Abstract
Background
Exposure to vitamin K antagonists (VKA) has been suggested to accelerate progression of chronic kidney disease (CKD) but robust clinical data are currently lacking.
Methods
We retrospectively evaluated the impact of VKA exposure on kidney function in patients with atrial fibrillation (AF) and CKD stage 3/4. Patients were prospectively followed within a primary care electronic database (median follow‐up of 1.45 years). The kidney function trajectory over time, defined as the annualized change in estimated glomerular filtration rate (eGFR), was analyzed with linear mixed‐effects regression including propensity score adjustment.
Results
14 432 patients (median age 78 years, median CHA 2 DS 2‐VASc score 4 points) contributed 97 792 eGFR measurements (mean 6.8 measurements/patient; range: 1‐197). Mean baseline eGFR was 50.3 mL/min/1.73 m2; and declined by 1.10 mL/min/1.73 m2/year (95% CI: 0.91‐1.28, P < 0.0001). In 7409 patients with VKA exposure, CKD progression was significantly faster compared to patients without VKA exposure (5‐year absolute eGFR loss from baseline: 6.0 mL/min/1.73 m2 vs 4.5 mL/min/1.73 m2, for an absolute 5‐year excess eGFR decline with VKA exposure of 1.5 mL/min/1.73 m2 (95% CI: 0.4‐2.7, P = 0.002). These results prevailed upon adjusting for CHA 2 DS 2‐VASc score and other potential imbalances in prognostic variables, and in several sensitivity analyses. In the group without documented VKA exposure, 1775 VKA patients (24%) and 1012 patients (14%) developed a 30% decline in eGFR during follow‐up (P < 0.0001).
Conclusions
In patients with AF and CKD, VKA use is associated with accelerated eGFR decline. Within the limitations of a retrospective analysis, this finding supports the “VKA‐renal‐calcification hypothesis.” However, although statistically significant, the excess loss in eGFR over 5 years with VKA was modest.
Keywords: anticoagulants, atrial fibrillation, chronic kidney disease, glomerular filtration rate, renal insufficiency
Essentials.
Vitamin K antagonist (VKA) exposure may accelerate chronic kidney disease (CKD).
A retrospective health database analysis evaluated the impact of VKA on renal function.
In 7409 patients with atrial fibrillation and CKD, exposure to VKA accelerated renal decline.
Findings support the concept of VKA nephropathy, although the absolute impact seemed small.
1. INTRODUCTION
Atrial fibrillation (AF) and chronic kidney disease (CKD) are common and often coexisting medical conditions in the elderly,1 with a prevalence of approximately 10% for AF and 30% for moderate‐to‐end‐stage CKD in the cohort of patients aged 75 years or above.2, 3, 4 Furthermore, AF has been shown to be a strong risk factor for developing incident CKD and vice versa, suggesting that AF and CKD are interdependent.1
Oral anticoagulation with vitamin K antagonists (VKA) or one of the newer direct oral agents inhibiting coagulation factors FII or FXa represents the mainstay of care for reducing the risk of cardioembolic stroke in AF patients with or without CKD.2, 5 However, it is currently hypothesized that the benefit of a reduction in stroke risk with VKA therapy may come at the cost of a higher propensity towards atherosclerotic plaque formation and vascular calcification,6 which may ultimately lead to a more rapid progression of CKD. Early evidence for this so‐called “renovascular calcification hypothesis” comes from basic and preclinical data as well as small clinical studies6, 7, 8 and raises concerns about the impact of long‐term VKA therapy on kidney disease progression, which may prevent physicians from prescribing otherwise indicated VKA to CKD patients with AF. Possibly the most suggestive evidence for a negative impact of VKA on renal function derives from a post‐hoc analysis of the RE‐LY trial, a large phase‐III trial that evaluated the direct oral anticoagulant (DOAC) dabigatran against VKA in 18 113 AF patients.9 Over an average observational period of 30 months, the mean decline in GFR was significantly greater with warfarin compared with dabigatran, and predictors for a more pronounced decline in GFR were presence of diabetes and previous warfarin use. Similarly, in ROCKET‐AF (median follow‐up 23.5 months) warfarin treatment was associated with a small, statistically significant decline in mean ± SD CrCl (−4.3 ± 14.6 mL/min) compared with patients receiving rivaroxaban (−3.5 ± 15.1 mL/min; P < 0.001).10 On the other hand, a recently published study in incident predialysis patients could not demonstrate an accelerated decline in renal function from VKA exposure.11
Therefore, further data on the impact of VKA on kidney function are needed. This retrospective study aimed to investigate within a large cohort whether exposure to VKA anticoagulants is associated with an accelerated renal decline in a real‐world cohort of patients with AF and stage 3/4 CKD.
1. MATERIALS AND METHODS
1.1. Study population and design
The current study is a retrospective analysis of prospectively collected, anonymized data from a validated longitudinal health records database, the IMS Disease Analyzer Germany (IMS‐DA).12 Data for this study were collected routinely by IMS outside the current study protocol. In IMS‐DA, clinical and laboratory data are collected longitudinally from ~1300 primary care physicians (PCPs) in Germany. For the current study, we retrospectively estimated glomerular filtration rate (eGFR) measurements, records on anticoagulation prescription, and clinical data to generate an outpatient cohort of patients with AF and stage 3/4 CKD. In detail, we used ICD‐10 codes to extract all patients who had a documented diagnosis of AF and stage 3/4 CKD between January 1, 2009 and August 31, 2015 in IMS‐DA, excluding patients exposed to any other anticoagulants than VKA or DOAC. From this initial data export obtained from IMS, we then excluded all patients with their AF or CKD diagnosis prior January 1, 2008 to reduce the impact of survivorship bias from patients with an exceptionally long time interval between their first AF and CKD diagnosis. The baseline date for our analysis was defined as the first time point with a valid eGFR measurement after January 1, 2008, at which both a diagnosis of AF and CKD stage 3/4 (“concomitant AF/CKD”) was documented. Patients who did not have an eGFR measurement at or after this baseline data were also excluded, as were patients who had: (a) at least one prescription of a DOAC, (b) missing baseline data for the CHA2DS2‐VASc score, (c) implausible eGFR measurements suspicious for data entry errors (ie, either 0 mL/min/1.73 m2 or ≥170 mL/min/1.73 m2), or (d) a diagnosis code of CKD stage 5 at baseline. The CHA2DS2‐VASc score and its items were constructed from comorbidity diagnosis codes that were documented prior the baseline date.
Patients with at least one documented prescription of a VKA (ascertained by prespecified Anatomical Therapeutic Chemical [ATC] drug codes) at or after baseline were assigned to the “VKA group,” whereas patients without such an exposure were assigned to the “No VKA group,” respectively. The concurrent use of low‐dose aspirin was permitted in both study groups.
1.2. Ethics
The IMS‐DA contains exclusively anonymized information. In accordance with German legal regulations (§3 of the German Federal Data Protection Act), neither an approval of an ethics committee nor consent from individual patients were required for this database study.
1.3. Statistical methods
The statistical analysis was performed according to best‐practice recommendations for the study of longitudinal eGFR data in patients with CKD.13 All analyses were performed using Stata 14.0 or later versions (Stata Corp., Houston, TX; full analysis code available on request from FP). Continuous variables were reported as medians (25th‐75th percentile), whereas count data were summarized as absolute frequencies (%). The distribution of baseline variables between patients in the two treatment groups were assessed with rank sum tests (continuous variables) and chi‐squared tests (categorical variables), respectively. Further, the magnitude of these differences was quantified with standardized mean differences (SMD), with SMDs ≥ 0.20 indicating potentially relevant differences between the two study groups.
The primary endpoint of this study was the longitudinal kidney function trajectory, defined as the annualized change in eGFR after baseline on both an absolute (in mL/min/1.73 m2/year) and relative (in percent/year) scale. The primary endpoint was modelled using uni‐ and multivariable linear mixed regression (Stata routine mixed).14 The choice for this model type was supported by the unbalanced distribution of number and timepoints of eGFR measurements in our cohorts, which is a typical for longitudinal kidney function studies.13, 15
Prior to studying the association between VKA exposure, covariates, and the primary endpoint, a “base” model reflecting eGFR patterns over time in our cohort was developed as a random‐intercept‐and‐slope model for the eGFR trajectory which included three fixed effects (linear, quadratic, and cubic specifications of follow‐up time), and two random effects (random intercept for the eGFR, and random slope for follow‐up time). The original eGFR values were used for analysis of the absolute change in eGFR, whereas loge‐transformed eGFR values were used for analyzing relative eGFR changes.15 Relative differences were expressed as percent change and computed by 100 × eβ. Furthermore, several sensitivity analyses were performed (Figure S2 and Table S2).
2. RESULTS
A total of 37 476 patients had a documented diagnosis of AF and stage 3/4 CKD between January 1, 2009 and August 31, 2015 in IMS‐DA. Of these, 14 432 patients fulfilled our inclusion criteria (Table 1, Figure S1) and were incorporated in the analysis. The 23 044 patients who were excluded had comparable baseline characteristics to the patients included in the final analysis (Table S1). At baseline, the median age of the analysis cohort was 78.4 years (25th‐75th percentile: 73‐84), the median CHA2DS2‐VASc score was 4 (25th‐75th percentile: 3‐5), and the median “baseline” eGFR was 48 mL/min/1.73 m2.
Table 1.
Baseline characteristics of the study population
| Variable | Overall (n = 14 432) | No VKA (n = 7023) | VKA (n = 7409) | P | SMD | SMDIPTW |
|---|---|---|---|---|---|---|
| Demographic variables | ||||||
| Age (y) | 78.4 [72.6‐83.8] | 79.7 [73.0‐85.6] | 77.4 [72.3‐82.4] | <0.0001 | 0.16 | 0.00 |
| Female sex | 6983 (48.4%) | 3678 (52.4%) | 3305 (44.6%) | <0.0001 | 0.16 | 0.00 |
| Insurance status | — | — | — | <0.0001 | — | — |
| Private | 1006 (7.0%) | 561 (8.0%) | 445 (6.0%) | — | 0.08 | 0.08 |
| Public | 13 425 (93.0%) | 6461 (92.0%) | 6964 (94.0%) | — | 0.08 | 0.08 |
| Unknown | 1 (0.0%) | 1 (0.0%) | 0 (0.0%) | — | 0.02 | 0.02 |
| Practice locationa | — | — | — | 0.85 | — | — |
| West Germany | 11 058 (76.6%) | 5386 (76.7%) | 5672 (76.6%) | — | 0.00 | 0.01 |
| East Germany | 3374 (23.4%) | 1637 (23.3%) | 1737 (23.4%) | — | 0.00 | 0.01 |
| CHA2DS2‐VASc score and its items | ||||||
| CHA2DS2‐VASc score | 4 [3‐5] | 4 [3‐5] | 4 [3‐5] | <0.0001 | 0.09 | 0.04 |
| Age <65 y | 1317 (9.1%) | 713 (10.2%) | 604 (8.2%) | — | 0.07 | 0.17 |
| Age 65‐74 y | 3679 (25.5%) | 1517 (21.6%) | 2162 (29.2%) | — | 0.17 | 0.11 |
| Age ≥ 75 y | 9436 (65.4%) | 4793 (68.3%) | 4643 (62.7%) | — | 0.12 | 0.00 |
| Hypertension | 12 118 (84.5%) | 5859 (83.4%) | 6329 (85.4%) | 0.001 | 0.06 | 0.00 |
| Congestive heart failure | 6731 (46.6%) | 3197 (45.5%) | 3534 (47.7%) | 0.009 | 0.04 | 0.00 |
| Stroke/TIA | 2400 (16.6%) | 1197 (17.0%) | 1203 (16.2%) | 0.19 | 0.02 | 0.00 |
| Diabetes mellitus | 7926 (54.9%) | 3908 (55.7%) | 4018 (54.2%) | 0.088 | 0.03 | 0.00 |
| Female sex | 6983 (48.4%) | 3678 (52.4%) | 3305 (44.6%) | <0.0001 | 0.16 | 0.00 |
| Vascular disease | 3051 (21.1%) | 1612 (23.0%) | 1439 (19.4%) | <0.0001 | 0.09 | 0.00 |
| Other variables | ||||||
| First eGFR at or after baseline (mL/min/1.73 m2) | 48 [36‐61] | 47 [35‐62] | 48 [37‐60] | 0.34 | 0.03 | 0.00 |
| Aspirin use at or after baseline | 4619 (32.0%) | 3064 (43.6%) | 1555 (21.0%) | <0.0001 | 0.50 | 0.17 |
Distribution overall and by exposure to VKA at or after baseline. Continuous variables are summarized as medians (25th percentile [Q1] through 75th percentile [Q3]), whereas categorical variables are reported as absolute frequencies and percentages.
eGFR, estimated glomerular filtration rate; IPTW, inverse‐probability‐of‐treatment‐weight; SMD, standardized mean difference; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
P‐values for difference between patients with and without documented exposure to VKA are from Pearson's chi‐squared tests (categorical variables) or Wilcoxon rank‐sum tests (continuous variables). SMDs ≥ 0.2 indicating a potentially relevant imbalance between the two study groups.
A total of 7409 patients were exposed to VKA and received a total of 36 610 prescriptions (98.5% phenprocoumon; 1.5% warfarin; 0.02% acenocoumarol). In contrast, 7023 patients (49%) did not have a documented exposure to VKA and served as the comparator “no VKA” group.
Patients with VKA exposure were significantly younger (median: 77.4 vs 79.7 years), less likely to be female (44.6% vs 52.4%), had lower CHA2DS2‐VASc scores (mean: 4.22 vs 4.35 points), and less likely to have a comedication with low‐dose aspirin (21.0% vs 43.6%; all P < 0.0001, Table 1) as compared to patients in the “no VKA” group. However, as indicated by SMDs, these differences were small on an absolute scale (all SMD < 0.20), except for low‐dose aspirin comedication where the SMD was 0.50.
2.1. Modeling the eGFR trajectory
After baseline, the 14 432 study patients contributed a total of 97 792 eGFR measurements to the analysis (mean 6 measurements/patient; range: 1‐197), and the median interval between first and last eGFR measurement was 1.4 years (25th‐75th percentile: 0.9‐3.3).
In the “base” linear mixed effects model, including three fixed effects for follow‐up time (linear, quadratic, and cubic) and a random effect for follow‐up time, the estimated eGFR at baseline was 50.3 mL/min/1.73 m2 (95% CI: 50.0‐50.6). The annualized absolute decline in eGFR was estimated at 1.10 mL/min/1.73 m2/year (95% CI: 0.91‐1.28, P < 0.0001, Table S3 [Model S1], Figure S3A). Fitting the same model to the log(eGFR) instead of the eGFR trajectory yielded a corresponding annualized decline on a relative scale of 2.8%/year (95% CI: 3.2‐2.4, P < 0.0001, Table S3 [Model S2], Figure S3B).
In multivariable extension of Model #S1 with CHA2DS2‐VASc score, age (centered at age 75), and the first eGFR measurement (centered at 50 mL/min/1.73 m2), the adjusted annualized absolute eGFR decline was estimated at 1.07 mL/min/1.73 m2/year (95% CI: 0.90‐1.24, P < 0.0001, Table S3 [Model S3]).
The mean baseline eGFR was lower in older patients (decrease in baseline eGFR = −0.2 mL/min/1.73 m2 for 5 years age increase above 75 years; 95% CI: −0.3 to −0.1, P < 0.0001), and lower in patients with higher CHA2DS2‐VASc scores (decrease in baseline eGFR = −0.1 for 1‐point increase in CHA2DS2‐VASc; 95% CI: −0.2 to 0.0, P = 0.01) A more pronounced absolute loss of kidney function over time was observed in older patients (increase in the annualized eGFR decline for 5 years increase in age = 0.1 mL/min/1.73 m2/year, 95% CI: 0.1‐0.2, P < 0.0001), in patients with higher CHA2DS2‐VASc scores (increase in the annualized eGFR decline for 1 point increase in CHA2DS2‐VASc = 0.1 mL/min/1.73 m2/year, 95% CI: 0.1‐0.2, P = 0.002), and in patients with higher baseline eGFR measurements (increase in the annualized eGFR decline for 5 mL/min/1.73 m2 increase in the first eGFR measurement = 0.3 mL/min/1.73 m2/year, 95% CI: 0.3‐0.4, P < 0.0001).
Similar results were obtained when fitting this model on the log(eGFR) scale to obtain relative estimates of kidney function decline (Table S3 [Model S4]).
2.2. eGFR trajectory in patients with and without documented exposure to VKA
Patients in the VKA group contributed more longitudinal eGFR measurements (n = 64 690, mean per patient: 9, range: 1‐158) than the group without documented VKA exposure (n = 33 102, mean per patient: 5, range: 1‐197), respectively (P < 0.0001). In univariable analysis, baseline eGFR was similar between patients in the “no VKA” group and the cohort with VKA exposure (estimated eGFR difference for being in the VKA group = −0.35 mL/min/1.73 m2, 95% CI: −0.99 to 0.29, P = 0.279).
Patients exposed to VKA demonstrated a more pronounced absolute eGFR decline from baseline than patients not exposed to VKA (estimated absolute difference 0.31 mL/min/1.73 m2/year, 95% CI: 0.08‐0.54, P = 0.009; Table 2 [Model 1]). In this model, patients exposed to VKA lost 6.0 mL/min/1.73 m2 (or 14.9%) of their eGFR over the 5 years after baseline, whereas the corresponding 5‐year loss in eGFR was 4.5 mL/min/1.73 m2/5 years (or 11.0%) in patients not exposed to VKA, respectively (absolute difference over 5 years = 1.9 mL/min/1.73 m2, P = 0.002, Figure 1A; Table 2 [Model 2], and Figure 1B).
Table 2.
Linear mixed models of kidney function and kidney function trajectory in patients with AF and CKD
| Model | Dependent variable | Independent variables | Coefficient (absolute difference, or % difference) | 95% CI | P |
|---|---|---|---|---|---|
| Model 1 | eGFR | Follow‐up time (per y) | −0.957 | −1.256 to −0.658 | <0.0001 |
| Follow‐up time (per y2) | 0.078 | −0.032 to 0.188 | 0.17 | ||
| Follow‐up time (per y3) | −0.015 | −0.030 to −0.003 | 0.017 | ||
| VKA exposure | −0.352 | −0.989 to 0.285 | 0.28 | ||
| VKA exposure # Follow‐up time | −0.309 | −0.542 to −0.077 | 0.009 | ||
| INTERCEPT | 50.5 | 50.0 to 50.9 | <0.0001 | ||
| Model 2 | log(eGFR) | Follow‐up time (per y) | −2.3% | −3.0 to −1.7 | <0.0001 |
| Follow‐up time (per y2) | 0.7% | −0.2 to 0.3 | 0.59 | ||
| Follow‐up time (per y3) | −0.3% | −0.6 to 0.0 | 0.054 | ||
| VKA exposure | 1.9% | 0.5 to 3.3 | 0.009 | ||
| VKA exposure # Follow‐up time | −0.9% | −1.4 to −0.3 | 0.002 | ||
| INTERCEPT | 3.8 | 3.8 to 3.8 | <0.0001 | ||
| Model 3 | eGFR | Follow‐up time (per y) | −1.012 | −1.313 to −0.711 | <0.0001 |
| Follow‐up time (per y2) | 0.093 | −0.017 to 0.203 | 0.097 | ||
| Follow‐up time (per y3) | −0.016 | −0.029 to −0.003 | 0.013 | ||
| VKA exposure | −1.313 | −1.924 to −0.703 | <0.0001 | ||
| VKA exposure # Follow‐up time | −0.294 | −0.526 to −0.062 | 0.013 | ||
| CHA2DS2‐VASc score (per 1 point increase) | −0.817 | −1.063 to −0.572 | <0.0001 | ||
| CHA2DS2‐VASc score # Follow‐up time | −0.113 | −0.204 to −0.022 | 0.015 | ||
| Age (per 1 y increase) | −0.571 | −0.610 to −0.532 | <0.0001 | ||
| Age # Follow‐up time | 0.007 | −0.008 to 0.022 | 0.36 | ||
| First eGFR # Follow‐up time | −0.001 | −0.007 to 0.005 | 0.81 | ||
| INTERCEPT | 52.6 | 52.2 to 53.1 | <0.0001 | ||
| Model 4 | eGFR | Follow‐up time (per y) | −2.6% | −3.2 to −1.9 | <0.0001 |
| Follow‐up time (per y2) | 0.4% | −0.2 to 0.3 | 0.74 | ||
| Follow‐up time (per y3) | 0.0% | −0.1 to 0.0 | 0.088 | ||
| VKA exposure | 0.0% | −1.3 to 1.3 | 0.99 | ||
| VKA exposure # Follow‐up time | −0.6% | −1.2 to −0.1 | 0.021 | ||
| CHA2DS2‐VASc score (per 1 point increase) | −1.9% | −2.4 to −1.4 | <0.0001 | ||
| CHA2DS2‐VASc score # Follow‐up time | −0.2% | −0.4 to 0.0 | 0.051 | ||
| Age (per 1‐y increase) | −1.0% | −1.1 to −0.9 | <0.0001 | ||
| Age # Follow‐up time | 0.0% | 0.0 to 0.1 | 0.037 | ||
| First eGFR # Follow‐up time | 0.1% | 0.1 to 0.1 | <0.0001 | ||
| INTERCEPT | 3.9 | 3.9 to 3.9 | <0.0001 |
Patients exposed to VKA during follow‐up had significantly faster progression of eGFR decline than patients without such an exposure. Models with “eGFR” as the dependent variable report coefficients on an absolute scale (ie, absolute differences in eGFR), whereas models with “log(eGFR)” as the dependent variables report relative coefficients (ie, relative differences in %). # indicates an interaction. Coefficients for interactions with follow‐up time indicate the association between the respective variable and the change in eGFR over time. All models included three fixed effects for follow‐up time (linear, quadratic, and cubic), a random intercept of the eGFR, and a random slope for the eGFR trajectory.
CI, confidence interval; eGFR, estimated glomerular filtration rate; VKA, vitamin K antagonist.
Figure 1.
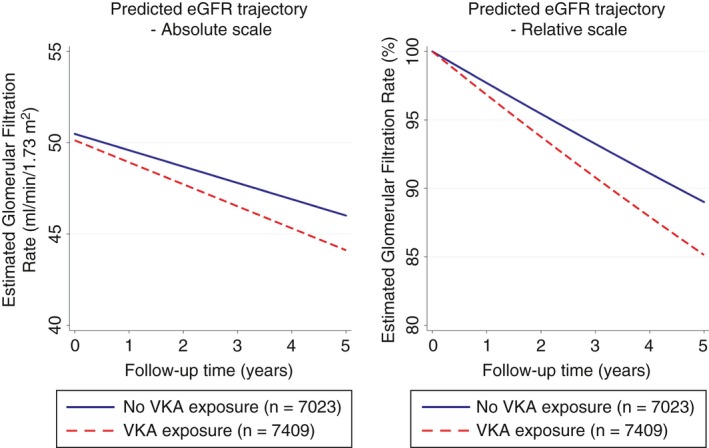
Absolute and relative kidney function trajectory over time in patients with and without a documented exposure to VKA during follow‐up. Panel A reports absolute changes, and Panel B relative changes. Patients with exposure to VKA had significantly increased absolute and relative declines of the eGFR. Quadratic and cubic terms were included in the slope of the eGFR trajectory. eGFR, estimated glomerular filtration rate; VKA, vitamin K antagonist
After multivariable adjustment for age, CHA2DS2‐VASc score, and the first eGFR value obtained after baseline, the association between VKA exposure and a faster decline in kidney function prevailed (absolute difference in annualized eGFR decline = −0.29 mL/min/1.73 m2/year [95% CI: −0.53 to −0.06], P = 0.01, Table 2 [Model 3]; corresponding relative decline = 0.6%/year [95% CI: 0.1‐1.2], P = 0.02, Table 2 [Model 4]).
2.3. Sensitivity analyses
We performed a propensity score (PS) analysis in order to account for putative differences between the two treatment groups potentially not removed by the multivariable analysis. Weighing the data with the inverse‐probability‐of‐treatment‐weights (IPTW) adequately reduced SMDs in variables between the two treatment groups (Table 1). When deciles of the PS were constructed and included as a third level into the linear mixed model (Level 1: eGFR measurements; Level 2: Patients; Level 3: PS deciles), the association between VKA exposure and a faster eGFR decline prevailed, with faster absolute (0.34 mL/min/1.73 m2/year (95% CI: 0.10‐0.57, P = 0.005) and relative annualized decline in eGFR (0.9%/year [95% CI: 0.4‐1.5, P = 0.001]) in the VKA group, respectively.
When only patients with at least 1 year of follow‐up were included in these PS‐adjusted models (n = 8821), the association between VKA exposure and a faster eGFR decline prevailed with faster absolute (0.28 mL/min/1.73 m2/year; 95% CI: 0.04‐0.52, P = 0.022) and relative decline of eGFR (0.7%/year [95% CI: 0.1‐1.3, P = 0.013]) in the VKA group.
In a further sensitivity analysis on the absolute eGFR scale, we did not observe evidence for a three‐way‐interaction between the CHA2DS2‐VASc score, VKA exposure and eGFR decline (P = 0.191), suggesting that the adverse association of VKA with eGFR decline applies to all risk levels of CHA2DS2‐VASc score, which translates into a larger relative impact on the eGFR trajectory in patients with higher CHA2DS2‐VASc scores in whom baseline eGFR is lower. Similarly, although patients with diabetes experienced a significantly faster decline in eGFR than nondiabetics (1.27 vs 0.69 mL/min/1.73 m2/year, P < 0.0001), the adverse association of VKA exposure with eGFR decline applied to both diabetics and nondiabetics (change in the eGFR trajectory with VKA exposure for patients that are documented diabetics = 0.20 mL/min/1.73 m2/year, 95% CI: −0.27 to −0.67, P = 0.395).
To gauge the sensitivity of our results towards inclusion of “prevalent” VKA users, we performed an analysis excluding all patients who had received at least one VKA prescription before the baseline date. In this subcohort of 8448 patients who were either never exposed to VKA at all (n = 5850) or only after baseline but not before baseline (n = 2598, ie, true “incident” VKA users), the adverse association between VKA exposure and an accelerated kidney function decline became more pronounced (absolute increase in eGFR decline for being exposed to VKA = 0.43 mL/min/1.73 m2/year, 95% CI: 0.15‐0.70, P = 0.002).
Next, we constructed an alternative VKA exposure variable, also assigning patients with at least one VKA prescription in the month preceding the baseline date to the VKA group. Here, a further 309 patients (2.1%) were reassigned to the VKA group. In this sensitivity analysis, the adverse association between VKA exposure and an accelerated kidney function decline prevailed on both an absolute (Table S4 [Model S5]) and relative scale (Table S4 [Model S6]). In a further sensitivity analysis, Models 3 and 4 of Table 2 were re‐fitted without the baseline eGFR as a predictor of the eGFR trajectory. Also here, the previously observed associations between VKA exposure, higher age, a higher CHA2DS2‐VASc score and accelerated kidney function decline remained highly similar with respect to magnitude and strength of association (Table S5 [Models S7 and 8]).
Out of the 7409 patients in the group with VKA exposure, 1775 (24.0%) experienced a 30% decline in eGFR during follow‐up at least once, as compared to 1012 (14.4%) out of 7023 patients in the group without documented VKA exposure, respectively (P < 0.0001). The median time‐to‐first 30% decline in eGFR was 6.7 years (95% CI: 6.2‐not reached). Five‐year 1‐Kaplan‐Meier risks of a 30% decline in eGFR were 41.5% in the VKA group, and 37.0% in the group without documented VKA exposure, respectively (log‐rank P < 0.0001, Figure 2). In univariable Cox regression, patient with documented VKA exposure experienced a 15% higher relative rate of developing a 30% decline in eGFR (hazard ratio [HR] = 1.15, 95% CI: 1.06‐1.24, P = 0.001), and this association prevailed upon multivariable adjustment for the CHA2DS2‐VASc score and the baseline eGFR (Table S5). When weighting this time‐to‐event data for the propensity score (using the inverse‐probability‐of‐treatment‐weight [IPTW]), the association between VKA exposure and a higher risk of this endpoint also prevailed (IPTW‐adjusted HR for VKA exposure = 1.20, 1.11‐1.30, P < 0.0001). Finally, sensitivity analyses using various specifications of VKA exposure as a time‐varying variable confirmed an adverse relationship between VKA and CKD progression (Table S5, Figure S4).
Figure 2.
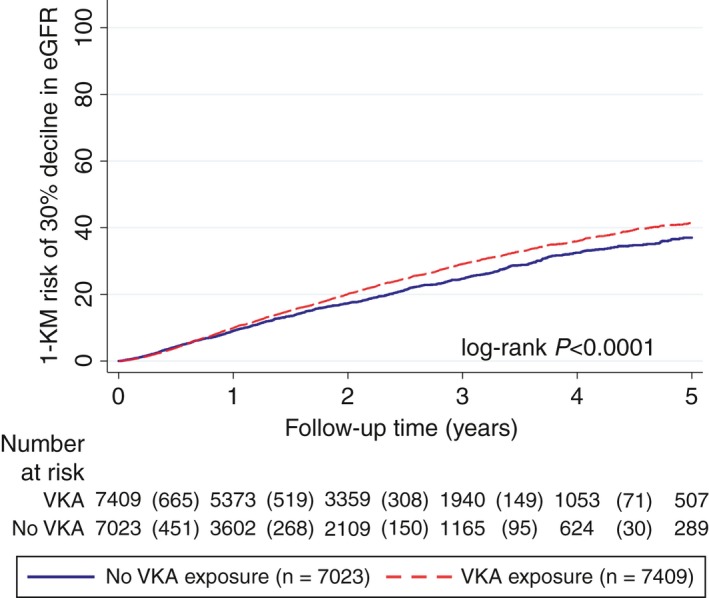
Higher risk of experiencing a 30% relative decline in eGFR during follow‐up in patients with a documented exposure to VKA.37 Curves were estimated with a 1‐Kaplan‐Meier estimator, and compared with a log‐rank test. Patients were censored at the last database date, which was the last date where either a diagnosis code, a prescription, or an eGFR measurement was recorded in IMS‐DA. Two hundred twelve patients in the group without documented VKA exposure did not have a follow‐up eGFR after baseline, and where thus censored at the day after baseline. The risk table reports the number of patients included in the respective study group 1, 2, 3, 4, and 5 years after baseline. The numbers in round brackets between these yearly intervals represent the number of patients who developed a 30% decline in eGFR within this interval. Note that the 1‐Kaplan‐Meier risks in this figure are higher than the crude proportions of patients with a 30% eGFR decline reported in the results section due to competing risk of death which leads to overestimation of event risks by the Kaplan‐Meier method.32 eGFR, estimated glomerular filtration rate; VKA, vitamin K antagonist
3. DISCUSSION
In this retrospective analysis of prospectively collected routine healthcare data in the primary care setting, we examined the impact of exposure to VKA on kidney function of 14 432 patients with AF and CKD stage 3/4. We found that patients with a documented exposure to VKA experienced a significantly accelerated decline in the eGFR over time as compared to patients without such an exposure. This finding was independent of age and comorbidities as summarized by the CHA2DS2‐VASc score, and baseline eGFR.
Our study was motivated by previous reports which have implicated VKA treatment in increased atherosclerotic calcification, plaque formation, and renal microvasculature damage.6, 8, 16 Although robust clinical data on this “renovascular calcification hypothesis” are limited, its implications are considerable because millions of patients globally are treated with VKA for prevention of stroke/systemic embolism in atrial fibrillation.2, 17 Our clinical data support this hypothesis by showing a statistically significant excess in kidney function loss in VKA patients, although the absolute impact was only small. Given that VKAs are usually given as a life‐long treatment for stroke prevention in AF, small adverse effects of VKA on kidney function may nonetheless cumulate towards clinically relevant dimensions over long exposure periods in the global population of patients with AF and CKD. Furthermore, VKA therapy in the real‐world setting is known to be associated with a high discontinuation rate and a trend towards insufficient dosing,18 effects that may lead to an underestimation of the harm in our analysis, since our observational period will include periods of underexposure to VKA from temporary or permanent treatment discontinuation and from using VKA in subtherapeutic dosages (INR < 2.0); thus, the adverse effect of VKA on kidney function more pronounced in long‐term exposure, which often includes phases of VKA overdosing.19
We addressed our study question using prospectively collected data from outpatients treated in the German primary care setting but such data are subject to a relevant selection bias.20 Nevertheless, in the absence of dedicated randomized trials, post‐hoc analyses from RCTs and large observational studies making using of advanced comparative effectiveness research methods such as PS may represent the best evidence available for generating new insights into this subject and for providing some guidance for clinical practice. Importantly, key aspects of our dataset are highly consistent with published data. For example, the absolute eGFR decline in our study population was approximately 1 mL/min/1.73 m2/year, which agrees with two large longitudinal kidney function trajectory studies.9, 21 Moreover, the relative eGFR decline of approximately 10%‐15% over 5 years is also in line with two recently published cohorts.15 In addition, the fact that only 50% of our study cohort had a documented exposure to VKA despite high CHA2DS2‐VASc scores is reflective of the well‐known discrepancy between guideline‐recommended anticoagulation for stroke prevention and implementation of this recommendation in elderly patients and real‐world settings.22 Finally, the association between VKA exposure and greater kidney disease progression was independent of comorbidities and also prevailed upon adjustment for a propensity score which reduced imbalances in baseline variables between the two treatment groups to negligible levels.
Patients with higher CHA2DS2‐VASc scores had a significantly faster eGFR decline, which was previously demonstrated by our group23 and illustrates the known impact of comorbidity on kidney disease progression. An important finding of the current study with respect to VKA and the CHA2DS2‐VASc score was that we did not observe an interaction between CHA2DS2‐VASc score and the magnitude of the adverse VKA‐kidney decline association. This suggests that VKA exposure is affecting kidney disease progression to the same absolute degree across all CHA2DS2‐VASc score levels. Considering that patients with higher CHA2DS2‐VASc scores have a lower eGFR to start with,23 such a similar absolute impact of VKA exposure entails a higher relative impact of VKA exposure on the eGFR trajectory in patients with higher CHA2DS2‐VASc scores. This may also explain, why warfarin was shown to have a negative effect on kidney function compared to rivaroxaban in ROCKET‐AF (mean CHADS2‐score 3.5) but not compared to apixaban in ARISTOTLE (mean CHADS2‐score 2.1).10, 24 On the other hand, it is also possible that different DOACs as warfarin comparators may have different effects on renal function.25, 26, 27 However, whether an excess decline in kidney function from VKA also leads to an increased risk of clinical outcomes such as stroke, bleeding, hospitalization, or death in AF patients with CKD cannot be answered by our study. In this context, it is important to highlight that warfarin has also been shown to prevent myocardial infarction in randomized controlled trials. It appears thus possible that a positive antithrombotic effect in different vascular beds might outweigh any negative effect of VKA on small vessel calcification and renal decline.
The eGFR trajectory as expressed by the annualized change in the eGFR was the primary analysis in this study but our findings were robust when we looked at other kidney endpoints proposed in the literature. Moreover, because VKA exposure is a dynamic rather than static variable, we also performed several sensitivity analyses that treated VKA exposure as a time‐varying variable28 which confirmed an impact from VKA on adverse kidney outcome. On the other hand, we provided estimates of eGFR trajectory for a horizon of 5 years after baseline, which may be somewhat extrapolated given the median follow‐up of our cohort was only 1.44 years.
Aspirin use was more frequent in the “no VKA” group compared to the VKA group and it seems reasonable to speculate that this difference may be explained by the fact that ASA has been regarded as a treatment alternative in AF patients in the past. Although a direct (positive or negative) impact of aspirin on kidney function has never been clearly demonstrated this difference in baseline characteristics can potentially be an important confounder. On the other hand, a reasonably large RCT of high risk patients (2173 Japanese patients with diabetes type II) did not indicate that long‐term ASA exposure (>8 years) had any effect on eGFR compared to no treatment.29
Some limitations of our study should be discussed. It is in the nature of retrospective administrative database analyses that confounding by indication is likely, since certain patient characteristics may drive prescribers towards or away from certain treatments or may impact dosing. In the present study, it cannot be fully excluded that prescribers anticipated renal effects when prescribing or withholding VKAs, in which case the patient selection itself could have had an important impact on our outcome analyses, because adjustments could only correct for measured confounding. On the other hand, the discussion of VKA‐related kidney injury has only started in recent years, as indicated by the work of Brodsky and coworkers in 2010 and 201119, 30 and the post‐hoc analyses of the DOAC trials that were published between 2015 and 2016.9, 10, 24 Therefore, it seems unlikely that such concerns had a major impact on treatment choices in our study population, which was treated between 2009 and 2015.
Due to the association between higher CHA2DS2‐VASc scores and mortality, our patient population will be “enriched” over time with patients that have a more favorable survival experience (“informative censoring”).13, 31 Ignoring competing mortality will also lead to an overestimation of 30% eGFR decline risks in our time‐to‐event analysis.32 Unfortunately, we could not take this time‐dependent and informative censoring into account since data on mortality, stroke/systemic embolism, or bleeding were not available. GFR values were supplied by IMS Germany without further details on the methods or formulae of estimation, which may add imprecision to our analyses.33 Nonetheless, we can carefully speculate that this potential heterogeneity in eGFR estimation methods in our dataset would likely only increase measurement error, and thus increase the width of confidence intervals of the VKA‐eGFR association (ie, a “conservative” bias in favor of the null hypothesis). Another limitation concerning potential informative censoring is that patients in the VKA group contributed significantly more eGFR records over time than patients without documented VKA exposure, which is consistent with the concept that patients on VKA were more frequently seen by their physicians and/or had their eGFR assessed more often. The impact of this between‐group difference on the results of our analysis remain uncertain for the time being. Patients with exposure to DOAC were excluded following prespecified exclusion criteria. This was necessary to restrict our analysis to truly “untreated” and “VKA exposed” patients, since the impact of DOAC on renal function is insufficiently understood at present and could have biased our analysis. However, such analyses are equally needed in the future. Prescription data on drugs that can impact kidney function, such as angiotensin‐converting‐enzyme inhibitors (ACE‐I),34 angiotensin‐1‐receptor‐blockers (ARBs),35 or nonsteroidal anti‐inflammatory drugs,36 were not available. Importantly, considering that VKA exposure may at least partly reflect an individual physician's “propensity to treat,” patients with VKA may have been more likely to also receive ACE‐Is and ARBs, which again would lead to an underestimation of the adverse VKA‐eGFR association. Assignment to the two treatment groups was ascertained retrospectively from prescription data, which has potential for misspecification of anticoagulation treatment. We addressed this issue by using the more cautious term of “VKA exposure” instead of “VKA treatment.” Moreover, we performed (A) two sensitivity analyses evaluating “incident” VKA users and VKA “never users” only or including patients who had a documented VKA prescription in the month before the baseline date to the “incident” VKA user group and the association between VKA exposure and an accelerated kidney function decline was even more pronounced in “incident users,” which adds another layer of support for the concept that VKAs impair kidney function over time, and (B) two sensitivity analyses treating VKA exposure as a time‐varying variable in which the previously observed associations also prevailed. The prespecified in‐ and exclusion criteria of our study selected 14 432 out of a total of 37 476 patients for analysis. Notably, summary statistics of the included and excluded patients were highly similar (Table S1), which suggests that our selection criteria did not introduce material selection bias. Nonetheless, we cannot rule out that our strategy of performing a so‐called complete case analysis may have led to bias in terms of selecting patients with observed covariables who may differ in terms of their outcome from patients without observed covariables. However, we opted for a complete case analysis because adding specific missing data techniques (such as multiple imputation) may have added another layer of complexity to the already complex analysis involving mixed modeling and propensity score analysis.
Data to specifically quantify renovascular calcification, such as kidney biopsies or computed tomography scans, were not available to us, and we hence cannot prove with certainty that renovascular calcification is the mechanism by which VKA exposure associates with an accelerated eGFR decline. Moreover, because by design we only included patients with stage 3/4 CKD, our results do not automatically generalize to other AF subpopulations. Because there is no a priori reason to assume that a potentially adverse association between VKA and eGFR decline is restricted to patients with stage 3/4 CKD, future studies should investigate the association between VKA and kidney function also in other AF or CKD patient groups. Data on the INR which may have allowed for a more refined analysis of VKA “dose” and kidney outcomes were not available to us. This is an important limitation since findings from case series suggest that over‐anticoagulation with warfarin, leading to INR values >3 may be associated with a faster decline in renal function.19, 30 Similarly, data on anti‐platelet agent comedication beyond aspirin (eg, clopidogrel or dipyridamole) which may have yielded a refined propensity score model were not available to us. Finally, the median follow‐up was significantly longer in patients exposed to VKA than in patients without documented exposure to VKA. This potential bias was addressed in a sensitivity analysis were we included only those patients who had at least 1 year of follow‐up. The association between VKA exposure and greater kidney disease progression prevailed in this sensitivity analysis. Considering the large sample size of the study, the large proportion of patients with long‐term follow‐up (25% had follow‐up intervals of at least 2.8% and 10% had follow‐up intervals of at least 4.3 years), and the robustness of our results towards multiple sensitivity and propensity score analyses, our eGFR trajectory data can be regarded as robust.
4. CONCLUSION
This retrospective analysis of prospectively collected routine healthcare data in the primary care setting supports the hypothesis that exposure to VKA has an adverse impact on kidney function in patients with AF and stage 3/4 CKD, leading to a more pronounced decline in absolute and relative eGFR values. However, the absolute magnitude of this association was modest. Our data confirm and extend previous observations in the real‐world setting on the “renovascular calcification hypothesis” within the largest cohort study on this topic to date but data on the impact of long‐term DOAC exposure on renal function are equally needed.
RELATIONSHIP DISCLOSURES
FP has received honoraria for lectures and consultancy from Eli Lilly, Daiichi Sankyo, Roche, and MSD Oncology. CA has received honoraria for lectures from Sanofi, Pfizer/BMS, Daiichi Sankyo, Boehringer Ingelheim, and Bayer. RK has received honoraria for consultancy, lectures, and support for research from AstraZeneca, Bayer AG, Berlin‐Chemie Menarini, Daiichi Sankyo, Sanofi, and Servier. JBW has received personal honoraria and institutional research support from Bayer, Boehringer‐Ingelheim, Daiichi‐Sankyo, Janssen, Pfizer, and Portola.
AUTHOR CONTRIBUTIONS
Conceived and designed the study: JBW, RK, FP. Performed statistical analyses: FP. Interpreted the results: all authors. Wrote the first draft of the manuscript: FP, JBW. Critical revision of the first draft of the manuscript: all authors. Contributed to the writing of the final manuscript: all authors. Agree with the manuscript's results and conclusions: all authors. ICMJE criteria for authorship read and met: all authors.
Supporting information
ACKNOWLEDGMENTS
We are very grateful to Dr. Karen Thomitzek from Bayer Pharma for supporting and handling the Investigator‐Initiated‐Research application with Bayer Pharma AG.
Posch F, Ay C, Stöger H, Kreutz R, Beyer‐Westendorf J. Exposure to vitamin k antagonists and kidney function decline in patients with atrial fibrillation and chronic kidney disease. Res Pract Thromb Haemost. 2019;3:207–216. 10.1002/rth2.12189
Funding information
Acquisition of data for this study was funded by an Investigator‐Initiated‐Research grant of Bayer Pharma AG (grant number: IIR‐DE‐2016‐3010) to the Gesellschaft für Wissens‐ und Technologietransfer der TU Dresden (GWT‐TUD GmbH) after review of the study protocol by the internal grant review board of Bayer Pharma AG. Bayer Pharma AG approved the manuscript prior submission but had no other role in the analysis and interpretation of the study.
REFERENCES
- 1. Boriani G, Savelieva I, Dan GA, Deharo JC, Ferro C, Israel CW, et al. Chronic kidney disease in patients with cardiac rhythm disturbances or implantable electrical devices: clinical significance and implications for decision making‐a position paper of the European Heart Rhythm Association endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace. 2015;17:1169–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- 3. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- 4. Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–69. [DOI] [PubMed] [Google Scholar]
- 5. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al. Updated European Heart Rhythm Association practical guide on the use of non‐vitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2015;17:1467–507. [DOI] [PubMed] [Google Scholar]
- 6. Schurgers LJ, Joosen IA, Laufer EM, Chatrou ML, Herfs M, Winkens MH, et al. Vitamin K‐antagonists accelerate atherosclerotic calcification and induce a vulnerable plaque phenotype. PLoS ONE. 2012;7:e43229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narasimha Krishna V, Warnock DG, Saxena N, Rizk DV. Oral anticoagulants and risk of nephropathy. Drug Saf. 2015;38:527–33. [DOI] [PubMed] [Google Scholar]
- 8. Cam G, Kwetcheu AT, Vigneau C, Siohan P, Queffeulou G, Gatault P, et al. Acute and chronic nephropathy induced by fluindione must be addressed. Nephrol Dial Transplant. 2012;27:1554–8. [DOI] [PubMed] [Google Scholar]
- 9. Bohm M, Ezekowitz MD, Connolly SJ, Eikelboom JW, Hohnloser SH, Reilly PA, et al. Changes in renal function in patients with atrial fibrillation: an analysis from the RE‐LY trial. J Am Coll Cardiol. 2015;65:2481–93. [DOI] [PubMed] [Google Scholar]
- 10. Fordyce CB, Hellkamp AS, Lokhnygina Y, Lindner SM, Piccini JP, Becker RC, et al. On‐treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: insights from ROCKET AF. Circulation. 2016;134:37–47. [DOI] [PubMed] [Google Scholar]
- 11. Voskamp PW, Dekker FW, Rookmaaker MB, Verhaar MC, Bos WJW, van Diepen M, et al. Vitamin K antagonist use and renal function in pre‐dialysis patients. Clin Epidemiol. 2018;10:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Becher H, Kostev K, Schroder‐Bernhardi D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47:617–26. [DOI] [PubMed] [Google Scholar]
- 13. Shou H, Hsu JY, Xie D, Yang W, Roy J, Anderson AH, et al. Analytic considerations for repeated measures of eGFR in cohort studies of CKD. Clin J Am Soc Nephrol. 2017;12(8):CJN.11311116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rabe‐Hesketh SS. Multilevel and longitudinal modeling using stata. College Station, TX: Stata Press; 2005. [Google Scholar]
- 15. Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rattazzi M, Faggin E, Bertacco E, Nardin C, Pagliani L, Plebani M, et al. Warfarin, but not rivaroxaban, promotes the calcification of the aortic valve in ApoE‐/‐ mice. Cardiovasc Ther. 2018;30:1755–5922. [DOI] [PubMed] [Google Scholar]
- 17. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr. , et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gallagher AM, Rietbrock S, Plumb J, van Staa TP. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost. 2008;6:1500–6. [DOI] [PubMed] [Google Scholar]
- 19. Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, et al. Warfarin‐related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beyer‐Westendorf J, Camm AJ, Coleman CI, Tamayo S. Rivaroxaban real‐world evidence: validating safety and effectiveness in clinical practice. Thromb Haemost. 2016;116(suppl 2):S13–23. [DOI] [PubMed] [Google Scholar]
- 21. Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, et al. Progression of kidney dysfunction in the community‐dwelling elderly. Kidney Int. 2006;69:2155–61. [DOI] [PubMed] [Google Scholar]
- 22. De Breucker S, Herzog G, Pepersack T. Could geriatric characteristics explain the under‐prescription of anticoagulation therapy for older patients admitted with atrial fibrillation? A retrospective observational study Drugs Aging. 2010;27:807–13. [DOI] [PubMed] [Google Scholar]
- 23. Beyer‐Westendorf J, Kreutz R, Posch F, Ay C. The CHA2DS2‐VASc score strongly correlates with glomerular filtration rate and predicts renal function decline over time in elderly patients with atrial fibrillation and chronic kidney disease. Int J Cardiol. 2018;253:71–7. [DOI] [PubMed] [Google Scholar]
- 24. Hijazi Z, Hohnloser SH, Andersson U, Alexander JH, Hanna M, Keltai M, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1:451–60. [DOI] [PubMed] [Google Scholar]
- 25. Yao X, Tangri N, Gersh BJ, Sangaralingham LR, Shah ND, Nath KA, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70:2621–32. [DOI] [PubMed] [Google Scholar]
- 26. Shin JI, Luo S, Alexander GC, Inker LA, Coresh J, Chang AR, et al. Direct oral anticoagulants and risk of acute kidney injury in patients with atrial fibrillation. J Am Coll Cardiol. 2018;71:251–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan YH, Yeh YH, See LC, Wang CL, Chang SH, Lee HF, et al. Acute kidney injury in asians with atrial fibrillation treated with dabigatran or warfarin. J Am Coll Cardiol. 2016;68:2272–83. [DOI] [PubMed] [Google Scholar]
- 28. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi‐state models. Stat Med. 2007;26:2389–430. [DOI] [PubMed] [Google Scholar]
- 29. Okada S, Morimoto T, Ogawa H, Sakuma M, Soejima H, Nakayama M, et al. Is long‐term low‐dose aspirin therapy associated with renal dysfunction in patients with type 2 diabetes? JPAD2 cohort study. PLoS ONE. 2016;11:e0147635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, et al. Warfarin therapy that results in an International Normalization Ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract. 2010;115:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crowther MJ, Andersson TM, Lambert PC, Abrams KR, Humphreys K. Joint modelling of longitudinal and survival data: incorporating delayed entry and an assessment of model misspecification. Stat Med. 2016;35:1193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ay C, Posch F, Kaider A, Zielinski C, Pabinger I. Estimating risk of venous thromboembolism in patients with cancer in the presence of competing mortality. J Thromb Haemost. 2015;13:390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD‐EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–62. [DOI] [PubMed] [Google Scholar]
- 35. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. [DOI] [PubMed] [Google Scholar]
- 36. Whelton A, Hamilton CW. Nonsteroidal anti‐inflammatory drugs: effects on kidney function. J Clin Pharmacol. 1991;31:588–98. [DOI] [PubMed] [Google Scholar]
- 37. Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64:821–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


