Abstract
Background
Arterial thrombosis models are important for preclinical evaluation of antithrombotics but how anesthetic protocol can influence experimental results is not studied.
Objectives
We studied how three most commonly used rodent anesthetics affect the induction of thrombosis and thrombus resolution with antiplatelet agent integrilin (Eptifibatide).
Methods
The Folts, electrolytic, and FeCl3 models of carotid artery thrombosis were evaluated. The extent of blood flow reduction required to elicit cyclic flow reductions (CFR) was examined in the Folts model. The occlusion time and stability following electrolytic or FeCl3 injury was assessed. The efficacy of Eptifibatide was studied in each cohort and clot composition following FeCl3 application was assessed histologically.
Results
Isoflurane and ketamine‐xylazine (ket‐x) elicited higher basal blood flow velocities. For reliable CFR in the Folts model, a higher degree of blood flow reduction was required under ket‐x and isoflurane. For the FeCl3 and electrolytic models, injury severity had to be increased in mice under ket‐x anesthesia to achieve rapid occlusion. FeCl3‐injured artery sections from ket‐x and isoflurane‐treated mice showed vessel dilatation and clots that were more fibrin/red‐cell rich compared to pentobarbitone. Integrilin led to cycle abolishment for all three Folts‐injury cohorts but for the electrolytic model a 2.5‐fold higher dose was required to restore blood flow under pentobarbitone. Integrilin after FeCl3 arterial injury was partially ineffective in isoflurane‐treated mice.
Conclusions
Anesthesia impacts rodent carotid artery occlusion experiments and alters integrilin efficacy. It is important to consider anesthetic protocols in animal experiments involving pharmacological agents for treatment of atherothrombosis.
Keywords: anesthesia, arteries, mice, thrombosis
1.
Essentials.
The Folts, electrolytic and FeCl3 models of carotid artery clot induction were studied.
Mice were placed under pentobarbitone, ketamine‐xylazine, or isoflurane anesthesia.
Clotting times and clot stability were variably affected by anesthesia in each model.
Efficacy of eptifibatide in each model was altered by anesthesia.
2. INTRODUCTION
Animal models have led to the discovery of new strategies for cardiovascular protection and the prevention and management of thrombosis. However, no single model can accurately recapitulate human vascular disease and therefore a range of different arterial thrombosis models have been developed. It has been reported that the same mice and same model in different laboratories have yielded different results,1 which has been attributed to differences in the surgical techniques utilized rather than the models themselves.1 Accordingly, it is advocated that the model of thrombosis be carefully selected to answer the research question.2
Topical application of FeCl3 on a vessel causes oxidative damage to and aggregation of red blood cells which mediate the adherence of platelets to the non‐denuded endothelium.3, 4 The time to cessation of blood flow can be affected by FeCl3 concentration, types of anesthesia, as well as surgical techniques.5 The Folts model is more clinically relevant as it encompasses the combined effects of deep‐vessel injury and high‐shear stress, thereby mimicking two key elements related to thrombus formation in atherosclerotic vessels.6, 7 In this model, thrombi are formed and then embolized in repetitive cycles, prior to administration of antithrombotic drugs which abolish these cycles.6 In the mouse electrolytic model, a thrombus is formed by applying a current to the external surface of the exposed carotid artery during stasis, causing disruption to the endothelial lining of the vessel.6
A survey conducted by the Biorheology subcommittee of the International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Committee (SSC) revealed that overall, the most commonly used murine models of thrombosis are ferric‐chloride and laser injury followed by mechanical and photochemical injury, then followed by electrolytic injury and other injury methods. Laser injuries are typically performed as a model of microvascular thrombosis and the site of injury is usually the cremaster and mesenteric arterioles.2 While this is common, laser injury to the carotid artery to simulate macrovascular thrombosis is seldom performed and therefore has not been included in this study.1 The same study also documented that type of anesthetic was reported as uncritical, and pentobarbitone and ketamine/xylazine (ket‐x) were the most commonly used.2 However, in countries like Australia pentobarbitone is being reclassified to a Schedule 8 (controlled) drug8 and is no longer recommended for use as a rodent anesthetic,9 and moreover, in the United States and many other countries, pentobarbitone is now unavailable.10 Therefore ket‐x and isoflurane are now the anesthetics of choice for laboratory animal experimentation. In rodents low concentrations of isoflurane (<1%) increase systemic blood flow while maintaining cardiac output and myocardial perfusion. Higher isoflurane concentrations (>2.5%) increase peripheral vasodilatation and decrease systemic vascular resistance.11 Anesthesia with ket‐x causes respiratory depression and decreases heart rate and arterial blood pressure; pentobarbitone also causes respiratory depression but does not affect heart rate to the same extent as ket‐x.12
Here, we studied how the three different anesthetics influence the experimental outcomes of the three abovementioned models of arterial thrombosis. The effect of anesthesia on hemodynamics, namely tail bleeding time and platelet aggregation in response to adenosine diphosphate (ADP) and thrombin was also studied. The αIIbβ3 pathway is platelet‐specific and mediates several aggregation‐dependent platelet roles in coagulation, fibrinolysis, etc. and therefore αIIbβ3 antagonists like eptifibatide/integrilin are globally effective in preventing thrombosis.13 Hence, we examined the efficacy of integrilin in minimizing arterial thrombosis initiated by each mode of injury, with all three anesthetic protocols.
3. METHODS
3.1. Animals
Eight‐ to 10‐week‐old male C57Bl/6 mice (22‐28 g) were used in these studies. Animals were held in the Alfred Medical Research and Education Precinct (AMREP) Animal Services facility, and kept on a 12 h light/dark cycle with ad libitum access to food and water. These studies were approved by the AMREP Animal Ethics Committee (E/1677/2016/M) in accordance with the Australian code for the care and use of animals for scientific purposes 8th edition, 2013.
3.2. Anesthesia
Mice were randomly chosen to be anesthetized with either pentobarbitone (60 mg/kg intraperitoneal (i.p.); Nembutal, Ceva Animal Health, Glenorie, New South Wales, Australia), ketamine/xylazine (85 mg/kg; Ketamav 100, MavLab Slacks Creek, Queensland, Australia/15 mg/kg i.p.; Ilium Xylazil‐20, Troy Laboratories, Glendenning, New South Wales, Australia), or isoflurane (5% for induction and 1.5% for maintenance in 1.0 L/min oxygen; Isothesia, Provet, Heatherton, Victoria, Australia). For ket‐x treated animals, a top up dose of 50% of the calculated dose for the animal was administered during the procedure. Mice were euthanized with an overdose of injectable anesthetic (Lethabarb, Virbac, Milperra, New South Wales, Australia) except for the platelet aggregation experiment‐control cohort of mice that were euthanized via CO2 inhalation.
3.3. Surgical set‐up
In mice receiving injectable anesthetics, a tracheotomy was performed and mice were mechanically ventilated with room air via a respiratory pump (MiniVent Type 845, Hugo Sachs Elektronik, Harvard Apparatus GmbH, March‐Hugstetten, Germany) and core body temperature was maintained at 37°C using a heat lamp. The left carotid artery was exposed via blunt dissection, and carefully dissected clear of the vagus nerve and surrounding tissue. A calibrated flow probe (0.5 mm in diameter) linked to a flow meter (TS420, Transonic Systems, Inc., Ithaca, NY, USA) was placed around the artery to measure blood flow velocity (mL/min), which was then corrected for body weight (mL/min/100 g). Blood flow data were acquired using a Powerlab device and analyzed using LabChart Pro., version 8.0 software (ADInstruments, Bella Vista, New South Wales, Australia). A ~2‐mm long injury was made on the common carotid artery ~3 to 5 mm below the bifurcation of common carotid into internal and external common carotid arteries. The right jugular vein was exposed for administration of drugs. Following the surgical procedures, the animals were allowed to stabilize for 10 minutes before continuation of the experiment.
3.4. Folts model
This was performed as published.14 Distal to the flow probe, a silk suture (6‐0) was tied loosely around the left carotid artery and subsequently tightened to cause a stenosis that decreased blood flow. The segment of the artery under the stenosis was then de‐endothelialized by pinching the artery over the suture five times with a pair of forceps. Carotid blood flow was monitored until it reached 0 mL/min, indicating an occlusive thrombus had formed at the site of stenosis.
After 30 seconds, the site of stenosis was gently agitated with a pair of forceps to embolize the thrombus, thus restoring blood flow. Once again, carotid blood flow was monitored until it reached 0 mL/min, and the time recorded. These phenomena are called cyclic flow reductions (CFRs), and are a consequence of recurrent platelet aggregation at the site of stenosis and injury, and subsequent dislodgement of the thrombus. Integrilin (Eptifibatide acetate, Sigma Aldrich, Castle Hill, New South Wales, Australia; 4 mg/kg) was administered during the fourth CFR at the point at which the thrombus had been embolized. Blood flow was then continuously monitored for 30 minutes post–drug administration. If an occlusive thrombus formed as indicated by a blood flow reading of 0 mL/min, CFRs were reinstated. The average amount of blood flowing through the site of stenosis during three cycles was determined by calculating the area under the blood flow curve.
3.5. Electrolytic model
Distal to the flow probe, a hook‐shaped electrode was placed around the left carotid artery. Integrilin (4 or 10 mg/kg) was administered 10 minutes before electrolytic injury. The artery was then clamped distally to the electrode using hemostatic clamps to occlude blood flow and a current (18‐22 mA) was delivered for a set period (2‐3 minutes) via the electrode using a lesion making device (Model 53500; Ugo Basile, Gemonio, Province of Varese, Italy). The artery clamp was then released immediately and blood flow monitored continuously for 30 minutes. The formation of an occlusive thrombus was indicated by a blood flow reading of 0 mL/min. Total blood flowing through the artery at the site of injury was determined by calculating the area under the blood flow curve.
3.6. Ferric chloride model
This method was carried out as described.15 Integrilin (4‐20 mg/kg) was administered 10 minutes before ferric chloride injury. The flow probe was then removed, and filter paper (1 mm diameter) soaked in 6% (0.36 M) or 10% (0.61 M) freshly prepared ferric chloride (45% Iron [III] chloride solution, Sigma Aldrich, diluted in saline) was placed on the external surface of the carotid artery for 3 minutes. After the 3‐minute period, the carotid artery was flushed with warmed saline and the flow probe reattached to the site of injury. Blood flow was recorded as above.
3.7. Carstairs staining
Approximately 3‐4 mm of the common carotid artery spanning the site of injury was dissected and stored in 4% phosphate buffered formalin and later processed, paraffin‐embedded, and 4‐μm thick sections were stained using the Carstairs stain, as described).4 Staining was performed by the Monash Histology Platform (Clayton, Australia). Using this staining technique, fibrin appears red, platelets appear blue/purple, red blood cells appear yellow, and collagen staining in the vessel wall appears blue. Images were visualized using an Olympus BX50 microscope and a 20X/0.46 NA objective, and the images were captured using an Olympus DP71 digital camera (Olympus, Tokyo, Japan). Image quantification is described in Supporting Information.
3.8. Statistical methods
Data are presented as mean ± SEM. GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla, California, USA (www.graphpad.com) was used for all analyses. Outliers were excluded based on the GraphPad Prism Robust regression and Outlier removal method. Basal blood flow as a function of time was analyzed using a two‐way repeated measures ANOVA with Tukey's post‐hoc analysis. Comparisons between the three different anesthesia groups were made using one‐way ANOVA followed by Tukey's multiple comparisons test. The effect of integrilin treatment was compared with a two‐way ANOVA with Tukey's post‐hoc analysis for multiple comparisons; for the Folt's model this was done using two‐way RM ANOVA with Sidak's post‐hoc correction. Histology data was assessed using one‐way ANOVA with Tukey's post‐hoc analysis and % fibrin area was assessed using Kruskal‐Wallis ANOVA with uncorrected Dunn's post‐hoc correction. In all cases, statistical significance was accepted if P < 0.05.
4. RESULTS
We first assessed whether anesthesia affects blood flow in the carotid artery. Pentobarbitone anesthesia reduced basal blood flow velocity (1.09 ± 0.01 mL/min/100 g) in the carotid artery when compared to ket‐x and isoflurane‐treated animals. In mice anesthetized with isoflurane pre‐experimental basal blood flow was 3.5 times higher than that of pentobarbitone treated mice (Figure 1A; n = 6 per group). Therefore, blood flow velocity in the carotid artery ranges from lowest to highest when mice are anesthetized with pentobarbitone, ket‐x and isoflurane, respectively.
Figure 1.
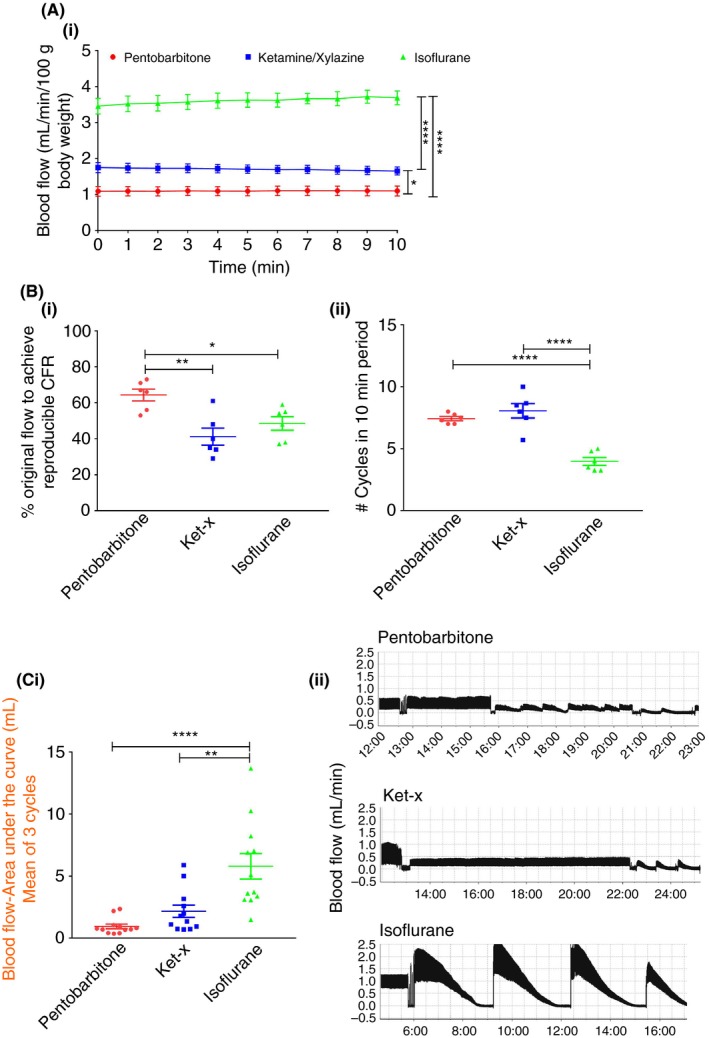
Folts model. (A) Basal blood flow in the carotid artery is significantly higher when mice are anesthetized with isoflurane and ket‐x than compared to pentobarbitone (n = 6). (B) Greater degree of blood flow restriction is necessary to initiate recurrent cycles of thrombus formation and embolism when mice are placed under anesthesia with ket‐x and isoflurane, compared to pentobarbitone (n = 5‐6). (ii) Significantly fewer cycles per 10 min are seen in the isoflurane group. (C) (i) Area under the curve analysis shows that blood flow over an average of three cycles is correspondingly increased in isoflurane‐treated animals as validated in (ii) a representative blood flow chart from one animal per cohort (representative of n = 12 animals). Data is mean ± SEM. *P < 0.05; **P < 0.01; **** P < 0.001 (one‐way analysis of variance (ANOVA) with Tukey post‐hoc analysis)
Next we studied how these changes in blood flow would affect the onset of CFR in the Folts model of mechanical injury. As seen in Figure 1Bi, blood flow had to be reduced to only 64.3 ± 8.1% of original flow rate to elicit ≥4 CFR in a 10‐minute period in mice anesthetized with pentobarbitone. In contrast, in ket‐x‐injected animals, flow rate had to be reduced to 41.2 ± 11.65% and a similar degree of restriction in flow was required to achieve periodic CFR in isoflurane‐treated mice (48.5%; n = 6 per group). Mice anesthetized with pentobarbitone and ket‐x show a greater number of cycles (~2x) within a 10‐minute period when compared to isoflurane (Figure 1Bii; n = 6 per group). Mice that were operated under inhaled isoflurane had significantly higher mean blood flow volume over three cycles (5.78 ± 1.03 mL; (Figure 1Ci; n = 12 per group) when compared to pentobarbitone and ket‐x injected animals. The blood flow traces for mice undergoing Folts preparation from one mouse per experiment, representative of n = 12 mice are shown in Figure 1Cii.
Electrolytic injury resulted in consistent occlusion times only in mice that were anesthetized with pentobarbitone. In fact, application of 18 mA current to the carotid artery had virtually no effect when mice were anesthetized with ket‐x and variable effect with isoflurane (Figure 2Ai). Accordingly, while stable occlusions were induced in a majority of pentobarbitone‐treated mice, only unstable occlusions, if any, were seen when the same current was applied to the carotid arteries of mice anesthetized with ket‐x and isoflurane (Figure 2Aii). Hence it was necessary to increase the injury severity by applying a current of 22 mA to the vessel in mice treated with ket‐x, as below. The occlusion times in the isoflurane‐treated cohort were still highly variable, with unstable thrombi evident in all mice even when the current was increased 26 and 28 mA (Figure S1; n = 3‐5 per group). In fact, even an injury induced at 28 mA current did not yield reproducible occlusive thrombi in isoflurane‐treated mice (Figure S1); in some instances, the vessel burst and in other instances unstable occlusions were seen (data not shown). Hence we discontinued electrolytic injury experiments in this cohort and concluded that isoflurane is a not a reliable anesthetic in this paradigm.
Figure 2.
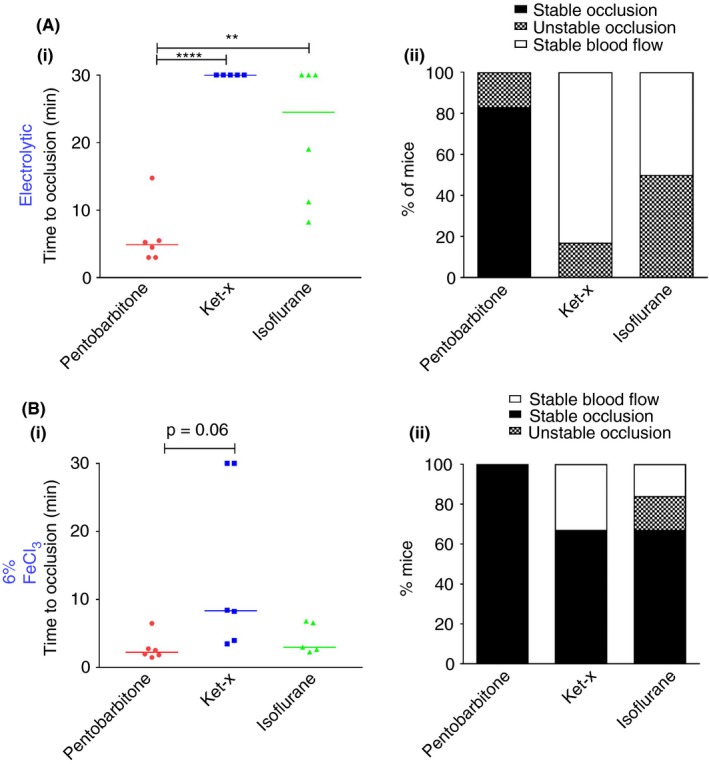
Electrolytic and FeCl3 thrombosis models. (A) (i) Electrolytic injury with 18 mA results in short occlusion times only in the pentobarbitone treated cohort and (ii) these occlusions are largely stable when compared to the ket‐x and isoflurane‐treated groups (n = 5‐6). Bar indicates median time. (B) Rapid vessel occlusion occurs when 6% FeCl3 is applied to the carotid artery under pentobarbitone and isoflurane anesthesia but not under ket‐x. Median times are shown. (ii) Thrombus stability is different in each of the three groups. *P < 0.05; **P < 0.01; **** P < 0.001 (one‐way ANOVA with Tukey post‐hoc analysis)
The time to cessation of blood flow with 6% FeCl3 was highly variable in the ket‐x (median time of 8.34 minutes) and isoflurane (median time of 3 minutes) treated groups although it was less variable in mice placed under pentobarbitone anesthesia (median time = 2.2 minutes) (Figure 2Bi). In ket‐x and isoflurane‐treated animals stable occlusive thrombi were only seen in 67% of animals (Figure 2Bii). Hence we proceeded to use 10% FeCl3 to induce thrombosis which provided more consistent results, as described below.
Tail bleeding time was not significantly different when mice were anesthetized with pentobarbitone, ket‐x, or isoflurane (n = 4 per group; Figure S2A). Furthermore, full blood evaluation revealed no anesthesia‐related changes in cell counts and hematocrit (Figure S2B; n = 6‐9 per group). When compared to control mice euthanized via CO2 inhalation, platelet function in response to 0.1 U/mL thrombin or 10 μmol/L ADP was similarly unaltered in wildtype mice placed under anesthesia with each of the three methods for a 20‐minute period (Figure S3; n = 3 per group). Therefore any changes in thrombosis and thrombus resolution seen in this study are unrelated to changes in platelet function.
Next we assessed clot composition in mice subjected to 10% FeCl3 injury to determine if there were any physical changes in the clots formed under each of the three anesthesia protocols. Figure 3A shows a representative image of Carstairs staining from one mouse per cohort of n = 3 mice per cohort. Vessel diameter (Figure 3Bi) and lumen area (Figure 3Bii) are less in pentobarbitone‐anesthetized mice while the vessel wall is significantly thicker (Figure 3Biii) in these mice. On the other hand, isoflurane‐treated mice had larger vessel diameters and areas together with reduced vessel wall thickness. In the ket‐x group, vessel thickness was significantly less than the pentobarbitone treated group (Figure 3Biii). These readouts align with published findings that isoflurane causes vasodilatation.16 Additionally, pentobarbitone‐treated mice had significantly less fibrin/red‐cell components in the clots compared to isoflurane‐treated mice and a similar trend was seen with mice treated with ket‐x (n = 3 per group; Figure 3Biv).
Figure 3.
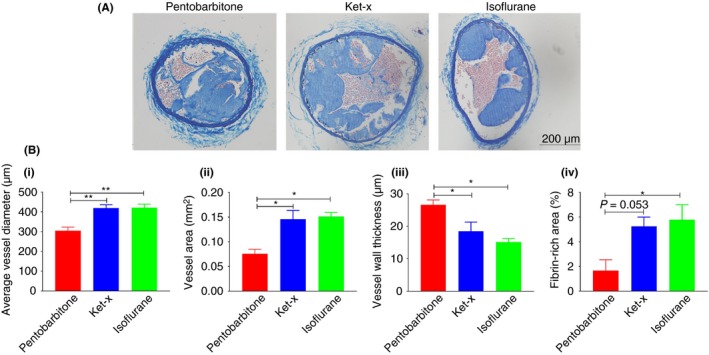
Carstairs staining of carotid artery sections following 10% FeCl3 injury. (A) Representative carotid artery sections dissected from mice subjected to FeCl3 injury under each of the three anesthetic protocols. (B) Quantification of N = 3‐5 sections of n = 3 animals per cohort shows that (i) average vessel diameter is significantly higher in ket‐x and isoflurane‐treated animals. **P < 0.01 (one‐way ANOVA with Tukey post‐hoc analysis). (ii) Vessel area is also similarly increased. (iii) Average thickness of the vessel wall is correspondingly reduced in both ket‐x and isoflurane animals compared to pentobarbitone treated animals *P < 0.05 (one‐way ANOVA with Tukey's post‐hoc analysis). (iv) the percent of fibrin in the clots formed under isoflurane anesthesia is significantly more than under ket‐x and pentobarbitone anesthesia. *P < 0.05 (Kruskal‐Wallis one‐way ANOVA with uncorrected Dunn's post‐hoc analysis). Data is mean ± SEM
We then investigated the efficacy of integrilin under each anesthetic protocol. For the Folts model, injection of integrilin after regular CFR were initiated significantly abolished ongoing CFR in all three cohorts (Figure 4Ai) while the duration of cycle abolishment was also consistent across all the three groups (Figure 4Aii; n = 6 per group). Therefore, despite anesthesia‐related changes in blood flow velocity and volume during the establishment of Folts injury, 4 mg/kg i.v. integrilin successfully restored blood flow regardless of anesthesia used.
Figure 4.
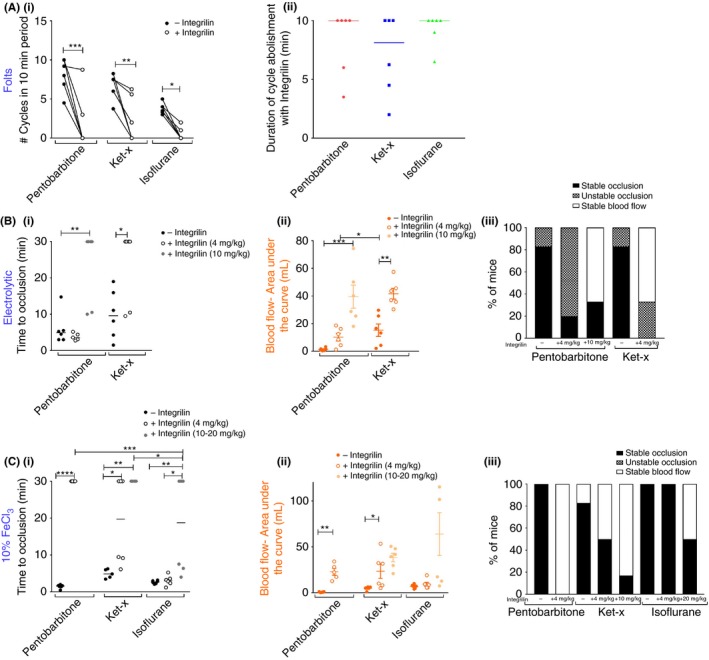
Anesthesia impacts the sensitivity to platelet inhibition that varies with thrombosis model. (A) Integrilin treatment (4 mg/kg) in the Folts model (i) significantly abolishes CFR in all three cohorts. *P < 0.05; **P < 0.01, *** P < 0.001 (two‐way RM ANOVA with Sidak's post‐hoc analysis) and (ii) CFR abolishment is maintained up to 10 min in all cohorts. Bars are median time. (B) Electrolytic model: (i) only at higher dose of 10 mg/kg does integrilin prolong median occlusion time in the pentobarbitone treated cohort when injury is applied at 18 mA. Increasing the applied current to 22 mA in mice anesthetized with ket‐x leads to median occlusion times of <10 min, and these thrombi can be resolved by 4 mg/kg integrilin injection. (ii) Area under curve analysis shows similar trends in that integrilin treatment promotes clot resolution in mice placed under pentobarbitone and ket‐x anesthesia with the latter cohort showing a better response to the lower dose of integrilin treatment (n = 5‐6). (iii) Thrombi are destabilized with 4 mg/kg integrilin in the pentobarbitone cohort and fully resolved with the higher dose of 10 mg/kg but only in four out of six mice. Four mg/kg integrilin leads to stable blood flow or destabilizes thrombi in all mice under ket‐x anesthesia (n = 5‐6). (C) 10% FeCl3 model: integrilin (4 mg/kg) is only effective at preventing vessel occlusion under pentobarbitone, and ket‐x to a lesser degree. Dose of 10 mg/kg is effective in ket‐x treated animals and in isoflurane‐treated animals, even a dose of 20 mg/kg only resolves thrombi in three out of six animals. Bars are median occlusion time (ii) area under curve show similar trends in that integrilin treatment mitigates arterial thrombosis in mice placed under pentobarbitone at a lower dose and in ket‐x anesthesia at 10 mg/kg but not isoflurane even at higher doses of 20 mg/kg. Data is mean ± SEM. (iii) Stable occlusive thrombi are resolved when integrilin is administered in mice that were anesthetized with pentobarbitone and in 50% and 87% of mice treated with ket‐x and doses of 4 and 10 mg/kg, respectively but in mice anesthetized with isoflurane even at doses of 20 mg/kg only 50% of mice showed thrombus dissolution (n = 5‐6). *P < 0.05; **P < 0.01; *** P < 0.001; **** P < 0.0001 (two‐way ANOVA with Sidak post‐hoc analysis). Second integrilin dataset: dose is 10 mg/kg for ket‐x and 20 mg/kg for isoflurane
In contrast, αIIbβ3 blockade prior to induction of electrolytic and 10% FeCl3 injury resulted in variable outcomes. Arterial thrombi formed by 18‐mA electrolytic injury in mice anesthetized with pentobarbitone could not be resolved by a dose of 4 mg/kg integrilin and neither occlusion times (Figure 4Bi) nor volume of blood flow in the vessel (Figure 4Bii) were significantly increased. Yet, integrilin appeared to markedly destabilize the thrombi that were formed (Figure 4Biii) even though vessel patency was not restored (data not shown). Only when the dose of integrilin was increased to 10 mg/kg was a robust antithrombotic effect evident in mice from the pentobarbitone cohort (Figure 4Bi; n = 6). In contrast, median occlusion time was significantly prolonged in 4 mg/kg integrilin‐treated mice that had been anesthetized with ket‐x albeit there were two mice in this group that did not respond to integrilin (Figure 3Bi). However, in this cohort, integrilin treatment restored blood flow (Figure 4Bii) and vessel patency (data not shown) and prevented the formation of stable occlusions (Figure 4Biii) in all animals. Therefore, while pentobarbitone and ket‐x anesthesia can be reliably utilized for electrolytic injury experiments, a robust anti‐thrombotic effect of αIIbβ3 blockade is only evident at a higher dose of 10 mg/kg in mice placed under pentobarbitone anesthesia.
Unlike for electrolytic injury, pretreatment with 4 mg/kg integrilin prevented vessel occlusion in all pentobarbitone anesthetized mice (n = 5) subjected to FeCl3 injury. This effect was only noted in 50% of the ket‐x treated cohort (n = 6), in which the median time to occlusion of 19.7 minutes. Upon increasing the dose to 10 mg/kg for ket‐x treated mice, vessel occlusion times were significantly prolonged. Surprisingly, 4 mg/kg integrilin was unable to resolve thrombi formed by 10% FeCl3 in mice anesthetized with isoflurane for the duration of the experiment (n = 6; Figure 4Ci). A dose of 10 mg/kg was then trialed with similar results (data not shown). When mice were treated with 20 mg/kg integrilin under isoflurane anesthesia three out of six animals showed successful prevention of thrombus formation (n = 6; Figure 4Ci). Similar trends were seen when we assessed the duration for which the vessels remained patent (data not shown) and the volume of blood flow in the vessel during the experiment (Figure 4Cii). In fact, stable occlusions remained in 50% of the ket‐x treated animals and in all of the isoflurane‐treated animals at the lower dose but in only one out of six animals treated with ket‐x at a dose of 10 mg/kg and 50% of the animals anesthetized with isoflurane and dosed with 20 mg/kg integrilin (Figure 4Ciii). Therefore integrilin has a high efficacy in the 10% FeCl3 model under pentobarbitone anesthesia but it is only effective at a higher dose (10 mg/kg) in mice under ket‐x anesthesia and is partially ineffective under isoflurane anesthesia even at a high dose of 20 mg/kg.
5. DISCUSSION
Carotid artery injury models have regular variations in severity, clot composition, and clot size and it is widely accepted that the results of a particular anti‐thrombotic drug being developed are model dependent.17 But here we show that the choice of anesthesia also needs to be carefully considered when testing new compounds for treatment of atherothrombosis.
Consistent with our findings, isoflurane is known to increase systemic blood flow but it does not increase blood pressure or cardiac output.18 Mice anesthetized with 100 mg/kg ket‐x have significantly lower heart rates and cardiac output than those treated with isoflurane,19 possibly due to peripheral α2‐adrenoceptor blockade.18 This may explain why ket‐x treated animals had lower basal blood flow velocities. We found that carotid artery basal blood flow was slowest under pentobarbitone anesthesia. This may be explained by earlier reports showing that pentobarbitone does not alter heart rate but reduces the cardiac index in mice due to a direct negative inotropic action on the myocardium or by inhibition of cardiac sympathetic tone.18 Shear rate is a function of velocity and is inversely proportional to vessel diameter.20 Isoflurane and ket‐x increase basal blood flow velocities but also appear to increase vessel diameter in our study, while pentobarbitone reduces blood flow velocity and results in reduced vessel diameter. Hence we hypothesize that within the context of each model of carotid artery thrombosis, vessel shear rates are comparable under each anesthetic protocol, although more specialized experiments are required to prove this hypothesis. A major limitation in this study is that hemodynamic parameters such as blood pressure, PO2, PCO2, blood glucose, and pH were not measured or controlled. However, it does not affect our observation that the choice of anesthesia changes results of the selected thrombotic models and that anesthesia also changes blood flow velocity without modulating tail bleeding time or altering platelet function. The doses of anesthesia used in this study are based on guidelines set out by the institutional ethics committee and align with recommended doses of anesthesia in mice. For pentobarbitone the dose is 40‐50 mg/kg i.p., which provides immobilization/anesthesia for 20‐40 minutes and sleep time of 120‐180 minutes. Recommended doses for ketamine + xylazine are 80‐100 mg/kg + 10 mg/kg i.p. that provides surgical anesthesia for 20‐30 minutes and sleep time of 60‐120 minutes.10
In a rabbit model of Folts‐like arterial stenosis, anesthesia using either pentobarbitone or isoflurane did not alter the number of CFR observed over a 45‐minute period.21 In our hands, to initiate regular CFR under anesthesia with both ket‐x and isoflurane, a higher degree of blood flow restriction was required. It should be noted that the percent reduction in blood flow in each cohort was not directly proportional to the basal blood flow, indicating that the blood flow rate for successful CFR induction under each anesthetic method is not merely a factor of basal blood flow rate. Once comparable CFR were initiated, there was a greater volume of blood flow in the isoflurane‐treated mice, which would promote the washout of clotting factors thereby justifying the reduced numbers of CFR noted in mice anesthetized with isoflurane and subjected to Folts‐stenosis injury.22
Multiple studies have shown that isoflurane has complex vasomotor effects.16, 18, 23 We attribute the lack of success of the electrolytic injury in formation of reproducible thrombi under isoflurane anesthesia to the observed physical changes in the vessel caused by this agent. Indeed the histological data strengthens the case that isoflurane results in the thinning of the vessel wall which could explain the increased risk of the vessel bursting under isoflurane in our experiments. The FeCl3 thrombosis model although convenient, is not clinically relevant, as the endothelium is not denuded by application of FeCl3. 4 Isoflurane appears to exert both pro‐dilatory and pro‐constrictive vasomotor properties. While it reduces endothelium‐mediated vessel dilatation by attenuating secretion of nitric oxide, when endothelial denudation occurs, such as during mechanical injury, this effect is lost and vasodilatation is increased in denuded vessels.23 Thus, future experiments should involve histological assessment of the carotid artery specimens of mice that have been subjected to electrolytic injury or Folts preparation to determine whether the vessels are more dilated under isoflurane anesthesia in these models.
The sensitivity to FeCl3 concentration is based on its intraluminal mass transport, which in turn is a function of blood velocity and vessel diameter both of which are increased under ket‐x and isoflurane anesthesia. Yet curiously application of 6% FeCl3 did not trigger the formation of stable occlusive thrombi only in the ket‐x treated group. This is similar to the electrolytic injury wherein a greater current had to be applied to initiate thrombus formation in the ket‐x treated group when compared to the pentobarbitone treated group.
Preventing the interaction of activated αIIbβ3 with its ligands represents the final common pathway for platelet aggregation and underpins our choice of integrilin in this study.24 Undoubtedly, it would be ideal to compare efficacies of alternative drugs hirudin or clopidogrel to integrilin under each anesthetic method. The in vivo efficacy of eptifibatide was first successfully examined in the canine model of Folts stenosis.7 αIIbβ3 antagonists have maximal efficacy in the Folts model, by abrogating platelet aggregation and in totally preventing CFRs, and maintaining arterial patency.25 Accordingly, we see a robust response of integrilin at 4 mg/kg under all three modes of anesthesia in the Folts model. Peptide inhibitors of αIIbβ3 such as integrilin, although effective at preventing platelet adhesion, are not as effective in preventing platelet activation when compared to αIIbβ3 receptor targeted antibodies such as abciximab.26 The current applied in the electrolytic injury procedure causes an endothelial lesion with platelet‐rich intravascular thrombi near the site of arterial stenosis.6 This may explain the need for higher doses of eptifibatide (10 mg/kg) to effectively recanalize the carotid artery after electrolytic vessel wall injury. Wang et al showed that clots formed with >5% FeCl3 are not amenable to antithrombotic therapy in rodents placed under isoflurane anesthesia.27 Further, clots formed by charge based aggregation in this model are not resolved by eptifibatide,4 which could explain why clots formed under isoflurane anesthesia were not entirely resolved by this agent even at a high dose of 20 mg/kg. The dose of 4 mg/kg i.v. integrilin has been applied in a mouse thromboembolism model under ketamine‐sedazine anesthesia.28 In fact, integrilin is effective at much lower doses (0.18 mg/kg) in a 10% FeCl3 injury model (anesthesia details unavailable),29 albeit effective thrombus resolution at a maximal dose of 20 mg/kg in mice anesthetized with ket‐x has also been reported.30 However, we show that under isoflurane anesthesia, even a dose of 20 mg/kg integrilin was only partially effective in the FeCl3 injury model. Magallon et al showed that a 0.18‐mg/kg dose of integrilin did not significantly prevent thrombus formation in a laser injury model, presumably under ket‐x anesthesia.31 This paper also showed that the IC50 value for eptifibatide was 25‐fold in mouse platelets compared to human platelets ex vivo; 1 μmol/L integrilin could achieve 50% inhibition of ADP‐induced mouse platelet aggregation. In our experiments integrilin was prepared at a concentration of 24 mmol/L and administered to mice at doses of 4‐20 mg/kg. A dose of 20 mg/kg is 100‐fold higher than the prescribed bolus dose in humans. Taken together with the finding that doses of up to 10 mg/kg were sufficient to achieve thrombus resolution in all paradigms barring the isoflurane‐treated mice under FeCl3 injury it stands to reason that the specific differences in efficacy of this drug are due to the anesthetic protocol and not the administered dose. Eptifibatide is a cyclic heptapeptide that competitively blocks the interaction of fibrinogen and the RGD binding site on integrin. Species differences in the ligand binding region of αIIbβ3 render rodent platelets less responsive to inhibition with RGD‐based compounds.32 Overall, the variability in efficacy of integrilin in the three models shows that the same anti‐thrombotic drug can elicit different effects simply by changing the anesthetic protocol. Further, within each of these models, anesthetics alter the efficacy of integrilin.
Here we present a comprehensive study wherein three anesthetic protocols have been compared under the same experimental conditions, from a single source of C57Bl6 wildtype mice, and by a single operator. The findings in our study raise the important question as to how many potentially efficacious antithrombotic drugs are disregarded in in vivo models owing to the choice of model and anesthesia protocol.
RELATIONSHIP DISCLOSURES
The authors report nothing to declare.
AUTHOR CONTRIBUTIONS
MS designed experiments, analyzed data, provided intellectual input, and wrote the manuscript. SS conducted all animal experiments and analyzed data. SF performed experiments and analyzed data. DDDC analyzed data and CS and SF assisted with the experiments. CJ and SHC assisted with image analysis and wrote the analysis macro. WSN and JRH provided intellectual input and assisted with drafting the manuscript. HHN designed experiments, provided intellectual input and financial support, and reviewed the manuscript.
Supporting information
ACKNOWLEDGMENTS
We acknowledge the assistance of Ms. Stephanie Jansen from Alfred Medical Research and Education Precinct (AMREP) Animal Services for blood collection via cardiac puncture. This study was supported by a National Health and Medical Research Council (NHMRC) project grant #APP1141046 awarded to HHN and by grant #1708 from the Bethlehem Griffiths Research Foundation awarded to HHN, MS, and SAS.
Sashindranath M, Sturgeon SA, French S, et al. The mode of anesthesia influences outcome in mouse models of arterial thrombosis. Res Pract Thromb Haemost. 2019;3:197–206. 10.1002/rth2.12184
Contributor Information
Maithili Sashindranath, Email: maithili.sashindranath@monash.edu.
Harshal H. Nandurkar, Email: harshal.nandurkar@monash.edu.
REFERENCES
- 1. Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (Eitzman series). Arterioscler Thromb Vasc Biol. 2007;27:2079–93. [DOI] [PubMed] [Google Scholar]
- 2. Denis CV, Dubois C, Brass LF, Heemskerk JW, Lenting PJ; Biorheology Subcommittee of the SSC of the ISTH . Towards standardization of in vivo thrombosis studies in mice. J Thromb Haemost. 2011;9:1641–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schoenwaelder SM, Jackson SP. Ferric chloride thrombosis model: unraveling the vascular effects of a highly corrosive oxidant. Blood. 2015;126:2652–3. [DOI] [PubMed] [Google Scholar]
- 4. Ciciliano JC, Sakurai Y, Myers DR, Fay ME, Hechler B, Meeks S, et al. Resolving the multifaceted mechanisms of the ferric chloride thrombosis model using an interdisciplinary microfluidic approach. Blood. 2015;126:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li W, McIntyre TM, Silverstein RL. Ferric chloride‐induced murine carotid arterial injury: a model of redox pathology. Redox Biol. 2013;1:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sturgeon SA, Jones C, Angus JA, Wright CE. Adaptation of the Folts and electrolytic methods of arterial thrombosis for the study of anti‐thrombotic molecules in small animals. J Pharmacol Toxicol Methods. 2006;53:20–9. [DOI] [PubMed] [Google Scholar]
- 7. Jagadeeswaran P, Cooley BC, Gross PL, Mackman N. Animal models of thrombosis from zebrafish to nonhuman primates: use in the elucidation of new pathologic pathways and the development of antithrombotic drugs. Circ Res. 2016;118:1363–79. [DOI] [PubMed] [Google Scholar]
- 8. Australia TGA . Joint Advisory Committee on Chemicals and Medicines Scheduling (ACCS‐ACMS #14): 2.2 Pentobarbital. Scheduling delegate's final decisions, March 2017. 2017.
- 9. Mayer J, Mans C. Chapter 9 ‐ Rodents In Exotic Animal Formulary. 5th ed London, UK: Elsevier Health Sciences, Saunders Imprint, 2018; 459–93. [Google Scholar]
- 10. Flecknell PA. Chapter 2 ‐ Anaesthesia In Laboratory Animal Anaesthesia. 3rd ed San Diego: Academic Press, 2009; 19–78. [Google Scholar]
- 11. Conzen PF, Vollmar B, Habazettl H, Frink EJ, Peter K, Messmer K. Systemic and regional hemodynamics of isoflurane and sevoflurane in rats. Anesth Analg. 1992;74:79–88. [DOI] [PubMed] [Google Scholar]
- 12. Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blumel G. A comparative study with various anesthetics in mice (pentobarbitone, ketamine‐xylazine, carfentanyl‐etomidate). Res Exp Med (Berl). 1984;184:159–69. [DOI] [PubMed] [Google Scholar]
- 13. Scarborough RM. Development of eptifibatide. Am Heart J. 1999;138:1093–104. [DOI] [PubMed] [Google Scholar]
- 14. Mangin P, Yap CL, Nonne C, Sturgeon SA, Goncalves I, Yuan Y, et al. Thrombin overcomes the thrombosis defect associated with platelet GPVI/FcRgamma deficiency. Blood. 2006;107:4346–53. [DOI] [PubMed] [Google Scholar]
- 15. Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990;60:269–80. [DOI] [PubMed] [Google Scholar]
- 16. Constantinides C, Mean R, Janssen BJ. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J. 2011;52:e21–31. [PMC free article] [PubMed] [Google Scholar]
- 17. Chi L, Rebello S, Lucchesi BR. In vivo models of thrombosis In: Uprichard ACG, Gallagher KP, editors Antithrombotics. Berlin Heidelberg: Springer, 1999; p. 101–27. [Google Scholar]
- 18. Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol. 2004;287:H1618–24. [DOI] [PubMed] [Google Scholar]
- 19. Kober F, Iltis I, Cozzone PJ, Bernard M. Cine‐MRI assessment of cardiac function in mice anesthetized with ketamine/xylazine and isoflurane. MAGMA. 2004;17:157–61. [DOI] [PubMed] [Google Scholar]
- 20. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow‐mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charbonneau S, Girard F, Boudreault D, Ruel M, Hardy J‐F. La technique anesthésique n'affecte pas la performance d'un modèle de réductions cycliques de flot chez le lapin: une étude pilote. Can J Anaesth. 2007;54:269. [DOI] [PubMed] [Google Scholar]
- 22. Brass LF, Diamond SL. Transport physics and biorheology in the setting of hemostasis and thrombosis. J Thromb Haemost. 2016;14:906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirstetter P, Lagneau F, Lucas O, Krupa Y, Marty J. Role of endothelium in the modulation of isoflurane‐induced vasodilatation in rat thoracic aorta. Br J Anaesth. 1997;79:84–7. [DOI] [PubMed] [Google Scholar]
- 24. Phillips DR, Conley PB, Sinha U, Andre P. Therapeutic approaches in arterial thrombosis. J Thromb Haemost. 2005;3:1577–89. [DOI] [PubMed] [Google Scholar]
- 25. Mousa SA, Bozarth JM, Forsythe MS, et al. Antiplatelet and antithrombotic efficacy of DMP 728, a novel platelet GPIIb/IIIa receptor antagonist. Circulation. 1994;89:3–12. [DOI] [PubMed] [Google Scholar]
- 26. Goto S, Tamura N, Li M, et al. Different effects of various anti‐GPIIb‐IIIa agents on shear‐induced platelet activation and expression of procoagulant activity. J Thromb Haemost. 2003;1:2022–30. [DOI] [PubMed] [Google Scholar]
- 27. Wang X, Xu L. An optimized murine model of ferric chloride‐induced arterial thrombosis for thrombosis research. Thromb Res. 2005;115:95–100. [DOI] [PubMed] [Google Scholar]
- 28. Przygodzki T, Talar M, Blazejczyk A, Kalchenko V, Watala C. Quantification of the blood platelet reactivity in the ADP‐induced model of non‐lethal pulmonary thromboembolism in mice with the use of laser doppler flowmetry. PLoS ONE. 2016;11:e0146346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwarz M, Meade G, Stoll P, et al. Conformation‐specific blockade of the integrin GPIIb/IIIa: a novel antiplatelet strategy that selectively targets activated platelets. Circ Res. 2006;99:25–33. [DOI] [PubMed] [Google Scholar]
- 30. Nonne C, Lenain N, Hechler B, et al. Importance of platelet phospholipase Cgamma2 signaling in arterial thrombosis as a function of lesion severity. Arterioscler Thromb Vasc Biol. 2005;25:1293–8. [DOI] [PubMed] [Google Scholar]
- 31. Magallon J, Chen J, Rabbani L, et al. Humanized mouse model of thrombosis is predictive of the clinical efficacy of antiplatelet agents. Circulation. 2011;123:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Basani RB, Zhu H, Thornton MA, et al. Species differences in small molecule binding to alpha IIb beta 3 are the result of sequence differences in 2 loops of the alpha IIb beta propeller. Blood. 2009;113:902–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


