SUMMARY
Mast cells can be found in close proximity to peripheral nerve endings where, upon activation, they release a broad range of pro-inflammatory cytokines and chemokines. However, the precise mechanism underlying this so-called neurogenic inflammation and associated pain has remained elusive. Here we report that the mast-cell-specific receptor Mrgprb2 mediates inflammatory mechanical and thermal hyperalgesia and is required for recruitment of innate immune cells at the injury site. We also found that the neuropeptide substance P (SP), an endogenous agonist of Mrgprb2, facilitates immune cells’ migration via Mrgprb2. Furthermore, SP activation of the human mast cell led to the release of multiple pro-inflammatory cytokines and chemokines via the human homolog MRGPRX2. Surprisingly, the SP-mediated inflammatory responses were independent of its canonical receptor, neurokinin-1 receptor (NK-1R). These results identify Mrgprb2/X2 as an important neuroimmune modulator and a potential target for treating inflammatory pain.
In Brief
Green et al. show that activation of the mast cell receptor Mrgprb2/X2 by the neuropeptide substance P leads to cytokine release and recruitment of immune cells contributing to inflammatory pain.
INTRODUCTION
As one of the key effector cells in the inflammatory process, mast cells are an important link between the nervous and immune systems. These immune cells can be found in close proximity to peripheral nerve endings and, due to their significant spatial advantages over other innate immune cells, are one of the first to respond to sensory nerve activation (Dothel et al., 2015). Upon activation by neuropeptides, mast cells can release a broad range of pro-inflammatory cytokines and chemokines (Héron and Dubayle, 2013). Mast cells are also involved in the recruitment of a variety of innate immune cells, further facilitating the inflammatory cascade and sensitization of peripheral afferents, which underlies the concept of neurogenic inflammation. This crosstalk between neurons and mast cells is implicated in many pathologies, including post-surgical pain (Yasuda et al., 2013), migraine, and arthritis (Ren and Dubner, 2010).
Activation of mast cells can heavily influence the subsequent inflammatory infiltrate, including recruitment of neutrophils, monocytes, and macrophages (Malaviya et al., 1996; Theoharides et al., 2007; Wezel et al., 2015). Concurrent with this immune cell recruitment are elevations in pro-inflammatory factors including tumor necrosis factor α (TNFα), interleukins, and the CCL family (Theoharides et al., 2012). Degranulation and cytokine release by mast cells are induced by activation of a variety of cell-surface receptors, including the Fc receptors and G-protein-coupled receptors (GPCRs) (Galli et al., 2005). However, the exact mechanism by which mast cells are activated after injury and release these inflammatory mediators is still unknown.
Mas-related G-protein-coupled receptors (Mrgprs) are a family of GPCRs expressed primarily on sensory neurons where they function as itch receptors (Liu and Dong, 2015). Recently, Mrgprb2 was identified as the mast cell receptor for basic secretagogues in mice (McNeil et al., 2015). Both Mrgprb2 and its human ortholog MRGPRX2 are selectively expressed on connective tissue mast cells where they can be activated by various basic secretagogues. Importantly, Mrgprb2 knockout does not impair the canonical IgE-enabled mast cell signaling, though mast cell activation via secretagogues such as compound 48/80 and substance P (SP) is abolished in Mrgprb2 mutant mice. Although Mrgprb2 has been shown to be activated by many peptidergic drugs, its activation by endogenous proinflammatory factors has yet to be elucidated.
Studies have shown that activation of mast cells by compound 48/80 leads to significant edema, weal, and flare that is marked by an influx of innate immune cells with a corresponding increase in peripheral afferent sensitivity (Chatterjea et al., 2012; Héron and Dubayle, 2013). To examine the role of Mrgprb2 in inflammation, we tested Mrgprb2-deficient mice (Mrgprb2−/−) in two inflammatory pain models, the incision model of postoperative pain and Complete Freund’s Adjuvant (CFA). We found that Mrgprb2−/− mice had reductions in pain hypersensitivity in both models. Moreover, Mrgprb2−/− mice had a significant reduction in recruitment of innate immune cells at the site of injury. SP activation of mast cells via Mrgprb2 promoted recruitment of innate immune cells and led to the release of multiple cytokines and chemokines. Using both NK-1 receptor knockout mice and its antagonists, both SP-mediated immune cell recruitment and cytokine release was found to be independent of the canonical SP receptor. The present study identifies the mast cell receptor Mrgprb2 as an important bridge between the nervous and immune systems through its role in innate immune cell recruitment via activation by the neuropeptide SP. Moreover, it challenges our current understanding of the NK-1 receptor as the primary facilitator of SP-generated peripheral neurogenic inflammation and pain.
RESULTS
Mrgprb2−/− Mice Are Resistant to Inflammation-Induced Hypersensitivity in Models of Inflammatory Pain
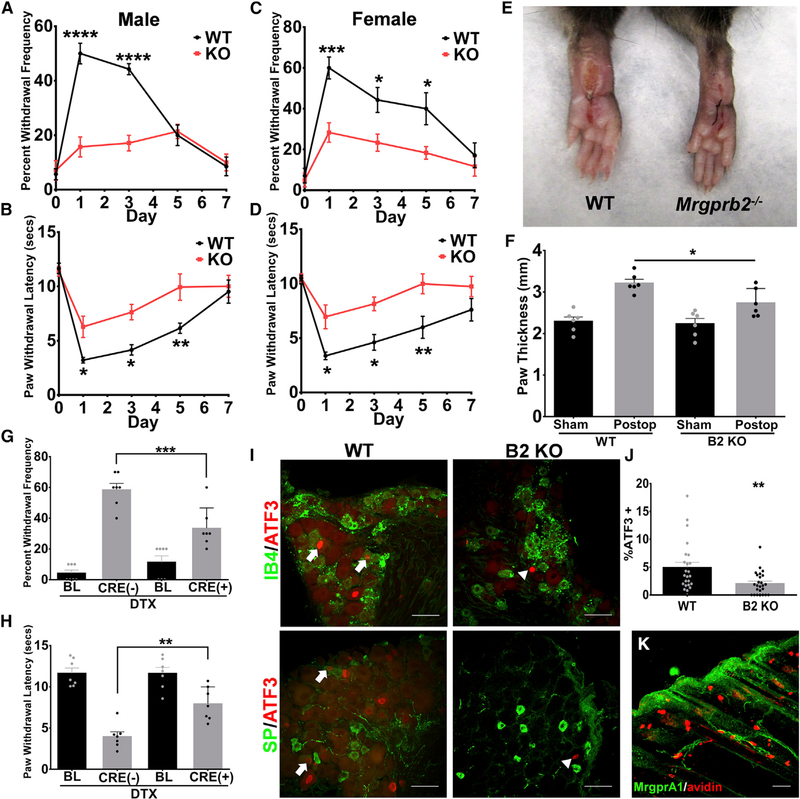
To evaluate the role of Mrgprb2 in inflammation, we utilized a preclinical model of inflammatory pain, the postoperative incision model (Pogatzki and Raja, 2003). At 24 h post hindpaw incision, there were significant increases in both mechanical and thermal hypersensitivity in wild-type (WT) animals compared to baseline (Figures 1A and 1B). However, compared to WT, Mrgprb2−/− male mice had significant reductions in both thermal and mechanical hypersensitivity. Both inflammation and pain hypersensitivity peaked at approximately 24 h post incision with animals returning to baseline at around day 7. These findings were also consistent in female mice that underwent the same incision injury (Figures 1C and 1D). Compared to sham, there was noticeable swelling 24 h post incision, but this was reduced in the Mrgprb2−/−mice (Figures 1E and 1F). Additionally, similar results were observed in the CFA model of inflammation, whereby both mechanical and thermal hypersensitivity were reduced in Mrgprb2−/− mice (Figure S1). To further define the role of Mrgprb2 in inflammatory pain, Mrgprb2+ mast cells were selectively ablated by injecting the cytotoxic protein diphtheria toxin (DTX) into Mrgprb2Cre transgenic mice crossed with a Cre-dependent ROSA26dtr line expressing the human diphtheria toxin receptor (DTR) (Han et al., 2013). Compared to DTX-treated Mrgprb2Cre(−) littermates, DTX-treated Mrgprb2Cre(+) mice had reductions in both mechanical (Figure 1G) and thermal hypersensitivity (Figure 1H)that were similar in magnitude to that found in Mrgprb2−/− mice, further pointing to the important role Mrgprb2-expressing mast cells have in this model of inflammatory pain.
Figure 1. Mrgprb2−/− Mice Exhibit Significantly Less Inflammation-Induced Hypersensitivity in an Incision Model of Inflammatory Pain.
(A–D) An incision-induced model of postoperative pain was performed on WT or Mrgprb2−/− mice. Mechanical (A) and thermal (B) hypersensitivity were measured starting 24 h after incision for up to 7 days. (BL, baseline, no difference was observed in baseline between WT and Mrgprb2−/− mice.) Female mice were also tested for mechanical (C) and thermal (D) hypersensitivity starting 24 h after incision for up to 7 days.
(E) Representative images of postoperative WT and Mrgprb2−/− hindpaws 24 h after incision.
(F) Quantification of incision-induced inflammation, measured by paw thickness, in WT and Mrgprb2−/− mice.
(G and H) Mechanical (G) and thermal (H) hypersensitivity was measured 24 h after incision in DTX-treated Mrgprb2Cre(−) DTR and Mrgprb2Cre(+) DTR mice.
(I) Confocal microscopy immunofluorescence images of DRG sections comparing WT to Mrgprb2−/− (B2 KO) mice in the incision model of postoperative pain. In the top panel, arrows point to ATF-positive nuclei (red) co-stained with IB4 (green). The bottom panel arrows point to ATF-positive nuclei (red) co-stained with substance P (SP, green). Scale bars indicate 50 μm.
(J) Graph of percentage of ATF+ DRG nuclei comparing postoperative WT and Mrgprb2−/− mice.
(K) Confocal image of a hindpaw skin section stained with anti-GFP antibody (green) to visualize MrgprA1-GFP expressing nerve fibers in the dermis. The section was counterstained with Avidin to label mast cells. Scale bar indicates 50 μm.
Data were analyzed using two-tailed Student’s t test and two-way ANOVA with Bonferroni’s post hoc test (> 2 groups); *p < 0.05, **p<0.01, ****p < 0.001, n = 6–7/group; error bar, SEM.
Because of mast cells proximity with sensory neurons, we next examined whether Mrgprb2−/− mice had reduced nerve injury in the incision model. After incision injury, neuronal activation was measured in lumbar 4 and 5 dorsal root ganglia (L4 and L5 DRG) via induction of activating transcription factor 3 (ATF3), a marker for nerve injury in the DRG (Tsujino et al., 2000). Although we observed low induction of ATF3 after incision, consistent with this type of injury model (Flatters, 2008), some ATF3+ neurons were co-stained with non-peptidergic neuron marker IB4 or peptidergic neuron marker SP (Figure 1I). We also saw significant decrease in ATF3 staining in Mrgprb2−/− mice compared to WT 24 h after incision injury (Figure 1J). These results further highlight the connection between mast cells and peripheral afferents and the significance Mrgprb2 has in mediating this link.
Although studies have implicated SP-activating Mrgpra1 (Azimi et al., 2017), we observed no expression of Mrgpral on mast cells in the dermis of MrgprA1-GFP-expressing mice (Figure 1K). Taken together, these findings underscore the importance of mast cells in inflammation and, for the first time, identify Mrgprb2 as contributing to mast-cell-mediated inflammatory pain.
Involvement of Mrgprb2 Mast Cell Receptor in Immune Cell Recruitment in a Postoperative Model of Inflammation and Pain
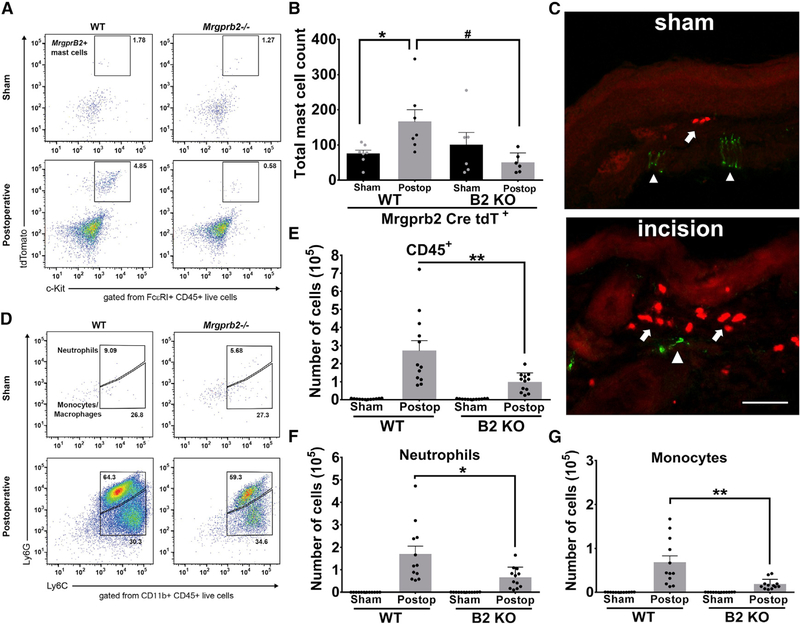
To ensure that the reductions in inflammatory pain were not due to loss of mast cells in Mrgprb2−/− mice, we used the tdTomato fluorescent protein under the Mrgprb2 promoter (Mrgprb2-Cre tdT). Flow cytometric analysis of tdTomato expressing c-Kit+ FcεRI+ mast cells found no difference between sham Mrgprb2-Cre tdT+ WT or Mrgprb2-Cre tdT+ Mrgprb2−/− mice (Figures 2A and 2B). At 24 h post incision, there was a significant increase in Mrgprb2-Cre tdT+ WT mast cells; however, Mrgprb2-Cre tdT+ Mrgprb2−/− mice had no significant increase in mast cells compared to sham controls (Figures 2A and 2B). In the hindpaw, mast cells were found near peripheral nerve endings, with concomitant accumulation at the incision site (Figure 2C).
Figure 2. Involvement of Mrgprb2 in Immune Cell Recruitment in a Postoperative Model of Inflammatory Pain.
(A) Representative flow cytometric profiles of biopsies taken from Mrgprb2-Cre tdT WT or Mrgprb2-Cre tdT Mrgprb2−/− hindpaw skin collected from sham or incision-injured animals. Numbers indicate the percentage of cells pre-gated on viability, CD45+, FcεRI+.
(B) The absolute number of Mrgprb2-Cre tdT + mast cells was calculated from the flow cytometric profile for each mouse.
(C) Confocal image of mouse hindpaw sections comparing sham to incision. Mrgprb2-Cre tdT + mast cells (red) are identified with arrows. Peripheral afferents (green) are stained with Neurofilament 200 and are identified with arrowheads. Scale bars indicate 100 μm.
(D) Representative flow cytometric profiles of biopsies taken from WT or Mrgprb2−/− hindpaw skin 24 h after incision injury. Numbers indicate the percentage of cells pre-gated on viability, CD45+, CD11b+.
(E-G) The absolute number of cells are shown for CD45+ cells (E), CD11b+Ly6G+ neutrophils (F), or CD11b+Ly6G-Ly6C+ monocytes (G).
Data were analyzed using two-tailed Student’s t test. *p < 0.05, **p < 0.01, n = 11/group; error bar, SEM.
As mast cells contribute in the recruitment of innate immune cells during the first phase of inflammation, we hypothesized that Mrgprb2 may also play a role in recruiting immune cells to the site of injury. To examine the leukocyte population, we used the pan leukocyte marker CD45. Analysis of CD45+ cell counts in sham hindpaw biopsies taken from WT and Mrgprb2−/− mice found no difference (Figure 2D). In contrast, incision injury and the resultant inflammation yielded a large increase in CD45+ cell counts in both WT and Mrgprb2−/− mice, but importantly, there was significantly fewer CD45+ cells in Mrgprb2−/− mice (Figure 2E). Moreover, we found the same immune cell recruitment pattern in (Ly)6G+CD11b+ neutrophils (Figure 2F) or CD11b+Ly6G− monocytes (Figure 2G). Of note, prior to incision injury, Mrgprb2−/− mice had no significant deficits in neutrophil or monocyte populations. Mast cells are important in the recruitment of inflammatory cells, and here we demonstrate that mast-cell-specific receptor Mrgprb2 is a major driver of that recruitment.
Substance P Promotes Innate Immune Cell Recruitment via Activation of Mrgprb2
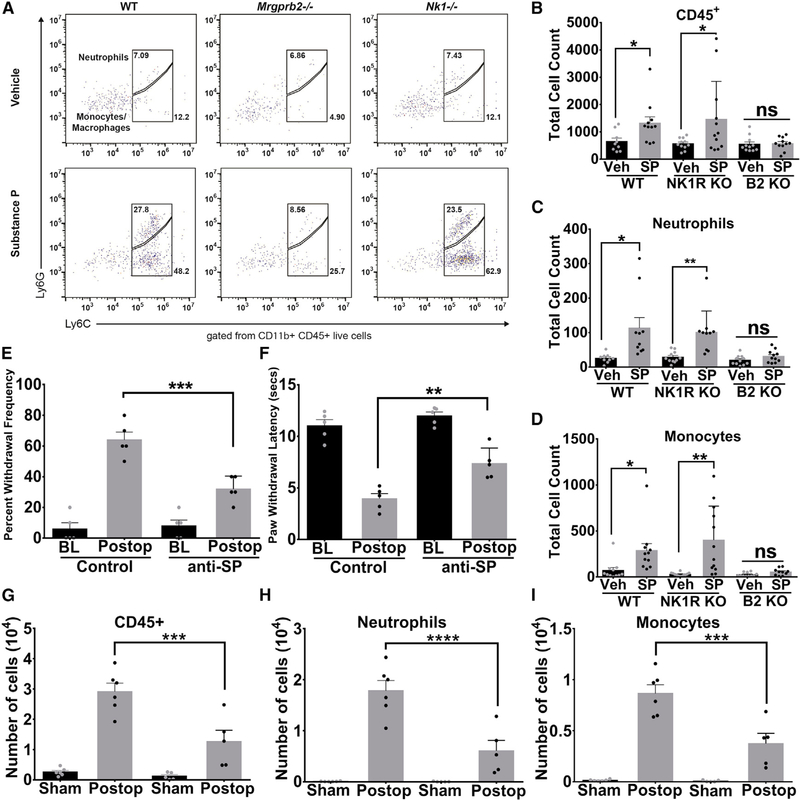
SP levels were found clinically to be elevated following orthopedic surgery, with increasing levels of SP correlating with increasing levels of acute pain intensity (Lisowska et al., 2016). Previously, we found SP to be an endogenous agonist of Mrgprb2 (McNeil et al., 2015), so we tested whether SP injected into the hindpaws of mice was capable of recruiting innate immune cells. Flow cytometric analysis (Figure 3A) of hindpaw biopsies taken 24 h after SP injection found significant increases in CD45+ cells (Figure 3B), neutrophils (Figure 3C), and monocytes (Figure 3D) in WT, but not Mrgprb2−/−, mice. The tachykinin receptor neurokinin-1 (NK-1R) is known to be the canonical receptor for SP. To test the effect of the NK-1 receptor on SP immune cell recruitment, we generated NK-1 receptor-deficient mice (NK-1R−/−). Interestingly we saw similar patterns of innate immune cell recruitment in NK-1R−/− mice as that of WT mice, whereby injections of SP caused significant influx of CD45+ cells, neutrophils, and monocytes (Figures 3B–3D). Therefore, deletion of NK-1 receptor does not have any effect on SP-induced immune cell recruitment. Consistent with this result, using NK-1R GFP knockin mice (Figure S2), we found the NK-1R is not expressed in mast cells (Figure S2A) or primary sensory neurons (Figure S1B). As a positive control, we observed many NK-1R+ neurons in the dorsal horn of the spinal cord as shown by previous studies (King et al., 2005) (Figure S2B). RT-PCR further confirmed that NK-1R is specifically expressed in the spinal cord and not in DRG and mast cells, whereas Mrgprb2 is specifically expressed in mast cells (Figure S2C). These results point to SP as a driver of immune cell recruitment via activation of Mrgprb2 rather than the NK-1 receptor. The finding that SP has a direct role in contributing to inflammation and pain is further supported by the result that immunoneutralization of peripherally released SP reduced both inflammatory mechanical (Figure 3E) and thermal (Figure 3F) hypersensitivity compared to isotype control in an incision model of postoperative pain. As Mrgprb2−/− mice had reduced immune cell infiltration after incision injury, we also examined whether immunoneutralization of peripherally released SP after injury influenced immune cell recruitment. Using flow cytometry (Figure S3A), we found that WT hindpaw biopsies taken 24 h after incision injury had significant increases in CD45+ cells (Figure 3G), neutrophils (Figure 3H), and monocytes (Figure 3I) that could be reversed with injection of anti-SP antibodies prior to surgery. This reduction in infiltrating immune cells due to SP immunoneutralization was concomitant with a reduction in paw swelling (Figure S3B).
Figure 3. Substance P Promotes Innate Immune Cell Recruitment via Mrgprb2.
(A) Representative flow cytometric profiles of biopsies taken from WT, NK-1R−/− or Mrgprb2−/− hindpaw skin injected with vehicle or substance P (50 μM in 10 μL). Numbers indicate the percentage of cells pre-gated on viability, CD45+, CD11b+.
(B-D) The absolute number of each immune cell was calculated from the flow cytometric profile for each mouse and is shown for CD45+ cells (B), CD11b+Ly6G+ neutrophils (C), or CD11b+Ly6G-Ly6C+ monocytes (D).
(E and F) Anti-substance-P antibodies (15 μg) or vehicle were injected into the hindpaws of WT and Mrgprb2−/− mice prior to incision-induced injury. At 23 h after incision, anti-substance-P antibodies (15 μg) were injected again, and mechanical (E) and thermal (F) hypersensitivity were measured 1 h later (BL, baseline). (G-I) Biopsies taken from WT hindpaw skin treated with anti-substance-P or isotype control antibodies 24 h after incision injury. The absolute number of cells are shown for CD45+ cells (G), CD11b+Ly6G+ neutrophils (H), or CD11b+Ly6G-Ly6C+ monocytes (I).
Data were analyzed using two-tailed Student’s t test. *p < 0.05, **p < 0.01, n = 13/group for WT, NK-1R−/−, and Mrgprb2−/− flow cytometry, n = 5/group for behavior, n = 6/group for flow analysis of anti-SP-treated tissue; error bar, SEM.
Substance-P-Treated Human Mast Cells Release Inflammatory Cytokines and Chemokines via MRGPRX2 Activation
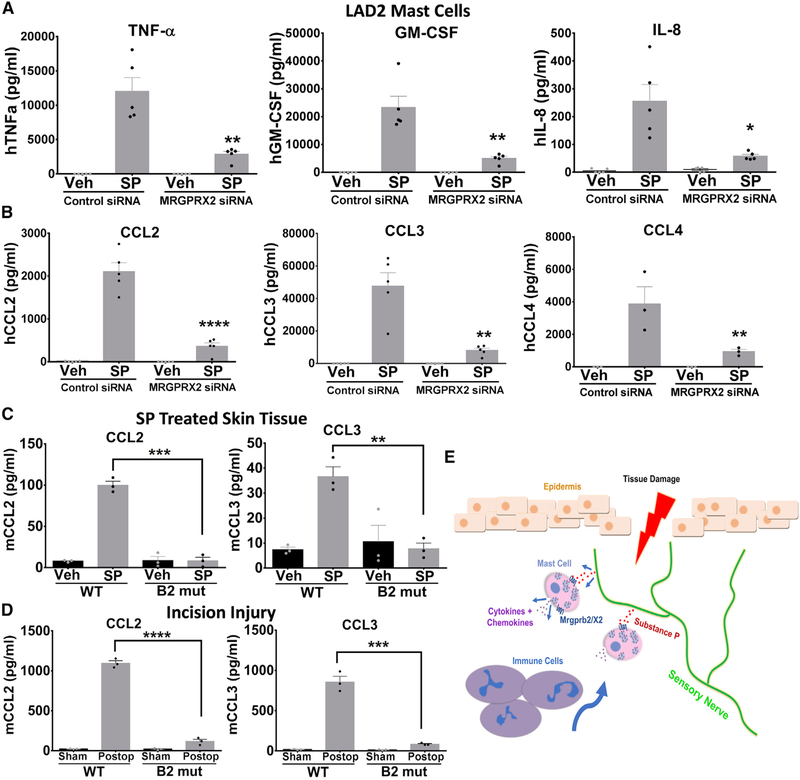
Our findings revealed that activation of resident mast cells by neuropeptides leads to the recruitment of innate immune cells, yet it was unclear which soluble mediators released were required for this effect. Therefore, we next explored the mechanism by which mast cells recruit immune cells to the periphery by examining the profile of cytokines and chemokines released. To test this, we applied SP or vehicle to human mast cell line LAD2 cells, shown previously to endogenously express MRGPRX2, the human homolog of mouse Mrgprb2, pre-treated with MRGPRX2-siRNA or control siRNA and measured the collected supernatant for common human inflammatory cytokines and chemokines using multi-analyte ELISAs. Among the cytokines that were tested—namely interleukin (IL)1α, IL1β, IL2, IL4, IL6, IL8, IL10, IL12, IL17A, interferon γ (IFNγ), TNFα, and granulocyte macrophage-colony stimulating factor (GM-CSF)—only TNFα, GM-CSF, and IL-8 were induced following stimulation with SP (Figure S4A, left panel). Additionally, an array of chemokines—CCL2, CCL3, CCL4, CCL5, C-X-C motif chemokine (CXCL)10, CXCL11, CXCL9, Eotaxin, CCL17, CCL22, and CXCL1—were measured, and SP only led to significant increases in CCL2, CCL3, and CCL4 (Figure S4B, right panel). We next followed these results up with a more detailed analysis examining each individual cytokine (Figure 4A) and chemokine (Figure 4B) that were found to be elevated in our array dataset. LAD2 cells in which MRGPRX2 was knocked down had significant reductions in all six cytokines and chemokines. Given that we observed the largest fold SP induction in chemokines CCL2 and CCL3, along with the previous findings that both can interact with one another to mediate neuronal inflammation (Chui and Dorovini-Zis, 2010; Youssef et al., 1998), we focused on these two chemokines for the in vivo studies. We found similar results in vivo, whereby SP injection into the hindpaw of WT mice evoked a significant increase in cytokines CCL2 and CCL3 that was abolished in Mrgprb2−/− mice (Figure 4C). Release in vivo of CCL2 and CCL3 was also decreased in Mrgprb2−/− hindpaw tissue taken 24 h after incision surgery compared to WT mice (Figure 4D).
Figure 4. Substance-P-Treated Human Mast Cells Release Inflammatory Cytokines and Chemokines via MRGPRX2 Activation.
(A and B) LAD2 mast cells were treated with siRNA against MRGPRX2 or control siRNA. Concentrations of (A) cytokines TNF-a, GM-CSF, IL-8, and (B) chemokines CCL2, CCL3, and CCL4 in supernatant taken from vehicle-treated or substance-P-treated (1 μM) LAD2 mast cells were determined by ELISA.
(C) Hindpaw tissue injected with vehicle or substance P (50 μM) was biopsied 24 h later, and CCL2 and CCL3 levels were determined by ELISA.
(D) 24 h after incision injury, hindpaw tissue was biopsied, and CCL2 and CCL3 levels were determined by ELISA.
(E) Diagram depicting the mechanism by which tissue damage and neuronal release of substance P activates MrgprX2/b2, leading to the release of cytokines and the subsequent recruitment of immune cells to the site of injury.
Data were analyzed using a one-way ANOVA. *p < 0.05, **p < 0.01, ****p < 0.001; error bar, SEM.
We next examined whether the NK-1 receptor was involved in SP-induced mediator release from mast cells. LAD2 mast cells were pre-treated with the potent NK-1 receptor antagonist SR 140333 (Jung et al., 1994), and SP-induced release of cytokines and chemokines was measured (Figure S4B). However, there was no significant decrease in SP-induced release of cytokines and chemokines from mast cells, indicating that MRGPRX2 as the primary receptor involved in mediating SP-induced release of these pro-inflammatory mediators.
DISCUSSION
Here we present results identifying the mast cell receptor Mrgprb2 as a crucial driver of neuropeptide-induced immune cell recruitment. Using a preclinical model of postoperative pain, we found reductions in both mechanical and thermal hypersensitivity in Mrgprb2−/− mice. The incision model of inflammatory pain has been shown to differ from that of CFA in regard to TRP channel expression and afferent fiber activation (Barabas and Stucky, 2013). With the incision model of postoperative pain being more clinically relevant, we chose it as the primary focus for the rest of the mechanistic studies. However, both incision and CFA models displayed similar reductions with regards to the magnitude of behavior in Mrgprb2−/− mice, indicating that both have similar mechanisms of activating Mrgprb2+ mast cells after inflammatory insult.
Similar to the studies with Mrgprb2−/− mice, ablation of mast cells positive for Mrgprb2 significantly attenuated but did not abolish incision-induced mechanical and thermal hypersensitivity. This finding could point to other downstream effectors of incision-induced allodynia and inflammation, including keratinocytes or basophils. However, both Mrgprb2−/− mice and mast cell ablation accounted for approximately 70% and 66% return to baseline for mechanical and thermal hypersensitivity, emphasizing the major role this mast cell receptor has in post-incision injury.
This reduction in inflammation observed was not due to loss of resident mast cells in the hindpaw, as there was no difference in tdTomato+ mast cell numbers between WT and Mrgprb2−/− mice. Interestingly, we saw an incision-induced increase in mast cells at only 24 h after injury that was abolished in Mrgprb2−/− mice, implicating non-IgE-mediated migration. Whether this is due to local proliferation, mobilization of mast cell progenitors in the skin, or recruitment from surrounding tissue will need further study.
Mrgprb2−/− mice presented no deficiencies in CD45+ cells, (Ly) 6G+CD11b+ neutrophils, or CD11b+Ly6G− monocytes compared to WT. However, post incision there was a massive increase in innate immune cells at the site of injury in WT animals that was significantly reduced in Mrgprb2−/− mice. Recent studies have shown that ablation of (Ly)6G+CD11b+ neutrophils is not required; rather, the CD11b+Ly6G− population of myeloid cells is sufficient to drive incision-induced inflammatory pain (Ghasemlou et al., 2015). After incision injury, we observed Mrgprb2-mediated reductions in recruitment of both populations. If Mrgprb2-driven infiltration of the CD11b+Ly6G− myeloid population is the main driver of post-incision inflammatory pain, further studies will have to address the role Mrgprb2-mediated recruitment of neutrophils has after injury. Nevertheless, these results point to Mrgprb2 as a key facilitator of immune cell recruitment in response to tissue damage.
The finding that NK-1−/− mice had no reduction in immune cells argues for Mrgprb2 as the primary receptor involved in SP-induced innate immune cell recruitment. Moreover, immuno-neutralization of SP decreased postoperative mechanical and thermal hypersensitivity and reduced immune cell infiltration, further implicating it directly in mediating incision-induced inflammatory pain. The discovery that SP contributes to pain and inflammation led to the development of multiple NK-1 receptor drugs over the past three decades. However, NK-1 receptor antagonists have failed to show analgesic efficacy in multiple clinical trials (Hill, 2000). Additionally, Azimi et al. (2016) have shown off target effects of certain NK-1 receptor antagonists with MRGPRX2. Thus, our findings that Mrgprb2 and the human ortholog MRGPRX2 underlie SP-induced inflammatory immune cell recruitment may provide a novel target for future drug development.
Lastly, we examined what mast cell mechanism initiates the SP-Mrgprb2-induced immune cell recruitment. Significant release of three inflammatory cytokines (TNFα, GM-CSF, and IL-8) due to SP activation of MRGPRX2 was found. Release of mast cell TNFα has also been shown to act as a positive autocrine feedback signal for the production of chemoattractant cytokines GM-CSF and IL-8 (Coward et al., 2002). Along with being a growth factor for monocytes and the recruitment of neutrophils (Francisco-Cruz et al., 2014), GM-CSF was shown to be required for arthritis inflammation and pain (Cook et al., 2013). IL-8 is primarily known for its role as a chemotactic factor for neutrophils and can also produce mechanical hypersensitivity when injected into rat hindpaws (Cunha et al., 1991).
Analysis of supernatants from SP-treated mast cells also uncovered MRGPRX2-mediated elevations in three chemokines, CCL2, CCL3, and CCL4. Although only a few studies have implicated CCL2 and CCL3 in directly sensitizing peripheral neurons, their role in monocytic recruitment may be the main driver in post-incision inflammatory pain (Abbadie et al., 2003; Xia and Sui, 2009). Furthermore, there was no reduction in these cytokines and chemokines after blockade of the NK-1 receptor, confirming MRGPRX2 as the primary receptor underlying their release. Injection of SP into the hindpaw evoked release of two of the primary cytokines elevated in the screening assay, CCL2 and CCL3, and a similar outcome was observed in tissue harvested after hindpaw incision injury. The observation that both cytokines were virtually abolished in Mrgprb2−/− mice further reinforces the LAD2 findings and provides in vivo confirmation for the importance of Mrgprb2 in post-injury immune cell recruitment. The in vivo role of cytokines TNFα, GM-CSF, and IL-8, along with chemokine CCL4, will be investigated in future studies.
Taken together, these studies reveal the mast cell receptor Mrgprb2 as a major downstream effector of neuronal signaling after tissue injury (Figure 4C). Mrgprb2 activation by the neuropeptide SP provides insight into how the peripheral nervous system modulates immune cells. The finding that SP promoted immune cell recruitment via Mrgprb2 rather than NK-1 receptor provides a novel target in treating inflammation and pain.
STAR ⋆ METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Xinzhong Dong (xdong2@jhmi.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse Lines
NK-1R-GFP knockin/knockout mice were generated with homologous recombination in mouse embryonic stem cells by replacing NK-1R open reading frame with GFP such that GFP expression is under the control of NK-1R promoter. NK-1R knockout mice are homozygous for GFP knockin.
Two to four-month old male and female mice backcrossed to C57BL/6 mice for more than 5 generations were tested. WT littermates were used in behavioral experiments with Mrgprb2−/− mice. All experiments were performed with the protocols approved by the Animal Care and Use Committee of Johns Hopkins University School of Medicine. The mice were housed in the vivarium with 12-h light/dark cycle and all the behavioral tests were performed from 9am to 2pm in the light cycle. The housing group was 5 at maximum.
Human mast cell culture
LAD2 cells used in this study were authenticated by measuring histamine and β-hexosaminidase release in response to activation by Compound 48/80. LAD2 (Laboratory of Allergic Diseases 2) male human mast cells were cultured in StemPro-34 SFM medium (Life Technologies) supplemented with 2 mM L-glutamine, 100 U perml penicillin, 50 μg per ml of streptomycin and 100 ng per ml recombinant human stem cell factor (Peprotech). The cell suspensions were seeded at a density of a million cells per ml and maintained at 37°C and 5% CO2.
METHOD DETAILS
Behavioral assays
The behavioral assays were performed with the personnel blinded to the genotypes or drugs injected.
Von Frey methods: Mice were placed in a transparent plastic box (4.5 × 5 × 10 cm) on a metal mesh and acclimatized for 30 min prior to testing. Each mouse was tested more than 5 times at a specific force manually, and the threshold was determined by the lowest force needed to elicit responses more than 50% of the time.
Hargreaves test: Mice were placed under a transparent plastic box (4.5 × 5 × 10 cm) on a glass platform (Plantar Test Apparatus, IITC Life Science). Radiant heat was adjusted to 18% of maximal output and shone on the center of the paws. Each mouse was tested more than 3 times, with each test performed 20 min apart.
CFA injection: Mice were injected with 5 μL of 50% emulsified Complete Freund’s Adjuvant (Sigma, F5881) in normal saline. Incision model: After baselines were measured, mice were anesthetized using 2% isoflurane. The right hind paw was prepared for incision by application of antiseptic betadine solution. The incision model in mice is created by a 5-mm incision beginning 2 mm from the proximal edge of the right heel. Curved forceps elevated the underlying muscle. A mattress suture of 8–0 nylon on a TG175–8 needle were then used to close the incision. Antibiotic ointment (Bacitracin Zinc Ointment) was then applied. Mice were tested 24 h after incision surgery.
Drugs
Complete Fruend’s Adjuvant was purchased from Sigma, Substance P and SR 140333 from Tocris, and Anti-Substance P Antibody for behavior studies was purchased from Millipore Sigma (AB1566) and isotype IgG was used as control.
Immunohistochemistry
Adult male mice up to 6 months of age were anesthetized with chloral hydrate (20 μL /gram of 25mg/mL solution) and perfused with 30 mL 0.1 M phosphate buffered saline (PBS) (pH 7.4, 4°C) followed with 30 mL of fixative (4% paraformaldehyde (vol/vol), 4°C). Spinal cord, dorsal root ganglia, and skin were dissected from the perfused mice. Tissues were post-fixed in fixative at 4° for 2 h. Tissues were cryoprotected in 20% sucrose (wt/vol) for up to 8 h followed by 30% sucrose for 24 h and then sectioned (25 μm width) with a cryostat. The sections on slides were dried at 37°C for 30 min, and fixed with 4% paraformaldehyde at room temperature (RT) for 10 min. The slides were pre-incubated in blocking solution (10% normal goat serum (vol/vol), 0.03% Triton X-100 (vol/vol) in PBS, pH 7.4) for 1 h at RT. The staining included the use of primary antibodies against Neurofilament 200 (mouse specific chicken polyclonal from Aves labs, 1:500 dilution, NFH), GFP (for NK1 and MrgprA1-GFP CR staining; mouse specific chicken polyclonal from Thermo Fisher, 1:500 dilution, A10262), and Substance P (rat monoclonal from Abcam, 1:250 dilution, M09205). To detect IB4 binding, sections were incubated with Griffonia simplicifolia isolectin GS-IB4 Alexa 568 from Invitrogen at 1:500 dilution (I-21412). Sections were incubated in secondary antibodies at 1:500 dilution, Alexa Fluor 488 from Invitrogen (A-11039) for NF 200 and NK1, Invitrogen 547 (A-21247) for Substance P. For avidin staining of mast cells, sections were incubated with avidin sulforhodamine 101 conjugate (Marker Gene Technologies, 1:500 dilution, M1124). Sections were washed three times with PBS and Fluoromount (Southern Biotech) was applied before coverslips were placed over section.
Flow cytometry
Hind paw tissue was collected using a 6mm biopsy punch. Tissue was placed in dissociation media containing Dispase 2 at 1.25 mg/mL (Sigma-Aldrich), Collagenase 2 at 2 mg/mL (Thermo Fisher Scientific) and Collagenase 4 at 2mg/mL (Thermo Fisher Scientific) and allowed to incubate at 37°C for approximately 75 mins. Live versus Dead cells were stained using Live/Dead Fixable Aqua Dead Cell Stain Kit (Thermo Fisher).
The following antibodies were from BioLegend: CD45-FITC (Cat. 103108), Cd11b-PE/Dazzle (Cat. 101256), Ly6C-APC (Cat. 128016), Ly6G-BV421 (Cat. 127628). Cells were treated with CD16/CD32 Fc Block (Cat.101320) 10 min before the addition of specific antibodies. Data were collected using a CytoFLEX LX (Beckman Coulter), and the data were analyzed using FlowJo (TreeStar). Mast cells were gated as live c-Kit+ FcεRI+, neutrophils were gates as live CD11b+Ly6G+, monocytes were gated as live CD11b+Ly6G−Ly6C+.
siRNA transfection of LAD2 cells
Expression of MRGPRX2 was downregulated with ON-TARGET plus SMARTpool siRNA against MRGPRX2 and control siRNA from Dharmacon. LAD2 cells were washed with medium, suspended at 0.5 × 106 cells per well, and transfected with 100 nm MRGPRX2 siRNA and control siRNA in antibiotic-free StemPro medium using Lipofectamine 3000 (Life Technologies) according to the manufacturer’s instruction at 37°C/5% CO2. Knockdown was confirmed by qPCR. Knockdown efficiency was 84.2% ± 2.1. At 48 h supernatant was collected and frozen at −80°C for later analysis.
Cytokine and Chemokine ELISAs
Cytokines and chemokines were measured using Human Common Cyokines Multi-Analyte ELISArray Kit and Human Common Chemokines Multi-Analyte ELISArray Kit (QIAGEN). IL-8, GM-CSF, TNFα, CCL2, CCL3, and CCL4 levels were measured using ELISA DuoSets (R&D Systems) according to manufacturer’s protocol. Briefly, cells were stimulated, and supernatants were harvested at 24 h time point and stored at −80C until used for ELISA. Data are representative of three independent experiments.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data are presented as standard error of the mean SEM and n represents the number of mice analyzed. The distribution of the variables in each experimental group was assumed normal. Most statistical comparisons were conducted by two-tailed, unpaired Student’s t test. or one- or two-way ANOVA with Bonferroni’s post hoc test (> 2 groups). Data were analyzed by GraphPad. A statistically significant difference was defined as p < 0.05. Error bars are SEM.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rat Anti-SP | Abcam | Cat# Ab7340; RRID: AB_305866 |
| Chicken anti-GFP | Life Technologies | Cat# A10262; RRID: AB_2534023 |
| Alexa Fluor 488 | Invitrogen | Cat# A11039; RRID: AB_142924 |
| Alexa Fluor 547 | Invitrogen | Cat# A21247; RRID: AB_141778 |
| GS-IB4 Alexa 568 | Invitrogen | Cat# I21412 |
| rabbit anti-NF200 | Chemicon | Cat# AB1982; RRID: AB_2313731 |
| Anti-mouse CD16/32 Fc Blocker | BioLegend | Cat# 101320; RRID: AB_1574975 |
| CD45-FITC | BioLegend | Cat# 103108; RRID: AB_312973 |
| PerCP/Cy5.5 anti-mouse CD8a | BioLegend | Cat# 100733; RRID: AB_2075239 |
| Anti-mouse CD45 PE | BioLegend | Cat# 103105; RRID: AB_312970 |
| Anti-mouse CD11b BV510 | BioLegend | Cat# 101245; RRID: AB_2561390 |
| Anti-mouse c-Kit BV605 | BioLegend | Cat# 135121; RRID: AB_2562040 |
| Anti-mouse FcεRI FITC | BioLegend | Cat# 134305; RRID: AB_1626102 |
| Anti-mouse IgE FITC | BioLegend | Cat# 406905; RRID: AB_493288 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| CFA | Sigma | Cat# F5881 |
| Substance P | Tocris | Cat# 1156 |
| SR 140333 | Tocris | Cat# 4012 |
| Anti-SP antibody for pain studies | Millipore Sigma | Cat# AB1566; RRID: AB_11213407 |
| Diphtheria Toxin | Sigma | Cat# D0564 |
| Isotype IgG | Millipore Sigma | Cat# NS01L |
| ZombieUV viability dye | BioLegend | Cat# 423107 |
| Human SCF | Peprotech | Cat# 300–07 |
| Avidin sulforhodamine | Marker Gene Technologies | Cat# M1124 |
| Goat Alexa Avidin | Sigma | Cat# 189727 |
| Critical Commercial Assays | ||
| Mouse CCL2 ELISA | R&D Systems | Cat# DY479 |
| Mouse CCL3 ELISA | R&D Systems | Cat# DY450 |
| Human TNF-alpha ELISA | R&D Systems | Cat# DY210 |
| Human CCL2 ELISA | R&D Systems | Cat# DY279 |
| Human CCL3 ELISA | R&D Systems | Cat# DY270 |
| Human CCL4 ELISA | R&D Systems | Cat# DY271 |
| Human GM-CSF ELISA | R&D Systems | Cat# DY215 |
| Human IL-8 ELISA | R&D Systems | Cat# DY208 |
| Human Inflammatory Cytokines Multi-Analyte ELISArray | QIAGEN | Cat# MEH-0004a |
| Experimental Models: Cell Lines | ||
| LAD2 | NIH | Metcalfe Lab |
| Experimental Models: Organisms/Strains | ||
| Mouse: Mrgprb2 Mut | McNeil et al., 2015 | N/A |
| Software and Algorithms | ||
| Prism 6.01 | GraphPad Software | N/A |
| FlowJo | Tree Star | N/A |
| Other | ||
| StemPro-34 SFM (1X) | Thermo Fisher Scientific | Cat# 10639011 |
| isoflurane Abbott Laboratories CAS# 26675–46–7 | Abbott Laboratories | Cas# 26675–46–7 |
| Lipofectamine 3000 | Life Technologies | Cat# L3000015 |
| SMARTpool: ON-TARGETplus MRGPRX2 siRNA | Dharmacon | Cat# L-005666–00–0005 |
| ON-TARGETplus Non-targeting Pool | Dharmacon | Cat# D-001810–10–05 |
Highlights.
The mast cell receptor Mrgprb2 is required for neurogenic inflammatory pain
Substance P (SP) recruits immune cells via Mrgprb2 independent of the NK-1 receptor
SP activation of Mrgprb2 and its human ortholog MRGPRX2 releases cytokines
Mrgprb2/X2 is a target for treating pain
ACKNOWLEDGMENT
We thank Dr. Qian Xu for his assistance with the NK-1R-GFP knockin/knockout mice and Mark Sabbagh for assistance in confocal imaging. The study was supported by grants from the US National Institutes of Health to X.D. (R01NS05479 and R01AI135186) and D.P.G. (T32NS070201 and F32GM121030). X.D. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interest.
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and can be found with this article online at https://doi.org/10.10167j.neuron.2019.01.012.
REFERENCES
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, and Forrest MJ (2003). Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc. Natl. Acad. Sci. USA 700, 7947–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi E, Reddy VB, Shade KC, Anthony RM, Talbot S, Pereira PJ, and Lerner EA (2016). Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight 7, e89362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, and Lerner EA (2017). Substance P activates Mas-related G protein-coupled receptors to induce itch. J. Allergy Clin. Immunol 740, 447–453.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas ME, and Stucky CL (2013). TRPV1, but notTRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity. Mol. Pain 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjea D, Wetzel A, Mack M, Engblom C, Allen J, Mora-Solano C, Paredes L, Balsells E, and Martinov T (2012). Mast cell degranulation mediates compound 48/80-induced hyperalgesia in mice. Biochem. Biophys. Res. Commun 425, 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui R, and Dorovini-Zis K (2010). Regulation ofCCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J. Neuroinflammation 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AD, Pobjoy J, Sarros S, Steidl S, Dürr M, Lacey DC, and Hamilton JA (2013). Granulocyte-macrophage colony-stimulating factor is a key mediator in inflammatory and arthritic pain. Ann. Rheum. Dis 72, 265–270. [DOI] [PubMed] [Google Scholar]
- Coward WR, Okayama Y, Sagara H, Wilson SJ, Holgate ST, and Church MK (2002). NF-kappa B and TNF-alpha: a positive autocrine loop in human lung mast cells? J. Immunol 769, 5287–5293. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Lorenzetti BB, Poole S, and Ferreira SH (1991). Interleukin-8 as a mediator of sympathetic pain. Br. J. Pharmacol 704, 765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dothel G, Barbaro MR, Boudin H, Vasina V, Cremon C, Gargano L, Bellacosa L, De Giorgio R, Le Berre-Scoul C, Aubert P, et al. (2015). Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 748, 1002–1011.e4. [DOI] [PubMed] [Google Scholar]
- Flatters SJ (2008). Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). Pain 735, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco-Cruz A, Aguilar-Santelises M, Ramos-Espinosa O, Mata-Espinosa D, Marquina-Castillo B, Barrios-Payan J, and Hernandez-Pando R (2014). Granulocyte-macrophage colony-stimulating factor: not just another haematopoietic growth factor. Med. Oncol 37, 774. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, and Tsai M (2005). Mast cells in the development of adaptive immune responses. Nat. Immunol 6, 135–142. [DOI] [PubMed] [Google Scholar]
- Ghasemlou N, Chiu IM, Julien JP, and Woolf CJ (2015). CD11b+Ly6G-myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc. Natl. Acad. Sci. USA 772, E6808–E6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. (2013). A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci 76, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron A, and Dubayle D (2013). A focus on mast cells and pain. J. Neuroimmunol 264, 1–7. [DOI] [PubMed] [Google Scholar]
- Hill R (2000). NK1 (substance P) receptorantagonists-why arethey not analgesic in humans? Trends Pharmacol. Sci 27, 244–246. [DOI] [PubMed] [Google Scholar]
- Jung M, Calassi R, Maruani J, Barnouin MC, Souilhac J, Poncelet M, Gueudet C, Emonds-Alt X, Soubrie P, Breliere JC, et al. (1994). Neuropharmacological characterization of SR 140333, a non peptide antagonist of NK1 receptors. Neuropharmacology 33, 167–179. [DOI] [PubMed] [Google Scholar]
- King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP Jr., Vanderah TW, Hunt SP, Hruby VJ, Lai J, and Porreca F (2005). Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain 776, 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowska B, Siewruk K, and Lisowski A (2016). SubstancePand acute pain in patients undergoing orthopedic surgery. PLoS ONE 77, e0146400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, and Dong X (2015). The role of the Mrgpr receptor family in itch. Handb. Exp. Pharmacol 226, 71–88. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Ross E, and Abraham SN (1996). Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 387, 77–80. [DOI] [PubMed] [Google Scholar]
- McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, and Dong X (2015). Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 579, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogatzki EM, and Raja SN (2003). A mouse model of incisional pain. Anesthesiology 99, 1023–1027. [DOI] [PubMed] [Google Scholar]
- Ren K, and Dubner R (2010). Interactions between the immune and nervous systems in pain. Nat. Med 76, 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Kempuraj D, Tagen M, Conti P, and Kalogeromitros D (2007). Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev 277, 65–78. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, and Kalogeromitros D (2012). Mast cells and inflammation. Biochim. Biophys. Acta 7822, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, and Noguchi K (2000). Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneuronsiAnovel neuronal markerof nerve injury. Mol. Cell. Neurosci 15, 170–182. [DOI] [PubMed] [Google Scholar]
- Wezel A, Lagraauw HM, van der Velden D, de Jager SC, Quax PH, Kuiper J, and Bot I (2015). Mast cells mediate neutrophil recruitment during atherosclerotic plaque progression. Atherosclerosis 241, 289–296. [DOI] [PubMed] [Google Scholar]
- Xia M, and Sui Z (2009). Recent developments in CCR2 antagonists. Expert Opin. Ther. Pat 19, 295–303. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Kido K, Ohtani N, and Masaki E (2013). Mast cell stabilization promotes antinociceptive effects in a mouse model of postoperative pain. J. Pain Res 6, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef S, Wildbaum G, Maor G, Lanir N, Gour-Lavie A, Grabie N, and Karin N (1998). Long-lasting protective immunity to experimental autoimmune encephalomyelitis following vaccination with naked DNA encoding C-C chemokines. J. Immunol 161, 3870–3879. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.