Abstract
Background
Venous thromboembolism (VTE) is a major cause of morbidity, mortality, and hospitalization in cancer patients.
Objectives
To evaluate the feasibility of an electronic alert to identify and screen at‐risk individuals and gather rates of early detection of deep vein thrombosis (DVT).
Patients/Methods
An alert was built into the electronic medical record based on a validated risk tool (Khorana Score [KS]) and outcomes evaluated in an initial silent phase. The alert functioned in real time to warn physicians of high‐risk patients (KS ≥ 3) and suggested lower extremity screening ultrasonography in a subsequent active phase.
Results
Of 194 consecutive patients identified as high risk in the silent phase, 14 (7.2%) developed subsequent DVT or pulmonary embolism (PE) over 90‐day follow‐up, with a median of 27 days. Mean 90‐day emergency room (ER) visits, all‐cause admissions, and length of stay (days) for patients with DVT were 1.2, 1.6, and 9.1 compared to 0.89, 0.93, and 5.1 for all patients, respectively. In the active phase, 197 consecutive alerts met inclusion criteria, and 40 patients (20.3%) received a screening ultrasound. Five (12.5%) had a DVT and were started on therapeutic anticoagulation. Of patients with alerts who had screening deferred, 13 (8.3%) were later diagnosed with DVT (median 50.5 days) and 7 (4.5%) with PE.
Conclusion
An automated alert may have value in early detection of DVT in high‐risk cancer patients leading to earlier intervention, and could potentially prevent VTE‐related morbidity.
Keywords: cancer, deep vein thrombosis, hypercoagulability, thromboembolism, thrombosis, venous thromboembolism
Essentials.
Cancer outpatients are at risk for thrombosis which can lead to urgent visits and hospitalization.
An electronic alert was built to identify high‐risk patients and suggest screening for early detection.
Of screened patients, 12.5% had deep vein thrombosis and were anticoagulated.
Screening ultrasonography in high‐risk cancer patients deserves further study to facilitate early detection.
1. INTRODUCTION
The incidence of venous thromboembolism (VTE) is about 7‐ to 28‐fold higher in cancer patients as compared to non‐cancer patients, making it an important contributor to morbidity and the second leading cause of mortality, accounting for 9% of deaths in cancer patients.1, 2, 3, 4 Cancer patients with VTE not only have a significant increase in the frequency and duration of hospitalizations, but also a delay in cancer‐directed therapy which can often lead to increased overall healthcare costs and financial toxicity.5
VTE in cancer patients is mostly diagnosed based on presenting symptoms or through incidental findings at the time of staging or surveillance imaging. There is a substantial variation in risk of VTE between individual cancer patients.6 Current guidelines from the American Society of Clinical Oncology (ASCO) recommend utilizing a validated risk tool for formal risk assessment.7 Recent data have shown that high‐risk patients are at a heightened risk of VTE and screening such patients with compression ultrasonography may identify subclinical VTE.2, 8, 9, 10, 11 Since both screen‐detected and incidental VTEs are identified based on imaging rather than symptoms, therapeutic anticoagulation is likely to be beneficial in both settings, although the evidence to support this is primarily for incidental pulmonary embolism (PE) and not for incidental deep vein thrombosis (DVT). Current guidelines also recommend that a screen‐detected (“incidental”) VTE be treated the same as symptomatic VTE.7 A prior healthcare process innovation project at Ottawa Hospital Regional Cancer Center utilized the Khorana score to identify high‐risk patients and target them for greater education regarding risk of VTE.12 However, utilizing such an electronic alert of high‐risk patients for screening or early detection has not been formally evaluated in a clinical setting.
The purpose of this study was to create an automated alert system for early detection of VTE in high‐risk cancer patients (as defined by the Khorana score) through lower extremity ultrasonography screening in the ambulatory setting. We developed and implemented an automated alert in the electronic medical record and evaluated rates of subsequent VTE, compliance with screening recommendations, and rate of subsequent VTE in unscreened patients.
2. METHODS
We developed a novel electronic medical record alert to identify consecutive ambulatory cancer patients at increased risk for VTE based on the Khorana score.3 We chose this score as it was the only validated risk tool in this setting at the time and remains the only one recommended by multiple guidelines. The alert was integrated into the electronic health record (EPIC) of Cleveland Clinic. The study was approved by the Institutional Research Ethics Committee. The requirement for informed consent was waived as this was a pilot study for better utilization of an already validated risk tool recommended by national guidelines. This pilot study was conducted exclusively at Taussig Cancer Center at the main campus of Cleveland Clinic from August 2016 through January 2018. All providers were educated regarding the pilot at scheduled physician and advanced practice provider meetings. This information was also distributed by the institute's monthly e‐newsletter.
2.1. Patient eligibility
Patient eligibility was determined upon histological or cytological confirmation of a cancer diagnosis with targeted enrollment for specific tumor types (gastric, pancreatic, lung, lymphoma, bladder, and testicular) in the initial silent phase and later expanded to all tumor types in the active phase of the study. Diagnoses were identified through ICD‐9 codes by disease group clusters, which were manually screened to remove any diagnoses within those clusters that were not appropriate for a KS of 1 or 2. Inclusion required patients to be at least 18 years of age with a follow‐up visit after diagnosis of active malignancy. Patients were excluded if (a) they had primary gynecologic malignancy (due to geographic distribution of ambulatory clinics); (b) they had a confirmed VTE within 3 months prior to diagnosis; or, (c) they were receiving therapeutic anticoagulation for any other medical indications (eg, arterial embolism, left ventricular thrombus, atrial fibrillation, pulmonary hypertension).
2.2. Identification of patients at risk for VTE
The automated alert used five risk factors that were independently predictive of symptomatic VTE in cancer patients.3 Each risk factor was weighted according to a point scale based on KS. The very high risk sites of cancer (stomach and pancreas) were assigned a score of 2, and high‐risk cancer sites (lung, lymphoma, bladder, testicular) were assigned a score of 1. A platelet count of 350 × 109/L or more, hemoglobin less than 100 g/L (10 g/dL), leukocyte count more than 11 × 109/L, and body mass index of 35 kg/m2 or more were assigned a score of 1 each. High risk of VTE was defined as a cumulative risk score of at least 3. The program utilized the current patient problem list to identify actively treated malignancies in order to assign scores of 0, 1, or 2, if appropriate. In addition, the diagnoses were screened for cancers coded according to the International Classification of Diseases, 9th Revision (ICD‐9). Blood counts measured greater than 2 weeks prior to clinic visit were not utilized for calculation of score, but patients remained eligible for alert based on remaining variables such as very high‐risk cancer diagnosis and BMI. The score was calculated at all new and established patient visits, but if it led to an alert, this was not repeated at subsequent visits.
Physicians, including fellows in training and mid‐level providers were educated about the upcoming pilot study in face‐to‐face meetings. The initial silent phase (conducted from August 2016 through January 2017) was the lead‐in to evaluate accuracy of the alert in patient risk stratification, who were then prospectively followed for VTE events over a 3‐month period. After completion of silent phase, physicians and other providers were again re‐educated about findings from this phase and an upcoming active phase. The active phase (conducted from June 2017 through December 2017) introduced a notification to clinicians of high‐risk patients in real time and suggested an option to order lower extremity ultrasonography (US) to screen for DVT. The automated alert of the active phase was generated when a high‐risk patient chart was opened during an outpatient oncology encounter. The alert suggested a bilateral lower extremity screening ultrasound, firing only once per provider for each patient, with the option to accept, ignore, or to repeat at a later time. Providers were educated to consider the order in any patient on active cancer therapy, unless otherwise deemed that results of the screening study would not alter medical management (ie, limited life expectancy, contraindications to medical/interventional management of diagnosed VTE, or if already on anticoagulation).
2.3. Follow‐up
Patients from both phases were followed for 3 months following their initial alert date or until date of death. Patients were excluded if lost to follow‐up within 90 days of alert. A confirmatory ultrasound was required for diagnosis of DVT.
2.4. Data collection and study end points
The primary end‐point was to describe acute‐appearing proximal or distal DVT rates in active phase patients within the high‐risk category (KS ≥ 3) through screening ultrasound testing ordered at the time of automated alert. Secondary end‐points included VTE (DVT and/or pulmonary embolism) event rate in high‐risk group (KS ≥ 3) at 3 months follow‐up regardless of screening study in both phases; VTE event rate in low and intermediate risk (KS < 3) at 3 months follow‐up in phase I; emergency room (ER) visits, hospital admissions, and length of stay (LOS) in both phases; and proportion of patients that subsequently started on therapeutic anticoagulation after a positive screening ultrasound in all patients. For patients with more than one alert event, only the index event was evaluated. Inpatient and outpatient records were checked to identify patients with a personal history of DVT or pulmonary embolism as indicated by ICD‐9 codes as well as by manual chart review.
Descriptive statistics were used to present patient baseline characteristics; Kaplan‐Meier failure function was used to estimate cumulative incidence of VTE.
3. RESULTS
3.1. Silent phase
During the silent phase, the alert screened 194 consecutive patients with the six oncologic diagnoses listed below, and risk stratified by KS, with median age of 63 years and 54% males. The most prevalent cancer types were lung (23.2%) and pancreatic (21.6%) cancers. Other malignancies included bladder, lymphoma, gastric, and testicular cancers (Table 1). The 90‐day cumulative VTE rates varied by KS. Patients with KS of 2 exhibited a DVT rate of 3.2% (2/63), KS 3 was 9.2% (6/65), and KS of 4 18.8% (3/16). Two patients with DVT also had PE. Occurrence of PE in this population exhibited rates of 2% (1/50), 1.6% (1/63), 1.5% (1/65), and 0% (0/16) in KS 1 to 4, respectively (Figure 1).
Table 1.
Silent and active phase patient characteristics
| All silent phase patients | Silent phase patients with VTE | All active phase patients | All active phase patients eligible for US screen | Active phase patients with VTE | |
|---|---|---|---|---|---|
| N = 194 | N = 14 | N = 289 | N = 197 | N = 25 | |
| Age (median) | 63 (19‐89) | 61.5 (40‐76) | 63 (22‐92) | 62 (22‐92) | 63 (28‐90) |
| Male | 105 (54%) | 10 (71.4%) | 133 (46%) | 112 (56.9%) | 7 (28%) |
| Female | 89 (46%) | 4 (28.6%) | 156 (54%) | 85 (43.4%) | 18 (72%) |
| Caucasian | 160 (82.5%) | 12 (85.7%) | 227 (79%) | 150 (76.1%) | 19 (76%) |
| African‐American | 24 (12.4%) | 2 (14.3%) | 52 (18%) | 39 (20.0%) | 5 (20%) |
| Lung | 45 (23.2%) | 3 (21.4%) | 42 (14.5%) | 35 (17.8%) | 2 (8%) |
| Pancreatic | 42 (21.6%) | 5 (35.7%) | 88 (30.4%) | 71 (36%) | 8 (32%) |
| Bladder | 11 (5.7%) | 2 (14.3%) | 19 (6.5%) | 13 (6.6%) | 3 (12%) |
| Lymphoma | 10 (5.2%) | 1 (7.1%) | 51 (17.6%) | 39 (20%) | 6 (24%) |
| Gastric | 9 (4.6%) | 1 (7.1%) | 27 (9.3%) | 20 (10.2) | 2 (8%) |
| Testicular | 8 (4.1%) | 0 (0%) | 1 (0.3%) | 1 (0.5%) | 1 (4%) |
| Other | 69 (35.6%) | 2 (14.3%) | 61 (21.4%) | 18 (9.1%) | 3 (12%) |
US, ultrasound; VTE, venous thromboembolism.
Figure 1.
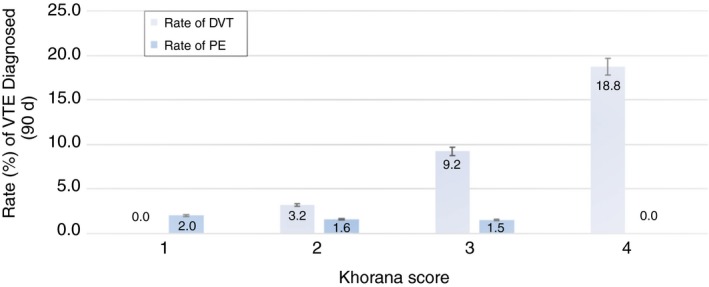
Rate of VTE by risk score during silent phase. The (n = 194) patient population exhibited a DVT rate of 0% (0/50), 3.2% (2/63), 9.2% (6/65), and 18.8% (3/16) based on those with Khorana scores of 1, 2, 3, and 4, respectively. Rates of PE based on score were 2.0% (1/50), 1.6% (1/63), 1.5% (1/65) and 0% (0/16), respectively. DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism
None of the 50 patients with a KS of 1 developed DVT during the 90‐day follow up. There were no patients with a score of 0 given that the silent phase was limited to higher risk oncology tumor types described by KS. No patient was lost to follow‐up in the silent phase of the study, and 33 patients had expired during the 90‐day period (Tables 2 and 3). Seven of the 13 DVT events in the silent phase were proximal thrombi, and two of three PE in this phase were segmental while one was subsegmental. The median time from alert to DVT and PE were 27 and 66 days, respectively. Of patients diagnosed with a DVT, 54.5% were identified during their initial or second clinic visit by an oncology provider. The cumulative incidence of VTE in this phase is shown Table 4. An equal number of silent phase patients were diagnosed with a DVT in the inpatient and ER settings (36.4% each). Mean ER visits over 90 days for patients with DVT were 1.2 compared to 0.89 for all patients. Mean all‐cause admissions and length of stay during this 90‐day period were 1.6 and 9.1 days for patients with a DVT, compared to 0.93 and 5.1 days for all 194 patients. Ninety‐day mortality rates were 36.4% (95% CI: [10.9%‐69.2%] for patients with DVT compared to 15.9% (15.9%, 95% CI: [10.9%‐22.0%] for the patients without DVT (Table 2).
Table 2.
Outcomes in silent phase patients with and without VTE
| All silent phase patients | Silent phase patients without VTE | Silent phase patients with DVT | Silent phase patients with PE | |
|---|---|---|---|---|
| N = 194 | N = 180 | N = 11 | N = 3 | |
| Median time from alert to VTE (range) | N/A | N/A | 27 d (0‐65) | 66 d (0‐82) |
| Visit type | ||||
| New patients or first follow‐up | 59 (30%) | 52 (28.9%) | 6 (54.5%) | 1 (33.3%) |
| Subsequent | 135 (70%) | 128 (71.1%) | 5 (45.5%) | 2 (66.6%) |
| Anticoagulation at time of alert | 32 (16.5%) | 30 (16.7%) | 2 (18.2%) | 0% |
| Where VTE was diagnosed | 0 (0%) | |||
| Inpatient | – | – | 4 (36.4%) | 0% |
| Emergency room | – | – | 4 (36.4%) | 1 (33.3%) |
| Ambulatory | – | – | 3 (27.3%) | 2 (66.6%) |
| Mean ER visits within 90 d of alert | 0.9 | 0.9 | 1.2 | 0.3 |
| Mean admissions within 90 d of alert | 0.9 | 0.9 | 1.6 | 0.3 |
| Mean LOS within 90 d of alert | 5.1 | 4.8 | 9.1 | 1 |
| Expired within 90 d of alert (N,%) [95% CI]a | 33 (17.0%) (95% CI: 12.0%‐23.0%) | 29 (16.1%) (95% CI: 11.1%‐22.3%) | 4 (36.4%) (95% CI: 10.9%‐69.2%) | 0 (0.0%) N/A |
CI, confidence interval; DVT, deep vein thrombosis; LOS, length of stay; PE, pulmonary embolism; VTE, venous thromboembolism.
95% CI calculated using binomial distribution method
Table 3.
Outcomes in active phase patients with deferred ultrasound screening (n = 157)
| DVT diagnosed within 30 d of alert | DVT diagnosed within 60 d of alert | DVT diagnosed within 90 d of alert | PE diagnosed within 30 d of alert | PE diagnosed within 60 d of alert | PE diagnosed within 90 d of alert | |
|---|---|---|---|---|---|---|
| N = 6 | N = 8 | N = 13 | N = 3 | N = 4 | N = 7 | |
| Median time from alert to VTE (range) | 23 d (0‐28) | 24.5 d (0‐60) | 50.5 d (0‐85) | 7 d (6‐18) | 12.5 d (6‐43) | 43 d (6‐90) |
| Mean time from alert to VTE | 18.8 d (SD 10.3) | 26.8 d (SD 12.6) | 50 d (SD 29.2) | 10.3 d (SD 6.7) | 18.5 d (SD 17.2) | 50 d (SD 39.8) |
| New or first follow‐up visit | 1 (16.7%) | 1 (12.5%) | 2 (15.4%) | 0% | 0 (0%) | 0% |
| Subsequent visit | 5 (83.3%) | 7 (87.5%) | 11 (84.6%) | 3 (100%) | 4 (100%) | 7 (100%) |
| Anticoagulation at the time of alert | 0% | 0% | 0% | 1 (33.3%) | 1 (25%) | 2 (28.6%) |
| Anticoagulation at the time of diagnosis | 0% | 0% | 0% | 1 (33.3%) | 1 (25%) | 2 (28.6%) |
| Inpatient | 2 (33.3%) | 3 (37.5%) | 5 (27.8%) | 1 (33.3%) | 1 (25%) | 2 (28.6%) |
| Emergency room | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Ambulatory | 4 (66.7%) | 5 (62.5%) | 8 (72.2%) | 2 (66.7%) | 3 (75%) | 5 (71.4%) |
DVT, deep vein thrombosis; PE, pulmonary embolism; SD, standard deviation; VTE, venous thromboembolism.
Table 4.
Incidence of VTE in silent phase (n = 194), and active phase with deferred ultrasound screening (n = 157)
| Incidence | Silent phase (N = 194) | Active phase without US screening (N = 157) |
|---|---|---|
| DVT (30‐d) | 3.1% (95% CI: 1.4%‐6.7%) | 3.2% (95% CI: 1.4%‐7.5%) |
| DVT (60‐d) | 5.2% (95% CI: 2.8%‐9.4%) | 4.5% (95% CI: 2.2%‐9.2%) |
| DVT (90‐d) | 5.7% (95% CI: 3.2%‐10%) | 7.7% (95% CI: 4.4%‐13.1%) |
| PE (30‐d) | 0.5% (95% CI: 0.1%‐3.6%) | 1.9% (95% CI: 0.6%‐5.8%) |
| PE (60‐d) | 0.5% (95% CI: 0.1%‐3.6%) | 2.6% (95% CI: 0.96%‐6.6%) |
| PE (90‐d) | 1% (95% CI: 0.3%‐4.1%) | 4.5% (95% CI: 2.2%‐9.1%) |
| VTE (30‐d) | 3.1% (95% CI: 1.4%‐6.8%) | 5.1% (95% CI: 2.6%‐10%) |
| VTE (60‐d) | 5.2% (95% CI: 2.8%‐9.4%) | 7.1% (95% CI: 4.0%‐12.4%) |
| VTE (90‐d) | 5.7% (95% CI: 3.2%‐10%) | 12.2% (95% CI: 8%‐18.4%) |
CI, confidence interval; DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
3.2. Active phase
In the active phase, 233 patients were identified as high risk by the computerized alert system during the 6‐month pilot screening period. Of these, 36 were excluded from analysis because they were already on therapeutic anticoagulation or had a recent history of VTE diagnosis. Only three patients were lost to follow‐up. Of the remainder (N = 197), the alert was accepted and screening lower extremity ultrasonography ordered on 40 patients (20.3%). Of these, five (12.5%) were found to have a lower extremity DVT and were all started on therapeutic anticoagulation. Of the remaining 157 patients that had alerts but were not screened, 13 (8.3%) were later diagnosed with symptomatic DVT after a median period of 50.5 days (0‐85) from the date of clinical alert (Figure 2). Of these 13 non‐screened patients, six patients (46.2%) were diagnosed with a symptomatic DVT within 30 days of their alert, and eight patients (61.5%) were diagnosed within 60 days (Table 3). Four patients with DVT also had PE. The cumulative incidence of VTE in the alerted but unscreened patients of the active phase is shown in Table 4. The seven (4.5%) additional non‐screened patients were diagnosed with a PE (median time from alert of 43 days [6‐90]). There were a total of 25 (12.7%) individual VTE events during active phase analysis of all screened and unscreened patients with an alert. Two of the five screened patients, while six of the 13 unscreened patients exhibited proximal lower extremity thrombi, while the rest had distal thrombi. Six of seven PE events were segmental thrombotic events, while one was subsegmental. Finally, 35 of 40 patients who did not have DVT on screening remained without documented VTE events during the 90‐day follow‐up period.
Figure 2.
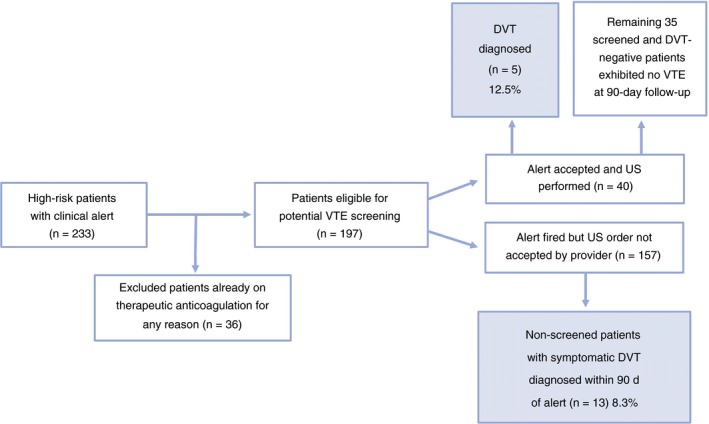
Rate of DVT at 90‐d follow up within the high‐risk (KS ≥ 3) patient population of active phase. Of 40 patients screened by US, 12.5% (n = 5) were found to have DVT, and the remainder exhibited no VTE events by 90 d. Of the non‐screened group, 8.3% (n = 13) developed symptoms with a confirmed DVT, and 7 of 157 patients (4.5%) were diagnosed with PE within 90 d (data not shown). This led to a total of 25 (12.7%) VTE events in the active phase high‐risk patient population. DVT, deep vein thrombosis; VTE, venous thromboembolism
4. DISCUSSION
In this real‐world clinical practice study, we demonstrate that creating an electronic health record alert to identify cancer patients at risk of DVT (based on a validated risk tool) is feasible, and impacts clinician behavior. We additionally demonstrate that suggesting the use of screening ultrasonography leads to identification of preexisting DVT in a significant portion of patients undergoing active cancer directed therapy. Our findings suggest that the use of electronic health record (EHR) alerts could lead to earlier detection of VTEs and treatment with anticoagulants.
In our study, the identified rates of DVT on screening US were higher than previously reported rates of around 9% on baseline screening (which included CT chest).10 This may be related to the known higher “real‐world” rates compared to patients on clinical trials, which were the basis of the two prior reports.10, 13 Our rates were similar to a prior clinical practice study at Ottawa Hospital Regional Cancer Center, although that study expanded the definition of high‐risk to a score of 2 or higher.12 It should be noted that there was a low rate of ordering the suggested screening ultrasonography, and it is possible that the clinicians use of additional discretion in determining risk of subclinical VTE beyond the electronic alert may have contributed to this higher than expected rate.6, 14, 15 We also observed that 8.3% of the patients who had their suggested ultrasound order deferred, subsequently developed a symptomatic DVT. The active phase alerted patient population that were eligible but not screened accounted for 13 symptomatic DVTs and an additional seven PE events during the 90‐day period. As opposed to this, none of the 35 patients with a confirmed negative screening ultrasound (of the 40 screened patients) had a documented DVT or PE during the 90‐day follow‐up period. This suggests that many of the 13 diagnosed DVTs could have been diagnosed by screening ultrasound on index alert prior to developing the signs/symptoms that subsequently required a diagnostic study. Further, it is possible that some PE events could have been avoided with early detection of DVT and institution of anticoagulation.
One finding of our study was low provider compliance to accept the suggested screening order, as only 20% of alerted high‐risk patients received ultrasonography. This is not unexpected given that the recommendation for screening was only a suggestion and not a mandate in this pilot study. We believe behavior may change with available data from this pilot supplemented by additional validation studies. The compliance may also have been limited by patient deferral due to time constraints of their clinical visit or disrupting patient schedules for those with additional appointments on the day of their alert encounter.
Our study certainly had several limitations. One limitation is the timing of the alert. Our current electronic medical record (EMR) does not clearly define a “pre‐chemotherapy” visit and so we were unable to devise the alert to fire during a specific time period. Future iterations of the alert or of the EMR may allow us to do so. Another limitation is that we are potentially over‐treating cancer patients with screen‐detected DVTs. However, unlike other settings, cancer is a persistent hypercoagulable state, and it is likely that DVTs may extend without anticoagulation. Treatment of incidentally discovered VTE is recommended by all current guidelines, although the data are primarily based on outcomes for PE with little evidence for incidentally discovered DVTs. Our study is a pilot and does not answer important questions such as whether earlier detection leads to better response to anticoagulation, reduces the likelihood of complications such as post‐thrombotic syndrome, and reduces number of VTE‐related hospitalization or deaths. Additionally, it does not address lead‐time bias. Further studies would be needed to better assess overall net clinical benefit of reducing overall VTE related morbidity from therapeutic anticoagulation without imposing unwanted bleeding complications.
An automated alert to trigger real‐time recommendations of screening ultrasonography for early detection of subclinical/asymptomatic DVT could be a valuable clinical strategy in initiating early treatment while potentially reducing the severity of sequelae associated with VTEs as well as reducing health care burden of ER visits and hospitalizations. The implementation of a computerized clinical decision system may lead to better‐informed care plans that provide improved patient outcomes in cancer‐associated thrombosis. Although acceptance of ultrasound orders was lower than anticipated, there appears to be a tangible benefit for early detection in lower‐extremity DVTs. Our alert thus far only “suggests” an ultrasound, but this could be altered in future iterations to a stronger “recommendation.” We plan to further focus on provider education by dissemination of internal data to improve compliance and increase acceptance of the automated alert system. However, “alert fatigue” can be an issue with provider compliance and needs to be addressed in future plans.16
Moving forward, we believe our findings are particularly relevant in the context of two ongoing trials of thromboprophylaxis—CASSINI and AVERT.17, 18 While both studies are evaluating the benefit of thromboprophylaxis in high‐risk patients (defined as KS ≥ 2), the study design of CASSINI introduces a baseline ultrasound prior to randomization whereas AVERT does not. Data from these studies should further clarify the benefit of screening ultrasonography in high‐risk cancer patients. Given that these screening techniques rely on parameters widely available electronically on most cancer patients, these alerts should be easy to implement if the available data continues to demonstrate benefit for patients, therefore and would allow early detection approaches to complement successful prophylaxis strategies in the future. As the project is currently ongoing with full support from the institutional leadership, we plan to revisit provider education during department staff meetings incorporating these data. Our approach may also be affected based on, results from ongoing prophylaxis studies.
In conclusion, in a pilot study at a large academic cancer center, we were able to successfully introduce an electronic medical alert that identified cancer patients at high risk for VTE. When appropriately utilized, this alert led to early detection of lower‐extremity DVT, providing the potential to avoid urgent visits and hospitalizations due to subsequent symptomatic events, including PE. Further studies are needed to increase compliance with the alert and to more completely evaluate its impact on clinical outcomes.
RELATIONSHIP DISCLOSURES
Dr. Khorana reports consulting fees and honoraria from Bayer, Janssen, Pfizer, Halozyme, Seattle Genetics, and AngioDynamics that are outside the scope of the submitted work.
AUTHOR CONTRIBUTIONS
Guarantor of integrity of entire study, A. Khorana.; study concepts/study design or data acquisition or data analysis/interpretation, G. Kunapareddy, B. Switzer, P. Jain, M. Conces, Y. Chen. A. Khorana; manuscript drafting or manuscript revision for important intellectual content, P. Jain, G. Kunapareddy, B. Switzer; manuscript final version approval, all authors; literature research, G. Kunapareddy, P. Jain, B. Switzer; statistical analysis, G. Kunapareddy, B. Switzer, P. Jain; and manuscript editing, all authors.
Supporting information
ACKNOWLEDGMENTS
Research reported in this publication was supported by the Sondra and Stephen Hardis Chair in Oncology Research (AAK). Dr. Khorana acknowledges additional research support from the National Heart, Lung, and Blood Institute (U01HL143402, R34 HL127156).
Kunapareddy G, Switzer B, Jain P, et al. Implementation of an electronic medical record tool for early detection of deep vein thrombosis in the ambulatory oncology setting. Res Pract Thromb Haemost. 2019;3:226–233. 10.1002/rth2.12176
Contributor Information
Girish Kunapareddy, Girishkuna.
Alok A. Khorana, Email: khorana@ccf.org, aakonc.
REFERENCES
- 1. Zwicker JI, Furie BC, Furie B. Cancer‐associated thrombosis. Crit Rev Oncol Hematol. 2007;62:126–36. [DOI] [PubMed] [Google Scholar]
- 2. Louzada ML, Majeed H, Dao V, Wells PS. Risk of recurrent venous thromboembolism according to malignancy characteristics in patients with cancer‐associated thrombosis: a systematic review of observational and intervention studies. Blood Coagul Fibrinolysis. 2011;22:86–91. [DOI] [PubMed] [Google Scholar]
- 3. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. [DOI] [PubMed] [Google Scholar]
- 5. Khorana AA, Dalal MR, Lin J, Connolly GC. Health care costs associated with venous thromboembolism in selected high‐risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clinicoecon Outcomes Res. 2013;5:101–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost. 2002;87:575–9. [PubMed] [Google Scholar]
- 7. Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33:654–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beck‐Razi N, Kuzmin A, Koren D, et al. Asymptomatic deep vein thrombosis in advanced cancer patients: the value of venous sonography. J Clin Ultrasound. 2010;38:232–7. [DOI] [PubMed] [Google Scholar]
- 9. Bernstein R, Haim N, Brenner B, Sarig G, Bar‐Sela G, Gaitini D. Venous sonography for the diagnosis of asymptomatic deep vein thrombosis in patients with cancer undergoing chemotherapy. J Ultrasound Med. 2004;23:655–233. [DOI] [PubMed] [Google Scholar]
- 10. Khorana AA, Rubens D, Francis CW. Screening high‐risk cancer patients for VTE: a prospective observational study. Thromb Res. 2014;134:1205–7. [DOI] [PubMed] [Google Scholar]
- 11. Satoh T, Matsumoto K, Uno K, et al. Silent venous thromboembolism before treatment in endometrial cancer and the risk factors. Br J Cancer. 2008;99:1034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lustig DB, Rodriguez R, Wells PS. Implementation and validation of a risk stratification method at The Ottawa Hospital to guide thromboprophylaxis in ambulatory cancer patients at intermediate‐high risk for venous thrombosis. Thromb Res. 2015;136:1099–102. [DOI] [PubMed] [Google Scholar]
- 13. Khorana AA, Francis CW, Kuderer NM, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Thromb Res. 2017;151:89–95. [DOI] [PubMed] [Google Scholar]
- 14. Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high‐risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–55. [DOI] [PubMed] [Google Scholar]
- 15. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377–82. [DOI] [PubMed] [Google Scholar]
- 16. Kucher N, Puck M, Blaser J, Bucklar G, Eschmann E, Luscher TF. Physician compliance with advanced electronic alerts for preventing venous thromboembolism among hospitalized medical patients. J Thromb Haemost. 2009;7:1291–6. [DOI] [PubMed] [Google Scholar]
- 17. Angelini D, Khorana AA. Risk assessment scores for cancer‐associated venous thromboembolic disease. Semin Thromb Hemost. 2017;43:469–78. [DOI] [PubMed] [Google Scholar]
- 18. Kimpton M, Wells PS, Carrier M. Apixaban for the prevention of venous thromboembolism in high‐risk ambulatory cancer patients receiving chemotherapy: rational and design of the AVERT trial. Thromb Res. 2018;164(suppl 1):S124–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


