Abstract
Background
Oral Submucous fibrosis (OSF) is a chronic inflammatory mucosal disease of unknown etiology. Statistics show cases of OSF which has a high rate of overall prevalence and increase the chance of malignant transformation. As we know malignant cells is situated in a very complex microenvironment with altered metabolic pathway including intermediates which participate in oxidative stress process which enhances metabolic rewiring and promotes tumor progression. This study aims to evaluate the tumor microenvironment and their role in metabolic reprogramming.
Methods
This study was conducted on the serum sample of OSF (n = 20) compared to the healthy group (n = 20) using ELISA. The serum levels of intermediate by-products of metabolic pathway and oxidative stress induced biomolecular damage products were determined. The sensitivity of results was analyzed by correlating it with markers of metabolic status (Glucose, Total cholesterol, Total protein).
Results
Metabolic pathway intermediates molecules like Fatty Acids (FAA), Ascorbic acid, Citrate, Oxaloacetate (OAA), levels were significantly high in the serum of OSF cases. This indicated that intermediates act as a metabolic switch that drives cells to adapt malignant transformation pathway. Markers related to oxidative DNA damage (8-hydroxy-2' -deoxyguanosine), Oxidative lipid peroxidation (8-epi-Prostaglandin F2α), and Protein carbonyl were significantly up-regulated. This significant increase in oxidative stress marker revealed the reprogramming of the metabolic pathway for fulfilling the nutritional requirement of cancer cells. A further significant correlation was observed with metabolic products confirmed altered metabolic status.
Conclusion
Our findings could identify the differentiating intermediate pathway metabolites and oxidative damage to biomolecules that are leading to rewiring of metabolism in the OSF group. Findings described in the study can be helpful to explain further the molecular aspects that lead to the progression of OSF towards carcinogenesis.
Keywords: Cancer research, Systems biology
1. Introduction
Oxidative stress (OS) renders a very important pathological role in the development of various diseases including oral cancer. OS is now considered as the outcome of an imbalance between metabolic end products/intermediates, oxidants and derivatives production [1]. A very complex microenvironment is found in cancer cells with many small intermediate metabolites that participate in enhancing OS to promote cancer development. OS is always co-related with all stages of cancer, from pre-cancerous to cancerous [2]. Oral submucous fibrosis (OSF) is a disease with increased of malignant conversion in more than 80 percent cases. Since the disease has been described, the malignant transformation of OSF has been linked with a histopathological aspect i.e (abnormal collagen metabolism), molecular aspect (aberrant expression of extracellular matrix (ECM)) as well as altered metabolic pathways. Studies correlated the pathogenesis of disease with extracellular matrix and fibroblast changes, role of trace metals, immune system, antioxidant status, changes in gene expression Still, the pathogenesis of this disease is still not clear and is believed to be multifactorial in origin [3]. According to studies reported biological matrix is constantly under OS arising from exogenous factors (e.g., ultraviolet rays) and endogenous factors (at the cellular level where mitochondria are involved) [4]. Research literature report that hypoxia and OS influence metabolic reprogramming of cancer cells in the tumor microenvironment [5]. Many changes on enzymatic and intermediate levels are responsible for the fulfillment of nutritional demand of cancer cells for proliferation, which may directly affect, regulation, and activity of essential components of metabolic pathways and exert an impact on metabolism, on glucose, proteins, and lipids [6]. Cancer cells show a wide range of metabolic profiles, [7]. Many studies have reported that OS plays vital role from a biological point of view and it is correlated to a wide variety of human diseases including cancer. The cancer cells unveiling the warburg effect leads to high level of lactate which causes acidosis in cancer microenvironment. This increase metabolic activity leads to over production of ROS. Therefore, the increase ROS level acts on several active metabolites that regulate a wide range of cellular processes. Keeping all this in mind studies have reported that metabolic changes and the effect of reactive oxygen species (ROS) are intertwined in cancer [8, 9].
Many kinds of literature define the role of ROS in the cellular metabolic pathway and define the modification of biological macromolecules including carbohydrates, lipids, and proteins. ROS (free radicles) are formed due to a disturbance in by-products and intermediates molecules of various biological cycle. Due to the presence of unpaired electron, they are unstable and highly reactive which affects the function of core metabolic enzymes when they react easily. Increased number of reported evidence suggests an interconnection between cancer metabolic pathway, intermediates, and OS [10, 11]. We first sought to investigate the oxidative damage status in metabolic pathway intermediates molecules (Fatty acids (FAA), Citrate, Ascorbic acid (vitamin C)) in OSF. These intermediate molecules are universally found in the body which plays an important role in cellular synthesis and energy metabolism [12]. Next, we focused on deciphering the oxidative damage caused to biomolecules, i.e. nucleic acids (8-hydroxydeoxyguanosine (8-OHdG)), proteins (Protein carbonyl), and lipids (8-iso-Prostaglandin F2α) and its interrelationship with metabolic markers (Glucose, total cholesterol, and total protein). Biomolecules are the most significant common target of the oxidative attack, which leads to mutations in DNA, Lipid and Protein [13, 14]. This study has first documented, oxidative stress-induced alteration in serum metabolic intermediates and biomolecules of the major metabolic pathway in OSF has been investigated and compared. The preliminary findings of oxidative stress markers and intermediates molecules change status OSF at a very early stage will provide valuable information and can be perhaps the most useful approach to the development of diagnostic, screening and therapeutic techniques.
2. Materials & methods
2.1. Subject selection and sample preparation
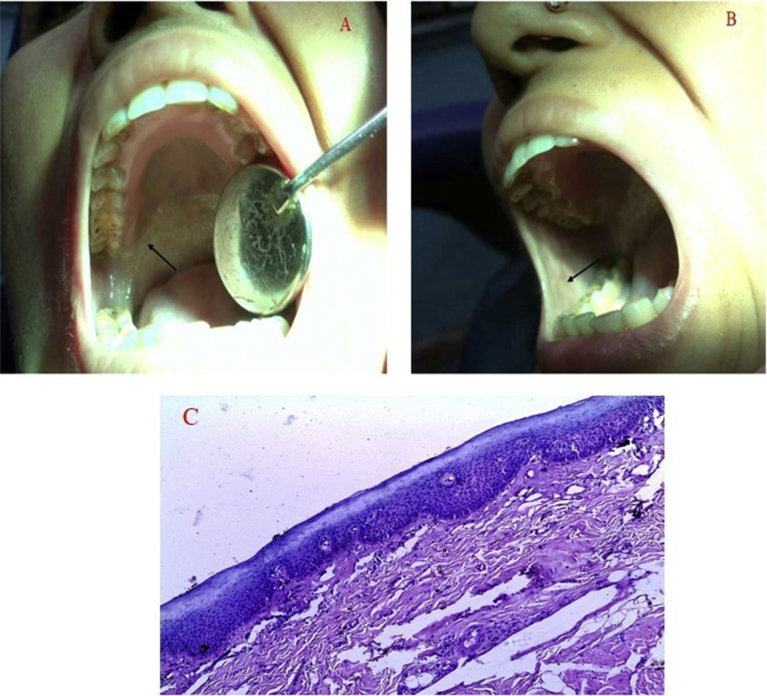
Subjects attending Barasat Cancer Research Hospital, Kolkata, India were enrolled in the study. A total of 40 subjects were enrolled in the study and categorized into two groups; (Oral submucous fibrosis with dysplasia (OSFW) (Group I, n = 20, Fig. 1(A–C)) and controls (Group II, n = 20). Subjects with an abnormality were excluded in the study. Institute ethics committee of Indian Institute of Technology, Kharagpur approved this case-control study [IIT/SRIC/SAO/2015]. Informed consent was obtained from all participants recruited in the study. The experiment was carried out by the approved Indian council of medical research guidelines and Helsinki declaration.
Fig. 1.
Photographs showing OSF case (A) involving soft palate (B) involving buccal mucosa (C) depicting H&E staining (10X) in tissue: (C) oral submucous fibrosis with dysplasia (OSFW).
2.2. Haematoxylin-eosin Staining (H& E)
Biopsy tissues were stained by H&E staining for confirmation. All tissue samples were snap-frozen within 30 minutes of biopsy and stored at -80 °C for further analysis. All the specimens were fixed in 10 % neutral buffered formalin for 24 hrs. 3 μm thick tissue sections were obtained from paraffin-embedded tissue using Microtome. Followed by deparaffinization in xylene and hydrated through 100% ethanol, and washed thoroughly in deionized water for 10 minutes. The sections were stained according to the methods specific for H & E staining respectively [6] (Fig. 1).
2.3. Enzyme-linked immunosorbent assays (ELISA)
The level of oxidative stress parameters in serum was analyzed by Enzyme-linked immunosorbent assay (ELISA). ELISA is an antigen-antibody based assay designed for identifying and quantifying biomolecules. In ELISA, an antigen is coated to microplate wells and then react to make a compound with an antibody that is linked with an enzyme. Then the antigen-antibody complex is detected by an enzyme-linked secondary antibody which is detected through colorimetric assay after incubation with a substrate [15]. The level of OS parameters in serum was analyzed by Enzyme-linked immunosorbent assay (ELISA). The concentrations of in serum of FAA [EnzyChromTM Free Fatty Acid Assay Kit (EFFA-100)], Ascorbic acid [EnzyChromTM Ascorbic Acid Assay Kit (EASC-100)], Citrate kits [EnzyChromTM Citrate Assay Kit (ECIT-100) ], Oxaloacetate (OAA) [EnzyChromTM Oxaloacetate Assay Kit (EOAA-100) ], Oxidative DNA damage (8-OHdG) [OxiSelect™, Cat no, STA-320-T], Oxidative lipid peroxidation (8-epi-PGF2α) [OxiSelect™, Cat no, STA-337], Protein carbonyl [OxiSelect™, Cat no,STA-310 ] were measured by commercially available ELISA kits and protocol mentioned accordingly. Data were analyzed concerning the standard curve of individual molecules. Estimation of Blood Biochemical Parameters Serum glucose, total protein, total cholesterol [TC ] concentrations was measured with commercially available enzymatic colorimetric diagnostic kits [Siemens, Gujrat, India; Coral clinical systems, Goa, India; Span Diagnostics Ltd., Surat, India]
2.4. Statistical analysis
Statistical analysis was performed using SPSS software (Version 11; SPSS, Inc., Chicago, IL). Data were analyzed by t-Test. Data are expressed as mean ± SEM for two groups. Statistical significance was determined at P < 0.05.
3. Results
3.1. Validation of disease through haematoxylin and eosin staining
H&E staining was done for confirmation of the disease. The histological changes (rete-ridges, inflammatory cells, the appearance of the epithelium) was considered by the pathologist for the presence of the disease. Fig. 1A and B depicts the photo of the OSF case included in the study. Fig. 1C depicted the H&E stained features of OSFW case with no reti-ridges, atrophic epithelium.
3.2. Oxidative damage status in metabolic pathway intermediates molecules
Molecular pathway Intermediates are small molecules which are precursors or metabolites of biologically significant molecules of metabolic pathways [16]. It is now increasingly reported that molecular pathway intermediates play a vital role, besides their function (anabolic and catabolic), in the regulation of enzymes which effects on the cellular redox state or rewiring of the metabolic pathway in a disease like cancer [16]. So in this present study effect of oxidative stress is studied on the metabolic intermediates.
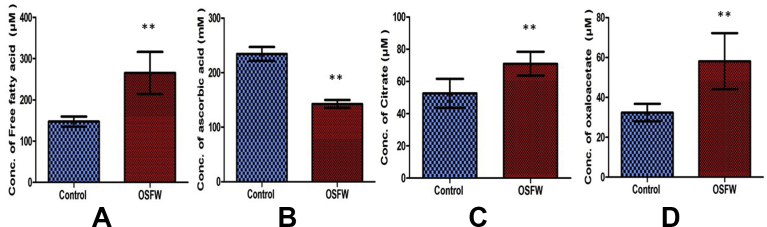
FAA play significant roles in energy metabolism and cellular synthesis, are implicated in a wide range of disorders including various types of cancers. Many kinds of literature reported the involvement of the FAA in oxidative stress [17, 18]. Moreover, the FAA is an important constituent of cellular membranes [17]. The cellular membrane, consists of FAA which plays an anti-inflammatory, indirectly an antioxidant role favoring physiological defense processes against ROS [19]. Fig. 2 A, Table 1 shows that FFA levels are significantly higher in the OSF group [265 ± 51.0] in comparison to the control group [147 ± 12.0]. Ascorbic acid an antioxidant plays a major role by protecting the cell from oxidative damages [20]. Fig. 2B, Table 1 depicts that the ascorbic acid level is low in the OSFW group [142 ± 7.2] in comparison to control [234 ± 12.8].
Fig. 2.
Comparison of serum levels of A) Free fatty acid, B) Ascorbic acid C) Citrate and D) oxaloacetate in OSFW and controls; Mean ± SEM; *p ≤ 0.001.
Table 1.
Comparison of serum levels of oxidative stress parameters in control and OSF [Results are expressed as Mean ± SEM].
| Parameters | Control (Mean ± SEM) | OSFW (Mean ± SEM | p-value |
|---|---|---|---|
| Levels of oxidative stress-induced metabolic intermediates in serum | |||
| Free Fatty Acid [μM] | 147 ± 12.0 | 265 ± 51.0 | *0.003 |
| Ascorbic Acid [mM] | 234 ± 12.8 | 142 ± 7.2 | *0.0001 |
| Citrate [μM] | 52 ± 8.9 | 70 ± 7.4 | 0.160 |
| Oxaloacetate [μM] | 32 ± 4.3 | 58 ± 14.1 | *0.0001 |
| Levels of oxidative stress-induced metabolic end products in serum | |||
| 8- Hydroxydeoxyguanosine (8OHdG) [ng/ml] | 12 ± 2.6 | 26 ± 3.0 | *0.001 |
| 8-iso-Prostaglandin F2α [pg/ml] | 270 ± 29.2 | 355 ± 9.0 | *0.001 |
| Protein carbonyl [nm/mg] | 7.4 ± 1.6 | 15.0 ± 1.3 | *0.001 |
Many studies have reported citrate pathway activates oxygen radical production during inflammation. The citrate is an important intermediate metabolite and a signal molecule regulating energy metabolism and the functional state of cells [21]. Fig. 2C, Table 1 findings show that serum citrate level is significantly higher in the OSF group [70 ± 7.4] than comparison to control [52 ± 8.9]. Hence our preliminary data can be considered as the involvement of citrate pathway activation and oxidative stress. Oxaloacetate (OAA) is an important intermediate molecule in the citric acid cycle which participates in the metabolic cycle. OAA is formed by the oxidation of malate, by deamidation of aspartate or by condensation of CO2 with pyruvate or phosphoenolpyruvate. It is involved in fatty acid synthesis; amino acid synthesis plays an important role as an intermediate in the cycle. Fig. 2D, Table 1 depicts that serum OAA level in the OSFW group [58 ± 14.1] is significantly higher in comparison to control [32 ± 4.3].
3.3. Oxidative stress-mediated bimolecular damage and metabolic rewiring
Increase in OS level is correlated with various kinds of diseases, including inflammatory condition, and carcinogenesis. High levels of OS are noted when the ability to eliminate the production of free radicals in the cells is low, resulting in the damage of most cellular biomolecules, including DNA, sugars, lipids, and proteins [22].
The high level of ROS plays a very vital role in carcinogenesis. The most common modification in DNA base occurs is 8-oxo-7,8-dihydroguanine (8-oHdG) [23]. Many kinds of literature have reported that in a cell, about 105 oxidative lesions per day are formed [14, 24]. 8-OHdG and thymine glycol are found in abundance and are studied as well as reported as markers of OS [24].
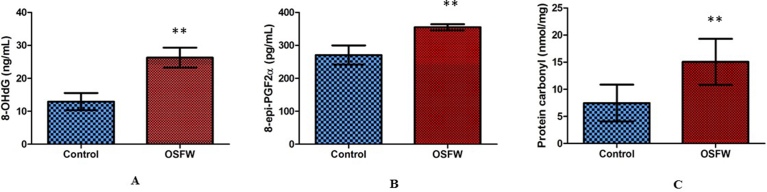
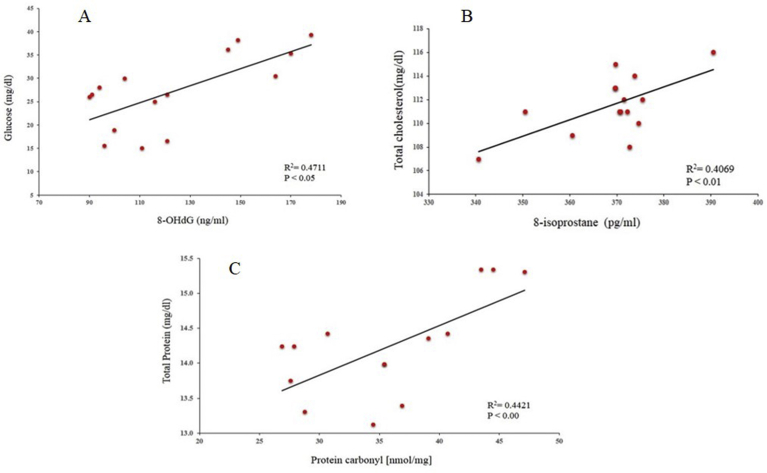
Fig. 3A shows significantly elevated levels of 8-OHdG [26 ± 3.0] in the OSF group in comparison to control [12 ± 2.6]. Studies have also reported a correlation between glucose metabolism and DNA damage Initiation of the DNA damage response (DDR) activates nucleotide synthesis and glucose metabolism [25]. A significant positive correlation in Fig. 4A R2 = 0.4711, p < 0.05 was seen between glucose and DNA damage in our results also. Increase glucose level can be related to the dependency of the DDR pathway on glucose.
Fig. 3.
Comparison of oxidative damage to biomolecules related to A) DNA (8-OHdG) B) Lipids (8-Isoprostane) C) protein (Protein carbonyl) in OSFW and controls; Mean ± SEM; *p ≤ 0.00.
Fig. 4.
Correlation between A) DNA Damage and glucose level (B) Total Cholesterol and 8-iso PGF2α (c) Total Protein and Protein carbonyl.
8-iso-Prostaglandin F2α (also known as 8-epi-PGF2α, 8-isoprostane, or 15-isoprostane F2t), is an isoprostane that is a useful marker for the assessment of OS. It is involved in pathophysiological changes in lipid metabolism [26]. Elevated levels are associated with carcinogenesis [27]. 8-iso PGF2α circulates as an esterified LDL Phospholipid and as free acid in biological fluids [28]. Significantly, elevated levels of 8-iso PGF2α were seen in the OSF group [355 ± 9.0] in comparison to control [270 ± 29.2] in Fig. 3B. Higher levels of lipid peroxidation products are reported in the progression of cancer which can be correlated with our results [29]. Moreover, Pearson correlation analysis between total cholesterol and 8-iso PGF2α [R2 = 0.4069, p < 0.01] in Fig. 4B showed a significantly positive correlation between each other. Studies reported that lipid peroxidation induces alterations in the lipid metabolism along with changes in properties of the biological membranes which leads to disease progression [29].
Proteins are the most immediate molecules affected by oxidative damage on cells [30]. Protein oxidation is defined as the covalent modification of a protein induced either directly by ROS or indirectly by reaction with intermediate products. Protein carbonyl derivatives of Pro, Arg, Lys, and Thr. are the most common oxidative modification which takes place [31]. These derivatives are chemically stable and serve as markers of OS. Fig. 3C indicates a significantly higher level of protein carbonyl level in the OSFW group [15.0 ± 1.3] compared to control [7.4 ± 1.6]. In comparison with our result, many studies have also suggested an increased level of protein carbonyl is reported in various types of cancers also [32, 33, 34]. Fig. 4C shows a strong correlation [R2 = 0.4421, p < 0.00] between total protein and protein carbonyl in OSFW. An increased level and a strong association of total protein and protein carbonyl can be considered as protein dysfunction which leads to poor prognosis in cancer [34].
4. Discussion
Numerous studies have reported that malignant transformation at the molecular level is the main pathological reason for carcinogenesis, often being associated with OS. ROS level in the cancer cells are higher in comparison to normal cell and are causative factors generally reported for mutation [35]. Many biochemical reactions use oxygen which generates ROS, and this reaction is initiated by intermediates products of the metabolic cycle. So in our study, we targeted some main intermediates metabolites which play an essential role during the metabolic reaction. According to our results increase in serum level of intermediate molecule FFA in the OSFW group can be regarded as an alteration in fatty acid metabolism [36]. As FAA is regarded as essential for cell proliferation, division, and metastasis and play an important role in cellular synthesis and energy metabolism. Literature also reports that OS is activated by free fatty acid [12]. It can be interpreted from results that it activates multiple signaling pathways for cell growth and proliferation which implies that targeting lipid metabolism could be a novel strategy for cancer prevention and treatment [37]. According to reported literature, the role of vitamin C said maintain redox balance (oxide/reduction). Low level of ascorbic acid was seen in OSFW group also. Literature reports that if the ascorbic acid level is low it can lead to oxidative damage to cells and tissues [38]. Nonetheless, there are strong and convincing reports that serum concentrations levels of ascorbic acid are positively co-related to health and vary inversely with disease and mortality [39]. It can be suggested that the serum ascorbic acid concentration may be a useful biomarker of disease as due to oxidative cell damage may be leading to progression of the disease. Moreover, this molecule may act as a co-substrate for several enzymes that are important for the functioning of the metabolism [40]. Alteration of intermediate serum levels in this study can be interpreted that metabolism consists of highly interconnected pathways through various intermediate molecules [41]. Alterations at an intermediate level in the metabolic pathway can act as the main pathological activators for carcinogenesis, and leading to metabolic rewiring with which may be leading the disease towards malignant transformation [35, 41]. Therefore, changes in intermediate molecules of pathways may lead to rigorous pressure on the cells and forcing cells to adapt to new conditions by metabolic rewiring [35].
Changes at intermediate by-products makes it necessary to investigate the oxidative damage caused to biomolecules also. Increase ROS levels always targets guanine bases in DNA efficiently and form 8-OHdG, based on which, the level of 8-OHdG is generally regarded as a biomarker of mutagenesis due to OS [42]. In our study higher level of serum 8-OHdG and significant correlation with blood glucose suggest oxidative DNA damage contributing to DNA repair pathways and nutritional dependencies in the tumor microenvironment.
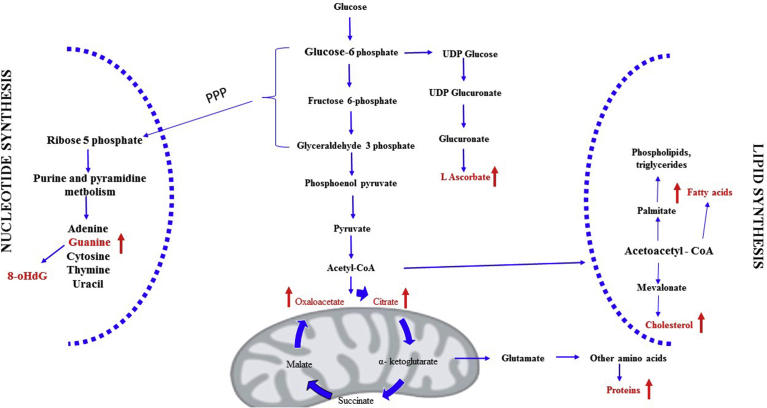
Lipid peroxidation is a well-defined mechanism of cellular damage in the human body. Lipid peroxides are indicators of OS that decompose to form more reactive and complex products such as isoprostanes. 8-iso-Prostaglandin F2α (commonly known as 8-epi-PGF2α) is an isoprostane that has been reported as a marker for the assessment of oxidative stress in serum [37]. Our study results show an increased level and positive correlation with total cholesterol in the serum of OSFW group which reflects the change in membranes, lipoproteins, and altered lipid metabolism. As reported altered lipid metabolism promotes cancer development, invasion, and metastasis via multiple signaling pathways [37]. As we know that severe OS induces protein carbonyl, an irreversible post-translational modification which plays important roles in both cancer progression and cancer suppression [43]. Fig. 5 depicts the altered intermediate metabolites and biomolecules representing metabolic rewiring. In our results increase the level of protein carbonyl and significant correlation with total protein can be lead to protein dysfunction and contribute to a poor prognosis of the disease. Finally, summarising the results, we can conclude that there are metabolic rewiring and OS happening in OSFW. Both these features are enabling cancer cells to achieve a more aggressive nature and initiating cell divisions for progression of the disease.
Fig. 5.
Representation of metabolic rewiring in oral submucous fibrosis.
5. Conclusion
These findings strongly suggest that in OSFW a long-lasting OS-induced metabolic dysfunction is initiated that leads to damage in protein, lipid, DNA damage. This effect of OS may be associated with the progression of the disease. Moreover, from the above results, we can conclude that due to OS, the metabolic alteration is initiated in the metabolic system (Fig. 5) and helping cancer cells for proliferation. The present study is unique in its way by demonstrating a statistically significant positive correlation between OS parameters and biochemical parameters for OSF. Further, treatment can be improved once inhibiting the underlying action of metabolite or enzyme. This may be helpful for the successful management of the disease.
Declarations
Author contribution statement
Vertika Rai: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Surajit Bose: Contributed reagents, materials, analysis tools or data.
Chandan Chakrabory: Analyzed and interpreted the data.
Satadal Saha: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Martinez-Outschoorn U.E. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9(16):3276–3296. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai V. Delineating metabolic dysfunction in cellular metabolism of oral submucous fibrosis using 1H nuclear magnetic resonance spectroscopy. Arch. Oral Biol. 2019;97:102–108. doi: 10.1016/j.archoralbio.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Rai V. “Omics” in oral cancer: new approaches for biomarker discovery. Arch. Oral Biol. 2017;87:15–34. doi: 10.1016/j.archoralbio.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Anderson R.A. The relationships between post-prandial lipaemia, endothelial function and oxidative stress in healthy individuals and patients with type 2 diabetes. Atherosclerosis. 2001;154(2):475–483. doi: 10.1016/s0021-9150(00)00499-8. [DOI] [PubMed] [Google Scholar]
- 5.Cairns R.A., Harris I.S., Mak T.W. Regulation of cancer cell metabolism. Nat. Rev. Canc. 2011;11(2):85. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 6.Rai V. Evaluation of aberrant metabolism related proteins in oral submucous fibrosis: a pilot study. J. Oral Biosci. 2018;60:87–91. [Google Scholar]
- 7.Rai V. Serum-based diagnostic prediction of oral submucous fibrosis using FTIR spectrometry. Spectrochim. Acta Mol. Biomol. Spectrosc. 2018;189:322–329. doi: 10.1016/j.saa.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Hess J.A., Khasawneh M.K. Cancer metabolism and oxidative stress: insights into carcinogenesis and chemotherapy via the non-dihydrofolate reductase effects of methotrexate. BBA Clin. 2015;3:152–161. doi: 10.1016/j.bbacli.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bose S. Evaluating an alternative cost effective protocol to screen and detect oral pre-cancerous and cancerous lesions. IJADS. 2017;3(4):178–184. [Google Scholar]
- 10.DeBerardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016;2(5) doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhari S.K. Oxidative and antioxidative mechanisms in oral cancer and precancer: a review. Oral Oncol. 2014;50(1):10–18. doi: 10.1016/j.oraloncology.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Soardo G. Oxidative stress is activated by free fatty acids in cultured human hepatocytes. Metab. Syndr. Relat. Disord. 2011;9(5):397–401. doi: 10.1089/met.2010.0140. [DOI] [PubMed] [Google Scholar]
- 13.Bartsch H., Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbeck's Arch. Surg. 2006;391(5):499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 14.Valko M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Dikalov S., Griendling K.K., Harrison D.G. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49(4):717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas R. Intermediates of metabolism: from bystanders to signalling molecules. Trends Biochem. Sci. 2016;41(5):460–471. doi: 10.1016/j.tibs.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Tsaluchidu S., Puri B.K. Fatty acids and oxidative stress. Ann. Gen. Psychiatr. 2008;7(S1):S86. doi: 10.1186/1471-244X-8-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piro S. Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stress. Metab. Clin. Exp. 2002;51(10):1340–1347. doi: 10.1053/meta.2002.35200. [DOI] [PubMed] [Google Scholar]
- 19.Hernández I. Free fatty acids enhance the oxidative damage induced by ethanol metabolism in an in vitro model. Food Chem. Toxicol. 2015;76:109–115. doi: 10.1016/j.fct.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Padayatty S.J. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 21.Convertini P. The contribution of the citrate pathway to oxidative stress in down syndrome. Immunology. 2016;149(4):423–431. doi: 10.1111/imm.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sesti F. Oxidative stress-mediated biomolecular damage and inflammation in tumorigenesis. In Vivo. 2012;26(3):395–402. [PubMed] [Google Scholar]
- 23.Aitken R.J., Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122(4):497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 24.Fraga C.G. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc. Natl. Acad. Sci. Unit. States Am. 1990;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turgeon M.-O., Perry N.J., Poulogiannis G. DNA damage, repair, and cancer metabolism. Front. Oncol. 2018;8:15. doi: 10.3389/fonc.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller T. Serum total 8-iso-prostaglandin F2α: a new and independent predictor of peripheral arterial disease. J. Vasc. Surg. 2004;40(4):768–773. doi: 10.1016/j.jvs.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 27.van't Erve T.J. Reinterpreting the best biomarker of oxidative stress: the 8-iso-prostaglandin F2α/prostaglandin F2α ratio shows complex origins of lipid peroxidation biomarkers in animal models. Free Radic. Biol. Med. 2016;95:65–73. doi: 10.1016/j.freeradbiomed.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basu S. Metabolism of 8-iso-prostaglandin F2α. FEBS Lett. 1998;428(1–2):32–36. doi: 10.1016/s0014-5793(98)00481-5. [DOI] [PubMed] [Google Scholar]
- 29.Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012:2012. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalle-Donne I. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003;329(1–2):23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 31.Buss H. Protein carbonyl measurement by a sensitive ELISA method. Free Radic. Biol. Med. 1997;23(3):361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 32.Aryal B., Rao V.A. Specific protein carbonylation in human breast cancer tissue compared to adjacent healthy epithelial tissue. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0194164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berlett B.S., Stadtman E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 34.Mannello F., Tonti G.A., Medda V. Protein oxidation in breast microenvironment: nipple aspirate fluid collected from breast cancer women contains increased protein carbonyl concentration. Anal. Cell Pathol. 2009;31(5):383–392. doi: 10.3233/CLO-2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrisic L. Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol. 2018;14:47–58. doi: 10.1016/j.redox.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Currie E. Cellular fatty acid metabolism and cancer. Cell Metabol. 2013;18(2):153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long J. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018;8(5):778. [PMC free article] [PubMed] [Google Scholar]
- 38.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 39.Chung W.Y. Plasma ascorbic acid: measurement, stability and clinical utility revisited. Clin. Biochem. 2001;34(8):623–627. doi: 10.1016/s0009-9120(01)00270-3. [DOI] [PubMed] [Google Scholar]
- 40.Figueroa-Méndez R., Rivas-Arancibia S. Vitamin C in health and disease: its role in the metabolism of cells and redox state in the brain. Front. Physiol. 2015;6:397. doi: 10.3389/fphys.2015.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berg J., Tymoczko J., Stryer L. WH Freeman; New York: 2002. Metabolism Consist of Highly Interconnected Pathways. Biochemistry. [Google Scholar]
- 42.Yakes F.M., Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. Unit. States Am. 1997;94(2):514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossner P., Jr. Plasma protein carbonyl levels and breast cancer risk. J. Cell Mol. Med. 2007;11(5):1138–1148. doi: 10.1111/j.1582-4934.2007.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]