Abstract
Background and Objective
Nonacog beta pegol (N9‐GP) and recombinant factor IX‐Fc fusion protein (rFIXFc) are extended half‐life rFIX compounds. We report the first single‐dose pharmacokinetic trial of N9‐GP and rFIXFc.
Patients/Methods
Paradigm 7 was a multicenter, open‐label, randomized, crossover trial in previously treated (>150 exposure days) adults with congenital hemophilia B (FIX activity ≤2%). Patients received single intravenous injections (50 IU/kg) of N9‐GP and rFIXFc with at least 21 days between doses. Plasma FIX activity, predose, and at serial time points up to 240 hours postdose, was measured using validated one‐stage clotting assays (SynthAFax for N9‐GP; Actin FSL for rFIXFc) and a chromogenic assay (ROX factor IX) with normal human plasma as calibrator. The primary endpoint was area under the FIX activity–time curve from 0 to infinity, dose‐normalized to 50 IU/kg (AUC0‐inf,norm).
Results
Fifteen patients received study treatment. Based on FIX activity results from the one‐stage clotting assays, estimated AUC0‐inf,norm was significantly greater for N9‐GP than rFIXFc (ratio: 4.39; P < 0.0001, based on a two‐sided test on 5% significance level). In addition, N9‐GP had a longer terminal half‐life, two times higher incremental recovery at 30 minutes and maximum FIX activity (dose‐normalized to 50 IU/kg) and six times higher FIX activity at 168 hours than rFIXFc. These findings were largely comparable with the chromogenic assay data and are consistent with published data for each compound.
Conclusions
In this comparison, N9‐GP demonstrated favorable pharmacokinetic characteristics versus rFIXFc, helping clinicians to understand differences between N9‐GP and rFIXFc.
Registration
This trial is registered with clinicaltrials.gov (NCT03075670) and the European Clinical Trials Database (EudraCT: 2016‐001149‐25).
Keywords: crossover trial, factor IX, hemophilia B, pharmacokinetics, phase I, randomized clinical trial
Essentials.
Published PK data for N9‐GP and rFIXFc (both EHL rFIX drugs) are not directly comparable.
paradigm 7 was the first randomized, single‐dose trial between two EHL compounds.
N9‐GP demonstrated consistently favorable PK characteristics versus rFIXFc.
These findings will help clinicians understand PK differences between EHL rFIX products.
1. INTRODUCTION
Prophylactic administration of coagulation factor is widely considered to be the current standard of care for patients with severe hemophilia.1, 2, 3, 4 To ensure optimal prophylaxis and cost‐effective factor use, dosing should be tailored according to the pharmacokinetic (PK) properties of the factor IX (FIX) product used, as well as each patient's clinical situation and disease severity.5, 6
Pharmacokinetic parameters are known to vary among different FIX products;7 it is important to understand these differences to ensure successful bleed prevention and control. With the advent of extended half‐life (EHL) recombinant FIX (rFIX) concentrates, which are expected to impact the hemophilia B treatment landscape profoundly, PK characteristics will remain a vital influence in treatment decisions.1
Nonacog beta pegol (N9‐GP, REFIXIA/REBINYN; Novo Nordisk A/S, Bagsværd, Denmark) and eftrenonacog alfa (rFIX‐Fc fusion protein [rFIXFc], ALPROLIX; Bioverativ Therapeutics, Inc., Waltham, MA) are EHL rFIX products that use different technologies to prolong the half‐life of FIX. N9‐GP uses site‐directed glycoPEGylation of FIX,8 while rFIXFc involves fusion of rFIX to the Fc domain of human immunoglobulin G1 (IgG1).9
Both N9‐GP10, 11, 12, 13 and rFIXFc14, 15, 16, 17 showed clinical efficacy and favorable safety profiles in their respective clinical development programs. Both products also show improved PK characteristics when compared with non‐modified (standard half‐life) FIX products;10, 11, 14, 15, 17, 18 these improvements are expected to decrease the burden of treatment and potentially allow patients to live a more physically active life.
However, published PK data for N9‐GP and rFIXFc are not directly comparable due to key differences in trial designs and PK analysis methodologies.19 Therefore, the aim of this trial was to perform a direct, single‐dose PK comparison between N9‐GP and rFIXFc. The findings from the trial are intended to help clinicians define optimal treatment strategies for patients with hemophilia B.
2. METHODS
2.1. Trial design
paradigm 7 was a multicenter, multinational, open‐label, randomized, crossover trial (Figure 1), conducted between March 7, and December 8, 2017. Total trial duration for each patient was approximately 32 days, excluding a 28‐day screening period. The trial consisted of one screening visit and two PK sessions, each of which comprised eight visits. Randomization was performed via the Interactive Web Response System and was balanced with respect to period (1:1, block size of 2). At visit 2, after randomization, patients received a single 50‐IU/kg dose of either N9‐GP or rFIXFc; at least 21 days after this first dose, patients received a single dose of the other product. Each dose administration was preceded by a minimum 96‐hour washout period from nonmodified FIX products (if administered); the use of commercial EHL rFIX products was not permitted during the trial.
Figure 1.
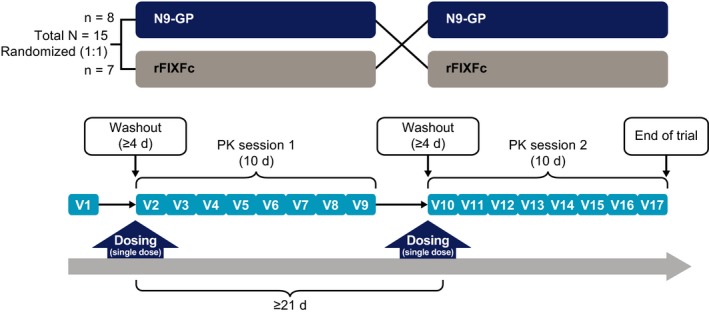
Trial design and pharmacokinetic sampling. Doses of each trial product were separated by at least 21 d; each dose administration was preceded by a 96‐h washout from non‐modified FIX products. FIX, factor IX; PK, pharmacokinetic; rFIXFc, recombinant factor IX‐Fc fusion protein; V, visit
The trial was conducted in accordance with Good Clinical Practice regulations and the ethical principles stated in the Declaration of Helsinki.20, 21 Approval from relevant institutional review boards and independent ethics committees was obtained and all patients provided written informed consent before the start of the trial.
2.2. Trial objectives
The primary objective of paradigm 7 was to compare the single‐dose PK of N9‐GP and rFIXFc in patients with hemophilia B. The secondary objective was to evaluate the safety of both treatments.
2.3. Patients
Investigators enrolled male patients aged 18‐70 years with congenital hemophilia B (FIX 2% or less) and more than 150 exposure days to any standard half‐life FIX product. Patients who had current or past history of FIX inhibitors (at least 0.6 Bethesda Units), were immunocompromised (CD4+ T cells 200/μL or fewer), or had a body mass index greater than 35 kg/m2 were excluded from the trial.
2.4. PK assessments
Each PK session was 10 days long and consisted of eight visits (Figure 1). Blood samples were collected for PK assessment at 14 time‐points over each 10‐day PK session: predose, 10 and 30 minutes, and 1, 3, 6, 8, 24, 48, 96, 144, 168, 192, and 240 hours postdose.
FIX activity was measured using the one‐stage clotting and chromogenic assays performed on a Siemens BCS‐XP analyzer (Siemens, Marburg, Germany). Normal human plasma (NHP) standards, calibrated against the World Health Organization international FIX standard, were used to calibrate all assays. For the one‐stage clotting assays, the activated partial thromboplastin time (aPTT) reagents were selected by referring to the product label and/or published literature recommendations for each product;8, 22, 23 SynthAFax (Instrumentation Laboratory, Bedford, MA) was used for N9‐GP and Actin FSL (Siemens, Marburg, Germany) for rFIXFc. To date, chromogenic assays had not been used in any clinical trials with rFIXFc; however, given that the ROX factor IX (Rossix AB, Mölndal, Sweden) kit has been previously qualified for measuring N9‐GP,24 it was subsequently qualified for measuring rFIXFc in the current trial and selected as the chromogenic assay for both products.
2.5. Endpoints
The primary endpoint was area under the FIX activity–time curve from 0 to infinity, dose‐normalized to 50 IU/kg (AUC0‐inf,norm). Secondary PK endpoints included maximum FIX activity dose‐normalized to 50 IU/kg (Cmax,norm), terminal half‐life (t ½), incremental recovery at 30 minutes (IR30min), clearance (CL), FIX activity at 168 hours (C168h), and apparent volume of distribution at steady state (V ss). FIX activity at 240 hours (C240h) was assessed as an additional endpoint. All PK endpoints were calculated based on plasma FIX activity measured from time 0 to 240 hours postdose. Dose normalization to 50 IU/kg (for AUC0‐inf,norm and Cmax,norm) was performed by multiplying the value for the non‐normalized parameter by 50 and dividing by the actual injected dose. The secondary safety endpoint was number of adverse events (AEs).
2.6. Statistical analyses
Based on data from the pivotal, phase III trial of N9‐GP,11 the within‐patient variation in terms of geometric coefficient of variation (CV) was ≤20%.
Assuming that data are log‐normal distributed, the 90% confidence interval (CI) of the AUC ratio between N9‐GP and rFIXFc would be [0.87; 1.15] times the estimated AUC ratio with 12 patients completing. Thus, 12 patients completing both PK sessions was considered necessary to provide an adequate comparison of the PK between N9‐GP and rFIXFc.
All PK endpoints were log‐transformed before being analyzed using a mixed‐effects model that included product and period as fixed effects and patients as a random effect. Such a model allowed adjustments to be made for patient variability, enabled inclusion of patients with data for only one product/period and precluded the requirement for a pairwise comparison between the two periods for each patient. Estimates with two‐sided 95% CIs were provided for each treatment, back‐transformed to the original scale. The two‐sided 95% CIs for the comparison between treatments (expressed as a ratio) were provided together with the P‐value. All PK endpoints were derived using noncompartmental methods and all analyses were performed at the 5% significance level.
All patients exposed to the trial products were included in the safety analysis set; all patients with at least one evaluable PK profile were included in the full analysis set (used for the primary PK analysis). AEs were summarized and listed descriptively.
2.7. Data‐sharing statement
The trial sponsor's policy on data sharing may be found at https://www.novonordisk-trials.com/how-access-clinical-trial-datasets.
3. RESULTS
3.1. Patient population and demographics
Patient demographics are presented in Table 1. In total, 15 patients were recruited from three countries (Germany, Switzerland, and the United States) and received single doses of N9‐GP and rFIXFc. Eight patients received N9‐GP first, then rFIXFc; seven received rFIXFc first, then N9‐GP. Mean age was 39.7 years (standard deviation: 15.5 years) and most patients were Caucasian (11 of 15; 73.3%).
Table 1.
Patient demographics
| Characteristic | Value |
|---|---|
| Number of patients, N | 15 |
| Age, y | |
| Mean (SD) | 39.7 (15.5) |
| Range | 21‐65 |
| Height, m | |
| Mean (SD) | 1.76 (0.08) |
| Range | 1.65‐1.89 |
| Body weight, kg | |
| Mean (SD) | 79.0 (16.7) |
| Range | 57.3‐117 |
| BMI, kg/m2 | |
| Mean (SD) | 25.3 (4.0) |
| Range | 19.2‐32.9 |
| Race, N (%) | |
| White | 11 (73.3) |
| Black/African American | 1 (6.7) |
| Asian | 1 (6.7) |
| Other | 2 (13.3) |
| Country, N (%) | |
| Germany | 6 (40.0) |
| Switzerland | 2 (13.3) |
| USA | 7 (46.7) |
BMI, body mass index; SD, standard deviation.
One patient experienced a breakthrough bleed after dosing with rFIXFc, which he self‐treated with the commercial rFIXFc product (prohibited per the trial protocol). After his PK profile was shown to be affected, this patient was excluded from the PK analysis; thus, the full analysis set comprised 14 patients. In addition, one patient missed the last two sampling time‐points (192 and 240 hours) for the N9‐GP PK session and another patient missed the last two sampling time‐points for the rFIXFc PK session. These patients were excluded from the analyses for AUC0‐inf,norm, t ½, C168h, C240h, CL, and V ss because the missing values could have potentially affected the derivation of these endpoints. No patients were withdrawn from the trial.
3.2. PK assessment
Mean FIX activity profiles for N9‐GP and rFIXFc, measured using the one‐stage clotting and chromogenic assays calibrated with NHP, are presented in Figure 2. Interpatient variability was low for both products and, for N9‐GP, the FIX activity profile measured using the one‐stage clotting assay was similar when calibrated by either NHP or a product‐specific standard (data not shown).
Figure 2.
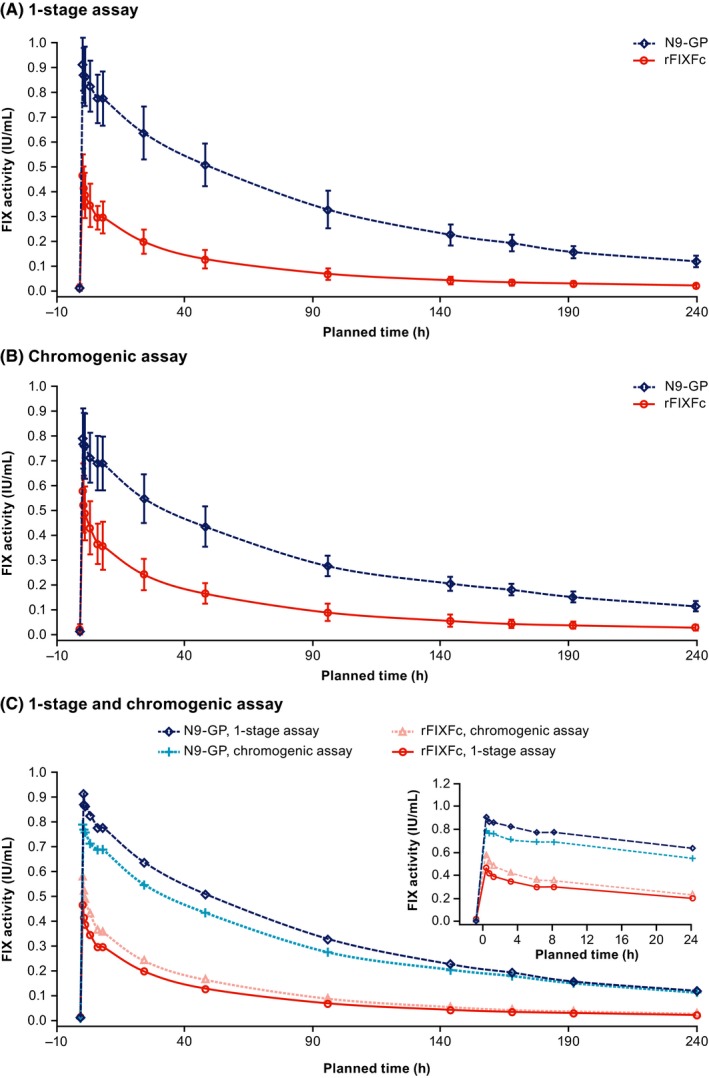
Mean FIX activity profiles following single 50‐IU/kg doses of N9‐GP and rFIXFc, measured using one‐stage clotting and chromogenic assays. Mean (±SD) FIX activity profiles following single 50‐IU/kg doses of N9‐GP and rFIXFc are shown, measured using one‐stage clotting assays (A) and the chromogenic assay (B). Panel (C) collates the mean plots with an insert magnifying FIX activity over the first 24 h. The aPTT reagents used for the one‐stage clotting assay were SynthAFax and Actin FSL for N9‐GP and rFIXFc, respectively. The ROX factor IX kit was used for the chromogenic assay with both treatments. All assays were calibrated using NHP. aPTT, activated partial thromboplastin time; FIX, factor IX; NHP, normal human plasma; PK, pharmacokinetic, rFIXFc, recombinant factor IX‐Fc fusion protein; SD, standard deviation
Geometric mean values and geometric coefficients of variation for pre‐dose FIX activity and the derived PK endpoints are shown in Table 2. The results from the mixed‐effects model are presented in Figure 3.
Table 2.
Pharmacokinetic endpoints
| Pharmacokinetic parameters | N9‐GP | rFIXFc | ||
|---|---|---|---|---|
| One‐stage clotting assay | Chromogenic assay | One‐stage clotting assay | Chromogenic assay | |
| Pre‐dose FIX activity (IU/mL) | ||||
| Geometric mean (CV) | 0.011 (72.2) | 0.011 (62.1) | 0.011 (91.8) | 0.015 (106.3) |
| Min; max | 0.005; 0.027 | 0.005; 0.025 | 0.003; 0.048 | 0.003; 0.084 |
| AUC0‐inf,norm, IU*h/mL* | ||||
| Geometric mean (CV) | 96.6 (16.7) | 89.2 (17.4) | 22.0 (23.7) | 27.5 (23.5) |
| Min; max | 67.4; 123.7 | 59.8; 109.6 | 16.0; 35.3 | 19.4; 44.0 |
| Cmax,norm, IU/mL | ||||
| Geometric mean (CV) | 0.91 (11.8) | 0.79 (14.5) | 0.45 (18.0) | 0.56 (17.6) |
| Min; max | 0.75; 1.08 | 0.63; 1.05 | 0.35; 0.62 | 0.41; 0.83 |
| t ½, h* | ||||
| Geometric mean (CV) | 103.2 (11.5) | 116.2 (12.3) | 84.9 (12.7) | 85.8 (16.8) |
| Min; max | 86.0; 130.5 | 95.5; 138.9 | 73.9; 116.5 | 59.7; 120.6 |
| IR30min, (IU/mL)/(IU/kg) | ||||
| Geometric mean (CV) | 0.017 (12.3) | 0.015 (16.9) | 0.008 (18.8) | 0.010 (20.3) |
| Min; max | 0.013; 0.021 | 0.010; 0.020 | 0.006; 0.011 | 0.006; 0.014 |
| CL, mL/h/kg* | ||||
| Geometric mean (CV) | 0.52 (16.7) | 0.56 (17.3) | 2.25 (25.1) | 1.78 (25.8) |
| Min; max | 0.40; 0.74 | 0.46; 0.84 | 1.32; 3.13 | 1.02; 2.58 |
| C168h, IU/mL* | ||||
| Geometric mean (CV) | 0.19 (18.1) | 0.18 (15.7) | 0.03 (31.2) | 0.04 (29.7) |
| Min; max | 0.12; 0.24 | 0.12; 0.22 | 0.02; 0.06 | 0.03; 0.08 |
| C240h, IU/mL* | ||||
| Geometric mean (CV) | 0.116 (17.1) | 0.114 (18.2) | 0.019 (32.1) | 0.024 (34.3) |
| Min; max | 0.084; 0.143 | 0.078; 0.140 | 0.013; 0.039 | 0.016; 0.054 |
| V ss, mL/kg* | ||||
| Geometric mean (CV) | 70.4 (20.6) | 85.7 (17.8) | 213.7 (23.7) | 172.7 (30.2) |
| Min; max | 54.1; 116.6 | 66.0; 125.1 | 134.2; 281.0 | 97.5; 277.9 |
The aPTT reagents used for the one‐stage clotting assay were SynthAFax and Actin FSL for N9‐GP and rFIXFc, respectively. The ROX factor IX kit was used for the chromogenic assay with both treatments. All assays were calibrated using NHP.
aPTT, activated partial thromboplastin time; AUC0‐inf,norm, area under the FIX activity–time curve from 0 to infinity, dose‐normalized to 50 IU/kg; C168h, FIX activity at 168 h; C240h, FIX activity at 240 h; CL, clearance; Cmax,norm, maximum FIX activity dose‐normalized to 50 IU/kg; IR30min, incremental recovery at 30 min; CV, geometric coefficient of variation; NHP, normal human plasma; PK, pharmacokinetic; rFIXFc, recombinant factor IX‐Fc fusion protein; t ½, terminal half‐life; V ss, apparent volume of distribution at steady state.
N = 12; two patients missed the last two PK time‐points and were excluded from the analysis for these parameters.
Figure 3.
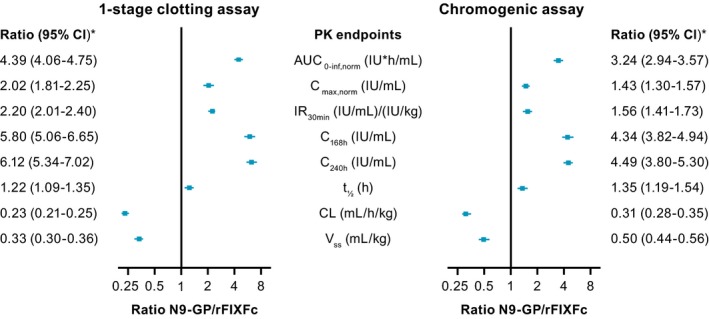
Analysis of PK endpoints for N9‐GP versus rFIXFc, derived from FIX activity measured using one‐stage clotting and chromogenic assays. All PK endpoints were log‐transformed before being analyzed using a mixed‐effects model that included product and period as fixed effects and patients as a random effect. Estimates with two‐sided 95% CIs were provided for each treatment, back‐transformed to the original scale; the two‐sided 95% CIs for the comparisons between treatments were expressed as ratios and provided together with the P‐values. The aPTT reagents used for the one‐stage clotting assay were SynthAFax and Actin FSL for N9‐GP and rFIXFc, respectively. The ROX factor IX kit was used for the chromogenic assay with both treatments. All assays were calibrated using NHP. *P < 0.0001 for all comparisons, except for t ½, for which P < 0.001 for both assays. aPTT, activated partial thromboplastin time; AUC0‐inf,norm, area under the FIX activity–time curve from 0 to infinity, dose‐normalized to 50 IU/kg; C168h, FIX activity at 168 h; C240h, FIX activity at 240 h; CI, confidence interval; CL, clearance; Cmax,norm, maximum FIX activity dose‐normalized to 50 IU/kg; FIX, factor IX; IR30min, incremental recovery at 30 min; NHP, normal human plasma; PK, pharmacokinetic; rFIXFc, recombinant factor IX‐Fc fusion protein; t ½, terminal half‐life; V ss, apparent volume of distribution at steady state
Based on FIX activity results from one‐stage clotting assays (Figure 2A), estimated AUC0–inf,norm was more than four times larger for N9‐GP versus rFIXFc (96.6 vs 22.0 IU*h/mL; estimated ratio: 4.39 [95% CI: 4.06‐4.75]; P < 0.001) (Figure 3). Results obtained with the chromogenic assay (Figure 2B,C) were comparable (3.24 [95% CI: 2.94‐3.57]; P < 0.0001).
Overall, N9‐GP demonstrated favorable PK characteristics compared with rFIXFc (Figure 3). Based on results using the one‐stage clotting assay, Cmax,norm, and IR30min were both twice as high with N9‐GP versus rFIXFc (estimated ratio: 2.02 and 2.20, respectively; P < 0.0001 for both), t ½ was slightly longer (estimated ratio: 1.22; P < 0.001) and FIX activity was approximately six times higher at both 168 hours (0.187 vs 0.032 IU/mL; estimated ratio: 5.80; P < 0.0001), and 240 hours (0.116 vs 0.019 IU/mL; estimated ratio: 6.12; P < 0.0001). Moreover, CL was four times lower (estimated ratio: 0.23; P < 0.0001) and V ss was three times lower (estimated ratio: 0.33; P < 0.0001). For all secondary and additional PK endpoints, results obtained using the chromogenic assay were comparable with results from the one‐stage clotting assay.
For each patient, N9‐GP had a higher IR30min, C168h, and C240h, as well as a greater AUC0–inf,norm, than rFIXFc.
3.3. Safety
No serious AEs were reported in the trial and no patients developed inhibitors. Overall, five AEs were reported in four patients during the PK sessions, while three AEs occurred in two patients during the washout period between PK sessions. Three AEs were reported in two patients during the N9‐GP PK session: fatigue and headache in one patient and fatigue in the other patient; all three AEs were considered mild in severity. Two AEs were reported in two patients after receiving rFIXFc: mild nasopharyngitis and moderate left elbow pain. No AEs were judged to be related to trial products.
4. DISCUSSION
Extended half‐life rFIX concentrates offer several potential benefits to patients with hemophilia B, particularly for chronic prophylactic use, including reduced dosing frequency, higher FIX activity levels, improved patient adherence, and enhanced quality of life.1 However, the availability of EHL rFIX concentrates, which differ from one another in terms of half‐life extension technology and manufacturing processes, raises key questions for clinicians; these include how to choose the most suitable product for their patients and what the cost implications will be.1
A thorough knowledge of product PK will not only help clinicians to choose a suitable EHL product, but will also help to determine the most suitable treatment regimen for each patient depending on an individual's needs, providing the most effective hemostatic coverage.25, 26
Previously published data from clinical trials have suggested a superior PK profile for N9‐GP versus rFIXFc. In the respective phase III trials that assessed once‐weekly prophylaxis regimens for each compound,8, 9, 11, 15 greater AUC0‐inf, and longer t ½ were reported for N9‐GP 40 IU/kg than for rFIXFc 50 IU/kg (arithmetic means: 86.89 vs 16.19 IU*h/mL and 85.1 vs 82.1 hours, respectively). Furthermore, a higher IR30min was reported for N9‐GP than for rFIXFc (0.02 vs 0.009 [IU/mL]/[IU/kg]), while reported CL was lower (0.43 vs 3.2 mL/h/kg).
However, due to key differences in the trial designs for each of these previous trials, the data are not directly comparable.11 Therefore, this first direct comparison offers valuable insight for clinicians seeking to understand the differences in PK characteristics between the two EHL rFIX products. In the current trial, the favorable PK characteristics of N9‐GP relative to rFIXFc, as suggested by previous trials, appear to have been confirmed. Compared with rFIXFc, N9‐GP had approximately four times larger AUC0‐inf,norm, two times higher IR30min and Cmax,norm, four times lower CL, slightly longer t ½, and six times higher C168h and C240h. Results for all PK endpoints were consistent using both the one‐stage clotting and chromogenic assays, as well as consistent with previously published findings for each product.10, 11, 14, 15
This confirmation of the favorable PK characteristics of N9‐GP, together with the positive hemostatic efficacy reported throughout the paradigm clinical program,11, 12, 13 suggest that N9‐GP may enable patients to follow similar physical activity regimens to the normal population, as weekly prophylaxis with 40 IU/kg elevates FIX levels to nonhemophilia ranges for most of the week with FIX trough levels in the mild hemophilic range.
Log‐scale comparison of the FIX activity profiles for each product (Figure 4) demonstrated that the N9‐GP data fit well into a one‐compartment model, while the rFIXFc data are best described by a two‐ or three‐compartment PK model. This emphasizes the different intercompartment distributions of each drug and is consistent with the observed difference in V ss, as well as the published data and known distribution phases for each product.14, 18 However, the clinical consequences of these findings are not known. After intravenous administration, N9‐GP is contained predominantly in the intravascular space,18 whereas rFIXFc shows a similar vascular distribution to native FIX.27 However, while some preclinical data suggest an association between FIX tissue distribution and hemostatic protection,28 other groups have demonstrated that extravascular FIX is not required for hemostasis and joint healing after an injury.29 To date, there are no clinical data to show that extravascular distribution of FIX manifests in any improvements in bleeding response and patient outcomes. The actual impact of FIX distribution on clinical effectiveness will require further investigation.
Figure 4.
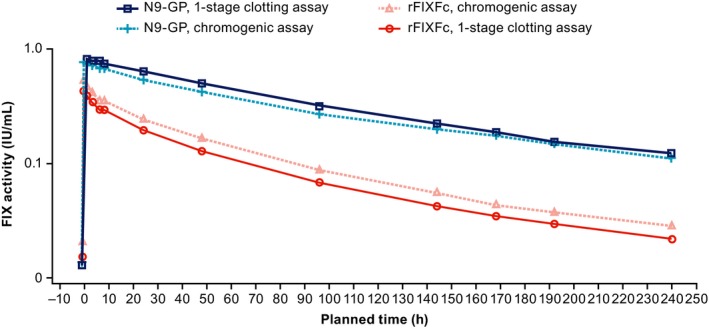
Log‐scale mean FIX activity profiles following single 50‐IU/kg doses of N9‐GP and rFIXFc, measured using one‐stage clotting and chromogenic assays. Log‐scale mean FIX activity profiles following single 50‐IU/kg doses of N9‐GP and rFIXFc are shown, measured using one‐stage clotting assays and the chromogenic assay. The aPTT reagents used for the one‐stage clotting assay were SynthAFax and Actin FSL for N9‐GP and rFIXFc, respectively. The ROX factor IX kit was used for the chromogenic assay with both treatments. All assays were calibrated using NHP. aPTT, activated partial thromboplastin time; FIX, factor IX; NHP, normal human plasma; rFIXFc, recombinant factor IX‐Fc fusion protein
The current trial provides a direct PK comparison of N9‐GP and rFIXFc; however, it should be noted that the single‐dose design of the current trial limits correlation of the findings with previous observations for treatment outcomes on routine prophylaxis. Due to the demanding nature of the trial protocol, it would have been difficult to expand the duration, patient sample size, and scope of the trial to include bleeding rates and patient outcomes.
The authors also acknowledge that it would have been ideal to base the primary analysis on a one‐stage clotting assay that used the same aPTT reagent for both products; however, unfortunately, there is not a single aPTT reagent that could be used to measure both products and allow a more robust head‐to‐head comparison. A previously published field study by Sommer et al showed that a one‐stage clotting assay using SynthAFax overestimates FIX activity for rFIXFc in spiked hemophilia B plasma samples,22 and a recent study by Kershaw et al also reported over‐recovery for rFIXFc with this aPTT reagent.23 Meanwhile, the field study by Sommer et al demonstrated that a one‐stage clotting assay with Actin FSL accurately reproduces the activity of rFIXFc.22 Therefore, assay reagents and kits were identified by available product label information and recommendations from published literature, and were ultimately selected based on successful assay qualification at the trial's onset. Furthermore, the PK characteristics observed for each product were consistent for one‐stage clotting and chromogenic assays used in the trial, as well as with previously published information.8, 9
5. CONCLUSIONS
This direct PK comparison demonstrated that N9‐GP had a higher IR30min and longer t ½ than rFIXFc, which resulted in significantly larger AUC0‐inf,norm and higher C168h, indicative of a favorable PK profile. No safety concerns were observed during the trial for either treatment. These findings will help clinicians understand differences in PK characteristics between N9‐GP and rFIXFc.
RELATIONSHIP DISCLOSURES
C. Escuriola Ettingshausen has acted as a consultant, received speaker's fees, and/or research funding from Alnylam, Bayer, Biotest, CSL Behring, Freeline, Grifols, LFB, Novo Nordisk, Octapharma, Roche, Shire, and SOBI. I. Hegemann has received travel funding and consultant fees from Baxter, Bayer, Biotest, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, and SOBI. M.L. Simpson has received consulting honoraria (for attending advisory boards) for Bayer, Bioverativ, and CSL Behring; Rush University receives money on her behalf for participating in clinical trials with Bayer, Bioverativ, Genentech, Novo Nordisk, Octapharma, and Shire. A. Cuker has received research funding from Alexion, Bayer, Bioverativ, Novo Nordisk, Pfizer, Shire, Spark Therapeutics, and Syntimmune, and has acted as a consultant/advisory board member for Genzyme, Kedrion, and Synergy. R. Kulkarni has served on advisory boards for Bioverativ, BPL, Kedrion, Novo Nordisk, Pfizer, and Shire, and participated as an investigator in clinical trials for Bayer, BioMarin, Bioverativ, Novo Nordisk, Pfizer, and Shire. R.K. Pruthi has received honoraria from Bayer, CSL Behring, Genentech, HEMA Biologics, and Novo Nordisk. M.‐L. Garly, R. M. Meldgaard and P. Persson are employees of Novo Nordisk A/S. R. Klamroth has received research support and honoraria from Bayer, Biotest, CSL Behring, Grifols, LEO Pharma, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, and SOBI. None of the authors received honoraria for activities related to the development of this manuscript.
AUTHOR CONTRIBUTIONS
C. Escuriola Ettingshausen, I. Hegemann, M.L. Simpson, A. Cuker, R. Kulkarni, R.K. Pruthi, and R. Klamroth were principal investigators who enrolled and cared for patients during the trial. M.‐L. Garly and P. Persson contributed to the design of the trial protocol. R. M. Meldgaard performed the data analysis. All authors had access to the primary clinical trial data and wrote the manuscript. The sponsor was responsible for trial operations, including data analysis. All authors were involved in interpretation of the trial results and preparation of the manuscript outline, providing input during the review stages and approval of the final manuscript. The authors assume full responsibility for the accuracy and completeness of the present work.
ACKNOWLEDGMENTS
We thank the investigators and trial staff, as well as the patients and their families for participating in the trial. This trial was sponsored by Novo Nordisk A/S (Bagsværd, Denmark). William Pickering and Mirella Ezban, of Novo Nordisk A/S, contributed to the selection and development of the FIX activity assays used in the trial. Safeer Mughal (PAREXEL), a medical writer supported by funding from Novo Nordisk A/S, provided drafts and editorial assistance to the authors during preparation of this manuscript.
Escuriola Ettingshausen C, Hegemann I, Simpson ML, et al. Favorable pharmacokinetics in hemophilia B for nonacog beta pegol versus recombinant factor IX‐Fc fusion protein: A randomized trial. Res Pract Thromb Haemost. 2019;3:268–276. 10.1002/rth2.12192
Presented in abstract form at the World Federation of Hemophilia, Glasgow, Scotland, May 20‐24, 2018.
REFERENCES
- 1. Carcao M. Changing paradigm of prophylaxis with longer acting factor concentrates. Haemophilia. 2014;20:99–105. [DOI] [PubMed] [Google Scholar]
- 2. Manco‐Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44. [DOI] [PubMed] [Google Scholar]
- 3. Gringeri A, Lundin B, von Mackensen S, Mantovani L, Mannucci PM; ESPRIT Study Group . A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost. 2011;9:700–10. [DOI] [PubMed] [Google Scholar]
- 4. Iorio A, Marchesini E, Marcucci M, Stobart K, Chan AK. Clotting factor concentrates given to prevent bleeding and bleeding‐related complications in people with hemophilia A or B. Cochrane Database Syst Rev. 2011:CD003429. [DOI] [PubMed] [Google Scholar]
- 5. Iorio A, Blanchette V, Blatny J, Collins P, Fischer K, Neufeld E. Estimating and interpreting the pharmacokinetic profiles of individual patients with hemophilia A or B using a population pharmacokinetic approach: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15:2461–5. [DOI] [PubMed] [Google Scholar]
- 6. Collins PW, Fischer K, Morfini M, Blanchette VS, Bjorkman S; International Prophylaxis Study Group Pharmacokinetics Expert Working Group . Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011;17:2–10. [DOI] [PubMed] [Google Scholar]
- 7. Lissitchkov T, Matysiak M, Zavilska K, Laguna P, Gercheva L, Antonov A, et al. Head‐to‐head comparison of the pharmacokinetic profiles of a high‐purity factor IX concentrate (AlphaNine®) and a recombinant factor IX (BeneFIX®) in patients with severe haemophilia B. Haemophilia. 2013;19:674–8. [DOI] [PubMed] [Google Scholar]
- 8. Refixia . [summary of product characteristics], Novo Nordisk. Bagsværd, Denmark. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004178/WC500232816.pdf. Accessed March 6, 2018.
- 9. Alprolix . [summary of product characteristics], Swedish Orphan Biovitrum Ltd. Stockholm, Sweden. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004142/WC500207015.pdf. Accessed March 6, 2018.
- 10. Negrier C, Knobe K, Tiede A, Giangrande P, Moss J. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood. 2011;118:2695–701. [DOI] [PubMed] [Google Scholar]
- 11. Collins PW, Young G, Knobe K, Karim FA, Angchaisuksiri P, Banner C, et al.; paradigm 2 Investigators . Recombinant long‐acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood. 2014;124:3880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young G, Collins PW, Colberg T, Chuansumrit A, Hanabusa H, Lentz SR, et al. Nonacog beta pegol (N9‐GP) in haemophilia B: a multinational phase III safety and efficacy extension trial (paradigm4). Thromb Res. 2016;141:69–76. [DOI] [PubMed] [Google Scholar]
- 13. Carcao M, Zak M, Abdul Karim F, Hanabusa H, Kearney S, Lu MY, et al. Nonacog beta pegol in previously treated children with hemophilia B: results from an international open‐label phase 3 trial. J Thromb Haemost. 2016;14:1521–276. [DOI] [PubMed] [Google Scholar]
- 14. Shapiro AD, Ragni MV, Valentino LA, Key NS, Josephson NC, Powell JS, et al. Recombinant factor IX‐Fc fusion protein (rFIXFc) demonstrates safety and prolonged activity in a phase 1/2a study in hemophilia B patients. Blood. 2012;119:666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Powell JS, Pasi KJ, Ragni MV, Ozelo MC, Valentino LA, Mahlangu JN, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369:2313–23. [DOI] [PubMed] [Google Scholar]
- 16. Powell JS, Apte S, Chambost H, Hermans C, Jackson S, Josephson NC, et al. Long‐acting recombinant factor IX Fc fusion protein (rFIXFc) for perioperative management of subjects with haemophilia B in the phase 3 B‐LONG study. Br J Haematol. 2015;168:124–34. [DOI] [PubMed] [Google Scholar]
- 17. Fischer K, Kulkarni R, Nolan B, Mahlangu J, Rangarajan S, Gambino G, et al. Recombinant factor IX Fc fusion protein in children with haemophilia B (Kids B‐LONG): results from a multicentre, non‐randomised phase 3 study. Lancet Haematol. 2017;4:e75–82. [DOI] [PubMed] [Google Scholar]
- 18. Tiede A, Abdul‐Karim F, Carcao M, Persson P, Clausen WHO, Kearney S, et al. Pharmacokinetics of a novel extended half‐life glycoPEGylated factor IX, nonacog beta pegol (N9‐GP) in previously treated patients with haemophilia B: results from two phase 3 clinical trials. Haemophilia. 2017;23:547–55. [DOI] [PubMed] [Google Scholar]
- 19. Iorio A, Edginton AN, Blanchette V, Blatny J, Boban A, Cnossen M, et al. Performing and interpreting individual pharmacokinetic profiles in patients with Hemophilia A or B: rationale and general considerations. Res Pract Thromb Haemost. 2018;2:535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. International Conference on Harmonisation ICH harmonised tripartite guideline . Guideline for good clinical practice E6(R1). Current Step 4 version. 1996. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed March 6, 2018.
- 21. World Medical Association . WMA Declaration of Helsinki ‐ ethical principles for medical research involving human subjects. Last amended by the 64th WMA General Assembly, Fortaleza, Brazil. Available at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed March 6, 2018.
- 22. Sommer JM, Buyue Y, Bardan S, Peters RT, Jiang H, Kamphaus GD, et al. Comparative field study: impact of laboratory assay variability on the assessment of recombinant factor IX Fc fusion protein (rFIXFc) activity. Thromb Haemost. 2014;112:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kershaw GW, Dissanayake K, Chen VM, Khoo TL. Evaluation of chromogenic factor IX assays by automated protocols. Haemophilia. 2018;24:492–501. [DOI] [PubMed] [Google Scholar]
- 24. Tiefenbacher S, Bohra R, Amiral J, Bowyer A, Kitchen S, Lochu A, et al. Qualification of a select one‐stage activated partial thromboplastin time‐based clotting assay and two chromogenic assays for the post‐administration monitoring of nonacog beta pegol. J Thromb Haemost. 2017;15:1901–12. [DOI] [PubMed] [Google Scholar]
- 25. Dargaud Y, Delavenne X, Hart DP, Meunier S, Mismetti P. Individualized PK‐based prophylaxis in severe haemophilia. Haemophilia. 2018;24:3–17. [DOI] [PubMed] [Google Scholar]
- 26. Fischer K, Ljung R. Primary prophylaxis in haemophilia care: guideline update 2016. Blood Cells Mol Dis. 2017;67:81–5. [DOI] [PubMed] [Google Scholar]
- 27. Diao L, Li S, Ludden T, Gobburu J, Nestorov I, Jiang H. Population pharmacokinetic modelling of recombinant factor IX Fc fusion protein (rFIXFc) in patients with haemophilia B. Clin Pharmacokinet. 2014;53:467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng D, Stafford KA, Broze GJ, Stafford DW. Evidence of clinically significant extravascular stores of factor IX. J Thromb Haemost. 2013;11:2176–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun J, Hua B, Livingston EW, Taves S, Johansen PB, Hoffman M, et al. Abnormal joint and bone wound healing in hemophilia mice is improved by extending factor IX activity after hemarthrosis. Blood. 2017;129:2161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


