAF. We did not identify any reports on systematic registries or clinical trials investigating AAF. All our knowledge on AFF is therefore currently based on case-reports. Based on the reported case reports, we conclude that:
Small AAFs can be asymptomatic and may be conservatively approached with the reduction of cardiac afterload and the use of diuretics. In these cases, it is highly recommended to closely observe the patient over time and if clinical conditions deteriorate, active closure of the fistula should be considered.
Large AAFs require immediate closure either percutaneously or via a surgical approach. Spontaneous closure of an AAF is very rare and conservative treatment must be strongly discouraged in cases with large fistulas or clinical symptoms ensue.
Although the case volume is low, compared to surgery, percutaneous closure has shown comparable outcome.
The surgical approach to close the fistula often entails suturing or the use of a patch. Percutaneous closure of AAF has been employed more often in the last few years. There are no specific devices for transcatheter closure of fistulas, but devices like the Amplatzer Septal Occluder, used for closing atrial septal defects, have proven their applicability for this purpose. Overall treatment success rates are at least 70% with a mortality rate of around 15%.
There are a number of limitations to our study, with the major limitation being that reported data came from case reports or case series. For these reasons, it is likely that there is some publication bias as it is highly likely that not all cases have been published. Furthermore, cases of patients who were unsuccessfully treated are less likely to be reported. Data from larger populations is lacking, meaning that specific data regarding incidence and prevalence does currently not exist. This review provides us with a number of insights into the occurrence and pathophysiology of AAF, as well as the current treatment options for this rare, but potentially life threatening, condition.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Nihoyannopoulos P, Sapsford R, Oakley CM. Congenital fistula between the aorta and left atrium. Br Heart J 1987;57:387-90. 10.1136/hrt.57.4.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarkar AK, Sanjeeva NG, Waghmare NS. Association of congenital descending aorto-left atrial fistula with the aortopulmonary window and atrial septal defect. Cardiol Young 2014;24:143-4. 10.1017/S1047951112002156 [DOI] [PubMed] [Google Scholar]
- 3.Elwatidy AF, Galal AN, Rhydderch D, et al. Aorto-right atrial fistula. Ann Thorac Surg 2003;76:929-31. 10.1016/S0003-4975(03)00448-X [DOI] [PubMed] [Google Scholar]
- 4.Dalla Pozza R, Kozlik-Feldmann R, Le TP, et al. Interventional closure of two fistulas after aortic valve surgery. Clin Res Cardiol 2009;98:451-4. 10.1007/s00392-009-0019-z [DOI] [PubMed] [Google Scholar]
- 5.Sabzi F, Heidari A, Faraji R. A rare case of aortic sinuses of valsalva fistula to multiple cardiac chambers secondary to periannular aortic abscess formation from underlying Brucella endocarditis. GMS Hyg Infect Control 2015;10:Doc14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moral S, Aboal J, Morales M. Multiple aorto-right cavitary fistula: a rare complication of prosthetic valvular endocarditis in intravenous drug users. Eur J Echocardiogr 2009;10:374-5. 10.1093/ejechocard/jen328 [DOI] [PubMed] [Google Scholar]
- 7.Gunarathne A, Hunt J, Gershlick A. Aorto-right atrial and right ventricular fistulae: a very rare complication of native bicuspid aortic valve endocarditis. Heart 2013;99:1708. 10.1136/heartjnl-2013-303846 [DOI] [PubMed] [Google Scholar]
- 8.Patel V, Fountain A, Guglin M, et al. Three-dimensional transthoracic echocardiography in identification of aorto-right atrial fistula and aorto-right ventricular fistulas. Echocardiography 2010;27:E105-8. 10.1111/j.1540-8175.2010.01225.x [DOI] [PubMed] [Google Scholar]
- 9.Hachida M, Koyanagi H, Hanayama N, et al. Successful reconstruction of aorto-left atrial fistula following aortic valve replacement and root enlargement by the Manouguian procedure. J Card Surg 1994;9:392-8. 10.1111/j.1540-8191.1994.tb00867.x [DOI] [PubMed] [Google Scholar]