Abstract
Anesthetics, especially propofol, are discussed to influence ischemic preconditioning. We investigated whether cardioprotection by milrinone or levosimendan is influenced by the clinically used anesthetics propofol, sevoflurane or dexmedetomidine. Hearts of male Wistar rats were randomised, placed on a Langendorff system and perfused with Krebs–Henseleit buffer (KHB) at a constant pressure of 80 mmHg. All hearts underwent 33 min of global ischemia and 60 min of reperfusion. Three different anesthetic regimens were conducted throughout the experiments: propofol (11 μM), sevoflurane (2.5 Vol%) and dexmedetomidine (1.5 nM). Under each anesthetic regimen, pharmacological preconditioning was induced by administration of milrinone (1 μM) or levosimendan (0.3 μM) 10 min before ischemia. Infarct size was determined by TTC staining. Infarct sizes in control groups were comparable (KHB-Con: 53 ± 9%, Prop-Con: 56 ± 9%, Sevo-Con: 56 ± 8%, Dex-Con: 53 ± 9%; ns). Propofol completely abolished preconditioning by milrinone and levosimendan (Prop-Mil: 52 ± 8%, Prop-Lev: 52 ± 8%; ns versus Prop-Con), while sevoflurane did not (Sevo-Mil: 31 ± 9%, Sevo-Lev: 33 ± 7%; p < 0.05 versus Sevo-Con). Under dexmedetomidine, results were inconsistent; levosimendan induced infarct size reduction (Dex-Lev: 36 ± 6%; p < 0.05 versus Dex-Con) but not milrinone (Dex-Mil: 51 ± 8%; ns versus Dex-Con). The choice of the anesthetic regimen has an impact on infarct size reduction by pharmacological preconditioning.
Keywords: preconditioning, myocardial infarction, propofol, sevoflurane, dexmedetomidine, milrinone, levosimendan
1. Introduction
Preconditioning interventions, ischemically or pharmacologically, are promising procedures against ischemia and reperfusion injury [1,2,3,4,5,6] that miss clinical routine use because of contradictive clinical data. Over 30 years ago, Murry and colleagues described the phenomenon of ischemic preconditioning; short cycles of non-lethal myocardial ischemia and reperfusion that were able to reduce infarct size significantly before a prolonged ischemic period [5]. A disadvantage of ischemic preconditioning is the massive invasiveness of this intervention, which makes it impracticable for clinical use. Cardioprotection can also be induced by remote preconditioning via tourniquet at the upper or lower limb, for example. It is assumed that humoral factors are released to the blood, which confers myocardial protection. This intervention was shown to induce a strong infarct size reduction [6,7,8]. Because of the lower invasiveness of the intervention compared to direct ischemic preconditioning, effects of remote preconditioning were investigated in clinical trials [9,10]. Unfortunately, the results from large multi-centre trials, e.g., RIPHeart [11] and ERRICA [12], could not show any cardioprotective effect of remote preconditioning in cardiothoracic patients. Propofol, the anesthetic used in these studies, was determined to be an influencing factor for the observed lack of cardioprotection. We previously showed that propofol completely abolished the cardioprotective effect of remote preconditioning [2], but the effect of direct ischemic preconditioning was unaffected [4]. Furthermore, we demonstrated that the time of preconditioning and propofol application is crucial [4].
The cardioprotective effects of ischemic preconditioning can be imitated pharmacologically (e.g., volatile anesthetics, opioids) [13,14,15]. For the phosphodiesterase-3 inhibitor milrinone [1] and the calcium-sensitiser levosimendan [16], infarct size-reducing effects were shown. The infarct size-reducing effects of both drugs were concentration-dependent [3]. Here, we set out to determine the influence of anesthetics on pharmacological preconditioning. We hypothesise that the continuous administration of the different clinically used anesthetics, including propofol, sevoflurane and dexmedetomidine, might have an impact on the preconditioning-induced infarct size reduction of milrinone and levosimendan, respectively.
2. Material and Methods
The study was conducted on the baseline of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (Publication number 85–23, revised 1996). The approval of the Animal Ethics Committee of the University of Duesseldorf, Germany has been granted (O27/12).
2.1. Surgical Preparation
The surgical preparation was performed as described previously [17]. Male Wistar rats were anesthetised by intraperitoneal injection of pentobarbital (90 mg/kg). Thereafter, animals were thoracotomised for the removal of the hearts. The hearts were mounted on a Langendorff system and perfused with a Krebs–Henseleit buffer (KHB). During the experiment, a constant pressure (80 mmHg) and temperature (37 °C) was maintained [18]. We inserted a 0.1–0.2 mL normal saline-filled balloon into the left ventricle via the left atrium and kept an end-diastolic pressure of 4–7 mmHg. The balloon was coupled to a pressure transducer, which was plugged into an analogue-to-digital converter. The hearts underwent an equilibration period for 20 min. The heart rate, left ventricular end-systolic pressure (LVESP) and left ventricular end-diastolic pressure (LVEDP) were measured continuously and digitised using an analogue-to-digital converter (PowerLab/8SP, ADInstruments Pty Ltd., Castle Hill, Australia) at a sampling rate of 500 Hz. The data were continuously recorded on a personal computer using Chart for Windows v5.0 (ADInstruments Pty Ltd., Castle Hill, Australia). The coronary flow was detected by time-dependent collection of the coronary effluent. The maximal contracture and the time point of maximal contracture were measured in each experiment during ischemia [18].
2.2. Experimental Protocol
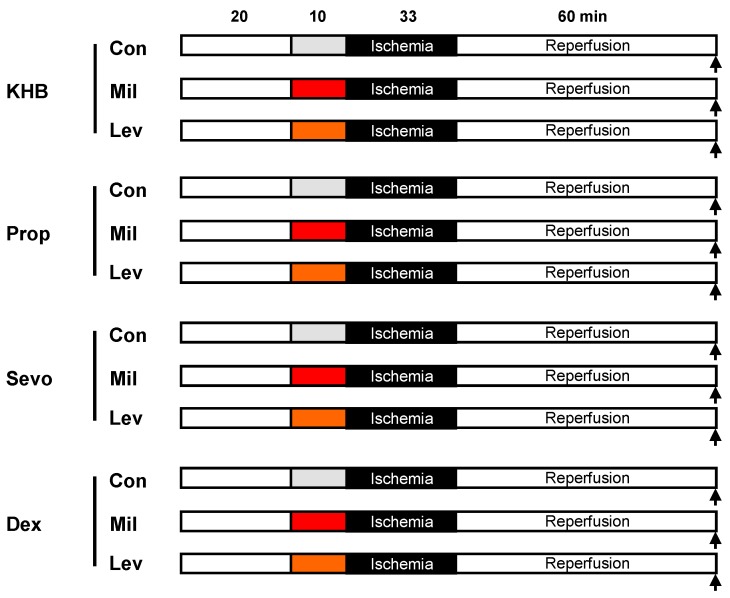
After surgical preparation, all hearts underwent baseline conditions for 20 min and 33 min of global ischemia, followed by 60 min of reperfusion (Figure 1). Global ischemia was achieved by stopping coronary perfusion of the heart with KHB. We randomly assigned the hearts into twelve groups (n = 7–8 per group). According to the group, hearts were perfused with either KHB only, propofol (11 µM, Prop), sevoflurane (2.5 Vol.%, Sevo) or dexmedetomidine (1.5 nM, Dex). Propofol and dexmedetomidine were continuously administered via a syringe pump. Sevoflurane was added to the oxygen-nitrogen-gas-mixture via an agent-specific vaporiser and bubbled into the KHB [19]. The sevoflurane concentration was measured using a Datex Ohmeda Capnomac Ultima gas monitor.
Figure 1.
Experimental protocol. KHB, Krebs–Henseleit buffer; Prop, propofol; Sevo, sevoflurane; Dex, dexmedetomidine; Con, control; Mil, milrinone preconditioning (red); Lev, levosimendan preconditioning (orange).
Control groups (KHB-Con, Prop-Con, Sevo-Con, Dex-Con): After surgical preparation, rats received KHB or the corresponding anesthetics continuously with no further treatment.
Milrinone (Mil) groups (KHB-Mil, Prop-Mil, Sevo-Mil, Dex-Mil): After surgical preparation, rats received KHB or the corresponding anesthetics continuously and Mil over 10 min before global ischemia. From our own unpublished data, we knew that the lowest cardioprotective concentration of Mil is 1 µM. Therefore, we used this concentration for induction of preconditioning.
Levosimendan (Lev) groups (KHB-Lev, Prop-Lev, Sevo-Lev, Dex-Lev): After surgical preparation, rats received KHB or the corresponding anesthetics continuously and Lev over 10 min before global ischemia. We have previously shown that Lev in a concentration of 0.3 µM is the lowest cardioprotective concentration conferring infarct size reduction [3].
Hearts were dyed with 0.75% triphenyltetrazoliumchloride (TTC) solution at the end of the experiments. The infarct size measurement was carried out using planimetry [18].
2.3. Statistical Analysis
Calculation of sample size was done using GraphPad StatMate™ (GraphPad Software, San Diego, CA, USA) and resulted in a group size of n = 8, which detected a 25% mean difference and a standard deviation of 11% in infarct size with a power of 80% (α < 0.05 (two-tailed)). Hemodynamic variables were measured continuously and detected during baseline, ischemia and reperfusion. Normality of data was tested with a Kolmogorov–Smirnov Test. To compare hemodynamic variables between groups or between different time points within groups, we used a two-way analysis of variance (ANOVA) and a Tukey post hoc test (GraphPad Software, San Diego, CA, USA). The infarct sizes were determined by an investigator blinded to the experimental groups. A one-way analysis (ANOVA) was chosen, followed by a Tukey post hoc test to analyse infarct size. Statistical analysis of hemodynamic variables and infarct sizes was performed in groups for the respective anesthetic (e.g., all groups with Prop, all groups with Sevo, etc.). Data are presented as mean ± standard deviation (SD). Changes were regarded as statistically significant if p < 0.05.
3. Results
3.1. Animal Characteristics
Table 1 shows body weight, wet and dry heart weight and level and time of maximal ischemic contracture of the experimental groups.
Table 1.
Weights and ischemic contracture.
| n | Body Weight (g) | Heart Weight Dry (g) | Heart Weight Wet (g) | Time of Max. Ischemic Contracture (min) | Level of Max. Ischemic Contracture (min) | ||
|---|---|---|---|---|---|---|---|
| KHB | Con | 8 | 301 ± 10 | 0.14 ± 0.02 | 1.47 ± 0.12 | 15:46 ± 1:42 | 54 ± 7 |
| Mil | 7 | 299 ± 23 | 0.15 ± 0.01 | 1.46 ± 0.11 | 16:27 ± 1:18 | 54 ± 11 | |
| Lev | 7 | 291 ± 21 | 0.14 ± 0.01 | 1.32 ± 0.14 | 15:39 ± 0:39 | 56 ± 12 | |
| Prop | Con | 8 | 286 ± 17 | 0.13 ± 0.02 | 1.44 ± 0.11 | 16:17 ± 1:05 | 59 ± 12 |
| Mil | 8 | 303 ± 23 | 0.16 ± 0.02 | 1.46 ± 0.08 | 16:00 ± 1:10 | 55 ± 4 | |
| Lev | 8 | 299 ± 26 | 0.15 ± 0.01 | 1.47 ± 0.11 | 16:26 ± 1:59 | 61 ± 11 | |
| Sevo | Con | 7 | 300 ± 08 | 0.12 ± 0.01 | 1.34 ± 0.06 | 14:26 ± 0:23 | 82 ± 13 |
| Mil | 7 | 298 ± 19 | 0.13 ± 0.01 | 1.30 ± 0.06 | 15:05 ± 1:53 | 70 ± 13 | |
| Lev | 7 | 297 ± 11 | 0.13 ± 0.01 | 1.29 ± 0.07 | 14:40 ± 1:07 | 81 ± 11 | |
| Dex | Con | 8 | 293 ± 18 | 0.14 ± 0.03 | 1.42 ± 0.09 | 15:21 ± 2:10 | 67 ± 17 |
| Mil | 8 | 290 ± 19 | 0.14 ± 0.02 | 1.41 ± 0.07 | 16:16 ± 1:54 | 55 ± 9 | |
| Lev | 7 | 296 ± 11 | 0.15 ± 0.02 | 1.42 ± 0.09 | 16:14 ± 1:23 | 55 ± 10 |
Data are mean ± standard deviation (SD). KHB, Krebs–Henseleit buffer; Prop, propofol; Sevo, sevoflurane; Dex, dexmedetomidine; Con, control; Mil, milrinone; Lev, levosimendan.
3.2. Infarct Size
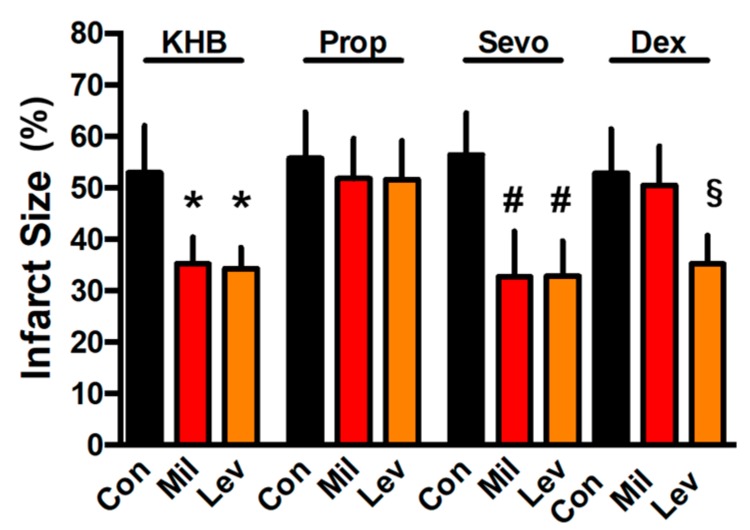
Infarct size in KHB control hearts was 53 ± 9% of the whole heart (Figure 2). Mil and Lev both induced a significant infarct size reduction (KHB-Mil: 35 ± 5%, KHB-Lev: 34 ± 4%; p < 0.05 versus KHB-Con). In propofol perfused control hearts, infarct size was 56 ± 9% (Figure 2). Pharmacological preconditioning with Mil or Lev was completely abolished under propofol treatment (Prop-Mil: 52 ± 8%, Prop-Lev: 52 ± 8%; ns versus Prop-Con). In contrast to propofol, Mil and Lev showed significant infarct size reduction under sevoflurane (Sevo-Mil: 31 ± 9%, Sevo-Lev: 33 ± 7%; p < 0.05 versus Sevo-Con: 56 ± 8%). Perfusion with dexmedetomidine led to an infarct size of 53 ± 9% in control hearts (Figure 2). Preconditioning with Mil did not induce cardioprotection (Dex-Mil: 51 ± 8%; ns versus Dex-Con). In contrast with Lev-induced preconditioning, infarct size was reduced to 35 ± 5% (p < 0.05 versus Dex-Con). There was no difference between control groups of the investigated anesthetic regimens (all p > 0.05).
Figure 2.
Infarct size measurement. Figure shows the infarct size of controls (Con), preconditioning with milrinone (Mil) and preconditioning with levosimendan (Lev) under different anesthetic regimens. Data are mean ± SD. * p < 0.05 versus KHB-Con, # p < 0.05 versus Sevo-Con and § p < 0.05 versus Dex-Con, respectively.
3.3. Hemodynamics
Hemodynamic data are shown in Table 2. After ischemia and during reperfusion, LVEDP and coronary flow were statistically different from baseline (Table 2).
Table 2.
Hemodynamic variables.
| Group | Baseline | PC | Reperfusion | ||
|---|---|---|---|---|---|
| 30 | 60 | ||||
| Heart Rate (bpm) | |||||
| KHB | Con | 333 ± 40 | 314 ± 42 | 279 ± 92 | 275 ± 62 |
| Mil | 327 ± 48 | 347 ± 44 | 242 ± 61 | 275 ± 43 | |
| Lev | 318 ± 39 | 312 ± 33 | 274 ± 34 | 258 ± 36 | |
| Prop | Con | 308 ± 32 | 294 ± 32 | 183 ± 69 * | 180 ± 67 * |
| Mil | 313 ± 30 | 293 ± 27 | 237 ± 46 | 212 ± 54 | |
| Lev | 316 ± 50 | 300 ± 51 | 208 ± 77 | 212 ± 74 | |
| Sevo | Con | 296 ± 23 | 271 ± 28 | 246 ± 20 | 215 ± 45 |
| Mil | 345 ± 38 | 367 ± 32 | 221 ± 60 | 266 ± 63 | |
| Lev | 304 ± 37 | 300 ± 27 | 271 ± 37 | 253 ± 32 | |
| Dex | Con | 308 ± 27 | 286 ± 15 | 248 ± 53 | 247 ± 28 |
| Mil | 318 ± 43 | 309 ± 39 | 257 ± 46 | 264 ± 26 | |
| Lev | 331 ± 54 | 328 ± 36 | 304 ± 36 | 265 ± 54 | |
| Phasic LVP (mmHg) | |||||
| KHB | Con | 117 ± 16 | 122 ± 10 | 17 ± 8 * | 22 ± 7 * |
| Mil | 130 ± 16 | 115 ± 13 | 15 ± 5 * | 19 ± 6 * | |
| Lev | 109 ± 17 | 116 ± 17 | 25 ± 15 * | 25 ± 11 * | |
| Prop | Con | 132 ± 20 | 126 ± 20 | 17 ± 8 * | 28 ± 14 * |
| Mil | 118 ± 18 | 119 ± 19 | 20 ± 4 * | 31 ± 11 * | |
| Lev | 130 ± 12 | 131 ± 13 | 20 ± 9 * | 27 ± 6 * | |
| Sevo | Con | 144 ± 17 | 145 ± 23 | 25 ± 14 * | 32 ± 10 * |
| Mil | 129 ± 19 | 143 ± 24 | 26 ± 13 * | 39 ± 18 * | |
| Lev | 136 ± 24 | 139 ± 11 | 27 ± 12 * | 35 ± 10 * | |
| Dex | Con | 124 ± 16 | 113 ± 20 | 21 ± 10 * | 29 ± 12 * |
| Mil | 123 ± 15 | 125 ± 17 | 27 ± 13 * | 30 ± 8 * | |
| Lev | 126 ± 11 | 127 ± 10 | 24 ± 14 * | 24 ± 10 * | |
| CF (mL·min−1) | |||||
| KHB | Con | 17 ± 3 | 15 ± 4 | 10 ± 1 * | 8 ± 1 * |
| Mil | 18 ± 2 | 16 ± 2 | 9 ± 2 * | 8 ± 2 * | |
| Lev | 15 ± 2 | 17 ± 2 | 7 ± 2 * | 5 ± 2 * | |
| Prop | Con | 17 ± 1 | 16 ± 1 | 9 ± 1 * | 7 ± 1 * |
| Mil | 16 ± 2 | 18 ± 3 | 10 ± 1 * | 8 ± 2 * | |
| Lev | 16 ± 1 | 19 ± 2 | 10 ± 2 * | 8 ± 2 * | |
| Sevo | Con | 15 ± 3 | 14 ± 3 * | 9 ± 2 * | 7 ± 2 * |
| Mil | 17 ± 3 | 18 ± 3 * | 11 ± 4 * | 9 ± 3 * | |
| Lev | 14 ± 3 | 17 ± 2 | 8 ± 3 * | 7 ± 3 * | |
| Dex | Con | 16 ± 3 | 14 ± 4 | 8 ± 1 * | 6 ± 1 * |
| Mil | 16 ± 2 | 14 ± 2 | 7 ± 3 * | 7 ± 1 * | |
| Lev | 19 ± 2 | 18 ± 3 | 9 ± 2 * | 7 ± 2 * | |
Data are mean ± SD. KHB, Krebs–Henseleit buffer; Prop, propofol; Sevo, sevoflurane; Dex, dexmedetomidine; Con, control; Mil, milrinone; Lev, levosimendan. * p < 0.05 versus baseline.
4. Discussion
The results of the present study show that (1) propofol abolished cardioprotection induced by pharmacological preconditioning with milrinone or levosimendan, (2) sevoflurane had no influence on milrinone- or levosimendan-induced infarct size reduction and (3) dexmedetomidine blocked protection of milrinone, but did not affect levosimendan-induced preconditioning.
Since the disappointing results from clinical trials investigating cardioprotective properties of remote preconditioning came up [11,12,20], the anesthetic regimen, and here especially propofol, was discussed as an influencing factor for the lack of cardioprotection in those studies. In the RIPHeart trial [11], anesthesia was performed 100% with propofol and in the ERRICA trial [12] about 90% of the patients received propofol. Recently, we demonstrated in the rat heart in vivo that propofol completely abolished the infarct size reduction of remote preconditioning, supporting the thesis of propofol being counterproductive in the context of remote preconditioning [2]. In the same study, we showed that the volatile anesthetic sevoflurane did not affect cardioprotection [2]. Direct myocardial ischemic preconditioning under propofol anesthesia was still present [4], but as aforementioned ischemic preconditioning is an extremely invasive and impractical intervention that is not suitable for clinical routine, with the exception of cardiac surgery or cardiac catheterisation. Fortunately, the effect of ischemic preconditioning can be mimicked pharmacologically, e.g., with milrinone or levosimendan [1,3,16]. The calcium sensitiser levosimendan and the phosphodiesterase-3 inhibitor milrinone are drugs indicated for treatment of acute heart failure [21,22]. Both drugs are routinely used in the operating room or the intensive care unit, so the cardioprotective properties of these substances are of interest just like possible negative influencing factors, e.g., anesthetics.
For milrinone and levosimendan, in each case we used the lowest concentration to induce the strongest cardioprotective effect. Recently, we demonstrated that 0.3 µM levosimendan is the lowest concentration that conferred the strongest infarct size reduction [3]. We know from own unpublished data that for milrinone, 1 µM is the concentration that leads to pronounced cardioprotective effects. Higher concentrations of milrinone could not maximise the infarct size reduction.
Propofol abolished the cardioprotective effect of both pharmacological preconditioning interventions, whereas sevoflurane had no impact on levosimendan- or milrinone-induced preconditioning. These results support the hypothesis of propofol being a negative influencing factor on conditioning strategies. Kottenberg et al. demonstrated that remote preconditioning under anesthesia with isoflurane decreased troponin levels in cardiac surgical patients after cardiopulmonary bypass, but this effect was abrogated under propofol anesthesia [23]. When solely administered, there was no difference in troponin levels between isoflurane and propofol. One has to distinguish between a continuous administration and a preconditioning stimulus of the volatile anesthetic sevoflurane. Preconditioning can be induced through three cycles of intermittent administration of sevoflurane alternated with phases of reperfusion before ischemia. Previously, we showed that continuous administration of sevoflurane for the whole study period (baseline, ischemia and reperfusion) had no effect on infarct size in the rat heart in vivo [2,4]. In a recent study, we demonstrated that intermittent sevoflurane administration (preconditioning stimulus) reduced infarct size significantly, whereas the continuous application of sevoflurane had no effect on infarct size [24]. In line with our experimental findings, Bein et al. [25] reported that in coronary surgery, patients that received a continuous administration of 1 MAC sevoflurane from induction to start of cardiopulmonary bypass did not result in any additional protection, compared with the control group. However, when the administration of sevoflurane before cardiopulmonary bypass was interrupted for 10 min, an improved myocardial performance and decreased postoperative troponin T were observed. These data suggest that the interrupted administration is crucial for clinically relevant cardioprotective effects. Cardioprotective strategies can be influenced by confounding factors, such as aging or diabetes. We previously showed that as a variable, aging abolished the infarct size reduction of ischemic and pharmacological preconditioning [26,27,28,29]. Co-morbidities, such as diabetes [30], or medications, such as beta-blockers [30], impact cardioprotection. By using young and healthy rat hearts, all possible confounding factors were excluded in the present study to investigate exclusively the effect of various anesthetics on pharmacological preconditioning.
However, the results from levosimendan- and milrinone-induced preconditioning under dexmedetomidine administration are inconsistent. While the cardioprotective effect of levosimendan was unaffected, milrinone-induced preconditioning was completely abolished. A clinical interaction and mutual influence between milrinone and dexmedetomidine is described [31]. We assume that this interaction is responsible for the loss of the milrinone effect under dexmedetomidine. However, we were not aware of this interaction when we initiated the present study, and an elucidation of this drug interaction was beyond the scope of the study. To the best of our knowledge, there is no study addressing the underlying mechanism and/or interaction of both substances in more detail. We can only speculate that signaling pathways induced by both dexmedetomidine and milrinone might interfere with each other. One possible candidate pathway is the p38MAPK/ERK signaling cascade, which is a well-described underlying mechanism in preconditioning interventions. Yeda et al. [32] showed that dexmedetomidine abolished the ischemia/reperfusion (I/R) injury-induced increase in p38 MAPK in diabetic rats, whereas Sanada et al. [33] demonstrated activation of p38 MAPK by milrinone-induced preconditioning in dogs. Thus, it is conceivable that combining both drugs might exert a neutralising effect on each other. Furthermore, levosimendan is 10–30 times more potent an inotropic agent than milrinone, albeit with a lower myocardial oxygen consumption [34]. Thus, levosimendan exerts a positive inotropic effect without disturbing the energy balance of the heart, which could be an advantage during I/R injury.
Furthermore, we did not investigate whether the sole application of milrinone with dexmedetomidine administration before and after the preconditioning stimulus would lead to different results. However, this approach would not represent the clinical scenario.
The present data have a purely descriptive manner and it is a limitation of the study that we did not establish the underlying blockade mechanism, which was beyond the scope of our study. The aim of our study was to elucidate anesthetic drugs as a possible confounding factor for conditioning strategies.
5. Conclusions
The results of our current study show that the choice of the anesthetic had an impact on the cardioprotective effect of pharmacological preconditioning. While protection was completely blocked by propofol, it was unaffected by the use of sevoflurane and inconsistent with dexmedetomidine. Thus, the anesthetic regimen might be an important influencing factor that should be considered when cardioprotective agents are used.
Author Contributions
Conceptualization, S.B., A.R. and R.H.; methodology, S.B. and A.R.; formal analysis, S.B., M.S., A.H. and R.H.; investigation, T.L., M.F. and S.B.; writing—original draft preparation, M.S. and R.H.; writing—review and editing, A.M. and M.W.H. (in partial fulfillment of the requirements for an MD thesis (T.L. and M.F.)).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Behmenburg F., Trefz L., Dorsch M., Strothoff M., Mathes A., Raupach A., Heinen A., Hollmann M.W., Berger M.M., Huhn R. Milrinone-Induced Postconditioning Requires Activation of Mitochondrial Ca2+-Sensitive Potassium (Mbkca) Channels. J. Cardiothorac. Vasc. Anesth. 2017 doi: 10.1053/j.jvca.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 2.Behmenburg F., van Caster P., Bunte S., Brsandenburger T., Heinen A., Hollmann M.W., Huhn R. Impact of Anesthetic Regimen on Remote Ischemic Preconditioning in the Rat Heart in Vivo. Anesth. Analg. 2018;126:1377–1380. doi: 10.1213/ANE.0000000000002563. [DOI] [PubMed] [Google Scholar]
- 3.Bunte S., Behmenburg F., Bongartz A., Stroethoff M., Raupach A., Heinen A., Minol J.P., Hollmann M.W., Huhn R., Sixt S.U. Preconditioning by Levosimendan Is Mediated by Activation of Mitochondrial Ca2+-Sensitive Potassium (Mbkca) Channels. Cardiovasc. Drugs Ther. 2018 doi: 10.1007/s10557-018-6819-5. [DOI] [PubMed] [Google Scholar]
- 4.Bunte S., Behmenburg F., Eckelskemper F., Mohr F., Stroethoff M., Raupach A., Heinen A., Hollmann M.W., Huhn R. Cardioprotection by Humoral Factors Released after Remote Ischemic Preconditioning Depends on Anesthetic Regimen. Crit. Care Med. 2019 doi: 10.1097/CCM.0000000000003629. [DOI] [PubMed] [Google Scholar]
- 5.Murry C.E., Jennings R.B., Reimer K.A. Preconditioning with Ischemia: A Delay of Lethal Cell Injury in Ischemic Myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 6.Przyklenk K., Bauer B., Ovize M., Kloner R.A., Whittaker P. Regional Ischemic ‘Preconditioning’ Protects Remote Virgin Myocardium from Subsequent Sustained Coronary Occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.CIR.87.3.893. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M., Tropak M., Diaz R.J., Suto F., Surendra H., Kuzmin E., Li J., Gross G., Wilson G.J., Callahan J., et al. Transient Limb Ischaemia Remotely Preconditions through a Humoral Mechanism Acting Directly on the Myocardium: Evidence Suggesting Cross-Species Protection. Clin. Sci. (Lond.) 2009;117:191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J., Zhang J., Yu P., Chen M., Peng Q., Wang Z., Dong N. Remote Ischaemic Preconditioning and Sevoflurane Postconditioning Synergistically Protect Rats from Myocardial Injury Induced by Ischemia and Reperfusion Partly Via Inhibition Tlr4/Myd88/Nf-Kappab Signaling Pathway. Cell. Physiol. Biochem. 2017;41:22–32. doi: 10.1159/000455815. [DOI] [PubMed] [Google Scholar]
- 9.Thielmann M., Kottenberg E., Kleinbongard P., Wendt D., Gedik N., Pasa S., Price V., Tsagakis K., Neuhauser M., Peters J., et al. Cardioprotective and Prognostic Effects of Remote Ischaemic Preconditioning in Patients Undergoing Coronary Artery Bypass Surgery: A Single-Centre Randomised, Double-Blind, Controlled Trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 10.Botker H.E., Kharbanda R., Schmidt M.R., Bottcher M., Kaltoft A.K., Terkelsen C.J., Munk K., Andersen N.H., Hansen T.M., Trautner S., et al. Remote Ischaemic Conditioning before Hospital Admission, as a Complement to Angioplasty, and Effect on Myocardial Salvage in Patients with Acute Myocardial Infarction: A Randomised Trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 11.Meybohm P., Bein B., Brosteanu O., Cremer J., Gruenewald M., Stoppe C., Coburn M., Schaelte G., Boning A., Niemann B., et al. A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N. Engl. J. Med. 2015;373:1397–1407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 12.Hausenloy D.J., Candilio L., Evans R., Ariti C., Jenkins D.P., Kolvekar S., Knight R., Kunst G., Laing C., Nicholas J., et al. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N. Engl. J. Med. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 13.Behmenburg F., Boekholt Y., van Caster P., Dorsch M., Heinen A., Hollmann M.W., Huhn R. Extended Second Window of Protection of Sevoflurane-Induced Preconditioning. J. Cardiovasc. Pharmacol. 2017;70:284–289. doi: 10.1097/FJC.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 14.Dorsch M., Behmenburg F., Raible M., Blase D., Grievink H., Hollmann M.W., Heinen A., Huhn R. Morphine-Induced Preconditioning: Involvement of Protein Kinase a and Mitochondrial Permeability Transition Pore. PLoS ONE. 2016;11:e0151025. doi: 10.1371/journal.pone.0151025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frassdorf J., Huhn R., Niersmann C., Weber N.C., Schlack W., Preckel B., Hollmann M.W. Morphine Induces Preconditioning Via Activation of Mitochondrial K(Ca) Channels. Can. J. Anaesth. 2010;57:767–773. doi: 10.1007/s12630-010-9325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepran I., Pollesello P., Vajda S., Varro A., Papp J.G. Preconditioning Effects of Levosimendan in a Rabbit Cardiac Ischemia-Reperfusion Model. J. Cardiovasc. Pharmacol. 2006;48:148–152. doi: 10.1097/01.fjc.0000246151.39758.2a. [DOI] [PubMed] [Google Scholar]
- 17.Huhn R., Heinen A., Weber N.C., Schlack W., Preckel B., Hollmann M.W. Ischaemic and Morphine-Induced Post-Conditioning: Impact of Mk(Ca) Channels. Br. J. Anaesth. 2010;105:589–595. doi: 10.1093/bja/aeq213. [DOI] [PubMed] [Google Scholar]
- 18.Behmenburg F., Dorsch M., Huhn R., Mally D., Heinen A., Hollmann M.W., Berger M.M. Impact of Mitochondrial Ca2+-Sensitive Potassium (Mbkca) Channels in Sildenafil-Induced Cardioprotection in Rats. PLoS ONE. 2015;10:e0144737. doi: 10.1371/journal.pone.0144737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riess M.L., Kevin L.G., Camara A.K., Heisner J.S., Stowe D.F. Dual Exposure to Sevoflurane Improves Anesthetic Preconditioning in Intact Hearts. Anesthesiology. 2004;100:569–574. doi: 10.1097/00000542-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Coverdale N.S., Hamilton A., Petsikas D., McClure R.S., Malik P., Milne B., Saha T., Zelt D., Brown P., Payne D.M. Remote Ischemic Preconditioning in High-Risk Cardiovascular Surgery Patients: A Randomized-Controlled Trial. Semin. Thorac. Cardiovasc. Surg. 2017 doi: 10.1053/j.semtcvs.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Ayres J.K., Maani C.V. Statpearls. StatPearls Publishing LLC.; Treasure Island, FL, USA: 2018. Milrinone. [Google Scholar]
- 22.Harjola V.P., Giannakoulas G., von Lewinski D., Matskeplishvili S., Mebazaa A., Papp Z., Schwinger R.H.G., Pollesello P., Parissis J.T. Use of Levosimendan in Acute Heart Failure. Eur. Heart J. Suppl. 2018;20:I2–I10. doi: 10.1093/eurheartj/suy039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kottenberg E., Thielmann M., Bergmann L., Heine T., Jakob H., Heusch G., Peters J. Protection by Remote Ischemic Preconditioning During Coronary Artery Bypass Graft Surgery with Isoflurane but Not Propofol—A Clinical Trial. Acta Anaesthesiol. Scand. 2012;56:30–38. doi: 10.1111/j.1399-6576.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 24.Berger M.M., Huhn R., Oei G.T., Heinen A., Winzer A., Bauer I., Preckel B., Weber N.C., Schlack W., Hollmann M.W. Hypoxia Induces Late Preconditioning in the Rat Heart in Vivo. Anesthesiology. 2010;113:1351–1360. doi: 10.1097/ALN.0b013e3181fce7ea. [DOI] [PubMed] [Google Scholar]
- 25.Bein B., Renner J., Caliebe D., Hanss R., Bauer M., Fraund S., Scholz J. The Effects of Interrupted or Continuous Administration of Sevoflurane on Preconditioning before Cardio-Pulmonary Bypass in Coronary Artery Surgery: Comparison with Continuous Propofol. Anaesthesia. 2008;63:1046–1055. doi: 10.1111/j.1365-2044.2008.05563.x. [DOI] [PubMed] [Google Scholar]
- 26.Behmenburg F., Heinen A., Bruch L.V., Hollmann M.W., Huhn R. Cardioprotection by Remote Ischemic Preconditioning Is Blocked in the Aged Rat Heart in Vivo. J. Cardiothorac. Vasc. Anesth. 2017;31:1223–1226. doi: 10.1053/j.jvca.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Heinen A., Behmenburg F., Aytulun A., Dierkes M., Zerbin L., Kaisers W., Schaefer M., Meyer-Treschan T., Feit S., Bauer I., et al. The Release of Cardioprotective Humoral Factors after Remote Ischemic Preconditioning in Humans Is Age- and Sex-Dependent. J. Transl. Med. 2018;16:112. doi: 10.1186/s12967-018-1480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinen A., Winning A., Schlack W., Hollmann M.W., Preckel B., Frassdorf J., Weber N.C. The Regulation of Mitochondrial Respiration by Opening of Mkca Channels Is Age-Dependent. Eur. J. Pharmacol. 2008;578:108–113. doi: 10.1016/j.ejphar.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Huhn R., Weber N.C., Preckel B., Schlack W., Bauer I., Hollmann M.W., Heinen A. Age-Related Loss of Cardiac Preconditioning: Impact of Protein Kinase A. Exp. Gerontol. 2012;47:116–121. doi: 10.1016/j.exger.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Ferdinandy P., Hausenloy D.J., Heusch G., Baxter G.F., Schulz R. Interaction of Risk Factors, Comorbidities, and Comedications with Ischemia/Reperfusion Injury and Cardioprotection by Preconditioning, Postconditioning, and Remote Conditioning. Pharmacol. Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 31.Canadian Institutes of Health Research. Alberta Innovates - Health Solutions. The Metabolomics Innovation Centre (TMIC) Drugbank Database. [(accessed on 20 December 2018)]; Available online: https://www.drugbank.ca/drugs/DB00235.
- 32.Xiao Y., Lei S., Huang Y., Zhao Bo., Wang H., Cao H., Xia Z. Dexmedetomidine Protects against Renal Ischemia and Reperfusion Injury by Inhibiting the P38-Mapk/Txnip Signaling Activation in Streptozotocin Induced Diabetic Rats. Acta Cir. Bras. 2017;32:429–439. doi: 10.1590/s0102-865020170060000003. [DOI] [PubMed] [Google Scholar]
- 33.Sanada S., Kitakaze M., Papst P.J., Asanuma H., Node K., Takashima S., Asakura M., Ogita H., Liao Y., Sakata Y., et al. Cardioprotective Effect Afforded by Transient Exposure to Phosphodiesterase Iii Inhibitors: The Role of Protein Kinase a and P38 Mitogen-Activated Protein Kinase. Circulation. 2001;104:705–710. doi: 10.1161/hc3201.092216. [DOI] [PubMed] [Google Scholar]
- 34.Kaheinen P., Pollesello P., Levijoki J., Haikala H. Effects of Levosimendan and Milrinone on Oxygen Consumption in Isolated Guinea-Pig Heart. J. Cardiovasc. Pharmacol. 2004;43:555–561. doi: 10.1097/00005344-200404000-00011. [DOI] [PubMed] [Google Scholar]