Abstract
Background
Current treatment for severe hemophilia A is replacement of deficient factor. Although replacement therapy has improved life expectancy and quality, limitations include frequent infusions and high costs. Gene therapy is a potential alternative that utilizes an adeno‐associated virus (AAV) vector containing the human genetic code for factor 8 (FVIII) that transduces the liver, enabling endogenous production of FVIII. Individuals with preexisting immunity to AAV serotypes may be less likely to benefit from this treatment.
Objectives
This study measured seroprevalence of antibodies to AAV5 and 8 in an UK adult hemophilia A cohort.
Patients/Methods
Patients were recruited from seven hemophilia centres in the UK. Citrated plasma samples from 100 patients were tested for preexisting activities against AAV5 and 8 using AAV transduction inhibition and total antibodies assays.
Results
Twent‐one percent of patients had antibodies against AAV5 and 23% had antibodies against AAV8. Twenty‐five percent and 38% of patients exhibited inhibitors of AAV5 or AAV8 cellular transduction respectively. Overall seroprevalence using either assay against AAV5 was 30% and against AAV8 was 40% in this cohort of hemophilia A patients. Seropositivity for both AAV5 and AAV8 was seen in 24% of participants.
Conclusions
Screening for preexisting immunity may be important in identifying patients most likely to benefit from gene therapy. Clinical studies may be needed to evaluate the impact of preexisting immunity on the safety and efficacy of AAV mediated gene therapy.
Keywords: adeno‐associated viral vectors, hemophilia A, seroprevalence
Essentials.
Gene therapy is a potential long‐term treatment for hemophilia A.
Hemophilia A patients were recruited in the UK and tested for preexisting immunity to AAV5 and AAV8.
21% of patients had antibodies against AAV5 and 23% had antibodies against AAV8.
25% of patients had inhibitors to AAV5 and 38% of patients had inhibitors to AAV8.
1. INTRODUCTION
Current treatment for hemophilia A involves administration of factor concentrates to prevent or treat bleeds. Although replacement of FVIII has improved life expectancy and quality, limitations include frequent infusions at high costs and the risk of inhibitor formation.1, 2 Gene therapy is a potential alternative long‐term treatment option that works by producing endogenous FVIII following a single intravenous infusion of a vector containing the appropriate genetic code that has trophism for the liver.3 This enables the liver to produce relevant factor and even modest increases (plasma level of 2 ng/mL resulting in an increase in activity of 1%) can ameliorate severe forms of the disease.4 To date, gene therapy has been successfully used to treat hemophilia B5, 6, 7, 8, 9 and recently successful gene transfer has been reported in hemophilia A.10
The adeno‐associated virus (AAV) serves as a promising gene delivery system as it can transduce dividing and non‐dividing cells and has not been associated with any disease.11, 12, 13 Adeno‐associated viruses are small, non‐enveloped single stranded, DNA viruses belonging to the Parvoviridae family and Dependovirus genus that cannot replicate autonomously and require a helper virus such as herpes simplex or adeno virus.14 Preexisting immunity against AAV vectors may represent a major barrier in gene transfer which could potentially result in clearance of the vector before it reaches the target cell.9, 15 The impact of preexisting immunity suggests that screening patients for seroprevalence may help identify those most likely to benefit from gene transfer.
Seroprevalence to different AAV serotypes is measured by either: (a) total antibody binding to the AAV capsid via immunoassay, or (b) detection of inhibitors that neutralize in vitro and in vivo the ability of AAV vectors to transduce.16 Immunoassay is a capture‐based method to detect antibodies capable of binding to the AAV capsid. The AAV capsid or peptide is coated on a plate, plasma or serum added, and antibodies detected with a secondary reagent.17 In vitro cell‐based assays use a reporter AAV vector that is incubated with the test sample before transduction of a cell line. These are amongst the most widely used methods of determining anti‐AAV neutralizing factors and the transduction inhibition assay is considered a standard.18
With the clinical application of gene therapy using AAV5 and AAV8 in hemophilia,7, 19 this study aimed to measure the prevalence of these serotypes using assays that measure transduction inhibition and total antibody level in the UK hemophilia A population. Secondary aims included measuring differences in the prevalence of AAV5 and AAV8 in those who were exposed to plasma derived products and those who were not. Furthermore, differences in seroprevalence of AAV5 and AAV8 based on human immunodeficiency virus (HIV) and hepatitis C status as well as exposure were also assessed.
2. MATERIALS AND METHODS
Plasma samples from a total of 101 hemophilia A patients recruited from seven UK hemophilia centers were tested for preexisting neutralizing factors to AAV5 and AAV8 using transduction inhibition (TI) activity and total antibody assay (TAb). Favourable ethical opinion for this study was obtained from the National Research Ethics Committee North West–Liverpool Central, study number 15/NW/0469. The AAV5 assays were developed by the department of Bioanalytical Sciences at Biomarin Pharmaceutical Inc and the AAV 8 assays were developed by Genosafe. The information on HIV and hepatitis C was obtained from historical medical records.
2.1. AAV5 and AAV8 total antibodies assay for human plasma
Total antibodies against AAV5 were measured in human plasma using a validated sequential bridging electrochemiluminescence (ECL) assay on the MSD platform as described previously.20 Sample results were expressed as an signal of noise (S/N) value, calculated by dividing sample ECL units by negative control ECL units. Samples that had S/N values >1.15 were considered positive. AAV5 TAb titers were determined as the reciprocal dilution of plasma samples at the titer cut point, S/N = 1.30. Average sensitivity for measuring antibodies to AAV5 was 4.5 ng/mL.
Total IgG antibodies to AAV8 were measured using a previously published ELISA technique.18 All samples with a mean optical density of >0.506 were considered and results reported as the reciprocal titer at the cut point. As there are no human anti‐AAV8 monoclonal antibodies available, the AAV8 limit of detection was determined to be 18.8 μg/mL using human intravenous immunoglobulins (IVIg) solution.
2.2. Cell‐based AAV5 and AAV8 transduction inhibition titer assay for human plasma
A validated AAV5 TI assay for human plasma samples has been previously described.20 AAV5 TI titers were determined as the reciprocal dilution of plasma samples at the titer cut point, 44.9% transduction of the negative control. The method used to measure the neutralising effect of AAV8 has also been previously described.18 AAV8 TI titers were determined as the first reciprocal dilution at which >38.4% inhibition of transduction by comparison with the negative control.
2.3. Statistical analysis
Continuous variables are presented as median and interquartile range (IQR) and categorical data as frequency and percentages. To evaluate differences between groups, Pearsons's chi‐square test was used when the expected cell frequencies were equal to or greater than 5 and Fisher's exact test was used when the expected numbers were less than 5. Univariate analysis was performed to identify significant variables and those significant were used in the multivariate logistic regression analysis to identify the independent predictive variables. P values (two‐tailed) <0.050 was considered statistically significant. Data were analyzed using SPSS version 23 (IBM, Armonk, New York).
3. RESULTS
Of the 101 patients recruited, one was excluded as the patient was not previously treated with FVIII. Results were analysed for 100 patients and descriptive characteristics of the population are presented in Table 1.
Table 1.
Demographics of the hemophilia A cohort
| Median (IQR) | |
|---|---|
| Age | 38 (27‐57) |
| Weight (kg) | 78 (70‐88.8) |
| Baseline FVIII level | No. of patients |
| <1 IU/dL | 60 |
| 1‐5 IU/dL | 14 |
| >5 IU/dL | 26 |
| Exposure to plasma products | 86 |
| Treatment | |
| Prophylaxis | 45 |
| On‐demand | 55 |
| HIV positive | 6 |
| Hepatitis C exposed | 42 |
| Hepatitis C positive | 22 |
The number of positive and negative patients for AAV5 and AAV8 based on the TI and TAb assays are presented in Table 2. In this study, positivity in either or both assays were used as a measure of seroprevalence. Seropositivity using TI and TAb assays are presented in Table 3.
Table 2.
Seroprevalence of AAV5 and AAV8 in the United Kindgom based on transduction inhibition and total antibodies assays. Percentage of patients who were positive for both AAV5 and AAV8 as measured by transduction inhibition and total antibodies assays
| % Positive patients | ||||
|---|---|---|---|---|
| TAb | TI | Either | Both | |
| AAV 5 | 21 | 25 | 30 | 16 |
| AAV 8 | 23 | 38 | 40 | 21 |
| AAV 5 & AAV 8 | 15 | 23 | 24 | 15 |
Table 3.
Comparison (%) between the transduction inhibition and total antibodies assays for AAV5 and AAV8
| Total antibody (TAb)/transduction Inhibition (TI) assay | AAV 5 (%) | AAV 8 (%) |
|---|---|---|
| TAb− TI− | 70 | 60 |
| TAb+ TI+ | 16 | 21 |
| TAb+ TI− | 5 | 2 |
| TAb− TI+ | 9 | 17 |
In patients positive for AAV5 the TI assay titers ranged from <2 to >256 and for the TAb assay the range was 57 to 2246. In AAV8 positive patients TAb assay titers ranged from 125 to 11 949 and for the TI assay the ranges were 3.35 to >256. No significant correlation was observed between TAb and TI titers for AAV5 (r = 0.68, P = 0.064), TAb and TI titers for AAV8 (r = 0.62, P = 0.070) and TI titers between AAV5 and AAV8 (r = 0.56, P = 0.087). A significant correlation (r = 0.77, P = 0.001) was observed in TAb titers between AAV5 and AAV8 (Figure 1). Relationships between exposure to plasma products, HIV and hepatitis C status, hepatitis C exposure and treatment were assessed, results were available for all 100 patients and are presented in Table 4. Mutlivariate analysis of increasing age and seroprevalence of both AAV5 and AAV8 are presented in Table 5.
Figure 1.
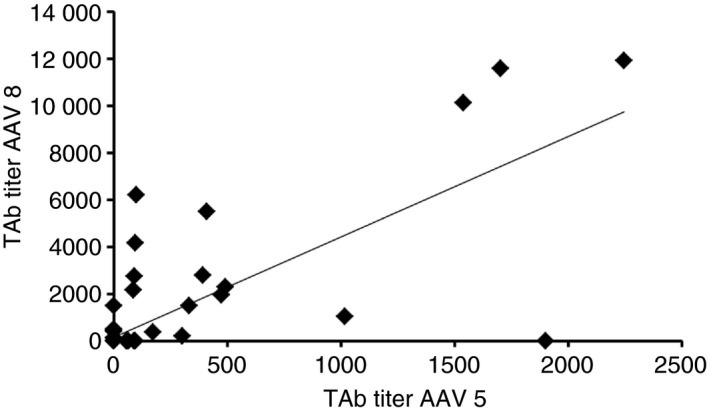
Correlation of total antibodies titers of AAV5 and AAV8 in the adult UK hemophilia A population (r = −0.77, P = 0.001)
Table 4.
Relationships of AAV5 and AAV8 seroprevalence with plasma product use, human immunodeficiency virus status and hepatitis C (exposure and status) (% positive)
| Plasma (% positive) | HIV (% positive) | Hepatitis C exposure (% positive) | Hepatitis C status (% positive) | |
|---|---|---|---|---|
| AAV5 TI | 28 | 17 | 40* | 36 |
| AAV5 TAb | 22 | 17 | 33* | 41* |
| AAV8 TI | 41 | 17 | 48 | 50 |
| AAV8 TAb | 27* | 0 | 33* | 36 |
*P < 0.05.
Table 5.
Multivariate analysis of increasing age and seroprevalence of AAV5 and AAV8
| Age <38 y | Age ≥38 y | Number of patients | OR (95% CI) | |
|---|---|---|---|---|
| AAV5 TI | ||||
| Negative | 47 | 32 | 79 | 0.16 (0.04‐0.52) |
| Positive | 4 | 17 | 21 | |
| AAV5 TA | ||||
| Negative | 45 | 30 | 75 | 0.21 (0.08‐0.56) |
| Positive | 6 | 19 | 25 | |
| AAV8 TI | ||||
| Negative | 46 | 31 | 77 | 0.16 (0.05‐0.52) |
| Positive | 4 | 17 | 21 | |
| Negative | 38 | 21 | 59 | 0.32 (0.13‐0.74) |
| Positive | 12 | 25 | 37 | |
4. DISCUSSION
In the present study, patient samples were tested for inhibitors of transduction and total antibodies for AAV5 and 8. We found that 21% of patients had antibodies to AAV5 and 23% of patients had antibodies to AAV8. Twenty‐five percent of patients exhibited inhibitors to AAV5 transduction and 38% of patients had inhibitors of AAV8 transduction. Thirty percent of patients were positive in either assay for AAV5 and 40% for AAV8. Fifteen percent had antibodies against both AAV5 and AAV8, 23% had inhibitors against both capsids, and 24% were positive in both assays for both AAV5 and AAV8.
Previous studies on AAV seroprevalence have indicated that following exposure there is production of antibodies against AAV from all four IgG subclasses which harbor neutralizing properties but other unidentified factors within individuals may also be present.21 Therefore, current estimates of seroprevalence encompass measures of antibodies against AAV or other neutralizing factors (Table 6).
Table 6.
Current estimates of the seroprevalence of AAV 5 and AAV 8
| Authors | Region | n | Population | AAV 5 antibodies (%) | AAV 8 antibodies (%) | AAV 5 neutralizing factors (%) | AAV 8 neutralizing factors (%) |
|---|---|---|---|---|---|---|---|
| Boutin et al21 | France | 226 | Healthy adults | 40 | 38 | 3.2 | 19 |
| Calcedo et al44 | Australia, Europe, Africa, USA, | 100 | Healthy adults | — | 5‐32 | — | — |
| Liu et al45 | China | 500 | Healthy children and adults | 40.2 | 82 | — | — |
| Liu et al45 | China | 270 | HIV‐1 infected | — | — | 37 | — |
| Li et al26, 43 | Thailand, USA | 62 | Paediatric hemophilia A | 25.8 | 22.6 | — | — |
| Mimuro et al47 | Japan | 85 | Healthy adults | 37.6 | 32.9 | — | — |
| Mimuro et al47 | Japan | 59 | Hemophilia | 35.6 | 32.9 | — | — |
| Falese et al20 | USA | 100 | Healthy adults | 5 | — | 24 | — |
| Falese et al20 | USA | 24 | Hemophilia A | 8 | — | 8 | — |
Currently, inhibitors of transduction to different AAV serotypes are determined by either in vitro or in vivo methods.16 Previous studies have demonstrated that several factors impact results including: assay sensitivity based on starting dilution, cell lines used for in vitro transduction, quantity of input AAV vector per cell, temperature, incubation time, volume of human sample used for AAV interaction, reporter transgene, eg, luciferase, LacZ etc., culture duration after addition of AAV transduction and heat inactivation of complement proteins.5, 15, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 In vitro assays were used in this study and the cut‐off titer values at which patients were considered positive for total antibodies was set at >1.15 for AAV5 and >0.506 for AAV8. Transduction inhibiton positive cut‐off titers were 44.9% for AAV5 and 38.4% for AAV8. These cut‐off values were determined by statistically analysing sufficient numbers of samples from healthy popluations, ensure distribution was normal by removing outliers and setting the desired rate of false positive results, generally 1%‐5%.18, 20 No significant correlations were observed between the TAb and TI titers against either capsid, specifically AAV5 (r = 0.68) or AAV8 (r = 0.62) nor when comparing the inhibitor titer values between AAV5 vs AAV8 (r = 0.56). It is noteworthy that in comparing antibody or TI titers between the two AAVs, differences in the quantity of virus employed in the cell‐based assays is likely to result in different sensitivities of the AAV5 vs AAV8 assays and hence should be taken into account. A significant correlation was observed between TAb titers of AAV5 and AAV8 confirming the existence of cross reactivity which has been observed in previous studies.21, 44
A previous study in hemophilia B demonstrated that neutralizing antibodies at titers lower than 1:10 could completely neutralize large vector doses with undetectable transgene expression.9 Currently, there is a lack of standardization of assays that measure neutralizing activity and total antibodies, therefore there is a variation in cut‐off titer values where participants are considered positive. Previous studies set the cut‐off positive titer at ≥50%,21, 26, 45 which was higher than the positive cut‐off values set in this study, and may influence the results. It is also important to note that for previous studies26, 28, 29 the cut‐off was determined in healthy US donors. In addition, the cut‐off threshold for determining seroprevalence may change with geographical location and/or the utilization of patient‐specific samples during validation of an assay. It is imperative therefore to standardize measurement of seroprevalence of AAV in hemophilia patients, not only as a single measurement but in a longitudional study as this may impact the eligibility and outcome of gene therapy.
We report a significant increase in seroprevalence of AAV8 in patients exposed to plasma products. There was also a significant increase in seroprevalence of both AAV5 and AAV8 in patients who were exposed to hepatitis C. Further investigation is required to establish the cause of increased seroprevalence due to plasma exposure and hepatitis C. However, transfusion transmitted infection cannot be discounted.
Seroprevalence was higher with increasing age in this study, which has been observed in previously. Evidence from healthy individuals in Japan showed that AAV infection occurred in childhood and seropositivity subsequently decreased, however there was a second increase of seropositivity after 30 years of age.46 Similarly, another Japanese study of healthy as well as hemophilia patients observed an increase in seropositivity with age.47
4.1. Limitations
As discussed previously, various factors influence the sensitivity of assays for measuring TAb and TI to AAV and these need to be considered while interpreting results. The number of positive patients differed depending on the type of assay and therefore both should be considered at this stage to screen potential patients for gene therapy. With currently available tests there is a variation in positive cut‐off values, therefore there needs to be a focus on standardising these assays. Measurement of AAV antibodies was only done at a single time point and evaluation of varying titer levels over time should be considered for future studies. Divergent methodology of the assays employed to measure AAV5 and AAV8 must be taken into consideration when interpreting results.
5. CONCLUSION
Despite progression in the current treatment of hemophilia A from plasma derived to recombinant products, natural exposure to AAV occurs at an early age, as shown in seroprevalence studies in both healthy and paediatric hemophilia populations.20, 26, 43 Therefore, screening may need to be considered for those most likely to respond to gene therapy with AAV vectors. It is currently unknown whether some level of seropositivity to AAV may be tolerated and clinical studies will need to evaluate the effect of preexisting immunity on the safety and efficacy of AAV mediated gene therapy. Further investigation is required to explore the link between increased seroprevalence in patients exposed to plasma products and Hepatitis C.
RELATIONSHIP DISCLOSURES
Dr S. Rangarajan is a study investigator, Prof J. Pasi has received grant support, personal fees, and non‐financial support and Drs G. Hayes and S. Fong are employees of Biomarin Pharmaceutical Inc. Dr G. Hayes also has a patent null pending. Ms R. Pink and Dr S. Stanford report grants from Biomarin Pharmaceutical Inc. during the conduct of the study. The remaining authors stated that they had no interests which might be perceived as posing a conflict or bias.
AUTHOR CONTRIBUTIONS
Dr S. Rangarajan designed the research. Dr S.N. Stanford wrote the paper. Mr K. Chandrakumaran and S.N. Stanford analyzed the data. Mrs R. Pink coordinated the study. All other authors reviewed and provided expert comments on the paper.
ACKNOWLEDGMENTS
This study was funded by a grant from BioMarin Pharmaceutical Inc. Methodologies for assays were provided by Biomarin Pharmaceutical Inc and Genosafe Pharmaceutical Company. Our sincere thanks to the research teams at all the recruiting sites for obtaining and processing blood samples and to all the patients who participated in the study.
Stanford S, Pink R, Creagh D, et al. Adenovirus‐associated antibodies in UK cohort of hemophilia patients: A seroprevalence study of the presence of adenovirus‐associated virus vector–serotypes AAV5 and AAV8 neutralizing activity and antibodies in patients with hemophilia A. Res Pract Thromb Haemost. 2019;3:261–267. 10.1002/rth2.12177
REFERENCES
- 1. Petrini P. Identifying and overcoming barriers to prophylaxis in the management of hemophilia. Hemophilia. 2007;13(Suppl 2):16–22. [DOI] [PubMed] [Google Scholar]
- 2. Farrugia A, Cassar J, Kimber MC, Bansal M, Fischer K, Auserswald G, et al. Treatment for life for severe hemophilia A‐ A cost‐utility model for prophylaxis vs. on‐demand treatment. Hemophilia. 2013;19:e228–38. [DOI] [PubMed] [Google Scholar]
- 3. Chuah MK, Evens H, VandenDriessche T. Gene therapy for hemophilia. J Thromb Haemost. 2013;11:99–110. [DOI] [PubMed] [Google Scholar]
- 4. Srivastava A, Brewer AK, Mauser‐Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Hemophilia. 2013;19:e1–47. [DOI] [PubMed] [Google Scholar]
- 5. Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus‐associated virus vector‐mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson‐Jones BJ, Ducore J, et al. Hemophilia B gene therapy with a high‐specific‐activity factor IX variant. N Engl J Med. 2017;377:2215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, et al. Long‐term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in hemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–61. [DOI] [PubMed] [Google Scholar]
- 9. Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV‐Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–267. [DOI] [PubMed] [Google Scholar]
- 10. Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. AAV5–factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377:2519–30. [DOI] [PubMed] [Google Scholar]
- 11. Smith‐Arica JR, Thomson AJ, Ansell R, Chiorini J, Davidson B, Mcwhir J. Infection efficiency of human and mouse embryonic stem cells using adenoviral and adeno‐associated viral vectors. Cloning Stem Cells. 2003;5:51–62. [DOI] [PubMed] [Google Scholar]
- 12. Friedman‐Einat M, Grossman Z, Mileguir F, Smetana Z, Ashkenazi M, Barkai G, et al. Detection of adeno‐associated virus type 2 sequences in the human genital tract. J Clin Microbiol. 1997;35:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Auricchio A, Rolling F. Adeno‐associated viral vectors for retinal gene transfer and treatment of retinal diseases. Curr Gene Ther. 2005;5:339–48. [DOI] [PubMed] [Google Scholar]
- 14. Daya S, Berns KI. Gene therapy using adeno‐associated virus vectors. Clin Microbiol Rev. 2008;21:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang H, Couto LB, Patarroyo‐White S, Liu T, Nagy D, Vargas JA, et al. Effects of transient immunosuppression on adenoassociated, virus‐mediated, liver‐directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masat E, Pavani G, Mingozzi F. Humoral immunity to AAV vectors in gene therapy: challenges and potential solutions. Discov Med. 2013;15:379–89. [PubMed] [Google Scholar]
- 17. Rincon MY, Prada CE, Lopez M, Castillo V, Echeverria LE, Serrano N. Determination of anti‐adeno‐associated viral vector neutralizing antibodies in patients with heart failure in the Cardiovascular Foundation of Colombia (ANVIAS): study protocol. JMIR Res Protoc. 2016;5:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meliani A, Leborgne C, Triffault S, Jeanson‐Leh L, Veron P, Mingozzi F. Determination of anti‐adeno‐associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum Gene Ther Methods. 2015;26:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sen D, Balakrishnan B, Gabriel N, Agrawal P, Roshini V, Samuel R, et al. Improved adeno‐associated virus (AAV) serotype 1 and 5 vectors for gene therapy. Sci Rep. 2013;6:1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Falese L, Sandza K, Yates B, Triffault S, Gangar S, Long B, et al. Strategy to detect pre‐existing immunity to AAV gene therapy. Gene Ther. 2017;24:768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, et al. Prevalence of serum IgG and neutralizing factors against adeno‐associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21:704–12. [DOI] [PubMed] [Google Scholar]
- 22. Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blacklow NR, Hoggan MD, Rowe WP. Serologic evidence for human infection with adenovirus‐associated viruses. J Natl Cancer Inst. 1968;40:319–27. [PubMed] [Google Scholar]
- 24. Georg‐Fries B, Biederlack S, Wolf J, zur Hausen H. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology. 1984;134:64–71. [DOI] [PubMed] [Google Scholar]
- 25. Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno‐associated virus type 1. J Virol. 1999;73:3994–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li C, Narkbunnam N, Samulski RJ, Asokan A, Hu G, Jacobson LJ, et al. Neutralizing antibodies against adeno‐associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012;19:288–94. [DOI] [PubMed] [Google Scholar]
- 27. Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, et al. Recombinant adeno‐associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–12. [DOI] [PubMed] [Google Scholar]
- 28. Halbert CL, Standaert TA, Aitken ML, Alexander IE, Russell DW, Miller AD. Transduction by adeno‐associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manning WC, Zhou S, Bland MP, Escobedo JA, Dwarki V. Transient immunosuppression allows transgene expression following readministration of adeno‐associated viral vectors. Hum Gene Ther. 1998;9:477–85. [DOI] [PubMed] [Google Scholar]
- 30. Halbert CL, Standaert TA, Wilson CB, Miller AD. Successful readministration of adeno‐associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hernandez YJ, Wang J, Kearns WG, Loiler S, Poirier A, Flotte TR. Latent adeno‐associated virus infection elicits humoral but not cell‐mediated immune responses in a nonhuman primate model. J Virol. 1999;73:8549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beck SE, Jones LA, Chesnut K, Walsh SM, Reynolds TC, Carter BJ, et al. Repeated delivery of adeno‐associated virus vectors to the rabbit airway. J Virol. 1999;73:9446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chao H, Samulski R, Bellinger D, Monahan P, Nichols T, Walsh C. Persistent expression of canine factor IX in hemophilia B canines. Gene Ther. 1999;6:1695–704. [DOI] [PubMed] [Google Scholar]
- 34. Cottard V, Mulleman D, Bouille P, Mezzina M, Boissier MC, Bessis N. Adeno‐associated virus‐mediated delivery of IL‐4 prevents collagen‐induced arthritis. Gene Ther. 2000;7:1930–9. [DOI] [PubMed] [Google Scholar]
- 35. Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P, et al. Humoral immunity to adeno‐associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol. 2000;74:2420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anand V, Chirmule N, Fersh M, Maguire AM, Bennett J. Additional transduction events after subretinal readministration of recombinant adeno‐associated virus. Hum Gene Ther. 2000;11:449–57. [DOI] [PubMed] [Google Scholar]
- 37. Nathwani AC, Davidoff A, Hanawa H, Zhou JF, Vanin EF, Nienhuis AW. Factors influencing in vivo transduction by recombinant adeno‐associated viral vectors expressing the human factor IX cDNA. Blood. 2001;97:1258–65. [DOI] [PubMed] [Google Scholar]
- 38. Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S, et al. A phase II, double‐blind, randomized, placebo‐controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther. 2002;13:1349–59. [DOI] [PubMed] [Google Scholar]
- 39. Huttner NA, Girod A, Perabo L, Edbauer D, Kleinschmidt JA, Buning H, et al. Genetic modifications of the adeno‐associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther. 2003;10:2139–47. [DOI] [PubMed] [Google Scholar]
- 40. Peden CS, Burger C, Muzyczka N, Mandel RJ. Circulating anti‐wild‐type adeno‐associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)‐mediated, but not rAAV5‐mediated, gene transfer in the brain. J Virol. 2004;78:6344–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le HT, Yu QC, Wilson JM, Croyle MA. Utility of PEGylated recombinant adeno‐associated viruses for gene transfer. J Control Release. 2005;108:161–77. [DOI] [PubMed] [Google Scholar]
- 42. Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B, et al. Prevalence of neutralizing antibodies against adeno‐associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: implications for gene therapy using AAV vectors. Hum Gene Ther. 2006;17:440–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li C, Diprimio N, Bowles DE, Hirsch ML, Monahan PE, Asokan A, et al. Single amino acid modification of adeno‐associated virus capsid changes transduction and humoral immune profiles. J Virol. 2012;86:7752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno‐associated viruses. J Infect Dis. 2009;199:381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu Q, Huang W, Zhang H, Wang Y, Zhao J, Song A, et al. Neutralizing antibodies against AAV2, AAV5 and AAV8 in healthy and HIV‐1‐infected subjects in China: implications for gene therapy using AAV vectors. Gene Ther. 2014;21:732–8. [DOI] [PubMed] [Google Scholar]
- 46. Erles K, Sebokova P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno‐associated virus (AAV). J Med Virol. 1999;59:406–11. [DOI] [PubMed] [Google Scholar]
- 47. Mimuro J, Mizukami H, Shima M, Matsushita T, Taki M, Muto S, et al. The prevalence of neutralizing antibodies against adeno‐associated virus capsids is reduced in young Japanese individuals. J Med Virol. 2014;86:1990–267. [DOI] [PubMed] [Google Scholar]


