Abstract
Background
Venous thromboembolism (VTE) is relatively common in children with acute lymphoblastic leukemia (ALL). Thrombotic risk factors in ALL are asparaginase and steroids. However, within the ALL populations treated on the same regimen, it is less clear which other risk factors play a role. Furthermore, few data are available on the effect of VTE on ALL outcomes.
Methods
In 778 children (1‐18 years) with newly diagnosed precursor‐B‐lineage or T‐lineage ALL, treated in the Dutch Childhood Oncology Group (DCOG) ALL‐10 protocol in the Netherlands (October 2004 to April 2013), we conducted a nested case control study with 59 VTE cases and 118 controls to identify risk factors for VTE.
Results
Fifty‐nine of 778 ALL patients developed VTE (7.6%), with cerebral venous sinus thrombosis (CVST) in 26 of 59 patients (44.1%). VTE occurred during induction treatment in 59.3% (n = 35) and in 40.7% (n = 24) during medium risk intensification. Conditional multivariable logistic regression analysis showed that age and ALL subtype were significantly associated with VTE (age ≥7 years: OR 2.72, 95% CI 1.33‐5.57; ALL subtype T‐ALL: OR 2.95, 95% CI 1.02‐8.57). A multivariable Cox model showed no association between the occurrence of VTE and event free survival. In CVST patients, permanent disability was present in 34.6%.
Conclusion
Within this large pediatric ALL cohort, we demonstrated a high morbidity in CVST patients. Age ≥7 years at diagnosis and T‐ALL subtype were the main risk factors for VTE, and should be considered in preventive strategies.
Keywords: acute lymphoblastic leukemia, incidence, pediatric, risk factors, venous thromboembolism
Essentials.
Venous thromboembolism (VTE) is a common complication in pediatric acute lymphoblastic leukemia (ALL).
This Dutch study identified incidence and risk factors for VTE.
7.6% of patients developed VTE, of which 44.1% were cerebral venous sinus thrombosis (CVST). Age and ALL subtype were risk factors for VTE.
1. INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common form of cancer in children.1 Over the past decades, survival rates increased tremendously to a 5‐year overall survival of >90%2, 3 due to more insight in the pathogenesis of ALL and optimized treatment strategies.4 The potential drawback of these treatment regimens is the burden of toxic effects. A severe and well‐described complication in ALL patients is venous thromboembolism (VTE).5 Reported incidences of VTE in childhood ALL varies from 1.1% to 36.7%.6, 7
The overall mortality rate in ALL patients as a result of VTE varies between 0% and 4.8%.6 A substantial proportion of VTE in ALL is due to cerebral venous sinus thrombosis (CVST) with a reported mortality between 0% and 28%.8, 9, 10, 11 Morbidity includes recurrent thrombosis, neurologic changes, catheter removal, bleeding due to antithrombotic agents, and the development of the postthrombotic syndrome.12, 13, 14, 15 Furthermore, VTE can lead to suboptimal ALL therapy, due to the necessity to interrupt, delay, or even discontinue chemotherapy, and thereby to inferior disease outcomes.16
Mechanisms underlying the increased VTE risk in ALL are not completely understood. Previous studies have shown that the disease itself, as well as treatment components, and patient characteristics, such as age, and thrombophilia, may contribute to a prothrombotic state. 6,8,12, 13, 14,17,18 The highest VTE risk arises in the first weeks of ALL treatment, especially during the use of asparaginase, in combination with corticosteroids. 13 , 17 , 19 Asparaginase is one of the most important risk factors for VTE, as it can induce coagulation disorders by decreasing natural anticoagulant proteins such as antithrombin (AT), protein C and protein S. However, it is also an essential component in modern ALL treatment, as insufficient exposure to asparaginase was shown to lead to decreased survival. 3 , 16 , 20 A better understanding of specific clinical risk factors for VTE during asparaginase treatment in ALL patients will be helpful to identify patients, in whom preventive measures might be beneficial, thereby improving the quality of ALL treatment.
The aim of this study is to assess the risk of symptomatic VTE during ALL treatment and identify clinical risk factors for VTE during asparaginase treatment in a large, well‐defined large cohort of children with ALL treated in the Dutch Childhood Oncology Group (DCOG) ALL‐10 study, using a case‐control design. In addition, we explored the effect of the development of VTE on the event free survival (EFS).
2. METHODS
2.1. Study population
We retrospectively analyzed children with ALL treated in the DCOG ALL‐10 protocol.21 Briefly, the study included 778 children with newly diagnosed ALL (precursor‐B lineage and T‐lineage ALL) between the ages of 1 and 18 years between October 2004 and April 2013 in six Dutch pediatric cancer centers. For each VTE case, we randomly selected two control patients without VTE from the ALL‐10 cohort (1:2 ratio). Controls were matched by treatment center only. The study was performed in accordance with the Declaration of Helsinki, and the institutional medical ethics committee approved this study.
2.2. Treatment
All children received the same induction chemotherapy containing prednisone 60 mg/m2/day (days 1‐28), and eight doses of native E.coli asparaginase (5000 IU/m2 per dose) every 3 days (Figure 1). Patients were stratified into three risk groups after induction treatment: standard risk, medium risk, and high risk. Medium risk patients received intensification therapy containing dexamethasone (6 mg/m2/day, 5 days every 3 weeks) and PEG‐asparaginase (2500 IU/m2, 15 doses in 30 weeks).
Figure 1.
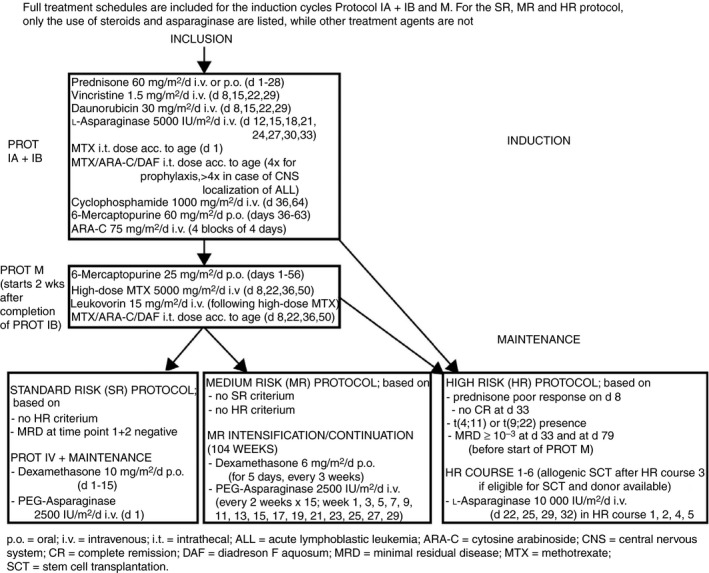
Treatment protocol of the Dutch Childhood Oncology Group (DCOG) ALL‐10 study
In all patients, a CVC was placed upon treatment initiation. None of the patients received low‐molecular‐weight heparin (LMWH) or AT supplementation routinely as thrombosis prophylaxis during ALL treatment. Children received fresh frozen plasma (FFP) if there was a bleeding tendency and fibrinogen levels dropped below 0.6 g/L on discretion of the treating physician, and platelet transfusions during induction phase, if platelet levels dropped below 10 × 109/L. Patients also received small amounts of unfractionated heparin (3 mL of 100 IU/mL) for prophylaxis of CVC blockage according to local standard of care.
2.3. Outcome definitions and data collection
VTE events were prospectively recorded centrally in the DCOG ALL‐10 study on toxicity reports. Patients with symptomatic VTE were identified from these reports (grade 3, 4, and 5 only, based on Common Terminology Criteria for Adverse Events (CTCAE), version 3.0; 2006). Screening for asymptomatic VTE was not performed. We did not include patients with incidentally found asymptomatic VTE, to avoid introduction of bias as result of missed asymptomatic thrombi. Detailed patient and treatment information was systematically obtained from patient records using standardized data collection forms for all patients with VTE.
VTE was defined as symptomatic venous thrombosis or pulmonary embolism (PE), including CVC‐associated VTE requiring intervention, objectively confirmed by appropriate imaging tests (computerized tomography [CT] or magnetic resonance imaging [MRI] for CVST, compression [Doppler] ultrasonography or venography for DVT of the leg or upper extremity vein thrombosis [UEVT] and CVC‐VTE, and [spiral] CT for pulmonary embolism).
VTE was considered CVC‐associated when it was located in the same vein as the catheter.
Central nervous system (CNS) involvement of ALL was defined as >5 leukocytes/μL cerebrospinal fluid (CSF) with identifiable leukemic cells, or intracerebral or meningeal mass seen on MRI or CT scans, or cranial nerve palsy (irrespective of CSF or imaging findings), or retinal involvement (irrespective of CSF findings). Sepsis was defined according to the Laboratory‐confirmed bloodstream infection (LCBI) criteria 1 and 2 of the Centers for Disease Control (CDC) and Prevention.22
Part of the children with VTE were tested for congenital thrombophilia, eg, Factor V Leiden or prothrombin G20210A mutation, or a reduced AT, protein C or S level according to local protocol.
2.4. Statistical analysis
VTE was expressed as percentage. Differences between children with and without VTE were calculated using Mann‐Whitney U, Chi2‐, or Fisher's exact tests. We used a case‐control design to identify potential clinical risk factors for VTE. Potential risk factors for VTE evaluated were age, sex, ALL subtype (B‐ALL or T‐ALL), ALL risk group (medium risk vs standard risk or high risk), presence of thrombophilia, CNS involvement in ALL and baseline absolute number of blasts in peripheral blood. Age and baseline absolute number of blasts in peripheral blood were explored as dichotomous variables, using the median value of all cases and controls (7 years and 42.5 × 109/L, respectively). The cumulative VTE‐free survival after ALL diagnosis was estimated with Kaplan Meier methodology. To study the association between risk factors and VTE univariable and multivariable conditional logistic regression models were estimated to take into account the case‐control design models. 23 Results are expressed as odds ratio (OR) with corresponding 95% confidence intervals (CI).
The effect of VTE on EFS (defined as non‐response, relapse, secondary malignancy, or death), was investigated by using an multivariable Cox regression analysis, with risk factors for VTE as age, ALL subtype, risk group. Results are presented as hazard ratio (HR) with corresponding 95% CIs. A two‐sided P < 0.05 was considered statistically significant. IBM SPSS Statistics 22.0 for Windows (IBM, IBM, Armonk, NY) was used for descriptive and estimation of time to VTE and Cox proportional hazard model. Analysis concerning the conditional logistic regression models is performed in R‐software environment.
3. RESULTS
3.1. Patients and VTE events
VTE events occurred in 59 of 778 included children (7.6%) during treatment in the ALL‐10 study. Of 59 children with VTE, 26 (44.1%) experienced CVST, 12 patients (20.3%) DVT of the leg, three patients (5.1%) PE, and 18 patients (30.5%) ULVT or CVC‐related thrombosis. None of these patients had previously experienced VTE (Table 1). Forty of 59 children were tested for congenital thrombophilia; in only two children, thrombophilia was found (one heterozygous prothrombin G20210A mutation, one heterozygous Factor V Leiden mutation).
Table 1.
Patient characteristics
| Cases with VTE (N = 59) | Controls without VTE (N = 118) | |
|---|---|---|
| Male, n (%) | 35 (59.3) | 76 (64.4) |
| Median age at inclusion, y (IQR) | 11 (6.0‐14.0) | 5 (2.0‐9.0) |
| ALL subtype | ||
| B‐ALL, n (%) | 43 (72.9) | 100 (84.7) |
| T‐ALL, n (%) | 16 (27.1) | 12 (10.2) |
| Unknown, n (%) | — | 6 (5.1) |
| Risk groupa | ||
| Standard risk, n (%) | 5 (8.5) | 27 (22.9) |
| Medium risk, n (%) | 43 (72.9) | 64 (54.2) |
| High risk, n (%) | 7 (11.9) | 4 (3.4) |
| Unknown/other, n (%) | 4 (6.8) | 23 (19.5) |
| Thrombophilic mutationsb, n (%) | 2 (3.4) | 1 (0.8) |
| CNS involvement of ALLc, n (%) | 4 (6.8) | 3 (2.5) |
| Median baseline leukocyte count (IQR) | 9.3 (5.1‐50.1) | 9.4 (3.9‐33.0) |
| Median baseline platelet count (IQR) | 66 (34.0‐136.0) | 60 (28.0‐119.0) |
| Median baseline absolute number of blasts (IQR) | ||
| In peripheral blood | 47 (10.3‐76.8) | 39.5 (12.3‐78.0) |
| In bone marrow | 87 (80.0‐94.0) | 89 (80.0‐94.0) |
| Sepsis, n (%) | 6 (10.2) | 13 (11.0) |
| Mortality, n (%) | 8 (13.6) | 13 (11.0) |
| Extramedullary disease, n (%) | 7 (11.9) | 11 (9.3) |
ALL, acute lymphoblastic leukemia; CNS, central nervous system; IQR, interquartile range; NS, not significant; VTE, venous thromboembolism.
Risk group is based on response to chemotherapy, initial CNS and testis involvement, presence of specific chromosomal abnormalities in the leukemic cells (see Methods).
Percent of tested patients; defined as presence of the Factor V Leiden or prothrombin G20210A mutation.
Extramedullary disease was defined as presence of a mediastinal mass, testicular involvement, or CNS involvement.
3.2. VTE events and ALL treatment
VTE events occurred during induction treatment of the DCOG ALL‐10 treatment protocol in 35 patients (59.3%), and during medium risk intensification treatment in 24 patients (40.7%). Patients stratified as standard risk or high risk (n = 16) did not experience any episode of VTE beyond induction treatment. The cumulative VTE‐free survival after ALL diagnosis was 95.5%, 92.7% and 92.4% after 9, 55, and 120 weeks, respectively (Figure 2).
Figure 2.
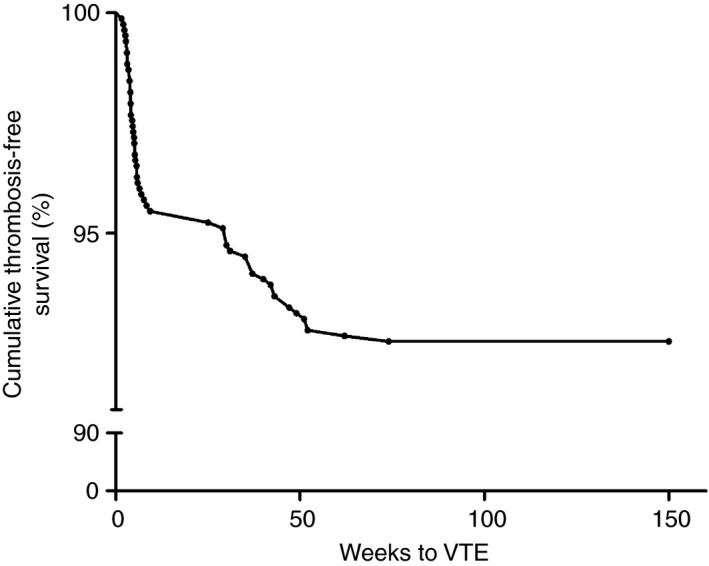
Cumulative thrombosis‐free survival after diagnosis of acute lymphoblastic leukemia
Fifty‐three of 59 patients (89.8%) with VTE had received asparaginase therapy <21 days prior to VTE occurrence. Thirty‐eight of 59 patients (64%) received corticosteroids <5 days before development of VTE. All these patients had concomitant use of asparaginase. CVST, occurred shortly after asparaginase administration (median time 3 days; range 1‐60), compared with other types of VTE (median time 7.5 days; range 2‐177). Twenty‐two of 26 (87.5%) CVST patients received simultaneous administration of corticosteroids, compared to 17/33 (51.5%) in other VTE patients (P = 0.008).
3.3. Identification of clinical risk factors for VTE in the case control study
Baseline characteristics for cases and controls are shown in Table 1. Univariate conditional logistic regression analysis showed that age as a dichotomous variable (age ≥7) ALL subtype (T‐ALL) and ALL medium risk group were statistically significantly associated with VTE (Table 2). In a multivariable conditional logistic regression analysis age and ALL subtype remained statistically significantly associated with VTE. Of all VTE, 24.1% occurred in patients aged 1‐5 years, 48.3% in patients aged 6‐12 years and 27.6% in patients aged ≥13 years.
Table 2.
Risk factors for VTE
| Variable | Univariate OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|
| Age dichotomous (≥7 vs <7 y) | 3.41 (1.72‐6.75) | 2.72 (1.33‐5.57) |
| Male versus female | 0.86 (0.64‐2.12) | |
| T‐ALL versus B‐ALL | 3.75 (1.40‐10.04) | 2.95 (1.02‐8.57) |
| MR group versus SR, HR, or unknown | 2.40 (1.17‐4.89) | 1.82 (0.85‐3.92) |
| High baseline absolute number of blasts in PB (≥42.5 vs <42.5) | 1.39 (0.71‐2.73) | |
| CNS involvement of ALL | 2.83(0.63‐12.69) |
ALL, acute lymphoblastic leukemia; BM, bone marrow; CI, confidence interval; CNS, central nervous system; HR, high risk; MR, medium risk; OR, odds ratio; PB, peripheral blood; SR, standard risk; VTE, venous thromboembolism.
Extramedullary disease was defined as presence of a mediastinal mass, testicular involvement, or CNS involvement.
In a univariate conditional logistic regression subanalysis for CVST, including age, ALL subtype, and ALL risk group, age ≥7 years and ALL medium risk group were the only significantly associated risk factor for CVST (Table 3). In a conditional multivariable logistic regression analysis none of these factors remained statistically significantly associated with CVST. Age distribution in patients with CVST was 16%, 56%, and 28% in age groups 1‐5, 6‐12, and ≥13 years, respectively.
Table 3.
Risk factors for CVST
| Variable | Univariate OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|
| Age dichotomous (≥7 vs <7 y) | 3.12 (1.07‐9.10) | 2.31 |
| Male versus female | 1.06 (0.41‐2.71) | |
| T‐ALL versus B‐ALL | 1.80 (0.43‐7.46) | 1.60 (0.44‐7.30) |
| MR group versus SR, HR, or unknown | 4.14 (1.10‐15.61) | 2.64 (0.66‐10.55) |
| High baseline absolute number of blasts in PB (≥42.5 vs <42.5) | 1.05 (0.04‐3.04) |
ALL, acute lymphoblastic leukemia; BM, bone marrow; CI, confidence interval; CVST, cerebral venous sinus thrombosis; HR, high risk; MR, medium risk; PB, peripheral blood; SR, standard risk; OR, odds ratio.
Extramedullary disease was defined as presence of a mediastinal mass, testicular involvement, or CNS involvement.
3.4. Treatment and outcome of VTE
Median follow‐up of the 59 VTE patients was 4.9 years (range 0.7‐9.6 years). Fifty‐three patients were treated with LMWH. Three patients with CVST did not receive LMWH due to concurrent bleeding. In one child with CVC related VTE the CVC was removed without anticoagulant treatment, and one patient had successful local low‐dose thrombolytic therapy. In one patient, the reason for withholding anticoagulant treatment was unknown. None of these patients experienced progression of their clot.
Median duration of LMWH treatment was 17 weeks (range 2‐103). One patient with cerebral aspergillosis experienced an intracerebral bleeding 2 days after the start of anticoagulant therapy. Five patients (8.5%) had a recurrent VTE. Four out of five patients used LMWH while developing recurrent VTE, three therapeutic and one prophylactic dosages. In the fifth patient, information on the use of LMWH was not known. Two of the patients with therapeutic LWMH had an invasive aspergillosis infection at the time of recurrent VTE. The patient with prophylactic LMWH died due to recurrent VTE (PE).
In 25 of 59 patients (42.4%), VTE occurrence led to ALL treatment protocol changes, such as delay or premature cessation of asparaginase therapy (n = 10), intrathecal therapy (n = 1), or both asparaginase and intrathecal therapy (n = 12). Details of withheld therapy doses were not retrieved. In one patient, only dexamethasone was cancelled, and in one patient treatment adjustments were unknown.
Eight of 59 patients (13.6%) with VTE died, but none directly due to the initial thrombotic event, compared to 13 of 118 control patients (11%). In the CVST subgroup, no patients died directly due to their thrombosis. Overall, four of 26 patients (15.4%) with CVST died, compared to three of 52 control CVST patients (5.8%).
In patients with CVST, acquired permanent disability as a result of the CVST was present in nine patients (34.6%). Most common was symptomatic epilepsy. Two patients had symptomatic epilepsy, focal motor deficits and cognitive disabilities. One patient had focal motor deficits. Six patients (30.8%) had symptomatic epilepsy and were treated with anti‐epileptic treatment for more than 1 year.
3.5. VTE and outcome of ALL
Multivariable Cox model showed no association between the occurrence of all types of VTE and event free survival (HR = 1.4, 95% CI 0.69‐2.72.
4. DISCUSSION
This study demonstrated that VTE occurred in 7.6% of children during ALL treatment. Multivariable analyses using a case‐control design showed that age ≥7 years was a main risk factor for VTE, thus confirming other studies. 24 , 25 Accordingly, it is recommended to include age in preventive strategies for VTE during ALL treatment. An analysis of the developmental hemostatic system showed a relative protective effect against VTE in young infants, compared with adults, with decreased levels of several critical procoagulant factors and increased levels of certain natural anticoagulants. 26 As the hemostatic system matures with increasing age in children, this may also increase their baseline VTE risk towards the level of young adults. Older children may, therefore, represent a subgroup of pediatric ALL patients in whom thromboprophylaxis with LMWH might be beneficial, and safe. 27 , 28 FFP or antithrombin supplementation have been described, but results on efficacy are inconsistent and inconclusive. 29 , 30 , 31 , 32 , 33 , 34
In the univariate conditional logistic regression model, T‐ALL subtype (OR 3.75; 95% CI 1.40‐10.04) and ALL medium risk group (OR 2.40; 95% CI 1.17‐4.89) were also significantly associated with VTE in addition to age. In the multivariable analysis T‐ALL subtype appeared to be an independent risk factor, as in the study of Giordano. That study described higher thrombin levels in T‐ALL patients. 35 ALL medium risk group was no longer significantly associated with VTE.
In concordance with other studies, asparaginase therapy is an important risk factor for VTE, during ALL treatment. 7 , 15 , 18 In our study, a majority of patients received asparaginase prior to VTE. Although the standard and high risk protocols both include one or more singular doses of asparaginase, it is likely that the repetitive and long‐term use of asparaginase in the induction cycles and medium risk protocol particularly predispose to a high VTE risk. This is in line with previous studies that demonstrated that length of exposure to, but not the dosages of asparaginase correlates with VTE risk. 7 , 8 In addition, a previous study showed no correlation between the level of asparaginase activity and the occurrence of VTE. 36
We also observed that CVST occurred very shortly after administration of asparaginase therapy in comparison with other types of VTE. This might be an explanation for the observation that CVST events occurred significantly earlier during treatment compared with other types of VTE. It is possible that patients with CVST represent a subgroup of patients at highest risk of VTE. However, specific risk factors and mechanisms explaining the predisposition for CVST during ALL treatment are still unclear. 10 , 37 One risk factor might be the simultaneous administration of steroids as combination therapy (asparaginase and steroids) occurred twice as much in patients with CSVT than in patients with other VTE. 11
In over one‐third (40%) of the VTE patients, VTE led to dose adjustments or interruption of asparaginase therapy. Asparaginase is a central component of ALL treatment, and not completing the full total dosage, could lead to a significantly worse outcome. 16 In our study, no statistical significance association was found between occurrence of VTE and EFS. It is ambiguous if reintroduction of asparaginase after VTE is safe. Two studies described reintroduction of asparaginase, in which most patients received LMWH prophylaxis and no recurrent thrombosis occurred. 38 , 39 However, another study described a high VTE recurrence rate, despite anticoagulant therapy. 18 Therefore, the efficacy and safety of thromboprophylaxis during ALL treatment remain unclear, and future studies are required for further assessment. In the meantime, identifying risk factors and those patients at highest risk may help to prepare such studies.
Although VTE events were collected prospectively, complete data retrieval was not possible for some variables, such as withheld therapy after VTE diagnosis. Although treatment protocols were standardized in the involved treatment centers, differences in care and awareness may have influenced VTE incidence. Moreover, we used a case‐control design instead of the entire ALL‐10 cohort for our analysis; despite using a 1:2 ratio for cases and controls, this may have affected the power of our results. In addition, we did not systemically study the presence of congenital thrombophilia in the ALL patients.
In conclusion, age ≥7 years at diagnosis and T‐ALL subtype are the main risk factors for VTE in children with ALL treated within a well‐defined setting including steroids and asparaginase. This underlines the importance of preventive strategies in those children with ALL at high‐risk for VTE.
RELATIONSHIP DISCLOSURE
The authors report nothing to disclose.
AUTHOR CONTRIBUTIONS
IK, HO, and MvdW, were involved in drafting the conception and design of the study. MF performed statistical analysis. HdGK performed data collection and assembly. All other authors were involved in the in the implementation of the study. IK, HO,ML, and MvdW drafted the manuscript and all other authors read, edited and approved the final manuscript.
Supporting information
Klaassen ILM, Lauw MN, Fiocco M, et al. Venous thromboembolism in a large cohort of children with acute lymphoblastic leukemia: Risk factors and effect on prognosis. Res Pract Thromb Haemost. 2019;3:234–241. 10.1002/rth2.12182
Contributor Information
Irene L. M. Klaassen, Email: i.l.klaassen@amc.uva.nl.
Mandy N. Lauw, @MandyNLauw.
Saskia Middeldorp, @MiddeldorpS.
C. Heleen van Ommen, @heleenvanommen.
REFERENCES
- 1. Kaatsch P. Epidemiology in childhood cancer. Cancer Treat Rev. 2010;36:277–85. [DOI] [PubMed] [Google Scholar]
- 2. Gatta G, Botta L, Rossi S, Aareleid T, Bielska‐Lasota M, Clavel J, et al. Childhood cancer survival in Europe 1999‐2007: results of EUROCARE‐5—a population‐based study. Lancet Oncol. 2014;15:35–47. [DOI] [PubMed] [Google Scholar]
- 3. Kamps WA, van der Pal‐de Bruin KM, Veerman AJ, Fiocco M, Bierings M, Pieters R. Long‐term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia. 2010;24:309–19. [DOI] [PubMed] [Google Scholar]
- 4. Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33:2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmiegelow K, Attarbaschi A, Barzilai S, Escherich G, Frandsen TL, Halsey C, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17:e231–9. [DOI] [PubMed] [Google Scholar]
- 6. Athale UH, Chan AK. Thrombosis in children with acute lymphoblastic leukemia: part I. Epidemiology of thrombosis in children with acute lymphoblastic leukemia. Thromb Res. 2003;111:125–31. [DOI] [PubMed] [Google Scholar]
- 7. Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Mariani G, de Gaetano G, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta‐analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108:2216–22. [DOI] [PubMed] [Google Scholar]
- 8. Payne JH, Vora AJ. Thrombosis and acute lymphoblastic leukaemia. Br J Haematol. 2007;138:430–45. [DOI] [PubMed] [Google Scholar]
- 9. Malhotra P, Jain S, Kapoor G. Symptomatic cerebral sinovenous thrombosis associated with l‐asparaginase in children with acute lymphoblastic leukemia: a single institution experience over 17 years. J Pediatr Hematol Oncol. 2018;40:e450–3. [DOI] [PubMed] [Google Scholar]
- 10. Zuurbier SM, Lauw MN, Coutinho JM, Majoie CB, van der Holt B, Cornelissen JJ, et al. Clinical course of cerebral venous thrombosis in adult acute lymphoblastic leukemia. J Stroke Cerebrovasc Dis. 2015;24:1679–84. [DOI] [PubMed] [Google Scholar]
- 11. Ghanem KM, Dhayni RM, Al‐Aridi C, Tarek N, Tamim H, Chan AKC, et al. Cerebral sinus venous thrombosis during childhood acute lymphoblastic leukemia therapy: risk factors and management. Pediatr Blood Cancer. 2017;64(12):e26694. [DOI] [PubMed] [Google Scholar]
- 12. Nowak‐Gottl U, Kenet G, Mitchell LG. Thrombosis in childhood acute lymphoblastic leukaemia: epidemiology, aetiology, diagnosis, prevention and treatment. Best Pract Res Clin Haematol. 2009;22:103–14. [DOI] [PubMed] [Google Scholar]
- 13. Athale UH, Chan AK. Thrombosis in children with acute lymphoblastic leukemia. Part II. Pathogenesis of thrombosis in children with acute lymphoblastic leukemia: effects of the disease and therapy. Thromb Res. 2003;111:199–212. [DOI] [PubMed] [Google Scholar]
- 14. Athale UH, Chan AK. Thrombosis in children with acute lymphoblastic leukemia Part III. Pathogenesis of thrombosis in children with acute lymphoblastic leukemia: effects of host environment. Thromb Res. 2003;111:321–7. [DOI] [PubMed] [Google Scholar]
- 15. Grace RF, Dahlberg SE, Neuberg D, Sallan SE, Connors JM, Neufeld EJ, et al. The frequency and management of asparaginase‐related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana‐Farber Cancer Institute consortium protocols. Br J Haematol. 2011;152:452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana‐Farber Consortium Protocol 91‐01. Blood. 2001;97:1211–241. [DOI] [PubMed] [Google Scholar]
- 17. Appel IM, Hop WC, van Kessel‐Bakvis C, Stigter R, Pieters R. L‐Asparaginase and the effect of age on coagulation and fibrinolysis in childhood acute lymphoblastic leukemia. Thromb Haemost. 2008;100:330–7. [PubMed] [Google Scholar]
- 18. Klaassen ILM, van Els AE, van de Wetering MD, van Ommen CH. Increasing incidence and recurrence rate of venous thromboembolism in paediatric oncology patients in one single centre over 25 years. Thromb Haemost. 2017;117:2156–62. [DOI] [PubMed] [Google Scholar]
- 19. Appel IM, van Kessel‐Bakvis C, Stigter R, Pieters R. Influence of two different regimens of concomitant treatment with asparaginase and dexamethasone on hemostasis in childhood acute lymphoblastic leukemia. Leukemia. 2007;21:2377–80. [DOI] [PubMed] [Google Scholar]
- 20. Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, et al. L‐asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117:238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pieters R, de Groot‐Kruseman H, Van der Velden V, Fiocco M, van den Berg H, de Bont E, et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol. 2016;34:2591–601. [DOI] [PubMed] [Google Scholar]
- 22. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. [DOI] [PubMed] [Google Scholar]
- 23. Langholz B, Goldstein L. Conditional logistic analysis of case‐control studies with complex sampling. Biostatistics. 2001;2:63–84. [DOI] [PubMed] [Google Scholar]
- 24. Athale UH, Mizrahi T, Laverdière C, Nayiager T, Delva YL, Foster G, et al. Impact of baseline clinical and laboratory features on the risk of thrombosis in children with acute lymphoblastic leukemia: a prospective evaluation. Pediatr Blood Cancer. 2018;65:e26938. [DOI] [PubMed] [Google Scholar]
- 25. Athale UH, Siciliano SA, Crowther M, Barr RD, Chan AK. Thromboembolism in children with acute lymphoblastic leukaemia treated on Dana‐Farber Cancer Institute protocols: effect of age and risk stratification of disease. Br J Haematol. 2005;129:803–10. [DOI] [PubMed] [Google Scholar]
- 26. Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998–2005. [PubMed] [Google Scholar]
- 27. Elhasid R, Lanir N, Sharon R, Weyl Ben Arush M, Levin C, Postovsky S, et al. Prophylactic therapy with enoxaparin during L‐asparaginase treatment in children with acute lymphoblastic leukemia. Blood Coagul Fibrinolysis. 2001;12:367–70. [DOI] [PubMed] [Google Scholar]
- 28. Harlev D, Zaidman I, Sarig G, Ben Arush MW, Brenner B, Elhasid R. Prophylactic therapy with enoxaparin in children with acute lymphoblastic leukemia and inherited thrombophilia during L‐asparaginase treatment. Thromb Res. 2010;126:93–7. [DOI] [PubMed] [Google Scholar]
- 29. Abbott LS, Deevska M, Fernandez CV, Dix D, Price VE, Wang H, et al. The impact of prophylactic fresh frozen plasma and cryoprecipitate on the incidence of CNS thrombosis and hemorrhage in children with acute lymphoblastic leukemia receiving asparaginase. Blood. 2009;114:5146–51. [DOI] [PubMed] [Google Scholar]
- 30. Imamura T, Morimoto A, Kato R, Izumi M, Murakami A, Matuo S, et al. Cerebral thrombotic complications in adolescent leukemia/lymphoma patients treated with L‐asparaginase‐containing chemotherapy. Leuk Lymphoma. 2005;46:729–35. [DOI] [PubMed] [Google Scholar]
- 31. Nowak‐Göttl U, Rath B, Binder M, Hassel JU, Wolff J, Husemann S, et al. Inefficacy of fresh frozen plasma in the treatment of L‐asparaginase‐induced coagulation factor deficiencies during ALL induction therapy. Haematologica. 1995;80:451–3. [PubMed] [Google Scholar]
- 32. Zaunschirm A, Muntean W. Correction of hemostatic imbalances induced by L‐asparaginase therapy in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1986;3:19–25. [DOI] [PubMed] [Google Scholar]
- 33. Ishii H, Oh H, Ishizuka N, Matsuura Y, Nakamura H, Asai T, et al. Cerebral infarction in a patient with acute lymphoblastic leukemia after fresh‐frozen plasma replacement during L‐asparaginase therapy. Am J Hematol. 1992;41:295–6. [DOI] [PubMed] [Google Scholar]
- 34. Sutor AH, Mall V, Thomas KB. Bleeding and thrombosis in children with acute lymphoblastic leukaemia, treated according to the ALL‐BFM‐90 protocol. Klin Padiatr. 1999;211:201–4. [DOI] [PubMed] [Google Scholar]
- 35. Giordano P, Santoro N, Del Vecchio GC, Rizzari C, Masera G, De Mattia D. T‐immunophenotype is associated with an increased prevalence of thrombosis in children with acute lymphoblastic leukemia. A retrospective study. Haematologica. 2003;88:1079–80. [PubMed] [Google Scholar]
- 36. Tong WH, Pieters R, de Groot‐Kruseman HA, Hop WCJ, Boos J, Tissing WJE, et al. The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia. Haematologica. 2014;99:1716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ranta S, Tuckuviene R, Makipernaa A, Albertsen BK, Frisk T, Tedgard U, et al. Cerebral sinus venous thromboses in children with acute lymphoblastic leukaemia—a multicentre study from the Nordic Society of Paediatric Haematology and Oncology. Br J Haematol. 2015;168:547–52. [DOI] [PubMed] [Google Scholar]
- 38. Musgrave KM, van Delft FW, Avery PJ, Clack RM, Chalmers EA, Qureshi A, et al. Cerebral sinovenous thrombosis in children and young adults with acute lymphoblastic leukaemia—a cohort study from the United Kingdom. Br J Haematol. 2017;179:667–88. [DOI] [PubMed] [Google Scholar]
- 39. Qureshi A, Mitchell C, Richards S, Vora A, Goulden N. Asparaginase‐related venous thrombosis in UKALL 2003‐ re‐exposure to asparaginase is feasible and safe. Br J Haematol. 2010;149:410–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


