Abstract
Introduction
Despite the central role of caregivers in managing HIV treatment for children living with HIV, viral suppression within caregiver–child dyads in which both members are living with HIV is not well described.
Methods
We conducted a retrospective analysis of children living with HIV <15 years of age and their caregivers living with HIV attending HIV clinics affiliated with the Academic Model Providing Access to Healthcare (AMPATH) in Kenya between 2015 and 2017. To be included in the analysis, children and caregivers must have had ≥1 viral load (VL) during the study period while receiving antiretroviral therapy (ART) for ≥6 months, and the date of the caregiver's VL must have occurred ±90 days from the date of the child's VL. The characteristics of children, caregivers and dyads were descriptively summarized. Multivariable logistic regression was used to estimate the odds of viral non‐suppression (≥ 1000 copies/mL) in children, adjusting for caregiver and child characteristics.
Results
Of 7667 children who received care at AMPATH during the study period, 1698 were linked to a caregiver living with HIV and included as caregiver–child dyads. For caregivers, 94% were mothers, median age at ART initiation 32.8 years, median CD4 count at ART initiation 164 cells/mm3 and 23% were not virally suppressed. For children, 52% were female, median age at ART initiation 4.2 years, median CD4 values at ART initiation were 15% (age < 5 years) and 396 cells/mm3 (age ≥ 5 years), and 38% were not virally suppressed. In the multivariable model, children were found more likely to not be virally suppressed if their caregivers were not suppressed compared to children with suppressed caregivers (aOR = 2.40, 95% CI: 1.86 to 3.10). Other characteristics associated with child viral non‐suppression included caregiver ART regimen change prior to the VL, caregiver receipt of a non‐NNRTI‐based regimen at the time of the VL, younger child age at ART initiation and child tuberculosis treatment at the time of the VL.
Conclusions
Children were at higher risk of viral non‐suppression if their caregivers were not virally suppressed compared to children with suppressed caregivers. A child's viral suppression status should be closely monitored if his or her caregiver is not suppressed.
Keywords: HIV, child, caregiver, viraemia, adherence, sub‐Saharan Africa
Abbreviations
- AMPATH
Academic Model Providing Access to Healthcare
- aOR
adjusted odds ratio
- ART
antiretroviral therapy
- ARV
antiretroviral
- CDC
Centers for Disease Control
- CI
confidence interval
- II
integrase inhibitor
- IQR
interquartile range
- MTRH
Moi Teaching and Referral Hospital
- NNRTI
non‐nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- TB
tuberculosis
- VL
viral load
- WHO
World Health Organization
1. Introduction
The scale‐up of antiretroviral therapy (ART) in sub‐Saharan Africa has improved survival of both children living with HIV and their parents and caregivers living with HIV 1. In these relationships, caregivers must assume responsibility for their own HIV management as well as their child's management. However, both individuals may be vulnerable to medical, economic and psychosocial stressors that may act as barriers to maintaining viral suppression and health for each individual and the relationship as a whole, suggesting the potential importance of family‐centred HIV services for these caregiver–child dyads 2, 3. Yet, despite the central role of caregivers in the management of children living with HIV and the common barriers to viral suppression they may both encounter, the association between viral non‐suppression in caregiver–child dyads is not well understood.
Various studies describing caregiver–child interactions in the context of HIV, particularly in resource‐limited settings, suggest the possibility that viral suppression in children and their caregivers is associated. Poverty, substance abuse, physical and mental illness, and perceived lack of social support may jeopardize adherence to ART for both caregivers and children 2, 4. Having a caregiver other than the mother, experiencing a change in caregiver, and not having a caregiver attend a child's clinic appointment have been associated with lower adherence and higher odds of virologic failure among children and adolescents 5, 6, 7, 8. A mother's attitude and behaviour regarding her own ART adherence may also influence her adherence practices towards her child 9. Such influences could be positive (e.g. modelling good adherence and beliefs about ART efficacy) or negative (e.g. externalizing feelings of stigma and guilt, concerns over ART side effects, forgetting ART doses or medication fatigue), and dynamic over time as children gain independence and family structures evolve 10, 11. Children may miss ART doses if their caregivers are unavailable or struggling to manage their own HIV infection and associated conditions 2, 12. HIV‐related stigma and disclosure concerns may further inhibit caregivers from administering ART to their children or bringing them to clinic 13, 14, 15, 16, 17.
HIV viral load (VL) testing has recently been introduced in Kenya and other countries in sub‐Saharan Africa for routine treatment monitoring 18, 19, 20. This presents a novel opportunity to examine caregiver–child viral suppression in this region. Given the risk of HIV drug resistance and HIV disease progression in the setting of HIV viraemia, characterizing the drivers of viral non‐suppression in children is important 21. The objective of this study is to describe the association between child and caregiver viral suppression and the factors that influence viral non‐suppression in children.
2. Methods
2.1. Study design
This retrospective cohort study used electronic medical record data from children living with HIV and their adult caregivers living with HIV who received HIV care at the Academic Model Providing Access to Healthcare (AMPATH) programme in western Kenya between 2015 and 2017. This study was approved by the Institutional Research and Ethics Committee at Moi University in Kenya and Indiana University IRB. Patient‐level consent was waived by the regulatory bodies because the data were collected as part of routine care and were de‐identified prior to analysis.
2.2. Study setting and population
AMPATH is a USAID‐funded HIV care and treatment programme situated in a generalized HIV epidemic setting in western Kenya 22. AMPATH is a participating site in the International Epidemiology Databases to Evaluate AIDS East Africa consortium 23. The county‐level HIV prevalence in the AMPATH catchment ranges from 1.6% to 20.7% among adults ≥15 years of age. In these counties, there are an estimated 446,693 HIV‐positive adults ≥15 years of age and 36,743 HIV‐positive children 0 to 14 years of age, with ART coverage ranging from 47% to 100% for adults and 49% to 98% for children as of 2017 24. Since 2001, AMPATH has enrolled over 150,000 patients living with HIV at Ministry of Health facilities across western Kenya and currently provides HIV care to approximately 85,000 patients, including over 7500 children 25, 26. All facilities provide standard of care HIV treatment services based on national guidelines, which in 2015 recommended ART initiation for all children ≤10 years of age and adolescents and adults with CD4 count <500 cells/mm3 19. The WHO Option B+ policy recommending lifelong ART for all pregnant women living with HIV was adopted by the Kenya Ministry of Health and AMPATH in 2014 19. In 2016, AMPATH transitioned to universal ART eligibility 27. Recommended first‐line ART at the time of the study was lopinavir‐based for children <3 years of age and efavirenz‐based for children three to fourteen years of age and adults ≥15 years of age.
Within the AMPATH programme there is a dedicated paediatric HIV clinic at Moi Teaching and Referral Hospital with paediatric‐dedicated clinical officers and paediatricians. However, at all other facilities, the same clinical officers treat both children and adults either in separate or combined adult/paediatric clinics. The study population included all children living with HIV and their caregivers living with HIV who attended an AMPATH affiliated HIV clinic at any time from 1 January 2015 to 14 February 2017. Routine VL monitoring was implemented for all patients at AMPATH during this period, replacing the previous recommendation for immunologic monitoring. At the time of the study, WHO and Kenyan HIV treatment guidelines recommended VL testing six months after ART initiation, and if ≤1000 copies/mL, annually thereafter 27, 28. Individuals with a VL ≥1000 copies/mL were recommended to have the VL test repeated after a minimum of three months of enhanced adherence counselling and support. CD4 testing was recommended at baseline, but not routinely thereafter, for individuals on ART with access to VL testing 19.
Caregiver–child dyads were selected for the study according to the following criteria: First, children were included in the study if they were: (1) <15 years of age on or after 1 January 2015 (study start date); (2) living with HIV and enrolled in care at AMPATH; (3) receiving ART for at least six months prior to 14 February 2017 (database closure); and (4) documented to have at least one VL measure while receiving ART for at least six months. We then excluded all children who were not linked to any caregiver in the medical record. Caregiver was defined as any individual living with HIV and categorized as mother, father, aunt, uncle, grandparent, stepparent, foster parent, guardian or caretaker. Although siblings could also act as caregivers in the Kenya context (e.g. for orphaned children whose parent(s) had died of HIV), the medical record did not contain information to substantiate siblings’ status as caregivers so were not included in the caregiver definition 29. Subsequently, we included caregivers according to the above criteria 2 to 4 used for children, along with the additional criterion that each caregiver has at least one VL measure ±90 days from the date of the child's VL measure during the study period. In the event that there was more than one eligible VL pair for a given dyad during the study period, we sampled the first chronological caregiver–child VL pair to serve as the unit of analysis for each individual. For caregivers linked to more than one child, we included each child in the analysis so that, for example, a caregiver linked to two children would be considered two caregiver–child dyads with the caregiver counted twice. For children linked to multiple caregivers, the mother was preferentially selected, followed by the father. In western Kenya and other contexts in sub‐Saharan Africa, mothers are commonly the primary caregivers for children living with HIV 5, 30, 31. We elected to restrict our sample to those caregiver–child VLs that occurred within 90 days of one another, as we assumed this to be a reasonable period in which a temporal association between caregiver–child viral suppression could be evaluated. Our decision to include VL measures that occur at least six months after initiating ART is also consistent with World Health Organization (WHO) and Kenyan HIV treatment guidelines, as elevated VL measures within six months may represent normal values along the continuum of VL decline for patients with adequate adherence after initiating ART 27, 32. Finally, we included children that were <15 years of age because age ≥15 is an established cutoff used to define “young adults” or “adults” according to the Kenyan Ministry of Health and other international health agencies 27, 33, 34, 35.
2.3. Data management
We used clinical and viral load data available in the AMPATH electronic medical record (EMR) that was collected during routine care initially on paper‐based forms and from 2016 by point‐of‐care data entry 36. The demographic section of the EMR contains a “relationships” function that enables the user to manually enter the names and medical record numbers of other AMPATH patients (e.g. spouses, children, other family members/caregivers) and designate the type of relationship the linkage represents (e.g. parent/child, sibling/sibling, grandparent/grandchild, etc.). These relationship links are recorded in the EMR by clinicians as part of routine clinical documentation. We used this linkage data to identify the caregiver–child dyads in our study. These data were de‐identified prior to analysis.
2.4. Statistical analysis
The primary outcome measure was viral non‐suppression among children, defined as ≥1000 copies/mL. The following independent variables were included for caregivers and children: Enrolment in HIV care – sex, mother/father vital status, caregiver type, total number of children each caregiver has (includes HIV‐positive children included and excluded from the study, as well as children who are HIV‐negative); ART initiation – age, WHO stage/CDC class, CD4 count/per cent; Characteristics during the study period – proportion of caregivers that attended same clinic as their linked children, total number of VL measures available for each individual, facility type (Moi Teaching and Referral Hospital vs. other); Characteristics at the time of the VL pair selected for analysis – age, pregnancy status (caregiver only), tuberculosis (TB) treatment status, number of days between caregiver and child VLs selected for analysis, antiretroviral (ARV) base class, ART line (according to Kenyan national treatment guidelines 27), whether an ARV base class switch occurred due to treatment failure at any time prior to the VL during the study period, and whether an ART regimen change occurred for any reason (except dose changes) at any time prior to the VL during the study period.
Logistic regression model fitted using generalized estimating equations (GEE) was used to calculate unadjusted odds ratios (OR) and 95% confidence intervals (CI) for independent variables to assess their associations with viral non‐suppression in children. A multivariable logistic regression model fitted using the same approach was then constructed to estimate the adjusted association between child viral non‐suppression and caregiver and child characteristics that were significant in the bivariable model 37, 38, 39. The bivariable and multivariable models were set‐up so that the child viral suppression status was the dependent variable and the caregiver suppression status was the independent variable. The effect of the caregiver suppression status was adjusted for the following caregiver and child characteristics: sex, age (years) at ART initiation, time (years) on ART, being on TB treatment at the time of the VL, WHO stage/CDC class at ART initiation, CD4 count or % at ART initiation, NNRTI base class at the time of the VL, ART regimen change for any reason before the VL, VL not suppressed, type of caregiver, number of children at the time of the VL (caregiver only), pregnant at the time of the VL (caregiver only), MTRH facility, caregiver vital status (child only) and the interaction between age at ART initiation and time on ART. GEE using exchangeable correlation structure and robust variance estimation was used to handle the effect of clustering of children within caregiver units in the model.
3. Results
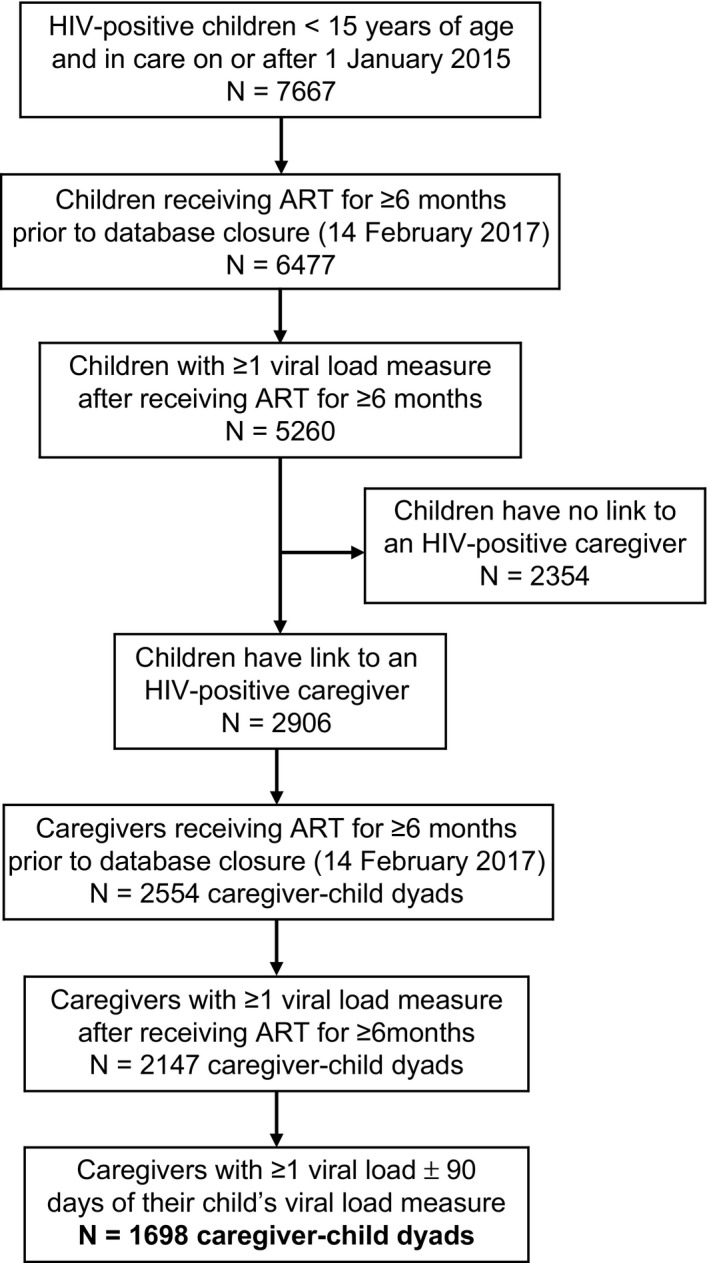
A total of 7667 children living with HIV received care at an AMPATH clinic at least once during the study period (Figure 1). Of these, 5260 met the inclusion criteria, and of those, 2906 were linked to a caregiver living with HIV who also received care at an AMPATH clinic during the study period. After eliminating linked caregivers who did not meet the inclusion criteria (37% of caregivers were eliminated because they did not have a VL ±90 days of the child VL), a total of 1698 caregiver–child dyads were eligible for the analysis. The age at ART initiation and proportion of females were similar between children included in the analysis and all children excluded from the analysis, as well as among the subset of children excluded because they did not have a link to a caregiver living with HIV. The distribution of dyad types was: mother–daughter (49%), mother–son (45%), father–son (3%) and father–daughter (2%).
Figure 1. Caregiver–child dyad selection.
3.1. Caregiver characteristics
There were 1639 unique caregivers included in the analysis, among whom 94% were mothers (Table 1). At enrolment, 3% (n = 54) of caregivers were linked to more than one child living with HIV, with 50 linked to two children, three linked to three children, and one linked to four children. Caregivers had a median of three children in total (with and without HIV). At ART initiation, the median caregiver age was 32.8 years. Caregivers’ median CD4 count (interquartile range [IQR]) at ART initiation was 164 (86 to 258) cells/mm3 among those with available data and the majority (88%) had a CD4 count ≤350 cells/mm3. Among caregivers with available WHO stage at ART initiation, 36% had Stage 3 or 4 disease. During the study period, caregivers attended the same clinic as their linked child in 99% of cases, with the caregiver bringing the child to clinic at least once during the study period in 94% of cases. Prior to the date of the caregiver VL during the study period, less than 1% of caregivers experienced an ARV base class switch due to treatment failure, while 8% experienced an ART regimen change for any reason. At the time of the caregiver VL, caregivers’ median age was 38.6 years, 0.7% were pregnant and 0.7% were on TB treatment. The median time on ART at the time of the VL was 5.7 years. A total of 89% of caregivers were receiving non‐nucleoside reverse transcriptase inhibitor (NNRTI)‐based ART and 89% of ART regimens were categorized as first line. The VL was not suppressed in 23% of caregivers.
Table 1.
Characteristics of caregivers
| Characteristic | N = 1639, n (%) |
|---|---|
| Female | 1551 (95%) |
| Age at ART initiation, median years (IQR) | 32.8 (28.5 to 37.2) |
| Age at VL, median years (IQR) | 38.6 (33.7 to 43.3) |
| Time on ART, median years (IQR) | 5.7 (3.6 to 7.9) |
| Type of caregiver | |
| Mother | 1535 (94%) |
| Father | 81 (4.9%) |
| Othera | 23 (1.4%) |
| Number of children at enrolment, median (IQR) | 3 (2 to 5) |
| Pregnant at VLb | 11 (0.7%) |
| On TB treatment at VL | 11 (0.7%) |
| Facility | |
| MTRH | 333 (20%) |
| Other | 1306 (80%) |
| WHO stage at ART initiation | |
| Stage 1 or 2 | 985 (60%) |
| Stage 3 or 4 | 550 (34%) |
| Missing | 104 (6.4%) |
| CD4 count at ART initiation, median (IQR) | 164 (86 to 258) |
| ≤350 | 875 (53%) |
| >350 | 119 (7.3%) |
| Missing | 645 (39%) |
| ARV base class at VL | |
| NNRTI | 1459 (89%) |
| PI | 178 (11%) |
| Other (i.e. PI + NNRTI or PI + II) | 2 (0.1%) |
| ART line at VL | |
| First line | 1461 (89%) |
| Second line | 177 (11%) |
| Third line | 1 (0.1%) |
| ARV base class switch for treatment failure before VLc | 7 (0.4%) |
| ART regimen change for any reason before VLd | 134 (8.2%) |
| VL not suppressed | 376 (23%) |
ART, antiretroviral therapy; ARV, antiretroviral; II, integrase inhibitor; IQR, interquartile range; MTRH, Moi Teaching and Referral Hospital; NNRTI, non‐nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TB, tuberculosis; VL, viral load; WHO, World Health Organization.
aOther caregiver types include: step‐parent (n = 16), guardian (n = 5), grandparent (n = 1), uncle (n = 1); bproportion expressed among female caregivers only (n = 1551); cindicates any ART switch due to failure from first‐ to second‐line or from second‐ to third‐line before the VL date according to Kenya HIV treatment guidelines; dexcludes dose change.
3.2. Child characteristics
For children, 52% of 1698 were female (Table 2). At ART initiation, the median child age was four years and 93% were <10 years of age, and 3% (n = 52) were linked to more than one caregiver. Among children with available parental vital status data at enrolment, the mother was deceased in 6% and father was deceased in 27%. At ART initiation, the WHO stage/CDC class was Stage 3 or 4/class B or C in 46% of children with available data. Among children <5 years of age, the CD4% at ART initiation was 15% (IQR 10 to 22); for children ≥5 years of age, the median CD4 count was 396 cells/mm3 (IQR 234 to 686). Two‐thirds of children with available data had a CD4% >25% (<5 years) or >350 cells/mm3 (>5 years) at ART initiation. Prior to the date of the child VL, 0.8% of children experienced an ARV base class switch due to treatment failure and 5.2% experienced an ART regimen change for any reason. At the time of the child VL used in the analysis, the median child age was 9.7 years and 0.8% were on TB treatment. The median time on ART for children at the time of the VL ranged from 1.2 years for children 0 to 2 years of age to 5.7 years for children 10 to 14 years of age. A total of 94% of children were receiving NNRTI‐based ART and 96% of children were categorized as receiving first‐line ART. The VL was not suppressed in 38% of children. Among children on ART ≥6 months and with ≥1 VL available measure who were excluded from the analysis because they did not have a link to an HIV‐positive caregiver (n = 2354; see Figure 1), the VL was not suppressed in 33% (n = 1576; p < 0.001 compared to children included in the analysis). Additionally, viral suppression among excluded children with documented orphan status (n = 1439, defined as mother or both parents deceased and using the earliest VL measure available during the study period) was 68%, compared to 64% for children without documented orphan status (n = 885) (p = 0.04).
Table 2.
Characteristics of children
| Characteristic | N = 1698, n (%) |
|---|---|
| Female | 885 (52%) |
| Age at ART initiation | |
| 0 to 2 years | 621 (36%) |
| 3 to 5 years | 502 (30%) |
| 6 to 9 years | 463 (27%) |
| 10 to 14 years | 112 (6.6%) |
| Age at VL | |
| 0 to 2 years | 85 (5.0%) |
| 3 to 5 years | 236 (14%) |
| 6 to 9 years | 586 (34%) |
| 10 to 14 years | 791 (47%) |
| Time on ART, median years (IQR) | |
| 0 to 2 years | 1.2 (0.8 to 1.7) |
| 3 to 5 years | 3.0 (1.9 to 4.0) |
| 6 to 9 years | 4.9 (2.8 to 6.1) |
| 10 to 14 years | 5.7 (3.7 to 7.6) |
| Caregiver vital status | |
| Mother deceased (n = 1093 with available data) | 68 (6.2%) |
| Father deceased (n = 1063 with available data) | 288 (27%) |
| Mother or father deceased (n = 1189 with available data) | 326 (27%) |
| On TB treatment at VL | 14 (0.8%) |
| WHO stage/CDC class at ART initiation | |
| Stage 1 or 2/Class N or A | 700 (41%) |
| Stage 3 or 4/Class B or C | 602 (36%) |
| Missing | 396 (23%) |
| CD4 % at ART initiation for children <5 years, median (IQR) (n = 450 with available data) | 15 (10 to 22) |
| CD4 count at ART initiation for children ≥5 years, median (IQR) (n = 349 with available data) | 396 (234 to 686) |
| CD4 % at ART initiation >25 for children <5 years|CD4 count at ART initiation >350 for children aged ≥5 years | |
| No | 519 (31%) |
| Yes | 280 (17%) |
| Missing | 899 (53%) |
| ARV base class at VL | |
| NNRTI | 1590 (94%) |
| PI | 108 (6.4%) |
| ART line at VL | |
| First line | 1626 (96%) |
| Second line | 72 (4.2%) |
| ARV base class switch for treatment failure before VLa | 14 (0.8%) |
| ARV regimen change for any reason before VLb | 89 (5.2%) |
| VL not suppressed | 652 (38%) |
ART, antiretroviral therapy; ARV, antiretroviral; CDC, Centers for Disease Control; II, integrase inhibitor; NNRTI, non‐nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TB, tuberculosis; VL, viral load; WHO, World Health Organization.
aIndicates any ART switch from first‐ to second‐line or from second‐ to third‐line before the VL date according to Kenya HIV treatment guidelines; bexcludes dose changes.
3.3. Viral load characteristics
There was a median (IQR) of 2 (2 to 3) available VL measures each for caregivers and children during the study period. The median (IQR) number of days between the caregiver and child VL measures used for analysis was 5 (0 to 55) days out of a maximum of 90 days according to the inclusion criteria. The viral suppression status (<1000 copies/mL) for all 1698 caregiver–child dyads was: caregiver and child both suppressed (n = 880 dyads, 52%), caregiver suppressed and child not suppressed (n = 430 dyads, 25%), caregiver not suppressed and child suppressed (n = 166 dyads, 10%), and caregiver and child both not suppressed (n = 222 dyads, 13%).
3.4. Associations with viral non‐suppression in children
In Table 3, caregiver and child characteristics that were significantly associated (p ≤ 0.05) with child viral non‐suppression in the bivariable model were included in the multivariable model. The sex of the caregiver and child was also included in the multivariable model despite not being statistically significant in the bivariable model given previously observed sex differences in HIV treatment outcomes 40, 41. Children with caregivers who were not virally suppressed were more than twice as likely to not be virally suppressed themselves compared to children with suppressed caregivers (adjusted odds ratio [aOR]=2.40, 95% CI: 1.86 to 3.10; Table 3). Characteristics associated with a higher adjusted odds of viral non‐suppression in children included caregiver ART regimen change for any reason before the VL (aOR = 1.82, 95% CI: 1.21 to 2.72), the child being on TB treatment at the time of the VL (aOR = 3.32, 95% CI: 1.13 to 9.81). Characteristics associated with a lower adjusted odds of viral non‐suppression in children included caregiver receipt of an NNRTI‐based regimen at the time of the VL (aOR = 0.69, 95% CI 0.50 to 0.97), and child age six to nine years (aOR = 0.49, 95% CI 0.27 to 0.89) and 10 to 14 years (aOR = 0.30, 95% CI 0.11 to 0.82) compared to zero to two years.
Table 3.
Unadjusted and adjusted odds ratios for factors associated with viral non‐suppression in children
| Characteristic | N | Unadjusted OR (95% CI) | Adjusted OR (95% CI) N = 1608 |
|---|---|---|---|
| Caregiver characteristics | |||
| Female | 1639 | 1.14 (0.73 to 1.78) | 0.84 (0.52 to 1.36) |
| Age (years) at ART initiation | 1635 | 0.97 (0.96 to 0.99) | 0.98 (0.96 to 1.00) |
| Time (years) on ART | 1635 | 1.00 (0.97 to 1.04) | 0.99 (0.94 to 1.04) |
| Type of caregiver | |||
| Mother versus other | 1639 | 0.82 (0.36 to 1.87) | |
| Father versus other | 0.66 (0.26 to 1.70) | ||
| Number of children at VL | 1504 | 0.98 (0.93 to 1.04) | |
| Pregnant at VL | 1551 | 2.80 (0.82 to 9.63) | |
| On TB treatment at VL | 1639 | 2.83 (0.82 to 9.69) | 1.70 (0.49 to 5.84) |
| MTRH facility | 1639 | 1.00 (0.78 to 1.28) | |
| WHO Stage 3 to 4 at ART initiation | |||
| Stage 3 or 4 versus Stage 1 or 2 | 1639 | 0.93 (0.75 to 1.15) | |
| Missing versus Stage 1 or 2 | 1.05 (0.70 to 1.58) | ||
| CD4 count at ART initiation >350 | |||
| Yes versus no | 1639 | 0.99 (0.67 to 1.46) | 1.23 (0.81 to 1.86) |
| Missing versus no | 1.21 (0.98 to 1.49) | 1.17 (0.94 to 1.47) | |
| NNRTI base class at VL (vs. PI/other regimen) | 1639 | 0.65 (0.47 to 0.88) | 0.69 (0.50 to 0.97) |
| ART regimen change for any reason before VL | 1639 | 2.89 (2.02 to 4.13) | 1.82 (1.21 to 2.72) |
| VL not suppressed | 1639 | 2.72 (2.15 to 3.43) | 2.40 (1.86 to 3.10) |
| Children characteristics | |||
| Female | 1639 | 0.89 (0.73 to 1.08) | 0.86 (0.70 to 1.06) |
| Age (years) at ART initiation | |||
| 0 to 2 years | 1639 | Ref. | Ref. |
| 3 to 5 years | 0.59 (0.33 to 1.04) | 0.71 (0.39 to 1.30) | |
| 6 to 9 years | 0.39 (0.22 to 0.68) | 0.49 (0.27 to 0.89) | |
| 10 to 14 years | 0.24 (0.09 to 0.63) | 0.30 (0.11 to 0.82) | |
| Time (years) on ART | 1639 | 0.98 (0.92 to 1.04) | 1.01 (0.93 to 1.10) |
| Caregiver vital status | |||
| Mother or father deceased versus neither mother nor father deceased | 1531 | 0.96 (0.73 to 1.25) | |
| Missing versus none | 0.95 (0.75 to 1.19) | ||
| On TB treatment at VL | 1639 | 3.36 (1.01 to 11.22) | 3.32 (1.13 to 9.81) |
| WHO stage at ART initiation | |||
| Stage 3/4 & CDC Class B/C versus Stage 1/2 & Class A/N | 1639 | 1.06 (0.85 to 1.32) | |
| Missing versus Stage 1/2 & Class A/N | 1.13 (0.88 to 1.46) | ||
| CD4 % at ART initiation >25 for children <5 years|CD4 count at ART initiation >350 for children aged >5 years | |||
| Yes versus no | 1612 | 0.77 (0.58 to 1.04) | 0.76 (0.55 to 1.05) |
| Missing versus no | 0.98 (0.79 to 1.22) | 0.97 (0.76 to 1.24) | |
| NNRTI base class at VL (vs. PI/other regimen) | 1639 | 1.15 (0.77 to 1.72) | |
| ART regimen change for any reason before VL | 1639 | 0.89 (0.57 to 1.38) | |
| Age at ART initiation × time (years) on ART | |||
| 0 to 2 years | 1639 | Ref. | Ref. |
| 3 to 5 years | 1.05 (0.95 to 1.15) | 1.03 (0.93 to 1.14) | |
| 6 to 9 years | 1.14 (1.01 to 1.28) | 1.13 (1.00 to 1.28) | |
| 10 to 14 years | 1.66 (1.14 to 2.40) | 1.68 (1.16 to 2.43) | |
ART, antiretroviral therapy; ARV, antiretroviral; NNRTI, non‐nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TB, tuberculosis; VL, viral load; WHO, World Health Organization.
4. Discussion
In our study of children and their caregivers living with HIV, we found that children had more than twice the odds of not being virally suppressed if their caregivers were not virally suppressed, compared to children with suppressed caregivers. Caregivers experiencing an ART regimen change for any reason before the VL and receiving a non‐NNRTI‐based regimen at the time of the VL were both associated with viral non‐suppression in children. In Kenya, non‐NNRTI‐based regimens (e.g. protease or integrase inhibitor‐based regimens) typically constitute second‐ or third‐line ART for patients with HIV viraemia on first‐line, NNRTI‐based ART 27. Inadequate adherence is a common cause of HIV viraemia among adults receiving first‐ and second‐line ART in sub‐Saharan Africa, and adherence to first‐line ART has been shown to be a strong predictor of adherence to second‐line ART 42, 43, 44. Thus, although our study did not directly measure adherence, it is possible that experiencing an ART regimen change or being on a non‐NNRTI regimen are markers of prior inadequate adherence among caregivers that increased the risk of future inadequate adherence (i.e. at the time of the VL) for them and their children 42, 45. Young children are uniquely dependent on caregivers for their ART management, and it is plausible that caregivers’ adherence practices extended to their children, increasing the risk of viral non‐suppression for both individuals 9. Further research is needed to understand the patient and site‐level factors (e.g. availability of counselling and other interventions to enhance adherence) that contribute to viral non‐suppression among dyads, as well as the potential influence of adherence and drug resistance 46.
Overall viral suppression among caregivers and children in our study was suboptimal. The VL was suppressed for both the caregiver and child in only half of dyads, while 62% of children and 77% of caregivers were suppressed overall. These suppression estimates are lower than current national, facility‐based estimates for children and adults receiving ART in Kenya at 68% and 86%, respectively, underscoring the vulnerability of this population and the importance of understanding the barriers to viral suppression it experiences 47, 48. Achieving viral suppression for children in sub‐Saharan Africa is especially challenging. Studies have reported lower viral suppression rates for children and adolescents compared to adults in Kenya (57% to 66% vs. 63% to 87%) and in other low‐ and middle‐income countries (60% to 75% vs. 85%), as well as compared to children and adolescents in high‐income countries (≥ 90%) 49, 50, 51, 52, 53. A range of factors can influence adherence and viral suppression for children including the child's age, familial and socio‐economic environment, stigma, disclosure, and the physical and mental health status of children and caregivers 3, 10, 54, 55. Consistent with prior studies, we found that older child age was protective against viral non‐suppression compared to child age ≤2 years 56, 57, 58. This could reflect behavioural changes during childhood such as younger children refusing to take medications or the positive effects of HIV disclosure to older children, the effect of ART dosing frequency (i.e. twice daily dosing of lopinavir‐based ART in children <3 years vs. once daily efavirenz‐based ART for children ≥3 years and ≥35 kg), or biologic factors such as slower rates of viral suppression in infants compared to older children.
Additionally, children in our study who were receiving TB treatment at the time of the VL were three times more likely to not be virally suppressed compared to children not receiving TB treatment. This finding may be a statistical artefact given that only 0.8% of children were on TB treatment at the time of the VL (with this low percentage related at least in part the cross‐sectional nature of this variable among individuals enrolled in HIV care and on ART for ≥6 months). However, treatment for TB has been associated with a higher risk of viral non‐suppression in two studies of children living with HIV in South Africa, which may be due to the child receiving protease inhibitor‐based ART (e.g. pharmacologic interactions between ritonavir and rifampicin), inadequate ART adherence due to higher toxicities or pill burden, or biologic factors (e.g. immune activation in the setting of active TB causing HIV viraemia) 59, 60, 61, 62. Alternatively, the risk of incident TB may have been higher among children with worsening immune function due to ongoing viral replication, or among children with late‐onset immune reconstitution inflammatory syndrome following ART initiation 63.
Our findings suggest that a child's viral suppression status should be carefully assessed at the time his or her caregiver is found not to be virally suppressed. Some barriers to adherence and viral suppression may be common to both individuals, and a family‐centred management approach, rather than an individual approach, may be needed to address these barriers effectively. Differentiated care models that focus on the needs of families living with HIV are emerging 64, 65, 66, 67, 68, 69. In South Africa, family clubs are being used as ART distribution alternatives 64. In Uganda, implementing weekly clinics where children and adolescents were treated together with their families improved appointment adherence, and similar models have been implemented in urban centres in Kenya and through community groups in Namibia 65, 70. These care models may offer services that are better tailored to family issues and more efficient for programmes and patients 64, 71, 72, 73. To date, however, existing models have focused on clinically stable families and children with reliable caregivers, and knowledge gaps exist regarding models for at‐risk families 65, 72, 74, 75. Psychosocial interventions that address stigma, disclosure and mental health issues will likely play an important role in these models 3, 10, 54, 55, 76.
Our study has strengths and limitations. This study is, to our knowledge, the first to identify an association between viral suppression in children and their caregivers who were both living with HIV. Although others have investigated caregiver–child interactions that could influence adherence, our large cohort of caregiver–child dyads enabled us to directly evaluate the outcome of viral suppression 7, 8, 9, 77. The use of observational programme data carries limitations. Although the proportions of children included and excluded from the analysis were similar in terms of sex and age at ART initiation, subject selection may have been biased. Linking caregivers and children in the AMPATH medical record is an active process performed by clinicians or counsellors during routine care, and these individuals may have been less inclined to link non‐mother caregivers to their children in the medical record. We had limited insight into the roles caregivers played in their children's HIV care in our retrospective study (e.g. who was responsible for administering a child's medications), though understanding these roles is ultimately essential to understanding the meaning of the viral suppression association we identified. Despite this limited insight, it is likely that mothers, who comprised the large majority of caregivers in our study, were in fact responsible for their children's HIV management, which is consistent with other studies in sub‐Saharan Africa 5, 31. Finally, adherence and the factors associated with it are dynamic processes that can vary over time 78. We plan to examine the longitudinal nature of viral suppression among child–caregiver dyads in future research.
5. Conclusions
Among caregiver–child dyads in which both members were living with HIV, children were more likely to not be virally suppressed if their caregivers were not virally suppressed, compared to children with suppressed caregivers. A child's viral suppression status should be closely monitored if his or her caregiver is not virally suppressed.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
J.M.H, E.A, B.L.G, A.G, J.W.H and K.W conceptualized and designed the study, and had primary responsibility for interpretation of the data. A.K and B.M analysed the data. J.M.H wrote the paper with assistance from E.A, B.G, A.G, J.W.H and K.K.W. All authors have read and approved the final manuscript.
Acknowledgements
We thank the AMPATH IeDEA site principal investigators Dr. Samuel Ayaya and Dr. Lameck Diero, and AMPATH data manager Mr. Edwin Sang, for contributing data to this study and for providing helpful comments. We thank Dr. Tau Liu for input during the analysis.
Funding
The research reported in this publication was supported by the Indiana Center for AIDS Research Junior Investigator Pilot Grant Program, National Institute Of Allergy And Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Drug Abuse (NIDA), National Cancer Institute (NCI) and the National Institute of Mental Health (NIMH), in accordance with the regulatory requirements of the National Institutes of Health under Award Number U01AI069911 East Africa IeDEA Consortium.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Humphrey, J. M. , Genberg, B. L. , Keter, A. , Musick, B. , Apondi, E. , Gardner, A. , Hogan, J. W. and Wools‐Kaloustian, K. Viral suppression among children and their caregivers living with HIV in western Kenya. J Int AIDS Soc. 2019;22(4):e25272
References
- 1. UNAIDS . Global AIDS Update 2016. Geneva: UNAIDS; 2016. [cited 2018 May 1]. Available from: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf [Google Scholar]
- 2. Smith Fawzi MC, Eustache E, Oswald C, Surkan P, Louis E, Scanlan F, et al. Psychosocial functioning among HIV‐affected youth and their caregivers in Haiti: implications for family‐focused service provision in high HIV burden settings. AIDS Patient Care STDS. 2010;24(3):147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vreeman RC, Wiehe SE, Pearce EC, Nyandiko WM. A systematic review of pediatric adherence to antiretroviral therapy in low‐ and middle‐income countries. Pediatr Infect Dis J. 2008;27(8):686–91. [DOI] [PubMed] [Google Scholar]
- 4. Ji G, Li L, Lin C, Sun S. The impact of HIV/AIDS on families and children–a study in China. AIDS. 2007;21 Suppl 8:S157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahourou DL, Benghezal M, Amorissani‐Folquet M, Yonaba C, Malateste K, Toni T, et al. Longitudinal cluster analysis of viral suppression during 25 months on antiretroviral therapy, adherence and factors associated in young West‐African children, in the MONOD ANRS 12206 cohort. 9th IAS Conference on HIV Science; 2017. July 23‐26; Paris. [Google Scholar]
- 6. Amani‐Bosse C, Dahourou DL, Malateste K, Amorissani‐Folquet M, Coulibaly M, Dattez S, et al. Virological response and resistances over 12 months among HIV‐infected children less than two years receiving first‐line lopinavir/ritonavir‐based antiretroviral therapy in Cote d'Ivoire and Burkina Faso: the MONOD ANRS 12206 cohort. J Int AIDS Soc. 2017;20(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gross R, Bandason T, Langhaug L, Mujuru H, Lowenthal E, Ferrand R. Factors associated with self‐reported adherence among adolescents on antiretroviral therapy in Zimbabwe. AIDS Care. 2015;27(3):322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lowenthal ED, Marukutira T, Tshume O, Chapman J, Nachega JB, Anabwani G, et al. Parental absence from clinic predicts human immunodeficiency virus treatment failure in adolescents. JAMA Pediatr. 2015;169(5):498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wrubel J, Moskowitz JT, Richards TA, Prakke H, Acree M, Folkman S. Pediatric adherence: perspectives of mothers of children with HIV. Soc Sci Med. 2005;61(11):2423–33. [DOI] [PubMed] [Google Scholar]
- 10. Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2009;6(4):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gichane MW, Sullivan KA, Shayo AM, Mmbaga BT, O'Donnell K, Cunningham CK, et al. Caregiver role in HIV medication adherence among HIV‐infected orphans in Tanzania. AIDS Care. 2018;30:701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cluver LD, Hodes RJ, Toska E, Kidia KK, Orkin FM, Sherr L, et al. ‘HIV is like a tsotsi. ARVs are your guns’: associations between HIV‐disclosure and adherence to antiretroviral treatment among adolescents in South Africa. AIDS. 2015;29 Suppl 1:S57–65. [DOI] [PubMed] [Google Scholar]
- 13. Vreeman RC, Nyandiko WM, Ayaya SO, Walumbe EG, Marrero DG, Inui TS. Factors sustaining pediatric adherence to antiretroviral therapy in western Kenya. Qual Health Res. 2009;19(12):1716–29. [DOI] [PubMed] [Google Scholar]
- 14. Poindexter CC. ‘It don't matter what people say as long as I love you’: experiencing stigma when raising an HIV‐infected grandchild. J Mental Health Aging. 2002;8(4):331–48. [Google Scholar]
- 15. Nabukeera‐Barungi N, Kalyesubula I, Kekitiinwa A, Byakika‐Tusiime J, Musoke P. Adherence to antiretroviral therapy in children attending Mulago Hospital, Kampala. Ann Trop Paediatr. 2007;27(2):123–31. [DOI] [PubMed] [Google Scholar]
- 16. Beima‐Sofie KM, Brandt L, Hamunime N, Shepard M, Uusiku J, John‐Stewart GC, et al. Pediatric HIV disclosure intervention improves knowledge and clinical outcomes in HIV‐infected children in Namibia. J Acquir Immune Defic Syndr. 2017;75(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feinstein L, Moultrie H, Myers T, Rie A. Effect of disclosure on HIV status to children receiving ART on six‐month virologic suppression. Am J Epidemiol. 2010;171 Suppl:S134. [Google Scholar]
- 18. WHO . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: WHO; 2013. [cited 2018 May 1]. Available from: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf [PubMed] [Google Scholar]
- 19. Kenya Ministry of Health; National AIDS and STI Control Program (NASCOP) . Guidelines on use of antiretroviral drugs for treating and preventing HIV infection: a rapid advice, 2014. Nairobi, Kenya; 2014. [cited 2018 May 1]. Available from: www.nascop.or.ke [Google Scholar]
- 20. Lecher S, Ellenberger D, Kim AA, Fonjungo PN, Agolory S, Borget MY, et al. Scale‐up of HIV viral load monitoring‐seven Sub‐Saharan African countries. MMWR Morb Mortal Wkly Rep. 2015;64(46):1287–90. [DOI] [PubMed] [Google Scholar]
- 21. Fitzgerald F, Penazzato M, Gibb D. Development of antiretroviral resistance in children with HIV in low‐ and middle‐income countries. J Infect Dis. 2013;207 Suppl 2:S85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Academic Model Providing Access to Healthcare Program [Internet]. Indianapolis; 2018. [cited 2018 Aug 12]. Available from: www.ampathkenya.org [Google Scholar]
- 23. Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub‐Saharan Africa. Int J Epidemiol. 2012;41(5):1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kenya Ministry of Health; National AIDS and STI Control Program (NASCOP) . Kenya HIV Estimates Report 2018. Nairobi, Kenya; 2016. [cited 2018 May 26]. Available from: https://nacc.or.ke/wp-content/uploads/2018/11/HIV-estimates-report-Kenya-20182.pdf [Google Scholar]
- 25. Einterz RM, Kimaiyo S, Mengech HN, Khwa‐Otsyula BO, Esamai F, Quigley F, et al. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med. 2007;82(8):812–8. [DOI] [PubMed] [Google Scholar]
- 26. Wools‐Kaloustian K, Kimaiyo S, Musick B, Sidle J, Siika A, Nyandiko W, et al. The impact of the President's Emergency Plan for AIDS Relief on expansion of HIV care services for adult patients in western Kenya. AIDS. 2009;23(2):195–201. [DOI] [PubMed] [Google Scholar]
- 27. Kenya Ministry of Health; National AIDS and STI Control Program (NASCOP) . Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya. Nairobi, Kenya; 2016. [cited 2018 May 26]. Available from: www.nascop.or.ke [Google Scholar]
- 28. WHO . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: WHO; 2016. [cited 2018 May 1]. Available from: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf [PubMed] [Google Scholar]
- 29. Yanagisawa S, Poudel KC, Jimba M. Sibling caregiving among children orphaned by AIDS: synthesis of recent studies for policy implications. Health Policy. 2010;98(2–3):121–30. [DOI] [PubMed] [Google Scholar]
- 30. McHenry MS, Oyungu E, McAteer CI, Ombitsa AR, Cheng ER, Ayaya SO, et al. Early childhood development in children born to HIV‐infected mothers: perspectives from Kenyan clinical providers and caregivers. Glob Pediatr Health. 2018;5:2333794X18811795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kigen HT, Galgalo T, Wamicwe J. Predictors of loss to follow‐up among HIV exposed children within HIV prevention of mother to child transmission cascade, Kericho County, Kenya, 2016. 9th IAS Conference on HIV Science; 2017. July 23‐26; Paris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Department of Health and Human Services; 2018. [cited 2018 May 26]. Available from: https://aidsinfo.nih.gov/guidelines/html/2/pediatric-arv-guidelines/83/adherence-to-antiretroviral-therapy-in-hiv-infected-children-and-adolescents [Google Scholar]
- 33. UNICEF . The AIDS epidemic continues to take a staggering toll, especially in sub‐Saharan Africa 2016. Geneva: UNICEF; 2016. [cited 2018 May 20]. Available from: http://data.unicef.org/hiv-aids/global-trends.html [Google Scholar]
- 34. WHO . Maternal, newborn, child and adolescent health: Adolescent Development. Geneva: WHO; 2016. [cited 2018 June 26]. Available from: http://www.who.int/maternal_child_adolescent/topics/adolescence/dev/en/ [Google Scholar]
- 35. CDC . STDs in Adolescents and Young Adults. Atlanta: CDC; 2014. [cited 2018 June 26]. Available from: https://www.cdc.gov/std/stats14/adol.htm#foot1 [Google Scholar]
- 36. Tierney WM, Rotich JK, Hannan TJ, Siika AM, Biondich PG, Mamlin BW, et al. The AMPATH medical record system: creating, implementing, and sustaining an electronic medical record system to support HIV/AIDS care in western Kenya. Stud Health Technol Inform. 2007;129(Pt 1):372–6. [PubMed] [Google Scholar]
- 37. Højsgaard SHU, Yan J. The R package geepack for generalized estimating equations. J Stat Soft. 2006;15(2):1–11. [Google Scholar]
- 38. Yan J, Fine J. Estimating equations for association structures. Stat Med. 2004;23(6):859–74. [DOI] [PubMed] [Google Scholar]
- 39. Yan J. Geepack: yet another package for generalized estimating equations. R‐News. 2002;2(3):12–4. [Google Scholar]
- 40. Kipp W, Alibhai A, Saunders LD, Senthilselvan A, Kaler A, Konde‐Lule J, et al. Gender differences in antiretroviral treatment outcomes of HIV patients in rural Uganda. AIDS Care. 2010;22(3):271–8. [DOI] [PubMed] [Google Scholar]
- 41. Scully EP. Sex differences in HIV infection. Current HIV/AIDS Rep. 2018;15(2):136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramadhani HO, Bartlett JA, Thielman NM, Pence BW, Kimani SM, Maro VP, et al. Association of first‐line and second‐line antiretroviral therapy adherence. Open Forum Infect Dis. 2014;1(2):ofu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Zyl GU, van Mens TE, McIlleron H, Zeier M, Nachega JB, Decloedt E, et al. Low lopinavir plasma or hair concentrations explain second‐line protease inhibitor failures in a resource‐limited setting. J Acquir Immune Defic Syndr. 2011;56(4):333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murphy RA, Sunpath H, Castilla C, Ebrahim S, Court R, Nguyen H, et al. Second‐line antiretroviral therapy: long‐term outcomes in South Africa. J Acquir Immune Defic Syndr. 2012;61(2):158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maartens G, Meintjes G. Resistance matters in EARNEST. Lancet HIV. 2017;4(8):e323–4. [DOI] [PubMed] [Google Scholar]
- 46. Haberer JE, Sabin L, Amico KR, Orrell C, Galarraga O, Tsai AC, et al. Improving antiretroviral therapy adherence in resource‐limited settings at scale: a discussion of interventions and recommendations. J Int AIDS Soc. 2017;20(1):21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. UNAIDS . 90‐90‐90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [cited 2018 May 1]. Available from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf [Google Scholar]
- 48. Kenya Ministry of Health; National AIDS and STI Control Program (NASCOP) . Viral Load System. Nairobi, Kenya; 2018. [cited 2018 July 15]. Available from: https://viralload.nascop.org [Google Scholar]
- 49. Mwau M, Syeunda CA, Adhiambo M, Bwana P, Kithinji L, Mwende J, et al. Scale‐up of Kenya's national HIV viral load program: findings and lessons learned. PLoS ONE. 2018;13(1):e0190659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boerma RS, Boender TS, Bussink AP, Calis JC, Bertagnolio S, Rinke de Wit TF, et al. Suboptimal viral suppression rates among HIV‐infected children in low‐ and middle‐income countries: a meta‐analysis. Clin Infect Dis. 2016;63(12):1645–54. [DOI] [PubMed] [Google Scholar]
- 51. Boender TS, Sigaloff KC, McMahon JH, Kiertiburanakul S, Jordan MR, Barcarolo J, et al. Long‐term virological outcomes of first‐line antiretroviral therapy for HIV‐1 in low‐ and middle‐income countries: a systematic review and meta‐analysis. Clin Infect Dis. 2015;61(9):1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cohen S, Smit C, van Rossum AM, Fraaij PL, Wolfs TF, Geelen SP, et al. Long‐term response to combination antiretroviral therapy in HIV‐infected children in the Netherlands registered from 1996 to 2012. AIDS. 2013;27(16):2567–75. [DOI] [PubMed] [Google Scholar]
- 53. Duong T, Judd A, Collins IJ, Doerholt K, Lyall H, Foster C, et al. Long‐term virological outcome in children on antiretroviral therapy in the UK and Ireland. AIDS. 2014;28(16):2395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vreeman RC, Ayaya SO, Musick BS, Yiannoutsos CT, Cohen CR, Nash D, et al. Adherence to antiretroviral therapy in a clinical cohort of HIV‐infected children in East Africa. PLoS ONE. 2018;13(2):e0191848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vreeman RC, Nyandiko WM, Liu H, Tu W, Scanlon ML, Slaven JE, et al. Measuring adherence to antiretroviral therapy in children and adolescents in western Kenya. J Int AIDS Soc. 2014;17:19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Musoke PM, Mudiope P, Barlow‐Mosha LN, Ajuna P, Bagenda D, Mubiru MM, et al. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr. 2010;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mehta K, Ekstrand ML, Heylen E, Sanjeeva GN, Shet A. Adherence to antiretroviral therapy among children living with HIV in South India. AIDS Behav. 2016;20(5):1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Asbjornsdottir KH, Hughes JP, Wamalwa D, Langat A, Slyker JA, Okinyi HM, et al. Differences in virologic and immunologic response to antiretroviral therapy among HIV‐1‐infected infants and children. AIDS. 2016;30(18):2835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Meyers TM, Yotebieng M, Kuhn L, Moultrie H. Antiretroviral therapy responses among children attending a large public clinic in Soweto, South Africa. Pediatr Infect Dis J. 2011;30(11):974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zanoni BC, Phungula T, Zanoni HM, France H, Feeney ME. Impact of tuberculosis cotreatment on viral suppression rates among HIV‐positive children initiating HAART. AIDS. 2011;25(1):49–55. [DOI] [PubMed] [Google Scholar]
- 61. Ren Y, Nuttall JJ, Egbers C, Eley BS, Meyers TM, Smith PJ, et al. Effect of rifampicin on lopinavir pharmacokinetics in HIV‐infected children with tuberculosis. J Acquir Immune Defic Syndr. 2008;47(5):566–9. [DOI] [PubMed] [Google Scholar]
- 62. Kalou M, Sassan‐Morokro M, Abouya L, Bile C, Maurice C, Maran M, et al. Changes in HIV RNA viral load, CD4+ T‐cell counts, and levels of immune activation markers associated with anti‐tuberculosis therapy and cotrimoxazole prophylaxis among HIV‐infected tuberculosis patients in Abidjan, Cote d'Ivoire. J Med Virol. 2005;75(2):202–8. [DOI] [PubMed] [Google Scholar]
- 63. Boulware DR, Callens S, Pahwa S. Pediatric HIV immune reconstitution inflammatory syndrome. Curr Opin HIV AIDS. 2008;3(4):461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tsondai P Wilkinson L, Wilkinson L, Henwood R, Ullauri A, Cassidy T, Tutu S, et al. Retention and viral suppression outcomes of patients enrolled in family ART adherence clubs in Cape Town, South Africa. 9th IAS Conference on HIV Science; 2017. July 23‐26; Paris. [Google Scholar]
- 65. International AIDS Society (IAS) . Differentiated care for HIV: a decision framework for differentiated antiretroviral therapy delivery for children, adolescents, and pregnant and breastfeeding women. Durban: IAS; 2017. [cited 2018 June 15]. Available from: http://www.differentiatedcare.org. [Google Scholar]
- 66. Romero‐Daza N, Ruth A, Denis‐Luque M, Luque JS. An alternative model for the provision of services to HIV‐positive orphans in Haiti. J Health Care Poor Underserved. 2009;20 4 Suppl:36–40. [DOI] [PubMed] [Google Scholar]
- 67. Tsondai PR, Wilkinson LS, Grimsrud A, Mdlalo PT, Ullauri A, Boulle A. High rates of retention and viral suppression in the scale‐up of antiretroviral therapy adherence clubs in Cape Town, South Africa. J Int AIDS Soc. 2017;20 Suppl 4:21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Venables E, Towriss C, Rini Z, Nxiba X, Solomon S, Cassidy T, et al. “If I'm not in the club, I have to move from one chair to another.” A qualitative evaluation of patient experiences of adherence clubs in Khayelitsha and Gugulethu, South Africa. 9th IAS Conference on HIV Science; 2017. July 23‐26; Paris. [Google Scholar]
- 69. International AIDS Society (IAS) . Differentiated care for HIV: a decision framework for antiretroviral therapy delivery. Durban: IAS; 2017. [cited 2018 June 24]. Available from: http://www.differentiatedcare.org [Google Scholar]
- 70. Graves JC, Elyanu P, Schellack CJ, Asire B, Prust ML, Prescott MR, et al. Impact of a Family Clinic Day intervention on paediatric and adolescent appointment adherence and retention in antiretroviral therapy: a cluster randomized controlled trial in Uganda. PLoS ONE. 2018;13(3):e0192068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Richter L. An introduction to family‐centred services for children affected by HIV and AIDS. J Int AIDS Soc. 2010;13 Suppl 2:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Myer L, Abrams EJ, Zhang Y, Duong J, El‐Sadr WM, Carter RJ. Family matters: co‐enrollment of family members into care is associated with improved outcomes for HIV‐infected women initiating antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;67 Suppl 4:S243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tolle MA. A package of primary health care services for comprehensive family‐centred HIV/AIDS care and treatment programs in low‐income settings. Trop Med Int Health. 2009;14(6):663–72. [DOI] [PubMed] [Google Scholar]
- 74. Tonwe‐Gold B, Ekouevi DK, Bosse CA, Toure S, Kone M, Becquet R, et al. Implementing family‐focused HIV care and treatment: the first 2 years’ experience of the mother‐to‐child transmission‐plus program in Abidjan, Cote d'Ivoire. Trop Med Int Health. 2009;14(2):204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McNairy ML, Abrams EJ, Rabkin M, El‐Sadr WM. Clinical decision tools are needed to identify HIV‐positive patients at high risk for poor outcomes after initiation of antiretroviral therapy. PLoS Med. 2017;14(4):e1002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ferrand RA, Simms V, Dauya E, Bandason T, McHugh G, Mujuru H, et al. The effect of community‐based support for caregivers on the risk of virological failure in children and adolescents with HIV in Harare, Zimbabwe (ZENITH): an open‐label, randomised controlled trial. Lancet Child Adolesc Health. 2017;1(3):175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Teasdale CA, Abrams EJ, Coovadia A, Strehlau R, Martens L, Kuhn L. Adherence and viral suppression among infants and young children initiating protease inhibitor‐based antiretroviral therapy. Pediatr Infect Dis J. 2013;32(5):489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Giannattasio A, Albano F, Giacomet V, Guarino A. The changing pattern of adherence to antiretroviral therapy assessed at two time points, 12 months apart, in a cohort of HIV‐infected children. Expert Opin Pharmacother. 2009;10(17):2773–8. [DOI] [PubMed] [Google Scholar]


