Abstract
Background & Aims
Serotonin (5-hydroxytryptamine [5-HT]) is synthesized mainly within enterochromaffin (EC) cells in the gut, and tryptophan hydroxylase 1 (Tph1) is the rate-limiting enzyme for 5-HT synthesis in EC cells. Accumulating evidence suggests the importance of gut microbiota in intestinal inflammation. Considering the close proximity of EC cells and the microbes, we investigated the influence of gut-derived 5-HT on the microbiota and the susceptibility to colitis.
Methods
Gut microbiota of Tph1-/- and Tph1+/- mice were investigated by deep sequencing. Direct influence of 5-HT on bacteria was assessed by using in vitro system of isolated commensals. The indirect influence of 5-HT on microbiota was assessed by measuring antimicrobial peptides, specifically β-defensins, in the colon of mice and HT-29 colonic epithelial cells. The impact of gut microbiota on the development of dextran sulfate sodium–induced colitis was assessed by transferring gut microbiota from Tph1-/- mice to Tph1+/- littermates and vice versa, as well as in germ-free mice.
Results
A significant difference in microbial composition between Tph1-/- and Tph1+/- littermates was observed. 5-HT directly stimulated and inhibited the growth of commensal bacteria in vitro, exhibiting a concentration-dependent and species-specific effect. 5-HT also inhibited β-defensin production by HT-29 cells. Microbial transfer from Tph1-/- to Tph1+/- littermates and vice versa altered colitis severity, with microbiota from Tph1-/- mice mediating the protective effects. Furthermore, germ-free mice colonized with microbiota from Tph1-/- mice exhibited less severe dextran sulfate sodium–induced colitis.
Conclusions
These findings demonstrate a novel role of gut-derived 5-HT in shaping gut microbiota composition in relation to susceptibility to colitis, identifying 5-HT–microbiota axis as a potential new therapeutic target in intestinal inflammatory disorders.
Keywords: 5-Hydroxytryptamine (5-HT), Tph1, Microbiota, β-defensins, Colitis
Abbreviations used in this paper: Abx, antibiotics; AMP, antimicrobial peptide; CD, Crohn’s disease; DMSO, dimethyl sulfoxide; DNBS, dinitrobenzene sulfonic acid; DSS, dextran sulfate sodium; EC, enterochromaffin; EEC, enteric endocrine cell; ELISA, enzyme-linked immunosorbent assay; ERK1/2, extracellular signal-regulated kinase-1 and -2; FDR, false discovery rate; GF, germ-free; GI, gastrointestinal; GIL, gut isolate library; hBD, human β-defensin; IBD, inflammatory bowel disease; IL, interleukin; mBD, mouse β-defensin; MPO, myeloperoxidase; OD, optical density; OTU, operational taxonomic unit; PBS, phosphate-buffered saline; PCoA, principal coordinate ordination; PPAR-γ, peroxisome proliferator-activated receptor gamma; qRT-PCR, quantitative real-time polymerase chain reaction; SCFA, short-chain fatty acid; Tph, tryptophan hydroxylase; UC, ulcerative colitis; WT, wild-type; 5-HT, 5-hydroxytryptamine; 5-HTP, 5-hydroxytryptophan
Graphical abstract

Summary.
Serotonin directly regulates bacterial growth in a species-dependent manner and indirectly via β-defensins. Higher gut mucosal serotonin levels select for a more colitogenic microbiota, resulting in increased severity of colitis. We show that serotonin-microbiota axis plays an important role in gut inflammation.
Serotonin, also known as 5-hydroxytryptamine (5-HT), is a biogenic amine that has been widely studied for its neuropsychological and cognitive roles in the central nervous system. What is often underappreciated is that the vast majority of 5-HT in the body is found in the gastrointestinal (GI) tract. The GI tract contains about 95% of the body's 5-HT, which is synthesized mainly within the enteric endocrine cells (EECs).1 Enterochromaffin (EC) cells are the best characterized subset of EECs and are the main source of 5-HT in the gut. EC cells are dispersed among epithelial cells in the mucosal layer of the GI tract and release 5-HT apically into the gut lumen, as well as basolaterally, in response to various mechanical and chemical stimuli.2, 3, 4 EC cells synthesize 5-HT from its precursor L-tryptophan. Tryptophan hydroxylase (Tph) catalyzes the synthesis of 5-HT.5 Two isoforms of Tph enzymes regulate 5-HT synthesis; these areTph1, mainly present in EC cells,5 whereas Tph2 predominates in the brain stem and enteric neurons.6, 7, 8
Changes in EC cell numbers and intestinal 5-HT content have been observed in experimental colitis and the 2 major forms of inflammatory bowel disease (IBD), Crohn’s disease (CD) and ulcerative colitis (UC).9, 10, 11, 12, 13, 14 In a seminal study, we demonstrated that Tph1-deficient (Tph1-/-) mice, which have significantly reduced 5-HT amount in the gut, exhibit reduced severity of colitis in 2 well-defined models of colitis (dextran sulfate sodium [DSS] and dinitrobenzene sulfonic acid [DNBS]).12 We also revealed that 5-HT plays a key role in the activation of immune cells to produce proinflammatory cytokines.12, 15 These findings are supported by findings that the severity of chemical-induced colitis or spontaneous colitis associated with interleukin (IL) 10 deficiency is increased when combined with 5-HT enhancing effects of serotonin reuptake transporter deficiency,16 highlighting 5-HT as an important signaling molecule in the pathogenesis of colitis. However, the precise mechanisms by which 5-HT influences the disease pathogenesis remain to be determined.
The mammalian GI tract is colonized by a complex, heterogeneous, and dynamic microbial ecosystem, and in humans, the GI tract contains up to 1 × 1014 colony-forming units of bacteria,17 with colonization occurring soon after birth. Commensal microorganisms within the GI tract play crucial roles in GI physiology, aid in digestion, provide competitive barriers to pathogen invasion, and contribute to the development of the host immune system.18, 19 In addition, gut microbiota are located at the complex interface of the epithelial barrier, and they are sensitive to changes in response to environmental factors, such as diet and drugs, and signals derived from the intestinal immune system, such as antimicrobial peptides (AMPs).18
There is now growing evidence that gut microbiota plays an important role in the pathophysiology of IBD. Fecal and intestinal mucosa-associated microbiota of IBD patients are characterized by decreased biodiversity and disruption of the microbe-host equilibrium.20 Contribution of intestinal microbiota in the disease pathogenesis is further demonstrated by using gnotobiotic mice, whereby colitis is not induced in the absence of microbes.21 Because of strategic location of EC cells in the epithelial lining of the mucosa and the emerging role of 5-HT in gut pathology, it is very likely that 5-HT from EC cells plays an important role in the modulation of gut microbial composition in the context of gut pathology and pathophysiology. However, little is known regarding the precise relationship between 5-HT signaling and gut microbiota.
In this study, by using in vitro system of commensal bacteria culture and in vivo system using Tph1-/- and germ-free (GF) mice, we investigate the role of gut-derived 5-HT in the regulation of gut microbiota composition and highlight a key role for 5-HT–microbiota axis in the pathogenesis of experimental colitis. Our study demonstrates that 5-HT selects for a more colitogenic microbiota directly by regulating the growth of bacteria in a species-dependent manner as well as indirectly by inhibiting β-defensin production from colonic epithelial cells, which altogether leads to perpetuation of gut inflammation.
Results
Tph1-/- Mice Have Altered Gut Microbiota
To determine whether mucosal 5-HT plays a role in selecting the microbiota, we analyzed the microbial composition of Tph1-/- and Tph1+/- mice, which have different levels of 5-HT in gut, with Tph1-/- mice having the lower amount. To minimize the genetic influence, we used Tph1+/- mice. We compared the cecal bacterial profiles of Tph1-/- and Tph1+/- offspring (F1 mice) from crosses of Tph1+/- offspring parents, as well as Tph1-/- mice from a breeding colony of Tph1-/- mice (Inbred). The 3 groups of mice were separated into distinct clusters as shown by visualization of Bray-Curtis diversity by principal coordinate ordination (PCoA) (Figure 1A). Although the 2 groups of Tph1-/- mice (Inbred and F1 offspring of Tph1+/- crosses) separated into distinct clusters, they appeared more similar in composition (Figure 1B). To confirm the functional effect of the altered microbiota in Tph1-/- mice, we next analyzed the short-chain fatty acid (SCFA) concentrations within the feces of naive Tph1-/- and Tph1+/- mice by using gas chromatography–mass spectrometry. We observed lower levels of acetate, butyrate, and propionate in Tph1-/- mice (Figure 1C).
Figure 1.
Tph1-/-mice have an altered gut microbiota compared with heterozygous Tph1+/-mice. 16S partial sequencing profiling analysis of cecal content of 3 groups of mice was carried out. Heterozygous mice (Tph1+/- (F1)) bred from heterozygous parents were compared with homozygous knockout mice (Tph1-/- (F1)) also bred from the same colony of heterozygous parents and mice from a breeding colony of knockout mice [Tph1-/- (inbred)]. (A) PCoA of Bray-Curtis dissimilarity revealed each group of mice had distinct microbiota. The 2 groups of Tph1-/- mice separated from Tph1+/- mice along the PCoA1 axis and from each other along the PCoA2 axis. (B) Taxonomic summaries (average of each group) at the genus level revealing greater similarity between the 2 groups of Tph1-/- mice. (C) Concentration of acetate, butyrate, propionate, and lactate in the feces of Tph1+/- and Tph1-/- mice. Data are from 1 representative experiment of 2 independent experiments performed. Data are presented as mean ± standard deviation from 4 mice per group; *P < .05 by Student t test.
To predict microbiota that is likely to be strongly influenced by the Tph1-/- genotype, we identified those taxa that were significantly different from the Tph1+/- heterozygous mice shared by both groups of Tph1-/- mice. Using Kruskal-Wallis non-parametric test, 32 operational taxonomic units (OTUs) are significantly different (P < .025, adjusted for false discovery rate [FDR]). This included 10 of the top 50 most abundant OTUs. Nine of 10 of these differences were increased in the relative abundance on the Tph1-/- mice relative to the Tph1+/- mice, including an increase in the 5 OTUs within the Bacteroidetes (OTUs representing Prevotella, Bacteroides, and 3 OTUs classified only to Bacteroidales), 2 taxa in the Firmicutes (Oscillospira, Lachnospiraceae), and 1 Proteobacteria OTU (Helicobacter). Within the Tenericutes, one OTU of Allobaculum was increased and another decreased (Figure 2). Further investigations will be required to validate these findings and determine the mechanisms that alter these taxa in the knockout mice.
Figure 2.
OTUs differ significantly between the heterozygous mice and the 2 groups of Tph1-/-mice. Those OTUs that differed with the Tph1+/- mice and shared between each group were selected, and only those present in the top 50 most abundant OTUs are shown (n = 10). Kruskal-Wallis non-parametric test was used, with FDR corrected P values <.025 used as a threshold. Box-whisker plots of relative abundance and log (relative abundance) for visualization purposes for each OTU are presented. Note that for the log transformed plots only 0 values were converted to a relative abundance of 10–6. Only OTU 2 (Tph1+/-) and OTU 12 (Tph1-/- inbred) were affected by this conversion.
5-Hydroxytryptamine Directly Influences Gut Bacteria Growth In Vitro
EC cells release 5-HT apically into the gut lumen as well as basolaterally. To explore whether EC cell–derived 5-HT can directly modulate gut microbial composition, we next sought to explore the direct effect of 5-HT on the growth of gut bacteria. We assessed the growth rate by using in vitro growth of 12 bacterial strains representing the major gut phyla groups: Bacteroidetes, Firmicutes, and Proteobacteria (Figure 3). According to the literature, 0.01 mg/L is the physiological concentration of 5-HT in the gut lumen.22 Among the 12 strains tested, we observed a concentration-dependent modulation of bacterial growth by 5-HT, and the effect was species-specific in 10 strains. In general, the anaerobic Bacteroides were more sensitive to 5-HT than the facultative anaerobes, although specific strains may exhibit enhanced growth at low concentrations. No significant effect on the growth of the strain of Clostridium bolteae or C ramosum was observed. These findings demonstrate that 5-HT can directly alter gut microbiota composition.
Figure 3.
Direct effect of serotonin (5-HT) on gut microbial communities. In vitro growth of 10 gut commensals in the presence of serotonin (5-HT) at 0.01, 0.1, and 1 mg/mL was measured by OD at 650 nm relative to control (without 5-HT) at 24 and 72 hours for aerobic and anaerobic bacteria, respectively. Concentration-dependent stimulation and inhibition of bacterial growth by 5-HT are species-specific. Data are from 1 representative experiment of 3 independent experiments with quadruplicates. Data are represented as mean ± standard error of the mean. *P < .05 by Student t test. Significant differences from negative control (no 5-HT) are indicated by *.
5-Hydroxytryptamine Attenuates β-defensin Production From Colonic Epithelial Cells
On the basis of our previous finding that Tph1-/- mice exhibit attenuated severity of DSS-induced colitis,12 we explored whether 5-HT can influence gut microbiota indirectly via AMPs. We found total β-defensin levels in naive Tph1-/- mice, which have reduced 5-HT in gut, are higher, compared with wild-type (WT) (Tph1+/+) littermates (Figure 4A). Restoration of 5-HT levels by 5-HT precursor, 5-hydroxytryptophan (5-HTP), in Tph1-/- mice12 reduced β-defensin production in the colon (Figure 4A). Total β-defensin levels were also higher in the colon of Tph1-/- mice as compared with WT littermates post-DSS (57.8215 ± 2.970896 and 35.70425 ± 2.672975, respectively).
Figure 4.
5-HT down-regulates PPAR-γ via 5-HT7receptors and subsequently inhibits β-defensin production. (A) Levels of total β-defensins in the colon of Tph1+/+, Tph1-/-, and 5-HTP–treated Tph1-/- mice. (B) Quantification of mDefb1 (left) and mDefb3 (right) mRNA expression in the colon of Tph1+/+, Tph1-/-, and 5-HTP–treated Tph1-/- mice. (C) Quantification of defb1 mRNA expression (left) and measurement of hBD-1 peptides (right) after 5-HT7 receptor antagonist (SB-269970; 1 μmol/L) treatment in HT-29 cells. (D) Quantification of defb4 mRNA expression (left) and measurement of hBD-2 peptides (right) after 5-HT7 receptor antagonist treatment. (E) Quantification of mDefb1 (left) and mDefb3 (right) mRNA expression in the colon of 5-HT7R-/- mice. (F) Quantification of PPAR-γ mRNA expression in the colon of Tph1+/+, Tph1-/-, and 5-HTP–treated Tph1-/- mice. (G) Quantification of PPAR-γ mRNA expression after 5-HT7 receptor antagonist (SB-269970; 1 μmol/L) treatment in HT-29 cells. (H) Quantification of mdefb1 (left) and mdefb3 (right) mRNA expression in Tph1-/- mice treated with PPAR-γ antagonist (GW-9662; 2 mg/kg intraperitoneally per day for 5 days), compared with vehicle (DMSO)-treated mice. (I) Quantification of defb1 (left) and defb4 (right) mRNA expression in HT-29 cells treated with MEK inhibitor (PD98059; 40 μmol/L). In vitro qRT-PCR data are representative of 3 individual experiments with quadruplicates. 5-HT concentration is 10–7 mol/L. In vitro data are represented as mean ± standard error of the mean, whereas in vivo data are represented as mean ± standard deviation from 4 to 6 mice per group; *P < .05, **P < .01, and ****P < .0001 by Student t test or 1-way analysis of variance, with Bonferroni multiple comparison test.
Because mouse β-defensin (mBD)-1 and mBD-3 levels were decreased on 5-HTP administration in the colon of Tph1-/- mice (Figure 4B), we decided to further investigate the role of 5-HT in β-defensin production by using HT-29 colonic epithelial cells. Human β-defensin (hBD)-1 (human orthologue of mBD-1) and hBD-2 (human orthologue of mBD-3) have been investigated extensively, which are expressed constitutively or induced under inflammatory conditions, respectively.23 In addition, we have previously found reduced severity of DSS-induced colitis in mice on inhibition of 5-HT7 receptor activation by a selective antagonist (SB-269970).24 In the present study, treatment with SB-269970 prevented 5-HT–induced down-regulation of β-defensin 1 and 2 (Figure 4C and D). We also used 5-HT7 receptor deficient (5-HT7R-/-) mice and found that these mice exhibit higher levels of mBD-1 and mBD-3 in the colon (Figure 4E).
5-HT inhibits peroxisome proliferator-activated receptor gamma (PPAR-γ) expression in various cells,25, 26 whereas PPAR-γ activates β-defensin in human colonic epithelial cells.27 We investigated PPAR-γ expression in Tph1-/- mice. These mice expressed higher PPAR-γ expression in the colon, whereas 5-HTP administration attenuated the expression (Figure 4F). In HT-29 cells, 5-HT inhibited PPAR-γ expression, whereas SB-269970 restored the expression (Figure 4G). To examine whether an increased expression of mBD-1 and mBD-3 is mediated through PPAR-γ, Tph1-/- mice were intraperitoneally treated with either vehicle (dimethyl sulfoxide [DMSO]) or PPAR-γ antagonist, GW-9662. The antagonist-treated mice showed lower levels of both β-defensins (Figure 4H). It has been shown that 5-HT inhibits PPAR-γ expression through extracellular signal-regulated kinase-1 and -2 (ERK1/2) pathway in pulmonary artery smooth muscle cells.28 We observed similar finding in HT-29 cells that pre-treatment with MEK inhibitor (PD98059) masked the inhibitory effect of 5-HT (Figure 4I). These findings altogether suggest that 5-HT down-regulates PPAR-γ via 5-HT7 receptors and subsequently inhibits the production of β-defensins from colonic epithelial cells.
Mucosal 5-Hydroxytryptamine Induced Changes in Gut Microbiota Alter Susceptibility to Colitis
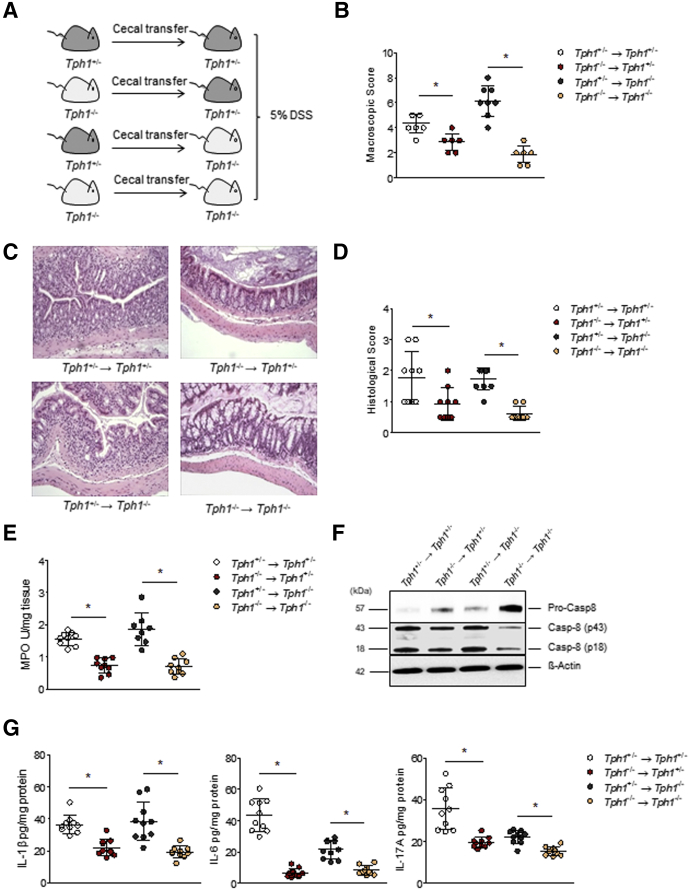
Tph1-/- mice exhibit reduced severity of colitis.12 We thus hypothesized that differences in gut microbiota composition between Tph1-/- and Tph1+/- mice play a role in the altered susceptibility to colitis, and that Tph1+/- microbiota confer a colitogenic effect. To study the effect of gut microbiota changes induced by 5-HT, we gavaged cecal contents from naive Tph1-/- into Tph1+/- mice and vice versa and induced colitis with 5% DSS (Figure 5A). Adoptive transfer of microbiota from Tph1+/- to Tph1-/- mice increased the severity of colitis of the recipient mice as reflected by increase in macroscopic (Figure 5B) and histologic damage scores (Figure 5C and D) and myeloperoxidase (MPO) activity (Figure 5E), compared with Tph1+/- mice that received microbiota from Tph1-/- mice. Notably, IL1β is a mucosal inflammatory marker in IBD, and recent studies reveal caspase-8, in addition to caspase-1, is involved in IL1β regulation.29, 30, 31, 32 We found an increased cleavage of caspase 8, when microbiota from Tph1+/- mice was transferred to the recipient mice (Figure 5F), which correlated to an increase in IL1β levels (Figure 5G). Moreover, there were higher levels of IL6 and IL17A in the colon of the recipient mice that received microbiota from Tph1+/- mice (Figure 5G).
Figure 5.
Microbiota from Tph1-/-mice attenuates DSS-induced colitis. (A) Schematic of cecal microbiota transfer, followed by colitis induction with 5% DSS in sterile drinking H2O. (B) Macroscopic scores on day 5 post-DSS. (C) Representative photomicrographs of H&E stained colon cross sections on day 5 post-DSS visualized using Nikon Eclipse 80i microscope. Original magnification, ×10. (D) Histologic damage scores. (E) MPO activity on day 5 post-DSS. (F) Western blot analysis performed on protein extracts obtained from the colon homogenates of the recipient mice administered with DSS. Representative Western blot with β-actin is presented as loading controls. Pro-Casp8, pro-caspase-8; Casp-8, caspase-8. Each lane represents an individual mouse. Results are the representative of 3 independent experiments performed on at least 3 mice per group. (G) Cytokine levels in the colon of Tph1-/- and Tph1+/- littermates on day 5 post-DSS after transfer of gut microbiota from Tph1+/- mice to Tph1-/- mice and vice versa. Data are from 1 representative experiment of 2 independent experiments performed. Data are represented as mean ± standard deviation from 8 to 10 mice per group; *P < .05 by Student t test. (→) denotes microbiota transfer.
To further confirm the colitogenic effect of Tph1+/- microbiota in increasing the susceptibility to DSS-induced colitis, we treated both Tph1+/- and Tph1-/- mice with broad-spectrum antibiotics (Abx) in drinking water for 10 days before the induction of colitis (Figure 6A). Abx treatment abrogated the differences in the colitis susceptibility between these mice as shown by similar macroscopic (Figure 6B) and histologic scores (Figure 6C and D), MPO activity (Figure 6E), as well as proinflammatory cytokine levels (Figure 6F). Altogether, these findings suggest that 5-HT perturbs and configures microbiota to a colitogenic microbiota, which subsequently increases host susceptibility to colitis.
Figure 6.
Pretreatment with broad-spectrum Abx abrogates differential susceptibility to DSS-induced colitis between Tph1+/-and Tph1-/-mice. (A) Schematic of representation of the treatments. (B) Macroscopic scores on day 5 post-DSS. (C) Histologic scores on day 5 post-DSS. (D) Representative photomicrographs of H&E stained colon cross sections on day 5 post-DSS visualized using Nikon Eclipse 80i microscope. Original magnification, ×10. (E) MPO activity on day 5 post-DSS. (F) IL1β (left) and IL6 (right) levels in the colon on day 5 post-DSS. Data are from 1 representative experiment of 2 independent experiments performed. Data are represented as mean ± standard deviation from 4 to 6 mice per group; *P < .05 by Student t test.
Transfer of Gut Microbiota From Tph1-/- Mice Exhibits Up-regulation of Gut Barrier Integrity and Down-regulation of Inflammation in Germ-free Mice
To further elucidate the role of gut 5-HT–microbiota axis in the pathogenesis of colitis, we transferred microbiota from either Tph1-/- or Tph1+/- littermates into GF mice and examined the development of DSS-colitis (Figure 7A). Although there was no difference in EC cell number and 5-HT levels on day 5 post-DSS (Figure 7B and C), investigations on the parameters of colitis revealed lower macroscopic scores, histologic damage score, MPO activity, and proinflammatory cytokines (IL1β and IL6) in GF mice colonized with microbiota from the Tph1-/- mice, as compared with those colonized with microbiota from Tph1+/- mice (Figure 7D–H). Recently, it has been shown that IL17C produced by epithelial cells plays an important role in the protection of DSS-colitis by inducing hBD-2.33 There was an increase in IL17C levels in GF mice with microbiota from Tph1-/-, supporting a protective role of IL17C in DSS-induced colitis (Figure 7I). However, we did not observe difference in IL23 levels (Figure 7J). Next, we investigated the expression of gut barrier components. There was a higher expression of mBD-3 (Figure 8A), ZO-1 (Figure 8B), but not occludin (Figure 8C), in GF mice with Tph1-/- microbiota, as compared with the GF mice with microbiota from Tph1+/- littermates. GF mice with Tph1-/- microbiota exhibited up-regulated Muc2 and Muc5ac expression, compared with GF mice with Tph1+/- microbiota (Figure 8D). Together, these findings reveal microbiota of Tph1-/- mice have the ability not only in maintaining the gut barrier integrity but also in reducing the severity of colitis.
Figure 7.
GF mice colonized by microbiota from Tph1-/-mice are resistant to DSS-induced colitis. (A) Schematic of cecal microbiota transfer, followed by DSS treatment. (B) 5-HT expressing EC cells on day 5 post-DSS. (C) 5-HT levels in the colon on day 5 post-DSS. (D) Macroscopic scores on day 5 post-DSS. (E) Representative photomicrographs (left) of H&E stained colon sections on day 5 post-DSS visualized using Nikon Eclipse 80i microscope, original magnification, ×10, and histologic scores (right) on day 5 post-DSS. (F) MPO activity on day 5 post-DSS. (G–J) Cytokines (IL1β, IL6, IL17C, and IL23) in the colon on day 5 post-DSS, respectively. Data are from 1 representative experiment of 2 independent experiments performed. Data are represented as mean ± standard deviation from 5 mice per group; *P < .05 and **P < .01 by Student t test. (→) denotes microbiota transfer.
Figure 8.
Components of intestinal barrier function in GF mice after microbiota transfer. (A) mDefb3, (B) Tjp1, and (C) Ocln mRNA expression in GF mice after transfer of gut microbiota from either Tph1+/- or Tph1-/- mice. (D) Western blot analysis of Muc2 and Muc5ac performed on protein extracts obtained from the colon homogenates of the recipient mice administered with DSS. Representative blots (left) of 3 independent experiments performed on at least 3 mice per group are illustrated. Western blot with β-actin is presented as loading controls. Each lane represents an individual mouse. Results are representative of 3 independent experiments. Bar graph (right) representing Muc2 and Muc5ac protein levels normalized for total protein levels and β-actin. Data are from 1 representative experiment of 2 independent experiments performed. Data are represented as mean ± standard deviation from 4 to 5 mice per group; *P < .05, **P < .01, and ***P < .001 by 1-way analysis of variance, with Neuman-Keuls multiple comparison test. (→) denotes microbiota transfer.
Tph1-/- and Tph1+/- Microbiota Transferred to Germ-free Mice Result in Distinct Microbiota Before and After Dextran Sulfate Sodium Administration
Analysis of microbial composition in GF mice colonized with gut microbiota from Tph1+/- and Tph1-/- mice revealed distinct microbiota before and after DSS treatment. Principal coordinate analysis (PCoA) of Bray-Curtis dissimilarity revealed that the microbiota of the Tph1+/- and Tph1-/- mice is separated into 2 distinct groups (permutational multivariate analysis of variance, P < .01). DSS administration shifted the microbial communities as expected, but GF mice colonized by Tph1+/- microbiota still exhibited distinct microbiota, compared with that in GF mice colonized by microbiota from Tph1-/- mice (Figure 9A). At the phylum level, DSS administration induced an expansion of Proteobacteria in both groups and reduction in Bacteroidetes/expansion of Firmicutes in the GF mice receiving microbiota from Tph1+/- mice, but GF mice receiving Tph1-/- microbiota were protected from this microbial shift (Figure 9B). In addition, investigations at the OTU level revealed reduction of 2 distinct Bacteroidales OTUs in the GF mice colonized by Tph1-/- microbiota compared with the GF mice colonized by Tph1+/- microbiota, whereas the GF mice colonized by the Tph1+/- microbiota exhibited less Akkermansia (Figure 9C). The increased abundance of Akkermansia in the GF mice colonized by Tph1-/- microbiota was further confirmed by testing the direct effect of 5-HT on the growth rate of the bacterium, whereby 5-HT inhibited the growth in a concentration-dependent manner (Figure 9D).
Figure 9.
Analysis of microbial composition in GF mice with or without DSS colitis after transfer of gut microbiota from Tph1+/-and Tph1-/-mice. (A) PCoA of Bray-Curtis dissimilarity showing distinct microbiota of Tph1+/-, Tph1-/-, and GF mice with or without DSS after colonization by either Tph1+/- or Tph1-/- microbiota. GF mice after receiving microbiota from Tph1+/- mice (red) versus Tph1-/- mice (cyan) without DSS (P = .009). GF mice after receiving microbiota from Tph1+/- mice (light green) versus microbiota from Tph1-/- mice (blue) with DSS colitis (P = .023). (B) Taxonomic summaries at the phylum level in GF mice with or without DSS after colonization by either Tph1+/- or Tph1-/- microbiota. (C) Examples of significant changes at the OTU level in GF mice with or without DSS after colonization by either Tph1+/- or Tph1-/- microbiota. n = 5 mice per group. (D) Concentration-dependent effect of 5-HT on the growth of A muciniphila. OD at 650 nm relative to control (without 5-HT) at 72 hours in the presence of serotonin (5-HT) at 0.01, 0.1, and 1 mg/mL. In vitro bacterial killing assay data are combined from 2 independent experiments with triplicates. Data are represented as means ± standard deviation. *P < .05 by Student t test. Significant difference from negative control (no 5-HT) is indicated by *.
Discussion
5-HT is a key enteric mucosal signaling molecule influencing gut physiology (motor and secretory function) and thus maintaining GI homeostasis. Dysregulated 5-HT signaling is observed in many GI diseases including IBD, functional disorders such as irritable bowel syndrome, colorectal cancer, and in various enteric infections.2, 9, 10, 11, 12, 13, 14 During the past decade, more studies are enlightening gut function as well as pathology rely on interactions with gut microbiota. Healthy microbiota is thought to collaborate with host to maintain the intestinal barrier, and disruption of this relationship can compromise the gut function. Because of close proximity of gut microbiota and 5-HT producing EC cells in the gut mucosal layer, cross-talk between them is likely to play a critical role in maintaining intestinal homeostasis. Whereas recently gut bacteria have been shown to stimulate the release of 5-HT from EC cells,34 the converse effect of 5-HT on microbiota remained to be determined. This study illustrates that 5-HT plays a key role in the regulation of gut microbial composition and that the direct and indirect influence of 5-HT on microbial composition affect the susceptibility to experimental colitis.
In recent years, gut microbiota has emerged as a topic of great interest in biomedical research. Many studies have demonstrated that disruption of the balanced composition of the gut microbiota is associated with both GI and non-GI diseases.35, 36, 37 In general, gut microbiota performs several vital functions for host health, including digestion of complex host-indigestible polysaccharides, pathogen displacement, synthesis of vitamins, and development of immune system.38 Two major bacterial phyla, Firmicutes and Bacteroidetes, and 5 minor bacterial phyla, Proteobacteria, Actinobacteria, Fusobacteria, Cyanobacteria, and Verrucomicrobia, comprise the gut microbiota in adult humans.39 EC cells, which are dispersed among the epithelial cells, lie in close proximity to gut microbiota and react to changes in gut contents by releasing biologically active molecules including 5-HT.1, 40, 41 Recently, it has been shown that microbial-derived metabolites, such as SCFAs (ie, butyrate and acetate) or secondary bile acids, especially deoxycholate, act on EC cells and up-regulate the expression of Tph1.34, 42, 43 In addition, bacterial toxins including cholera toxin44 and Escherichia coli lipopolysaccharide45 have been shown to stimulate 5-HT release from EC cells. Taken together, there is now evidence to postulate a role of microbiota in 5-HT production from EC cells. In addition to the effect of gut microbiota on 5-HT production from EC cells, it is also possible that 5-HT may influence microbiota in relation to gut function. Indeed, in our studies, we observed gut microbial composition differs between Tph1-/- and Tph1+/- littermates, which have different levels of gut 5-HT, with Tph1-/- mice having the lower amount.
On the basis of previous studies that revealed importance of littermates in defining host genetic effect on the gut microbiota composition as well as subsequent microbial effect on the host susceptibility of DSS-induced colitis,46, 47 we controlled for non-genetic confounders by generating littermates from Tph1+/- parents to investigate whether the impact of Tph1 genotype on gut microbiota is dominant over both parentage and housing conditions. Here we observed altered microbial composition in Tph1-/- mice, along with altered SCFA concentrations. Interestingly, we observed lower acetate, butyrate, and propionate levels in the feces of Tph1-/- mice compared with Tph1+/- mice. The precise reason for these lower levels is not clear, but it seems possible that the lower levels in Tph1-/- mice reflect the differences in microbial composition between the Tph1-/- and Tph1+/- mice.
Increased mucosal 5-HT content and EC cell hyperplasia are associated with experimental colitis and IBD.9, 10, 11, 12, 14, 48 Moreover, IBD patients have dysbiotic microbiota with a decrease in obligate anaerobes.49, 50 In our in vitro study using diverse commensal bacterial strains, there was a significant growth inhibition in most of the bacteria tested. According to the literature, 0.01 mg/L is the physiological concentration of 5-HT in the gut lumen.22 When we used higher concentration, there was significant growth inhibition in most of the bacteria tested, and when affected, the obligate anaerobes were more sensitive to 5-HT. This provides evidence that high levels of 5-HT can directly alter the configuration of gut microbiota.
AMPs shape the composition of the microbiota and help maintain gut homeostasis. Defensins constitute a major class and are necessary to fend off microorganisms in the mucosal layer.51 Abnormal β-defensin production has been implicated in a number of GI disorders including IBD,27, 51, 52 whereby diminished antimicrobial activity due to attenuated hBD-1 and hBD-2 expression is associated with colonic CD patients.52 In addition, 5-HT7 receptor expression is increased in the inflamed regions of CD patients.53 5-HT7 receptor is also up-regulated in the murine intestine post-DSS, compared with the controls,24, 53 whereas blocking 5-HT signaling with a selective 5-HT7R antagonist or genetic deletion of the receptor alleviates colitis in DSS- and DNBS-colitis.24 Previously, Guseva et al53 found that blocking 5-HT7 receptor exacerbates severity of DSS-colitis. The authors state that the dose, route of administration, and housing of animals may account for the difference in the results.53 In our study, inhibition of 5-HT7 receptors by selective antagonist restored β-defensin production in HT-29 cells. 5-HT7R-/- mice also expressed higher levels of mBD-1 and mBD-3 in the colon. Recently, it has been demonstrated that 5-HT4 receptor stimulation via enema administration has a protective effect in the experimental colitis but not via intraperitoneal injection, which is shown to be associated with increased motility.54 Further studies are warranted to elucidate the role of other 5-HT receptors expressed on intestinal epithelial cells in β-defensin production.
PPAR-γ is essential for maintaining β-defensin expression in the colon.27 There is now substantial evidence from experimental models of colitis and IBD patients that PPAR-γ agonists play a role as a key inhibitor of colitis by regulating immune activation and inflammation.55, 56 We found GW-9662 reduced mBD-1 and mBD-3 expression in Tph1-/- mice, whereas 5-HT inhibited β-defensin production by attenuating PPAR-γ via 5-HT7 receptors through ERK1/2-dependent pathway in HT-29 cells. There are studies showing that Bacteroides thetaiotaomicron and Enterococcus faecalis activate intestinal epithelial PPAR-γ, which decreases IL8 and increases IL10 production, respectively.57, 58 Our in vitro study using bacterial strains illustrated the concentration- and species-dependent effect of 5-HT on the growth of B thetaiotaomicron and E faecalis, providing further support that 5-HT can also inhibit PPAR-γ in a microbiota-dependent manner. Altogether, these findings suggest 5-HT released from EC cells directly and indirectly (via modulation of β-defensin production) plays a crucial role in regulation of gut microbial composition. These findings are further supported by the observations of different gut microbial composition in Tph1-/- and Tph1+/- mice.
There is now abundant evidence to postulate a link between gut microbiota and IBD.59 In CD, fecal stream diversion reduces inflammation and induces mucosal healing in the excluded intestinal segment, whereas infusion of intestinal contents reactivates the disease. In UC, short-term treatment with broad-spectrum Abx rapidly reduces mucosal inflammation.60 Recently, a randomized controlled trial has shown that fecal microbiota transplantation induces remission in a significantly greater percentage of patients with active UC than placebo, with no difference in adverse events.61 Adoptive transfer of microbiota from Tph1+/- to Tph1-/- and vice versa and the studies using GF mice provide evidence for the important role of 5-HT–microbiota axis in the pathogenesis of colitis, with Tph1-/- microbiota mediating protective effect in GF mice. GF mice express Tph1 and secrete 5-HT at a much lower level than SPF mice.34 Our finding that there was no difference in EC cell number and 5-HT levels after transfer of either Tph1+/- or Tph1-/- microbiota into GF mice post-DSS suggests that the difference in the disease severity can be attributed to the difference in the microbiota composition.
Consistent with our finding that Tph1+/- and Tph1-/- mice carry different microbiota composition, we found distinct differences in microbiota between GF mice with Tph1-/- microbiota and GF mice with microbiota from Tph1+/- littermates pre- and post-DSS. Deep sequencing at the genus level revealed that GF mice colonized by Tph1+/- microbiota exhibit low abundance of A muciniphila on DSS treatment, compared with that in GF mice with microbiota from Tph1-/- mice. We also observed concentration-dependent direct inhibitory effect of 5-HT on the growth rate of the A muciniphila. Although the role of A muciniphila in the pathogenesis of colitis is still unclear, it has been shown that A muciniphila–derived extracellular vesicles mediate protective effects in the development of DSS-induced colitis.62 In addition, A muciniphila adheres to colonic epithelial cells and strengthens an impaired gut barrier63 by stimulating enterocyte proliferation and promoting wound restitution,64 thereby suggesting an important role of this bacterium in mediating protection in intestinal inflammation. Higher abundance of A muciniphila in Tph1-/- microbiota along with the inhibitory effect of high 5-HT in the growth of the bacterium further provide evidence to postulate the influence of 5-HT–microbiota axis in the pathogenesis of colitis. Altogether, these results highlight the importance of 5-HT in regulating gut microbial composition and ultimately altering susceptibility to DSS-induced colitis.
In summary, this study not only provides novel information on 5-HT–microbiota axis in relation to intestinal immune responses and the pathogenesis of colitis but also shed light on the bidirectional relationship between EC cells and microbiota in gut function. Identifying the specific bacterial species associated with alteration in gut 5-HT levels in inflammation may ultimately lead to improved therapeutic strategies using the bacterial species or targeting 5-HT signaling in various intestinal inflammatory disorders including IBD.
Methods
All authors had access to the study data and reviewed and approved the final manuscript.
Mice
All mice used in this study were male and 6–8 weeks old, except for GF mice, which were male and 10–12 weeks old. Breeding pairs of Tph1+/+ (WT) and Tph1-/- mice on C57BL/6 background were obtained from CNRS, Paris, France. Tph1-/- mice on C57BL/6 background were originally produced by gene mutation as previously described.65 Briefly, Tph1-/- mice have been generated by substituting exon 2 of the Tph1 locus by the nlslacZneopolyA cassette. These mice are viable, express normal amounts of 5-HT in the brain, and show no observed differences in food intake or body weight as compared with Tph1+/+ mice. Tph1+/- and Tph1-/- offspring (F1 mice) were generated from crosses of Tph1+/- offspring parents, as well as mice from a breeding colony of Tph1-/- mice (Inbred). Breeding pairs of 5-HT7 receptor–deficient (5-HT7R-/-) mice on C57BL/6 background, originally generated as described by Hedlund et al,66 were provided by Peter B. Hedlund (Scripps Research Institute, La Jolla, CA). C57BL/6 mice were purchased from Taconic Biosciences, Rensselaer, NY. GF mice on the C57BL/6 background were derived and maintained under gnotobiotic conditions in the Axenic/Gnotobiotic Unit at McMaster University. All experiments were approved by the McMaster University animal ethics committee and conducted under the Canadian guidelines for animal research.
Experimental Protocol
As previously described,12 5-HTP (Cat. # H9772; Sigma-Aldrich, St Louis, MO) was administered subcutaneously at a dosage of 50 mg/kg twice a day for 8 days; control mice received saline. Tph1-/- mice received daily intraperitoneal injection of GW-9662 at 2 mg/kg/day for 5 days, and control mice received DMSO. DSS (molecular mass 40 kDa; Cat. # 02160110; MP Biomedicals Incorporated, Solon, OH) was added to drinking water at a final concentration of 5% (w/v) and 2.5% (w/v) for SPF and GF mice, respectively, for 5 days. Mean DSS consumption was noted per cage each day. Mice were killed 5 days after the beginning of DSS administration. Macroscopic damage scores were performed by using a previously published scoring system for DSS-colitis.67 Colonic damage was scored on the basis of a published scoring system that considers architectural derangements, goblet cell depletion, edema/ulceration, and degree of inflammatory cell infiltrate.67 MPO (an index of granulocytes infiltration and inflammation) activity was determined by using a published protocol.67 Tph1+/- and Tph1-/- mice were administered ad libitum with broad-spectrum Abx, consisting of neomycin (0.5 g l-1), ampicillin (0.5 g l-1), vancomycin (0.5 g l-1), and metronidazole (0.5 g l-1) in sterile drinking water for 10 days before the start of DSS (5%)-colitis and continuing until day 5 post-colitis; their control groups received sterile drinking water before the induction of colitis.
Adoptive Microbiota Transfer
For adoptive microbiota transfer experiments, 200-μL cecal samples from Tph1-/- mice were diluted in sterile phosphate-buffered saline (PBS) and gavaged to Tph1+/- littermates and vice versa for 7 days beginning 2 days before induction of DSS (5%)-colitis and continuing until day 5 post-colitis. GF mice received microbiota (cecal) by oral gavage from Tph1-/- or Tph1+/- littermates 7 days before the induction of DSS (2.5%) colitis.
Bacterial Culture
To determine the effect of 5-HT on bacterial growth, the growth rate of diverse commensal bacterial strains from human gut isolate library (GIL) in Dr Michael Surette lab were studied in the presence of 5-HT at varying concentrations (0.01 mg/mL, 0.1 mg/mL, 1 mg/mL). Obligate anaerobic strains included Akkermansia muciniphila (ATCC BAA-835), Bacteroides fragilis (GIL83), Bacteroides intestinalis (GIL98), Bacteroides thetaiotaomicron (GIL179), Clostridium bolteae (GIL94), Clostridium ramosum (GIL107), Eubacterium limosum (GIL141), Flavonifractor plautii (GIL193), and Ruminococcus gnavus (GIL116). Facultative anaerobic strains included Enterocococcus faecalis (GIL6), Streptococcus salivarius (GIL9), and Streptococcus australis (GIL58). Escherichia coli DH5α was used as a control. Strains were grown in brain-heart infusion broth overnight and diluted 1:100 into 96-well microplates containing 150 μL of media supplemented with 5-HT as indicated. Anaerobes were incubated at 37°C in an anaerobic environment (5% CO2, 5% H2, 90% N2) for 72 hours, whereas facultative anaerobes were incubated at 37°C in 5% CO2 for 24 hours. Microbial growth was measured by optical density (OD) at 650 nm and normalized to control culture (no 5-HT).
Microbiome Profiling and Analysis
Bacterial profiling was carried out by amplification of the V3 region of the 16SrRNA gene as described previously.68, 69 Amplification products were sequenced on an Illumina MiSeq Illumina (Farncombe Institute) with 2 × 250 nt paired end reads. The data were processed by an in-house bioinformatics pipeline,70 and analysis was carried using QIIME71 and PhyloSeq.72 The OTU table was filtered to remove singletons and those not assigned to bacteria. After filtering, the minimal number of reads per sample was 21,975, and the samples were rarified to 21,000 reads.
Gas Chromatography–Mass Spectrometry Analysis of Fecal Short-Chain Fatty Acids
SCFA concentrations of fecal contents were determined by mass spectrometry. Briefly, fecal samples were acidified with weight equivalent amount of 3.7% hydrochloric acid. The tubes were sonicated in methanol for 20 minutes before use. Internal standards (14.72 mmol/L butyric acid-d7) were added to the acidified samples, followed by diethyl ether to obtain diethyl ether-fecal extract. The ether extracts were then mixed with N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA), followed by derivatization by incubating the organic extract-MTBSTFA mixture at room temperature for 1 hour. The derivatized samples were run through 6890N Network GC system (Agilent Technologies, Mississauga, Canada) equipped with DB-17HT (30 m × 0.25 mm ID, 0.15 mm film) and 5973N Mass Selective Detector (Agilent Technologies). Acetic acid, propionic acid, and butyric acid were quantified and reported as nmol/mg of fecal sample.
Immunohistochemistry
Formalin-fixed, paraffin-embedded colonic segments were stained for detection of 5-HT by using a previously published method.73, 74 Colonic tissue sections were deparaffinized with CitriSolv (Cat. # 04355121; Fisher Scientific, Markham, Canada) and rehydrated in graded concentrations of ethanol. Sections were subjected to heat-induced epitope retrieval, blocked with 3% bovine serum albumin, and incubated with a polyclonal antibody raised against rabbit anti-mouse 5-HT (1:5000 dilution; Cat. # 20080; ImmunoStar, Hudson, WI) for 1 hour at room temperature. Sections were washed with PBS/0.5% Tween-20 and incubated with DakoEnVision+ System-HRP (Cat. # K4003; Dako, Burlington, Canada). Sections were developed by using 3,3’-diaminobenzidine solution (SIGMA FAST; Cat. # 079K8208; Sigma-Aldrich), and counterstained with Mayer’s hematoxylin solution (Cat. # MHS1; Sigma-Aldrich). Sections were visualized by using a Nikon Eclipse 80i microscope (Nikon Instruments Inc, Melville, NY). The number of 5-HT positive cells per 10 crypts was counted in 4 different areas for each section.
Drugs and Reagents
5-HT (Cat. # H9523; Sigma-Aldrich), IL1β (Cat. # 200-01B; Peprotech, Rocky Hill, NJ), rabbit polyclonal anti-β actin (Cat. # ab8227; Abcam, Cambridge, MA), SB-269970 (5-HT7 receptor antagonist; Cat. # 1612; Tocris Bioscience, Bristol, UK), GW-9662 (PPAR-γ antagonist; Cat. # 22978-25-2; Caymen Chemicals, Ann Arbor, MI), and PD98059 (MEK inhibitor; Cat. # 9900L; Cell Signaling Technology, Danvers, MA) were prepared according to the manufacturer's manual.
Cell Culture
The human colonic adenocarcinoma HT-29 cells (ATCC HTB-38) were maintained in Dulbecco modified Eagle medium/F12 (1:1) with 10% (v/v) heat-inactivated fetal bovine serum, supplemented with modified Eagle medium and HEPES buffer (pH 7.5) as well as penicillin and streptomycin at 37°C in a humidified 5% CO2 atmosphere. Cell media were changed every other day. HT-29 cells were seeded in a 12-well culture plate at a density of 5.0 × 105 cells/mL. Cells were allowed to attach for overnight, which were then washed twice with PBS and subsequently replenished with the serum-free media. Cells were stimulated with 5-HT (10–7 mol/L), IL1β (40 ng/mL), or the medium alone. After the treatment, cells remained viable as revealed by trypan blue exclusion assay. HT-29 cells were used between passage numbers 17 and 22.
Quantitative Real-time Polymerase Chain Reaction
Total RNA from HT-29 cell lines and colonic tissues from naive Tph1+/- and Tph1-/- mice, GF and DSS-induced GF mice colonized by cecal microbiota from either Tph1-/- or Tph1+/- mice was extracted by using TRIzol Reagent (Cat. # 15596026; ThermoFisher, Waltham, MA). Complementary DNA was prepared from 1 μg total RNA using iScript cDNA Synthesis Kit (Cat. # 1708891; Bio-Rad Laboratories, Mississauga, Canada). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed by using SsoFast Evagreen SYBR Green PCR Master Mix (Cat. # 1725201; Bio-Rad Laboratories) and CFX96 qRT-PCR system (Bio-Rad). Primers were used at a concentration of 10 μmol/L (Tables 1 and 2). Each reaction mixture contained cDNA, SsoFast Evagreen SYBR Green PCR Master Mix, and 1 μmol/L of primers. Values of target mRNA were corrected relative to the housekeeping gene coding for human glyceraldehyde 3-phosphate dehydrogenase and mouse 18S. The data were analyzed according to the 2-ΔΔCT method and expressed as relative abundances (mean ± standard error of the mean).
Table 1.
Quantitative Real-time Polymerase Chain Reaction Human Primers
| Forward (5'-3') | Reverse (5'-3') | |
|---|---|---|
| Gapdh | CTTAGCACCCCTGGCCAAG | TGGTCATGAGTCCTTCCACG |
| Pparg | AAGGCCATTTTCTCAAACGA | AGGAGTGGGAGTGGTCTTCC |
| Defb1 | Bio-Rad qHsaCID0015106, PrimePCR SYBR Green Assay | Bio-Rad qHsaCID0015106, PrimePCR SYBR Green Assay |
| Defb4 | Bio-Rad qHsaCID0038951, PrimePCR SYBR Green Assay | Bio-Rad qHsaCID0038951, PrimePCR SYBR Green Assay |
Table 2.
Quantitative Real-time Polymerase Chain Reaction Mouse Primers
| Forward (5'-3') | Reverse (5'-3') | |
|---|---|---|
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| Defb1 | GGTGTTGGCATTCTCACAAG | ACAAGCCATCGCTCGTCCTTTATG |
| Defb3 | GGATCCATTACCTTCTGTTTGC | ATTTGAGGAAAGGAACTCCAC |
| Ocln | ATGTCCGGCCGATGCTCTCTC | CTTTGGCTGCTCTTGGGTCTGTAT |
| Pparg | CTGCTCAAGTATGGTGTCCATGA | ATGAGGACTCCATCTTTATTCA |
| Tjp1 | ACCCGAAACTGCTGCTGTGGATAG | AAATGGCGGGCAGAACTTGTGTA |
Enzyme-Linked Immunosorbent Assay
5-HT levels were measured by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Cat. # IM1749; Beckman Coulter, Fullerton, CA). Briefly, colonic tissues were weighed and were homogenized in 0.2 N perchloric acid. After centrifugation at 10,000g for 5 minutes, the supernatants were collected, and the pH was neutralized by using 1 mol/L borate buffer. The supernatants were used for analysis of 5-HT levels using commercially available ELISA kit (Beckman Coulter). 5-HT content was expressed as a function of tissue weight (ng/mg). For intestinal cytokine and β-defensin measurement, colonic tissues were homogenized in Tris-buffered saline containing a protease inhibitor mixture (Cat. # P8340; Sigma-Aldrich, Oakville, Canada). Samples were centrifuged for 5 minutes at 3300g, and the resulting supernatants were frozen at –80°C until use. Total protein levels were quantified in the colon homogenates by using DC Protein Assay Kit (Cat. # 5000111; Bio-Rad Laboratories). Cytokine levels (IL1β, Cat. # SMLB00C; IL6, Cat. # SM6000B; IL17A, Cat. # SM1700; and IL23, Cat. # M2300) were determined according to the manufacturer’s instructions (Quantikine Murine; R&D Systems, Minneapolis, MN). IL17C levels were measured by using a commercially available ELISA kit (Cat. # SED347Mu; Cloud Clone Corp, Katy, TX). Levels of mouse total β-defensins, human β-defensin 1 and 2 peptide were measured by using commercially available ELISA kits (Cat. # MBS9315750, MBS052463, and MBS703403, respectively; Mybiosource, Cedarlane, Burlington, Canada).
Western Blot
Colons isolated from mice were homogenized in Tris-buffered saline containing protease inhibitor (Cat. # P8340; Sigma-Aldrich). Equal amounts of protein homogenates from each group were loaded and electrophoresed onto 7-20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane by using Transblot turbo transfer system (Bio-Rad) as per manufacturer’s instructions. Membranes were blocked with 3% bovine serum albumin blocking buffer for 1 hour at room temperature and incubated with primary antibodies against Procaspase-8 (1:1000) (Cat # 4927; Cell Signaling Technology), Cleaved Caspase-8 (1:1000) (Cat # 8592; Cell Signaling Technology), Muc2 (0.2 μg/mL) (Cat # sc-15334; Santa Cruz Biotechnology, Santa Cruz, CA), and Muc5ac (1:1000) (Cat. # M5293; Sigma-Aldrich) for overnight at 4°C. Membranes were washed, incubated with either anti-rabbit horseradish peroxidase–linked antibody (1:5000, Cat. # 7074; Cell Signaling Technology) or anti-mouse horseradish peroxidase–linked antibody (0.08 μg/mL, Cat. # sc-2318; Santa Cruz Biotechnology) for 1 hour at room temperature. Proteins were visualized by use of SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific). β-actin was used as a loading control. Densitometric analysis was performed on Western blots with ImageJ software (version 1.48), normalized to total actin. Total protein concentration of homogenized tissue was determined by using DC Protein Assay Kit (Bio-Rad).
Statistical Analysis
Data are represented as means ± standard deviation or means ± standard error of the mean. Where appropriate, data were analyzed by using unpaired Student t test, 1-way analysis of variance, followed by Newman-Keuls, Bonferroni multiple comparison post hoc tests, or Mann-Whitney tests using GraphPad Prism version 5 (GraphPad Software, La Jolla, CA). Bacterial community structures were assessed by using Bray-Curtis beta diversity measures after rarefication to normalize for variable number of reads per sample. Permutational multivariate analysis of variance was used to analyze statistical differences in beta diversity using the vegan package in R.75 Results were visualized by using PCoA plots. Calculations of taxa that differed significantly between mice groups were computed by using DESeq276 (considered significant, if the P value was <.01 after adjustment for multiple testing via DESeq2's implementation of the Benjamini-Hochberg multiple testing adjustment procedure). An associated P value <.05 was considered statistically significant in this study.
Acknowledgments
The authors thank Dr Francine Côté (CNRS, France) for providing Tph1+/+ and Tph1-/- mice and Dr Peter Hedlund (Scripps Research Institute) for 5-HT7-/- mice. The authors also thank Janice Kim, Dr Premsyl Bercik, Dr Elena Verdu, and the staffs of the Axenic Gnotobiotic Unit (AGU) at McMaster University for technical assistance.
Footnotes
Author contributions Y.H.K., H.W., E.D., and W.I.K: conception and design of research. Y.H.K., H.W., J-E.G., L.R., M.S., S.B., M.S.S., and S.B. performed experiments. Y.H.K., H.W., E.D., L.R., J-E.G., and M.G.S. analyzed data. Y.H.K., E.D., J-E.G., M.G.S., and W.I.K. interpreted results of experiments. Y.H.K, E.D., and W.I.K. drafted the manuscript. Y.H.K, S.M.C., M.G.S., and W.I.K. edited and revised manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding Supported by a grant (#93732) from the Canadian Institutes of Health Research (CIHR) to W.I.K.
References
- 1.Kim D.Y., Camilleri M. Serotonin: a mediator of the brain–gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 2.Gershon M. Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13:15–30. [PubMed] [Google Scholar]
- 3.Lundgren O. Enteric nerves and diarrhoea. Basic Clin Pharmacol Toxicol. 2002;90:109–120. doi: 10.1034/j.1600-0773.2002.900301.x. [DOI] [PubMed] [Google Scholar]
- 4.Racke K., Reimann A., Schwörer H., Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res. 1995;73:83–87. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick P.F. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem. 1999;68:355–381. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- 6.Margolis K.G., Stevanovic K., Li Z., Yang Q.M., Oraveca T., Zambrowicz B., Jhaver K.G., Diacou A., Gershon M.D. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut. 2013;63:928–937. doi: 10.1136/gutjnl-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walther D.J., Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 8.Walther D.J., Peter J.U., Bashammakh S., Hörtnagl H., Voits M., Fink H., Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 9.Ahonen A., Kyösola K., Penttilä O. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res. 1976;8:1–7. [PubMed] [Google Scholar]
- 10.Belai A., Boulos P., Robson T., Burnstock G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut. 1997;40:767–774. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coates M.D., Mahoney C.R., Linden D.R., Sampson J.E., Chen J., Blaszyk H., Crowell M.D., Sharkey K.A., Gershon M.D., Mawe G.M. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome 1. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Ghia J.E., Li N., Wang H., Collins M., Deng Y., El–Sharkawy R.T., Côté F., Mallet J., Khan W.I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 13.Linden D.R., Chen J.-X., Gershon M.D., Sharkey K.A., Mawe G.M. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 14.Manocha M., Khan W.I. Serotonin and GI disorders: an update on clinical and experimental studies. Clin Transl Gastroenterol. 2012;3:e13. doi: 10.1038/ctg.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N., Ghia J.E., Wang H., McClemens J., Cote F., Suehiro Y., Mallet J., Khan W.I. Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol. 2011;178:662–671. doi: 10.1016/j.ajpath.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haub S., Ritze Y., Bergheim I., Pabst O., Gershon M., Bischoff S. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol Motil. 2010;22:826–834. doi: 10.1111/j.1365-2982.2010.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liévin-Le Moal V., Servin A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill D.A., Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2009;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manichanh C., Borruel N., Casellas F., Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy R., Hoper M., Deodhar K., Erwin P., Kirk S., Gardiner K. Interleukin 10-deficient colitis: new similarities to human inflammatory bowel disease. Br J Surg. 2000;87:1346–1351. doi: 10.1046/j.1365-2168.2000.01615.x. [DOI] [PubMed] [Google Scholar]
- 22.Tsukamoto K., Ariga H., Mantyh C., Pappas T.N., Yanagi H., Yamamura T., Takahashi T. Luminally released serotonin stimulates colonic motility and accelerates colonic transit in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R64–R69. doi: 10.1152/ajpregu.00856.2006. [DOI] [PubMed] [Google Scholar]
- 23.O’Neil D.A., Porter E.M., Elewaut D., Anderson G.M., Eckmann L., Ganz T., Kagnoff M.F. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–6724. [PubMed] [Google Scholar]
- 24.Kim J.J., Bridle B.W., Ghia J.E., Wang H., Syed S.N., Manocha M.M., Rengasamy P., Shajib M.S., Wan Y., Hedlund P.B. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol. 2013;190:4795–4804. doi: 10.4049/jimmunol.1201887. [DOI] [PubMed] [Google Scholar]
- 25.Ke R., Xie X., Li S., Pan Y., Wang J., Yan X., Zang W., Gao L., Li M. 5-HT induces PPAR γ reduction and proliferation of pulmonary artery smooth muscle cells via modulating GSK-3β/β-catenin pathway. Oncotarget. 2017;8:72910–72920. doi: 10.18632/oncotarget.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Tian X.Y., Mao G., Fang X., Fung M.L., Shyy J.Y.-J., Huang Y., Wang N. Peroxisome proliferator-activated receptor-γ ameliorates pulmonary arterial hypertension by inhibiting 5-hydroxytryptamine 2B receptor. Hypertension. 2012;60:1471–1478. doi: 10.1161/HYPERTENSIONAHA.112.198887. [DOI] [PubMed] [Google Scholar]
- 27.Peyrin-Biroulet L., Beisner J., Wang G., Nuding S., Oommen S.T., Kelly D., Parmentier-Decrucq E., Dessein R., Merour E., Chavatte P. Peroxisome proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. Proc Natl Acad Sci U S A. 2010;107:8772–8777. doi: 10.1073/pnas.0905745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X., Chen C., Cheng G., Liang L., Yao X., Yang G., You P., Shou X. Peroxisome proliferator-activated receptor γ attenuates serotonin-induced pulmonary artery smooth muscle cell proliferation and apoptosis inhibition involving ERK1/2 pathway. Microvasc Res. 2015;100:17–24. doi: 10.1016/j.mvr.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Becker C., Watson A.J., Neurath M.F. Complex roles of caspases in the pathogenesis of inflammatory bowel disease. Gastroenterology. 2013;144:283–293. doi: 10.1053/j.gastro.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 30.Gringhuis S.I., Kaptein T.M., Wevers B.A., Theelen B., Van Der Vlist M., Boekhout T., Geijtenbeek T.B. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 31.Gurung P., Kanneganti T.D. Novel roles for caspase-8 in IL-1β and inflammasome regulation. Am J Pathol. 2015;185:17–25. doi: 10.1016/j.ajpath.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monie T.P., Bryant C.E. Caspase-8 functions as a key mediator of inflammation and pro-IL-1β processing via both canonical and non-canonical pathways. Immuno Rev. 2015;265:181–193. doi: 10.1111/imr.12284. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez-Carrozzi V., Sambandam A., Luis E., Lin Z., Jeet S., Lesch J., Hackney J., Kim J., Zhou M., Lai J. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 34.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buttó L.F., Haller D. Dysbiosis in intestinal inflammation: cause or consequence. Int J Med Microbiol. 2016;306:302–309. doi: 10.1016/j.ijmm.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 37.Umbrello G., Esposito S. Microbiota and neurologic diseases: potential effects of probiotics. J Transl Med. 2016;14:298. doi: 10.1186/s12967-016-1058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bäckhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 39.Palmer C., Bik E.M., DiGiulio D.B., Relman D.A., Brown P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan W., Ghia J. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19–27. doi: 10.1111/j.1365-2249.2010.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharkey K.A., Mawe G.M. Neuroimmune and epithelial interactions in intestinal inflammation. Curr Opin Pharmacol. 2002;2:669–677. doi: 10.1016/s1471-4892(02)00215-1. [DOI] [PubMed] [Google Scholar]
- 42.Fukumoto S., Tatewaki M., Yamada T., Fujimiya M., Mantyh C., Voss M., Eubanks S., Harris M., Pappas T.N., Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 43.Reigstad C.S., Salmonson C.E., Rainey J.F., Szurszewski J.H., Linden D.R., Sonnenburg J.L., Farrugia G., Kashyap P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bearcroft C., Perrett D., Farthing M. 5-hydroxytryptamine release into human jejunum by cholera toxin. Gut. 1996;39:528–531. doi: 10.1136/gut.39.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kidd M., Gustafsson B., Drozdov I., Modlin I. IL1β- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol Motil. 2009;21:439–450. doi: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemire P., Robertson S.J., Maughan H., Tattoli I., Streutker C.J., Platnich J.M., Muruve D.A., Philpott D.J., Girardin S.E. The NLR protein NLRP6 does not impact gut microbiota composition. Cell Rep. 2017;21:3653–3661. doi: 10.1016/j.celrep.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 47.Mamantopoulos M., Ronchi F., Van Hauwermeiren F., Vieira-Silva S., Yilmaz B., Martens L., Saeys Y., Drexler S.K., Yazdi A.S., Raes J. Nlrp6-and ASC-dependent inflammasomes do not shape the commensal gut microbiota composition. Immunity. 2017;47:339–348. doi: 10.1016/j.immuni.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Bishop A., Pietroletti R., Taat C., Brummelkamp W., Polak J. Increased populations of endocrine cells in Crohn's ileitis. Virchows Arch. 1987;410:391–396. doi: 10.1007/BF00712758. [DOI] [PubMed] [Google Scholar]
- 49.Frank D.N., Amand A.L.S., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ott S., Musfeldt M., Wenderoth D., Hampe J., Brant O., Fölsch U., Timmis K., Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muniz L.R., Knosp C., Yeretssian G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Frontiers in Immunology. 2012;3:310. doi: 10.3389/fimmu.2012.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wehkamp J., Harder J., Weichenthal M., Mueller O., Herrlinger K.R., Fellermann K., Schroeder J.M., Stange E.F. Inducible and constitutive β-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215–223. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Guseva D., Holst K., Kaune B., Meier M., Keubler L., Glage S., Buettner M., Bleich A., Pabst O., Bachmann O. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis. 2014;20:1516–1529. doi: 10.1097/MIB.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 54.Spohn S.N., Bianco F., Scott R.B., Keenan C.M., Linton A.A., O'Neill C.H., Bonora E., Dicay M., Lavoie B., Wilcox R.L. Protective actions of epithelial 5-hydroxytryptamine 4 receptors in normal and inflamed colon. Gastroenterology. 2016;151:933–944. doi: 10.1053/j.gastro.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubuquoy L., Jansson E.Å., Deeb S., Rakotobe S., Karoui M., Colombel J.-F., Auwerx J., Pettersson S., Desreumaux P. Impaired expression of peroxisome proliferator-activated receptor γ in ulcerative colitis. Gastroenterology. 2003;124:1265–1276. doi: 10.1016/s0016-5085(03)00271-3. [DOI] [PubMed] [Google Scholar]
- 56.Su C.G., Wen X., Bailey S.T., Jiang W., Rangwala S.M., Keilbaugh S.A., Flanigan A., Murthy S., Lazar M.A., Wu G.D. A novel therapy for colitis utilizing PPAR-γ ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383–389. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Are A., Aronsson L., Wang S., Greicius G., Lee Y.K., Gustafsson J.-Å., Pettersson S., Arulampalam V. Enterococcus faecalis from newborn babies regulate endogenous PPARγ activity and IL-10 levels in colonic epithelial cells. Proc Natl Acad Sci U S A. 2008;105:1943–1948. doi: 10.1073/pnas.0711734105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly D., Campbell J.I., King T.P., Grant G., Jansson E.A., Coutts A.G., Pettersson S., Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat Immnuol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 59.Campieri M., Gionchetti P. Bacteria as the cause of ulcerative colitis. Gut. 2001;48:132–135. doi: 10.1136/gut.48.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohkusa T., Kato K., Terao S., Chiba T., Mabe K., Murakami K., Mizokami Y., Sugiyama T., Yanaka A., Takeuchi Y. Newly developed antibiotic combination therapy for ulcerative colitis: a double-blind placebo-controlled multicenter trial. Am J Gastroenterol. 2010;105:1820–1829. doi: 10.1038/ajg.2010.84. [DOI] [PubMed] [Google Scholar]
- 61.Moayyedi P., Surette M.G., Kim P.T., Libertucci J., Wolfe M., Onischi C., Armstrong D., Marshall J.K., Kassam Z., Reinisch W. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Kang C.S., Ban M., Choi E.J., Moon H.G., Jeon J.S., Kim D.K., Park S.K., Jeon S.G., Roh T.-Y., Myung S.J. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PloS One. 2013;8:e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reunanen J., Kainulainen V., Huuskonen L., Ottman N., Belzer C., Huhtinen H., de Vos W.M., Satokari R. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of epithelial cell layer. Appl Environ Microbiol. 2015;81:3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alam A., Leoni G., Quiros M., Wu H., Desai C., Nishio H., Jones R.M., Nusrat A., Neish A.S. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol. 2016;1:15021. doi: 10.1038/nmicrobiol.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Côté F., Thévenot E., Fligny C., Fromes Y., Darmon M., Ripoche M.-A., Bayard E., Hanoun N., Saurini F., Lechat P. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hedlund P., Danielson P., Thomas E., Slanina K., Carson M., Sutcliffe J. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim J.J., Shajib M.S., Manocha M.M., Khan W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp. 2012:3678. doi: 10.3791/3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartram A.K., Lynch M.D., Stearns J.C., Moreno-Hagelsieb G., Neufeld J.D. Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end Illumina reads. Appl Environ Microbiol. 2011;77:3846–3852. doi: 10.1128/AEM.02772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stearns J.C., Davidson C.J., McKeon S., Whelan F.J., Fontes M.E., Schryvers A.B., Bowdish D.M., Kellner J.D., Surette M.G. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISEM J. 2015;9:1246–1259. doi: 10.1038/ismej.2014.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whelan F.J., Surette M.G. A comprehensive evaluation of the sl1p pipeline for 16S rRNA gene sequencing analysis. Microbiome. 2017;5:100. doi: 10.1186/s40168-017-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McMurdie P.J., Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan W.I., Motomura Y., Wang H., El-Sharkawy R.T., Verdu E.F., Verma-Gandhu M., Rollins B.J., Collins S.M. Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2006;291:G803–G811. doi: 10.1152/ajpgi.00069.2006. [DOI] [PubMed] [Google Scholar]
- 74.Manocha M., Shajib M., Rahman M., Wang H., Rengasamy P., Bogunovic M., Jordana M., Mayer L., Khan W. IL-13-mediated immunological control of enterochromaffin cell hyperplasia and serotonin production in the gut. Mucosal Immunol. 2013;6:146–155. doi: 10.1038/mi.2012.58. [DOI] [PubMed] [Google Scholar]
- 75.Oksanen J. Multivariate analysis of ecological communities in R: vegan tutorial. R package version. 2011;1:11–12. [Google Scholar]
- 76.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]