Abstract
Aim, materials & methods:
Urinary cortisol profile has the potential as a diagnostic biomarker. We therefore developed a stable-isotope dilution ultraperformance chromatography multistage MS-based method to quantify cortisol and 16 metabolites in human urines.
Results & conclusion:
The LOD for cortisol and its metabolites ranges from 0.02 to 5.81 pg/μl urine. The inter- and intraday variations were 3.7–12.9% and 3.5–15.6%, respectively. Among the metabolites analyzed, significant person-to-person heterogeneity was observed, demonstrating the need for comprehensive metabolite profiling in diagnosis. Nevertheless, the glucuronides of dihydrocortisol, dihydrocortisone, tetrahydrocortisol, allo-tetrahydrocortisol and tetrahydrocortisone are the major ones. The sum of the glucuronidated and free forms constitute >93% of the metabolites analyzed, which is termed as total cortisol equivalent. Total cortisol equivalent may serve as a surrogate of cortisol secretion.
Clinical trial registration number: NCT02500472
Keywords: : cortisol metabolism, high-resolution accurate-mass MS, stress, tobacco, urine biomarker
Cortisol has the potential to serve as a biomarker to estimate stress level [1–3]. Plasma or salivary cortisol as a biomarker is challenging, because its abundance is subjected to diurnal fluctuation [4]. Urinary measurement, on the other hand, captures cortisol secretion over a period of time and may be more reliable [5]. Since cortisol is extensively metabolized with only about 1% of plasma cortisol excreted into urine in its parent form [6–8], both cortisol and its metabolites need to be quantified, particularly given the potential person-to-person heterogeneity of cortisol metabolism, which has never been quantitatively estimated.
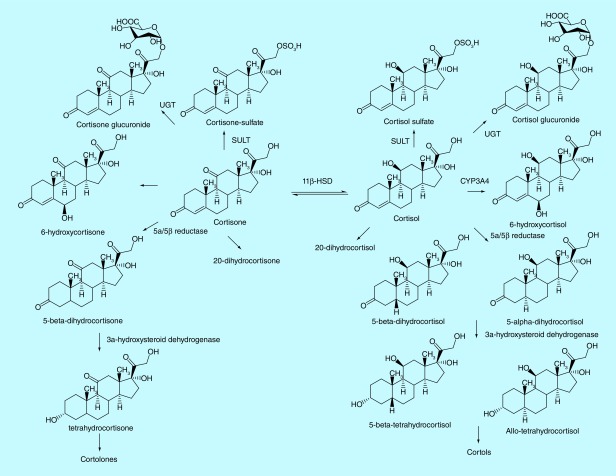
The major metabolic pathways of cortisol in human are summarized in Figure 1 [9]. Various methods have been developed to quantify cortisol and its metabolites in human urines, including ELISA, HPLC, GC–MS and HPLC–MS/MS [10]. GC–MS has been extensively employed to measure cortisol metabolites in human biofluids, which have reported reference values for cortisol metabolites in human urine [11–13]; however, derivatization was typically needed prior to analyses due to the low volatility of cortisol and its metabolites [11,14,15]. Lack of high selectivity is another limit for GC–MS and LC–MS-based methods. Although MS/MS-based methods exhibited superior selectivity [9,10,16], measurement of ion transitions with low-resolution MS, such as multiple reaction monitoring with triple quadrupoles MS, typically suffers from interfering metabolites from matrices, which co-elute with targeted metabolites and exhibit the same transition at the nominal mass. The interference sometimes can cause problems in peak integration and quantification of targeted metabolites. In contrast, high-resolution accurate-mass measurement MS at MSn scan stage enables us to extract the product ions at a mass tolerance of 5–10 p.p.m. window, which greatly reduces the interfering peaks from a complex matrix and produces cleaner chromatograms for reliable quantification. In addition, it also remains to be determined the relative abundances of free, glucurodinated and sulfate forms of cortisol metabolites in a particular subject and how heterogeneous they are among subjects. The major metabolites may serve as a better surrogate of total cortisol secretion than cortisol itself to estimate stress level. The relative abundance of cortisol metabolites may have diagnostic values since it would reflect the activities of the key enzymes involved in cortisol metabolism [17,18].
Figure 1. . Major metabolic pathways of cortisol in human.
In this study, we developed a sensitive stable-isotope dilution ultra-high performance liquid chromatography multistage MS (UHPLC–MSn)-based method with high-resolution accurate-mass Orbitrap to quantify cortisol and its 16 metabolites (Figure 1) in human urines. We also applied this method to evaluate the impact of tobacco smoking on the urinary profiling of cortisol metabolites in humans. By profiling these metabolites among nonsmokers (n = 14) and smokers (n = 21), we identified the major metabolites and characterized the level of person-to-person heterogeneity. The sum of five cortisol metabolites and their glucuronides accounted for >93% of the total metabolites quantified herein. We defined the sum of free, glucuronidated and sulfate forms of cortisol metabolites as the total cortisol equivalent (TCE). TCE provides a better estimate of total cortisol excretion than cortisol alone. Its level in smokers was found four-times of that in nonsmokers.
Materials & methods
Chemicals & materials
Cortisol, [9,11,12,12-2H4]-cortisol, 5α-dihydrocortisol, 5β-dihydrocortisol, 6α-hydroxycortisol, 6β-hydroxycortisol, [9,11,12,12-2H4]-6β-hydroxycortisol, allo-3α-tetrahydrocortisol (A-THF), 5β-tetrahydrocortisol (THF), [2,2,4,4,21,21-2H6]-allo-3α-tetrahydrocortisol, cortisone, [2,2,4,6,6,9,21,21-2H8]-cortisone, 5β-tetrahydrocortisone (THE) and [2,2,4,4,21,21-2H6]-tetrahydrocortisone were purchased from Toronto Research Chemicals (TRC, ON, Canada). [9,11,12,12-2H4]-cortisol-21-sulfate was purchased from Sigma-Aldrich (MO, USA). The chemical purity and isotope purity were above 99% as provided by TRC (MO, USA). Identification of those standards was confirmed with high-resolution MS and H-NMR by TRC. Stability tests of isotope-labeled standards were performed biweekly during their storage at -20°C. Water, formic acid, methanol and acetonitrile were Optima LC/MS grade from Fisher Scientific (NJ, USA). β-Glucuronidase was from Helix pomatia and purchased from Sigma-Aldrich. Oasis MCX and WAX SPE cartridges (30 mg) were purchased from Waters (MA, USA). StrataX reverse-phase cartridges (33 μm, 10 mg/1 ml) were purchased from Phenomenex (CA, USA). Other chemicals were purchased from Sigma-Aldrich unless stated otherwise. Cortisol- and cortisone-sulfates were synthesized in house (details in Supplementary Data).
Human urine samples
Overnight urine samples of human smokers (n = 21) were obtained from a clinical trial conducted at the University of Minnesota. The study was approved by the Institutional Review Board at the University of Minnesota and all subjects provided informed, written consent. Urine samples of human nonsmokers (n = 14) were purchased from BioreclamationIVT (Bioreclamation, Inc., MD, USA). The details of urine sample collection of nonsmokers were unknown.
Enrichment of cortisol metabolites from urine
For free form of cortisol metabolites, human urine (100 μl) was mixed with H2O (100 μl) and dichloromethane (800 μl). For the sum of free and gluc forms of cortisol metabolites, urine (100 μl) and H2O (100 μl) were first treated with β-glucuronidase (500 units) at 37°C for 6 h followed by the addition of dichloromethane (800 μl). For both types of analyses, [2H4]-cortisol and [2H8]-cortisone were spiked at a level of 2 pg/μl urine; [2H4]-6-hydroxycortisol was spiked at 50 pg/μl urine; [2H6]-A-THF and [2H6]-THE were spiked at 100 pg/μl urine. The dichloromethane fraction was speed vacuumed to dryness, re-suspended in 10% CH3OH (1 ml), applied to a StrataX cartridge, and washed by 10% CH3OH (1 ml) and H2O (4 ml). The StrataX cartridge was preconditioned with CH3OH (1 ml) followed by H2O (2 ml). The metabolites were eluted with CH3OH (1 ml), speed vacuumed to dryness and re-suspended in 10% CH3OH (50 μl) for analysis.
For cortisol- and cortisone-sulfate, human urine (100 μl) was mixed with CH3OH (-20°C, 900 μl) to precipitate the proteins. [2H4]-cortisol-21-sulfate was spiked at a level of 5 pg/μl urine. The mixture was centrifuged at 13,000 × g for 20 min. The supernatant was transferred to an Eppendorf tube, acidified with HCO2H (0.5%, final concentration), applied to a WAX cartridge, and washed with 0.5% HCO2H in H2O (2 ml) followed by CH3OH (1 ml). The WAX cartridge was preconditioned with 2.5% NH4OH in CH3OH (1 ml) and 0.5% HCO2H (2 ml). The metabolites were eluted with 2.5% NH4OH in CH3OH (1 ml), speed vacuumed to dryness and re-suspended in 10% CH3OH (50 μl) for analysis.
Measurement of cortisol metabolites by ultraperformance LC ESI multistage MS
Mass spectral data of each cortisol and cortisone metabolites were acquired by direct infusion with a Q Exactive Hybrid Quadrupole Orbitrap Mass Spectrometer (Thermo Fisher, CA, USA) and a Heated ESI (HESI-II) source (Thermo Fisher). The flow rate of direct infusion was set as 10 μl/min. Instrument tuning parameters were as follows: sheath gas, 5; auxiliary gas, 0; auxiliary gas temperature, 50°C; capillary temperature, 300°C; spray voltage, 4 kV; 1μ scan; maximum injection time, 100 and 200 ms for full MS and MSn, respectively; in source collision-induced dissociation, 10 eV; and higher-energy collisional dissociation (HCD), 20 for MS2. Resolution was set as 70,000 and 17,500 at m/z 200 for full MS and MSn, respectively. The isolation width was set at m/z 1 for MS2 scan modes. All analyses were conducted in the positive ionization mode.
The urinary samples following SPE were assayed by targeted UHPLC–MS2 or pseudo UHPLC–MS3 employing a Dionex Ultimate 3000 RS (Thermo Fisher) and a Q Exactive Hybrid Quadrupole Orbitrap Mass Spectrometer. The samples were resolved through an Atlantis dc18 column (150 × 2.1 mm, 3 μm particle size, 100 Å; Waters) with a 25-min linear gradient from 99% A (H2O with 1% CH3CN and 0.01% HCO2H) to 99% B (CH3CN with 5% H2O and 0.01% HCO2H) at a flow rate of 150 μl/min. The same UHPLC system was employed for cortisol- and cortisone-sulfate, but employed a 25-min linear gradient from 99% A (5 mM ammonium acetate in 10% CH3CN) to 50% B (5 mM ammonium acetate in CH3CN with 10% H2O) at a flow rate of 150 μl/min. Chromeleon 7.2 Chromatography Data System was used for the UHPLC management. Parallel reaction monitoring was employed to measure the cortisol metabolites. The parameters for HESI-II were set as follows: sheath gas, 15; auxiliary gas, 5; auxiliary gas temperature, 200°C; capillary temperature, 300°C; spray voltage, 4 kV; 1μ scan; maximum injection time, 200 ms for MSn; and HCD, 20. In-source CID collision energy was set as 10 eV for pseudo UHPLC–MS3. Resolution was set as 17,500 at m/z 200 for MSn. The isolation width was set at m/z 1 for MSn scan mode. Automated gain control was set at 50,000 for Orbitrap MS2. The Orbitrap was routinely calibrated in the positive-ion mode using Pierce LTQ Velos ESI Positive Ion Calibration Solution (2 μg/ml caffeine, 1 μg/ml MRFA, 0.001% Ultramark 1621 and 0.00005% n-butylamine; Thermo Fisher).
Calibration curves
The postextraction spike method was employed. Since the levels of endogenous cortisol metabolites in the human urine were well above the LOD, a synthetic urine was used as the blank matrix [19]. Nine-point calibration curves (0–10,000 fg/μl urine) were constructed for cortisol and cortisone. Ten-point calibration curves (0–200 pg/μl urine) were constructed for 6-hydroxycortisol. Ten-point calibration curves (0–1000 and 0–2000 pg/μl urine) were constructed for THF/A-THF and THE, respectively. Ten-point calibration curves (0–10,000 and 0–20,000 pg/μl urine) were constructed for dihydrocortisol and dihydrocortisone, respectively. [2H4]-cortisol, [2H4]-6-hydroxycortisol, [2H6]-A-THF, [2H4]-cortisol sulfate, [2H8]-cortisone and [2H6]-THE were spiked as internal standards at levels of 2, 50, 100, 5, 2 and 200 pg/μl, respectively. LOD and LOQ were estimated by 3.3 σ/s and 10 σ/s above the background level signal of a blank matrix, respectively (σ is the SD of the slope [s] of the calibration curve) [20].
Method validation
Method validation follows the US FDA guideline [21]. Performance of this method was validated by specificity, precision, accuracy, within-day and between-day reproducibility using the pure standards of cortisol metabolites that were spiked into the blank urine matrix at four different concentrations on 3 different days. Matrix effect was evaluated by the ratios of ion signals of cortisol standards that were spiked to the urine matrix after sample preparation to the ion signals of standards that were spiked directly to the solvent. Accuracy was expressed as the percentage of the mean of measured values to theoretical values. Short-term stability of cortisol metabolites in the matrix was evaluated after three freeze and thaw cycles, and 1-, 6- and 24-h incubation in the blank urine at 37°C.
Total nicotine equivalents & urinary creatinine
The smoking status of nonsmokers and smokers was confirmed by the measurement of total nicotine equivalents (TNE) in the urine, which is the sum of nicotine, cotinine, 3-hydroxycotine, their glucuronides and nicotine-N-oxide [22]. Creatinine was measured using a modified Jaffe reaction method [23] for urine volume adjustment. The level of urinary cortisol metabolites was expressed as pmol/mg creatinine.
Statistical analysis
Two-tailed t-test was performed for the analysis of the results between smokers and nonsmokers using GraphPad Prism (v. 7.00). p < 0.05 was considered statistically significant.
Results & discussion
Mass spectrometric characterization of cortisol metabolites
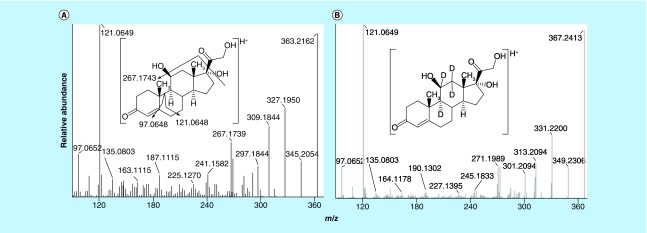
Mass spectra of cortisol and its metabolites at MS2 and pseudo MS3 [24] scan stages, which were characterized by direct infusion of pure standards with accurate mass measurements by Orbitrap, exhibited excellent mass accuracy. The observed masses of product ions were within 3 p.p.m. window of the calculated m/z values. As an example, product ion mass spectra of cortisol (m/z 363.2162 vs calculated m/z 363.2166, Δ1.1 p.p.m.) and [2H4]-cortisol (m/z 367.2413 vs calculated m/z 367.2417, Δ1.1 p.p.m.) contained ions at m/z 97.0652 (C6H9O, vs calculated m/z 97.0648, Δ4.1 p.p.m.), m/z 121.0649 (C8H9O, vs calculated m/z 121.0648, Δ0.8 p.p.m.), m/z 135.0803 (C9H11O, vs calculated m/z 135.0804, Δ0.7 p.p.m.), m/z 267.1739 and 271.1989 ([M+H-C2H8O4]+), m/z 309.1844 and 313.2094 ([M+H-3H2O]+), m/z 327.1950 and 331.2200 ([M+H-2H2O]+) and m/z 345.2054 and 349.2306 ([M+H-H2O]+) (Figures 2A & B).
Figure 2. . Product ion mass spectra of (A) cortisol (m/z 363.2 >) and (B) [2H4]-cortisol (m/z 367.2 >) at MS2 scan stage.
Production mass spectra of cortisol metabolites and corresponding isotope-labeled standards are shown in Supplementary Figures 1–3. THF and A-THF (m/z 367.2479) tend to undergo in-source fragmentation by ESI in full MS scan, losing one to three molecules of water to produce a base peak at m/z 331.2268. THF/A-THF and [2H6]-A-THF exhibited different relative abundances of product ions at m/z 135.1166, m/z 147.1167, m/z 139.1415 and m/z 151.1415 (Supplementary Figure 2). After confirmed their identification by H-NMR and UHPLC–MS, we suspected that the positioning of the deuterium atoms might cause the different relative abundance in ionization. In-source fragmentation of parent ions in full MS scan was also observed for THE and dihydrocortisone. Therefore, we employed a pseudo-MS3, which was to use the in-source fragmentation by applying a 10 eV in-source CID followed by product-ion scanning in the Orbitrap, for quantification of THF, A-THF, THE and dihydrocortisone (Supplementary Figures 2E–G & 3A & B). Atmospheric pressure chemical ionization-based ionization has been employed for cortisol metabolites; however, we aimed to develop a method to simultaneously measure different cortisol metabolites, instead of switching the sources between ESI and atmospheric pressure chemical ionization for different compounds. By employing the cation exchange SPE cartridge, we extracted the cortisol- and cortisone-sulfates from the urine and directly measured them by UHPLC-MS/MS (Supplementary Figures 2C & 3D).
Supplementary Figure 4 shows the UHPLC–MS2 and UHPLC–MS3 profiles of the pure standards of cortisol, their metabolites and corresponding isotope-labeled standards. We assayed both α- and β-anomers of 6-hydroxycortisol, 5-tetrahydrocortisol and 5-dihydrocortisol, but did not achieve chromatographic separation of the two anomers under current UHPLC conditions. Therefore, the integration of the peaks of 6-hydroxycortisol, 5-tetrahydrocortisol and 5-dihydrocortisol included both α- and β-anomers. Deuterium isotope effect caused [2H6]-A-THF to elute 0.5 min earlier than the unlabeled THF and A-THF (Supplementary Figure 4A). Previous studies reported a partial separation of THF and A-THF by using chromatographic condition with 0.1% acetic acid or formic acid (FA) [9,25]. However, we observed considerable ion suppression and lost 40–80% ion signals of cortisol metabolites when the concentration of FA increased from 0.01 to 0.05%, and 0.1% during optimization of chromatographic conditions. No deuterium isotope effects were observed for the isotope-labeled standards of other cortisol metabolites.
Method validation
Because the endogenous levels of urinary cortisol, 6-hydroxycortisol and cortisone are above the LOD, a synthetic urine sample was employed as the blank matrix for calibration curve construction. Human urine samples from nonsmokers were used as blank matrices for the other cortisol metabolites. In the blank urine sample spiked with LLOQ level of cortisol metabolites, six to nine scans were acquired across the full width of the peaks (Supplementary Figure 5). At the high spike level of standards, 14–22 scans were acquired across the full width of the peak (Supplementary Figure 5).
The calibration curves of cortisol metabolites are shown in Supplementary Figure 6. Because dihydrocortisol and dihydrocortisone, which were estimated by [2H6]-THF and [2H6]-THE, exhibited different MS/MS fragmentation, slopes of their calibration curves were not close to 1. We estimated LOD and LOQ by 3.3 σ/s and 10 σ/s, respectively (σ is the standard deviation of the slope [s] of the calibration curve) [26], because there was no measurable background signal at the MS2 and MS3 scan stage in the blank sample acquired by the high-resolution Orbitrap. LOD and LOQ values of cortisol metabolites were summarized in Table 1. Sensitivities were achieved for cortisol and cortisone in the urine with LOD as low as 0.05 and 0.02 pg/μl urine, respectively, 10–75-times more sensitive than the methods in previous reports [9,25]. LOD and LOQ estimates were higher for A-THF/THF, 6-hydroxycortisol, dihydrocortisol, THE and dihydrocortisone, likely because these compounds were labile under ESI and underwent in-source fragmentation to lose H2O molecules in the full MS scan, which caused a drop of ion signals measured at MS2 and MS3 scan stages. Previous studies also reported LOD values to be 0.2–1 pg/μl for A-THF, THF and THE using low-resolution MS [9]. In our study, A-THF, THF and THE were not detectable at a mass tolerance of 5 p.p.m. when spiked at 0.2 pg/μl urine. However, when the mass tolerance increased to 500 p.p.m., interfering peaks from the matrix were observed that co-eluted with A-THF/THF and THE (Supplementary Figure 7).
Table 1. . Estimates of LOD and LOQ for cortisol metabolites in human urines.
| Metabolites | LOD (pg/μl urine) | LOQ (pg/μl urine) |
|---|---|---|
| Cortisol | 0.05 | 0.16 |
| 6-Hydroxycortisol | 1.20 | 3.63 |
| A-THF/THF | 0.79 | 2.39 |
| Dihydrocortisol | 1.56 | 4.74 |
| Cortisol sulfate | 0.23 | 0.77 |
| Cortisone | 0.02 | 0.08 |
| THE | 1.72 | 5.21 |
| Dihydrocortisone | 5.81 | 17.61 |
| Cortisone-sulfate | 0.28 | 0.84 |
[2H4]-cortisol, [2H4]-6β-hydroxycortisol, [2H6]-A-THF, [2H8]-cortisone, [2H6]-THE and [2H4]-cortisol sulfate were employed as internal standards.
A-THF: Allo-3α-tetrahydrocortisol; THE: 5β-tetrahydrocortisone; THF: 5β-tetrahydrocortisol.
The accuracy, precision and reproducibility of our methods were summarized in Supplementary Tables 1 & 2. The within day and between day variations were 2.8–12.9% and 3.2–15.6%, respectively. Recovery rates ranged 50–92% after sample workup, depending on each metabolite. The matrix effect for cortisol metabolites at LLOQ and high range is summarized in Supplementary Table 3. Matrix effect values, which were calculated as the ratios of peak areas of pure standards spiked to the blank urine after sample preparation to the peak areas of the standards spiked to the pure solvents, ranged from 82 to 142% and 95 to 120%, respectively (Supplementary Table 3). These data suggest the sample preparation by tandem liquid–liquid extraction with dichloromethane and offline SPE greatly reduced the ion suppression from the urine. Freeze-and-thaw stability and short-term in-matrix stability of cortisol metabolites were also examined and summarized in Supplementary Table 4. Freeze-thaw caused relatively small loss of signals except for A-THF/THF at the LLOQ level and dihydrocortisone at the high range. Short-term in-matrix stability experiment showed that the signals of cortisol metabolites at the high spiked level after a 24-h test were 71–114% of the control with dihydrocortisone being the lowest one while there was no appreciable loss within 1 h for any analyte. At the LLOQ- spiked level, signals of most metabolites decreased significantly after the 24-h test except for the two sulfates. It should be noted that the endogenous level of the free and gluc forms of these metabolites in the human urine are in the range of the high spiked level, thus the stability at the high spike level is more clinically relevant.
UHPLC–MSn analysis of cortisol metabolites in human urine
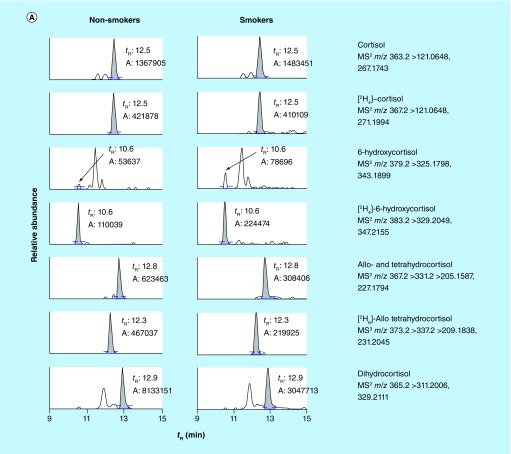
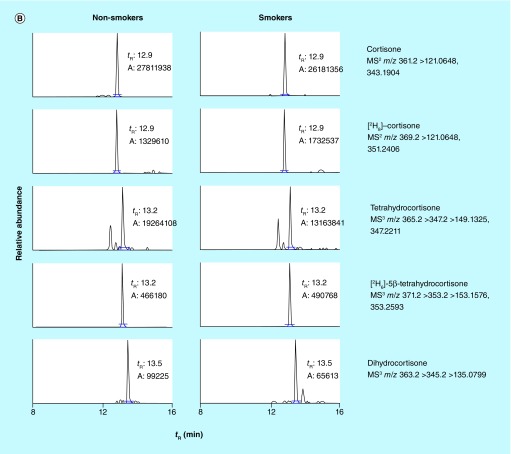
We then employed this targeted UHPLC–MSn method to quantify cortisol metabolites in the urine samples of 14 nonsmokers and 21 smokers. Their smoking status was confirmed by quantifying the urinary TNE. With the comprehensive understanding of nicotine metabolism, TNE (the sum of nicotine, cotinine, 3-hydroxycotinine, their glucuronide conjugates and nicotine N-oxide, which captures 80–90% of nicotine metabolites) has been demonstrated as an excellent surrogate to estimate human smoking status [22,27–29]. The average TNE values in smokers and nonsmokers are 158 ± 85 (40–423) and 4 ± 2 (2–8) nmol/mg creatinine, respectively (Supplementary Figure 8). Figure 3 shows examples of the reconstructed ion chromatograms of cortisol metabolites in the urine of a nonsmoker and a smoker. Elution time and mass spectra of these metabolites in the urine sample were in agreement with the data acquired by the pure standards. High-resolution accurate-mass measurement of Orbitrap at MSn scan stage enabled us to extract the product ions at a mass tolerance of 5 p.p.m. window, which greatly reduced the interfering peaks from the matrix and produced cleaner ion chromatograms for reliable quantification in comparison to the low-resolution MS-based method (Supplementary Figure 7). Although other peaks were seen on the chromatograms of 6-hydroxycortisol, dihydrocortisol, THE and dihydrocortisone even at a mass tolerance of 5 p.p.m., those peaks did not co-elute with targeted metabolites (Figure 3). We also observed the level of 6-hydroxycortisol decreased by 14–30% after β-glucuronidase treatment (data not shown), which suggested that 6-hydroxycortisol was not stable, consistent with the result of its stability test (Supplementary Table 4). Therefore, we only quantified the free form of 6-hydroxycortisol.
Figure 3. . Reconstructed ion chromatograms of (A) cortisol and (B) cortisone metabolites in the urine samples of human nonsmokers and smokers.
The mass extraction window was ±5 p.p.m.
The estimates of cortisol and its metabolites in the urine of 14 nonsmokers and 21 smokers upon creatinine correction were summarized in Table 2. The levels of urinary total cortisol and cortisone were comparable to the values reported in a previous study (12–80 μg/24 h for cortisol; 25–341 μg/24 h for cortisone) [16,25]; however, the abundance of total dihydrocortisol, A-THF/THF, dihydrocortisone and THE determined in our study was 10–100 fold higher [16]. The level of those metabolites may be underestimated in the previous study, because they were estimated based on [2H4]-cortisol and [2H4]-hydroxycortisol being the internal standards. As demonstrated in Table 1, dihydrocortisol, A-THF/THF, dihydrocortisone and THE were labile and underwent in-source fragmentation under ESI, which caused their signals to decrease by 15–130-fold compared with cortisol and cortisone. The free forms of dihydrocortisol, THE and dihydrocortisone in some subjects were below LOD. In all the samples, cortisol and cortisol gluc were the low abundant metabolites, accounting for not >0.1% of the metabolites quantified, raising the question of them as the surrogate of cortisol secretion. On the other hand, the sum of the free and glucuronide forms of dihydrocortione, dihydrocortisol, A-THF/THF and THE accounted for >92% of the cortisol metabolites in all 35 urines was analyzed. These data suggest that the sum of these five metabolites and their glucuronides, which can be obtained from a single analysis with β-glucuronidase treatment, offers a close approximation of the total amount of cortisol and metabolites in urine, which is termed as urinary TCE. There is a difference in the values of total 5-dihydrocortisol measured in our study and those reported values in the literature [30]. However, most, if not all, of previous reports only quantified either the free form or the total form. In addition, they rarely performed the quantitative analysis to each individual subject. Of course, our results remain to be validated in samples from larger populations.
Table 2. . Quantities of cortisol metabolites in the urine of 14 nonsmokers and 21 smokers.
| Metabolites | Smokers | Nonsmokers | ||
|---|---|---|---|---|
| Level (pmol/mg creatinine) | Relative abundance | Level (pmol/mg creatinine) | Relative abundance | |
| Cortisol | 3–58 | <0.07% | 0.4–61† | <0.04% |
| Cortisol gluc | 8–565 | <0.02% | 1–17 | <0.03% |
| THF/A-THF | 1–71 | <0.03% | 30–1635 | <0.94% |
| THF/A-THF gluc | 612–9139 | 0.14–9.50% | 352–11,584 | 0.29–5.38% |
| Dihydrocortisol | 4–3628 | <0.01–2.52% | 0.4–56† | <0.18% |
| Dihydrocortisol gluc | 14,542–280,266 | 2.88–83.40% | 14,064–408,434 | 13.72–92.34% |
| Cortisol sulfate | 28–179 | <0.11% | 4–148 | <0.03% |
| 6-hydroxycortisol | 5–616 | <0.33% | 1–1871† | <0.01–6.08% |
| Cortisone | 18–184 | <0.17% | 11–231 | <0.18% |
| Cortisone gluc | 18–2132 | <0.01–2.34% | 12–562 | <0.31% |
| THE | 1–243† | <0.17% | 0.5–217† | <0.08% |
| THE gluc | 2559–34,770 | 0.57–9.55% | 988–41,734 | 2.35–14.85% |
| Dihydrocortisone | 14–5213 | <0.01–5.15% | 2–2405† | <0.01–1.38% |
| Dihydrocortisone gluc | 19,447–1,868,017 | 9.39–95.39% | 4460–196,412 | 1.41–69.85% |
| Cortisone sulfate | 1–380† | <0.14% | 1–217† | <0.08% |
| Total dihydrocortisol + dihydrocortisone + THF/A-THF + THE | 97.04–99.96% | 92.91–99.82% | ||
†When the measured levels were below the LOD, half of the LOD values of the metabolites were reported herein.
A-THF: Allo-3α-tetrahydrocortisol; THE: 5β-tetrahydrocortisone; THF: 5β-tetrahydrocortisol.
More importantly, there was significant abundance heterogeneity of these metabolites among individuals. For instance, the abundance of dihydrocortisol gluc varied between 2.9 and 83.4% among smokers, and 13.7 and 92.3% among nonsmokers. Likewise, the abundance of dihydrocortisone gluc varied between 9.4 and 95.4% among smokers, and 1.4 and 69.9% among nonsmokers. Similar extent of variation was observed among other metabolites. These variations may be due to the biological and physiological differences among the participants, particularly the UDP-glucuronosyltransferase activity, which have significant human heterogeneity [31]. The large variations were not likely due to differential reaction kinetics, because smoker urine collection was well controlled and similar degrees of variation were observed with the nonsmoker urines. Further investigations are warranted to determine the source for such variations and whether these variations are of clinical diagnosis values.
Cigarette smoking & urinary total cortisol equivalent
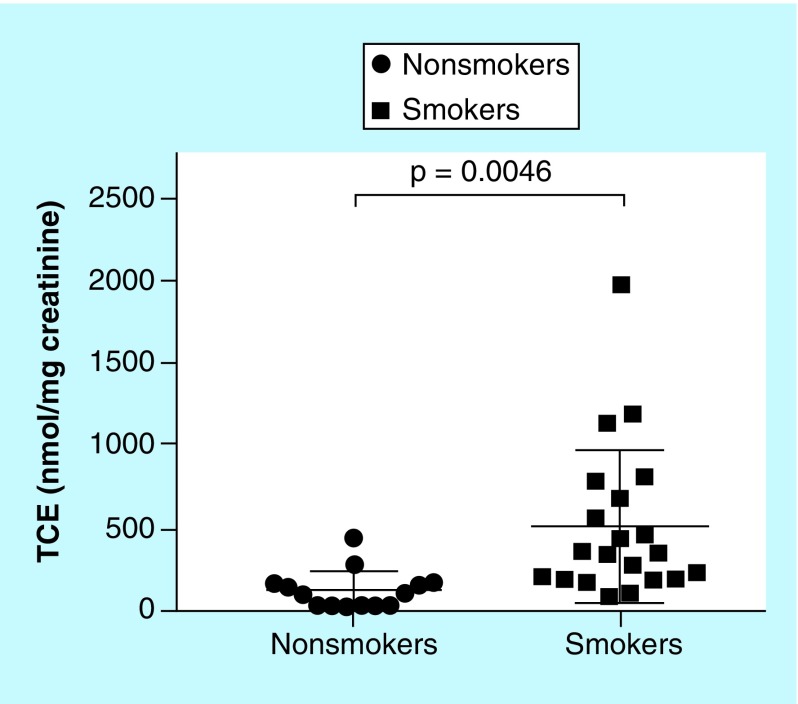
It is well known that cigarette smoking helps smokers cope with stress [32,33]. However, nicotine addiction could lead to increased long-term stress [34]. Indeed, cigarette smoking has been reported to increase cortisol level in human plasma and saliva [35,36]. A positive correlation was observed as well between the content of nicotine in different cigarettes and the magnitude of increase in plasma cortisol [37]. In this study, we therefore explored the association between urinary TCE and smoking status. The average TCE in smokers was 512 ± 101 nmol/mg creatinine, which is fourfold that in nonsmokers (128 ± 31 nmol/mg creatinine; p = 0.0046) (Figure 4). Consistent with previous plasma and saliva observations [35–37], smoking appeared to increase the cortisol secretion in human urine.
Figure 4. . Estimates of total cortisol equivalent in the urines of nonsmokers and smokers.
Unpaired two-tailed t-test.
TCE: Total cortisol equivalent.
Conclusion
We developed a UHPLC–MSn method using high-resolution accurate-mass Orbitrap. The method can detect and quantify cortisol and 16 of its metabolite in human urine with a sensitivity as low as 55 amol (20 fg) cortisol metabolite per μl urine in 100 μl urine. We also used this method to profile cortisol metabolites in human nonsmokers and smoker urines. The sum of the free and glucuronide forms of dihydrocortisol, A-THF, THF, dihydrocortisone and THE, termed TCE, offers an approximation of the total cortisol metabolites. Interestingly the TCE values are fourfold in the urines of smokers to nonsmokers, consistent with the previous reports that tobacco use may increase cortisol secretion. Besides stress, urinary cortisol metabolite profiles may be useful for disease diagnostics. For instance, the ratio of cortisol to cortisone, an estimate of 11β-hydroxysteroid dehydrogenase activity, has been proposed to evaluate children with adrenal diseases and Cushing syndrome [17,18]. Previous clinical studies also reported the ratio of allo-tetrahydrocortisol and THF to tetrahydrocortisone as an indicator of hypertension and depression [38–40]. Our method, which exhibits excellent sensitivity and selectivity to measure free and total forms of cortisol, cortisone, A-THF, THF and THE, has the potential in disease diagnosis clinically.
There are also some limitations of our method. We did not quantify 6-hydroxycortisone, 20-dihydrocortisol, 20-dihydrocortisone, cortols and cortolones (Figure 1), which were estimated to be at the comparable levels of cortisol and cortisone by GC–MS in urine [13]. Efforts are needed in the future to quantify these metabolites and their conjugates. In addition, we used in-source fragmentation coupled with MS/MS in Orbitrap to quantify THF, A-THF, THE and dihydrocortisone; however, there is a possibility that the ions from in-source fragmentation may come from other steroids, which happen to co-elute with our targeted compounds on the chromatogram, due to their structural and physicochemical similarities. We also did not have the detailed information on the commercially purchased urine samples of nonsmokers, such as collection time, subject health conditions, which may introduce effects to the TCE quantified. Thus, a more systematic study is needed to confirm the impact of tobacco smoking on the quantitative profiling of cortisol metabolites in human urine in the future.
Future perspective
Because of the advancement of mass spectrometers and commercially available isotope-labeled standards of cortisol metabolites, the LC–MS/MS-based method with high-resolution accurate-mass mass spectrometers provides better accuracy, selectivity and sensitivity in quantification of cortisol metabolites in human biofluids. Urine can be a great matrix for identification of biomarkers for disease diagnosis because of the noninvasive collection, large volumes and cumulative information of a period of time. Our method can be adapted to diagnostic laboratories and clinics for disease diagnosis.
Summary points.
Method sensitivity & validation
The method is sensitive to detect attomole cortisol metabolites per microliters human urine samples.
First report to directly assay cortisol- and cortisone-sulfates by SPE following online UHPLC–MS/MS.
Application of the method
First study to report the free, glucuronide and sulfate forms of major cortisol metabolites and the relative abundance of cortisol metabolites in human urines.
Urinary cortisol metabolites level in smokers is 4× greater than nonsmokers.
Our method can be applied for disease diagnosis.
Supplementary Material
Acknowledgements
The authors would like to thank J Paladino, SC Narayanapillai and V Nguyen for the help of sample collection and preparation. The authors would also thank the Department of Medicinal Chemistry at University of Florida for the Orbitrap MS usage. The authors also thank J Lu of Department of Pharmacology, Penn State College of Medicine, Hershey for data discussion and manuscript editing.
Footnotes
Supplementary data
See online at: https://www.future-science.com/doi/10.4155/bio-2018-0182
Data sharing statement
Deidentified individual data that underlie the results reported in this article (text, tables, figures and appendices), along with the study protocol, will be available indefinitely for anyone who wants access to them.
Financial & competing interests disclosure
The work was funded by the University of Minnesota Masonic Cancer Center pilot grant, University of Florida Medicinal Chemistry Department, College of Pharmacy Frank Duckworth Endowment, University of Florida Health Cancer Center Startup fund and NIH R01CA193286. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37(5):589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]; •• A review of cortisol as a stress biomarker.
- 2.Butts CD, Bloom MS, Frye CA, et al. Urine cortisol concentration as a biomarker of stress is unrelated to IVF outcomes in women and men. J. Assist. Reprod. Genet. 2014;31(12):1647–1653. doi: 10.1007/s10815-014-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Rubin RT, Heist EK, McGeoy SS, Hanada K, Lesser IM. Neuroendocrine aspects of primary endogenous depression. XI. Serum melatonin measures in patients and matched control subjects. Arch. Gen. Psychiatry. 1992;49(7):558–567. doi: 10.1001/archpsyc.1992.01820070052008. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Morato J, Pozo OJ, Marcos J. Targeting human urinary metabolome by LC–MS/MS: a review. Bioanalysis. 2018;10(7):489–516. doi: 10.4155/bio-2017-0285. [DOI] [PubMed] [Google Scholar]
- 6.Tomlinson JW, Walker EA, Bujalska IJ, et al. 11β-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr. Rev. 2004;25(5):831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 7.Gathercole LL, Lavery GG, Morgan SA, et al. 11β-hydroxysteroid dehydrogenase 1: translational and therapeutic aspects. Endocr. Rev. 2013;34(4):525–555. doi: 10.1210/er.2012-1050. [DOI] [PubMed] [Google Scholar]
- 8.Hatz HJ. Glucocorticoide: immunologische Grundlagen, Pharmakologie und Therapierichtlinien; mit 116 Tabellen. Wiss. Verlag-Ges. 2005 [Google Scholar]
- 9.Cho HJ, Kim JD, Lee WY, Chung BC, Choi MH. Quantitative metabolic profiling of 21 endogenous corticosteroids in urine by liquid chromatography–triple quadrupole-mass spectrometry. Anal. Chim. Acta. 2009;632(1):101–108. doi: 10.1016/j.aca.2008.10.059. [DOI] [PubMed] [Google Scholar]; • LC–MS/MS-based method to quantify cortisol metabolites in human urine.
- 10.Gatti R, Antonelli G, Prearo M, Spinella P, Cappellin E, De Palo EF. Cortisol assays and diagnostic laboratory procedures in human biological fluids. Clin. Biochem. 2009;42(12):1205–1217. doi: 10.1016/j.clinbiochem.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Weykamp CW, Penders TJ, Schmidt NA, Borburgh AJ, Van De Calseyde JF, Wolthers BJ. Steroid profile for urine: reference values. Clin. Chem. 1989;35(12):2281–2284. [PubMed] [Google Scholar]
- 12.Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CHL. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS) J. Steroid Biochem. Mol. Biol. 2010;121(3-5):496–504. doi: 10.1016/j.jsbmb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer DA, editor. The Quest Diagnostic Manual, Endocrinology. Test Selection and Interpretation. Quest Diagnostics; CA, USA: 2007. [Google Scholar]
- 14.Shibasaki H, Nakayama H, Furuta T, et al. Simultaneous determination of prednisolone, prednisone, cortisol, and cortisone in plasma by GC–MS: estimating unbound prednisolone concentration in patients with nephrotic syndrome during oral prednisolone therapy. J. Chromatogr. B. 2008;870(2):164–169. doi: 10.1016/j.jchromb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Palermo M, Gomez-Sanchez C, Roitman E, Shackleton CH. Quantitation of cortisol and related 3-oxo-4-ene steroids in urine using gas chromatography/mass spectrometry with stable isotope-labeled internal standards. Steroids. 1996;61(10):583–589. doi: 10.1016/s0039-128x(96)00118-9. [DOI] [PubMed] [Google Scholar]
- 16.Marcos J, Renau N, Casals G, Segura J, Ventura R, Pozo OJ. Investigation of endogenous corticosteroids profiles in human urine based on liquid chromatography tandem mass spectrometry. Anal. Chim. Acta. 2014;812:92–104. doi: 10.1016/j.aca.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Nomura S, Fujitaka M, Jinno K, Sakura N, Ueda K. Clinical significance of cortisone and cortisone/cortisol ratio in evaluating children with adrenal diseases. Clin. Chim. Acta. 1996;256(1):1–11. doi: 10.1016/s0009-8981(96)06392-9. [DOI] [PubMed] [Google Scholar]
- 18.Lin C-L, Wu T-J, Machacek DA, Jiang N-S, Kao PC. Urinary free cortisol and cortisone determined by high performance liquid chromatography in the diagnosis of Cushing's syndrome. J. Clin. Endocrinol. Metab. 1997;82(1):151–155. doi: 10.1210/jcem.82.1.3687. [DOI] [PubMed] [Google Scholar]
- 19.Chutipongtanate S, Thongboonkerd V. Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal. Biochem. 2010;402(1):110–112. doi: 10.1016/j.ab.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Fan H, Shao ZY, Xiao YY, et al. Incidence and survival of non-small cell lung cancer in Shanghai: a population-based cohort study. BMJ Open. 2015;5(12):e009419. doi: 10.1136/bmjopen-2015-009419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US FDA. Guidance for industry on bioanalytical method validation. Fed. Regist. 2001;66(100):28526. [Google Scholar]
- 22.Murphy SE, Park SS, Thompson EF, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35(11):2526–2533. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand. J. Clin. Lab. Invest. 1965;17(4):381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 24.Perez S, Eichhorn P, Barcelo D, editors. Applications of Time-of-Flight and Orbitrap Mass Spectrometry in Environmental, Food, Doping, and Forensic Analysis. Elsevier; MA, USA: 2016. [Google Scholar]
- 25.Allende F, Solari S, Campino C, et al. LC–MS/MS method for the simultaneous determination of free urinary steroids. Chromatographia. 2014;77(7-8):637–642. doi: 10.1007/s10337-014-2638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; • LC–MS/MS-based method to quantify cortisol metabolites in human urine.
- 26.International Conference on Harmonization. Validation of analytical procedures: text and methodology Q2(R1) 2005. http://www.ich.org/products/guidelines/quality/quality-single/article/validation-of-analytical-procedures-text-and-methodology.html
- 27.Wang J, Liang Q, Mendes P, Sarkar M. Is 24h nicotine equivalents a surrogate for smoke exposure based on its relationship with other biomarkers of exposure? Biomarkers. 2011;16(2):144–154. doi: 10.3109/1354750X.2010.536257. [DOI] [PubMed] [Google Scholar]
- 28.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul. Toxicol. Pharmacol. 2007;47(2):171–183. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Murphy SE. Nicotine metabolism and smoking: ethnic differences in the role of P450 2A6. Chem. Res. Toxicol. 2017;30(1):410–419. doi: 10.1021/acs.chemrestox.6b00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenschmid B, Heilmann P, Oelkers W, Rejaibi R, Schoneshofer M. 20-Dihydroisomers of cortisol and cortisone in human urine: excretion rates under different physiological conditions. J. Clin. Chem. Clin. Biochem. 1987;25(6):345–349. doi: 10.1515/cclm.1987.25.6.345. [DOI] [PubMed] [Google Scholar]
- 31.Guillemette C, Ritter JK, Auyeung DJ, Kessler FK, Housman DE. Structural heterogeneity at the UDP-glucuronosyltransferase 1 locus: functional consequences of three novel missense mutations in the human UGT1A7 gene. Pharmacogenetics. 2000;10(7):629–644. doi: 10.1097/00008571-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Nesbitt PD. Smoking, physiological arousal, and emotional response. J. Pers. Soc. Psychol. 1973;25(1):137–144. doi: 10.1037/h0034256. [DOI] [PubMed] [Google Scholar]
- 33.Parrott AC. Stress modulation over the day in cigarette smokers. Addiction. 1995;90(2):233–244. doi: 10.1046/j.1360-0443.1995.9022339.x. [DOI] [PubMed] [Google Scholar]
- 34.Moylan S, Jacka FN, Pasco JA, Berk M. How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways. Brain Behav. 2013;3(3):302–326. doi: 10.1002/brb3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tweed JO, Hsia SH, Lutfy K, Friedman TC. The endocrine effects of nicotine and cigarette smoke. Trends Endocrinol. Metab. 2012;23(7):334–342. doi: 10.1016/j.tem.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J. Clin. Endocrinol. Metab. 2007;92(3):819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- 37.Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persky H. Tetrahydrocortisol/tetrahydrocortisone ratio (H4F/H4E) as an indicator of depressive feelings. Psychosom. Med. 1976;38(1):13–18. doi: 10.1097/00006842-197601000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Olivieri O, Pizzolo F, Ravagnani V, et al. Urinary cortisol to cortisone metabolites ratio in prednisone-treated and spontaneously hypertensive patients. J. Hypertens. 2008;26(3):486–493. doi: 10.1097/HJH.0b013e3282f2d35e. [DOI] [PubMed] [Google Scholar]; •• Urinary cortisol metabolites in disease diagnosis.
- 40.Romer B, Lewicka S, Kopf D, et al. Cortisol metabolism in depressed patients and healthy controls. Neuroendocrinology. 2009;90(3):301–306. doi: 10.1159/000235904. [DOI] [PubMed] [Google Scholar]; •• Urinary cortisol metabolites in disease diagnosis.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.