Abstract
Background
The aim of this retrospective study was to assess the influence of the sequence of pulmonary vessel ligation during video-assisted thoracoscopic lobectomy on long-term survival in patients with non-small cell lung cancer (NSCLC).
Methods
This retrospective study included 60 patients treated surgically with lobectomy and standard lymphadenectomy between 2012 and 2013. Patients had primary ligation of the pulmonary vein or veins (PV group, 33 patients) or of the pulmonary artery or arteries (PA group, 27 patients). Patients were excluded if they had undergone pulmonary wedge resection before lobectomy. Subgroup and sensitivity analyses were also used to investigate the effect of clinical characteristics of interest on survival.
Results
Median follow-up was 54.5 months. Baseline characteristics of the two groups were statistically comparable regarding gender, histology, type of resection, T stage, and overall stage (all P>0.05). Overall, 5-year survival reached 66.67% in the PV group and 44.44% in the PA group (P=0.084). There were no differences between two groups regarding overall survival (OS) (P=0.063, HR: 2.093; 95% CI: 0.960–4.564), disease-free survival (DFS) (P=0.180, HR: 1.539; 95% CI: 0.820–2.889), or cancer-specific deaths (P=0.227, 14/33 vs. 17/27). Subgroup analyses showed no significant difference of OS (P=0.374, HR: 1.541; 95% CI: 0.594–3.997) or DFS (PLog-rank=0.746) for isolated adenocarcinoma, but significant differences in OS (PLog-Rank=0.036; HR: 3.992; 95% CI: 0.987–16.139) and DFS (P=0.044, HR: 3.011; 95% CI: 1.031–8.795) between the two groups of patients in whom squamous cell carcinomas. Sensitivity analysis showed that the differences were not statistically significant between the two groups regarding OS (P=0.140, HR: 1.944; 95% CI: 0.804–4.700) and DFS (P=0.190, HR: 1.605; 95% CI: 0.791–3.255) for patients with a tumour diameter of greater than 3 cm.
Conclusions
In summary, the sequence of pulmonary vessel ligation during video-assisted thoracoscopic lobectomy for NSCLC has no different effects on long-term survival, but for patients with squamous cell carcinoma, venous ligation should be preferred first since it may bring survival advantage after surgery.
Keywords: Non-small cell lung cancer (NSCLC), lobectomy, pulmonary artery (PA), pulmonary vein (PV), survival
Introduction
The incidence of lung cancer has been increasing each year (1). In recent years, with the encouragement of the WHO, greater attention has been paid to lung cancer screening, which increases the probability of early detection of lung cancer (2). In developed countries, breakthroughs have been made in basic research on lung cancer, and new therapeutic methods have been continuously applied in clinical practice, such as TKI drugs (3), PD-1 blockade (4), and CAR-T treatment (5). Although these efforts have made an impact on overall survival (OS) of lung cancer patients, surgery still holds an irreplaceable position in the treatment of lung cancer (6). Constant developments in the field have led to advances in minimally invasive video-assisted thoracoscopic surgery (VATS) techniques, which have shown favourable results in terms of rehabilitation effect (7,8).
This study considered the different characteristics of lobectomy between lung cancer under thoracoscopic assistance and thoracotomy. The effects of the sequence of pulmonary vascular ligation on patients’ long-term survival after surgery were analysed.
Methods
Retrospective analysis was performed on 60 patients who underwent lobectomy in the thoracic surgery department between October 2012 and September 2013. General clinical data were collected, including age, gender, and tumour size based on preoperative CT, tumour pathological type, and TNM stage postoperatively. According to the needs of the study, 67 eligible patients were screened for inclusion in this study. The followed-up data included overall survival (OS) and disease-free survival (DFS).
Data collection
Patient inclusion criteria were lobectomy plus mediastinal lymph node radical dissection under VATS for primary non-small cell lung cancer (NSCLC). Exclusion criteria were preoperative examination indicating distant metastasis, sublobar resection, including wedge-shaped resection and segmental resection, pulmonary lobectomies by thoracotomy, wedge resection of a tumour lesion before lobectomy, postoperative pathology-confirmed small cell lung cancer, the order of vascular ligation (partial arterial branch, vein, and residual arterial branch), or the missing order of ligation of pulmonary vessels. Sixty-seven patients were eligible for the study, and 7 of the 67 patients were lost to follow-up. Finally, 60 patients were included in this study. According to the sequence of pulmonary artery ligation in the surgical records, patients were divided into primary ligation of the pulmonary vein or veins (PV group, 33 patients) or primary ligation of the pulmonary artery or arteries (PA group, 27 patients). None of those patients died perioperatively. All patients included in our study underwent radical lymph node dissection.
Statistical analysis
Statistical analysis was performed using SPSS20.0 software. Mann-Whitney U test was used for continuous data, and χ2 or Fisher’s exact test was used for categorical variables. Cox proportional hazard model was used to analyze the relationship between the pattern of primary ligation and survival. The Kaplan-Meier (Log-rank test) curve was constructed to assess the survival of the two groups. Subgroup and sensitivity analyses were also used to investigate the effect of clinical characteristics of interest on survivals. A P value <0.05 was considered significant.
Results
Baseline and clinical characteristics
General data of patients in the PV and PA groups are shown in Table 1. There were no significant differences in age, sex, TNM stage, and tumour pathological type between the two groups. For early stage lung cancer, especially for adenocarcinoma in situ and micro-invasive carcinoma, wedge resection or pulmonary segmentectomy is the most common choice, which does not meet our inclusion criteria, so the proportion of patients with early T stage was relatively low. There is significant difference in lesion location, because compared with the upper lobe of the right lung, there were more branches of the pulmonary artery in the upper lobe of the left lung and the priority ligation of veins is more dangerous, therefore, surgeons are accustomed to ligate part or all of the arterial branches first while performing lobectomy.
Table 1. General data of patients in the PV and PA groups.
| Characteristics | PV (n=33) | PA (n=27) | P values |
|---|---|---|---|
| Gender (male) | 66.67% (22/33) | 70.37% (19/27) | 0.761 |
| Age (year) | 59.55 | 62.15 | 0.327 |
| Combine with COPD | 0.458 | ||
| Yes | 7 | 8 | |
| No | 26 | 19 | |
| Lesion location | 0.004 | ||
| Right upper lobe | 18 | 6 | |
| Right middle lobe | 5 | 1 | |
| Right lower lobe | 2 | 10 | |
| Left upper lobe | 8 | 3 | |
| Left lower lobe | 0 | 7 | |
| Tumour pathological type | 0.955 | ||
| Squamous cell carcinomas | 5 | 7 | |
| Adenocarcinoma | 22 | 18 | |
| Gland scale cancer | 4 | 1 | |
| Others | 2* | 1△ | |
| T stage of tumour pathological | 0.288 | ||
| T1 | 10 | 2 | |
| T2 | 15 | 21 | |
| T3 | 7 | 4 | |
| T4 | 1 | 0 | |
| TNM stage of tumour pathological | 0.471 | ||
| IA | 8 | 1 | |
| IB | 6 | 9 | |
| IIA | 2 | 4 | |
| IIB | 6 | 5 | |
| IIIA | 10 | 5 | |
| IIIB | 0 | 3 | |
| IVA | 1 | 0 | |
| TKIs treatment | 0.368 | ||
| Yes | 8 | 4 | |
| No | 25 | 23 | |
| Tumour-related death | 14 | 17 | 0.227 |
| 5-year survival | |||
| DFS | 39.39% (13/33) | 29.63% (8/27) | 0.430 |
| OS | 66.67% (22/33) | 44.44% (12/27) | 0.084 |
*, one case of large cell carcinoma with squamous cell carcinoma, the other case of large cell carcinoma with adenocarcinoma; △, the case of adenocarcinoma complicated with carcinoid. PV, pulmonary vein; PA, pulmonary artery; COPD, chronic obstructive pulmonary disease; DFS, disease-free survival; OS, overall survival.
Primary survival comparison
Overall, 5-year survival reached 66.67% (22/33) in the PV group and 44.44% (12/27) in the PA group (P=0.084). Kaplan-Meier survival curves showed the differences were not statistically significant regarding OS [PLog-Rank=0.056, 66.67% (22/33) vs. 44.44% (12/27), respectively], DFS [PLog-Rank= 0.176, 39.40% (13/33) vs. 29.63% (8/27), respectively] (Figures 1,2), or cancer-specific deaths (P=0.227, 14/33 vs. 17/27, respectively). Cox model analysis further confirmed that the differences of OS (P=0.063, HR: 2.093; 95% CI: 0.960–4.564) and DFS (P=0.180, HR: 1.539; 95% CI: 0.820–2.889) were not statistically significant.
Figure 1.
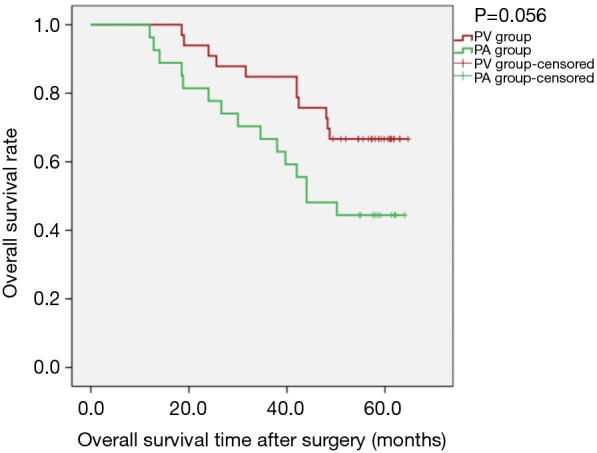
Kaplan-Meier estimates of overall survival by overall cohort; PV group: 33 patients; PA group: 27 patients (PLog-Rank=0.056).
Figure 2.
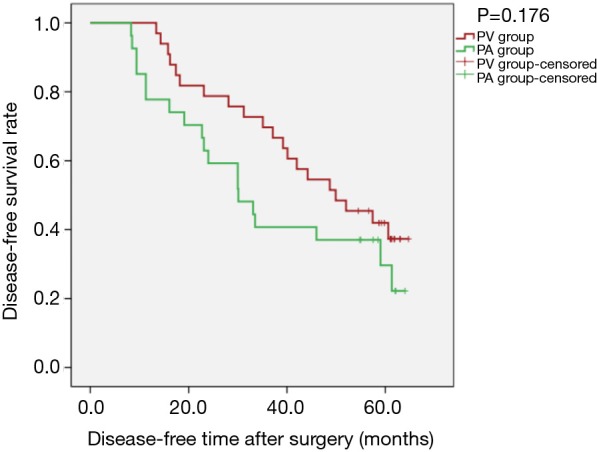
Kaplan-Meier estimates of disease-free survival by overall cohort; PV group: 33 patients; PA group: 27 patients (PLog-Rank=0.176).
Additional survival analysis
Kaplan-Meier survival curves applied for subgroup analysis showed no significant difference between the PV and PA groups in OS [PLog-Rank=0.369, 65.22% (15/23) vs. 52.63% (10/19), respectively] or DFS [PLog-Rank=0.746, 39.13% (9/23) vs. 42.11% (8/19), respectively] between the two groups for patients with isolated adenocarcinoma (Figures 3,4). Cox model analysis confirmed that the differences in OS (P=0.374, HR: 1.541; 95% CI: 0.594–3.997) and DFS (P=0.746, HR: 1.139; 95% CI: 0.517–2.513) were not statistically significant. However, there was a statistically significant difference in OS [PLog-Rank=0.036, 70.00% (7/10) vs. 25.00% (2/8), respectively] and DFS [PLog-Rank=0.035, 40.00% (4/10) vs. 0% (0/8), respectively] between the two groups of patients with squamous cell carcinomas (Figures 5,6). Cox model showed no statistically significant difference in OS (P=0.052, HR: 3.992; 95% CI: 0.987–16.139) but a statistically significant difference in DFS (P=0.044, HR: 3.011; 95% CI: 1.031–8.795) in these groups (Table 2).
Figure 3.
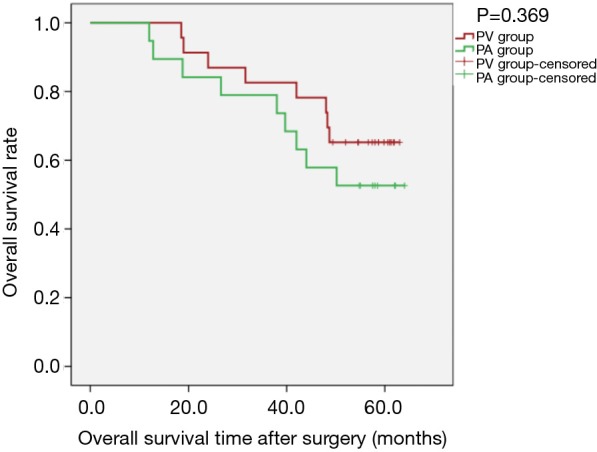
Kaplan-Meier estimates of overall survival for patients with adenocarcinoma; PV group: 23 patients; PA group: 19 patients (PLog-Rank=0.369).
Figure 4.
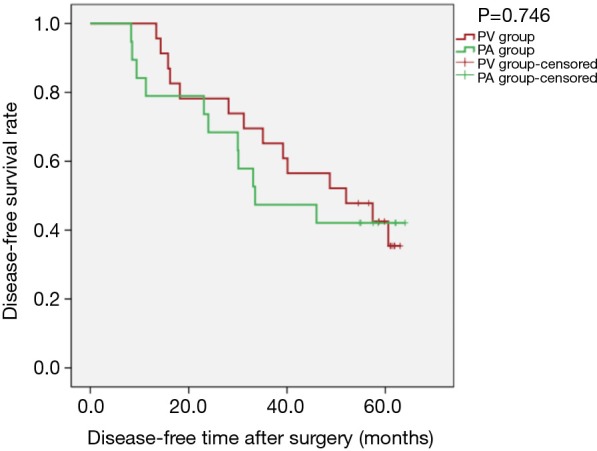
Kaplan-Meier estimates of disease-free survival for patients with adenocarcinoma; PV group: 23 patients; PA group: 19 patients (PLog-Rank=0.746).
Figure 5.
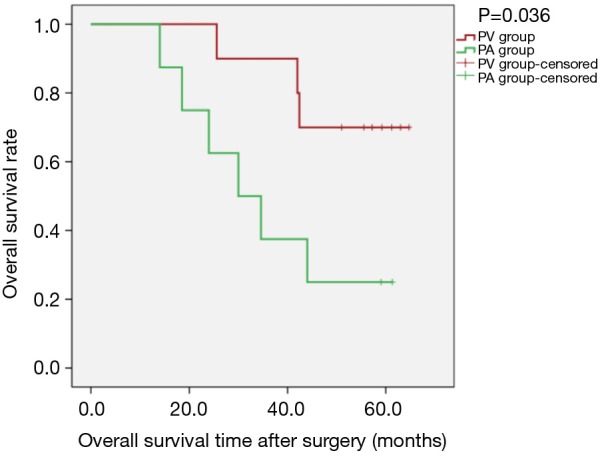
Kaplan-Meier estimates of overall survivals for patients with pathological squamous cell carcinomas; PV group: 10 patients; PA group: 8 patients (PLog-Rank=0.036).
Figure 6.
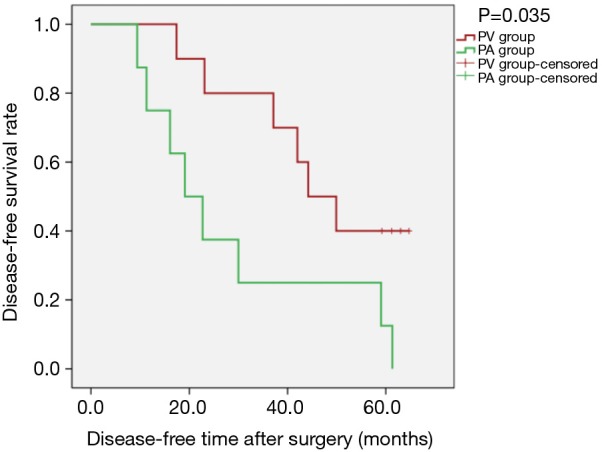
Kaplan-Meier estimates of disease-free survival for patients with pathological squamous cell carcinomas; PV group: 10 patients; PA group: 8 patients (PLog-Rank=0.035).
Table 2. Gender and age data of patients in the PV and PA subgroups.
| Characteristics | PV | PA | P values |
|---|---|---|---|
| Adenocarcinoma (cases) | 23 | 19 | |
| Gender (male) | 60.87% (14/23) | 68.42% (13/19) | 0.611 |
| Age (year) | 56.13 | 60.21 | 0.144 |
| Squamous cell carcinomas (cases) | 10 | 8 | |
| Gender (male) | 80.00% (8/10) | 75.00% (6/8) | 0.872 |
| Age (year) | 62.42 | 64.09 | 0.427 |
| Tumour diameter >3 cm (cases) | 27 | 21 | |
| Gender (male) | 70.37% (19/27) | 76.19% (16/21) | 0.653 |
| Age (year) | 58.73 | 61.69 | 0.396 |
PV, pulmonary vein; PA, pulmonary artery.
Sensitivity analysis revealed that, for patients with tumour diameter greater than 3 cm, there was no statistically significant difference between the two groups regarding OS [PLog-Rank=0.131, 66.67% (18/27) vs. 47.62% (10/21, respectively)] or DFS (PLog-Rank=0.185, 40.74% (11/27) vs. 28.57% (6/21), respectively] (Figures 7,8). Cox model confirmed that the differences were not statistically significant in terms of OS (P=0.140, HR: 1.944; 95% CI: 0.804–4.700) and DFS (P=0.190, HR: 1.605; 95% CI: 0.791–3.255).
Figure 7.
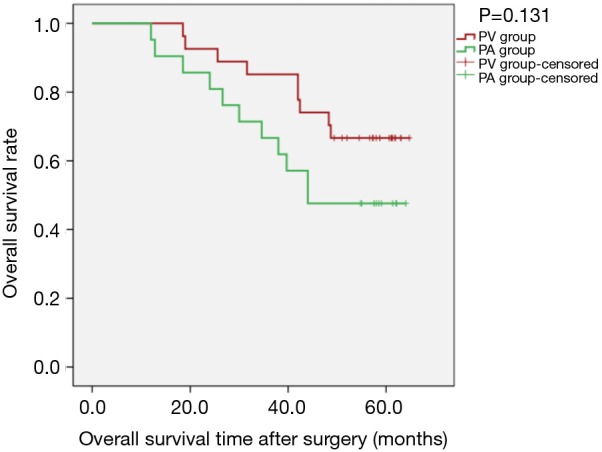
Kaplan-Meier estimates of overall survivals for patients with tumour diameter greater than 3 cm; PV group: 27 patients; PA group: 21 patients (PLog-Rank=0.131).
Figure 8.
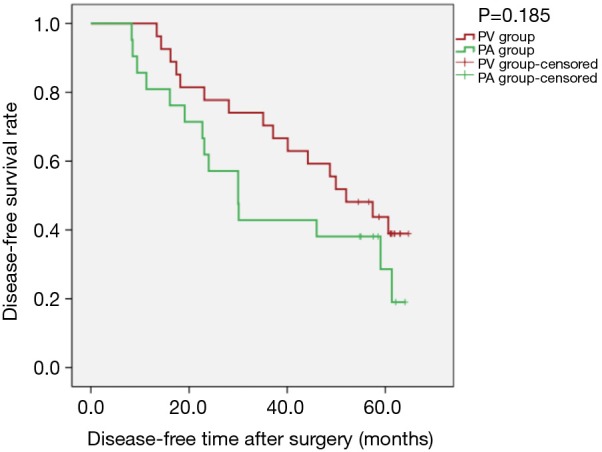
Kaplan-Meier estimates of disease-free survival for patients with tumour diameter greater than 3 cm; PV group: 27 patients; PA group: 21 patients (PLog-Rank=0.185).
Discussion
Regarding the application of lobectomy for lung cancer patients, most surgeons now advocate primacy of ligation of the pulmonary vein (9), Schneiderman reported a case in 1989 (10) indicating that tumour cells may fall off during surgery and enter vein. Kozak and others previously conducted a prospective study (11) in which 5-year survival reached 50% in the PA group and 54% in the PV group (P=0.82) and did not differ significantly between cancer-related and non-cancer-related deaths (P=0.67 and P=0.26, respectively). This is the most representative prospective study on this topic; however, this prospective study did not take into account the effect of pulmonary wedge resection on tumour site before lobectomy. Because the priority of pulmonary vein ligation is to block tumour cell metastasis through the pulmonary vein, wedge resection of lung tumours before lobectomy can also achieve this goal. Again, this prospective study analysed the effect of T staging on overall patient survival. However, there was no further analysis of the possible differences in T stage between the two groups of patients. The smaller the tumour diameter is, the less likely it is that the tumour cells will fall off and enter venous blood circulation which confirmed by Li’s study (12) that the ligation sequence of intraoperative pulmonary veins and arteries had no significant effect on the long-term survival of early-stage small-cell lung cancer patients.
In previous retrospective studies (11-13), there were no further stratified analysis of the effects of thoracoscopy and tumour pathological type under investigation. The effect of thoracotomy and thoracoscopic surgery on the results of study was also ignored. Thoracotomy lobectomy allows surgeons to dissect the hilum layer by layer in one direction. VATS lobectomy may require dissection of the hilum in different directions and an assistant or the main surgeon to pull the lung lobe with the tumour to different locations. This recurrent traction increases the likelihood of tumour cells shedding into the bloodstream.
Combined with previous research methods and results, we designed this retrospective study. In view of the differences in tumour pathological types, squamous cell carcinoma and adenocarcinoma have significant differences in location and prognosis. In this study, we further isolated patients with squamous cell carcinoma (squamous cell carcinoma, adenosquamous cell carcinoma, large cell lung cancer and partial squamous cell carcinoma) from adenocarcinoma. The reason why patients with squamous cell carcinoma were considered separately is that most squamous cell carcinoma tumours are located near the central part of the hilum of the lung, from which it is easier to access the main pulmonary vein, and thus tumour cells were more likely to be shed into the blood. Due to the limitations of the retrospective study itself and the limited sample size, some patients’ preoperative CT and other imaging data have been lost. So, we have no way to clarify the type of central or peripheral tumour in every patient; this is one of the defects of the study. For our study, it was found that, for isolated adenocarcinoma, there was no statistically significant difference in the effect of vascular dissection sequence on postoperative survival during thoracoscopic lobectomy (Figures 3,4); however, in patients with squamous cell carcinoma, there was an obvious statistical significance by K-M curves (PLog-Rank=0.036 and 0.035 for OS and DFS, respectively). The study found that patients with squamous cell carcinoma in the PV group had an advantages over those in the PA group in terms of postoperative survival (Figures 5,6).
Considering the research of Li et al. (12), in our study, T staging was considered independently, and postoperative survival of patients with tumour diameter greater than 3 cm was the main outcome analysed. The results showed that in all patients with tumour diameter greater than 3 cm, the sequence of pulmonary vascular detachment had some effect on postoperative OS and DFS. The Kaplan-Meier curve is shown in Figures 7,8, with obvious separation. The PV group was superior to the PA group for both OS and DFS. However, the difference was not significantly different (PLog-Rank=0.131 and 0.185 for OS and DFS, respectively).
Some studies have indirectly confirmed the importance of priority ligation of pulmonary veins in lobectomy of lung cancer. Hashimoto et al. (14) confirmed the number of circulating tumour cells (CTCs) in pulmonary veins increases as a result of surgery during lobectomy. The pulmonary vein is an overflow channel for tumour cell metastasis, even though the increase in pvCTC count was not significantly associated with surgical procedure, including the sequence of vessel interruption. Song et al. conducted a prospective randomised study (15) related to lCD44v6 (reflecting the invasiveness and adhesiveness of malignant tumour cells), suggesting that ligation of the PV should be performed first during lobectomy. Surgical manipulation itself may stimulate the occurrence of blood micro-metastases. Ligation of the PV first during surgery may help prevent blood micro-metastases.
Kurusu (16) examined the level of CEA mRNA in the blood before and after surgery in two different sequences of vascular dissection, suggesting that delay in ligation of the PV leads to shedding of cancer cells in the bloodstream. It was also confirmed that patients undergoing lobectomy for primary lung cancer the PV should be ligated first by pin1 mRNA (17) and perioperative CK19 mRNA (18) expression. These significant differences were not directly reflective of better or worse OS and PFS; it may be that tumour metastasis requires not only CTC cells but also micro-environmental support (19).
The order of priority ligation of blood vessels may affect intraoperative bleeding (12,20), which is considered by many surgeons. A study by Yellin et al. had confirmed that (20), in straightforward lobectomy, the amount of blood retained in the resected lobe is small. This amount was not affected by the sequence of hilar vessel interruption. Since this may be related to the surgeon’s surgical skills, intraoperative adhesion, and the development of pulmonary fissures, our retrospective data were limited, so we did not perform further analysis.
Besides, due to the retrospective nature of the study and thus the limited data, we were unable to analyse the influence of the time interval of pulmonary arteriovenous ligation and non touch technique on postoperative survival. Large-scale, randomised clinical trials are needed to confirm our findings.
Conclusions
In summary, the sequence of pulmonary vessel ligation during video-assisted thoracoscopic lobectomy for NSCLC has no different effects on long-term survival, but for patients with squamous cell carcinoma, venous ligation should be preferred first, since it may bring survival advantage after surgery. The present findings should be further confirmed by randomised, controlled clinical trials.
Acknowledgements
None.
Ethical Statement: Approval was obtained to review the data from Institutional Ethical Committee of The First Affiliated Hospital of Guangzhou Medical University.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bunn PA., Jr Early-stage non-small-cell lung cancer: current perspectives in combined-modality therapy. Clin Lung Cancer 2004;6:85-98. 10.3816/CLC.2004.n.022 [DOI] [PubMed] [Google Scholar]
- 2.Mulshine JL, D’Amico TA. Issues with implementing a high-quality lung cancer screening program. CA Cancer J Clin 2014;64:352-63. 10.3322/caac.21239 [DOI] [PubMed] [Google Scholar]
- 3.Schrank Z, Chhabra G, Lin L, et al. Current Molecular-Targeted Therapies in NSCLC and Their Mechanism of Resistance. Cancers (Basel) 2018;10. doi: . 10.3390/cancers10070224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishijima TF, Shachar SS, Nyrop KA, et al. Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist 2017;22:470-9. 10.1634/theoncologist.2016-0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiesgen S, Chicaybam L, Chintala NK, et al. Chimeric Antigen Receptor (CAR) T-Cell Therapy for Thoracic Malignancies. J Thorac Oncol 2018;13:16-26. 10.1016/j.jtho.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taioli E, Lee DS, Lesser M, et al. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. Eur J Cardiothorac Surg 2013;44:591-7. 10.1093/ejcts/ezt051 [DOI] [PubMed] [Google Scholar]
- 7.Jones JC, Meyer BW, Robinson JL. Indications for lobectomy in the treatment of carcinoma of the lung. J Thorac Surg 1956;32:500-7. [PubMed] [Google Scholar]
- 8.D’Andrilli A, Rendina EA. Enhanced recovery after surgery (ERAS) and fast-track in video-assisted thoracic surgery (VATS) lobectomy: preoperative optimisation and care-plans. J Vis Surg 2018;4:4. 10.21037/jovs.2017.12.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minnich DJ, Bryant AS, Ashley DH, et al. Inflow and outflow occlusion technique of the pulmonary artery and veins for the technically difficult left upper lobectomy. Eur J Cardiothorac Surg 2012;41:353-6. 10.1016/j.ejcts.2011.05.036 [DOI] [PubMed] [Google Scholar]
- 10.Schneiderman J, Lieberman Y, Adar R. Multiple tumor emboli after lung resection. J Cardiovasc Surg (Torino) 1989;30:496-8. [PubMed] [Google Scholar]
- 11.Kozak A, Alchimowicz J, Safranow K, et al. The impact of the sequence of pulmonary vessel ligation during anatomic resection for lung cancer on long-term survival—a prospective randomized trial. Adv Med Sci 2013;58:156-63. 10.2478/v10039-012-0061-3 [DOI] [PubMed] [Google Scholar]
- 12.Li F, Jiang G, Chen Y, et al. Curative Effects of Different Sequences of Vessel Interruption During the Completely Thoracoscopic Lobectomy on Early Stage Non-Small Cell Lung Cancer. Ann Thorac Cardiovasc Surg 2015;21:536-43. 10.5761/atcs.oa.15-00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Refaely Y, Sadetzki S, Chetrit A, et al. The sequence of vessel interruption during lobectomy for non-small cell lung cancer: is it indeed important? J Thorac Cardiovasc Surg 2003;125:1313-20. 10.1016/S0022-5223(03)00022-9 [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Tanaka F, Yoneda K, et al. Positive correlation between postoperative tumor recurrence and changes in circulating tumor cell counts in pulmonary venous blood (pvCTC) during surgical manipulation in non-small cell lung cancer. J Thorac Dis 2018;10:298-306. 10.21037/jtd.2017.12.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song PP, Zhang W, Zhang B, et al. Effects of different sequences of pulmonary artery and vein ligations during pulmonary lobectomy on blood micrometastasis of non-small cell lung cancer. Oncol Lett 2013;5:463-8. 10.3892/ol.2012.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurusu Y, Yamashita J, Hayashi N, et al. The sequence of vessel ligation affects tumor release into the circulation. J Thorac Cardiovasc Surg 1998;116:107-13. 10.1016/S0022-5223(98)70248-X [DOI] [PubMed] [Google Scholar]
- 17.Ai ZH, Zhang WX. Expression of pin1 mRNA in the circulation of non-small cell lung cancer patients and influence of sequence of vessel ligation. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2008;33:1132. [PubMed] [Google Scholar]
- 18.Ge MJ, Shi D, Wu QC, et al. Observation of circulating tumour cells in patients with non-small cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in peroperative period. J Cancer Res Clin Oncol 2006;132:248. 10.1007/s00432-005-0059-3 [DOI] [PubMed] [Google Scholar]
- 19.Robichaud N, Hsu BE, Istomine R, et al. Translational control in the tumor microenvironment promotes lung metastasis: Phosphorylation of eIF4E in neutrophils. Proc Natl Acad Sci U S A 2018;115:E2202-9. 10.1073/pnas.1717439115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yellin A, Sadetzki S, Simansky DA, et al. The sequence of vessel interruption during lobectomy: does it affect the amount of blood retained in the lobe? Eur J Cardiothorac Surg 2007;31:711-3. 10.1016/j.ejcts.2007.01.019 [DOI] [PubMed] [Google Scholar]


