Abstract
Background
Veno-arterial (VA) extracorporeal membrane oxygenation (ECMO) is used in various cardiogenic shocks. In severe myocardial dysfunction, left heart (LH) distension may occur and aggravate pulmonary edema. Despite the recent case reports on various venting catheter insertion methods for LH decompression, the necessity and efficacy of LH venting procedure are still controversial. Therefore, we focused on evaluating efficacy of LH venting catheter insertion for LH decompression.
Methods
In total, 373 patients received VA ECMO at our institution from May 2012 to January 2016. Of these, 25 patients underwent LH venting catheter insertion. Indication for the procedure included pulmonary congestion observed on chest radiogram, with arterial pulse pressure ≤10 mmHg. The control group comprised of 45 patients with peripheral VA ECMO having arterial pulse pressure ≤ for ≥24 hours during the same study period who did not undergo LH venting procedure. Finally, 70 patients were compared and analyzed.
Results
Mean age of the patients was 52.6±17.1 years. The ECMO running time in each group was 7.2±7.1 days in the vent (−) group and 9.2±8.5 days in the vent (+) group. Successful weaning rate was higher in the LH vent (+) group (P=0.08). Moreover, LH venting catheter insertion was identified as a predictor of weaning success with marginal significance (OR =2.47; 95% CI: 0.90–6.72; P=0.07).
Conclusions
LH decompression by venting catheter insertion in patients on VA ECMO may be more effective and helpful for successful ECMO weaning than conventional medical management without survival benefit.
Keywords: Veno-arterial extracorporeal membrane oxygenation (VA ECMO), left heart decompression (LH decompression), trans-septal venting catheter insertion
Introduction
Veno-arterial (VA) extracorporeal membrane oxygenation (ECMO) is used for various etiologies of cardiogenic shock, such as postoperative myocardial dysfunction, acute heart failure secondary to myocarditis, etc. (1). VA ECMO in the setting of severe myocardial dysfunction, may result in loss of pulsatility and left heart (LH) distension which leads to pulmonary edema and hemorrhage. Therefore, the LH distension in patients on VA ECMO should be managed aggressively by all means. Recently, successful LH decompression achieved by percutaneous insertion of trans-septal catheter has been reported (1-3). However, due to the invasive nature and risk complication involved the procedure, as well as lack of data favoring the efficacy of the LH venting catheter compared to conventional management, the LH venting catheter insertion still remains controversial. This study was aimed to evaluate the efficacy and outcomes of the LH venting catheter insertion for the LH decompression.
Methods
Study population
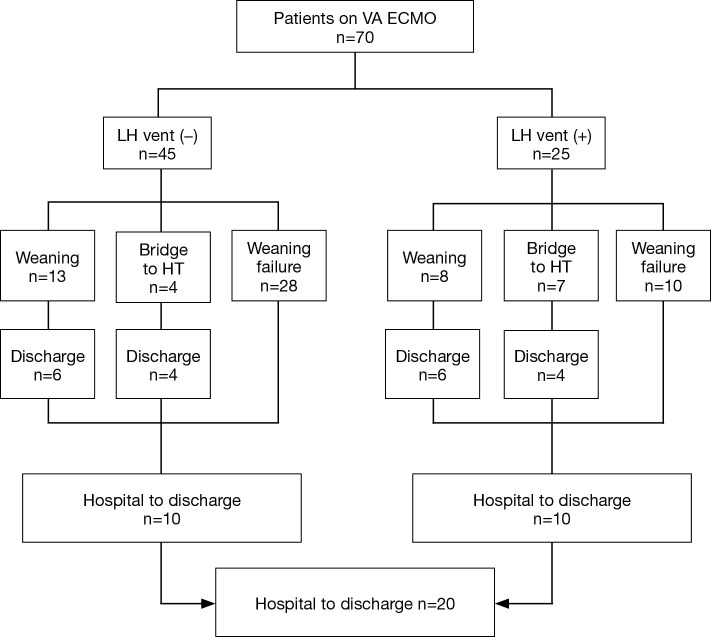
This study was based on retrospective review of medical records of patients at our institution from May 2012 to January 2016. A total of 373 patients were kept on the VA ECMO support and among them, 25 patients underwent the LH venting catheter insertion. The LH venting catheter insertion was performed under central VA ECMO in 5 patients. Among these, 3 patients received venting procedure because of post cardiotomy shock and 2 patients received minimal invasive surgical LH venting in a peripheral VA ECMO. Percutaneous trans-septal catheter insertion was performed in the remaining 20 patients with peripheral VA ECMO. Indication for the LH venting catheter insertion was based on the presence of pulmonary congestion on chest radiogram or the signs of pulmonary edema or hemorrhage with narrow arterial pulse pressure ≤10 mmHg. As a control group, we enrolled 45 patients with peripheral VA ECMO support during the same study period who manifested arterial pulse pressure below 10 mmHg at least over 24 hours with pulmonary congestion on chest radiogram, but did not undergo the LH venting procedure. These patients were managed with conventional inotropic supports or intra-aortic balloon pump (IABP) per physician’s preference. As a result, a total of 70 patients were analyzed in this study (Figure 1). This study was approved by the institutional review board (IRB) of our institution, and informed consent was waived due to its retrospective nature (IRB number: 2018-0035).
Figure 1.
Flowchart of 70 patients on VA ECMO. VA, veno-arterial; ECMO, extracorporeal membrane oxygenation; HT, heart transplantation; LH, left heart.
Establishment of extracorporeal circuit and management
The ECMO was established by either peripheral or central cannulation. Before cannulation, a bolus of heparin (50 or 100 units per kilogram) was infused according to the patient’s coagulation state at the time of insertion. Vascular access for central VA ECMO was achieved through ascending aorta and right atrium. Peripheral VA ECMO cannulation was accessed through femoral artery and femoral vein. Size of the arterial cannula was chosen according to patient’s body surface area, ranging from 14 to 25 Fr, and of the venous cannula, ranging from 17 to 34 Fr.
We maintained the ECMO flow to remain at least 80% of the cardiac output of the patients which was calculated by the body surface area of the patients. Blood pressure was continuously monitored from the arterial line at the right arm and the targeted mean blood pressure was 60–70 mmHg. If the blood pressure became low, we added continuous infusion of norepinephrine and vasopressin as a second line vasoactive agent attain the targeted blood pressure. When the pulse contour became narrow, inotropic agent such as dobutamine or low dose epinephrine was added to increase the contractility. Volume replacement was performed in cases of hypovolemia when observed on transthoracic echocardiography to enhance the preload and contractility. Intra-aortic balloon pump was inserted through the contralateral femoral artery per physician’s preference.
Weaning trial was performed based on the hemodynamic stability, wide pulse pressure, and echocardiographic findings. Inotropic supports were initiated or increased while reducing 10–20% of ECMO flow every 6–12 hours. When the flow reached the less than 1.5 L/min after a short period of time, the circuit was clamped. If vital signs were maintained with circuit clamping, cannulae were removed.
Venting catheter insertion procedure
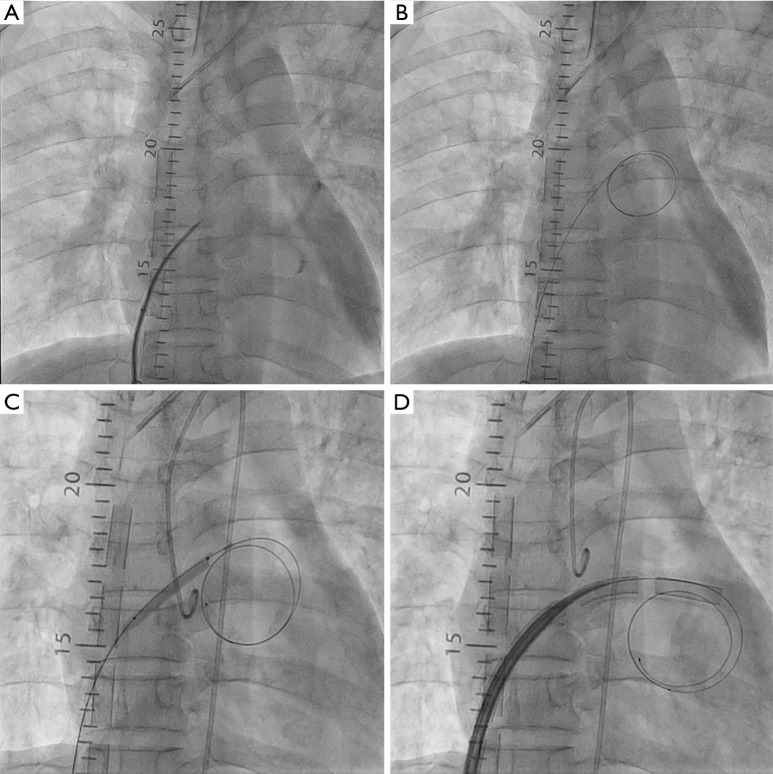
The central LH venting catheter insertion procedure was performed inside the operating room and the catheter was accessed to the left atrium (LA) or LV apex directly. Catheterization for trans-septal venting was accessed via the femoral vein in all the cases. The contralateral femoral vein at previously cannulated site was punctured using the modified Seldinger technique, and a venous sheath was placed. The trans-septal puncture was performed with a 71-cm trans-septal needle (BRK Trans-septal needle, St. Jude Medical, Minneapolis, MN, USA) and a guidewire (Toray Medical Co. Ltd., Tokyo, Japan) was positioned in the LA. The interatrial septum was dilated with an Inoue dilator (Toray Medical Co. Ltd., Tokyo, Japan). A 20 to 28-Fr venous cannula was used and introduced into the LA by a guidewire. After removing the guidewire, the cannula was connected to the ECMO drain circuit (Figure 2).
Figure 2.
Venting catheter insertion procedure. (A) The trans-septal puncture was performed with a trans-septal needle; (B) a guidewire was positioned in the LA through septal puncture; (C) the punctured interatrial septum was dilated with a dilator; (D) a venous cannula was used and introduced into the left atrium by a guidewire. LA, left atrium.
Statistical analysis
Statistical analyses were performed by using a SPSS version 21 (IBM institute, Cary, NC, USA). In addition, each value was compared by performing independent sample t-test, cross-tabulation analysis (χ2-analysis or Fisher’s exact test). To identify the predictor for successful ECMO weaning, univariable analysis was performed using a logistic regression model. P<0.05 was considered statistically significant.
Results
Patient’s demographics and procedural data are summarized in Table 1. The mean ages of the patients without the LH vent and with the LH vent were 57.4±16.8 and 43.8±13.9 years, respectively, with a significant difference of P=0.001. There was no significant difference between the two groups in terms of prior cardiopulmonary cerebral resuscitation (CPCR), ventilator support and IABP before the insertion of the ECMO cannula. Creatinine was significantly higher in the LH vent (+) group. In the LH vent (+) group, the mean time interval from the initiation of the ECMO to the LH venting catheter insertion was 3±4.14 days. Complication related to the insertion procedure occurred in 1 patient, in whom the LA was perforated but without much hemodynamic significance. In all other patients, the procedures were performed successfully without any complications. Vent drainage ranged from 1,859 to 3,940 mL/min.
Table 1. Patients’ demographics and procedural data.
| Variables | LH vent (−) (n=45) | LH vent (+) (n=25) | P |
|---|---|---|---|
| Age, years | 57.4±16.8 | 43.8±13.9 | 0.001 |
| Gender (male) | 25 (55.6) | 14 (56.0) | 0.97 |
| BSA | 1.6±0.2 | 1.6±0.2 | 0.36 |
| Prior CPCR | 22 (48.9) | 9 (36.0) | 0.29 |
| Etiology of RCS | 0.08 | ||
| ACS | 16 (35.6) | 4 (16.0) | |
| ADHF | 5 (11.1) | 11 (44.0) | |
| PCS | 13 (28.9) | 3 (12.0) | |
| Others | 11 (24.4) | 7 (28.0) | |
| Laboratory values | |||
| Hemoglobin, g/dL | 11.6±2.6 | 12.5±3.1 | 0.24 |
| Creatinine, mg/dL | 1.4±0.6 | 2.2±2.0 | 0.02 |
| Total bilirubin, mg/dL | 1.4±1.4 | 2.7±4.2 | 0.09 |
| AST | 534.1±185.7 | 156.6±188.8 | 0.39 |
| ALT | 339.7±116.4 | 133.0±172.1 | 0.45 |
| Lactate | 8.6±4.5 | 8.8±5.1 | 0.90 |
| Ventilator support | 21 (46.7) | 8 (32.0) | 0.23 |
| IABP | 4 (8.9) | 0 | 0.29 |
| Vasoactive inotropic support*, µg/kg/min | |||
| Epinephrine | 0.05±0.08 | 0.04±0.07 | 0.56 |
| Norepinephrine | 0.18±0.19 | 0.15±0.19 | 0.65 |
| Dopamine | 4.3±6.6 | 5.2±8.9 | 0.68 |
| Dobutamine | 2.7±4.9 | 5.8±6.2 | 0.04 |
| Vasopressin | 0.04±0.02 | 0.008±0.01 | 0.32 |
Results are presented as mean ± SD, or n (%). *, value of the highest dosage of each vasoactive inotropic agent during narrow pulse pressure. LH, left heart BSA, body surface area; CPCR, cardio-pulmonary cerebral resuscitation; RCS, refractory cardiogenic shock; ACS, acute coronary syndrome; ADHF, acute decompensated heart failure; PCS, postcardiotomy shock; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IABP, intra-aortic balloon pumping.
The outcomes of the ECMO support are summarized in Table 2. The successful weaning rate was higher in the LH vent (+) group in contrast to that in the LH vent (−) group with marginal statistical significance of P=0.08. The hospital mortality and morbidity rates were 50%, which were not statistically significant between the vent (+) group and the vent (−) group.
Table 2. Outcomes.
| Variables | LH vent (−) (n=45) | LH vent (+) (n=25) | P |
|---|---|---|---|
| ECMO running time, days | 7.2±7.1 | 9.2±8.5 | 0.24 |
| Ventilator support, days | 13.9±16.9 | 16.4±11.2 | 0.46 |
| ICU LOS, days | 17.5±21.6 | 25.6±19.8 | 0.11 |
| Hospital LOS, days | 24.0±37.6 | 45.9±48.5 | 0.08 |
| Complications, n (%) | 4 (8.9) | 5 (20.0) | 0.26 |
| Bleeding | 2 (4.4) | 2 (8.0) | |
| Distal malperfusion2 (4.4)3 (12.0)Weaning success, n (%) | 17 (37.8) | 15 (60.0) | 0.08 |
| Survival to discharge, n (%) | 10 (22.2) | 10 (40.0) | 0.11 |
LH, left heart; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LOS, length of stay.
As for the analysis of the predictors for the successful weaning, LH vent was identified as a predictor with marginal significance (OR =2.47; 95% CI: 0.90–6.72; P=0.07). The age was the risk factor for the successful weaning (OR =0.96; 95% CI: 0.93–0.99; P=0.03) (Table 3).
Table 3. Predictors for weaning success.
| Variables | Odds ratio | 95% CI | P |
|---|---|---|---|
| Age | 0.96 | 0.93–0.99 | 0.03 |
| Gender, male | 0.82 | 0.32–2.12 | 0.68 |
| BSA | 6.23 | 0.60–64.02 | 0.12 |
| Prior CPCR | 0.60 | 0.23–1.56 | 0.29 |
| Etiology of RCS | 0.64 | ||
| ACS | 0.83 | 0.22–3.02 | |
| ADHF | 1.25 | 0.32–4.82 | |
| PCS | 1.25 | 0.32–4.83 | |
| Others | Reference | Reference | |
| Hemoglobin | 1.14 | 0.95–1.36 | 0.16 |
| Creatinine | 1.22 | 0.80–1.87 | 0.34 |
| Total bilirubin | 0.94 | 0.76–1.16 | 0.59 |
| AST | 0.99 | 0.99–1.00 | 0.26 |
| ALT | 1.00 | 0.99–1.00 | 0.49 |
| Lactate | 0.94 | 0.84–1.04 | 0.27 |
| Ventilator support | 0.94 | 0.36–2.44 | 0.90 |
| IABP | 3.72 | 0.36–37.72 | 0.26 |
| LH venting | 2.47 | 0.90–6.72 | 0.07 |
BSA, body surface area; PCR, cardio-pulmonary cerebral resuscitation; RCS, refractory cardiogenic shock; ACS, acute coronary syndrome; ADHF, acute decompensated heart failure; PCS, postcardiotomy shock; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IABP, intra-aortic balloon pumping; LH, left heart.
Discussion
In the VA ECMO, the pulse pressure tends to decrease due to the drainage of the venous blood from the right atrium, and thus, less blood is ejected from the left ventricle. When the extracorporeal flow reaches 100% of the venous return and the systemic pulse contour becomes flat, it may cause the flow stagnation and clotting in the pulmonary vessels as well as the heart chambers. Therefore, the Extracorporeal Life Support Organization (ELSO) recommends maintaining 80% of the total ECMO flow, so that the pulse pressure remains about 10 mmHg while the heart maintains its function at the minimal level (4). However, the most important hemodynamic changes occurring with peripheral VA ECMO is the marked increase in the LV afterload as the result of the retrograde aortic flow (5). Therefore, if the heart is completely nonfunctional, the pulse pressure remains less than 10 mmHg or flat, despite the use of inotropic supports. This situation leads to the LH distension as the blood tends to fill the left chambers, and this eventually leads to the pulmonary edema. Thus, the LH distention must be treated either by restoring the pulse pressure or by draining the blood from the left chambers (6).
Regarding LH unloading during VA ECMO, several different percutaneous and surgical approaches have been proven to be useful in clinical practice. Surgical venting of LH is usually adopted in postcardiotomy heart failure or pediatric patients (7). In a central configuration of ECMO, LH venting can be achieved by inserting a cannula through the LA or LV apex, and pulmonary vein (8,9). In cases of a peripheral VA ECMO configuration, LH venting may be performed using minimally invasive techniques (10). LH unloading can be also achieved by a percutaneous approach, using several techniques: pulmonary artery drainage by inserting a venous cannula into the main pulmonary artery (11,12), trans-aortic catheter venting (13), and trans-septal venting either by atrial septostomy or by placing a drain cannula in LA (1-3,14-16). Minimally invasive implanted extracorporeal LVAD also provides the significant unloading of LV. Impella (Abiomed Inc., USA) as a trans-aortic axial flow pump, is placed percutaneously through a femoral artery. Some reports have been published about the efficacy of LH unloading in the concomitant use with VA ECMO (17,18). However, this Impella device is not available in our country yet. We used the trans-septal catheter insertion as a technique for LH decompression in most cases (Figure 3), because this procedure is less invasive in contrast to central venting procedure; thus, avoiding a surgery performed under general anesthesia. Even though, advances in these techniques have helped in achieving provided less invasive but more effective LH decompression, several issues regarding LH unloading such as proper indications, timing, and the efficacy remain still controversial (19). Camboni et al. suggested the possibility that by adding another mechanical therapy to ECMO the complexity of the case increases as well as the rate of complications and insisted that prospective randomized study is necessary to demonstrate the clinical relevance of LH (20).
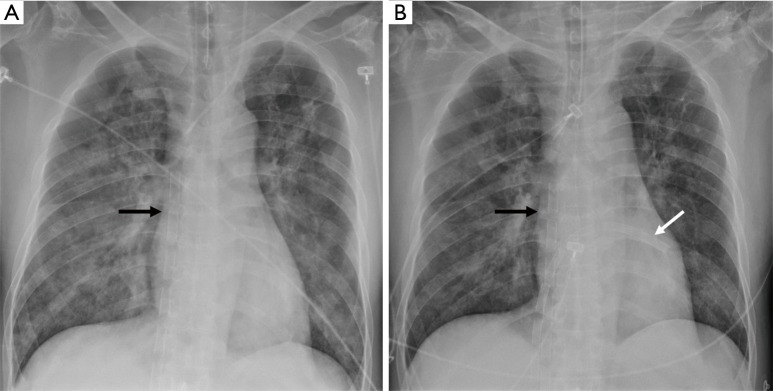
Figure 3.
Drainage cannulae configuration after LH venting on chest X-ray. (A) Chest X-ray before LH venting cannula insertion. Venous drainage cannula was placed in RA (see black arrow); (B) chest X-ray after LH venting cannula insertion. Another venous drainage cannula was placed in LA (see white arrow). LH, left heart; RA, right atrium; LA, left atrium.
A retrospective study by Truby et al. reported the poor outcomes of LV distention compared to non-distended patients and suggested the potential benefit of early decompression (21). Our study also aimed to demonstrate the efficacy of the LH venting catheter insertion for the decompression of the LH by comparing the LH vent (+) group with the LH vent (−) group. Our study showed that the successful weaning rate was higher in the LH vent (+) group in contrast to that in the LH vent (−) group. Moreover, the LH venting catheter insertion was identified as the predictors for the successful ECMO weaning with marginal statistical significance.
However, this study has several major limitations. This was a single-center, retrospective study and involved a small study population. As the study population was small, the outcomes might not show strong statistical significance. In addition, many other variables related to the patient's conditions and baseline characteristics might also have worked as confounders. Physician’s preference might also affect the decision for LH venting, which worked as an intrinsic bias. Finally, the inclusion criteria for the control group might be factitious. However, there have been no reliable criteria or definition for the LH decompression in literature and even in real practice. Therefore, we established the criteria with prolonged narrow pulse pressure which was the most reliable sign of possible LH distension.
Conclusions
LH decompression by venting catheter insertion in patients on VA ECMO may be more effective and helpful for successful ECMO weaning in contrast to conventional medical management. However, LH decompression was not related to survival benefit. Further prospective study with a large cohort may be necessary to validate this issue.
Acknowledgements
None.
Ethical Statement: This study was approved by the institutional review board (IRB) of our institution (IRB number: 2018-0035), and informed consent was waived due to its retrospective nature.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Aiyagari RM, Rocchini AP, Remenapp RT, et al. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit Care Med 2006;34:2603-6. 10.1097/01.CCM.0000239113.02836.F1 [DOI] [PubMed] [Google Scholar]
- 2.Alkhouli M, Narins CR, Lehoux J, et al. Percutaneous Decompression of the Left Ventricle in Cardiogenic Shock Patients on Venoarterial Extracorporeal Membrane Oxygenation. J Card Surg 2016;31:177-82. 10.1111/jocs.12696 [DOI] [PubMed] [Google Scholar]
- 3.Swartz MF, Smith F, Byrum CJ, et al. Transseptal catheter decompression of the left ventricle during extracorporeal membrane oxygenation. Pediatr Cardiol 2012;33:185-7. 10.1007/s00246-011-0113-7 [DOI] [PubMed] [Google Scholar]
- 4.Robert HB, Steven AC. The physiology of extracorporeal life support. In: Brogan T, MacLaren G, Lequier L, et al. Extracorporeal life support: The ELSO red book. 5th ed. Ann Arbor, MI: ELSO, Michigan University, 2017:36-7. [Google Scholar]
- 5.Burkhoff D, Sayer G, Doshi D, et al. Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol 2015;66:2663-74. 10.1016/j.jacc.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 6.Meani P, Pappalardo F. The step forward for VA ECMO: left ventricular unloading! J Thorac Dis 2017;9:4149-51. 10.21037/jtd.2017.10.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandrio S, Springer W, Karck M, et al. Extracorporeal life support with an integrated left ventricular vent in children with a low cardiac output. Cardiol Young 2014;24:654-60. 10.1017/S1047951113001017 [DOI] [PubMed] [Google Scholar]
- 8.Rescigno G, Aratari C, Matteucci ML, et al. Management of transapical left venting during adult peripheral extracorporeal membrane oxygenation. Mechanical Circulatory Support 2011;2:5981 10.3402/mcs.v2i0.5981 [DOI] [Google Scholar]
- 9.Weymann A, Schmack B, Sabashnikov A, et al. Central extracorporeal life support with left ventricular decompression for the treatment of refractory cardiogenic shock and lung failure. J Cardiothorac Surg 2014;9:60. 10.1186/1749-8090-9-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guirgis M, Kumar K, Menkis AH, et al. Minimally invasive left-heart decompression during venoarterial extracorporeal membrane oxygenation: an alternative to a percutaneous approach. Interact Cardiovasc Thorac Surg 2010;10:672-4. 10.1510/icvts.2009.228346 [DOI] [PubMed] [Google Scholar]
- 11.von Segesser LK, Kwang K, Tozzi P, et al. A simple way to decompress the left ventricle during venoarterial bypass. Thorac Cardiovasc Surg 2008;56:337-41. 10.1055/s-2008-1038664 [DOI] [PubMed] [Google Scholar]
- 12.Avalli L, Maggioni E, Sangalli F, et al. Percutaneous left-heart decompression during extracorporeal membrane oxygenation: an alternative to surgical and transeptal venting in adult patients. ASAIO J 2011;57:38-40. 10.1097/MAT.0b013e3181fe5d0b [DOI] [PubMed] [Google Scholar]
- 13.Hong TH, Byun JH, Lee HM, et al. Initial Experience of Transaortic Catheter Venting in Patients with Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. ASAIO J 2016;62:117-22. [DOI] [PubMed] [Google Scholar]
- 14.Hlavacek AM, Atz AM, Bradley SM, et al. Left atrial decompression by percutaneous cannula placement while on extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2005;130:595-6. 10.1016/j.jtcvs.2004.12.029 [DOI] [PubMed] [Google Scholar]
- 15.Eastaugh LJ, Thiagarajan RR, Darst JR, et al. Percutaneous left atrial decompression in patients supported with extracorporeal membrane oxygenation for cardiac disease. Pediatr Crit Care Med 2015;16:59-65. 10.1097/PCC.0000000000000276 [DOI] [PubMed] [Google Scholar]
- 16.Kotani Y, Chetan D, Rodrigues W, et al. Left atrial decompression during venoarterial extracorporeal membrane oxygenation for left ventricular failure in children: current strategy and clinical outcomes. Artif Organs 2013;37:29-36. 10.1111/j.1525-1594.2012.01534.x [DOI] [PubMed] [Google Scholar]
- 17.Akanni OJ, Takeda K, Truby LK, et al. EC-VAD: Combined Use of Extracorporeal Membrane Oxygenation and Percutaneous Microaxial Pump Left Ventricular Assist Device. ASAIO J 2019;65:219-26. 10.1097/MAT.0000000000000804 [DOI] [PubMed] [Google Scholar]
- 18.Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19:404-12. 10.1002/ejhf.668 [DOI] [PubMed] [Google Scholar]
- 19.Donker DW, Brodie D, Henriques JP, et al. Left ventricular unloading during veno-arterial ECMO: a review of percutaneous and surgical unloading interventions. Perfusion 2019;34:98-105. 10.1177/0267659118794112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camboni D, Schmid C. To vent or not on veno-arterial extracorporeal membrane oxygenation, does it improve myocardial recovery and outcome? J Thorac Dis 2017;9:4915-8. 10.21037/jtd.2017.11.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truby LK, Takeda K, Mauro C, et al. Incidence and Implications of Left Ventricular Distention During Venoarterial Extracorporeal Membrane Oxygenation Support. ASAIO J 2017;63:257-65. 10.1097/MAT.0000000000000553 [DOI] [PubMed] [Google Scholar]