Abstract
The gustatory system encodes information about chemical identity, nutritional value, and concentration of sensory stimuli before transmitting the signal from taste buds to central neurons that process and transform the signal. Deciphering the coding logic for taste quality requires examining responses at each level along the neural axis—from peripheral sensory organs to gustatory cortex. From the earliest single-fiber recordings, it was clear that some afferent neurons respond uniquely and others to stimuli of multiple qualities. There is frequently a “best stimulus” for a given neuron, leading to the suggestion that taste exhibits “labeled line coding.” In the extreme, a strict “labeled line” requires neurons and pathways dedicated to single qualities (e.g., sweet, bitter, etc.). At the other end of the spectrum, “across-fiber,” “combinatorial,” or “ensemble” coding requires minimal specific information to be imparted by a single neuron. Instead, taste quality information is encoded by simultaneous activity in ensembles of afferent fibers. Further, “temporal coding” models have proposed that certain features of taste quality may be embedded in the cadence of impulse activity. Taste receptor proteins are often expressed in nonoverlapping sets of cells in taste buds apparently supporting “labeled lines.” Yet, taste buds include both narrowly and broadly tuned cells. As gustatory signals proceed to the hindbrain and on to higher centers, coding becomes more distributed and temporal patterns of activity become important. Here, we present the conundrum of taste coding in the light of current electrophysiological and imaging techniques at several levels of the gustatory processing pathway.
Keywords: geniculate ganglion, gustatory coding, gustatory cortex, nucleus of solitary tract, taste bud, taste quality
Introduction
All sensory systems must address the problem of conveying information about the quality, intensity, and location of sensory stimulation from peripheral receptors to the brain. For both olfaction and taste, stimuli can be chemically diverse. The olfactory system is known to encode this chemical diversity, in part, through the use of hundreds of molecular receptors with overlapping receptive ranges. Olfactory signals from peripheral neurons are carried on circuits that exhibit convergence and distributed patterns at different stages along the neural axis to encode odor recognition and discrimination (Laurent 2002; Nara et al. 2011; Nunez-Parra et al. 2014; Srinivasan and Stevens 2018). The gustatory system, which serves to detect nutrients, minerals, and toxins, also identifies diverse chemical structures across broad concentration ranges. The logic of how the mammalian gustatory system encodes information on chemical identity, that is, quality coding, is the subject of active investigation using a variety of experimental approaches and resulting in competing models of taste coding. The present review examines some of the evidence, interpretations, and controversies regarding gustatory quality coding.
Most research on taste quality coding focuses on discriminating “sweet” (e.g., sugars), “salty” (Na+ salts), “sour” (acids), and so forth. Labeled line coding posits that quality-specific taste receptor cells (TRCs) (e.g., “sweet”-specific) synapse only with primary sensory afferent(s) that are dedicated to that same quality. This, then, establishes a dedicated transmission line from the taste bud cell to the brain that is “labeled” for a single quality. According to this coding, the different transmission lines (“sweet,” “salty,” “sour,” etc.) are separate, distinct, and parallel. The sensory afferent neurons are all highly tuned to transmit one given quality. They are all “specialists” for a given quality.
In contrast, combinatorial coding allows more flexibility in the responses of primary afferent fibers. Thus, a given taste compound can elicit impulses in an ensemble of several primary afferent fibers, each of which varies in their response profiles. That is, some fibers might be “sweet-best,” others might be “salt-best”; they respond robustly to sugars or Na+ salts, respectively, while retaining weaker responses to other tastes (“specialists”). Other fibers in the ensemble may respond quite broadly to many different taste compounds with no strong preference (“generalists”). However, when activated by a specific taste compound, the entire ensemble of afferent fibers generates a particular combinatorial signal that identifies that stimulus. Collectively, the combination of specialists and generalists, not any individual sensory afferent axon on its own, transmits the information about taste quality. Temporal coding conveys information in the pattern of impulses in individual primary sensory afferents. Different taste stimuli may elicit different patterns of action potentials in afferent fibers, which might lead to differential excitation/inhibition of neurons in the central nervous system (CNS).
For theorists, both models present a dilemma: how do multisensitive cells convey an unambiguous message that identifies taste quality? The labeled line and across-neuron pattern theories share the notion that spikes are integrated over time and ignore the dynamics of firing rate changes that occur during a taste response. These dynamic aspects of the response may also carry taste information, a form of signaling called “temporal coding.”
The origins of labeled line coding in the sensory nervous system might be said to come from René Descartes, who, in his classical drawing of the innocent cherub toasting his toes (Descartes 1664, p. 27), clearly outlined a labeled line (here, for painful heat) from peripheral sensory organ to the brain (Roper 2014). However, the first explicit statements of labeled line coding were by Sir Charles Bell (1811; see Bell and Shaw 1868) and Johannes Müller, who coined the concept law of specific nerve energies (LOSNE), according to which “each type of sensory nerve ending, however stimulated (electrically, mechanically, etc.), gives rise to its own specific sensation; moreover, each type of sensation depends not upon any special character of the different nerves but upon the part of the brain in which their fibers terminate” (Müller 1836). Since then, it has become clear that each modality is indeed “labeled” insofar as touch, temperature, taste, olfaction, vision, hearing, and so forth are each transmitted along separate neural pathways. The question, now, is whether such “labeling” extends to different qualities “within” a sensory modality, such as red versus green color, rose versus geranium scent, or sweet versus salty taste. That is the crux of the current debate. In certain sensory systems, such as vision and olfaction, the answer is clearly “no”; colors and odors unarguably display combinatorial quality coding.
In this review, we examine the evidence, primarily derived from electrophysiological and imaging studies at different levels of the taste system, of the responses of receptors and neurons to stimuli representing different taste qualities. We discuss what the responses at each level suggest about the logic of coding taste quality.
The detectors: coding taste quality in taste bud cells
A strict peripheral labeled line coding for taste qualities (sweet, salty, sour, etc.) has been strongly promoted by some researchers (Yarmolinsky et al. 2009; Chen et al. 2011b; Barretto et al. 2015). The strongest evidence for such a hard-wired logic for taste quality coding comes from the observation that taste bud cells express primarily or only one type of taste receptor. Some cells express a few to several members of the Tas2R family of receptors which are activated by bitter-tasting compounds (Mueller et al. 2005; Behrens et al. 2007). Other TRCs may express heterodimeric Tas1R family receptors, which are activated by either sweet- or umami-tasting compounds (Nelson et al. 2001, 2002; Dando et al. 2012). Yet other cells are dedicated for sour taste sensing (Huang et al. 2006). However, some fraction of taste cells do express taste receptors for more than one quality (Dando et al. 2012). The relatively nonoverlapping pattern of receptor expression led to the proposal that, similar to insects, mammals use a hard-wired logic for coding taste quality (Yarmolinsky et al. 2009). That is, for example, Tas2R-expressing TRCs, when stimulated, activate a dedicated subset of afferent fibers which would encode the bitter taste quality. Other dedicated TRCs and nerve fibers would convey sweet and so on. The taste-quality-dedicated TRCs constitute the beginning of a labeled line for “bitter” or “sweet” that is maintained along the taste axis to the gustatory cortex.
The question is how well do the responses of individual taste bud cells mirror the seemingly compartmentalized, nonoverlapping pattern of expression of the various taste receptors. The taste quality sensitivity and selectivity of specific populations of taste bud cells have been examined through both electrophysiological and Ca2+ imaging methods (Tomchik et al. 2007; Yoshida et al. 2009, 2018) using several distinct ex vivo preparations. Using the combination of transgenically identified taste bud cell types and apical stimulation with a variety of taste stimuli, the response profiles of taste bud cell types have been studied electrophysiologically (Yoshida et al. 2009) and via Ca2+ imaging (Caicedo et al. 2002; Tomchik et al. 2007). Very consistently, Type II cells respond best to sweet, bitter, or umami taste stimuli. “Bitter-best” taste cells are the most narrowly tuned and respond almost exclusively to bitter compounds (Yoshida et al. 2009b). In contrast, some “sweet-best” TRCs are more broadly tuned such that, in addition to sucrose, some also respond to salt (NaCl) and/or umami stimuli (monosodium glutamate, MSG). Type III cells from fungiform taste buds consistently respond to acid (sour) stimuli, and each cell typically responds to multiple acids (citric, acetic, or HCl). Thus, tuning, measured in the electrical responsivity of cells from fungiform taste buds (Yoshida et al. 2009), is generally similar to that measured by the Ca2+ responses of Type II cells from mouse circumvallate taste buds (Tomchik et al. 2007). Further, in both studies, responses to acids were limited to Type III cells.
Type III cells in mouse fungiform papillae fell into 2 groups with approximately 75% responding only to acids, the rest being broadly tuned, with responses to salty, umami, and/or bitter stimuli in addition to acids. This observation differed conspicuously the Ca2+ imaging study, which reported that all or most Type III cells in mouse circumvallate taste buds were both sour-responsive and broadly tuned (Tomchik et al. 2007). Whether these differences are attributable to differences in methodology or in the taste bud fields examined (fungiform vs. circumvallate) remains to be determined.
Another question that has been explored electrophysiologically in mouse fungiform taste bud cells is how diverse stimuli that produce similar taste perception are represented in the initial receptor cells. For example, many sugars (sucrose, fructose, etc.), artificial sweeteners (saccharin, sucralose, etc.), and certain proteins (Monellin, Thaumatin, Brazzein, etc.) all elicit sweet taste. Similarly, there are numerous chemically diverse compounds, all of which elicit bitter taste. To test whether TRCs respond identically to diverse stimuli of a given quality (e.g., “bitter”) or can discriminate among perceptually similar compounds, responses were recorded to a battery of bitter-tasting compounds (Yoshida et al. 2018). Type II TRCs from fungiform and circumvallate taste buds showed considerable heterogeneity in their responses to this battery of bitter chemicals. Some bitter stimuli elicited responses in 5–8 times as many taste cells as did other bitter compounds. That is, taste compounds that are perceived as having similar taste may produce very different patterns of activation among taste bud cells
Yoshida et al. (2018) also demonstrated that bitter-sensitive cells as a population displayed considerable heterogeneity. When tested with 10 bitter compounds, some were selective for only a single stimulus, whereas others responded broadly to as many as 9 of the 10 stimuli tested. Such heterogeneous responses among bitter-sensitive taste cells had also been demonstrated using functional imaging of rat and mouse circumvallate taste bud cells (Caicedo and Roper 2001; Caicedo et al. 2002). The family of bitter taste receptors Tas2Rs, includes ≈35 diverse members and each of these Tas2Rs is activated by a different complement of bitter compounds (Lossow et al. 2016). In both human and mouse, some Tas2rs are narrowly tuned and others can be activated by large numbers of bitter tasting compounds (Meyerhof et al. 2010; Lossow et al. 2016). Thus, the selectivity of bitter-sensitive TRCs would be defined by the expression of different combinations of Tas2Rs.
All molecular receptors for bitter tastants, Tas2Rs, were reported to be co-expressed in some TRCs with the interpretation that discrimination among bitter stimuli could not occur (Adler et al. 2000), More comprehensive analyses showed that only limited numbers of Tas2Rs are expressed per TRC and in various combinations (Matsunami et al. 2000; Behrens et al. 2007). The electrophysiological and Ca2+ imaging results above also demonstrate that the initial hypothesis (Mueller et al. 2005) for how bitter taste quality is coded in the periphery was likely incorrect. Combinatorial expression of Tas2Rs in individual TRCs could, in principle, form a basis for discriminating among different bitter compounds, but it is unclear whether such discrimination exists along the taste neural axis or even behaviorally.
Taken together, electrophysiological and Ca2+ imaging data indicate that taste buds contain many taste receptor cells dedicated to detect one of 5 basic taste qualities. These may provide the basis for discrimination across basic taste qualities. However, taste buds also contain TRCs that respond to multiple taste qualities (Caicedo et al. 2002; Tomchik et al. 2007; Yoshida et al. 2009). These multiply responsive cells may reflect information processing (divergence and convergence of signals) that occurs within taste buds via cell–cell synaptic interactions (Huang et al. 2007; Dando and Roper 2009; Huang et al. 2009; Chaudhari 2014). Moreover, some taste cells express multiple types of taste receptors. For instance, a subset of taste cells expresses all three T1R subunits and responds to both sweet and umami compounds (Dando et al. 2012; Kusuhara et al. 2013). Whether broadly tuned TRCs serve a distinct role from narrowly tuned TRCs as well as the contribution of broadly tuned TRCs to coding of taste signals remain, however, still unclear.
Taste quality coding begins with the sensitivities of individual receptor cells within taste buds. The synaptic connections between these cells and gustatory nerve fibers is a major unknown at present. Understanding convergence or divergence at these peripheral synapses will be key to understanding the initial coding of taste signals in the periphery.
Quality coding in the first neurons of the taste pathway
How do primary sensory afferent neurons transmit taste information to the CNS (see Figure 1) and how does activity in primary afferents represent taste quality (sweet, salty, sour, etc.)?
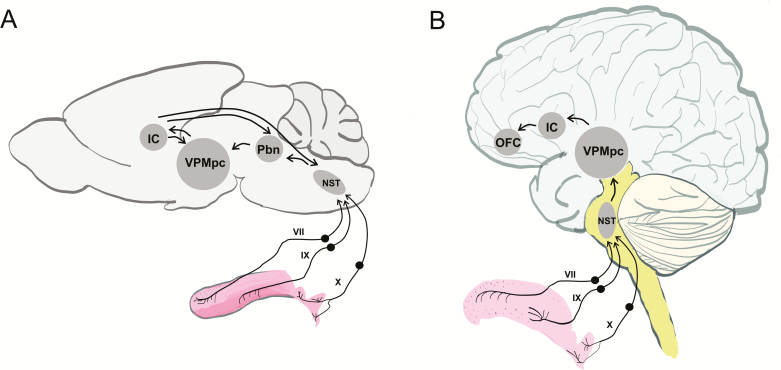
Figure 1.
Schematics of the (A) rodent and (B) human gustatory pathways with a focus on peripheral and thalamo-cortical relays. In both species, information is conveyed via cranial nerves VII, IX, and X from the tongue to the brainstem. NST, nucleus of the solitary tract; PbN, parabrachial nucleus; VPMpc, parvicellular portion of the ventroposteromedial nucleus of the thalamus; IC, insular cortex; OFC, orbitofrontal cortex.
Electrophysiological recordings and Ca2+ imaging studies from primary sensory afferent neurons (single fibers or ganglion neuron somata) have been carried out by several groups. Some form of combinatorial coding in taste was originally suggested by Pfaffmann (1941) based on early electrophysiological recordings from afferent fibers that innervated taste buds in the cat. Single units were found that responded to lingual stimulation with more than one taste compound (e.g., quinine or HCl or both). That many fibers were not limited to excitation by a single taste quality, which was inconsistent with a labeled line coding scheme. This led Pfaffmann (1941) to conclude “[…] sensory quality does not depend simply on the ‘all or nothing’ activation of some particular fiber group alone, but on the pattern of other fibers active.” Other investigators elaborated and extended this model to encompass the widespread co-activation of a large number of sensory afferent fibers, with different combinations of the same fibers constituting the code for different taste qualities. This was termed “cross-fiber coding” and was held as the polar opposite of labeled line coding (Erickson 2008). According to cross-fiber coding, activity in any single fiber on its own does not convey information about sweet, sour, salty, etc. Only the combined activity of many fibers generates the code. Some resolution of these two opposite concepts—labeled line versus combinatorial coding—was obtained by Frank and Pfaffmann (1969). They recorded from single sensory afferent fibers from the tongues of hamsters and observed that although many fibers did indeed respond to multiple taste stimuli, the most effective stimulus of a fiber was predictive of the relative effectiveness of the other stimuli. These observations suggested that there were fiber “types” organized according to the stimulus that evoked the “best” response. They termed these “sweet-best,” “salt-best,” etc. fibers. Although this has been interpreted as a form of labeled line coding, the fact is that activity in a single fiber could not unambiguously distinguish between (strong) excitation by the “best” stimulus versus (weak) excitation by other, less effective stimuli.
The observation of “best stimulus” for individual taste afferent fibers has been widely replicated in different laboratories and in mammalian species ranging from mice to monkeys (Sato et al. 1975; Tonosaki and Beidler 1989; Hellekant and Ninomiya 1994; Danilova et al. 1999). A further refinement of the distinctions between taste afferents was the recognition that some neurons respond principally or exclusively to one stimulus type, usually sugars—the so-called “specialist” neurons; other neurons responded to a variety of electrolytes that might produce sour, bitter, or salty tastes (reviewed by Frank et al. 2008). Specialist and generalist neurons have been detected electrophysiologically as single-fiber recordings on afferent nerves and by extracellular recordings in geniculate ganglia. A method applied more recently is functional imaging of sensory afferent neuron activity using genetically encoded Ca2+ indicators such as GCaMP. Barretto et al. (2015) and Wu et al. (2015) carried out functional imaging on geniculate ganglion neurons in the mouse and cataloged responses to a battery of different taste stimuli presented at different concentrations. Those studies verified that about half the ganglion neurons were “specialists” that responded best (and some solely) to a single taste compound, such as sucrose. Specialist neurons could be detected for each of the five “basic” taste qualities (sweet, sour, salty, bitter, umami). The geniculate ganglion also had “generalist” sensory neurons that responded much more broadly to taste stimuli, mirroring the electrophysiological recordings from the primary afferent axons (above).
The relative proportion of specialist and generalist neurons varied strongly depending on the concentrations of stimuli tested (Wu et al. 2015). Importantly, neurons that displayed a specialist profile with a low concentration stimulus were transformed to generalists when the same stimuli were tested at higher concentrations. At concentrations that produced maximal responses, half the neurons exhibited responses to multiple distinct stimuli. Unless half the information from the periphery is discarded, which seems unlikely, a resolution to the question of taste coding is that a cross-fiber code involving a combination of primary afferent axons that vary in their “tuning,” from specialists to generalists, encode taste.
In addition to encoding the basic taste qualities, there is a question of how stimuli that produce a similar quality may be discriminated from one another. For instance, in primates, individual afferent fibers that responded to one sweet stimulus typically also responded to several other sweets and minimally to bitter or sour tastants (Hellekant and Ninomiya 1994; Wang et al. 2009). This type of narrow tuning is much less prevalent for taste qualities other than sweet: individual neurons respond quite variably to different salts (Frank et al. 2008). However, this feature remains incompletely explored in the periphery as most studies have utilized only limited panels of taste stimuli.
Whether sensory afferent fibers and their parent ganglion neurons employ patterns of action potentials to encode stimulus identity has been explored to only a limited extent. Different taste stimuli appear to cause primary afferent fibers to fire action potentials with somewhat different patterns, though these differences are not marked (Ogawa et al. 1974; Nagai and Ueda 1981; Lawhern et al. 2011). Thus, spike discharge pattern may augment and refine the combinatorial coding described above (Nagai and Ueda 1981). Taste coding in the periphery most likely involves activating a combination of afferent fibers having varying tuning capabilities (from specialists to generalists) and subtly different firing patterns. All these factors together play a role in the transmission of information needed to discriminate sweet, sour, salty, bitter, and umami.
Parenthetically, a key point that should be noted is that to date, recordings from the primary afferent neurons have only been obtained in anesthetized animals. It is possible that some of the distinctions noted below in the response properties between peripheral afferents and higher level neurons may be attributable to anesthesia.
Hindbrain neurons: evidence for temporal coding
Gustatory afferents from the periphery project directly to the nucleus of the solitary tract (NST) in the brainstem where there is substantial convergence (Whitehead and Frank 1983; Whitehead 1986). Cells in the brainstem, NST, and parabrachial nucleus of the pons (PbN; the main target of projections from the NST), are generally more broadly tuned than peripheral fibers in both anesthetized (see Spector and Travers 2005, for a review) and awake (see Roussin et al. 2012, but see Nakamura and Norgren 1991) rodents, though there are still groups of neurons in each structure that are narrowly tuned to a single taste quality. Like fibers/cells in the periphery, neurons in the brainstem can become more broadly tuned with changes in stimulus concentration. Moreover, response profiles, defined as the subset of taste qualities that evokes a response, of NST and PbN cells can change over time (Sammons et al. 2016). This may be due to the changing inputs to these cells as taste receptor cells die and are replaced. Despite such turnover, the network obviously needs to remain stable in its output. It is possible that extensive convergence from neurons with different profiles of sensitivities may support this stability; that is, the loss or addition of a few inputs with different taste sensitivities would have minimal impact on the target cells if there were enough variety in the array of inputs. Further, simultaneous recordings from taste-responsive NST and PbN cells have shown that NST with a particular best stimulus are more effective in driving PbN cells with a similar best stimulus, though the same PbN cells receive input from NST cells with all types of best stimulus preferences (Di Lorenzo and Monroe 1997; Di Lorenzo et al. 2009). As a changing array of inputs to NST cells shift their response profiles from one best stimulus to another, simultaneous activation of enough inputs responding to a given best stimulus may also cause PbN cells upstream to shift their best stimulus in kind, as well as modifying the effectiveness of inputs that were activated. Thus, response profiles may change but the overall proportions of the constituents of the network encoding taste stimuli may remain consistent.
With a variety of response profiles in the taste-responsive portion of the NST and PbN, there remains the problem of how confusion among similar-tasting, but not identical, tastants is resolved. As discussed, the across-fiber/neuron patterns may offer one solution, but another might be response dynamics, that is, temporal coding. Variation in the temporal pattern of taste-evoked firing offers a way to disambiguate two tastants that evoke similar response magnitudes within the same cell (Di Lorenzo et al. 2009).
Both specialist and generalist neurons have been described in brainstem taste areas in electrophysiological studies with anesthetized animals. Perceptually similar stimuli evoke similar patterns of neuronal population activity, lending support to the combinatorial coding model discussed above (Smith et al. 2000; Simon et al. 2006; Geran and Travers 2009). However, unlike taste bud cells and sensory afferent neurons, gustatory neurons of the brainstem do exhibit evidence of temporal coding. “Metric space analysis” (MSA; Victor and Purpura 1996, 1997) has been used to quantify this. MSA begins by determining a “distance” between spike trains in terms of the “cost” of making them identical, via adding, deleting, or moving spikes. Adding or removing a spike costs one arbitrary unit. The cost of moving a spike in time by an amount t is given by qt, where q is a parameter that controls the sensitivity of the distance to spike timing. Based on these distances, calculated from repeated neural responses to presentations of several tastants, one can determine two information-theoretic quantities: Hcount and Hspike[q]. Hcount is the amount of information about taste quality conveyed by spike count alone, and Hspike[q] is the amount of information about taste quality when spike timing is taken into account.
In early work using anesthetized rats, spike timing was shown to convey a significant amount of information about taste stimuli in both the NST (Di Lorenzo and Victor 2003) and the PbN (Rosen et al. 2011), the first and second synapses, respectively, in the central gustatory pathway in rodents. Specifically, in about half of the taste-responsive cells in NST (Di Lorenzo and Victor 2003) and PbN (Rosen et al. 2011), spike timing contributes to taste quality discrimination–and in both NST and PbN, this contribution was largest in neurons that would appear to be broadly tuned if only spike count were considered. In addition, in the NST, spike timing contributes significant amounts of information to distinguishing among responses to the components of binary mixtures (Di Lorenzo et al. 2009), between tastants of different concentrations (Chen et al. 2011a) and tastants of the same taste quality but different chemical compositions (Roussin et al. 2008).
Although evidence for temporal coding of taste stimuli in brainstem structures has been obtained in the anesthetized animal, further studies asked whether there was similar evidence of temporal coding of taste in the alert animal (Roussin et al. 2012; Weiss and Di Lorenzo 2012). To that end, rats were implanted with 8-channel microwire electrode bundles aimed at either the NST or PbN. Following recovery from surgery, mildly water-deprived rats were placed in an experimental chamber with a drinking spout that allowed control of various fluids on a lick-by-lick basis. Taste responses in the NST and PbN of awake, freely licking rats differed in several ways from those recorded under anesthesia. For example, in addition to the typical phasic-tonic time course of response seen under anesthesia, brief lick-by-lick responses were also apparent in many NST and PbN cells recorded in awake rats. Of these, some cells had responses that progressively increased with successive licks. There were also many cells with very long latency (>2 s) taste responses that began long after the licks were completed (Roussin et al. 2012), which might be the result of stimulation of post-oral receptors during swallowing.
Recordings from the NST (Roussin et al. 2012) and PbN (Weiss et al. 2014) of awake, freely licking rats revealed a rich variety of cell types in addition to those that respond solely to taste. For example, many cells fire in phase with licking, with peak firing rates just at the time of the lick or between licks. In addition, there are cells that significantly decrease their firing rate during a lick bout. The relative silencing of such cells when the rat engages in consummatory behavior suggests that they may set the initial conditions for the network to acquire sensory information. Moreover, these data underscore the idea that sensory and motor components of gustation are inextricably linked.
In a separate series of experiments, the effects of pairing olfactory stimuli with tastants were tested (Escanilla et al. 2015). Widespread modulation of taste responses was observed, including changes in response magnitude and latency following taste–odor pairing. MSA of taste- and odor-evoked responses showed that NST cells were more competent at discriminating tastants when they were presented with odors than when presented alone. This applied for all taste qualities, and whether or not spike timing was taken into account, leading to the hypothesis that brainstem neurons may be most keenly tuned to respond to naturalistic stimuli, that is food, rather than pure chemical exemplars of taste qualities (Escanilla et al. 2015). This was tested by presenting complex, natural stimuli such as grape juice (sweet), clam juice (salty), lemon juice (sour), and coffee (bitter). Evoked spike trains in the PbN of awake, freely licking rats displayed conveyed significantly more information to naturalistic stimuli than those associated with single compounds (Weiss et al. 2014).
In conclusion, data from electrophysiological recordings from awake, freely licking rats underscores the role of the gustatory brainstem as an important node in the neural circuit that controls food identification and ingestion. In addition, dynamics—both intrinsic to the spike trains and related to the lick cycle—are prominent and functionally significant aspects of neural responses.
From the gustatory brainstem, afferents target the most medial portion of the ventral posteromedial thalamus. Taste-responsive thalamic neurons in this nucleus form an important source of input to gustatory cortex. Although this small region has been understudied relative to other taste areas, there is recent evidence that the gustatory thalamus may play important roles in taste quality and palatability coding, as well as stimulus expectation (Liu and Fontanini 2015).
Patterns of activity in the rodent gustatory cortex
Within gustatory cortex (GC), physiological studies demonstrate that taste-responsive cells are often multimodal, responding to other sensory stimuli in addition to taste (for review, see Maffei et al. 2012). When recordings are made in in either anesthetized or awake animals probed with only sapid stimuli, both narrowly and broad taste-responsive neurons are found, similar to those found in both peripheral and other central taste areas (e.g. Yamamoto et al. 1984, 1985, 1989; Ogawa et al. 1992a, 1992b; Katz et al. 2001; Spector and Travers 2005; Sadacca et al. 2016; Stapleton et al. 2006). The roles of these cell types are still ambiguous in terms of function, although there is evidence that some cortical taste neurons may respond broadly to sets of stimuli that can be classified as sharing a hedonic value (Yamamoto et al. 1989; Fontanini and Katz 2006).
An important and related, yet not well-understood, aspect of taste coding involves the spatial organization of taste neurons—are cells responsive to particular taste stimuli clustered together? Other sensory systems differ in this mode of organization; from the well-known somatotopy of barrel cortex to the apparent random overlap of odorant responses in piriform cortex (Petersen 2007; Stettler and Axel 2009). Chen et al. (2011b) used 2-photon imaging to describe a sharply segregated quality representation in mouse GC. Here, quality-specific clusters of singly responsive neurons were separated in space along the cortical surface by areas with only sparse taste-evoked activity. In contrast, the vast majority of work on taste cortex is entirely consistent in suggesting that there is little to no stimulus topography in how taste qualities are represented in the gustatory cortex. Across the anterior–posterior expanse of GC, mapping studies using different techniques have yielded very different conclusions. For instance, studies using either in vivo recordings, or intrinsic imaging, show a large degree of overlap among the basic taste stimuli, with bias toward overrepresentation of individual qualities at the anterior and posterior extremes (Yamamoto et al. 1985; Bahar et al. 2004; Accolla et al. 2007; Carleton et al. 2010). A genetically encoded trans-synaptic tracer similarly suggested that neurons receiving input for different taste qualities are intermingled in the gustatory cortex (Sugita and Shiba 2005).
More recently, 2-photon imaging was used to investigate taste responses to stimuli, representing four primary qualities (acid, bitter, salty, and sweet) in an area of mouse gustatory cortex defined by taste thalamic input (Fletcher et al. 2017). This “central” area, located just posterior to the landmark middle cerebral artery, possessed thalamic terminal labeling concentrated in the dysgranular subdivision. Using a virally expressed calcium indicator (GCaMP6s), taste imaging responses were collected in anesthetized mice in this delineated area. Not surprisingly, cortical taste cells were found to respond either best to individual stimuli or combinations of stimuli. Spatial mapping demonstrated that taste quality responses overlapped in this region, with no evidence of segregation of cells responding to a single quality. Principle components analysis of this aggregate data suggested that the primary taste qualities were distinctly represented in the population response, providing a basis for discrimination despite spatial overlap. Moreover, the stimuli were ordered along the first component in a way that suggested hedonic character may also be represented in the response.
The finding of an area of quality overlap in the center of mouse GC fits in nicely with the previously mentioned mapping studies in the rat (Yamamoto et al. 1985; Accolla et al. 2007) and other recent 2-photon approaches in mice (Livneh et al. 2017; Lavi et al. 2018). Still, these papers and the Chen et al. (2011b) study leave open the possibility that bitter taste responses and sweet taste responses may be overrepresented posteriorly and anteriorly, respectively, in GC. If so, any topographic representation of taste quality likely stems from the source of peripheral input. The glossopharyngeal (IX) nerve, which innervates posterior taste buds, is known to be more responsive to bitter-tasting stimuli than branches of the facial nerve (VII), which innervate taste buds on the anterior tongue and palate (Frank et al. 1983; Frank 1991). In rat taste cortex, information from the chorda tympani branch of VII projects to the anterior GC, whereas information from IX targets the posterior GC (Yamamoto et al. 1980; Hanamori et al. 1998). A similar “gradient” of taste quality representation that follows peripheral input has also been described in the parabrachial nucleus in the rodent brainstem (Halsell and Travers 1997; Geran and Travers 2006). In this discussion, however, it is important to consider the multimodal nature of GC as well as surrounding cortical areas. For example, there is also a prominent viscero-sensory representation found in posterior insular cortex, adjacent to GC (Cechetto and Saper 1987). Perhaps correspondingly, the hotspot for conditioned taste aversion learning is also found in posterior insular or GC (Schier et al. 2014, 2016). Furthermore, Hanamori et al. (1998) found that over 75% of taste-responsive neurons in posterior GC in rat were also responsive to a nociceptive stimulus (tail pinch).
In summary, reports (Chen et al. 2011b; Peng et al. 2015) from a single laboratory notwithstanding, the evidence is now quite strong that gustatory signals for taste quality are distributed and intermingled in the gustatory cortex.
Patterns of gustatory activity in the human cortex
Although taste processing in the periphery and also the CNS has gained considerable attention in animal models, these processes are still to be investigated in humans. Of particular interest are questions on how, when, and where taste information, in general, and specific taste attributes such as taste quality, intensity, and hedonics, in particular, are processed in the human brain. Human neuroimaging studies have shown that taste consistently activates a range of cortical areas including the anterior insula and frontal operculum (FOP), mid-dorsal insula and overlying Rolandic operculum, posterior insula and parietal operculum (POP), as well as the postcentral gyrus (cf. Veldhuizen et al. 2011; Yeung et al. 2018). Evidence suggests that the mid-dorsal insula and the adjacent FOP form GC (Small et al. 1999; O’Doherty et al. 2001; Bender et al. 2009; Small 2010; Iannilli et al. 2012), whereas the posterior insula and POP have been implicated in oral somatosensation and attention to the mouth rather than gustation (Veldhuizen et al. 2007). These findings are in line with macaque anatomy, where the anterior and mid insula and the FOP, but not the POP, receive taste afferents from the thalamus (Pritchard et al. 1986) but may not directly translate to human physiology. Observations that taste sensations can be elicited by electrical stimulation of the mid-dorsal insula (Mazzola et al. 2017), further corroborating its role as GC. Consistent with the anatomical evidence, scalp-level electrophysiological studies found pronounced activation of the bilateral anterior in mid insula and adjacent FOP in response to electric (Ohla et al. 2010) and sapid taste (Tzieropoulos et al. 2013; Crouzet et al. 2015) within 150 ms of taste delivery.
Functionally, insular activation has been linked with sensory taste features, such as taste intensity (Guest et al. 2007; Grabenhorst and Rolls 2008; Ohla et al. 2010; Spetter et al. 2010; Tzieropoulos et al. 2013) and taste quality (Schoenfeld et al. 2004; Crouzet et al. 2015); taste pleasantness and valuation, on the other hand, have been mostly associated with activity in the OFC, the anatomically later, secondary taste area (Guest et al. 2007; Grabenhorst and Rolls 2008). However, it has also been proposed that the GC jointly encodes both the chemical identity and palatability of a tastant (de Araujo et al. 2006), thereby suggesting a role of the insula in the evaluation of taste or its precursors beyond mere sensory processing. This notion is corroborated by observations that expectations about the value of a taste, induced by visual cues, modulate taste-related processing in the rodent (Grossman et al. 2008) and in the human (Nitschke et al. 2006; Ohla et al. 2012) insula.
In contrast to animal models, the mechanisms underlying taste quality coding have received little attention in humans mostly due to the limited spatial resolution of noninvasive brain imaging techniques such as functional magnetic resonance imaging (fMRI) yielding a spatial resolution of a few millimeters at best. Accordingly, only a few fMRI studies have addressed the question of a gustotopic organization of the human GC and their results failed to provide evidence for a clear spatial segregation of taste qualities but rather suggest a partial overlap of insular representations for different tastes (Schoenfeld et al. 2004; Dalenberg et al. 2015; Prinster et al. 2017). However, cortical activation patterns change rapidly, within milliseconds, rendering temporal information a candidate variable for taste quality coding. In fact, neuronal response patterns obtained from electrophysiological recordings at the scalp allow deciphering which taste participants tasted on a given trial. The onset of this discriminability coincided with the earliest taste-evoked responses that were localized in GC, signifying that quality is among the first attributes of a taste represented in the central gustatory system (Crouzet et al. 2015) in strong accord with electrophysiological studies in awake rodents (Stapleton et al. 2006; Graham et al. 2014; Pavao et al. 2014). The results also align with and add to observations that neuronal response patterns along the rodent gustatory neuroaxis, including the nucleus of the solitary tract (Di Lorenzo et al. 2009), parabrachial nucleus (Geran and Travers 2013), and insula (Jezzini et al. 2013), code taste quality.
More recent evidence linked the predictive value of gustatory neural response patterns and taste-related decision-making. For this, behavioral reports from different tasks were combined with multivariate analyses of large-scale electrophysiological recordings in a series of studies. Specifically, Crouzet et al. (2015) showed that the more alike the neural response patterns of any two tastes were, as indicated by poorer discriminative performance of a classifier, the more these tastes were confused by the participants. The results were surprising for the taste domain because they provide evidence for a mapping between neural and phenomenological rather than between neural and chemical spaces. Whether the information encoded in gustatory neural response patterns drives actual behavior was addressed in two further studies. In the study by Wallroth et al. (2018), participants were to detect the presence of a taste as quick as possible. They found that the onset of taste decoding (discriminable brain response patterns) indeed predicted “when” participants detected a given taste by button press and linked neuronal response patterns to the speed of simple gustatory perceptual decisions—a vital performance index of nutrient sensing. Interestingly, the onset of taste decoding was earlier in this study, where participants responded speedily, compared with the previous study, where participants performed a delayed response task suggesting that the timing of gustatory coding is in a way flexible and dependent on behavioral goals.
Although the mere detection of a taste in the oral cavity may prepare a nonspecific response, the regulation of nutrient uptake and expulsion of potential toxins calls for quick and reliable taste detection and identification. Whether taste detection and discrimination are sequential or parallel processes, that is, whether you know what it is as soon as you taste it, was addressed in another study (Wallroth and Ohla forthcoming). To uncover the sequence of processing steps involved in taste perceptual decisions, participants performed taste-detection and taste-discrimination tasks. Irrespective of taste quality and task, neural decoding onset and behavioral response times were strongly linked, demonstrating that differences between taste judgments are reflected early during chemosensory encoding. Moreover, neural and behavioral detection times were faster for the iso-hedonic salty and sour tastes than their discrimination time. No such latency difference was observed for sweet and bitter, which differ hedonically. These results indicate that the human gustatory system detects a taste faster than it discriminates between tastes, yet hedonic computations may run in parallel (Perez et al. 2013) and facilitate taste identification.
Together these studies clearly show that the information encoded in taste-related neural response patterns is also the foundation for gustatory decision-making and that the timing aligns with task-specific goals.
Cortical population coding of taste decisions and behavior
Taste quality is tightly linked to taste palatability or pleasantness. Whereas sweet taste is typically liked, bitter taste is commonly aversive to most mammals. Accordingly, the gustatory neuroaxis needs to represent both features as they, together, drive food-related decisions and allow adaptive behavior. In awake rats, taste administration is represented by complex temporal coding in single neurons: a brief period of nonspecific firing is followed by approximately 500 ms of identity-related firing, which is, in turn, replaced by firing that is reliably related to taste palatability (Katz et al. 2000; Sadacca et al. 2012). A series of studies have demonstrated that the palatability “epoch” can be independently manipulated, validating the characterization: changes in perceived palatability, such as that observed at the transition from an attentive to “withdrawn” state (Fontanini and Katz 2005, 2006) and across conditioned taste aversion learning (Grossman et al. 2008; Moran and Katz 2014), change palatability epoch coding although having no impact on the earlier approximately 800 ms of taste-induced activity.
CNS neural responses provide information about the identity of tastes on the tongue. Countless studies have demonstrated that sapid stimuli flowing across the tongue of anesthetized animals induce responses in neurons across the gustatory neuroaxis (for just a few examples, see (Azuma et al. 1984; Yamamoto 1984; Di Lorenzo 1988; Yamamoto et al. 1989; Nishijo and Norgren 1990; Erickson et al. 1994; Di Lorenzo and Victor 2003; Li et al. 2013). Perhaps the most discussed facet of these studies is the fact that taste responses vary vastly in breadth; a great deal of energy has been devoted to debating theories of gustatory coding that turn on these breadths of responsivity (e.g., Smith and St John 1999; Di Lorenzo 2000; Scott 2004; Spector and Travers 2005; Lemon and Katz 2007). Neural circuitry in general, and taste circuits in particular, are rife with cross-talk and feedback at both microcircuit (within region) and macrocircuit (between region) level (Jones et al. 2006). Empirical and theoretical work makes it clear that neural responses in such interactive networks should contain functionally interpretable dynamics that are most meaningful when examined at the ensemble rather than at the single cell level (e.g., see Abarbanel and Rabinovich 2001).
An independent set of studies have made use of analytic techniques specialized to interpret the real-time firing of multiple simultaneously recorded neurons (hidden Markov modeling, or HMM). This work reveals that firing rate modulations within taste responses, which appear gradual in across-trial averages of single-neuron firing, are in fact not gradual at all. Rather, they reflect sudden coherent shifts between ensemble states: at particular time points within individual trials, the firing rates of (on average) approximately 50% of the recorded neurons will change simultaneously; across-trial averages “smear” these changes, making them look more gradual, because they happen at different latencies in different trials (Jones et al. 2007; Miller and Katz 2010). Together, these two sets of results suggest the testable hypothesis that GC neural ensembles, far from simply coding what the taste is, may process that information to directly drive action. If in fact palatability-related firing appears suddenly in single trials (a possibility implied by but not directly demonstrated in the above-described work), it is possible to hypothesize that this appearance predicts the onset of consumption behavior. Our testing (Sadacca et al. 2016) proves this to be the case, in that analyses keyed to the onset of the ensemble state dominant during the palatability epoch (rather than to stimulus onset time, as is more typical) reveal that palatability coding does emerge suddenly—more suddenly than a range of ramping models, including the model used to explain primate perceptual decision-making (see Shadlen et al. 1996; Gold and Shadlen 2001) can explain and as fast as models assuming instantaneous state transitions (Sadacca et al. 2016).
Armed with the knowledge of precisely “when” decision-related information appears in GC on individual trials, the authors were then able to compare this information to within-trial latencies of palatability-related behavioral responses, measured through electromyography. This analysis specifically reveals that the sudden emergence of the “palatability-related state” in GC neural ensembles predicts both “whether” the rat will gape in response to taste stimulation and precisely “when” that gape will occur, in single trials, with correlation values of approximately 0.75 (Sadacca et al. 2016).
The above results, although robust, are phenomenological. Li and et al. (2016) performed two types of perturbation experiments to test whether GC ensemble transitions are causally linked to consumption behavior. In one experiment, arrival of an aversive taste was cued: as the rats learned the meaning of the cue across a full session, the latency with which they gaped in response to the taste decreased by approximately 150 ms; recordings showed that the cue had an almost identical impact on neural coding of that aversive taste. In the second experiment, optogenetic silencing of GC neurons was shown to change the likelihood of gaping. Together, these experiments confirm the general hypothesis that GC is a part of a distributed system responsible for transforming an incoming identity code into a taste decision.
These results, although perhaps surprising within the field of taste research, are consistent with a great deal of work on sensorimotor systems—and, more specifically, on work describing the top-down modulation of multirhythmic central pattern generators (Marder 2012).
Conclusion
When examined at each level of the nervous system—periphery, brainstem, and cortex—it is evident that individual taste-responsive receptor cells or neurons may respond either selectively or broadly to stimuli of different taste qualities. Recent approaches to rodent and human central taste also emphasize the importance of temporal response patterns, which likely underlie the progression of taste behavior, from detectability to discrimination. This response complexity supports the notion of combinatorial coding along the gustatory neuroaxis. The flexibility inherent in this type of coding for the sense of taste may be necessary for animals to exhibit adaptive behavior in food selection and consummatory behavior.
Acknowledgements
The authors acknowledge the following sources of support: Federal Ministry of Education and Research [BMBF; 01EA1408A-G to K.O.], National Institutes of Health [NIH; R01DC014420 and R21DC012746 to S.D.R. and N.C., R01DC007630 to S.D.R., R01DC016833 to J.B. and M.F., R01DC006914 to P.M.D. and J.D.V., R01DC00945, R01DC007708, and R01DC006666 to D.B.K., R01DC006308 to N.C.], and Japan Society for the Promotion of Science [JSPS; KAKENHI JP26462815 and 18K09507 to R.Y.]. The authors thank Iryna Ruda for help with the artwork in Figure 1.
References
- Abarbanel HD, Rabinovich MI. 2001. Neurodynamics: nonlinear dynamics and neurobiology. Curr Opin Neurobiol. 11:423–430. [DOI] [PubMed] [Google Scholar]
- Accolla R, Bathellier B, Petersen CC, Carleton A. 2007. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci. 27:1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. 2000. A novel family of mammalian taste receptors. Cell. 100:693–702. [DOI] [PubMed] [Google Scholar]
- Azuma S, Yamamoto T, Kawamura Y. 1984. Studies on gustatory responses of amygdaloid neurons in rats. Exp Brain Res. 56:12–22. [DOI] [PubMed] [Google Scholar]
- Bahar A, Dudai Y, Ahissar E. 2004. Neural signature of taste familiarity in the gustatory cortex of the freely behaving rat. J Neurophysiol. 92:3298–3308. [DOI] [PubMed] [Google Scholar]
- Barretto RP, Gillis-Smith S, Chandrashekar J, Yarmolinsky DA, Schnitzer MJ, Ryba NJ, Zuker CS. 2015. The neural representation of taste quality at the periphery. Nature. 517:373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M, Foerster S, Staehler F, Raguse JD, Meyerhof W. 2007. Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J Neurosci. 27:12630–12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Shaw A. 1868. Reprint of the “idea of a new anatomy of the brain,” with letters, &c. J Anat Physiol. 3:147–182. [PMC free article] [PubMed] [Google Scholar]
- Bender G, Veldhuizen MG, Meltzer JA, Gitelman DR, Small DM. 2009. Neural correlates of evaluative compared with passive tasting. Eur J Neurosci. 30:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Kim KN, Roper SD. 2002. Individual mouse taste cells respond to multiple chemical stimuli. J Physiol. 544:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Roper SD. 2001. Taste receptor cells that discriminate between bitter stimuli. Science. 291:1557–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton A, Accolla R, Simon SA. 2010. Coding in the mammalian gustatory system. Trends Neurosci. 33:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. 1987. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 262:27–45. [DOI] [PubMed] [Google Scholar]
- Chaudhari N. 2014. Synaptic communication and signal processing among sensory cells in taste buds. J Physiol. 592:3387–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Victor JD, Di Lorenzo PM. 2011a. Temporal coding of intensity of NaCl and HCl in the nucleus of the solitary tract of the rat. J Neurophysiol. 105:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gabitto M, Peng Y, Ryba NJ, Zuker CS. 2011b. A gustotopic map of taste qualities in the mammalian brain. Science. 333:1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet SM, Busch NA, Ohla K. 2015. Taste quality decoding parallels taste sensations. Curr Biol. 25:890–896. [DOI] [PubMed] [Google Scholar]
- Dalenberg JR, Hoogeveen HR, Renken RJ, Langers DR, ter Horst GJ. 2015. Functional specialization of the male insula during taste perception. Neuroimage. 119:210–220. [DOI] [PubMed] [Google Scholar]
- Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. 2012. Adenosine enhances sweet taste through A2B receptors in the taste bud. J Neurosci. 32:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Roper SD. 2009. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 587:5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova V, Roberts T, Hellekant G. 1999. Responses of single taste fibers and whole chorda tympani and glossopharyngeal nerve in the domestic pig, Sus scrofa. Chem Senses. 24:301–316. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A Jr, Nicolelis MA, Simon SA. 2006. Neural ensemble coding of satiety states. Neuron. 51:483–494. [DOI] [PubMed] [Google Scholar]
- Descartes R. 1664. L’ Homme. Paris: Charles Angot. [Google Scholar]
- Di Lorenzo PM. 1988. Taste responses in the parabrachial pons of decerebrate rats. J Neurophysiol. 59:1871–1887. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM. 2000. The neural code for taste in the brain stem: response profiles. Physiol Behav. 69:87–96. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Chen JY, Victor JD. 2009. Quality time: representation of a multidimensional sensory domain through temporal coding. J Neurosci. 29:9227–9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S. 1997. Transfer of information about taste from the nucleus of the solitary tract to the parabrachial nucleus of the pons. Brain Res. 763: 167–181. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Victor JD. 2003. Taste response variability and temporal coding in the nucleus of the solitary tract of the rat. J Neurophysiol. 90:1418–1431. [DOI] [PubMed] [Google Scholar]
- Erickson RP. 2008. A study of the science of taste: on the origins and influence of the core ideas. Behav Brain Sci. 31:59–75; discussion 75. [DOI] [PubMed] [Google Scholar]
- Erickson RP, Di Lorenzo PM, Woodbury MA. 1994. Classification of taste responses in brain stem: membership in fuzzy sets. J Neurophysiol. 71:2139–2150. [DOI] [PubMed] [Google Scholar]
- Escanilla OD, Victor JD, Di Lorenzo PM. 2015. Odor-taste convergence in the nucleus of the solitary tract of the awake freely licking rat. J Neurosci. 35:6284–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Ogg MC, Lu L, Ogg RJ, Boughter JD Jr. 2017. Overlapping representation of primary tastes in a defined region of the gustatory cortex. J Neurosci. 37:7595–7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. 2005. 7 to 12 Hz activity in rat gustatory cortex reflects disengagement from a fluid self-administration task. J Neurophysiol. 93:2832–2840. [DOI] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. 2006. State-dependent modulation of time-varying gustatory responses. J Neurophysiol. 96:3183–3193. [DOI] [PubMed] [Google Scholar]
- Frank M, Pfaffmann C. 1969. Taste nerve fibers: a random distribution of sensitivities to four tastes. Science. 164:1183–1185. [DOI] [PubMed] [Google Scholar]
- Frank ME. 1991. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J Neurophysiol. 65:1452–1463. [DOI] [PubMed] [Google Scholar]
- Frank ME, Contreras RJ, Hettinger TP. 1983. Nerve fibers sensitive to ionic taste stimuli in chorda tympani of the rat. J Neurophysiol. 50:941–960. [DOI] [PubMed] [Google Scholar]
- Frank ME, Lundy RF Jr, Contreras RJ. 2008. Cracking taste codes by tapping into sensory neuron impulse traffic. Prog Neurobiol. 86:245–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geran L, Travers S. 2013. Temporal characteristics of gustatory responses in rat parabrachial neurons vary by stimulus and chemosensitive neuron type. PLoS One. 8:e76828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geran LC, Travers SP. 2006. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol. 96:2513–2527. [DOI] [PubMed] [Google Scholar]
- Geran LC, Travers SP. 2009. Bitter-responsive gustatory neurons in the rat parabrachial nucleus. J Neurophysiol. 101:1598–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. 2001. Neural computations that underlie decisions about sensory stimuli. Trends Cogn Sci. 5:10–16. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. 2008. Selective attention to affective value alters how the brain processes taste stimuli. Eur J Neurosci. 27:723–729. [DOI] [PubMed] [Google Scholar]
- Graham DM, Sun C, Hill DL. 2014. Temporal signatures of taste quality driven by active sensing. J Neurosci. 34:7398–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SE, Fontanini A, Wieskopf JS, Katz DB. 2008. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci. 28:2864–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest S, Grabenhorst F, Essick G, Chen Y, Young M, McGlone F, de Araujo I, Rolls ET. 2007. Human cortical representation of oral temperature. Physiol Behav. 92:975–984. [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers SP. 1997. Anterior and posterior oral cavity responsive neurons are differentially distributed among parabrachial subnuclei in rat. J Neurophysiol. 78:920–938. [DOI] [PubMed] [Google Scholar]
- Hanamori T, Kunitake T, Kato K, Kannan H. 1998. Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J Neurophysiol. 79:2535–2545. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Ninomiya Y. 1994. Bitter taste in single chorda tympani taste fibers from chimpanzee. Physiol Behav. 56:1185–1188. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. 2006. The cells and logic for mammalian sour taste detection. Nature. 442:934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. 2009. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 29:13909–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. 2007. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA. 104: 6436–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannilli E, Singh PB, Schuster B, Gerber J, Hummel T. 2012. Taste laterality studied by means of umami and salt stimuli: an fMRI study. Neuroimage. 60:426–435. [DOI] [PubMed] [Google Scholar]
- Jezzini A, Mazzucato L, La Camera G, Fontanini A. 2013. Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks. J Neurosci. 33:18966–18978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LM, Fontanini A, Katz DB. 2006. Gustatory processing: a dynamic systems approach. Curr Opin Neurobiol. 16:420–428. [DOI] [PubMed] [Google Scholar]
- Jones LM, Fontanini A, Sadacca BF, Miller P, Katz DB. 2007. Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles. Proc Natl Acad Sci USA. 104:18772–18777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DB, Nicolelis MA, Simon SA. 2000. Nutrient tasting and signaling mechanisms in the gut. IV. There is more to taste than meets the tongue. Am J Physiol Gastrointest Liver Physiol. 278:G6–G9. [DOI] [PubMed] [Google Scholar]
- Katz DB, Simon SA, Nicolelis MA. 2001. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 21:4478–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuhara Y, Yoshida R, Ohkuri T, Yasumatsu K, Voigt A, Hübner S, Maeda K, Boehm U, Meyerhof W, Ninomiya Y. 2013. Taste responses in mice lacking taste receptor subunit T1R1. J Physiol. 591:1967–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. 2002. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 3:884–895. [DOI] [PubMed] [Google Scholar]
- Lavi K, Jacobson GA, Rosenblum K, Lüthi A. 2018. Encoding of conditioned taste aversion in cortico-amygdala circuits. Cell Rep. 24:278–283. [DOI] [PubMed] [Google Scholar]
- Lawhern V, Nikonov AA, Wu W, Contreras RJ. 2011. Spike rate and spike timing contributions to coding taste quality information in rat periphery. Front Integr Neurosci. 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon CH, Katz DB. 2007. The neural processing of taste. BMC Neurosci. 8(Suppl 3):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Maier JX, Reid EE, Katz DB. 2016. Sensory cortical activity is related to the selection of a rhythmic motor action pattern. J Neurosci. 36:5596–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Yoshida T, Monk KJ, Katz DB. 2013. Lateral hypothalamus contains two types of palatability-related taste responses with distinct dynamics. J Neurosci. 33:9462–9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fontanini A. 2015. State dependency of chemosensory coding in the gustatory thalamus (VPMpc) of alert rats. J Neurosci. 35:15479–15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, et al. 2017. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature. 546:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossow K, Hübner S, Roudnitzky N, Slack JP, Pollastro F, Behrens M, Meyerhof W. 2016. Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J Biol Chem. 291:15358–15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Haley M, Fontanini A. 2012. Neural processing of gustatory information in insular circuits. Curr Opin Neurobiol. 22:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. 2012. Neuromodulation of neuronal circuits: back to the future. Neuron. 76:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Montmayeur JP, Buck LB. 2000. A family of candidate taste receptors in human and mouse. Nature. 404:601–604. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Royet JP, Catenoix H, Montavont A, Isnard J, Mauguière F. 2017. Gustatory and olfactory responses to stimulation of the human insula. Ann Neurol. 82:360–370. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. 2010. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 35:157–170. [DOI] [PubMed] [Google Scholar]
- Miller P, Katz DB. 2010. Stochastic transitions between neural states in taste processing and decision-making. J Neurosci. 30:2559–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Katz DB. 2014. Sensory cortical population dynamics uniquely track behavior across learning and extinction. J Neurosci. 34:1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. 2005. The receptors and coding logic for bitter taste. Nature. 434:225–229. [DOI] [PubMed] [Google Scholar]
- Müller J. 1836. On the nerves supplying the cavernous structure of the penis; and their annexion with the hypogastric plexus of the sympathic. The London Medical Gazette. XVIII: 143. [Google Scholar]
- Nagai T, Ueda K. 1981. Stochastic properties of gustatory impulse discharges in rat chorda tympani fibers. J Neurophysiol. 45:574–592. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Norgren R. 1991. Gustatory responses of neurons in the nucleus of the solitary tract of behaving rats. J Neurophysiol. 66:1232–1248. [DOI] [PubMed] [Google Scholar]
- Nara K, Saraiva LR, Ye X, Buck LB. 2011. A large-scale analysis of odor coding in the olfactory epithelium. J Neurosci. 31:9179–9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. 2002. An amino-acid taste receptor. Nature. 416:199–202. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. 2001. Mammalian sweet taste receptors. Cell. 106:381–390. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Norgren R. 1990. Responses from parabrachial gustatory neurons in behaving rats. J Neurophysiol. 63:707–724. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Dixon GE, Sarinopoulos I, Short SJ, Cohen JD, Smith EE, Kosslyn SM, Rose RM, Davidson RJ. 2006. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nat Neurosci. 9:435–442. [DOI] [PubMed] [Google Scholar]
- Nunez-Parra A, Li A, Restrepo D. 2014. Coding odor identity and odor value in awake rodents. Prog Brain Res. 208:205–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. 2001. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 85:1315–1321. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Hasegawa K, Murayama N. 1992a. Difference in taste quality coding between two cortical taste areas, granular and dysgranular insular areas, in rats. Exp Brain Res. 91:415–424. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Murayama N, Hasegawa K. 1992b. Difference in receptive field features of taste neurons in rat granular and dysgranular insular cortices. Exp Brain Res. 91:408–414. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Yamashita S, Sato M. 1974. Variation in gustatory nerve fiber discharge pattern with change in stimulus concentration and quality. J Neurophysiol. 37:443–457. [DOI] [PubMed] [Google Scholar]
- Ohla K, Toepel U, le Coutre J, Hudry J. 2010. Electrical neuroimaging reveals intensity-dependent activation of human cortical gustatory and somatosensory areas by electric taste. Biol Psychol. 85:446–455. [DOI] [PubMed] [Google Scholar]
- Ohla K, Toepel U, le Coutre J, Hudry J. 2012. Visual-gustatory interaction: orbitofrontal and insular cortices mediate the effect of high-calorie visual food cues on taste pleasantness. PLoS One. 7:e32434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavão R, Piette CE, Lopes-dos-Santos V, Katz DB, Tort AB. 2014. Local field potentials in the gustatory cortex carry taste information. J Neurosci. 34:8778–8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Gillis-Smith S, Jin H, Tränkner D, Ryba NJ, Zuker CS. 2015. Sweet and bitter taste in the brain of awake behaving animals. Nature. 527:512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez IO, Villavicencio M, Simon SA, Gutierrez R. 2013. Speed and accuracy of taste identification and palatability: impact of learning, reward expectancy, and consummatory licking. Am J Physiol Regul Integr Comp Physiol. 305:R252–R270. [DOI] [PubMed] [Google Scholar]
- Petersen CC. 2007. The functional organization of the barrel cortex. Neuron. 56:339–355. [DOI] [PubMed] [Google Scholar]
- Pfaffmann C. 1941. Gustatory afferent impulses. J Cell Comp Physiol. 17: 243–258. [Google Scholar]
- Prinster A, Cantone E, Verlezza V, Magliulo M, Sarnelli G, Iengo M, Cuomo R, Di Salle F, Esposito F. 2017. Cortical representation of different taste modalities on the gustatory cortex: A pilot study. PLoS One. 12:e0190164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, Norgren R. 1986. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol. 244:213–228. [DOI] [PubMed] [Google Scholar]
- Roper SD. 2014. Sensory end-organs: signal processing in the periphery: a symposium presented at the 2013 Annual Meeting of the Society for Neuroscience, San Diego, CA, USA. J Physiol. 592:3383–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AM, Victor JD, Di Lorenzo PM. 2011. Temporal coding of taste in the parabrachial nucleus of the pons of the rat. J Neurophysiol. 105:1889–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussin AT, D’Agostino AE, Fooden AM, Victor JD, Di Lorenzo PM. 2012. Taste coding in the nucleus of the solitary tract of the awake, freely licking rat. J Neurosci. 32:10494–10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussin AT, Victor JD, Chen JY, Di Lorenzo PM. 2008. Variability in responses and temporal coding of tastants of similar quality in the nucleus of the solitary tract of the rat. J Neurophysiol. 99:644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadacca BF, Mukherjee N, Vladusich T, Li JX, Katz DB, Miller P. 2016. The behavioral relevance of cortical neural ensemble responses emerges suddenly. J Neurosci. 36:655–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadacca BF, Rothwax JT, Katz DB. 2012. Sodium concentration coding gives way to evaluative coding in cortex and amygdala. J Neurosci. 32:9999–10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons JD, Weiss MS, Escanilla OD, Fooden AF, Victor JD, Di Lorenzo PM. 2016. Spontaneous changes in taste sensitivity of single units recorded over consecutive days in the brainstem of the awake rat. PLoS One. 11:e0160143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Ogawa H, Yamashita S. 1975. Response properties of macaque monkey chorda tympani fibers. J Gen Physiol. 66:781–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier LA, Blonde GD, Spector AC. 2016. Bilateral lesions in a specific subregion of posterior insular cortex impair conditioned taste aversion expression in rats. J Comp Neurol. 524:54–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier LA, Hashimoto K, Bales MB, Blonde GD, Spector AC. 2014. High-resolution lesion-mapping strategy links a hot spot in rat insular cortex with impaired expression of taste aversion learning. Proc Natl Acad Sci USA. 111: 1162–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld MA, Neuer G, Tempelmann C, Schüssler K, Noesselt T, Hopf JM, Heinze HJ. 2004. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience. 127:347–353. [DOI] [PubMed] [Google Scholar]
- Scott K. 2004. The sweet and the bitter of mammalian taste. Curr Opin Neurobiol. 14:423–427. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Britten KH, Newsome WT, Movshon JA. 1996. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci. 16:1486–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. 2006. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci. 7:890–901. [DOI] [PubMed] [Google Scholar]
- Small DM. 2010. Taste representation in the human insula. Brain Struct Funct. 214:551–561. [DOI] [PubMed] [Google Scholar]
- Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M. 1999. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 10:7–14. [DOI] [PubMed] [Google Scholar]
- Smith DV, John SJ, Boughter JD. 2000. Neuronal cell types and taste quality coding. Physiol Behav. 69:77–85. [DOI] [PubMed] [Google Scholar]
- Smith DV, St John SJ. 1999. Neural coding of gustatory information. Curr Opin Neurobiol. 9:427–435. [DOI] [PubMed] [Google Scholar]
- Spector AC, Travers SP. 2005. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 4:143–191. [DOI] [PubMed] [Google Scholar]
- Spetter MS, Smeets PA, de Graaf C, Viergever MA. 2010. Representation of sweet and salty taste intensity in the brain. Chem Senses. 35:831–840. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Stevens CF. 2018. The distributed circuit within the piriform cortex makes odor discrimination robust. J Comp Neurol. 526:2725–2743. [DOI] [PubMed] [Google Scholar]
- Stapleton JR, Lavine ML, Wolpert RL, Nicolelis MA, Simon SA. 2006. Rapid taste responses in the gustatory cortex during licking. J Neurosci. 26:4126–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R. 2009. Representations of odor in the piriform cortex. Neuron. 63:854–864. [DOI] [PubMed] [Google Scholar]
- Sugita M, Shiba Y. 2005. Genetic tracing shows segregation of taste neuronal circuitries for bitter and sweet. Science. 309:781–785. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. 2007. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 27:10840–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonosaki K, Beidler LM. 1989. Sugar best single chorda tympani nerve fiber responses to various sugar stimuli in rat and hamster. Comp Biochem Physiol A Comp Physiol. 94:603–605. [PubMed] [Google Scholar]
- Tzieropoulos H, Rytz A, Hudry J, le Coutre J. 2013. Dietary fat induces sustained reward response in the human brain without primary taste cortex discrimination. Front Hum Neurosci. 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundström JN. 2011. Identification of human gustatory cortex by activation likelihood estimation. Hum Brain Mapp. 32:2256–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. 2007. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Senses. 32:569–581. [DOI] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. 1996. Nature and precision of temporal coding in visual cortex: a metric-space analysis. J Neurophysiol. 76:1310–1326. [DOI] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. 1997. Sensory coding in cortical neurons. Recent results and speculations. Ann N Y Acad Sci. 835:330–352. [DOI] [PubMed] [Google Scholar]
- Wallroth R, Höchenberger R, Ohla K. 2018. Delta activity encodes taste information in the human brain. Neuroimage. 181:471–479. [DOI] [PubMed] [Google Scholar]
- Wallroth R, Ohla K Forthcoming. As soon as you taste it - evidence for sequential and parallel processing of gustatory information. eNeuro. 5(5). doi: 10.1523/ENEURO.0269-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Danilova V, Cragin T, Roberts TW, Koposov A, Hellekant G. 2009. The sweet taste quality is linked to a cluster of taste fibers in primates: lactisole diminishes preference and responses to sweet in S fibers (sweet best) chorda tympani fibers of M. fascicularis monkey. BMC Physiol. 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MS, Di Lorenzo PM. 2012. Not so fast: taste stimulus coding time in the rat revisited. Front Integr Neurosci. 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MS, Victor JD, Di Lorenzo PM. 2014. Taste coding in the parabrachial nucleus of the pons in awake, freely licking rats and comparison with the nucleus of the solitary tract. J Neurophysiol. 111:1655–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead MC. 1986. Anatomy of the gustatory system in the hamster: synaptology of facial afferent terminals in the solitary nucleus. J Comp Neurol. 244:72–85. [DOI] [PubMed] [Google Scholar]
- Whitehead MC and Frank ME. 1983. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J Comp Neurol. 220: 378–395. [DOI] [PubMed] [Google Scholar]
- Wu A, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. 2015. Breadth of tuning in taste afferent neurons varies with stimulus strength. Nat Commun. 6:8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. 1984. Taste responses of cortical neurons. Prog Neurobiol. 23:273–315. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Kawamura Y. 1980. Localization of cortical gustatory area in rats and its role in taste discrimination. J Neurophysiol. 44:440–455. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. 1989. Taste responses of cortical neurons in freely ingesting rats. J Neurophysiol. 61:1244–1258. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, Kato T, Kawamura Y. 1984. Gustatory responses of cortical neurons in rats. I. Response characteristics. J Neurophysiol. 51:616–635. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, Kato T, Kawamura Y. 1985. Gustatory responses of cortical neurons in rats. II. Information processing of taste quality. J Neurophysiol. 53:1356–1369. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJ. 2009. Common sense about taste: from mammals to insects. Cell. 139:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung AWK, Goto TK, Leung WK. 2018. Affective value, intensity and quality of liquid tastants/food discernment in the human brain: an activation likelihood estimation meta-analysis. Neuroimage. 169:189–199. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, et al. 2009. Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol. 587:4425–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Takai S, Sanematsu K, Margolskee RF, Shigemura N, Ninomiya Y. 2018. Bitter taste responses of gustducin-positive taste cells in mouse fungiform and circumvallate papillae. Neuroscience. 369:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]