Abstract
Myocardial ischemia is considered the cornerstone of the treatment of patients with coronary artery disease (CAD). Although the deleterious effects of myocardial infarction, the maximum expression of ischemia, have been extensively studied and described, the clinical effects of chronic, documented myocardial ischemia are not completely clarified. The first studies that compared therapies for coronary disease focused on the presence of anatomical features and assessed ischemia based on the interpretation of the findings of obstructive atherosclerotic lesions. They suggested that revascularization interventions did not confer any clinical advantage over medical therapy (MT), in terms of cardiac or overall death. Other retrospective studies that were dedicated to assessing the impact of documented stress-induced ischemia on cardiovascular outcomes have suggested a prognostic impact of chronic ischemia. However, this has been questioned in recent studies. Moreover, the previous understanding that chronic ischemia could lead to worsening of ventricular function was not confirmed in a recent study. Thus, the prognostic significance of stress-induced ischemia has been questioned. Regarding treatment options, although some previous analyses have suggested that interventional therapies would reduce cardiovascular events in CAD patients with documented ischemia, recent post-hoc studies and metanalysis have shown distinct results. In this review article, the authors discuss myocardial ischemia, the different responses of the myocardium to ischemic insults, ischemic preconditioning, and the main findings of recent studies about the clinical aspects and treatment of patients with chronic, documented myocardial ischemia.
Keywords: Myocardial infarction, ischemia, myocardium, atherosclerosis
Introduction
The first reports of ischemic heart disease in the eighteenth century started with the description of patients with angina symptoms. Posteriorly, the identification of atherosclerotic obstructive lesions explained, at least in part, the pathogenesis of this complex disease. However, with the scientific evolution, it has been shown that coronary circulation has idiosyncratic properties that aim to protect the myocardium, in the chronic setting. Thus, despite the frequent findings of obstructive atherosclerotic lesions in the coronaries, they are not synonymous with chronic myocardial ischemia and may only represent part of a major problem. Meanwhile, the diagnosis of myocardial ischemia has many particularities, and treatment is a debatable issue and the rationale for ongoing studies. The previous thinking that treating ischemia by interventions would reduce cardiovascular events is still controversial.
Coronary circulation and pathophysiology of myocardial ischemia
Myocardial ischemia is a multifactorial pathophysiological condition that involves a complex and specific interaction between coronary vessels and the myocardium. Ultimately, it represents an imbalance between myocardial oxygen supply and demand that can occur in different situations. Myocardial supply depends on blood oxygen rates and coronary perfusion pressure, and oxygen demand depends on multiple variables, especially heart rate, contractility, and diastolic ventricular pressure.
The most frequent cause of coronary blood flow limitation is obstruction due to atherosclerosis. The nature of the coronary flow goes far beyond macrovascular epicardial disorders and consists of a multifaceted mechanism (1). Coronary circulation is anastomotic and not terminal, such as occurs in other organs like lungs and kidneys. Thus, myocardial vascular beds can be supplied by more than one coronary vessel. The different vessel systems might contribute in distinct ways to myocardial blood flow to a specific region.
Moreover, coronary blood flows from epicardial to endocardial layers, and the latter are more susceptible to flow variation according to ventricular pressures, hence more susceptible to myocardial ischemia. In addition, both stenotic and non-stenotic coronaries might be subject to vasospasm, which could intermittently interfere with symptoms and explain some patterns seen in chronic angina. Moreover, microvascular and endothelial dysfunction could also result in ischemia or represent additional factors, because they are responsible for vasodilation and coronary flow reserve. The smaller the vessel, the greater the repercussion of the increased left ventricular filling pressures on its fluid dynamics.
The presence of collaterals may also play a role in coronary circulation. Collateral vessels are a network of channels present in the heart that connect epicardial vascular branches from different regions. Under unstimulated conditions (no obstructive coronary lesions), the ability of these channels to permit blood flow is limited because their vascular resistance is high. It is believed that upon ischemic stimulation, these channels go through a process of arteriogenesis to form vascular vessels, with the 3 layers (intima, median layer, and adventitia) that are anatomically indistinguishable from other epicardial vessels (2). The collaterals undergo remodeling to a larger caliber with diameters expanding five-to-ten-fold compared with the unstimulated state (3), and, thus, vascular resistance is reduced, allowing blood flow, and, theoretically, the reduction of myocardial ischemia. It has been shown that the diameter of the collaterals in the absence of coronary artery disease (CAD) ranges from 10–200 µm, compared with 100–800 µm in the presence of CAD (4). Collaterals in humans are thought to develop by expansion of a preexisting collateral network. However, no studies have been conducted to exclude the growth of new vessels. The factors to induce the expansion of collateral vessels are likely a combination of mechanical (shear stress) and chemical factors. In clinical practice, collaterals are mostly visualized during coronary arteriography supplying myocardial areas of severe or occluded epicardial obstructive lesions. It is assumed that the presence of collaterals might reduce myocardial ischemia (5), but frequently they are responsible for areas of ischemia and effort angina. On the other hand, some studies have suggested that the presence of the collateral vessels might reduce infarct size (6).
Interestingly, microvascular collaterals have also been identified and postulated to influence myocardial perfusion and ischemia.
All these specific characteristics of coronary circulation, i.e., anastomotic in nature, flowing from epicardial to endocardial layers, and the presence of a great network of epicardial and microvascular collaterals, correlate with each other and form the complexity involved in the perfusion of the myocardium. They are believed to protect the myocardium from limiting-flow obstructions.
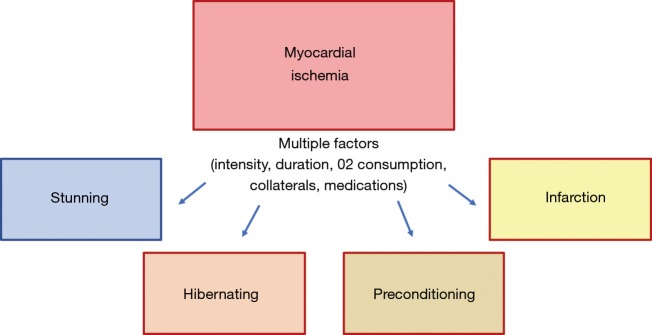
Once ischemia is triggered, anaerobic metabolism of myocytes begins, followed by tissue acidosis from generation of lactic acid and reduction of adenosine triphosphate (ATP) availability. In theory, primary changes initially affect diastolic and systolic function, evolving with worsening of end diastolic pressure and arterial pressure, therefore worsening ischemia. The presence of ischemia leads to the release of chemosensitive substances, such as lactate, adenosine, bradykinin, histamine, reactive oxygen species, responsible for stimulating neurologic receptors and provoking angina symptoms (7). If sustained and uninterrupted, ischemia will eventually cause rupture of cell membranes, elevating extracellular potassium levels, and promoting changes in electrocardiographic repolarization. Severe mitochondrial disarrangements will cause cell death. Interestingly, depending on many other complex factors, ischemic insults may trigger other different myocardial responses, such as stunning, hibernation, or even preconditioning (8).
Myocardium responses to ischemic insults (stunning, hibernation, preconditioning)
After an ischemic insult, and depending on its intensity, duration, and compensatory mechanisms [collateral circulation (9) and hypoxic hyperemia], the myocardium can exhibit distinct responses (Figure 1). Excluding the severe insults that lead to a complete depletion of ATP to mitochondrial functioning, leading to cell death, in theory, chronic ischemia can lead to myocardial stunning, hibernation, or activate cellular mechanisms to become less susceptible to further ischemic insults. This last myocardial response is called ischemic preconditioning.
Figure 1.
Schematic diagram showing the distinct myocardial responses to ischemic insults.
Conceptually, the stunning myocardium is completely distinct from hibernating myocardium, because stunning presumes that myocardial flow is restored, and the contractile deficit should reverse after a variable period. Myocardial stunning appears to result from the reperfusion injury that is believed to be primarily caused by the generation of reactive oxygen species, transient calcium overload associated with decreased responsiveness of the contractile apparatus to calcium and altered ion channel activity (10). The subsequent contractile deficit is believed to have a protective function to the myocardium, limiting the multiple and deleterious cellular disarrangements caused by ischemia and finally limiting the progression to irreversible cell damage during reperfusion (11).
The diagnosis of hibernating myocardium occurs after myocardial tissue reverses its contractile deficit after a revascularization procedure, confirming that ischemia was the etiology of the myocardial dysfunction. The preprocedural diagnosis of this condition is more challenging and could be theoretically done with the confirmation of myocardial ischemia and a contractile deficit, but it would still need the improvement in contraction after the resolution of ischemia.
Interestingly, brief and repetitive episodes of myocardial ischemia can activate complex cellular pathways, turning the mitochondria more resistant to further ischemic insults. This phenomenon termed ischemic preconditioning (IP) was first described by Charles Murry, who showed in a canine model that 4 cycles of 5 minutes of myocardial ischemia followed by reperfusion reduced by 75% the infarcted area produced by the occlusion of the circumflex artery for 40 minutes (12). Since Murry’s seminal work, several researchers have studied this cardioprotective phenomenon. These studies have suggested that IP is a cellular phenomenon with many complex pathways that are still only partially understood (13). The release of some substances especially endogenous adenosine during brief ischemia enhances the release of bradykinin, opioids, catecholamines, and more adenosine (14,15). These substances activate many redundant pathways, that ultimately phosphorylate the ATP-sensitive K channels. The opening of these last channels seems to play a fundamental role in the preconditioning phenomenon (16,17).
Other researchers have also proposed mechanisms such as remote ischemic preconditioning (18) and postconditioning (19), in order to recommend clinical strategies.
Although it is challenging to induce IP in humans, the phenomenon can be observed in studies with sequential exercise stress tests (20-22). These studies have shown that this cardioprotective mechanism might be influenced by diseases (23,24), medications (14,25-28), and one study (data under publication) has suggested lower cardiac events in 1-year follow-up in patients who had this phenomenon, compared with CAD patients without evidence of this mechanism. Interestingly, diabetic medications that block K-ATB channels in the pancreas in order to secret insulin, may also interfere with these channels in the heart. Because these channels are of upmost importance to trigger IP, their blockage has also been shown to negatively interfere with this cardioprotective mechanism (27,28).
Thus, depending on several intrinsic and extrinsic factors, the myocardium can respond in distinct ways when submitted to ischemic insults.
Identification of myocardial ischemia
The diagnosis of myocardial ischemia is based on clinical suspicion. And this is primarily influenced by the presence of angina pectoris, the main clinical manifestation of myocardial ischemia. In clinical practice, the characterization of patient’s symptoms and angina are of upmost importance, because it dictates the options of diagnostic procedures and also the intended treatment. Moreover, many CAD patients during follow-up evolve with chest pain complaints, and frequently these are due to other causes of chest pain, rather than ischemic heart disease.
William Heberden, in the eighteenth century, was the first physician to describe patients with clinical symptoms of angina pectoris (29). Typical angina is characterized as chest discomfort precipitated by exertion or emotional stress, whose intensity grows gradually and lasts a few minutes before dissipating. The symptoms should dissipate with rest. The most frequent described patterns are the sensations of constriction, suffocating, burning, or heaviness. Typical location is retrosternal, potentially irradiating to the ulnar surface of left, right, or both arms, and in some cases reaching the neck and mandible. Manifestations of epigastric discomfort are not uncommon and should not be underestimated. Elderly persons and women often present with atypical symptoms like exertional dyspnea or fatigue. The assumption that diabetic patients usually present with atypical symptoms (30,31) has not been confirmed in recent studies (32,33). Moreover, the fact that 82.1% of all diabetic patients in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial had symptoms of myocardial ischemia and of these 74% had typical angina at study entry does not support that previous assumption (34).
When these symptoms are triggered by the same effort intensity, the pattern is called “fixed threshold,” and usually has less coronary tonus affecting its occurrence. On the other hand, the patients who describe “good days and bad days” probably have dynamic stenosis according to coronary tonus and endothelial dysfunction, a pattern reported as “variable threshold.” Two other important phenomena are frequent among this population. The first one is the ability of reaching further distances following a resting period after an effort that triggered angina. This is described as “warm-up” angina, whose pathophysiological explanation is related to ischemic preconditioning (21,22,35). The second one is defined as the occurrence of mild angina during the first stages of exercise with the disappearance of chest pain at higher workloads. This is the walk-through angina, a phenomenon probably caused by a delayed vasodilation of collateral vessels (36).
Finally, the intensity of symptoms and effort that triggers them are also important prognostic factors and are the main indications for revascularization interventions in most patients with CAD.
The electrocardiographic manifestation of ischemia usually begins before clinical chest pain, and the main patterns are segment ST depression and T wave inversion. In stable CAD patients, the electrocardiogram (ECG) is frequently normal. However, during physical or pharmacological stress, ECG may show ischemic findings, and these are important diagnostic and prognostic tools in clinical practice.
Stress-induced tests have important diagnostic and prognostic roles. In populations of intermediate pretest probability of CAD, stress-induced tests may help in diagnosing myocardial ischemia. However, the interpretation of negative results has to be done cautiously because it does not rule out the diagnosis of CAD. Additionally, in patients with typical angina such tests may also indicate the amount of ischemia and presumably may indicate those patients with worse outcomes.
In clinical practice, different methods can help to investigate myocardial ischemia. The most performed ones are those that apply physical exertion, the use of inotropic or vasodilator medications. Physical exertion is the preferred method in most patients who can exercise and who do not have electrocardiographic changes that make the assessment of ischemic findings difficult. It is the most physiological way to increase myocardial oxygen consumption and provides prognostic information besides the presence or not of ischemia, such as exercise tolerance, clinical symptoms, exercise-induced arrhythmias, and arterial pressure during exercise. Inotropic drugs such as dobutamine are also effective, because they augment cardiac frequency and blood pressure, the two major components of cardiac oxygen consumption, but may induce arrhythmias due to the stimulation of cardiac adrenergic receptors. Vasodilators such as adenosine or dipyridamole (the second acts by inhibiting the enzyme that metabolizes adenosine) causes distinct vasodilation in the coronary arteries. Theoretically, the vascular territories with obstructive lesions have lower vasodilator reserve capacity than vascular territories with no obstructions. Thus, the lower reserve capacity to vasodilate these beds causes a lower concentration of the cardiac tracer during the infusion of these vasodilators compared with the concentration during rest. Tests with these vasodilators are especially indicated for patients with limitations to performing the exercise test. However, patients may experience unpleasant side effects of these medications, especially chest pain. Thus, one can assess ischemia after stress by electrocardiographic changes (treadmill test), left ventricle wall motion changes [stress-echo, stress-magnetic resonance imaging, single-photon emission computed tomography (SPECT), positron-emission tomography (PET)], or myocardial perfusion imaging (SPECT, PET). These techniques, by assessing myocardial function itself, consider other relevant factors, such as the presence of collateral vessels and repercussion of other possible variables influencing ischemia, such as left ventricular filling pressures, after-load, and contractility.
Invasive gradient flow measurements, such as coronary fractional flow reserve (FFR) or instant wave-free ratio (iFR), assess the pressure gradient caused by each coronary lesion. Because invasive gradient flow techniques measure pressure gradients along the supposed obstructive lesion, they do not directly assess information about myocardial ischemia. Moreover, the cut-off values used by FFR to determine ischemia are based on the information of other stress-induced ischemia methods, such as scintigraphy and echocardiography (37). These “gold-standard” methods used to define FFR cut-offs also have limited accuracy for identifying ischemia. Thus, although FFR has been increasingly used in clinical practice to guide revascularization, it has many limitations regarding extrapolation of coronary gradient pressures to predict the complexity of myocardial ischemia.
Myocardial ischemia versus anatomic disease
In 1974, a seminal work of Gould and Lipscomb studied coronary blood flow in dogs and assessed the effects of progressive coronary narrowing on resting and maximal hyperemic blood flow (38). These authors showed in these animal models that a reduction in the circumflex coronary diameter of ≥85% limited resting coronary blood flow and that reductions of ≥50% progressively limited maximal coronary flow reserve. Based on these results, obstructions ≥85% were considered to be critical stenosis, and then were further interpreted as ischemia-causing stenosis. This concept was then directly applied in clinical practice.
Although this study has largely contributed to the understanding of the impact of coronary obstructions on vascular blood flow, the authors themselves state that their findings should not be directly extrapolated to humans, because of the diversity and complexity of ischemic heart disease in man. In fact, much evidence suggests that the straightforward relation between chronic obstructive coronary atherosclerosis and myocardial ischemia might be misleading and only represent a simplified view of the problem. And this might also be one of the reasons why treating epicardial stenosis with percutaneous interventions has not been associated with lower cardiac events compared with MT.
Based on these controversies, it is important to emphasize that the methods used to study coronary anatomy (i.e., invasive coronary angiography and coronary tomography) fundamentally provide distinct information of functional methods that assess myocardial ischemia. The first assesses coronary arteries and their atherosclerotic lesions, whereas the other assesses the myocardium itself. Importantly, both methods (anatomic and functional) also have their own sensitivities, specificities, and accuracies, and cannot be interpreted as synonymous with the same problem. In this sense, it is frequent in clinical practice that multivessel CAD patients with severe stenotic atherosclerotic lesions have no evidence of myocardial ischemia on stress tests. Interestingly, in the Medicine, Angioplasty, or Surgery Study II (MASS II) trial (39), in the randomized group of patients with multivessel CAD (2 or 3-vessel disease with at least 70% obstruction in proximal branches), 55% of the patients had no evidence of myocardial ischemia on treadmill stress tests.
Studies using FFR have shown that the capacity of coronary angiography in predicting ischemic lesions is limited. Although this is known in intermediate lesions, it seems also quite limited in severe ones. In the Fractional Flow Reserve versus Angiography (FAME) for guiding percutaneous coronary intervention trial (40), of the 71–90% stenosis, 20% of the lesions were classified as hemodynamically insignificant by FFR (FFR >0.80), and, on the other hand, 35% of 50–70% stenosis was considered functionally significant by FFR (FFR <0.80). This is one perspective of the complexity that the identification of myocardial ischemia involves, with a substantial reclassification of lesions according to the method applied. Interestingly, a large multicenter registry study (41) showed that among 1,075 CAD patients who underwent coronary angiography and FFR, the FFR information changed the interpretation of disease severity and affected the initial therapy management based on angiography visual assessment in 43% of the patients. Thus, from the initial 1,075 patients, 1,028 were treated based on the FFR information. Comparing the chosen treatment based on angiography and the chosen treatment by FFR, these patients were stratified into those who were reclassified by FFR and those who were not reclassified. Whereas the authors concluded that this management based on the reclassification of lesions by FFR was safe, it showed similar one-year major cardiovascular event results compared with the patients managed based on angiographic visual assessment. On the other hand, FFR was useful to reclassify intermediate angiographically stenoses, and in these cases, might be an incremental tool in cases of interoperator discordance.
Regarding the indication for revascularization procedures, the interpretation of the anatomical and the functional tests are the basis for understanding the two essential conditions to indicate these procedures: the vessel and its atherosclerotic lesion and the myocardial functional information. Thus, in theory, both vessel and muscle specific conditions are mandatory to indicate revascularization. However, whereas coronary anatomy is an essential condition for treatment indication, the information about myocardial ischemia is frequently interpreted based on the symptoms of the patient, the results of the stress tests, and frequently only on the basis of the degree of the stenosis evaluated in the angiography.
Despite this paradox, the prognostic information of the presence of ischemia in stress tests and the indication of the best treatment approach is still a matter of intense debate and the rationale of the NHLBI-sponsored International Study of Comparative Health Effectiveness with Medical and Invasive approaches (ISCHEMIA) trial (42).
Myocardial ischemia, cardiovascular outcomes, and CAD treatments
The first studies that compared therapies for CAD focused on the presence of the number of anatomical diseased vessels. One of these first studies, the Coronary Artery Surgery Study (CASS), randomized patients with 1, 2, or 3-vessel coronary disease to MT or coronary bypass surgery (43). Interestingly, 21% of the CAD patients did not have angina symptoms and were selected only on the basis of the anatomical information. In the entire study population and in the population with angina symptoms, the authors found that revascularization interventions did not confer any clinical advantage over MT, in terms of overall death. In the Veterans Administration randomized trial, besides anatomical features, the inclusion of patients was also based on the presence of stable angina symptoms and electrocardiographic evidence of ischemia. In the general population, the authors did not find survival differences between surgery and MT in an 11-year follow-up (44). In the European Coronary Study, the authors also selected patients with angina symptoms, but they did not observe any relevance of angina on prognosis, and the evidence of reductions in death with bypass surgery was only observed in high-risk patients (45).
In the BARI 2D trial, diabetic patients were included if they had ≥50% coronary stenosis with a positive stress test or ≥70% obstruction with classic angina (34). Thus, the selection was based both on anatomical and functional features. The 5.3-year follow-up showed that the reduction of ischemia by revascularization interventions was not superior to MT for reducing overall death or the combined end point of death, myocardial infarction, or stroke. Moreover, a substudy of BARI 2D that assessed the prognostic impact of baseline angina symptoms did not find any association of angina with the occurrence of clinical events (46).
In the MASS II trial, besides the presence of 2- or 3-vessel CAD, inclusion criteria also ponder the presence of angina or positive stress tests. The 5-year follow-up study showed no difference in mortality among MT, percutaneous coronary interventions (PCI), or bypass surgery (39).
Assuming that the angina report is subjective, and its threshold may be variable and difficult to define, and that documented ischemia might impose worse prognosis to CAD patients, previous studies assessing only patients with positive stress tests suggested a prognostic impact of ischemia on clinical outcomes. However, many methodological concerns limit the precision of these studies. One of the most remarkable ones was conducted by Hachamovitch et al. (47) and suggested that documented ischemia is a factor associated with higher rates of cardiovascular death. The authors have also postulated that a myocardial ischemic area of more than 10% would be an indication for revascularization procedures, because of the observed lower mortality of the revascularization group compared with the group of patients managed only with MT. Despite these intuitive results, this analysis has many limitations, especially because of its retrospective nature and treatment selection bias. Because of its retrospective nature, it is impossible to determine the reasons why patients with more than 10% or even 20% of myocardial ischemia underwent MT alone, and not revascularization. Other variables probably influenced treatment choice by the attending physicians. Moreover, the groups assigned to MT or coronary interventions are completely distinct from each other, and even after statistical adjustments by propensity score matching, they probably kept unbalanced confounding factors. Despite all these issues, the interpretation of this work has been used as a reference to many CAD therapy guidelines. The lack of well-designed studies should not be the reason to justify referencing studies with major methodological concerns in clinical practice guidelines.
Other recent analyses have shown opposite results. The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial (48) compared MT alone with PCI plus MT in stable CAD patients with angina pectoris and/or documented stress-induced myocardial ischemia. The main finding of this study was that PCI did not reduce the rates of cardiovascular events compared with MT alone. A nuclear COURAGE substudy analyzed the outcome results of patients who underwent myocardial perfusion scintigraphy before treatment and 6 to 18 months after randomization (49). Only 314 of the initial 2,287 patients of the original trial were studied. The authors showed that PCI more often achieved a reduction in ischemia greater than 5% compared with MT alone, and that the group of patients who had this reduction of at least 5% (both in PCI or MT groups) was associated with a lower unadjusted risk for death or myocardial infarction. However, after multivariable adjustment, this benefit was not observed anymore. Another substudy of the COURAGE trial with 1,381 patients found similar event rates in both therapy arms, irrespective of the severity and extent of ischemia at baseline (50).
The Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2 (FAME 2) trial also compared MT alone with PCI in patients with CAD and significant stenosis based on the measurement of FFR <0.80 (51). In these patients, the FFR-guided PCI group had lower rates of the combined endpoint of death, myocardial infarction, or urgent revascularization, compared with MT alone. However, the results were driven solely by the need for revascularization, and not by death or myocardial infarction.
In our research group, a post-hoc analysis of the MASS II trial assessed cardiovascular event rates (death, MI, or coronary revascularizations) and evolution of left ventricular function after 10-year follow-up in patients with stable multivessel CAD with initial preserved systolic ventricular function (data under publication process). Patients were randomized to MT alone, PCI, or coronary artery bypass grafting (CABG), and were analyzed according to the presence or absence of exercise stress-induced ischemia at baseline. In this study, patients with documented myocardial ischemia had similar rates of cardiovascular events and evolution of ventricular function, compared with multivessel CAD patients with no myocardial ischemia.
A recent meta-analysis of contemporary trials also confirms the findings of these previous studies (52). This study compared the effects of PCI and MT with MT alone in patients with stable CAD and documented myocardial ischemia in clinical outcomes. The authors concluded that PCI was not associated with a reduction in death, nonfatal myocardial infarction, unplanned revascularization, or angina compared with MT alone, in this subset of patients with documented ischemia. Assuming that PCI is more effective for reducing ischemia than MT alone, the therapeutic goal of reducing chronic ischemia does not seem to be really effective in lowering cardiovascular event rates.
Another recent registry study evaluated myocardial ischemia by stress-perfusion cardiac magnetic resonance in all-comer patients with known or suspected CAD (53). The authors showed the combined event rates of cardiac death, MI, or revascularizations in patients stratified by the presence of ischemia. Patients were divided into 3 groups: no ischemia patients, <1.5 ischemic segments, and ≥1.5 ischemic segments. The authors showed that the group with ≥1.5 ischemic segments had higher rates of clinical events than the other 2 groups, in a mean 2.5-year follow-up. Despite these results, selection bias might strongly influence study findings, because it is possible that patients with no detected ischemia actually did not have CAD, whereas patients with ischemia had a greater chance of having obstructive, severe CAD. Thus, the selection of patients with angiographically documented CAD could solve, at least in part, this important methodological bias. Moreover, the classification of patients based on the arbitrary number of ischemic segments may also influence study results.
Even in the context of severe left ventricular dysfunction (ejection fraction ≤35%) and multivessel CAD, a substudy (54) of the Surgical Treatment for Ischemic Heart Failure (STICH) trial suggested that documented myocardial ischemia did not identify patients with worse prognosis or those with greater benefit from CABG over MT alone. However, only 399 of the 1,212 patients of the original trial had ischemia assessed by radionuclide stress test or dobutamine stress echocardiography.
Regarding the role of chronic myocardial ischemia in the development of ventricular arrhythmias or sudden cardiac death, a recent study (55) has also questioned the impact of documented ischemia in major arrhythmic events (MAEs). In this interesting, retrospective study of patients with left ventricular ejection fraction ≤35%, the findings of stress-rest PET showed that markers of myocardial ischemia on PET including global or peri-infarct ischemia, coronary flow reserve, and resting or hyperemic myocardial blood flow were not associated with arrhythmic events in univariable or multivariable analysis. On the other hand, transmural scar was independently associated with MAEs, even after adjustments for confounding factors, including resting left ventricular ejection fraction. Table 1 shows a summary of the results of the main studies that assessed myocardial ischemia and compared CAD treatment options.
Table 1. Summary of relevant studies on CAD, their anatomic definitions, methods to assess myocardial ischemia, percentage of documented myocardial ischemia and main results.
| Article | N | Angiographic criteria | Ischemia assessment | Documented ischemia on stress tests (%) | Main findings |
|---|---|---|---|---|---|
| CASS (MT × CABG) | 780 | Lesions >70%, or 50–70% if left main CAD | Angina CCS-1 or 2 | No | No differences in survival in 6- and 10-year follow-up. No difference between 1, 2 or 3-vessel disease. Benefit for surgical treatment in patients with reduced ejection fraction and 3-vessel disease in 10-year follow-up |
| MASS II (MT × PCI × CABG) | 611 | Proximal 2 or 3 multivessel >70% | Typical angina or positive treadmill test | 45% | No differences in 5-year follow-up regarding death. CABG superior to MT and PCI regarding major cardiovascular events, especially due to lower rates of new coronary interventions |
| BARI-2D (MT × PCI or CABG) | 2,368 | ≥50% with a positive stress test or ≥70% with typical angina | Typical angina or positive treadmill test | – | No difference in the rates of death and major cardiovascular events between patients undergoing prompt revascularization and those undergoing medical therapy |
| COURAGE (MT × PCI) | 2,287 | >70% and ischemia on resting ECG or stress test, or >80% and typical angina | Typical angina or positive stress test (treadmill or pharmacologic) | 85% | No differences in the risk of death, myocardial infarction, or other major cardiovascular events between MT and PCI |
| FAME-II | 888 | >50% in ≥1 vessel with FFR of 0.80 or less | Typical angina and FFR measures | 100% | Lower rates of the primary composite end point of death, MI, or urgent revascularization at 5 years for PCI group, due to lower rates of revascularizations. No significant difference in death |
| Hachamovitch et al. | 10,627 | No angiographic criteria | Nuclear study (exercise or pharmacologic stress) | 33% | Revascularization compared with MT had greater survival benefit in patients with moderate to large amounts of inducible ischemia |
| Garzillo et al. | 535 | Proximal multivessel >70% | Exercise stress testing | 50% | Presence of documented ischemia was not associated with the occurrence of major adverse events or changes in ejection fraction |
| Vincenti et al. | 1,024 | No angiographic criteria | Stress perfusion CMR | 33% | An ischemia burden of >1.5 ischemic segments on stress-perfusion CMR was associated with composite endpoints |
| Gupta et al. | 439 | No angiographic criteria | Stress/rest PET | 28% | Documented ischemia was not associated with appropriate ICD shocks and sudden cardiac death in patients with LVEF <35% |
PET, positron-emission tomography; CAD, coronary artery disease; CASS, Coronary Artery Surgery Study; MT, medical therapy; CABG, coronary artery bypass grafting; MASS II, Medicine, Angioplasty, or Surgery Study II; PCI, percutaneous coronary interventions; COURAGE, Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation; FAME, Fractional Flow Reserve versus Angiography; ECG, electrocardiogram; FFR, fractional flow reserve; CMR, cardiac magnetic resonance; ICD, implantable cardiac defibrillator; LVEF, left ventricular ejection fraction.
Although it seems reasonable that alleviating myocardial ischemia might be a therapy goal for CAD patients, despite their symptoms, it is uncertain whether documented myocardial ischemia is associated with higher endpoint rates, worsening of ventricular function, and even development of ventricular arrhythmias, as indicated in these recent articles. Moreover, the findings of these recent studies that interventional therapies do not lower cardiovascular risk compared with MT alone also suggest that alleviating chronic ischemia might not be a definitive therapeutic goal, at least for the majority of CAD patients. However, this is still a controversial issue and awaits further prospective, well-designed studies. Finally, the functional information of documented myocardial ischemia seems not to represent or predict atherosclerotic plaque instability, which is the major responsible for clinical events. Thus, the interpretation of such functional tests should be done cautiously, and not isolated from other clinical information, especially when revascularization strategies are being considered for the treatment of CAD patients.
We hope that results from the ongoing ISCHEMIA trial shall bring more reliable information regarding this important issue.
Acknowledgements
The study was supported by Zerbini Foundation.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Marzilli M, Merz CN, Boden WE, et al. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link! J Am Coll Cardiol 2012;60:951-6. 10.1016/j.jacc.2012.02.082 [DOI] [PubMed] [Google Scholar]
- 2.Schaper W, Ito WD. Molecular mechanisms of coronary collateral vessel growth. Circ Res 1996;79:911-9. 10.1161/01.RES.79.5.911 [DOI] [PubMed] [Google Scholar]
- 3.Chilian WM, Penn MS, Pung YF, et al. Coronary collateral growth--back to the future. J Mol Cell Cardiol 2012;52:905-11. 10.1016/j.yjmcc.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulton WF. Arterial anastomoses in the coronary circulation. I. Anatomical features in normal and diseased heart demonstrated by stereoarteriography. Scott Med J 1963;8:420-34. 10.1177/003693306300801102 [DOI] [PubMed] [Google Scholar]
- 5.Berry C, Balachandran KP, L'Allier PL, et al. Importance of collateral circulation in coronary heart disease. Eur Heart J 2007;28:278-91. 10.1093/eurheartj/ehl446 [DOI] [PubMed] [Google Scholar]
- 6.Habib GB, Heibig J, Forman SA, et al. Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. The TIMI Investigators. Circulation 1991;83:739-46. 10.1161/01.CIR.83.3.739 [DOI] [PubMed] [Google Scholar]
- 7.Longhurst JC, Tjen-A-Looi SC, Fu LW. Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes. Ann N Y Acad Sci 2001;940:74-95. 10.1111/j.1749-6632.2001.tb03668.x [DOI] [PubMed] [Google Scholar]
- 8.Kalogeris T, Baines CP, Krenz M, et al. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 2012;298:229-317. 10.1016/B978-0-12-394309-5.00006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khand A, Fisher M, Jones J, et al. The collateral circulation of the heart in coronary total arterial occlusions in man: systematic review of assessment and pathophysiology. Am Heart J 2013;166:941-52. 10.1016/j.ahj.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 10.Bolli R, Marbán E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 1999;79:609-34. 10.1152/physrev.1999.79.2.609 [DOI] [PubMed] [Google Scholar]
- 11.Depre C, Vatner SF. Cardioprotection in stunned and hibernating myocardium. Heart Fail Rev 2007;12:307-17. 10.1007/s10741-007-9040-3 [DOI] [PubMed] [Google Scholar]
- 12.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124-36. 10.1161/01.CIR.74.5.1124 [DOI] [PubMed] [Google Scholar]
- 13.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 2007;12:181-8. 10.1007/s10741-007-9025-2 [DOI] [PubMed] [Google Scholar]
- 14.Liu GS, Thornton J, Van Winkle DM, et al. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation 1991;84:350-6. 10.1161/01.CIR.84.1.350 [DOI] [PubMed] [Google Scholar]
- 15.Wall TM, Sheehy R, Hartman JC. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther 1994;270:681-9. [PubMed] [Google Scholar]
- 16.Grover GJ, Sleph PG, Dzwonczyk S. Role of myocardial ATP-sensitive potassium channels in mediating preconditioning in the dog heart and their possible interaction with adenosine A1-receptors. Circulation 1992;86:1310-6. 10.1161/01.CIR.86.4.1310 [DOI] [PubMed] [Google Scholar]
- 17.Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res 1992;70:223-33. 10.1161/01.RES.70.2.223 [DOI] [PubMed] [Google Scholar]
- 18.Przyklenk K, Bauer B, Ovize M, et al. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87:893-9. 10.1161/01.CIR.87.3.893 [DOI] [PubMed] [Google Scholar]
- 19.Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 2003;285:H579-88. 10.1152/ajpheart.01064.2002 [DOI] [PubMed] [Google Scholar]
- 20.MacAlpin RN, Kattus AA. Adaption to exercise in angina pectoris. The electrocardiogram during treadmill walking and coronary angiographic findings. Circulation 1966;33:183-201. 10.1161/01.CIR.33.2.183 [DOI] [PubMed] [Google Scholar]
- 21.Ylitalo K, Jama L, Raatikainen P, et al. Adaptation to myocardial ischemia during repeated dynamic exercise in relation to findings at cardiac catheterization. Am Heart J 1996;131:689-97. 10.1016/S0002-8703(96)90272-0 [DOI] [PubMed] [Google Scholar]
- 22.Maybaum S, Ilan M, Mogilevsky J, et al. Improvement in ischemic parameters during repeated exercise testing: a possible model for myocardial preconditioning. Am J Cardiol 1996;78:1087-91. 10.1016/S0002-9149(96)90057-0 [DOI] [PubMed] [Google Scholar]
- 23.Galiñanes M, Fowler AG. Role of clinical pathologies in myocardial injury following ischaemia and reperfusion. Cardiovasc Res 2004;61:512-21. 10.1016/j.cardiores.2003.11.028 [DOI] [PubMed] [Google Scholar]
- 24.Ferdinandy P, Hausenloy DJ, Heusch G, et al. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 2014;66:1142-74. 10.1124/pr.113.008300 [DOI] [PubMed] [Google Scholar]
- 25.Fryer RM, Auchampach JA, Gross GJ. Therapeutic receptor targets of ischemic preconditioning. Cardiovasc Res 2002;55:520-5. 10.1016/S0008-6363(02)00316-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Mieren G, Nevelsteen I, Vanderper A, et al. Angiotensin-converting enzyme inhibition and food restriction restore delayed preconditioning in diabetic mice. Cardiovasc Diabetol 2013;12:36. 10.1186/1475-2840-12-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahmi Garcia RM, Rezende PC, Hueb W. Impact of hypoglycemic agents on myocardial ischemic preconditioning. World J Diabetes 2014;5:258-66. 10.4239/wjd.v5.i3.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahmi RM, Uchida AH, Rezende PC, et al. Effect of hypoglycemic agents on ischemic preconditioning in patients with type 2 diabetes and symptomatic coronary artery disease. Diabetes Care 2013;36:1654-9. 10.2337/dc12-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman ME. William Heberden and some account of a disorder of the breast. Clin Cardiol 1987;10:211-3. 10.1002/clc.4960100314 [DOI] [PubMed] [Google Scholar]
- 30.Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000;283:3223-9. 10.1001/jama.283.24.3223 [DOI] [PubMed] [Google Scholar]
- 31.Junghans C, Sekhri N, Zaman MJ, et al. Atypical chest pain in diabetic patients with suspected stable angina: impact on diagnosis and coronary outcomes. Eur Heart J Qual Care Clin Outcomes 2015;1:37-43. 10.1093/ehjqcco/qcv003 [DOI] [PubMed] [Google Scholar]
- 32.Ängerud KH, Brulin C, Näslund U, et al. Patients with diabetes are not more likely to have atypical symptoms when seeking care of a first myocardial infarction. An analysis of 4028 patients in the Northern Sweden MONICA Study. Diabet Med 2012;29:e82-7. 10.1111/j.1464-5491.2011.03561.x [DOI] [PubMed] [Google Scholar]
- 33.Sharma A, Sekaran NK, Coles A, et al. Impact of diabetes mellitus on the evaluation of stable chest pain patients: insights from the PROMISE (Prospective multicenter imaging study for evaluation of chest pain) trial. J Am Heart Assoc 2017;6:e007019. 10.1161/JAHA.117.007019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.BARI 2D Study Group, Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503-15. [DOI] [PMC free article] [PubMed]
- 35.Williams RP, Manou-Stathopoulou V, Redwood SR, et al. 'Warm-up Angina': harnessing the benefits of exercise and myocardial ischaemia. Heart 2014;100:106-14. 10.1136/heartjnl-2013-304187 [DOI] [PubMed] [Google Scholar]
- 36.Gavazzi A, De Servi S, Cornalba C, et al. Significance of the walk-through angina phenomenon during exercise testing. Cardiology 1986;73:47-53. 10.1159/000173981 [DOI] [PubMed] [Google Scholar]
- 37.Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703-8. 10.1056/NEJM199606273342604 [DOI] [PubMed] [Google Scholar]
- 38.Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol 1974;34:48-55. 10.1016/0002-9149(74)90092-7 [DOI] [PubMed] [Google Scholar]
- 39.Hueb W, Lopes NH, Gersh BJ, et al. Five-year follow-up of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation 2007;115:1082-9. 10.1161/CIRCULATIONAHA.106.625475 [DOI] [PubMed] [Google Scholar]
- 40.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24. 10.1056/NEJMoa0807611 [DOI] [PubMed] [Google Scholar]
- 41.Van Belle E, Rioufol G, Pouillot C, et al. Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circulation 2014;129:173-85. 10.1161/CIRCULATIONAHA.113.006646 [DOI] [PubMed] [Google Scholar]
- 42.ISCHEMIA Trial Research Group , Maron DJ, Hochman JS, et al. International study of comparative health effectiveness with medical and invasive approaches (ISCHEMIA) trial: Rationale and design. Am Heart J 2018;201:124-35. 10.1016/j.ahj.2018.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coronary Artery Surgery Study (CASS) : a randomized trial of coronary artery bypass surgery. Survival data. Circulation 1983;68:939-50. 10.1161/01.CIR.68.5.939 [DOI] [PubMed] [Google Scholar]
- 44.Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group Eleven-year survival in the Veterans Administration randomized trial of coronary bypass surgery for stable angina. N Engl J Med 1984;311:1333-9. 10.1056/NEJM198411223112102 [DOI] [PubMed] [Google Scholar]
- 45.Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med 1988;319:332-7. 10.1056/NEJM198808113190603 [DOI] [PubMed] [Google Scholar]
- 46.Dagenais GR, Lu J, Faxon DP, et al. Prognostic impact of the presence and absence of angina on mortality and cardiovascular outcomes in patients with type 2 diabetes and stable coronary artery disease: results from the BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial. J Am Coll Cardiol 2013;61:702-11. 10.1016/j.jacc.2012.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011;32:1012-24. 10.1093/eurheartj/ehq500 [DOI] [PubMed] [Google Scholar]
- 48.Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503-16. 10.1056/NEJMoa070829 [DOI] [PubMed] [Google Scholar]
- 49.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283-91. 10.1161/CIRCULATIONAHA.107.743963 [DOI] [PubMed] [Google Scholar]
- 50.Shaw LJ, Weintraub WS, Maron DJ, et al. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J 2012;164:243-50. 10.1016/j.ahj.2012.05.018 [DOI] [PubMed] [Google Scholar]
- 51.Xaplanteris P, Fournier S, Pijls NHJ, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 2018;379:250-9. 10.1056/NEJMoa1803538 [DOI] [PubMed] [Google Scholar]
- 52.Stergiopoulos K, Boden WE, Hartigan P, et al. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med 2014;174:232-40. 10.1001/jamainternmed.2013.12855 [DOI] [PubMed] [Google Scholar]
- 53.Vincenti G, Masci PG, Monney P, et al. Stress perfusion CMR in patients with known and suspected CAD. Prognostic value and optimal ischemic threshold for revascularization. JACC Cardiovasc Imaging 2017;10:526-37. 10.1016/j.jcmg.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 54.Panza JA, Holly TA, Asch FM, et al. Inducible myocardial ischemia and outcomes in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol 2013;61:1860-70. 10.1016/j.jacc.2013.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta A, Harrington M, Albert CM, et al. Myocardial scar but not ischemia is associated with defibrillator shocks and sudden cardiac death in stable patients with reduced left ventricular ejection fraction. JACC Clin Electrophysiol 2018;4:1200-10. 10.1016/j.jacep.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]