Abstract
Durian (Durio zibethinus Murr.) is an energy-dense seasonal tropical fruit grown in Southeast Asia. It is one of the most expensive fruits in the region. It has a creamy texture and a sweet-bitter taste. The unique durian flavour is attributable to the presence of fat, sugar, and volatile compounds such as esters and sulphur-containing compounds such as thioacetals, thioesters, and thiolanes, as well as alcohols. This review shows that durian is also rich in flavonoids (i.e., flavanols, anthocyanins), ascorbic acid, and carotenoids. However, limited studies exist regarding the variation in bioactive and volatile components of different durian varieties from Malaysia, Thailand, and Indonesia. Experimental animal models have shown that durian beneficially reduces blood glucose and cholesterol levels. Durian extract possesses anti-proliferative and probiotics effects in in vitro models. These effects warrant further investigation in human interventional studies for the development of functional food.
Keywords: durian, esters, thioacetals, thioesters, volatile compounds, polyphenols, propionate
1. Introduction
Durio zibethinus Murr. (family Bombacaceae, genus Durio) is a seasonal tropical fruit grown in Southeast Asian countries such as Malaysia, Thailand, Indonesia, and the Philippines. There are nine edible Durio species, namely, D. lowianus, D. graveolens Becc., D. kutejensis Becc., D. oxleyanus Griff., D. testudinarum Becc., D. grandiflorus (Mast.) Kosterm. ET Soeg., D. dulcis Becc., Durio sp., and also D. zibethinus [1]. However, only Durio zibethinus species have been extensively grown and harvested [2]. In Malaysia, a few varieties have been recommended for commercial planting such as D24 (local name: Bukit Merah), D99 (local name: Kop Kecil), and D145 (local name: Beserah). In Thailand, durian species were registered based on local names such as Monthong, Kradum, and Puang Manee. There are similar varieties between Malaysian and Thailand but with different name as follows: D123 and Chanee, D158 and Kan Yao, and D169 and Monthong [3]. Similar to Thailand, durian varieties in Indonesia are registered based on their local names, such as Pelangi Atururi, Salisun, Nangan, Matahari, and Sitokong [1,4].
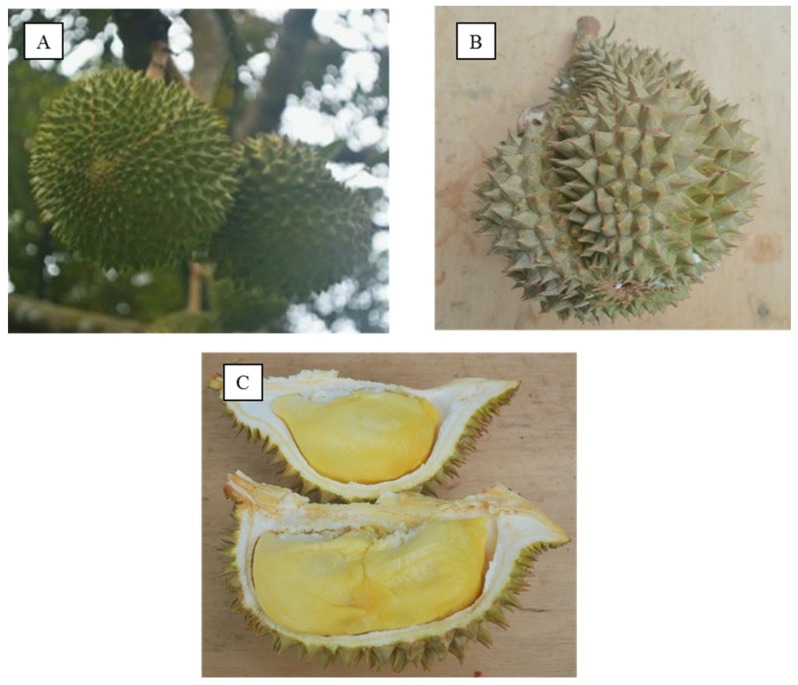
The durian fruit shape varies from globose, ovoid, obovoid, or oblong with pericarp colour ranging from green to brownish [1] (Figure 1). The colour of edible aril varies from one variety to the others and fall in between the following: yellow, white, golden-yellow or red [5]. It is eaten raw and has a short shelf-life, from two to five days [5,6]. Fully ripened durian fruit has a unique taste and aroma, and is dubbed “king of fruits” in Malaysia, Thailand, and Singapore. The unique taste and aroma is attributed to the presence of volatile compounds (esters, aldehydes, sulphurs, alcohols, and ketones) [6,7].
Figure 1.
(A) Durian tree with fruit. (B) Durian fruit with its spiny rind. (C) Durian aril (flesh).
Hundreds of volatile compounds have been identified in Malaysian, Thailand, and Indonesian durian varieties such as esters (ethyl propanoate, methyl-2-methylbutanoate, propyl propanoate), sulphur compounds (diethyl disulphide, diethyl trisulphide and ethanethiol), thioacetals (1-(methylthio)-propane), thioesters (1-(methylthio)-ethane), thiolanes (3,5-dimethyl-1,2,4-trithiolane isomers), and alcohol (ethanol) [6,7]. However, the bioactivity of these compounds has not yet been thoroughly explored. A study by Alhabeeb et al. (2014) showed that 10 g/day inulin propionate ester (a synthetic propionate) releases large amounts of propionate in the colon. This subsequently increases perceived satiety (increased satiety and fullness, decreased desire to eat) [8]. Chambers et al. (2015) showed that the same propionate ester (400 mmol/L) increased peptide YY (PYY) and glucagon-like peptide 1 (GLP-1) in primary cultured human colonic cells. This study also showed that 10 g/day of inulin-propionate ester reduced energy intake (14%) compared with the control (inulin) [9].
Durian is also rich in polyphenols such as flavonoids (flavanones, flavonols, flavones, flavanols, anthocyanins), phenolic acids (cinnamic acid and hydroxybenzoic acid), tannins, and other bioactive components such as carotenoids and ascorbic acid [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Current epidemiological studies have suggested that polyphenols decrease the risk of chronic diseases (e.g., cardiovascular diseases, cancers and diabetes) [26,27,28,29,30]. However, polyphenols might act synergistically with other phytochemicals [26]. However, currently, there are limited studies exploring the health benefits of bioactive components in durian. Hence, we aimed to review the nutritional and bioactive compounds present in durian varieties from Thailand, Indonesia, and Malaysia, as well as to explore the potential health benefits of durian.
2. Nutritional Composition of Different Durian Varieties
The energy content of durian is in the range of 84–185 kcal per 100 g fresh weight (FW) (Table 1) [6,18,19]. This range is somewhat similar to that of the United States Department of Agriculture (USDA), Malaysian, and Indonesian food composition databases [20,21,22]. Durian aril of the Thailand variety of Kradum showed the highest energy content at 185 kcal compared with other durian varieties [6,12,13]. Indonesian variety of Hejo showed the lowest energy content at 84 kcal per 100 g FW of durian aril [6]. The higher and lower energy contents are attributed to the difference in carbohydrate content. The carbohydrate content varies between different durian varieties in the range between 15.65 to 34.65 g per 100 g FW [6,12,13]. The range of carbohydrate content is similar to that of USDA, Malaysian and Indonesian food composition data, at 27.09 g, 27.90 g, and 28.00 g per 100 g FW, respectively [31,32,33]. The energy content of durian is the highest compared with other tropical fruits such as mango, jackfruit, papaya, and pineapple [31].
Table 1.
Nutritional composition of durian aril (flesh) of different durian varieties (g per 100 g fresh weight).
| Durian Variety | Indonesian Variety | Thailand Variety | Unknown Variety [31] | Unknown Variety [32] | Unknown Variety [33] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ajimah | Hejo | Matahari | Sukarno | Monthong | Chanee | Kradum | Kobtakam | ||||
| Nutrients | |||||||||||
| Energy (kcal) [6] * [31,32,33] |
151 | 84 | 163 | 134 | 134–162 | 145 | 185 | 145 | 147 | 153 | 134 |
| Carbohydrate (g) [6] * [12,13,31,32,33] |
28.90 | 15.65 | 34.65 | 27.30 | 21.70–27.10 | 20.13 | 29.15 | 21.15 | 27.09 | 27.90 | 28.00 |
| Protein (g) [6] * [12,13,31,32,33] |
2.36 | 1.76 | 2.33 | 2.13 | 1.40–2.33 | 3.10 | 3.50 | 2.86 | 1.47 | 2.70 | 2.50 |
| Fat (g) [6] * [12,13,31,32,33] |
2.92 | 1.59 | 1.69 | 1.86 | 3.10–5.39 | 4.48 | 4.67 | 4.40 | 5.33 | 3.40 | 3.00 |
Protein content of different durian varieties is in the range of 1.40 to 3.50 g per 100 g FW [6,12,13]. This range is similar to that of USDA, Malaysian, and Indonesian food composition data, at 1.47 g, 2.70 g, and 2.50 g per 100 g fresh weight (FW), respectively [31,32,33]. Durian contains a high amount of fat and is in the range of 1.59 to 5.39 g per 100 g FW, a figure comparable to the data from USDA, Malaysian, and Indonesian food composition databases at 5.33 g, 3.40 g, and 3.00 g of fat per 100 g FW, respectively [6,12,13,31,32,33]. The fat content of durian is somewhat comparable to one-third of ripe olives [31]. Total sugar of Malaysian, Thailand, and Indonesian durian varieties is in the range of 7.52 to 16.90 g, 14.83 to 19.97 g, and 3.10 to 14.05 g per 100 g FW, respectively (Table 2). The Thailand variety of Kradum showed the highest total sugar, at 19.97 g per 100 g FW. Sucrose was the predominant sugar in durian, with 5.57 to 17.89 g per 100 FW, followed by glucose, fructose, and maltose. However, the Malaysian variety of D24 contains higher amounts of fructose than glucose.
Table 2.
Sugar composition of different durian varieties (g per 100 g fresh weight).
| Sugars | Fructose [13,35,36] | Glucose [13,35,36] | Sucrose [13,35,36] | Maltose [13,35] | Total Sugar [6] * [13,35,36] |
|---|---|---|---|---|---|
| Malaysian Variety | |||||
| Durian Kampung | 1.60 | 2.21 | 12.58 | 0.51 | 16.90 |
| D2 | 1.66 | 2.51 | 7.70 | NA | 11.87 |
| D24 | 0.76 | 0.73 | 6.03 | NA | 7.52 |
| MDUR78 | 1.82 | 2.77 | 8.02 | NA | 12.61 |
| D101 | 1.29 | 1.97 | 5.57 | NA | 8.83 |
| Chuk | 1.28 | 1.87 | 10.65 | NA | 13.80 |
| Thailand Variety | |||||
| Monthong | 0.15 | 0.74 | 13.69 | 0.25 | 14.83 |
| Chanee | 0.26 | 0.58 | 15.71 | 0.00 | 16.55 |
| Kradum | 0.33 | 0.71 | 17.89 | 1.04 | 19.97 |
| Kobtakam | 0.10 | 0.45 | 17.30 | 0.26 | 18.11 |
| Indonesian Variety | |||||
| Ajimah | NA | NA | NA | NA | 14.05 |
| Hejo | NA | NA | NA | NA | 3.10 |
| Matahari | NA | NA | NA | NA | 8.14 |
| Sukarno | NA | NA | NA | NA | 8.12 |
* Total sugar is the sum of each individual sugar except for [6], NA, not available.
Table 3 shows fatty acid compositions of different durian varieties. Thailand durian varieties showed higher monounsaturated fatty acids (MUFA) than saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA), with exception of Monthong. Palmitic acid (16:0) was the major SFA, in the range of 84.57 to 1696.00 mg per 100 g FW, while oleic acid (18:1) was the major MUFA found in the matured or fully ripened durian (64.89 to 2343.30 mg per 100 g FW). However, each study used a different technique for fatty acid analysis. Gas chromatography was used by Charoenkiatkul et al. (2015) while high pressure liquid chromatography was used by Haruenkit et al. (2010) [13,14]. Both MUFA and SFA might be involved in various metabolic pathways, including the regulation of transcription factors and the expression of multiple genes related to inflammatory processes [37,38,39].
Table 3.
Fatty acid (FA) composition of different durian varieties (mg per 100 g fresh weight).
| Thailand Variety | Monthong | Chanee | Kradum | Kobtakam | |
|---|---|---|---|---|---|
| Fatty Acid Name | Nomenclature | Fatty Acids Composition | |||
| Decanoic (Capric) [14] | C 10:0 | 0.11–0.19 | NA | NA | NA |
| Dodecanoic (Lauric) [13] | C 12:0 | 3.07 | 16.00 | 16.68 | 9.63 |
| Tetradecanoic (Myristic) [13,14] | C 14:0 | 1.50–30.70 | 64.00 | 41.70 | 32.10 |
| Hexadecanoic (Palmitic) [13,14] | C 16:0 | 84.57–1473.60 | 1696.00 | 1626.30 | 1508.70 |
| cis-9-Hexadecenoic (Palmitoleic) [13] | C 16:1 | 122.80 | 192.00 | 125.10 | 160.50 |
| Octadecanoic (Stearic) [13,14] | C 18:0 | 3.48–61.40 | 64.00 | 83.40 | 96.30 |
| cis-9-Octadecenoic (Oleic) [13,14] | C 18:1 n-9 | 64.89–1074.50 | 1952.00 | 2376.90 | 2343.30 |
| cis-9,12-Octadecadienoic (Linoleic) [13,14] | C 18:2 n-6 | 10.78–184.20 | 128.00 | 125.10 | 160.50 |
| cis-6,9,12-Octadecatrienoic (γ-Linolenic) [13] | C 18:3 n-6 | 184.20 | 384.00 | 208.50 | 96.30 |
| Eicosanoic (arachidic) [14] | C 20:0 | 0.58 | NA | NA | NA |
| Saturated FA (SFA) [14] | 1565.70 | 1824.00 | 1751.40 | 1669.20 | |
| Monounsaturated FA (MUFA) [14] | 1228.00 | 2144.00 | 2543.70 | 2503.80 | |
| Polyunsaturated FA (PUFA) [14] | 337.70 | 480.00 | 375.30 | 256.80 | |
NA, not available.
Table 4 shows the mineral compositions of ripe Thailand durian. Durian is high in potassium in the range from 70.00 to 601.00 mg per 100 g FW [11,13,14,31,32,33]. This is comparable to potassium-rich fruit such as banana, with the value of 358.00 mg per 100 g FW [31]. Phosphorus, magnesium, and sodium are in the range of 25.79 to 44.00, 19.28 to 30.00, and 1.00 to 40.00 mg per 100 g FW, respectively. Durian is also a source of iron, copper, and zinc with the range of 0.18 to 1.90, 0.12 to 0.27 and 0.15 to 0.45 mg per 100 g FW, respectively. The Thailand variety of Chanee showed the highest level of iron, zinc and potassium among the studied durian [12,19,20,21,22,29]. Durian also contains vitamin A, different types of vitamin B, and vitamin E [13,14,15,31,32,33].
Table 4.
Mineral and vitamin contents of different durian varieties.
| Durian Variety | Thailand Variety | Malaysian Variety | Unknown Variety [31] | Unknown Variety [32] | Unknown Variety [33] | |||
|---|---|---|---|---|---|---|---|---|
| Monthong | Chanee | Kradum | Kobkatam | Unknown [15] | ||||
| Macrominerals (mg per 100 g fresh weight) | ||||||||
| Calcium [13,14,31,32,33] | 4.298–6.134 | 5.44 | 3.75 | 3.21 | NA | 6.00 | 40.00 | 7.00 |
| Phosphorus [13,14,31,32,33] | 25.79–33.59 | 32.96 | 36.70 | 37.56 | NA | 39.00 | 44.00 | 44.00 |
| Sodium [13,14,31,32,33] | 6.14–15.66 | 11.84 | 19.60 | 21.51 | NA | 2.00 | 40.00 | 1.00 |
| Potassium [13,14,31,32,33] | 377.00–489.42 | 539.20 | 439.52 | 438.17 | NA | 436.00 | 70.00 | 601.00 |
| Magnesium [13,14,31,32,33] | 19.28–24.87 | 23.36 | 23.35 | 22.79 | NA | 30.00 | NA | NA |
| Microminerals (mg per 100 g fresh weight) | ||||||||
| Iron [13,14,31,32,33] | 0.18–0.23 | 0.45 | 0.33 | 0.36 | NA | 0.43 | 1.90 | 1.30 |
| Copper [13,14,31,32,33] | 0.13–0.15 | 0.27 | 0.23 | 0.17 | NA | NA | NA | 0.12 |
| Manganese [14] | 0.23–0.26 | NA | NA | NA | NA | NA | NA | NA |
| Zinc [13,14,31,33] | 0.15–0.21 | 0.45 | 0.37 | 0.32 | NA | 0.28 | NA | 0.30 |
| Vitamins (μg per 100 g fresh weight) | ||||||||
| A (RAE) | NA | NA | NA | NA | NA | 2.00 | NA | NA |
| B1/Thiamine | NA | NA | NA | NA | NA | 374.00 | 100.00 | 100.00 |
| B2/Riboflavin | NA | NA | NA | NA | NA | 200.00 | 100.00 | 100.00 |
| B3/Niacin | NA | NA | NA | NA | NA | 1074.00 | NA | 13650.00 |
| B6/Pyridoxine | NA | NA | NA | NA | NA | 316.00 | NA | NA |
| E/Tocopherol or Tocotrienol (μg per 100 g fresh weight) | ||||||||
| α-tocopherol | NA | NA | NA | NA | 3774.00 | NA | NA | NA |
| γ-tocopherol | NA | NA | NA | NA | 1013.00 | NA | NA | NA |
| δ-tocopherol | NA | NA | NA | NA | 11.00 | NA | NA | NA |
| δ-tocotrienol | NA | NA | NA | NA | 1.00 | NA | NA | NA |
NA, not available; RAE, retinol activity equivalent.
Table 5 shows soluble, insoluble, and total dietary fibres in Thailand durian varieties. However, there are limited data available for Indonesian and Malaysian varieties. The total dietary fibre is in the range from 1.20 to 3.39 g per 100 g FW for Thailand Monthong variety. However, it must be noted that different analyses were used between studies. Soluble dietary fibre varied from 0.74 g (Puang Manee) to 1.40 g (Monthong) per 100 g FW while insoluble dietary fibre is in the range from 0.60 g (Kan Yao) to 2.44 g (Chanee) per 100 g FW [10,12,16].
Table 5.
Soluble, insoluble, and total dietary fibre in different durian variety (g per 100 g fresh weight).
| Type of Fibre | Soluble [10,12,16] | Insoluble [10,12,16] | Total Dietary Fibre [10,11,12,13,16,31,32,33] |
|---|---|---|---|
| Thailand Variety | |||
| Monthong | 0.40–1.40 | 0.80–1.92 | 1.20–3.39 |
| Chanee | 1.14 | 2.44 | 2.91–3.58 |
| Kradum | 0.77 | 1.64 | 2.41–3.17 |
| Kan Yao | 1.01 | 0.60 | 1.61 |
| Puang Manee | 0.74 | 1.95 | 2.69 |
| Kobtakam | NA | NA | 2.41 |
| Unknown variety | NA | NA | 3.80 |
| Unknown variety | NA | NA | 0.90 |
| Unknown variety | NA | NA | 3.50 |
NA, not available.
3. Bioactive Compounds and Antioxidant Capacity
Total polyphenols content of ripe durian is in the range of 21.44 to 374.30 mg gallic acid equivalent (GAE) per 100 fresh weight (FW) (Table 6). The Thailand variety of Monthong showed the highest polyphenols content with 374.30 mg GAE per 100 FW compared with other durian varieties [10,11,12,13,14,17,18,19,20,21]. Total flavonoid content of different durian varieties is in the range of 1.90 to 93.90 mg catechin equivalent (CE) per 100 g FW [10,11,12,14,16,17,18,19,20,21,22]. This review found three main flavonoids, namely flavanones (hesperetin and hesperidin), flavonols (morin, quercetin, rutin, kaempferol, myricetin), and flavones (luteolin and apigenin). Hesperetin was quantified in Thailand durian variety in the range of 260.99 to 1110.23 μg per 100 g FW [16]. The predominant flavonol was quantified in Monthong as quercetin with 2549.30 mg per 100 g FW [18,19,20]. Morin, a type of flavonol, was also detected in mature and ripe durian variety of Monthong in the range from 110.00 to 550.00 μg per 100 g FW [19]. Rutin and kaempferol were quantified in the range of 163.90 to 912.05 μg per 100 g FW and 131.64 to 2200.00 μg per 100 g FW, respectively [18]. Lowest and highest myricetin contents were quantified in Kradum and Monthong, at 320.00 μg and 2159.27 μg per 100 g FW, respectively [19,23]. The main flavones were identified in durian as luteolin and apigenin in the range of 279.29 to 509.09 µg and 509.09 to 791.94 µg per 100 g FW, respectively [16,18,19,23]. The total flavanol content is in the range of 0.13 mg to 5.18 mg CE per 100 g FW [11,12,14,17,18,19,20,21]. The anthocyanins content is in the range 0.32 to 633.44 mg cyanidin-3-glucoside equivalent (CGE) per 100 g FW [18,19,22].
Table 6.
Bioactive compounds of different durian varieties (mg/μg per 100 g fresh weight).
| Bioactive Compounds | Durian Variety | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malaysian Variety | Thailand Variety | Unknown Variety [23] | ||||||||||
| Chaer Phoy | Yah Kang | Ang Jin | D11 | Unknown | Chanee | Kan Yao | Puang Manee | Kradum | Monthong | Kobtakam | ||
| Polyphenols | ||||||||||||
| Total polyphenols [10,11,12,13,16,17,18,19,20,21,22] | 67.12 mg GAE | 80.45 mg GAE | 97.78 mg GAE | 71.13 mg GAE | 99.00 mg GAE | 21.44–321.20 mg GAE | 283.30 mg GAE | 310.50 mg GAE | 94.18–271.50 mg GAE | 56.18–374.30 mg GAE | 94.18 mg GAE | 79.15 mg GAE |
| Flavonoids | ||||||||||||
| Total flavonoids [10,12,18,29,32,34,35,36,37,38,39] | 22.56 mg CE | 22.22 mg CE | 22.50 mg CE | 20.58 mg CE | NA | 1.90–81.60 mg CE | 3.51–72.10 mg CE | 3.24–18.10 mg CE | 4.48–19.80 mg CE | 4.49–93.90 mg CE | NA | NA |
| Flavanone | ||||||||||||
| Hesperetin [16] | NA | NA | NA | NA | NA | 321.15 μg | 260.99 μg | 640.79 μg | 1110.23 μg | 562.98 μg | NA | NA |
| Hesperidin [19] | NA | NA | NA | NA | NA | NA | NA | NA | NA | 200.00 μg | NA | NA |
| Flavonol | ||||||||||||
| Quercetin [18,19,20] | NA | NA | NA | NA | NA | 2.22 mg | 2.44 mg | 2.18 mg | NA | 1.20–2549.30 mg | NA | NA |
| Morin [19] | NA | NA | NA | NA | NA | NA | NA | NA | NA | 110.00–550.00 μg | NA | NA |
| Rutin [18] | NA | NA | NA | NA | NA | 492.41 μg | NA | 733.20 μg | 163.90 μg | 912.05 μg | NA | NA |
| Kaempferol [16,19] | NA | NA | NA | NA | NA | 479.09 μg | 644.80 μg | 430.18 μg | 131.64 μg | 830.26–2200.00 μg | NA | 1310.00 mg |
| Myricetin [19] | NA | NA | NA | NA | NA | NA | 1559.56 μg | 964.47 μg | 2159.27 μg | 320.00–2087.83 μg | NA | 1010.00 mg |
| Bioactive Compounds | Durian Variety | |||||||||||
| Malaysian Variety | Thailand Variety | Unknown Variety [23] | ||||||||||
| Chaer Phoy | Yah Kang | Ang Jin | D11 | Chanee | Kan Yao | Puang Manee | Kradum | Monthong | Kobtakam | |||
| Flavonoids | ||||||||||||
| Flavone | ||||||||||||
| Luteolin [21] | NA | NA | NA | NA | 364.92 μg | 279.29 μg | 509.09 μg | 287.69 μg | 338.22 μg | NA | NA | |
| Apigenin [21] | NA | NA | NA | NA | 739.42 | 763.83 μg | 509.09 μg | 791.94 μg | 620.00–665.89 μg | NA | NA | |
| Total flavanols [11,12,14,17,18,19,20] | NA | NA | NA | NA | 0.15 mg CE | 0.13 mg CE | 0.15 mg CE | 0.13 mg CE | 0.18 mg CGE–5.18 mg CE | NA | NA | |
| Total anthocyanins [15,17,38] | NA | NA | NA | NA | 0.38 mg CGE | 0.34 mg CGE | 0.37 mg CGE | 0.32 mg CGE | 0.39–633.44mg CGE | NA | NA | |
| Phenolic Acids | ||||||||||||
| Cinnamic acid [19] | NA | NA | NA | NA | NA | NA | NA | NA | 600.00–660.00 μg | NA | 1510.00 mg | |
| Caffeic acid [19,21] | NA | NA | NA | NA | NA | NA | NA | NA | 31.08–490.00 μg | NA | NA | |
| p-Coumaric acid [19,21] | NA | NA | NA | NA | NA | NA | NA | NA | 29.22-600.00 μg | NA | NA | |
| Ferulic acid [18,21] | NA | NA | NA | NA | 215.95 μg | NA | 158.67 μg | NA | 414.40 μg | NA | NA | |
| p-Anisic acid [22] | NA | NA | NA | NA | NA | NA | NA | NA | 1.48 μg | NA | NA | |
| Gallic acid [18] | NA | NA | NA | NA | 1416.00 μg | NA | 4760.10 μg | NA | 2072.00 μg | NA | NA | |
| Vanillic acid [19,22] | NA | NA | NA | NA | NA | NA | NA | NA | 20.72–300.00 μg | NA | NA | |
| Bioactive Compounds | Durian Variety | |||||||||||
| Malaysian Variety | Thailand Variety | |||||||||||
| Chaer Phoy | Yah Kang | Ang Jin | D11 | Unknown [15] | Chanee | Kan Yao | Puang Manee | Kradum | Monthong | Kobtakam | ||
| Carotenoids | ||||||||||||
| Total carotenoids [11,17,24] | 7.10 μg BCE | 5.13 μg BCE | 6.02 μg BCE | 8.22 μg BCE | NA | 4400.00–6000.00 μg | NA | NA | NA | 222.88–1167.00 μg | NA | |
| β-Carotene [13,20,21,24,25] | NA | NA | NA | NA | 201.00 μg | 84.54–4429.00 μg | 54.17 μg | 320.87 μg | 232.44–250.20 μg | 35.92–4250.00 μg | 385.84 μg | |
| α-Carotene [13,20,21,24,25] | NA | NA | NA | NA | 37.00 μg | 47.23–1329.00 μg | 8.61 μg | 38.55 μg | 52.54–79.09 μg | 7.79–343.00 μg | 263.54 μg | |
| β-Crptoxanthin [13,16] | NA | NA | NA | NA | 7.00 μg | 17.58 μg | 4.87 μg | 17.80 μg | 26.80 μg | 5.85 μg | ND/NA | |
| Lycopene [13,16] | NA | NA | NA | NA | 12.00 μg | 11.62 μg | 1.38 μg | 17.47 μg | 6.91 μg | 2.80 μg | ND/NA | |
| Lutein [13,16,24,25] | NA | NA | NA | NA | 11.00 μg | 14.00–41.28 μg | 7.21 μg | 18.16 μg | 32.35–54.21 μg | 7.96–41.75 μg | 72.23 μg | |
| Zeaxanthin [13,16,24,25] | NA | NA | NA | NA | 37.00 μg | 0.09–37.47 μg | 11.37 μg | 20.21 μg | 49.44 μg | 0.14–11.95 μg | ND/NA | |
| Tannins [16,18,21,22] | NA | NA | NA | NA | NA | NA | NA | NA | NA | 29.60–296.00 μg | NA | |
| Ascorbic acid [11,14,17,20,21,22] | 2.41 mg | 2.21 mg | 1.93 mg | 2.56 mg | 25.18 mg | NA | NA | NA | NA | 54.76–347.80 mg | NA | |
NA, not available; GAE, gallic acid equivalent; CE, catechin equivalent; CGE, cyanidin-3-glucoside equivalent; ND, not detected; BCE, β-carotene equivalent.
Phenolic acids in durians belong to hydroxycinnamic acid (caffeic, p-coumaric, ferulic, p-anisic acid) and hydroxybenzoic acid (gallic and vanillic acid) derivatives. Cinnamic acid, caffeic acid, p-coumaric acid, and p-anisic acid were quantified in Monthong variety in the range of 600.00 to 660.00 μg, 31.08 to 490.00 μg, 29.22 to 600.00 μg, and 1.48 μg per 100 g FW, respectively [19,21]. Ferulic acid was identified in Chanee, Puang Manee, and Monthong in the range of 215.95 μg, 158.67 μg and 414.40 μg per 100 g FW, respectively [18,21]. Gallic acid is the main hydroxybenzoic acid identified in Chanee, Monthong, and Puang Manee, at 1416.00, 2072.00, and 4760.10 μg per 100 g FW respectively [18].
Total carotenoids content was higher in Thailand compared with Malaysian variety in the range of 222.88 µg to 6000.00 µg and 5.13 µg to 8.22 µg BCE per 100 g FW, respectively [11,17,24]. Thailand durian varieties contain minor amount of β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin [13,18,24,25]. Carotenoid content varies in durian and depending on factors such as variety, part of the plant, degree of maturity, climate, soil type, growing conditions and geographical area of production [40]. Tannins have been identified in Monthong variety in the range from 29.60 to 296.00 μg per 100 g FW [11,14,21,22]. Ascorbic acid content in the Malaysian variety is in the range from 1.93 to 8.62 mg per 100 g FW [17]. The Thailand variety of Monthong variety showed the highest ascorbic acid, with 347.80 mg per 100 g FW [14].
Durian is rich in bioactive polyphenols and hence, exerts antioxidant potential. Table 7 shows antioxidant capacity of durians based on 1-diphenyl-2-picrylhydrazyl radical (DPPH), ferric ion reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC), cupric reducing antioxidant capacity (CUPRAC), hydrophilic oxygen radical absorbance capacity (H-ORAC), and 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) assays [12,13,14,15,18,22,23,40,41]. Antioxidant activities of Thailand durian varieties were in the range from 97.93 to 1366.16 μM Trolox equivalents (TE) per 100 g FW for DPPH assay, 71.84 to 749.08 μM TE per 100 g FW for FRAP assay, 1903.40 to 2793.90 μM TE per 100 g FW for ORAC assay, 427.65 to 1075.60 μM TE per 100 g FW for CUPRAC assay, and from 265.86 to 2352.70 μM TE per 100 g FW for ABTS assay [12,13,14,18,41,42]. Antioxidant activity of the unknown Malaysian durian variety was 1838.00 μM TE per 100 g FW, as determined using H-ORAC assay [15]. Antioxidant activity of unknown durian (Chinese study) was 498.00 μM TE per 100 g FW as assayed using ABTS [23].
Table 7.
Antioxidant activities of different durian varieties (μM Trolox equivalents per 100 g fresh weight).
| Type of Antioxidant Activity Assay | DPPH [12,13,14,22,40,41] | FRAP [12,13,14,18,22] | ORAC[13] | CUPRAC[12,14,18,22] | ABTS [12,14,18,22,41] | H-ORAC[15] |
|---|---|---|---|---|---|---|
| Thailand Variety | ||||||
| Monthong | 97.93–1366.15 | 71.84–749.08 | 1903.40 | 427.65–1075.60 | 265.86–2352.70 | NA |
| Chanee | 128.00–245.60 | 232.10–457.43 | 2304.00 | 955.40 | 2091.40 | NA |
| Kradum | 250.20 | 667.20 | 2793.90 | 806.50 | 1773.20 | NA |
| Kan Yao | 209.09 | 204.70 | NA | 845.50 | 1843.60 | NA |
| Puang Manee | NA | 244.90 | NA | 924.90 | 2020.40 | NA |
| Kobtakam | 192.60 | 513.60 | 2343.30 | NA | NA | NA |
| Malaysian Variety | ||||||
| Unknown | NA | NA | NA | NA | NA | 1838.00 |
| Unknown Variety | ||||||
| Unknown [23] | NA | NA | NA | NA | 498.00 | NA |
NA, not available; DPPH, 1,1-diphenyl-2-picrylhydrazyl radical; FRAP, ferric ion reducing antioxidant power; ORAC, oxygen radical absorbance capacity; CUPRAC, cupric reducing antioxidant capacity; H-ORAC, hydrophilic oxygen radical absorbance capacity; ABTS, 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid.
4. Volatile Components
Durian is rich in volatiles esters, alcohols, ketones and sulphur (Table 8). These volatile compounds gave durian a unique flavour and taste. Chin et al. (2007) reported 39 volatile compounds in the three Malaysian durian varieties, D2, D24 and D101 [7]. A total of 44 volatile compounds were identified in Indonesian durian varieties of Ajimah, Hejo, Matahari, and Sukarno [42]. The main volatile component in durian is sulphur. Ethanethiol, propanethiol, diethyl disulphide, ethyl propyl disulphide, ethyl propyl disulphide, and diethyl trisulphide were the predominant sulphur compounds identified in Malaysian durian variety. The sulphur compounds in Malaysian varieties were 97% higher than Indonesian variety.
Table 8.
Mean relative amounts of volatiles identified in different durian varieties.
| Compounds | Relative Amount in ng per g fresh weight | ||||||
|---|---|---|---|---|---|---|---|
| Malaysian Variety | Indonesian Variety | ||||||
| D101 | D2 | D24 | Hejo | Matahari | Ajimah | Sukarno | |
| Sulphur compounds | |||||||
| Ethanethiol [7,42] | 5480.00 | 4260.00 | 3550.00 | ND | 5.40 | 50.70 | 36.40 |
| Propanethiol [7,42] | 5000.00 | 2720.00 | 2720.00 | ND | 18.00 | 31.10 | ND |
| Methyl ethyl disulphide [42] | NA | NA | NA | ND | ND | ND | 11.50 |
| Diethyl disulphide [7,42] | 12420.00 | 1585.00 | 18760.00 | 24.40 | 323.90 | 245.20 | 188.40 |
| Ethyl propyl disulphide [7,42] | 3630.00 | 3350.00 | 9040.00 | 2.30 | 43.20 | 11.30 | 4.60 |
| Bis(ethylthio)methane [42] | NA | NA | NA | 49.30 | 105.40 | 246.10 | 118.20 |
| Diethyl trisulphide [7,42] | 5970.00 | 14680.00 | 2520.00 | 10.20 | 185.50 | 213.60 | 72.30 |
| 3,5-Dimethyl-1,2,4- trithiolane (isomer 1) [7,42] | 470.00 | 1460.00 | 1740.00 | 1.50 | 10.60 | 20.80 | 2.00 |
| 3,5-Dimethyl-1,2,4- trithiolane (isomer 2) [7,42] | 590.00 | 1470.00 | 1710.00 | 1.00 | 10.60 | 17.70 | 1.10 |
| 1,1-Bis(methylthio)- ethane [7] | NA | NA | NA | 5.30 | 14.50 | 5.80 | 3.00 |
| 1,1-Bis(ethylthio)-ethane [7,42] | 420.00 | 490.00 | 710.00 | 1.90 | 15.80 | 5.90 | 10.20 |
| 3-Mercapto-2- methylpropanol[7] | NA | NA | NA | 2.50 | 21.90 | 2.80 | 4.70 |
| Dipropyl trisulphide [7] | 120.00 | 160.00 | 110.00 | NA | NA | NA | NA |
| Dipropyl disulphide [7] | 200.00 | 110.00 | 1030.00 | NA | NA | NA | NA |
| 1-(ethylthio)-1-(methylthio)-Ethane [7] | 660.00 | 140.00 | 660.00 | NA | NA | NA | NA |
| S-propyl ethanethioate [7] | 340.00 | 60.00 | 320.00 | NA | NA | NA | NA |
| S-ethyl ethanethioate [7] | 90.00 | ND | 310.00 | NA | NA | NA | NA |
| 1-(methylthio)-propane [7] | 270.00 | ND | 130.00 | NA | NA | NA | NA |
| Total | 35660.00 | 30485.00 | 43310.00 | 98.40 | 754.80 | 851.00 | 452.40 |
| Alcohols | |||||||
| Ethanol [7,42] | 720.00 | 1090.00 | 590.00 | 419.90 | 688.80 | 843.40 | 1091.30 |
| 2-Methyl-1-butanol [42] | NA | NA | NA | ND | ND | 17.40 | ND |
| 3-Methyl-1-butanol [42] | NA | NA | NA | 10.50 | ND | ND | 14.60 |
| 2,3-Butanediol [42] | NA | NA | NA | 4.60 | ND | ND | 11.70 |
| Total | 720.00 | 1090.00 | 590.00 | 435.00 | 688.80 | 860.80 | 1117.60 |
| Ketones | |||||||
| 3-Hydroxy-2-butanone [42] | NA | NA | NA | 56.20 | 84.20 | 71.30 | 64.10 |
| Aldehydes | |||||||
| Acetaldehyde [42] | NA | NA | NA | 44.90 | 62.20 | 33.90 | ND |
| Esters | |||||||
| Ethyl acetate [7,42] | 280.00 | 610.00 | 930.00 | 28.10 | 52.40 | 34.80 | 31.20 |
| Methyl propanoate [7,42] | 970.00 | 880.00 | 700.00 | 16.40 | 52.20 | ND | ND |
| Ethyl propanoate [7,42] | 3110.00 | 1850.00 | 2530.00 | 386.60 | 719.50 | 742.30 | 0.00 |
| Methyl-2-methylbutanoate [7,42] | 4070.00 | 2330.00 | 2290.00 | 86.20 | 105.60 | 85.40 | 24.90 |
| Ethyl butanoate [7,42] | 850.00 | 2220.00 | 40.00 | 73.20 | 131.90 | 252.30 | 83.20 |
| Propyl propanoate [7,42] | 4630.00 | 1740.00 | 3810.00 | ND | 88.40 | ND | ND |
| Ethyl 2-methylbutanoate [7,42] | 460.00 | 510.00 | 500.00 | 2938.40 | 2373.40 | 3846.70 | 1085.90 |
| Diethyl carbonate [42] | NA | NA | NA | ND | 9.70 | ND | 7.40 |
| Propyl 2-methylbutanoate [7,42] | 126.70 | 4770.00 | 113.00 | 109.30 | 208.50 | 192.0 | 11.80 |
| Propyl butanoate [7,42] | 950.00 | 630.00 | 950.00 | 3.50 | 16.30 | ND | ND |
| Propyl 3-methylbutanoate [7,42] | 19.00 | ND | 380.00 | 237.90 | ND | ND | ND |
| Ethyl 2-butenoate [7,42] | ND | 140.00 | ND | ND | 252.20 | 397.60 | 132.10 |
| Methyl hexanoate [7] | 320.00 | 1700.00 | ND | ND | ND | ND | ND |
| Ethyl (2E)-2-pentenoate [42] | NA | NA | NA | 4.50 | ND | ND | ND |
| Ethyl 3-hexanoate [42] | NA | NA | NA | ND | ND | 93.02 | 32.30 |
| Propyl hexanoate [7,42] | 580.00 | 500.00 | 310.00 | 3.10 | 24.20 | 3.90 | ND |
| Propyl tiglate [42] | NA | NA | NA | 12.80 | ND | ND | ND |
| Ethyl heptanoate [7,42] | 150.00 | 250.00 | 150.00 | 42.40 | 111.20 | 74.80 | ND |
| Methyl octanoate [7,42] | 220.00 | 100.00 | ND | 4.30 | 26.90 | ND | ND |
| Ethyl octanoate [7,42] | 550.00 | 550.00 | 260.00 | 91.0 | 174.60 | 108.40 | 45.90 |
| Ethyl (4Z)-4-octenoate [42] | NA | NA | NA | ND | 17.40 | ND | ND |
| Ethyl-2,4-hexadienoate [42] | NA | NA | NA | ND | ND | 3.10 | 2.60 |
| Ethyl-3-hydroxybutanoate [42] | NA | NA | NA | 6.10 | 12.20 | 23.40 | 16.80 |
| Propyl octanoate [42] | NA | NA | NA | ND | 14.50 | ND | ND |
| Ethyl-2-octenoate [42] | NA | NA | NA | 2.20 | ND | ND | ND |
| Ethyl decanoate [42] | NA | NA | NA | 10.90 | 8.20 | 10.40 | 11.1 |
| Ethyl 2-methylpropanoate [7] | 460.00 | 510.00 | 520.00 | NA | NA | NA | NA |
| Propyl acetate [7] | 190.00 | 90.00 | 560.00 | NA | NA | NA | NA |
| Methyl butanoate [7] | 300.00 | 450.00 | ND | NA | NA | NA | NA |
| Ethyl 3-methylbutanoate [7] | 190.00 | 220.00 | 220.00 | NA | NA | NA | NA |
| 3-methylbutyl propanoate [7] | 730.00 | ND | 600.00 | NA | NA | NA | NA |
| Total | 19155.70 | 20050.00 | 14863.00 | 3947.60 | 4399.30 | 5868.12 | 1429.10 |
NA, not available; ND, not detected.
The volatile sulphur compounds (VSCs) have a smell resembling onion [43]. Durians from Indonesia have lower VSCs and contributed to the less sulphuric odour in Hejo and Sukarno. Sukarno has sweet odour, while Hejo has the mildest sulphuric odour among the studied durians varieties in Indonesia [6]. There were an additional 12 VSCs identified in Indonesian variety of Cane, Kodak, and Bobo [44]. The VSCs were identified as S-ethyl thioacetate, 1-hydroxy-2-methylthioethane, methyl 2- methylthioacetate, dimethyl sulfone, S-ethyl thiobutyrate, ethyl 2-(methylthio) acetate, 2-isopropyl-4-methylthiazole, S-isopropyl 3-(methylthio), S-methyl thiohexanoate, 5-methyl-4-mercapto-2-hexanone benzothiazole, 3,4-dithia-2-ethylthiohexane, and S-methyl thiooctanoate, 3,5- dimethyltetrathiane.
Ethanol was the predominant alcohol compound in Malaysian and Indonesian varieties in the range from 590.00 to 720.00 ng per g and 419.90 to 1091.30 ng per g fresh weight (FW), respectively. Another three alcohols were detected in durian as 2-methyl-1-butanol, 3-methyl-1-butanol and 2,3-butanediol in Malaysian and Indonesian durian [7,45]. Weenen et al. (1996) detected additional alcohols in durian Cane, Kodak, and Bobo from Indonesia as hexadecanol, 9-octadecen-1-ol (cis and trans), and isobutyl alcohol [44]. Voon et al. (2007) and Chin et al. (2008) detected 1-propanol, 1-butanol and 1-hexanal in Malaysian variety of Chuk, D101, D2, MDUR78, and D24 [45,46].
3-Hydroxy-2-butanone (a ketone) was identified in the Indonesian variety in the range of 56.20 to 84.20 ng per g FW [7,42]. Durian Matahari showed the highest amount of 3-hydroxy-2-butanone with 84.20 ng per g FW and the lowest in Hejo with 56.20 ng per g FW [42]. 3-Hydroxy-2-butanone (common name: acetoin) has a pleasant yogurt creamy odour and a fatty butter taste. It is present in vinegar and alcoholic beverages [47]. Another ketone was identified as 2-hydroxy-3-pentanone in durian varieties from Indonesia, Kodak, Cane, and Bobo [44]. However, 2-hydroxy-3-pentanone has an unfavourable odour like fishy and earthy [48]. For aldehydes, acetaldehyde detected in Indonesian variety of Hejo, Matahari, and Ajimah in the range of 33.90 to 62.20 ng per g FW [42]. Acetaldehyde contributed to the fruity and sweet aroma in durian [49].
Esters are the second most abundant bioactive compounds in durian after sulphur. Esters were the volatiles that contributed to the sweet odour to durian, more than aldehyde, while aldehyde contributed more to the fruity note. The major ester compounds in Malaysian durian varieties (D101, D2, and D24) were characterized as propyl 2-methylbutanoate, ethyl propanoate, propyl propanoate and methyl 2-methylbutanoate [7]. Ethyl 2-methylbutanoate was detected as major ester in Indonesian varieties of Hejo, Matahari, and Ajimah [42]. Ethyl propanoate, methyl propanoate, propyl propanoate, ethyl 2-methyl propanoate and 3-methylbutyl propanoate volatiles have similar structure and differ only in the number of carbon atoms (ethyl, methyl) [50]. Ethyl propanoate is the major ester detected in Malaysian and Indonesian durians in the range of 0.00 to 3110.00 ng per g FW. It was noted that Malaysian durian variety showed much higher content of ethyl propanoate than Indonesian variety [7,42].
5. Health Benefits of Durian
Durian is rich in macronutrients (sugars and fat) and micronutrients (potassium), dietary fibres, and bioactive and volatile compounds. An intake of one serving size of durian aril (155 g) contributes to 130 to 253 kcal and is equivalent to one large pear and four small apples without skin, respectively [6,31,32,33]. Durian is energy-dense due to sugar and fat content and hence, might contribute to daily energy intake and will also increase postprandial blood glucose.
5.1. Effects of Durian on Blood Glucose
Durian is high in sugar, but supplementation of 5% freeze-dried Monthong (Thailand variety) in 1% cholesterol-enriched diets in rats for 30 days did not raise the plasma glucose level compared with control diet [41]. In humans, Robert et al. (2008) showed that durian had the lowest glycaemic index (GI = 49) compared with watermelon (GI = 55), papaya (GI = 58), and pineapple (GI = 90) [51]. The low GI value for durian might be due to the presence of fibre and fat. Fibre slows digestion in the digestive tract and will slow down the conversion of the carbohydrate to glucose, thus lower the GI of food [52]. Fat does not have a direct effect on blood glucose response, but it may influence glycaemic response indirectly by delaying gastric emptying, and thus slowing the rate of glucose absorption [53].
Durian is rich in potassium and is similar to potassium-rich fruit, i.e., banana [31]. A meta-analysis study showed that there was a linear dose-response between low serum potassium and risk of type 2 diabetes mellitus [54]. Chatterjee et al. (2017) demonstrated that potassium chloride supplementation reduced the worsening effect of fasting glucose in African-Americans compared with placebo [55]. Collectively, the evidence has shown that potassium content in durian might play a role in the regulation of blood glucose. The effect of durian on blood glucose has not been thoroughly explored both in animal and human studies, and hence, warrants further investigation. Potassium might play a role in glucose homeostasis but might also have negative implications in certain conditions. For instance, those with chronic kidney disease (CKD), diabetes mellitus (DM), and heart failure (HF) or on pharmacological therapies may develop hyperkalaemia [56].
5.2. Cholesterol-Lowering Properties of Durian
Anti-atherosclerotic properties of durian aril have been reported in experimental rat models [10,11,20,22,40,41]. Previous in vitro and in vivo studies investigated the health benefits of durian (Monthong variety) on lipid profiles [10,11,22]. Haruenkit et al. (2007) showed that rats fed with durian significantly (p < 0.05) reduced postprandial plasma total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) with 14.9% and 21.6%, respectively, compared with control group [10]. Gorinstein et al. (2011) showed a reduction in the levels of plasma TC (12.1%), LDL-C (13.3%), and triglycerides (TG) (14.1%) compared with the control group [11]. The results were consistent when tested with other durian from Thailand varieties (Chanee and Kan Yao) compared with control. Leontowicz et al. (2011) showed that rats supplemented with ripe durian had significantly lowered TG (26.3%), but not significant in TC (4.8%) and LDL-C (6.3%). Histological analysis demonstrated that ripe durian protected the liver and aorta from exogenous cholesterol loading and protected the intimal surface area of the aorta [20]. Durian also demonstrated the ability to hinder postprandial plasma lipids compared with snake fruit and mangosteen [10,11,22]. Previous studies have showed that propionate (0.6 mmol/L) inhibited fatty acid and cholesterol synthesis in isolated rat hepatocytes [57]. In our review, three different propionate esters were identified, i.e., ethyl propionate, methyl propionate and propyl propionate. These esters could be a potent inhibitor for free fatty acids and cholesterol synthesis but this warrants further investigations. However, these esters are highly volatile and could be easily vaporized during sample processing and storage [57].
5.3. Anti-Proliferative Activity
The polyphenol and flavonoid contents of durian are in the range of 21.44 to 374.30 mg GAE and 1.90 to 93.90 mg CE per 100 g FW. The mechanisms of action of polyphenols strongly relates to their antioxidant activity. Polyphenols are known to decrease the level of reactive oxygen species in the human body [58]. The phenolic groups present in the polyphenol structure can accept an electron to form relatively stable phenoxyl radicals, thereby disrupting chain oxidation reactions in cellular components [59]. On the other hand, polyphenols could induce apoptosis and inhibit cancer growth [60,61,62,63]. There are many studies pointing out an essential role of polyphenolic compounds as derived from vegetables, fruits, or herbs in the regulation of epigenetic modifications, resulting in the antiproliferative protection [64]. Jayakumar and Kanthimathi studied the anti-proliferative activity of durian using a breast cancer cell line (MCF-7). This study showed that durian fruit can be considered as potential sources of polyphenols with protective effects against nitric oxide-induced proliferation of MCF-7 cells, an oestrogen receptor-positive human breast cancer cell line [65]. At a concentration of 600 µg/mL, durian fruit extracts inhibited MCF-7 cell growth by 40%. However, an in vivo study is needed to confirm this effect.
5.4. Probiotic Effects
Durian aril is rich in sugar with total sugar content between 3.10 to 19.97 g per 100 g FW. The moisture content of durian aril is 56.1 g to 69.3 g per 100 g FW and pH between 6.9 to 7.6 [5,13,31]. These could be an optimum condition for bacteria fermentation. Durian aril is fermented after being left at room temperature for a few days and turns sour and watery. In Malaysia, underutilised durian aril is fermented (spontaneous and uncontrolled) to a product known as Tempoyak [66]. Tempoyak is widely used as seasoning in cooking. According to Leisner et al. (2001) lactic acid bacteria (LAB) are the predominant microorganisms in Tempoyak [67]. The LAB microorganisms were identified as Lactobacillus plantarum. However, other species including Lactobacillus fersantum, Lactobacillus corynebacterium, Lactobacillus brevis, Lactobacillus mali, Lactobacillus fermentum, Lactobacillus durianis, Lactobacillus casei, Lactobacillus collinoides, Lactobacillus paracasei and Lactobacillus fructivorans were also reported in Tempoyak [67,68,69,70]. Khalil et al. (2018) and Ahmad et al. (2018) recently demonstrated the potential of Tempoyak as a source of probiotics. The study by Khalil et al. (2018) isolated seven Lactobacillus strains that belonged to five different species of the genus Lactobacillus, including one Lactobacillus fermentum (DUR18), three Lactobacillus plantarum (DUR2, DUR5, DUR8), one Lactobacillus reutri (DUR12), one Lactobacillus crispatus (DUR4), and one Lactobacillus pentosus (DUR20) from Tempoyak. These strains were able to produce exopolysaccharide (EPS) and had great potency to withstand the extreme conditions, either at low pH 3.0, in 0.3% bile salts or in in vitro model of gastrointestinal conditions [69]. EPS has the prebiotic potential to positively affect the gastrointestinal (GIT) microbiome and may reduce cholesterol [70]. Ahmad et al. (2018) isolated Lactobacillus plantarum from Tempoyak and showed good probiotic properties including acid and bile salt tolerance, antioxidative, antiproliferative effects, and remarkable adhesion on colon adenocarcinoma cell line (HT-29 cell lines) [71].
6. Conclusions
Durian is rich in macronutrients (sugars and fat) and micronutrients (potassium), dietary fibre, and volatile compounds. Durian is an energy-dense fruit due to high sugar and fat content and, hence, might contribute to daily energy intake and increase postprandial blood glucose. Durian is also rich in bioactive polyphenols and hence possessed strong in vitro antioxidant capacity. However, the bioactivity of these polyphenols in animal or human studies is still scarce and needs further investigation. The major volatile compounds have been identified in the Malaysian, Thai, and Indonesian durian varieties as esters (ethyl propanoate, methyl-2- methylbutanoate, propyl propanoate), sulphurs (diethyl disulphide, diethyl trisulphide and ethanethiol), thioacetals (1-(methylthio)-propane), thioesters (1-(methylthio)-ethane), thiolanes (3,5-dimethyl-1,2,4-trithiolane isomers), and alcohol (ethanol). Both in vitro and in vivo animal studies showed that durian possessed anti-hyperglycaemic, anti-atherosclerotic, anti-proliferative, and probiotic effects. Durian is rich in bioactive compounds, and hence can be used as an active ingredient for the development of functional foods. Further human interventional studies are warranted to explore the health benefits of functional foods prepared with durian.
Author Contributions
N.A.A.A. and A.M.M.J. wrote and approved the paper.
Funding
This study and the Article Processing Charge (APC) were funded by the Fundamental Research Grant Scheme (FRGS) Ministry of Education Malaysia (grant number: FRGS/1/2017/SKK06/UNISZA/03/07).
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Idris S. Durio of Malaysia. 1st ed. Malaysian Agricultural Research and Development Institute (MARDI); Kuala Lumpur, Malaysia: 2011. pp. 1–130. [Google Scholar]
- 2.Brown M.J. Durio—A Bibliographic Review. 1st ed. The International Plant Genetic Resources Institute (IPGRI); New Delhi, India: 1997. pp. 2–87. [Google Scholar]
- 3.Husin N.A., Rahman S., Karunakaran R., Bhore S.J. A review on the nutritional, medicinal, molecular and genome attributes of Durian (Durio zibethinus L.), the King of fruits in Malaysia. Bioinformation. 2018;14:265–270. doi: 10.6026/97320630014265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tirtawinata M.R., Santoso P.J., Apriyanti L.H. DURIAN. Pengetahuan dasar untuk pencinta durian. 1st ed. Agriflo (Penebar Swadaya Grup); Jakarta, Indonesia: 2016. p. 31. [Google Scholar]
- 5.Ho L., Bhat R. Exploring the potential nutraceutical values of durian (Durio zibethinus L.)—An exotic tropical fruit. Food Chem. 2015;168:80–89. doi: 10.1016/j.foodchem.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Belgis M., Wijaya C.H., Apriyantono A., Kusbiantoro B., Yuliana N.D. Physicochemical differences and sensory profiling of six lai (Durio kutejensis) and four durian (Durio zibethinus) cultivars indigenous Indonesia. Int. Food Res. J. 2016;23:1466–1473. [Google Scholar]
- 7.Chin S.T., Nazimah S.A.H., Quek S.Y., Man Y.B.C., Rahman R.A., Hashim D.M. Analysis of volatile compounds from Malaysian durians (Durio zibethinus) using headspace SPME coupled to fast GC-MS. J. Food Compost. Anal. 2007;20:31–44. doi: 10.1016/j.jfca.2006.04.011. [DOI] [Google Scholar]
- 8.Alhabeeb H., Chambers E.S., Frost G., Morrison D.J., Preston T. Inulin propionate ester increases satiety and decreases appetite but does not affect gastric emptying in healthy humans. Proc. Nutr. Soc. 2014;73 doi: 10.1017/S0029665114000354. [DOI] [Google Scholar]
- 9.Chambers E.S., Viardot A., Psichas A., Morrison D.J., Murphy K.G., Zac-Varghese S.E.K., McDougall K., Preston T., Tedford C., Finlayson G.S., et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haruenkit R., Poovarodom S., Leontowicz M., Sajewicz M., Kowalska T., Delgado-Licon E., Delgado-Licon E., Rocha-Guzman N.E., Gallegos-Infante J., Trakhtenberg S., et al. Comparative study of health properties and nutritional value of durian, mangosteen, and snake fruit: Experiments In Vitro and In Vivo. J. Agric. Food Chem. 2007;55:5842–5849. doi: 10.1021/jf070475a. [DOI] [PubMed] [Google Scholar]
- 11.Gorinstein S., Poovarodom S., Leontowicz H., Leontowicz M., Namiesnik J., Vearasilp S., Haruenkit R., Ruamsuke P., Katrich E., Tashma Z. Antioxidant properties and bioactive constituents of some rare exotic Thai fruits and comparison with conventional fruits. In vitro and in vivo studies. Food Res. Int. 2011;44:2222–2232. doi: 10.1016/j.foodres.2010.10.009. [DOI] [Google Scholar]
- 12.Gorinstein S., Haruenkit R., Poovarodom S., Vearasilp S., Ruamsuke P., Namiesnik J., Leontowicz M., Leontowicz H., Suhaj M., Sheng G.P. Some analytical assays for the determination of bioactivity of exotic fruits. Phytochem. Anal. 2010;21:355–362. doi: 10.1002/pca.1207. [DOI] [PubMed] [Google Scholar]
- 13.Charoenkiatkul S., Thiyajai P., Judprasong K. Nutrients and bioactive compounds in popular and indigenous durian (Durio zibethinus murr.) Food Chem. 2015;193:181–186. doi: 10.1016/j.foodchem.2015.02.107. [DOI] [PubMed] [Google Scholar]
- 14.Haruenkit R., Poovarodom S., Vearasilp S., Namiesnik J., Sliwka-Kaszynska M., Park Y., Heo B., Cho J., Jang H.G., Gorinstein S. Comparison of bioactive compounds, antioxidant and antiproliferative activities of Mon Thong durian during ripening. Food Chem. 2010;118:540–547. doi: 10.1016/j.foodchem.2009.05.029. [DOI] [Google Scholar]
- 15.Isabelle M., Lee B.L., Koh W., Huang D., Ong C.N. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. 2010;123:77–84. doi: 10.1016/j.foodchem.2010.04.002. [DOI] [Google Scholar]
- 16.Kongkachuichai R., Charoensiri R., Sungpuag P. Carotenoid, flavonoid profiles and dietary fiber contents of fruits commonly consumed in Thailand. Int. J. Food Sci. Nutr. 2010;61:536–548. doi: 10.3109/09637481003677308. [DOI] [PubMed] [Google Scholar]
- 17.Ashraf M.A., Maah M.J., Yusoff I. Study of antioxidant potential of tropical fruit durian. Asian J. Chem. 2011;23:3357–3361. [Google Scholar]
- 18.Toledo F., Arancibia-Avila P., Park Y., Jung S., Kang S., Heo B.G., Drzewiecki J., Zachwieja Z., Zagrodzki P., Pasko P., et al. Screening of the antioxidant and nutritional properties, phenolic contents and proteins of five durian cultivars. Int. J. Food Sci. Nutr. 2008;59:415–427. doi: 10.1080/09637480701603082. [DOI] [PubMed] [Google Scholar]
- 19.Arancibia-avila P., Toledo F., Park Y., Jung S., Kang S., Heo B.G., Lee S., Sajewicz M., Kowalska T., Gorinstein S. Antioxidant properties of durian fruit as influenced by ripening. Food Sci. Technol. 2008;41:2118–2125. doi: 10.1016/j.lwt.2007.12.001. [DOI] [Google Scholar]
- 20.Leontowicz H., Leontowicz M., Jesion I., Bielecki W., Poovarodom S., Vearasilp S., Gonzalez-Aguilar G., Robles-Sanchez M., Trakhtenberg S., Gorinstein S. Positive effects of durian fruit at different stages of ripening on the hearts and livers of rats fed diets high in cholesterol. Eur. J. Integr. Med. 2011;3:e169–e181. doi: 10.1016/j.eujim.2011.08.005. [DOI] [Google Scholar]
- 21.Park Y., Cvikrova M., Martincova O., Ham K., Kang S., Park Y., Namiesnik J., Rambola A.D., Jastrzebski Z., Gorinstein S. In vitro antioxidative and binding properties of phenolics in traditional, citrus and exotic fruits. Food Res. Int. 2015;74:37–47. doi: 10.1016/j.foodres.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Poovarodom S., Haruenkit R., Vearasilp S., Ruamsuke P., Leontowicz H., Leontowicz M., Namiesnik J., Trakhtenberg S., Gorinstein S. Nutritional and pharmaceutical applications of bioactive compounds in tropical fruits. In: Poovarodom S., Yingjajaval, editors. International Symposium on Mineral Nutrition of Fruit Crops. 9th ed. Volume 1. International Society for Horticultural Science; Korbeek-Lo, Belgium: 2013. pp. 77–86. [Google Scholar]
- 23.Fu L., Xu B., Gan R., Zhang Y., Xia E., Li H. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 24.Wisutiamonkul A., Ampomah-Dwamena C., Allan A.C., Ketsa S. Carotenoid accumulation and gene expression during durian (Durio zibethinus) fruit growth and ripening. Sci. Hortic. 2017;220:233–242. doi: 10.1016/j.scienta.2017.03.048. [DOI] [PubMed] [Google Scholar]
- 25.Wistutiamonkul A., Promdang S., Ketsa S., Doorn W.G.V. Carotenoids in durian fruit pulp during growth and postharvest ripening. Food Chem. 2015;180:301–305. doi: 10.1016/j.foodchem.2015.01.129. [DOI] [PubMed] [Google Scholar]
- 26.Costa C., Tsatsakis A., Mamoulakis C., Teodoro M., Briguglio G., Caruso E., Tsoukalas D., Margina D., Efthimious D., Kouretas D., et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017;110:286–299. doi: 10.1016/j.fct.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Leifert W.R., Abeywardena M.Y. Grape seed and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5-lipoxygenase activity. Nutr. Res. 2008;28:842–850. doi: 10.1016/j.nutres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Mostofsky E., Johansen M.B., Tjønneland M.A., Chahal H.S., Mittleman M.A., Overvad K. Chocolate intake and risk of clinically apparent atrial fibrillation: The Danish Diet, Cancer, and Health Study. Heart. 2017;103:1163–1167. doi: 10.1136/heartjnl-2016-310357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmit S.L., Rennert H.S., Gruber S.B. Coffee consumption and the risk of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2016;25:634–639. doi: 10.1158/1055-9965.EPI-15-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oba S., Nagata C., Nakamura K., Fujii K., Kawachi T., Takatsuka N., Shimizu H. Consumption of coffee, green tea, oolong tea, black tea, chocolate snacks and the caffeine content in relation to risk of diabetes in Japanese men and women. Br. J. Nutr. 2010;103:453–459. doi: 10.1017/S0007114509991966. [DOI] [PubMed] [Google Scholar]
- 31.United States Department of Agriculture Agricultural Research Service. USDA Food Composition Data. [(accessed on 19 September 2018)]; Available online: https://ndb.nal.usda.gov/ndb/search/list?home=true.
- 32.MyFCD, Malaysian Food Composition Database. [(accessed on 19 September 2018)]; Available online: http://myfcd.moh.gov.my/index.php/1997-food-compositon-database.
- 33.Data Komposisi Pangan Indonesia. [(accessed on 19 September 2018)]; Available online: http://www.panganku.org/id-ID/beranda.
- 34.Merrill A.L., Watt B.K. Energy Value of Foods: Basis and Derivation. United States Government Publishing Office; Washington, WA, USA: 1973. [Google Scholar]
- 35.Wasnin R.M., Karim M.S.A., Ghazali H.M. Effect of temperature-controlled fermentation on physico-chemical properties and lactic acid bacterial count of durian (Durio zibethinus Murr.) pulp. J. Food Sci. Technol. 2014;51:2977–2989. doi: 10.1007/s13197-012-0869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voon Y.Y., Sheikh A.H.N., Rusul G., Osman A., Quek S.Y. Characterisation of Malaysian durian (Durio zibethinus Murr.) cultivars: Relationship of physicochemical and flavour properties with sensory properties. Food Chem. 2007;103:1217–1227. doi: 10.1016/j.foodchem.2006.10.038. [DOI] [Google Scholar]
- 37.Salter A.M., Tarling E.J. Regulation of gene transcription by fatty acids. Animal. 2007:1314–1320. doi: 10.1017/S1751731107000675. [DOI] [PubMed] [Google Scholar]
- 38.Weaver K.L., Ivester P., Seeds M., Case L.D., Arm J.P., Chilton F. Effect of Dietary Fatty Acids on Inflammatory Gene Expression in Healthy Humans. J. Biol. Chem. 2009;284:15400–15407. doi: 10.1074/jbc.M109.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denardin C.C., Hirsch G.E., Rocha R.F.D., Vizzotto M., Henriques A.T., Moreira J.C.F., Guma F.T.C.R., Emanuellli T. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J. Food Drug Anal. 2015;23:387–398. doi: 10.1016/j.jfda.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leontowicz H., Leontowicz M., Haruenkit R., Poovarodom S., Jastrzebski Z., Drzewiecki J., Ayala A.L.M., Jesion I., Trakhtenberg S., Gorinstein S. Durian (Durio zibethinus Murr.) cultivars as nutritional supplementation to rat’s diets. Food Chem. Toxicol. 2008;46:581–589. doi: 10.1016/j.fct.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 41.Leontowicz M., Leontowicz H., Jastrzebski Z., Jesion I., Haruenkit R., Poovarodom S., Katrich E., Tashma Z., Drzewiecki J., Trakhtenberg S., et al. The nutritional and metabolic indices in rats fed cholesterol-containing diets supplemented with durian at different stages of ripening. BioFactors. 2007;29:123–136. doi: 10.1002/biof.552029203. [DOI] [PubMed] [Google Scholar]
- 42.Belgis M., Hanny C., Apriyantono A., Kusbiantoro B., Dewi N. Volatiles and aroma characterization of several lai (Durio kutejensis) and durian (Durio zibethinus) cultivars grown in Indonesia. Sci. Hortic. 2017;220:291–298. doi: 10.1016/j.scienta.2017.03.041. [DOI] [Google Scholar]
- 43.Li J., Schieberle P., Steinhaus M. Insights into the key compounds of durian (Durio zibethinus L. ‘Monthong’) pulp odor by odorant quantitation and aroma simulation experiments. J. Agric. Food Chem. 2017;65:639–647. doi: 10.1021/acs.jafc.6b05299. [DOI] [PubMed] [Google Scholar]
- 44.Weenen H., Koolhaas W.E., Apriyantono A. Sulfur-containing volatiles of durian fruits (Durio zibethinus Murr.) J. Agric. Food Chem. 1996;44:3291–3293. doi: 10.1021/jf960191i. [DOI] [Google Scholar]
- 45.Voon Y.Y., Sheikh A.H.N., Rusul G., Osman A., Quek S.Y. Volatile flavour compounds and sensory properties of minimally processed durian (Durio zibethinus cv. D24) fruit during storage at 4 °C. Postharvest Biol. Technol. 2007;46:76–85. doi: 10.1016/j.postharvbio.2007.04.004. [DOI] [Google Scholar]
- 46.Chin S.T., Nazimah S.A.H., Quek S.Y., Man Y.C., Rahman R.A., Dzulkifly M.H. Changes of volatiles’ attribute in durian pulp during freeze- and spray-drying process. Int. J. Food Sci. Technol. 2008;41:1899–1905. doi: 10.1016/j.lwt.2008.01.014. [DOI] [Google Scholar]
- 47.Xiao Z., Lu J.R. Generation of acetoin and its derivatives in foods. J. Agric. Food Chem. 2014;62:6487–6497. doi: 10.1021/jf5013902. [DOI] [PubMed] [Google Scholar]
- 48.Chang Y., Hou H., Li B. Identification of Volatile Compounds in Codfish (Gadus) by a Combination of Two Extraction Methods Coupled with GC-MS Analysis. J. Ocean Univ. China. 2016;15:509–514. doi: 10.1007/s11802-016-2852-9. [DOI] [Google Scholar]
- 49.Li J., Schieberle P., Steinhaus M. Characterization of the Major Odor-Active Compounds in Thai Durian (Durio zibethinus L. ‘Monthong’) by Aroma Extract Dilution. Analysis and Headspace Gas Chromatography−Olfactometry. J. Agric. Food Chem. 2012;60:11253–11262. doi: 10.1021/jf303881k. [DOI] [PubMed] [Google Scholar]
- 50.The Metabolomics Innovation Centre The Human Metabolome Database. [(accessed on 19 September 2018)]; Available online: http://www.hmdb.ca/
- 51.Robert S.D., Ismail A.A., Winn T., Wolever T.M. Glycemic index of common Malaysian fruits. Asia Pac. Clin. Nutr. 2008;17:35–39. [PubMed] [Google Scholar]
- 52.Maćkowiak K., Torlińska-Walkowiak N., Torlińska B. Dietary fibre as an important constituent of the diet. Postȩpy Hig. Med. Dośw. 2016;70:104–109. doi: 10.5604/17322693.1195842. [DOI] [PubMed] [Google Scholar]
- 53.Hu F.B., Dam R.M.V., Liu S. Diet and risk of Type II diabetes: The role of types of fat and carbohydrate. Diabetologia. 2001;44:805–817. doi: 10.1007/s001250100547. [DOI] [PubMed] [Google Scholar]
- 54.Peng Y., Zhong G., Mi Q., Li K., Wang A., Li L., Liu H. Potassium measurements and risk of type 2 diabetes : A dose-response meta-analysis of prospective cohort studies. Oncotarget. 2017;8:100603–100613. doi: 10.18632/oncotarget.21823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chatterjee R., Slentz C., Davenport C.A., Johnson J., Lin P., Muehlbauer M., D’Alessio D., Svetkey L.P., Edelman D. Effects of potassium supplements on glucose metabolism in African Americans with prediabetes: A pilot trial. Am. J. Clin. Nutr. 2017:1–8. doi: 10.3945/ajcn.117.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lakkis J.I., Weir R.W. Hyperkalemia in the Hypertensive Patient. Curr. Cardiol. Rep. 2018;20:12. doi: 10.1007/s11886-018-0954-2. [DOI] [PubMed] [Google Scholar]
- 57.Demigne B.C., Morand C., Levrat M., Besson C., Moundras C., Remesy C. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br. J. Nutr. 1995;74:209–219. doi: 10.1079/BJN19950124. [DOI] [PubMed] [Google Scholar]
- 58.Gorzynik-Debicka M., Przychodzen P., Cappello F., Kuban-Jankowska A., Gammazza A.M., Knap N., Wozniak M., Gorska-Ponikowska M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018;19:547. doi: 10.3390/ijms19030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clifford M.N. Chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000;80:1033–1043. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1033::AID-JSFA595>3.0.CO;2-T. [DOI] [Google Scholar]
- 60.Borska S., Chmielewska M., Wysocka T., Drag-Zalesinska M., Zabel M., Dziegiel P. In vitro effect of quercetin on human gastric carcinoma: Targeting cancer cells death and MDR. Food Chem. Toxicol. 2012;50:3375–3383. doi: 10.1016/j.fct.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 61.Brown E.M., Gill C.I.R., McDougall G.J., Stewart D. Mechanisms underlying the anti-proliferative effects of berry components in In vitro models of colon cancer. Curr. Pharm. Biotechnol. 2012;13:200–209. doi: 10.2174/138920112798868773. [DOI] [PubMed] [Google Scholar]
- 62.Sergediene E., Jonsson K., Syzmsusiak H., Tyrakowska B., Rietjens I.M.C.M., Cenas N. Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: Description of quantitative structure-activity relationships. FEBS Lett. 1999;462:392–396. doi: 10.1016/S0014-5793(99)01561-6. [DOI] [PubMed] [Google Scholar]
- 63.Singh M., Singh R., Bhui K., Tyagi S., Mahmood Z., Shukla Y. Tea polyphenols induce apoptosis through mitochondrial pathway and by inhibiting nuclear factor-κB and Akt activation in human cervical cancer cells. Oncol. Res. 2011;19:245–257. doi: 10.3727/096504011X13021877989711. [DOI] [PubMed] [Google Scholar]
- 64.Stefanska B., Karlic H., Varga F., Fabianowska-Majeska K., Haslberger A.G. Epigenetic mechanisms in anti-cancer actions of bioactive food components—The implications in cancer prevention. Br. J. Pharmacol. 2012;167:279–297. doi: 10.1111/j.1476-5381.2012.02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jayakumar R., Kanthimathi M.S. Inhibitory effects of fruit extracts on nitric oxide-induced proliferation in MCF-7 cells. Food Chem. 2011;126:956–960. doi: 10.1016/j.foodchem.2010.11.093. [DOI] [Google Scholar]
- 66.Chuah L., Shamila-Syuhada A.K., Liong M.T., Rosma A., Thong K.L., Rusul G. Physio-chemical, microbiological properties of tempoyak and molecular characterisation of lactic acid bacteria isolated from tempoyak. Food Microbiol. 2016;58:95–104. doi: 10.1016/j.fm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Leisner J.J., Vancanneyt M., Rusul G., Pot B., Lefebvre K., Fresi A., Tee L.K. Identification of lactic acid bacteria constituting the predominating microflora in an acid-fermented condiment (tempoyak) popular in Malaysia. Int. J. Food Microbiol. 2001;63:149–157. doi: 10.1016/S0168-1605(00)00476-1. [DOI] [PubMed] [Google Scholar]
- 68.Leisner J.J., Vancanneyt M., Lefebvre K., Vandemeulebroecke K., Hoste B., Euras Vilalta N., Rusul G., Swings J. Lactobacillus durianis sp. nov., isolated from an acid-fermented condiment (tempoyak) in Malaysia. Int. J. Syst. Evol. Microbiol. 2002;52:927–931. doi: 10.1099/ijs.0.02091-0. [DOI] [PubMed] [Google Scholar]
- 69.Khalil E.S., Manap M.Y.A., Mustafa S., Alhelli A.M., Shokryazdan P. Probiotic properties of exopolysaccharide-producing lactobacillus strains isolated from tempoyak. Molecules. 2018;23:398. doi: 10.3390/molecules23020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmad A., Yap W.B., Kofli N.T., Ghazali A.R. Probiotic potentials of Lactobacillus plantarum isolated from fermented durian (Tempoyak), a Malaysian traditional condiment. Food Sci. Nutr. 2018;6:1370–1377. doi: 10.1002/fsn3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korcz E., Kerényi Z., Varga L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food Funct. 2018;9:3057–3068. doi: 10.1039/C8FO00118A. [DOI] [PubMed] [Google Scholar]