Abstract
Background
Adequate patient selection is the key to successful lung volume reduction in patients with pulmonary emphysema. Computed tomography (CT) enables a reliable detection of pulmonary emphysema and allows an accurate quantification of the severity. Our goal was to investigate the usefulness and reliability of color-coded (CC) CT images in classification of emphysema and preoperative lung volume reduction planning.
Methods
Fifty patients undergoing lung volume reduction surgery at our institution between September 2015 and February 2016 were retrospectively investigated. Three readers visually assessed the amount and distribution patterns of pulmonary emphysema on axial, multi-planar and CC CT images using the Goddard scoring system and a surgically oriented grading system (bilateral markedly heterogenous, bilateral intermediately heterogenous, bilateral homogenous and unilateral heterogenous emphysema). Observer dependency was investigated by using Fleiss’ kappa (κ) and the intraclass correlation coefficient (ICC). Results were compared to quantitative results from densitometry measurements and lung perfusion scintigraphy by using Spearman correlation. Recommendations for lung volume reduction sites based on emphysema amount and distribution of all readers were compared to removal sites from the surgical reports.
Results
Inter-rater agreement for emphysema distribution rating was substantial for CC images (κ=0.70; 95% CI, 0.64–0.80) and significantly better compared to axial and multiplanar images (P≤0.001). The inter-rater agreement for recommended segment removal was moderate for CC images (κ=0.56; 95% CI, 0.49–0.63) and significantly better compared to axial and multiplanar images (P<0.001). Visual emphysema rating correlated significantly with measurements from densitometry and perfusion scintigraphy in the upper and lower lung zones in all image types.
Conclusions
CC CT images allow a precise, less observer-dependent evaluation of distribution of pulmonary emphysema and resection recommendation compared to axial and multiplanar CT images and might therefore be useful in lung volume resection surgery planning.
Keywords: Pulmonary emphysema; densitometry; pneumonectomy; tomography, spiral computed; pulmonary disease; chronic obstructive
Introduction
Pulmonary emphysema is a long-term, irreversible pneumopathy defined as an abnormal permanent enlargement beyond the terminal bronchioles due to a destruction of lung parenchyma (1,2). Emphysema is one manifestation of a group of obstructive, chronic and often progressive lung diseases, i.e., chronic obstructive pulmonary disease (COPD) (3).
There are only limited treatment options available for patients with severe emphysema. Among them, lung volume reduction surgery (LVRS), may improve respiratory mechanics by removing damaged lung tissue (4). Since its introduction in 1990s, many studies investigated patient selection for LVRS and revealed that correct identification of the type of emphysema distribution is of great importance regarding success of this type of surgery (5-9).
Ever since, imaging modalities play an important role in the diagnosis of pulmonary diseases and computed tomography (CT) has evolved to be the principal and most widely used diagnostic tool for detailed imaging of lung parenchyma (10,11). Imaging of the lungs using CT enables a reliable detection of pulmonary emphysema and moreover allows an accurate quantification of the severity.
Basically, there are two different techniques available for the assessment and quantification of pulmonary emphysema in CT. First the semi-quantitative, where an experienced radiologist or clinician performs a subjective visual grading of the severity of pulmonary emphysema using a pre-defined numerical score chart. By contrast, the quantitative approach enables an automated assessment of the pulmonary emphysema depicting emphysematous areas as low attenuation areas (LAAs) in respect of a defined threshold value ranging from −900 Hounsfield unit (HU) to −950 HU (12,13). On the other hand, Technetium-99m macro aggregated albumin (99mTc-MAA) perfusion scintigraphy may further aid in identifying target areas of resection in LVRS patients with homogenous emphysema distribution (14).
Since the correct identification of the present type of emphysema distribution belongs to the most important factors to estimate the clinical outcome after LVRS, we developed a color-coded (CC) imaging of the emphysematous lung using the quantitative approach. The purpose of this study was to investigate the reliability of the new visualisation type on classification of emphysema and preoperative resection planning.
Methods
Subjects
We retrospectively investigated all patients undergoing thoracoscopic LVRS at our institution between September 2015 and February 2016. The local ethics committee approved the study (EC-No. 2014-0275). Inclusion criteria were availability of a preoperative CT-scan and Technetium 99mTC macro aggregated albumin (99mTc-MAA) perfusion scintigraphy.
CT protocol
All subjects underwent a preoperative CT at full inspiration and full expiration using a standard CT protocol. All scans were acquired with tube current modulation (mA modulation) to ensure correct patient exposure and reduce patient dosis and voltage was selected according to the patient size and ranged between 120–140 kVp. The protocol further included reconstructions with 2 mm slice-thickness and tissue convolution kernel as well as lung window settings (15). Our standard protocol did not include intravenous contrast medium since higher lung parenchyma densities are detected when contrast medium is administered (16,17). All CT scans were acquired during a single breath-hold.
Image processing
Three types of datasets were prepared for the readout: (I) axial high resolution CT slices with 2 mm slice thickness in lung window (W/L: 1,600/−700 HU), further called “axial images”; (II) sagittal and coronal reconstructed high resolution CT slices with 2 mm slice thickness in lung window, further called “MPR images” (multiplanar reconstruction images); and (III) CC images, using density mask technique, in all three dimensions depicting the subranges using a dedicated automated software (3viseon 3.5, 3mensio medical imaging, The Netherlands), further called “CC images”. The density mask technique settings were as follow: ranges of 40 HU per color starting from −1,000 to −760 HU, using red for the range from −1,000 to −960 HU corresponding severe emphysema and green for the range of −800 to −760 HU corresponding normal lung tissue.
Visual assessment of CT
Three readers (reader 1, radiologist with 9 years of experience in thoracic imaging; reader 2, radiologist with 5 years of experience in thoracic imaging, and reader 3, thoracic surgeon, with 5 years of experience) assessed all patients on axial, MPR and CC lung images. All images were displayed on PACS workstation (AGFA HealthCare, Mortsel, Belgium). The readers evaluated the images three times with an interval of 3 weeks starting with the axial images. All readers were blinded to information regarding the patients’ clinical data, the results of quantitative assessment and results from surgery. Emphysema distribution was visually assessed using a surgically oriented grading system, based on differences in the extent of lung destruction in adjacent lung segments: bilateral markedly heterogenous, bilateral intermediately heterogenous, bilateral homogenous and unilateral heterogenous emphysema (5,18,19). Each lung was divided into three zones: the upper zone (extending from the apices to the aortic arch), the mid zone (extending from the aortic arch to the level of the tracheal bifurcation), and the lower zone (extending from the tracheal bifurcation to the level of the diaphragm). The amount of emphysema was visually graded according to the modified Goddard scoring system (20)—0: no signs of emphysema; 1: 1–25%; 2: 26–50%; 3: 51–75%; and 4: >75% of emphysema, respectively. Each reader furthermore noted up to 6 lung zones (upper right/left including the apical upper lobe; middle right/left including the basal upper lobe, and the superior lower lobe; basal right/left including the basal lower lobe, middle lobe/lingula) per patient which he would recommend for removal during LVRS based to its emphysema amount and the global emphysema distribution.
Quantitative assessment of CT
The amount of pulmonary emphysema defined as the percentage of lung parenchyma below the predefined threshold of −950 (LAA%, LAA/total lung volume) was automatedly calculated using dedicated segmentation software (Ziostation2, Ziosoft, Tokyo, Japan). All larger airways were excluded from the analysis. A medical student assessed the segmentation and performed corrections if necessary.
Lung perfusion scintigraphy
Planar lung perfusion scintigraphy was performed using a dedicated scanner (Discovery NM/CT 670 or Infinia Hawkeye, GE Healthcare, Waukesha, WI, USA) after intravenous injection of 180±20 MBq by 99mTc-MAA (99mTc-Technetium-Macrosalb MAASOL, GE Healthcare) in anterior, posterior, right lateral, left lateral, left posterior oblique, and right posterior oblique view (140 keV ±10%, LEHR collimator; matrix: 256×256, acquisition time: 101 s) The amount of perfusion defined as % of the total measured 99mTc-MAA activity on 2D plane was calculated using three equal rectangular regions of interest (ROI) on the anterior and posterior views: top, middle, and bottom. The counts in each ROI were divided by the total counts over the lung measured from the anterior and posterior view.
LVRS surgery
All patients were operated by unilateral or bilateral video assisted thoracic surgery LVRS or by thoracotomy in the case of adhesions. Targeted lung tissue for resection was chosen based on preoperative radiological assessment using the above-mentioned images and intraoperative findings of trapped air and perfusion and were removed by using atypical resection and/or lobectomy (21,22). None of the readers were involved in the preoperative multidisciplinary emphysema treatment board, where all candidates for LVRS were discussed by thoracic surgeons, pulmonologists and radiologists.
Statistics
The statistical analysis was performed in R version 3.5 (R Foundation for Statistical Computing, Vienna, Austria). Inter-rater agreement for nominal data was assessed using Fleiss’ kappa (κ) and confidence intervals (CI) were calculated using resampling with bootstrapping (23). Inter-rater reliability for ordinal data was assessed with the intraclass correlation coefficient (ICC) (24). Inter-rater agreement was interpreted according to Landis and Koch—almost perfect: 0.8–1.00; substantial: 0.61–0.80; moderate: 0.41–0.60; fair: 0.21–0.40; slight: 0.00–0.20; poor: <0.00 (25). Inter-rater reliability was interpreted after the suggestion of Cicchetti and Sparrow—excellent agreement: >0.75; good agreement: 0.59–0.75; fair agreement: 0.40–0.58; and poor agreement: <0.40 (26). Association between nominal data was evaluated using χ2 and Cramer’s V coefficient. Spearman correlation coefficient (ρ) was used to assess correlation between ordinal and continuous data and among continuous data. A P value of less than 0.05 were considered statistically significant. Bonferroni correction was applied when appropriate.
Results
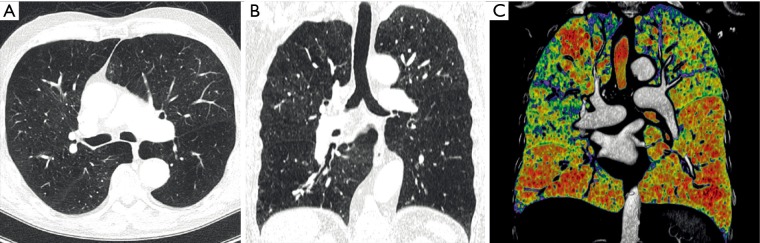
Fifty patients fulfilled all criteria and were included. Selection of the study population is shown in Figure 1. A total of 150 axial, MPR and CC CT image sets were assessed by the three readers. The analysis included a total of 133 lung zones involved in LVRS. Ninety-four percent (47/50) of the patients had atypical resection and 6% (3/50) had lobectomy and atypical resection. Eighteen percent (9/50) of the patients had a unilateral LVRS, 82% (41/50) had a bilateral LVRS. Demographics and further details of the study population is given in Table 1. Example of a preoperative CT scan including axial, multiplanar and CC images is depicted in Figure 2.
Figure 1.
Flow chart of study population selection. LVRS, lung volume reduction surgery.
Table 1. Demographics of study patients (n=50).
| Variable | Number |
|---|---|
| Female/male | 22/28 |
| Age at CT scan, years | 63 [42–84] |
| Time between CT and perfusion scintigraphy, days | 53 [0–418] |
| CT before scintigraphy | 28 (56%) |
| CT after scintigraphy | 7 (14%) |
| CT and scintigraphy on same day | 15 (30%) |
| Time between CT and LVRS, days | 49 [1–267] |
Data presented as n, mean [range], or n (%). CT, computed tomography; LVRS, lung volume reduction surgery.
Figure 2.
Preoperative CT of a 79-year-old subject with COPD Gold IV. (A) Axial, (B) multiplanar (MPR), and (C) color-coded (CC) CT images. Emphysema distribution scoring by three readers was as follows: 2× unilateral heterogenous and 1× bilateral intermediately heterogenous on axial images, 2× unilateral intermediately heterogenous and 1× bilateral intermediately heterogenous on MPR images and 3× bilateral heterogenous on CC images. COPD, chronic obstructive pulmonary disease.
Observer dependency
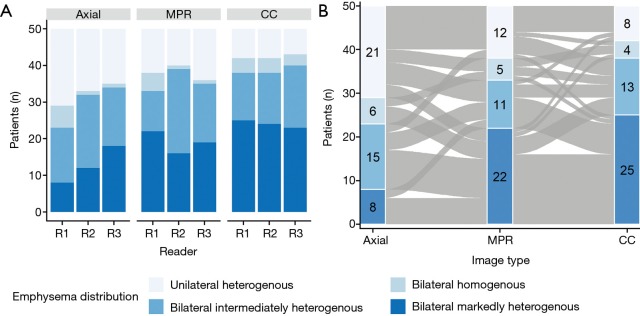
Inter-rater agreement for emphysema distribution rating was fair for axial and MPR (κ=0.26; 95% CI, 0.11–0.37 and κ=0.40; 95% CI, 0.22–0.50) and moderate for CC images (κ=0.70; 95% CI, 0.64–0.80), respectively. Inter-rater agreement was significantly better for CC images compared to axial (P≤0.001) and MPR images (P≤0.001). Results of the visual emphysema distribution rating is illustrated in Figure 3A. ICC for emphysema amount rating was good (0.77; 95% CI, 0.72–0.81) for axial, fair for MPR (0.60; 95% CI, 0.51–0.67) and good for CC images (0.74; 95% CI, 0.68–0.79), respectively. The inter-rater agreement for recommended segment removal was fair for axial and MPR images (κ=0.33; 95% CI, 0.26–0.39 and κ=0.39; 95% CI, 0.32–0.46, respectively) and moderate for CC images (κ=0.56; 95% CI, 0.49–0.63). Inter-rater agreement was significantly better for CC images compared to axial (P≤0.001) and MPR images (P≤0.001).
Figure 3.
Emphysema distribution rating results. (A) Stacked bar plots of the scores of the emphysema distribution readout [on axial, multiplanar (MRP) and color-coded (CC) images] for readers 1–3 (x-axis) and absolute count of the different emphysema distribution scores (y-axis). (B) Sankey network plot showing emphysema distribution scores of reader 1 per n patient (y-axis) for axial, MRP and CC images (x-axis). Note the detailed differences between the reformations (ribbons linking the reformations).
Association between image type and emphysema distribution
Visual emphysema distribution scores were significantly associated with the image type (χ2=24.149, df =6, P value <0.001). Emphysema was more frequently scored as bilateral markedly heterogenous emphysema on CC images compared to axial and MPR images (Cramer’s V =0.164, 95% CI, 0.12–0.24). Details of the readout of reader 1 are shown in Figure 3B. Combined contingency table of the emphysema distribution scores of readers 1–3 is shown in Table 2.
Table 2. Combined contingency table of emphysema distribution scores of readers 1–3.
| Image type | Scores | |||
|---|---|---|---|---|
| Bilateral markedly heterogenous | Bilateral intermediately heterogenous | Bilateral homogenous | Unilateral heterogenous emphysema | |
| Axial | 38 | 51 | 8 | 53 |
| MPR | 57 | 50 | 7 | 36 |
| CC | 72 | 44 | 11 | 23 |
CT, computed tomography; MPR, multiplanar reconstruction.
Correlation with quantitative CT
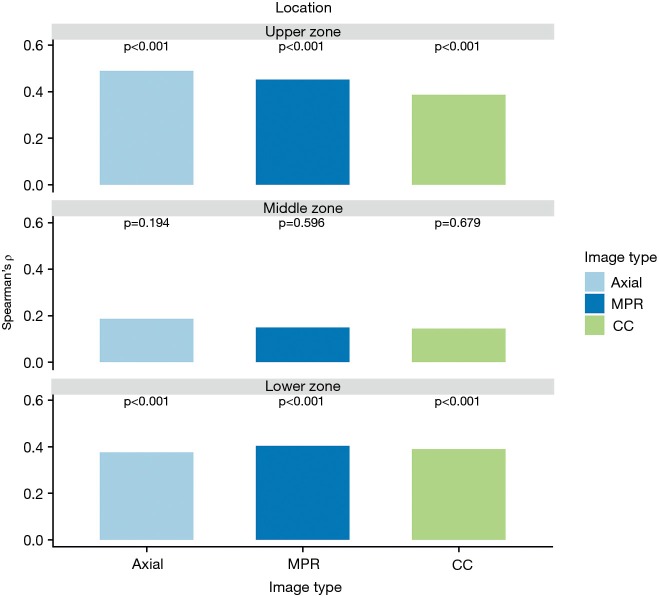
Visual emphysema amount of all images correlated significantly with the measured amount of emphysema in the upper and lower lung zones (ρ ranged between 0.39–0.48 in the upper lung zones and between 0.39–0.40 in the lower lung zones, all P value <0.001). There was no significant correlation between emphysema amount rating and measured amount of emphysema in middle zones (ρ ranged between 0.15–0.18, P value ranged from 0.194–0.679). All correlation results are visually summarized in Figure 4 for further details see Table S1. Emphysema amount was overestimated in most of all analysed zones on all images [in 79.3% (714/900) zones of axial, 80.7% (726/900) zones of MPR and 78.8% (709/900) of zones in CC images, respectively].
Figure 4.
Bar plots representing Spearman’s ρ (y-axis) of visual emphysema scores and measured emphysema amount per CT-reformation (x-axis) for different lung zones. Note the significant correlation between visual emphysema score and measured emphysema amount in the upper and lower zones for all reconstructions.
Table S1. Results of spearman correlation between visual emphysema score of all readers and calculated emphysema amount.
| Image-type | Location | Spearman ρ | P value |
|---|---|---|---|
| Axial | 1 | 0.48 | <0.001 |
| Axial | 2 | 0.18 | 0.194 |
| Axial | 3 | 0.39 | <0.001 |
| MPR | 1 | 0.45 | <0.001 |
| MPR | 2 | 0.15 | 0.596 |
| MPR | 3 | 0.40 | <0.001 |
| CC | 1 | 0.39 | <0.001 |
| CC | 2 | 0.15 | 0.679 |
| CC | 3 | 0.40 | <0.001 |
MPR, multiplanar reconstruction; CC, colour-coded; 1, upper zone; 2 middle zone; 3, lower zone.
Correlation with lung perfusion scintigraphy
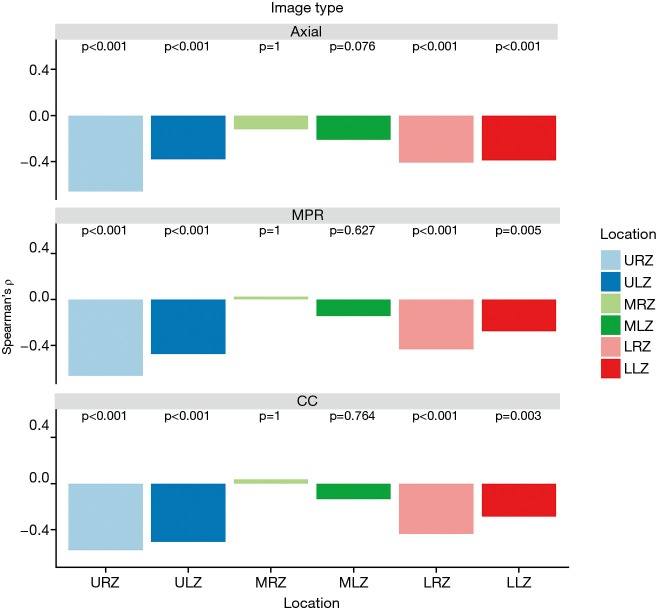
Visual emphysema amount of all image types showed a significantly negative correlation with perfusion measurements from lung perfusion scintigraphy in the upper and lower zones (P value ranged between 0.001 and <0.001 and 0.004 and <0.001, respectively). There was no significant correlation between visual emphysema amount and perfusion measurements for the middle zones (P value ranged between 0.105–1). Correlation results are depicted in Figure 5 and detailed results for each reader 1–3 are summarized in Table S2 in the electronic supplementary material.
Figure 5.
Bar plots representing Spearman’s ρ (y-axis) of visual emphysema scores of three readers on axial, multiplanar (MRP) and color-coded (CC) images and measured perfusion percent with scintigraphy per lung location. URZ, upper right zone; ULZ, upper left zone; MRZ, middle right zone; MLZ, middle left zone; LRZ, lower right zone; LLZ, lower left zone.
Table S2. Results of spearman correlation between visual emphysema score for each reader and calculated emphysema amount.
| Reader | Location | Image type | Spearman ρ | Statistic | P value |
|---|---|---|---|---|---|
| 1 | 1 | Axial | −0.18 | 24498.91 | 1 |
| 1 | 2 | Axial | −0.64 | 34147.17 | <0.001 |
| 1 | 3 | Axial | −0.29 | 26808.98 | 0.43 |
| 1 | 4 | Axial | −0.21 | 25212.2 | 1 |
| 1 | 5 | Axial | −0.42 | 29527.47 | 0.025 |
| 1 | 6 | Axial | −0.43 | 29873.99 | 0.016 |
| 1 | 1 | MPR | −0.56 | 32388.69 | <0.001 |
| 1 | 2 | MPR | −0.69 | 35154.75 | <0.001 |
| 1 | 3 | MPR | −0.28 | 26585.51 | 0.518 |
| 1 | 4 | MPR | −0.02 | 21203.65 | 1 |
| 1 | 5 | MPR | −0.21 | 25236.96 | 1 |
| 1 | 6 | MPR | −0.43 | 29844.09 | 0.017 |
| 1 | 1 | CC | −0.62 | 33754.92 | <0.001 |
| 1 | 2 | CC | −0.62 | 33758.54 | <0.001 |
| 1 | 3 | CC | −0.09 | 22796.74 | 1 |
| 1 | 4 | CC | −0.08 | 22473.02 | 1 |
| 1 | 5 | CC | −0.18 | 24644.27 | 1 |
| 1 | 6 | CC | −0.46 | 30419.63 | 0.008 |
| 2 | 1 | Axial | −0.5 | 31288.84 | 0.002 |
| 2 | 2 | Axial | −0.68 | 35015.31 | <0.001 |
| 2 | 3 | Axial | −0.22 | 25312.67 | 1 |
| 2 | 4 | Axial | −0.08 | 22554.7 | 1 |
| 2 | 5 | Axial | −0.42 | 29582.28 | 0.024 |
| 2 | 6 | Axial | −0.54 | 32090.78 | <0.001 |
| 2 | 1 | MPR | −0.55 | 32187.65 | <0.001 |
| 2 | 2 | MPR | −0.7 | 35445.46 | <0.001 |
| 2 | 3 | MPR | −0.26 | 26211.97 | 0.697 |
| 2 | 4 | MPR | 0.08 | 19106.2 | 1 |
| 2 | 5 | MPR | −0.34 | 28003.93 | 0.142 |
| 2 | 6 | MPR | −0.56 | 32449.89 | <0.001 |
| 2 | 1 | CC | −0.61 | 33425.26 | <0.001 |
| 2 | 2 | CC | −0.57 | 32618.88 | <0.001 |
| 2 | 3 | CC | −0.27 | 26423.82 | 0.59 |
| 2 | 4 | CC | 0.09 | 18898.96 | 1 |
| 2 | 5 | CC | −0.41 | 29400.57 | 0.03 |
| 2 | 6 | CC | −0.55 | 32277.25 | <0.001 |
| 3 | 1 | Axial | −0.49 | 31091.91 | 0.003 |
| 3 | 2 | Axial | −0.73 | 36131.37 | <0.001 |
| 3 | 3 | Axial | −0.16 | 24229.46 | 1 |
| 3 | 4 | Axial | −0.09 | 22745.62 | 1 |
| 3 | 5 | Axial | −0.49 | 30982.09 | 0.003 |
| 3 | 6 | Axial | −0.48 | 30735.86 | 0.005 |
| 3 | 1 | MPR | −0.43 | 29718.86 | 0.02 |
| 3 | 2 | MPR | −0.75 | 36436.97 | <0.001 |
| 3 | 3 | MPR | 0 | 20829.2 | 1 |
| 3 | 4 | MPR | 0 | 20728.45 | 1 |
| 3 | 5 | MPR | −0.34 | 27859.54 | 0.164 |
| 3 | 6 | MPR | −0.4 | 29246.28 | 0.036 |
| 3 | 1 | CC | −0.37 | 28527.54 | 0.082 |
| 3 | 2 | CC | −0.6 | 33308.99 | <0.001 |
| 3 | 3 | CC | −0.11 | 23103.52 | 1 |
| 3 | 4 | CC | 0.06 | 19656.54 | 1 |
| 3 | 5 | CC | −0.32 | 27497.17 | 0.233 |
| 3 | 6 | CC | −0.39 | 28892.66 | 0.054 |
MPR, multiplanar reconstruction; CC, colour-coded; 1, upper right zone; 2 upper left zone; 3, middle right zone; 4, lower right zone; 5, lower left zone.
Association between recommended zones for removal and surgery
CT-based, recommended zones for removal during LVRS were significantly associated with the removed segments during surgery for all types of images: axial (χ2=1,040, df =36, P value =0; Cramer’s V =0.59, 95% CI, 0.54–0.61), MPR (χ2=991.7, df =36, P value ≤0.001, Cramer’s V =0.57, 95% CI, 0.53–0.60) and CC (χ2=1,032.8, df =36, P value =0; Cramer’s V =0.58, 95% CI, 0.53–0.60). Agreement between preoperative suggestions and results from surgery is depicted in Table 3.
Table 3. Agreement of recommended resection zones of all 3 readers based on axial, MPR and CC images regarding resected zones during surgery.
| Image type | Surgery | ||||||
|---|---|---|---|---|---|---|---|
| 0 | URZ | ULZ | MRZ | MRZ | LRZ | LLZ | |
| Axial | |||||||
| 0 | 0 | 27 | 26 | 10 | 15 | 60 | 40 |
| URZ | 19 | 66 | 0 | 0 | 0 | 0 | 0 |
| ULZ | 18 | 0 | 67 | 0 | 0 | 0 | 0 |
| MRZ | 23 | 0 | 0 | 20 | 0 | 0 | 0 |
| MLZ | 21 | 0 | 0 | 0 | 24 | 0 | 0 |
| LRZ | 9 | 0 | 0 | 0 | 0 | 18 | 0 |
| LLZ | 11 | 0 | 0 | 0 | 0 | 0 | 26 |
| MPR | |||||||
| 0 | 0 | 23 | 27 | 15 | 16 | 59 | 44 |
| URZ | 17 | 70 | 0 | 0 | 0 | 0 | 0 |
| ULZ | 19 | 0 | 66 | 0 | 0 | 0 | 0 |
| MRZ | 20 | 0 | 0 | 15 | 0 | 0 | 0 |
| MLZ | 17 | 0 | 0 | 0 | 23 | 0 | 0 |
| LRZ | 12 | 0 | 0 | 0 | 0 | 19 | 0 |
| LLZ | 19 | 0 | 0 | 0 | 0 | 0 | 22 |
| CC | |||||||
| 0 | 0 | 19 | 23 | 12 | 23 | 56 | 40 |
| URZ | 19 | 75 | 0 | 0 | 0 | 0 | 0 |
| ULZ | 21 | 0 | 71 | 0 | 0 | 0 | 0 |
| MRZ | 18 | 0 | 0 | 18 | 0 | 0 | 0 |
| MLZ | 20 | 0 | 0 | 0 | 16 | 0 | 0 |
| LRZ | 14 | 0 | 0 | 0 | 0 | 22 | 0 |
| LLZ | 20 | 0 | 0 | 0 | 0 | 0 | 26 |
MPR, multiplanar reconstruction; CC, color-coded; 0, no agreement between CT-based recommendation/surgery; URZ, upper right zone; ULZ, upper left zone; MRZ, middle right zone; MLZ, middle left zone; LRZ, lower right zone; LLZ, lower left zone.
Discussion
Our results show that the use of CC images is less observer-dependent in characterizing emphysema distribution patterns and providing recommendations for resection compared to axial and multiplanar CT images. The CC image-based quantification of emphysema showed similar correlation to quantitative CT compared to axial or multiplanar CT images.
Early studies showed good correlation between patients with marked heterogeneity in the severity of pulmonary emphysema and functional outcome LVRS (27,28). The National Emphysema Treatment Trial (NETT) revealed patients with heterogenous emphysema in the upper lobes and low exercise capacity as best responders to LVRS (9,29). Since recent studies suggest a broader spectrum of patients with different distributions of emphysema suitable for LVRS (30-34) and case selection as well as a multidisciplinary approach play relevant roles in the outcome of LVRS programs (35,36), observer-independent imaging methods that can reliably depict areas with severe emphysema are of great importance.
Some evidence suggests, that also patients with homogeneous emphysema can profit from LVRS (30). However, it is important that harm and benefit is well balanced for this population group. Furthermore, a careful preoperative planning is crucial. Although current automated lung emphysema quantification software provides a wealth of quantitative information, they are not designed to provide a classification of the different types of emphysema distributions. The latter is important for LVRS planning and outcome (5,8,19,37). The surgically oriented grading system of emphysema distribution used in this study plays an important role at our institution for preoperative assessment and is well established, although it has not yet been investigated regarding inter-reader agreement.
For standard CT based assessment of emphysema Hersh et al. reported a poor interobserver agreement among 5 readers (radiologists and pulmonologists) in determination of upper lobe-predominant disease on CT scans of 30 patients with emphysema (38). Bankier et al. reported moderate interobserver agreement of visual grading of emphysema on grey-scale images (12). Mohsen et al. also found improved inter-rater agreement of visual quantitation of emphysema using a simple density mask compared to grayscale scale images, however, the authors did not take specific lung zones into consideration in this study (39). In the present study, CC images lead to a substantial inter-rater agreement regarding the grading of emphysema distribution compared to axial or MPR images. In addition, we observed a shift from initially homogeneous rated scans on axial images towards heterogeneous rated scans on CC images and the inter-reader agreement of emphysema distribution on CC images improved. These two facts are responsible for the third finding, which is the substantial inter-rater agreement in providing recommendations for lung segment resection for LVRS on CC images. Furthermore, the selected segments are well associated with the final resected lung. In consequence, the CC images allow readers to define the areas for resection more accurately than on axial images.
Our results showed a significant correlation between subjective emphysema ratings and quantitative CT measurements. This is in accordance with a previously published study, analyzing correlations of MPR reformations between subjective scores on axial CT-images and densitometric measurements, even though, this study did not investigate specific lung zones (12). There was no significant correlation between emphysema amount rating and measured amount of emphysema in the middle zones. The reason therefore might be by aggravated anatomical localisation of this region. In the present study the amount of emphysema was visually generally overestimated compared to emphysema measurements. This is in accordance with Bankier et al., reporting that radiologists tended to relatively overestimate emphysema in patient with severe emphysema, which could result from the fact, that readers have the tendency to err on the side of caution under test conditions (12).
When comparing the CT-based data with lung scintigraphy, the visual emphysema amount rating of all image types showed a significantly negative correlation with perfusion measurements from scintigraphy in the upper and lower zones, indicating that perfusion scintigraphy may help to identify target areas for resection in LVRS as suggested by Thurnheer et al. (14). Missing correlation between quantitative CT measurements and perfusion scintigraphy of the middle zones remains unclear. This result could be caused by aggravated anatomical localisation of these regions in combination by the fact that CT and scintigraphy measure different properties of the lungs, namely anatomy/structure and circulation, which is, of course, also correct for all other zones, but has more influence close to the perihilar structures. Furthermore, results from the perfusion scintigraphy presented in this study did not consider information from the CT and therefore differentiation of perfusion defects due to obstruction and von Euler-Liljestrand effects or irreversible lung destruction was limited in the perfusion scintigraphy.
The reason to develop a method that additionally illustrates the severity and distribution of the pulmonary emphysema was not to implement a competitive process against already implemented and accepted quantitative imaging approaches, but rather to develop a method that helps surgeons to determine their decision for an invasive therapeutic procedure. While it enables a less observer-dependent overview of the severity and distribution of pulmonary emphysema, it is easily and quickly to acquire by using any software providing density mask technique.
Our study has limitations that need to be considered. First, this is a retrospective single-center study with its inherent limitation, and therefor conclusions drawn from the present analysis await further proof in larger (and ideally multi-centric) observations. Nevertheless, all types of emphysema were represented in a sufficient number of cases. A second limitation is the missing consideration of other factors affecting the resection site during LVRS, such as scars or pulmonary nodules. However, none of the included patients had a malignancy. Third limitation is the lack of postoperative CT evaluation of patients to confirm the operation, respectively to reproduce the resection border. The association between recommended zones for removal and finally removed lung areas had to be done by reviewing the operative reports, which may have led to some discrepancy in defining the resection area.
In conclusion, our study suggests that CC CT images allow a precise, less observer-dependent quantitation of distribution of pulmonary emphysema and resection recommendation compared to axial and MPR CT images.
Acknowledgements
None.
Ethical Statement: The local ethics committee approved the study (EC-No. 2014-0275), and informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Webb WR. Radiology of obstructive pulmonary disease. AJR Am J Roentgenol 1997;169:637-47. 10.2214/ajr.169.3.9275869 [DOI] [PubMed] [Google Scholar]
- 2.The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis 1985;132:182-5. [DOI] [PubMed] [Google Scholar]
- 3.Taraseviciene-Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. J Clin Invest 2008;118:394-402. 10.1172/JCI31811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teschler H, Stamatis G, El-Raouf Farhat AA, et al. Effect of surgical lung volume reduction on respiratory muscle function in pulmonary emphysema. Eur Respir J 1996;9:1779-84. 10.1183/09031936.96.09091779 [DOI] [PubMed] [Google Scholar]
- 5.Weder W, Thurnheer R, Stammberger U, et al. Radiologic emphysema morphology is associated with outcome after surgical lung volume reduction. Ann Thorac Surg 1997;64:313-9; discussion 319-20. 10.1016/S0003-4975(97)00564-X [DOI] [PubMed] [Google Scholar]
- 6.Washko GR, Hoffman E, Reilly JJ. Radiographic Evaluation of the Potential Lung Volume Reduction Surgery Candidate. Proc Am Thorac Soc 2008;5:421-6. 10.1513/pats.200802-017ET [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Emphysema Treatment Trial Research Group , Fishman A, Fessler H, et al. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075-83 10.1056/NEJMoa11798 [DOI] [PubMed] [Google Scholar]
- 8.van Agteren JE, Carson KV, Tiong LU, Smith BJ. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev 2016;10:CD001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. 10.1056/NEJMoa030287 [DOI] [PubMed] [Google Scholar]
- 10.Naidich DP. Pulmonary parenchymal high-resolution CT: to be or not to be. Radiology 1989;171:22-4. 10.1148/radiology.171.1.2648472 [DOI] [PubMed] [Google Scholar]
- 11.Naidich DP. High-resolution Computed Tomography of the Pulmonary Parenchyma: Past, Present, and Future? J Thorac Imaging 2010;25:32-3. 10.1097/RTI.0b013e3181cc4de6 [DOI] [PubMed] [Google Scholar]
- 12.Bankier AA, De Maertelaer V, Keyzer C, et al. Pulmonary Emphysema: Subjective Visual Grading versus Objective Quantification with Macroscopic Morphometry and Thin-Section CT Densitometry. Radiology 1999;211:851-8. 10.1148/radiology.211.3.r99jn05851 [DOI] [PubMed] [Google Scholar]
- 13.Mascalchi M, Camiciottoli G, Diciotti S. Lung densitometry: why, how and when. J Thorac Dis 2017;9:3319-45. 10.21037/jtd.2017.08.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurnheer R, Engel H, Weder W, et al. Role of Lung Perfusion Scintigraphy in Relation to Chest Computed Tomography and Pulmonary Function in the Evaluation of Candidates for Lung Volume Reduction Surgery. Am J Respir Crit Care Med 1999;159:301-10. 10.1164/ajrccm.159.1.9711030 [DOI] [PubMed] [Google Scholar]
- 15.Boedeker KL, McNitt-Gray MF, Rogers SR, et al. Emphysema: Effect of Reconstruction Algorithm on CT Imaging Measures. Radiology 2004;232:295-301. 10.1148/radiol.2321030383 [DOI] [PubMed] [Google Scholar]
- 16.Heussel CP, Kappes J, Hantusch R, et al. Contrast enhanced CT-scans are not comparable to non-enhanced scans in emphysema quantification. Eur J Radiol 2010;74:473-8. 10.1016/j.ejrad.2009.03.023 [DOI] [PubMed] [Google Scholar]
- 17.Behrendt FF, Das M, Mahnken AH, et al. Computer-Aided Measurements of Pulmonary Emphysema in Chest Multidetector-Row Spiral Computed Tomography: Effect of Image Reconstruction Parameters. J Comput Assist Tomogr 2008;32:899-904. 10.1097/RCT.0b013e31815ade64 [DOI] [PubMed] [Google Scholar]
- 18.Russi EW, Stammberger U, Weder W. Lung volume reduction surgery for emphysema. Eur Respir J 1997;10:208-18. 10.1183/09031936.97.10010208 [DOI] [PubMed] [Google Scholar]
- 19.Caviezel C, Franzen D, Inci I, et al. Emphysemchirurgie – State of the Art 2016. Zentralbl Chir 2016;141:S26-34. 10.1055/s-0042-112026 [DOI] [PubMed] [Google Scholar]
- 20.Goddard PR, Nicholson EM, Laszlo G, et al. Computed tomography in pulmonary emphysema. Clin Radiol 1982;33:379-87. 10.1016/S0009-9260(82)80301-2 [DOI] [PubMed] [Google Scholar]
- 21.Verleden GM, Van Raemdonck D, Lerut T, et al. Surgery for non-neoplastic Disorders of the Chest: a clinical Update. Sheffield: European Respiratory Society, 2004. [Google Scholar]
- 22.Caviezel C, Franzen D, Weder W. Chirurgische Lungenvolumenreduktion. Pneumologie 2018;72:64-78. 10.1055/s-0043-103363 [DOI] [PubMed] [Google Scholar]
- 23.Zapf A, Castell S, Morawietz L, Karch A. Measuring inter-rater reliability for nominal data - which coefficients and confidence intervals are appropriate? BMC Med Res Methodol 2016;16:93. 10.1186/s12874-016-0200-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420-8. 10.1037/0033-2909.86.2.420 [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 26.Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 1981;86:127-37. [PubMed] [Google Scholar]
- 27.Cooper JD, Trulock EP, Triantafillou AN, et al. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995;109:106-16; discussion 116-9. 10.1016/S0022-5223(95)70426-4 [DOI] [PubMed] [Google Scholar]
- 28.Slone RM, Gierada DS. Radiology of pulmonary emphysema and lung volume reduction surgery. Semin Thorac Cardiovasc Surg 1996;8:61-82. [PubMed] [Google Scholar]
- 29.Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT): Part II: Lessons Learned about Lung Volume Reduction Surgery. Am J Respir Crit Care Med 2011;184:881-93. 10.1164/rccm.201103-0455CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weder W, Tutic M, Lardinois D, et al. Persistent benefit from lung volume reduction surgery in patients with homogeneous emphysema. Ann Thorac Surg 2009;87:229-36; discussion 236-7. 10.1016/j.athoracsur.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 31.Caviezel C, Aruldas C, Franzen D, et al. Lung volume reduction surgery in selected patients with emphysema and pulmonary hypertension. Eur J Cardiothorac Surg 2018;54:565-71. 10.1093/ejcts/ezy092 [DOI] [PubMed] [Google Scholar]
- 32.Inci I, Iskender I, Ehrsam J, et al. Previous lung volume reduction surgery does not negatively affect survival after lung transplantation. Eur J Cardiothorac Surg 2018;53:596-602. 10.1093/ejcts/ezx318 [DOI] [PubMed] [Google Scholar]
- 33.Opitz I, Ulrich S. Pulmonary hypertension in chronic obstructive pulmonary disease and emphysema patients: prevalence, therapeutic options and pulmonary circulatory effects of lung volume reduction surgery. J Thorac Dis 2018;10:S2763-74. 10.21037/jtd.2018.07.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caviezel C, Schneiter D, Opitz I, et al. Lung volume reduction surgery beyond the NETT selection criteria. J Thorac Dis 2018;10:S2748-53. 10.21037/jtd.2018.08.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathinam S, Oey I, Steiner M, et al. The role of the emphysema multidisciplinary team in a successful lung volume reduction surgery programme†. Eur J Cardiothorac Surg 2014;46:1021-6. 10.1093/ejcts/ezu129 [DOI] [PubMed] [Google Scholar]
- 36.Oey I, Waller D. The role of the multidisciplinary emphysema team meeting in the provision of lung volume reduction. J Thorac Dis 2018;10:S2824-9. 10.21037/jtd.2018.02.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russi EW, Bloch KE, Weder W. Functional and morphological heterogeneity of emphysema and its implication for selection of patients for lung volume reduction surgery. Eur Respir J 1999;14:230. 10.1034/j.1399-3003.1999.14a39.x [DOI] [PubMed] [Google Scholar]
- 38.Hersh CP, Washko GR, Jacobson FL, et al. Interobserver Variability in the Determination of Upper Lobe-Predominant Emphysema. Chest 2007;131:424-31. 10.1378/chest.06-1040 [DOI] [PubMed] [Google Scholar]
- 39.Mohsen LA, Gawad EA, Ibrahiem MA. CT quantification of emphysema: Is semi-quantitative scoring a reliable enough method? Egypt J Radiol Nuel Med 2014;45:673-8. 10.1016/j.ejrnm.2014.03.015 [DOI] [Google Scholar]