Abstract
In contrast to the large number of genetic studies on obesity, there has been significantly less nutrigenetics investigation of the interaction between diet and single nucleotide polymorphisms (SNPs) in obesity, especially within Eastern Mediterranean populations. The aim of this study was to evaluate the potential interactions between three candidate SNPs, namely, rs1558902 and rs9939609 in the fat mass and obesity (FTO) gene and the rs7903146 variant of the Transcription factor 7 like 2 (TCF7L2) gene, and macronutrient intake with regard to obesity, body fat, and muscle composition. Three hundred and eight healthy Lebanese adults were included in this study. Data collection included a questionnaire for demographics and lifestyle in addition to a detailed dietary assessment using a culture-specific 80-item semi-quantitative food frequency questionnaire. This was coupled with anthropometric measurements and peripheral blood withdrawal for DNA and genotyping using Taqman allele discrimination assays. The two FTO candidate SNPs were not associated with risk of obesity in this population sample, yet there was a trend, though not a significant one, towards lower muscle mass among carriers of the risk allele of either FTO SNPs. To our knowledge, these results have not been previously reported. As for the TCF7L2 rs7903146 variant, results were congruent with the literature, given that individuals who were homozygous for the risk allele had significantly higher body mass index (BMI) and body fat despite lower intakes of saturated fat. Similar interactions, though not significant, were shown with muscle mass, whereby individuals who were homozygous for the risk allele had lower muscle mass with higher intakes of saturated fat, a result that, to our knowledge, has not been previously reported.
Keywords: nutrigenetics, FTO, TCF7L2, body mass, body mass composition
1. Introduction
Obesity is increasingly recognized as a public health concern with its prevalence reaching alarming levels in several parts of the world, plaguing both high- and low-income countries and jeopardizing their ability to cope with the increasing cost of treating obesity-associated diseases [1,2,3,4,5,6]. In Lebanon, a small Arab country in the Eastern Mediterranean basin, data stemming from national cross-sectional surveys has indicated a significant increasing trend in the prevalence of obesity across all age groups [7,8]. Increases in adult mean body mass index (BMI) have been estimated at 1.4 kg/m2 for Lebanese women and 1.8 kg/m2 for men over the past decade. This exceeds BMI increases reported from high-income countries such as the USA (1.1–1.2 kg/m2 per decade) [9] and exceeds a recently reported worldwide estimate (0.4–0.5 kg/m2 per decade). These findings carry important public health implications given that the association between body mass index (BMI) and chronic diseases is continuous and entails a dose-response relationship [3,9].
The causes of weight gain and obesity are multifactorial, and efforts to identify these causes are crucial for the development of effective preventive strategies. In addition to high dietary energy intake and low energy expenditure, obesity is influenced by factors such as genetic predisposition. Although hereditability seems substantial, with at least a 40% contribution based on twin and family studies, genetic mechanisms predisposing people to obesity are still not very well understood and are at many times contradictory [10,11]. Recent studies have shown that genetic variants interact with environmental factors such as food intake and hence affect obesity. The evaluation of this interaction is termed nutrigenetics, the aim of which is to better understand an individual phenotype by genetic predisposition. It also applies to the concept of “personalized nutrition”, which aims to prevent the onset and development of chronic diseases by targeting dietary recommendations to an individual’s genetic profile [10,11].
In contrast to the large number of genetic studies on obesity [12] there has been significantly less nutrigenetics research on the interaction between diet and single nucleotide polymorphisms (SNPs) with regard to obesity. The highest evidence by far is for the two rs1558902 and rs9939609 variants in the fat mass and obesity (FTO) associated gene, with the A allele being consistently linked to increasing BMI [13] as well as being more prone to weight loss through diet or lifestyle interventions in comparison to non-carriers [14]. This risk allele has also been shown to modify associations/responses to protein, fat, and exercise for weight-related phenotypes. For example, homozygous AA genotypes have been seen to produce greater reductions in weight, increases in muscle mass and changes in fat distribution in response to a two-year high protein diet, with opposite effects having been observed in those who consumed low protein diets [15]. As for gene interaction with fat intake, AA individuals are also prone to significantly enhanced weight loss in response to consuming a diet that is low in saturated fat and high in polyunsaturated fat [16], and to more weight gain with high fat and carbohydrate diets [17]. Interestingly, participants with the AA genotype have an enhanced weight loss response with higher physical activity when compared to TT [18]. It is important to note that these SNPs are in high-linkage disequilibrium (LD) in Europeans, though it is not known if this is the case for the Eastern Mediterranean population. An additionally interesting fact is the implication of variants in the Transcription factor 7 like 2 (TCF7L2) gene with fat interactions. For example, in a 10-week randomized hypoenergetic diet, TCF7L2 rs7903146 homozygous TT participants were seen to significantly lose 44% more weight in response to a moderate- to low-fat diet [19]. A two-year weight loss intervention has also showed congruent results [20].
To our knowledge, there have been no diet-gene interaction evaluations for the Eastern Mediterranean population except for a couple of studies on dietary interaction with candidate SNPs in Melanocortin 4 Receptor (MC4R) and the Apolipoprotein gene family in Iranian populations [21,22,23]. Therefore, the aims of this nutrigenetics study were to evaluate the potential interaction between three candidate SNPs, rs1558902 and rs9939609 in the FTO gene and the rs7903146 variant of the TCF7L2 gene, and macronutrient intake with regard to obesity, body fat and muscle composition.
2. Materials and Methods
2.1. Study Participants and Data Collection
This cross-sectional study builds on a previously recruited and described community cohort of unrelated Lebanese individuals [24]. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Ethics Committee of the American University of Beirut (Pharmaco.NZ.23).
After signing their informed consent, 501 Lebanese subjects older than 18 years old were recruited from the Greater Beirut area between February and June 2014. A multi-component questionnaire was administered in an interview settin, to obtain information on demographic and socioeconomic characteristics, lifestyle habits, physical activity using the short version of the International Physical Activity Questionnaire (IPAC) [25], and medical history. Fasting blood samples were obtained for glucose, hemoglobin A1C (HbA1C), and lipid profile measurements. Sitting blood pressure was obtained twice at 10-min intervals using a digital sphygmanometer.
For the present study, participants who reported the diagnosis of a chronic disease or metabolic abnormality that could have affected their food consumption habits were excluded. The selection of subjects from the original survey (n = 501) was therefore performed based on the following criteria: Firstly, having complete anthropometric and dietary data, and secondly, having no known diagnosis of hypertension, dyslipidemia, diabetes, or hyperglycemia. The final sample size was 308.
2.2. Anthropometric Measurements
Anthropometric measurements were obtained using standardized protocols [26] and calibrated equipment. Height and weight measurements were collected using a portable stadiometer (Holtain, Crymych, UK) and a Seca calibrated electronic weighing scale (Hamburg, Germany), respectively. The measurements were taken twice and the average of the two values adopted. Body mass index was calculated as the ratio of weight (kilograms) to the square of height (meters). A tetrapolar single frequency (330 µA at 100 kHz) electrical bioimpedance analyzer (Inbody Body Composition Analyzer, Inbody 230, InBody Co., Ltd., Seoul, Korea) was used to measure body composition.
2.3. Dietary Data
Dietary intake was assessed using a culture-specific 80-item semi-quantitative food frequency questionnaire (FFQ) [27]. Participants were asked to specify the frequency of consumption as either per day, per week, per month, per year or never. Participants had the choice to report their intake either in terms of reference portion size or in grams. The reference portions of the two-dimensional food portion visual (Millen and Morgan, Nutrition Consulting Enterprises, Framingham, MA, USA) [28] were used, and a reference portion, representing one standard serving expressed in household measures, was defined for each food item. Common household measures used were measuring cups and spoons, in addition to real portion-size photographs. The reported frequency of each food item and beverage was then converted to a daily portion intake. The daily energy and macronutrient consumption of participants was computed using the food composition database of Nutritionist Pro™ software (Axxya Systems LLC, Stafford, TX, USA) and a food composition table of Middle-Eastern foods for local and traditional foods [29].
2.4. Genotyping
DNA was isolated from 300 µL peripheral whole blood using the Qiagen Flexigene® DNA kit (Qiagen, Hilden, Germany, cat # 51204) and stored at −20 °C until analysis. Genotyping for the three candidate SNPs was performed using Taqman allele discrimination assays on the CFX384 Touch real-time PCR detection system from BioRad (Hercules, CA, USA). The reaction mix contained 2× universal PCR Master Mix No AmpErase UNG (ThermoFisher Scientific, Applied Biosystems, Waltham, MA, USA) and 40× Taqman SNP genotyping assay (ThermoFisher Scientific, Waltham, MA, USA) diluted to 1×, DNA, and Nuclease-free water to make up a total volume of 10 µL/well. The PCR protocol was as described by the manufacturer: 95 °C for 10 min, then 40 cycles of 92 °C for 15 s followed by 60 °C for 1 min. Ten percent of the samples were run in duplicate and showed 100% reproducibility.
2.5. Statistical Analyses
Data were analyzed using Statistical Package for Social Sciences (SPSS) version 24.0 for Windows (Chicago, IL, USA). Since there were three groups of analyses (BMI, body fat, and muscle mass), Bonferroni’s correction was used for the p-value considered in this study and the statistical significance level was set at 0.016 (being the ratio of 0.05 over three). No power analysis was performed because the study aims were multifactorial and included interactions with a number of dietary factors on three outcomes with potential confounders. Moreover, there is little nutrigenetics literature on body fat and muscle mass, in addition to the fact that this study builds on an already available sample. Thus, this work is considered exploratory and hypothesis generating.
Allele frequencies were calculated and tested for Hardy Weinberg Equilibrium (HWE) using the chi square test. A Kappa statistic for agreement was calculated for the two FTO genotypes for assessment of LD. Note that all of the participants were Caucasian Arabs.
Data were described in terms of frequencies and percentages for categorical variables, and mean ± standard deviation (SD) for continuous ones. Means with associated 95% confidence intervals (CI) of continuous variables were also estimated where appropriate. Associations between genotypes and participant characteristics, anthropometric measurements, lifestyle factors, and diet were performed using chi square or one-way analysis of variance (ANOVA) as applicable.
Dietary intakes of macronutrients, expressed as percent energy intakes, were categorized into tertiles based on statistical grounds. A within-tertile trend analysis was performed using general linear models. The results were adjusted for potentially confounding factors, namely, age, sex and physical activity, as applicable. To account for any effect modification of dietary intake on the associations between the genotypes and each of the three outcomes, a multivariate analysis was carried out while including an interaction term of the genotypes and the different dietary intake tertiles. The same approach was carried out for the effect modification of physical activity on the various associations.
3. Results
3.1. Genotype Frequencies
The minor allele frequencies (MAFs) of the three SNPs were: FTO rs1558902 A allele (46%), FTO rs9939609 A allele (45%), and TCF7L2 rs7903146 T allele (35%). These were all in HWE and the frequencies were similar to those for reported MAFs [30]. As expected, the two FTO SNPs were in Linkage Disequilibrium (LD) (Kappa (95% CI) = 0.83(0.78–0.89)), and therefore, data for FTO rs9939609 are shown in supplementary tables only.
3.2. Associations of Baseline Characteristics, Lifestyle, and Dietary Habits with Genotypes
Table 1 shows the characteristics, lifestyle, and dietary habits of the study sample and the association of these characteristics with FTO rs1558902 and TCF7L2 rs7903146. The majority of the included participants were female (62.7%); they were relatively young with a mean ± SD age of 39.79 ± 14.00 years. The majority performed some type of exercise (85.7%). There was a relatively high proportion of current smokers (43.8% for cigarette and 32.8% for narghileh), and approximately one fifth of the study population currently consumed alcohol (21.4%).
Table 1.
Associations of baseline characteristics, lifestyle, and dietary habits for those with FTO (rs9939609) and TCF7L2 (rs7903146) genotypes.
| FTO (rs1558902) | TCF7L2 (rs7903146) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Among All | TT | AT | AA | p | CC | CT | TT | p | |
| N = 308 | N = 85 | N = 165 | N = 58 | N = 134 | N = 130 | N = 43 | |||
| Characteristics and lifestyle factors | |||||||||
| Gender, Female | 193 (62.7) | 50 (58.8) | 103 (62.4) | 40 (69.0) | 0.47 | 86 (35.8) | 82 (63.1) | 25 (58.1) | 0.77 |
| Age (years) | 39.79 ± 14.00 | 38.46 ± 13.59 | 41.85 ± 14.23 | 35.89 ± 13.04 | 0.011 | 38.80 ± 13.66 | 40.61 ± 14.39 | 40.70 ± 14.02 | 0.53 |
| Crowding index | 1.55 ± 0.85 | 1.55 ± 0.89 | 1.56 ± 0.87 | 1.49 ± 0.74 | 0.86 | 1.54 ± 0.82 | 1.57 ± 0.83 | 1.52 ± 1.04 | 0.93 |
| Levels of physical activity | |||||||||
| Low | 137 (44.5) | 35 (41.2) | 72 (43.6) | 30 (51.7) | 0.71 | 51 (38.1) | 68 (52.3) | 18 (41.9) | 0.20 |
| Moderate | 99 (32.1) | 27 (31.8) | 55 (33.3) | 17 (29.3) | 50 (37.3) | 34 (26.2) | 14 (32.6) | ||
| High | 72 (23.4) | 23 (27.1) | 38 (23.0) | 11 (19.0) | 33 (24.6) | 28 (21.5) | 11 (25.6) | ||
| Physical activity | |||||||||
| None | 44 (14.3) | 14 (16.5) | 20 (12.1) | 10 (17.2) | 0.50 | 17 (12.7) | 18 (13.9) | 9 (20.9) | 0.40 |
| Any | 264 (85.7) | 71 (83.5) | 145 (87.9) | 48 (82.8) | 117 (87.3) | 112 (86.2) | 34 (79.1) | ||
| Cigarette smoker | |||||||||
| Never | 148 (48.1) | 43 (50.6) | 73 (44.2) | 32 (55.2) | 0.23 | 65 (48.5) | 59 (45.4) | 24 (55.8) | 0.71 |
| Current | 135 (43.8) | 32 (37.7) | 79 (47.9) | 24 (41.4) | 60 (44.8) | 58 (44.6) | 16 (37.2) | ||
| Past | 25 (8.1) | 10 (11.8) | 13 (7.9) | 2 (3.5) | 9 (6.7) | 13 (10.0) | 3 (7.0) | ||
| Narghileh smoker | |||||||||
| Never | 177 (57.5) | 43 (50.6) | 103 (62.4) | 31 (53.5) | 0.28 | 81 (60.5) | 75 (57.7) | 20 (46.5) | 0.62 |
| Current | 101 (32.8) | 32 (37.7) | 46 (27.9) | 23 (39.7) | 41 (30.6) | 42 (32.3) | 18 (41.9) | ||
| Past | 30 (9.7) | 10 (11.8) | 16 (9.7) | 4 (6.9) | 12 (9.0) | 13 (10.0) | 5 (11.6) | ||
| Alcohol drinker | |||||||||
| Never | 223 (72.4) | 60 (70.6) | 119 (72.1) | 44 (75.9) | 0.38 | 100 (74.6) | 99 (76.2) | 24 (55.8) | 0.08 |
| Current | 66 (21.4) | 20 (23.5) | 38 (23.0) | 8 (13.8) | 28 (20.9) | 22 (16.9) | 15 (34.8) | ||
| Past | 19 (6.2) | 5 (5.9) | 8 (4.9) | 6 (10.3) | 6 (4.5) | 9 (6.9) | 4 (9.3) | ||
| Body mass and composition | |||||||||
| Body mass index (BMI) (kg/m²) | 27.78 ± 5.62 | 28.49 ± 5.70 | 27.35 ± 5.44 | 27.96 ± 6.00 | 0.31 | 27.57 ± 5.65 | 27.79 ± 5.44 | 28.37 ± 6.20 | 0.72 |
| Body fat (kg) | 26.24 ± 11.42 | 27.48 ± 11.72 | 25.30 ± 11.09 | 27.12 ± 11.84 | 0.29 | 26.27 ± 11.73 | 26.15 ± 10.91 | 26.32 ± 12.30 | 0.99 |
| Muscle mass (kg) | 26.43 ± 6.37 | 27.41 ± 6.71 | 26.35 ± 6.19 | 25.21 ± 6.25 | 0.13 | 26.32 ± 6.14 | 26.17 ± 6.33 | 27.25 ± 7.09 | 0.62 |
| Energy and macronutrient intake | |||||||||
| Total energy (Kcal/day) | 3600.55 ± 2029.45 | 3737.20 ± 1973.73 | 3491.42 ± 2048.33 | 3710.71 ± 2072.60 | 0.60 | 3728.28 ± 2359.00 | 3467.58 ± 1782.08 | 3632.36 ± 1608.44 | 0.58 |
| Carbohydrates (g/day) | 441.61 ± 254.55 | 475.47 ± 271.82 | 427.01 ± 253.47 | 437.90 ± 230.63 | 0.41 | 468.61 ± 317.18 | 416.61 ± 195.45 | 435.43 ± 182.07 | 0.25 |
| Percent Kcal from carbohydrates (%) | 50.07 ± 8.54 | 51.24 ± 8.85 | 49.89 ± 7.79 | 48.85 ± 9.95 | 0.24 | 50.98 ± 8.62 | 49.27 ± 8.40 | 49.47 ± 8.63 | 0.24 |
| Proteins (g/day) | 116.26 ± 77.07 | 113.44 ± 61.23 | 113.44 ± 68.02 | 129.24 ± 113.76 | 0.36 | 115.59 ± 75.04 | 117.14 ± 84.40 | 116.65 ± 60.46 | 0.99 |
| Percent Kcal from proteins (%) | 12.95 ± 3.66 | 12.32 ± 2.72 | 13.15 ± 3.44 | 13.32 ± 5.13 | 0.16 | 12.61 ± 2.85 | 13.41 ± 4.58 | 12.70 ± 2.58 | 0.18 |
| Sugar (g/day) | 132.55 ± 117.60 | 146.90 ± 173.91 | 125.58 ± 91.12 | 131.37 ± 74.63 | 0.40 | 147.88 ± 156.56 | 120.28 ± 73.09 | 123.54 ± 75.09 | 0.14 |
| Percent Kcal from sugar (%) | 14.79 ± 6.47 | 14.82 ± 7.30 | 14.62 ± 5.88 | 15.22 ± 6.90 | 0.83 | 15.59 ± 7.08 | 14.37 ± 6.03 | 13.70 ± 5.65 | 0.15 |
| Total fat (g/day) | 150.12 ± 90.40 | 147.92 ± 76.68 | 147.09 ± 91.08 | 162.00 ± 106.31 | 0.54 | 151.61 ± 97.79 | 148.71 ± 86.89 | 151.30 ± 78.34 | 0.96 |
| Percent Kcal from total fat (%) | 39.00 ± 7.89 | 37.65 ± 7.52 | 39.30 ± 7.43 | 40.14 ± 9.43 | 0.14 | 38.39 ± 7.90 | 39.76 ± 7.61 | 38.75 ± 8.72 | 0.36 |
| Saturated fat (g/day) | 42.97 ± 29.24 | 42.62 ± 24.57 | 42.07 ± 30.95 | 46.04 ± 30.78 | 0.67 | 44.06 ± 33.05 | 42.34 ± 26.84 | 42.03 ± 23.65 | 0.87 |
| Percent Kcal from saturated fat (%) | 10.38 ± 2.76 | 10.17 ± 2.66 | 10.39 ± 2.80 | 10.65 ± 2.84 | 0.60 | 10.22 ± 2.65 | 10.65 ± 2.98 | 10.11 ± 2.40 | 0.34 |
| Monounsaturated fat (MUFA) (g/day) | 55.33 ± 34.48 | 54.70 ± 29.58 | 54.35 ± 34.55 | 59.04 ± 40.76 | 0.66 | 55.43 ± 35.99 | 54.73 ± 33.52 | 57.29 ± 33.46 | 0.92 |
| Percent kcal from MUFA (%) | 13.82 ± 4.03 | 13.37 ± 3.78 | 13.98 ± 4.06 | 14.02 ± 4.32 | 0.49 | 13.47 ± 3.85 | 14.07 ± 3.81 | 14.17 ± 5.14 | 0.40 |
| Polyunsaturated fat (PUFA) (g/day) | 39.36 ± 25.06 | 38.07 ± 20.26 | 38.55 ± 24.34 | 43.52 ± 32.42 | 0.37 | 39.42 ± 24.49 | 39.36 ± 25.64 | 39.55 ± 25.80 | 1.00 |
| Percent kcal from PUFA (%) | 10.00 ± 3.76 | 9.40 ± 3.17 | 10.13 ± 3.78 | 10.54 ± 4.41 | 0.17 | 9.84 ± 3.60 | 10.18 ± 3.64 | 10.00 ± 4.63 | 0.77 |
| Cholesterol (mg/day) | 382.03 ± 373.27 | 398.50 ± 460.86 | 358.75 ± 295.23 | 424.11 ± 427.62 | 0.46 | 388.62 ± 403.55 | 382.10 ± 365.80 | 352.42 ± 295.41 | 0.86 |
Data are presented as mean ± standard deviation of the mean (SD) or N (%). p values: chi-square, t-test or one-way analysis of variance (ANOVA) as appropriate; p-values that are statistically significant are in bold.
Mean daily dietary energy intake was estimated at 3600.55 ± 2029.45 Kcal/day with carbohydrates making up the highest contribution (50.07%), followed by fat (39.00%), and protein (12.95%). Sugar was estimated to contribute 14.8% of energy intake, while the percent contributions of the various types of fat were as follows: 10.38% for saturated fat, 13.8% for monounsaturated fat (MUFA), and 10% for polyunsaturated fat (PUFA).
None of these factors differed between the genotypes except for age, which differed in terms of the FTO genotypes only (Table 1 and Table S1).
3.3. Associations of BMI and Body Fat and Muscle with Genotypes
As shown in Table 1, the average BMI for the study population was estimated at 27.78 ± 5.62 kg/m2. There was a high prevalence of being overweight or obese in the study sample, with 66.2% being overweight (BMI > 25 kg/m2) and 31.2% being obese (>30 kg/m2). Mean body fat was estimated at 26.24 ± 11.42 kg and muscle mass at 26.43 ± 6.37 kg. No significant differences were noted among the different genotypes (Table 1 and Table S1).
3.4. Interactions between Diet and Genotypes with BMI, Body Fat, and Muscle Mass
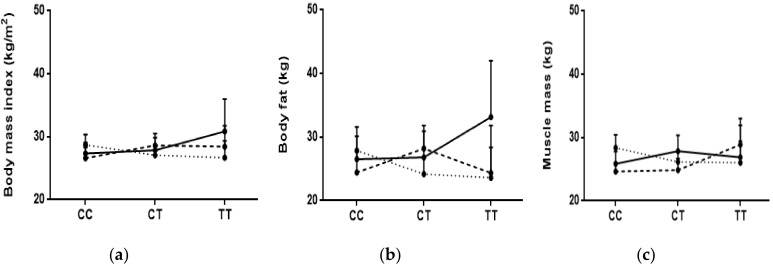
For BMI, and as shown in Table 2 and Table 3 and Table S2, there were trends with the TCF7L2 SNP whereby subjects who were homozygous for the risk allele (TT) and categorized in the second tertile of % of energy fat intake were significantly heavier (mean (95%CI): 31.03 (26.64–35.43)) even after adjustment for age, sex, and physical activity. More importantly, there was a significant interaction between % of energy saturated fat and the TCF7L2 genotypes, in which it appears that those homozygous for the risk allele had a higher BMI despite lower intakes of saturated fat (tertiles 1 and 2) (Table 3 and Figure 1a). This significance appeared after adjustment for age, sex, and physical activity. No significant trends or interactions were revealed with both FTO SNPs.
Table 2.
Interaction between daily dietary intake and FTO (rs1558902) genotypes with BMI (kg/m2).
| No. of Subjects by Genotype (TT/AT/AA) |
TT
Mean (95% CI) (N = 85) |
AT
Mean (95% CI) (N = 165) |
AA
Mean (95% CI) (N = 58) |
p-Trend 1 | p-Trend 2 | p-Trend 3 | p-Interaction 1 | p-Interaction 2 | p-Interaction 3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Among ALL | 85/165/58 | 28.49 (27.26–29.72) | 27.35 (26.52–28.19) | 27.96 (26.38–29.54) | 0.58 | 0.68 | 0.53 | - | - | - |
| Carbohydrates (% of energy) | ||||||||||
| Tertile 1 (<47.317) | 25/53/26 | 29.05 (26.88–31.22) | 27.63 (26.24–29.02) | 28.49 (25.93–31.04) | 0.71 | 0.43 | 0.44 | 0.58 | 0.76 | 0.73 |
| Tertile 2 (47.317–53.984) | 29/64/15 | 27.33 (25.30–29.35) | 27.03 (25.63–28.43) | 27.25 (24.04–30.45) | 0.96 | 0.70 | 0.76 | |||
| Tertile 3 (>53.984) | 31/48/17 | 29.12 (26.78–31.45) | 27.47 (25.81–29.13) | 27.78 (24.71–30.84) | 0.46 | 0.59 | 0.43 | |||
| Fat (% of energy) | ||||||||||
| Tertile 1 (<35.131) | 31/45/16 | 28.74 (26.31–31.17) | 26.92 (25.13–28.71) | 29.27 (25.79–32.76) | 0.79 | 0.72 | 0.94 | 0.86 | 0.98 | 0.81 |
| Tertile 2 (35.131–41.333) | 27/67/17 | 27.59 (25.60–29.57) | 28.08 (26.78–29.38) | 24.87 (23.07–26.67) | 0.08 | 0.24 | 0.21 | |||
| Tertile 3 (>41.333) | 27/53/25 | 29.09 (26.99–31.18) | 26.80 (25.39–28.21) | 29.21 (26.57–31.86) | 0.93 | 0.98 | 0.98 | |||
| Protein (% of energy) | ||||||||||
| Tertile 1 (<11.700) | 39/57/25 | 29.34 (27.93–30.75) | 27.10 (25.82–28.39) | 27.12 (24.53–29.70) | 0.09 | 0.10 | 0.06 | 0.55 | 0.44 | 0.41 |
| Tertile 2 (11.700–13.985) | 27/54/15 | 26.10 (23.98–28.29) | 26.78 (25.26–28.29) | 29.21 (26.19–32.23) | 0.08 | 0.06 | 0.06 | |||
| Tertile 3 (>13.985) | 19/54/18 | 30.13 (26.47–33.78) | 28.19 (26.59–29.80) | 28.08 (24.99–31.17) | 0.33 | 0.22 | 0.16 | |||
| Saturated fat (% of energy) | ||||||||||
| Tertile 1 (<8.595) | 26/42/17 | 28.11 (26.31–29.92) | 27.73 (25.80–29.65) | 28.65 (25.13–32.17) | 0.77 | 0.47 | 0.52 | 0.35 | 0.29 | 0.33 |
| Tertile 2 (8.595–11.034) | 28/65/11 | 28.18 (25.70–30.65) | 27.36 (25.95–28.78) | 28.46 (24.10–32.82) | 0.90 | 0.98 | 0.83 | |||
| Tertile 3 (>11.034) | 31/58/30 | 29.07 (26.84–31.31) | 27.07 (25.87–28.27) | 27.38 (25.34–29.42) | 0.21 | 0.20 | 0.19 | |||
| MUFA (% of energy) | ||||||||||
| Tertile 1 (<11.890) | 29/44/19 | 29.12 (26.60–31.65) | 26.54 (25.02–28.07) | 29.05 (26.16–31.94) | 0.97 | 0.72 | 0.89 | 0.89 | 0.95 | 0.92 |
| Tertile 2 (11.890–14.571) | 27/65/18 | 27.87 (25.82–29.92) | 28.05 (26.72–29.37) | 26.36 (23.40–29.33) | 0.36 | 0.54 | 0.63 | |||
| Tertile 3 (>14.571) | 29/56/21 | 28.42 (26.42–30.42) | 27.18 (25.62–28.75) | 28.33 (25.59–31.08) | 0.96 | 0.83 | 0.73 | |||
| PUFA (% of energy) | ||||||||||
| Tertile 1 (<8.426) | 36/51/21 | 28.81 (26.59–31.04) | 27.32 (25.46–29.19) | 27.62 (24.71–30.53) | 0.51 | 0.84 | 0.66 | 0.34 | 0.49 | 0.41 |
| Tertile 2 (8.426–10.930) | 26/66/16 | 28.45 (26.25–30.66) | 27.16 (26.01–28.01) | 27.30 (24.88–29.72) | 0.46 | 0.30 | 0.13 | |||
| Tertile 3 (>10.930) | 23/48/21 | 28.01 (26.03–29.98) | 27.64 (26.16–29.12) | 28.80 (25.73–31.86) | 0.63 | 0.64 | 0.66 | |||
1 p-value crude; 2 p-value adjusted for age and sex; 3 p-value adjusted for age, sex, and physical activity.
Table 3.
Interaction between daily dietary intake and TCF7L2 (rs7903146) genotypes with BMI (kg/m2).
| No. of Subjects by Genotype (CC/CT/TT) | CC Mean (95% CI) (N = 134) | CT Mean (95% CI) (N = 130) | TT Mean (95% CI) (N = 43) | p-Trend 1 | p-Trend 2 | p-Trend 3 | p-Interaction 1 | p-Interaction 2 | p-Interaction 3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Among ALL | 134/130/43 | 27.57 (26.61–28.54) | 27.79 (26.84–28.73) | 28.37 (26.46–30.28) | 0.42 | 0.47 | 0.48 | - | - | - |
| Carbohydrates (% of energy) | ||||||||||
| Tertile 1 (<47.317) | 43/46/15 | 28.96 (27.29–30.63) | 27.95 (26.44–29.46) | 26.69 (23.18–30.20) | 0.17 | 0.20 | 0.20 | 0.10 | 0.11 | 0.17 |
| Tertile 2 (47.317–53.984) | 44/49/15 | 26.07 (24.24–27.89) | 27.62 (26.13–29.10) | 28.74 (26.11–31.38) | 0.11 | 0.15 | 0.12 | |||
| Tertile 3 (>53.984) | 47/35/13 | 27.71 (26.16–29.27) | 27.82 (25.64–30.00) | 29.87 (25.39–34.36) | 0.25 | 0.30 | 0.33 | |||
| Fat (% of energy) | ||||||||||
| Tertile 1 (<35.131) | 47/28/16 | 27.69 (25.80–29.58) | 28.58 (25.80–31.35) | 27.53 (25.10–29.96) | 0.93 | 0.87 | 0.87 | 0.24 | 0.35 | 0.33 |
| Tertile 2 (35.131–41.333) | 46/51/14 | 26.10 (24.85–27.35) | 27.72 (26.45–29.00) | 31.03 (26.64–35.42) | 0.001 | 0.003 | 0.002 | |||
| Tertile 3 (>41.333) | 41/51/13 | 29.09 (27.26–30.93) | 27.42 (25.94–28.90) | 26.53 (23.06–30.01) | 0.15 | 0.21 | 0.19 | |||
| Protein (% of energy) | ||||||||||
| Tertile 1 (<11.700) | 53/50/18 | 28.01 (26.53–29.49) | 27.64 (26.26–29.02) | 27.81 (25.26–30.35) | 0.88 | 0.92 | 0.86 | 0.54 | 0.61 | 0.56 |
| Tertile 2 (11.700–13.985) | 45/36/14 | 26.54 (24.87–28.21) | 26.76 (25.17–28.36) | 28.70 (24.48–32.93) | 0.21 | 0.31 | 0.30 | |||
| Tertile 3 (>13.985) | 36/44/11 | 28.21 (26.14–30.28) | 28.80 (26.83–30.77) | 28.86 (24.30–33.43) | 0.77 | 0.66 | 0.63 | |||
| Saturated fat (% of energy) | ||||||||||
| Tertile 1 (<8.595) | 40/33/11 | 27.36 (25.63–29.09) | 27.87 (25.90–29.85) | 30.86 (25.74–35.99) | 0.08 | 0.08 | 0.09 | 0.020 | 0.015 | 0.016 |
| Tertile 2 (8.595–11.034) | 47/42/15 | 26.62 (24.92–28.32) | 28.64 (26.76–30.51) | 28.44 (25.16–31.73) | 0.30 | 0.17 | 0.16 | |||
| Tertile 3 (>11.034) | 47/55/17 | 28.71 (27.05–30.36) | 27.09 (25.77–28.41) | 26.69 (24.05–29.33) | 0.18 | 0.16 | 0.16 | |||
| MUFA (% of energy) | ||||||||||
| Tertile 1 (<11.890) | 43/37/12 | 27.70 (26.04–29.37) | 28.17 (25.93–30.40) | 27.58 (24.50–30.66) | 0.95 | 0.99 | 0.91 | 0.82 | 0.94 | 0.99 |
| Tertile 2 (11.890–14.571) | 54/39/16 | 27.26 (25.64–28.88) | 27.53 (26.13–28.93) | 29.72 (26.56–32.89) | 0.11 | 0.22 | 0.12 | |||
| Tertile 3 (>14.571) | 37/54/15 | 27.88 (25.98–29.77) | 27.72 (26.27–29.16) | 27.55 (23.42–31.69) | 0.85 | 0.95 | 0.99 | |||
| PUFA (% of energy) | ||||||||||
| Tertile 1 (<8.426) | 49/41/18 | 27.84 (25.94–29.74) | 28.10 (26.08–30.11) | 27.49 (23.97–31.00) | 0.85 | 0.89 | 0.82 | 0.79 | 0.68 | 0.72 |
| Tertile 2 (8.426–10.930) | 49/43/15 | 26.38 (25.12–27.64) | 28.06 (26.46–29.66) | 29.42 (26.74–32.11) | 0.033 | 0.037 | 0.034 | |||
| Tertile 3 (>10.930) | 36/46/10 | 28.83 (26.93–30.74) | 27.26 (25.83–28.69) | 28.37 (23.58–33.17) | 0.81 | 0.69 | 0.69 |
1 p-value crude; 2 p-value adjusted for age and sex; 3 p-value adjusted for age, sex, and physical activity; p-values that are statistically significant are in bold.
Figure 1.
Interactions between tertiles of daily % energy saturated fat intake and TCF7L2 (rs7903146) genotypes with (a) BMI (kg/m2), (b) body fat (kg) and (c) muscle mass (kg). Dots represent mean values and bars represent the upper 95% confidence interval: ____ <8.595 %; ---- 8.595–11.034%; …… >11.034%.
Concerning body fat, and as shown in Tables S3–S5, there was also a trend, though non-significant, with the TCF7L2 SNP, whereby subjects who were homozygous for the risk allele (TT) and categorized in the second tertile of % of energy fat intake were heavier (mean (95% CI): 31.34 (22.69–39.98)) (p-values between 0.05 and 0.016 after adjustment). More importantly, there was, again and similarly to BMI, a significant interaction between % of energy saturated fat and the TCF7L2 genotypes whereby those homozygous for the risk allele had higher body fat despite lower intakes of saturated fat (tertile 1) (Table S4 and Figure 1b). This significance appeared after adjustment for age, sex, and physical activity. No significant trends or interactions were revealed with both FTO SNPs.
With regard to muscle mass (Tables S6–S8), there was a non-significant trend (p = 0.04) with the FTO rs1558902 SNP, whereby participants who were homozygous for the risk allele (AA) had lower muscle mass when compared to those who were heterozygous or homozygous for the non-risk allele. This was true for the whole cohort and after categorization of dietary intake into tertiles (p-values between 0.05 and 0.016) (Table S6). Similar results appeared with FTO rs9939609 (Table S8). There was also a trend with the TCF7L2 SNP, though not significant, whereby subjects who were homozygous for the risk allele (TT) and categorized in the second tertile of % of energy polyunsaturated fat intake had higher muscle mass (mean (95%CI): 30.77 (26.34–35.20)) after adjustment (p-values between 0.05 and 0.016) (Table S7). Interestingly, there was an interaction, though not significant, between % of energy saturated fat and the TCF7L2 genotypes, whereby individuals who were homozygous for the risk allele had lower muscle mass with high intakes of saturated fat (tertile 3) (Table S7 and Figure 1c). No significant interactions were revealed with both FTO SNPs.
Of note is that no significant interactions were revealed between physical activity and the three SNPs concerning either BMI or body composition (Table S9).
4. Discussion
This is the first nutrigenetics study performed on a Lebanese population. We have shown that although the two FTO candidate SNPs are not associated with risk of obesity in this population sample, there was a trend, though not significant, towards lower muscle mass among carriers of the risk allele of both FTO SNPs. To our knowledge, these results have not been reported previously. As for the TCF7L2 rs7903146 variant, we have shown results that are congruent with the literature [19,20] whereby individuals who were homozygous for the risk allele had significantly higher BMI and body fat despite lower intakes of saturated fat. Similar interactions, though not significant, were shown with muscle mass whereby those homozygous for the risk allele had lower muscle mass with high intakes of saturated fat, a result that to our knowledge, has not been previously reported. Note that none of the three SNPs were associated with differences in macronutrient intake, a finding that was established in a recent genome-wide meta-analysis [31].
In addition to MC4R, loci in the FTO gene have been consistently linked with the risk of obesity [32,33,34,35,36,37] with FTO being highly expressed in the hypothalamus and playing a role in the ghrelin, leptin, and melanocortin pathways [38,39,40]. The most compelling data has been, however, mainly compiled from Europeans and individuals of European descent [32,33,34,35,36]. Interestingly, in a meta-analysis of genome-wide association studies of those from diverse ancestries (five Caucasians, one Chinese, one African-American, and one Hispanic), it was shown that the significant FTO SNPs that were revealed in Caucasians had no or weak evidence of association in non-Caucasian groups [41]. These findings and the interesting result of this study concerning a potentially lower muscle mass among the FTO risk allele carriers reinforce the potential for uniqueness in differences in genetic variations in various populations and their differential role in obesity risk, although the current study may be insufficiently able to detect a significant association between FTO SNPs and body mass and composition. As for the potential interaction between FTO SNPs and diet with regard to body mass and composition, a recent systematic review and meta-analysis of 14 studies with various intervention types and durations has shown that individuals who carry the FTO T risk allele may benefit more from diet and lifestyle interventions when compared to non-carriers. Nevertheless, it is important to note that the benefit is relatively small (0.18 to 0.20 kg additional weight loss). In addition, a meta-analysis of only randomized controlled trials (n = 8, some of which were included in the aforementioned analysis) has shown that the FTO risk allele was not associated with any differential change in adiposity after dietary weight loss interventions [42]. It is possible that the lack of interactions with the two FTO variants in the current study is due to the study design being cross-sectional with a relatively small sample size when compared to, for example, Sonestedt and colleagues’ [17] evaluation of close to 5000 subjects. An additional compelling explanation could be interethnic differences as recently suggested by Merritt et al. [43] who showed that protein intake modifies the effect of the FTO rs1558902 risk allele on body weight in East Asians but not in Caucasians or South Asians.
The TCF7L2 gene, which is known to play an important role in the transcription of the proglucagon gene and hence the synthesis of glucagon-like-peptide 1 (GLP-1), which is implicated in insulin regulation, appetite and food intake [44,45], has been studied much less in the context of obesity and more in diabetes and metabolic syndrome [46,47]. To our knowledge, only a few studies have evaluated the potential of macronutrient-TCF7L2 gene interaction with regard to obesity and weight loss [19,20,48,49]. Two of these have shown that individuals who are homozygous for the TCF7L2 rs7903146 T-risk allele are more sensitive to low fat weight-loss diets [19,20] and one has revealed that T risk allele carriers are more prone to weight loss when they consume a Mediterranean diet that is rich in sources of unsaturated fat [49]. Interestingly, no significant differences in baseline measures of body mass and composition by genotype groups were observed. Results of the current study, though cross sectional by design, concur with these findings and suggest that the T-allele confers a higher risk for obesity and adiposity in spite of lower saturated fat intakes. Of note is that it is unclear to us why only those who were categorized in the second tertile of percent energy from total fat and were homozygous for the risk allele (TT) had significantly higher BMI values. This is a finding that necessitates further investigation and may have appeared in the two other tertiles with a larger sample size. More importantly, this study is the first to show a potential link between lower muscle mass in individuals who are homozygous for the T-risk allele and who consume higher levels of saturated fat. It is unclear to us why subjects who were homozygous for the risk allele (TT) and were categorized in the second tertile of percent energy from polyunsaturated fat had significantly higher muscle mass. Increasing evidence, however, points towards differential effects of various types of fatty acids on metabolic, regulatory, and biological processes, with the concept of fatty acid sensing gaining more recognition [50]. It has in fact been suggested that the type of dietary fat may be an important determinant of body composition and lean body mass [51]. One supplementation trial conducted among postmenopausal women has shown that PUFAs (safflower oil) increase lean tissue and reduced trunk fat [52]. Similarly, a randomized trial conducted among normal weight adults has shown that, in comparison with saturated fat, PUFAs (sunflower oil) promote lean tissue accretion, with the increase in lean body mass being nearly three times greater in comparison with saturated fat feeding [51]. In our study, the intake of saturated fat was estimated at around 10.4% of energy intake, with 56% of the study subjects exceeding the upper limit specified by the World Health Organization (WHO) (i.e., 10% of energy intake) [53]. This high intake of saturated fat may be of concern, particularly in light of its potential interaction with the T-risk allele. The consumption of saturated fat has been on the rise in Lebanon as the nutrition transition unfolds [8]. The traditional Lebanese diet, which is rich in fruits, vegetables, pulses, and olive oil, and which is described as a variant of the Mediterranean diet [54], is in fact gradually eroding and shifting towards a Westernized dietary pattern which is characterized by high intakes of animal products and fast food, with significantly higher levels of saturated fat [27].
This study has several strengths. It was conducted on a random sample of the adult population living in Greater Beirut and followed a well-planned design, protocol, and methodology. Rather than estimating total fat alone, the dietary assessment performed in our study allowed us to differentiate between the intakes of various types of fatty acids and to contribute to the growing body of evidence on the potential differential effects of these fatty acids. In addition, we did not rely on BMI as the sole indicator of adiposity but rather performed a comprehensive body composition assessment where both components of fat mass and muscle mass were evaluated.
The results of this study should, however, be considered in light of the following limitations, on top of the relatively small sample size. First, the dietary assessment performed in this study was based on an FFQ, which may be limited by measurement errors, reliance on memory, and the number of food items included in the food list. FFQs may also overestimate energy intake, which, coupled with the high prevalence of being overweight and obesity in our sample, may explain the observed high energy intake in the study population [55]. Despite its potential limitations, the FFQ method has been reported as one of the most suitable dietary assessment tools in large epidemiological studies since it provides information on a subject’s habitual diet over long periods of time [56]. It is worth noting that although the FFQ used in the present study was not validated in the study population, it was previously adopted for the assessment of dietary intakes and their association with obesity and the metabolic syndrome in Lebanese adults, yielding plausible findings [27,57,58,59]. In addition, the FFQ was administered by trained nutritionists rather than being self-administered. The self-administration approach has several disadvantages, as self-administration of the FFQ necessitates a literate population, and could result in inconsistent interpretations of the food list and be associated with lower response and completion rates, all of which may jeopardize the validity of the data [56]. However, as is generally observed in most questionnaire-based surveys, the interview approach may engender a social desirability bias, whereby participating subjects may answer in a manner that they perceive as acceptable or favorable to the interviewer [60]. In the present study, the field workers who performed data collection received thorough training to decrease judgmental verbal and non-verbal communication in order to reduce social desirability bias.
5. Conclusions
In conclusion, the current results add to the growing literature on gene-nutrient interaction with obesity [10,11]. Although variants in the FTO gene do not appear to interact with various macronutrients with regard to body mass and composition, altering dietary fat composition with respect to the TCF7L2 rs7903146 variant may differentially affect body parameters. These results should, however, be confirmed with a larger population sample and preferably a prospective interventional cohort design should they be translated into a personalized nutrition practice.
Acknowledgments
The authors thank Steven McNamara for proofreading the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4426/9/1/11/s1. Table S1: Associations of baseline characteristics, lifestyle and dietary habits with FTO (rs9939609), Table S2: Interaction between daily dietary intake and FTO (rs9939609) genotypes on body mass index (BMI) (kg/m2), Table S3: Interaction between daily dietary intake and FTO (rs1558902) genotypes on body fat (kg), Table S4: Interaction between daily dietary intake and TCF7L2 (rs7903146) genotypes on body fat (kg), Table S5: Interaction between daily dietary intake and FTO (rs9939609) genotypes on body fat (kg), Table S6: Interaction between daily dietary intake and FTO (rs1558902) genotypes on muscle mass (kg), Table S7: Interaction between daily dietary intake and TCF7L2 (rs7903146) genotypes on muscle mass (kg), Table S8: Interaction between daily dietary intake and FTO (rs9939609) genotypes on muscle mass (kg), Table S9: Interaction between physical activity and the three SNPs on body mass index (BMI) (kg/m2), body fat (kg), and muscle mass (kg).
Author Contributions
Conceptualization, L.N., H.T., and N.K.Z.; Data curation, A.M. and H.T.; Formal analysis, R.A., A.M., and H.T.; Funding acquisition, L.N., H.T., and N.K.Z.; Investigation, R.A. and N.K.Z.; Methodology, L.N., H.T., and N.K.Z.; Project administration, L.N., H.T., and N.K.Z.; Resources, L.N., H.T., and N.K.Z.; Supervision, L.N., H.T., and N.K.Z.; Visualization, R.A. and A.M.; Writing—original draft preparation, L.N. and N.K.Z.; Writing—review and editing, L.N., R.A., A.M., H.T., and N.K.Z.
Funding
This research was funded by the American University of Beirut Faculty of Medicine Medical Practice Plan & Department of Nutrition & Food Sciences of the American University of Beirut Faculty of Agriculture and Food Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hossain P., Kawar B., El Nahas M. Obesity and diabetes in the developing world—A growing challenge. N. Engl. J. Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 2.Ni M.C., Rodgers A., Pan W.H., Gu D.F., Woodward M. Body mass index and cardiovascular disease in the Asia-Pacific Region: An overview of 33 cohorts involving 310000 participants. Int. J. Epidemiol. 2004;33:751–758. doi: 10.1093/ije/dyh163. [DOI] [PubMed] [Google Scholar]
- 3.Whitlock G., Lewington S., Sherliker P., Clarke R., Emberson J., Halsey J., Qizilbash N., Collins R., Peto R. Body-mass index and cause-specific mortality in 900000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wormser D., Kaptoge S., Di A.E., Wood A.M., Pennells L., Thompson A., Sarwar N., Kizer J.R., Lawlor D.A., Nordestgaard B.G., et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., Amann M., Anderson H.R., Andrews K.G., Aryee M., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasreddine L., Naja F., Chamieh M.C., Adra N., Sibai A.M., Hwalla N. Trends in overweight and obesity in Lebanon: Evidence from two national cross-sectional surveys (1997 and 2009) BMC Public Health. 2012;12:798. doi: 10.1186/1471-2458-12-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasreddine L., Naja F.A., Sibai A.M., Helou K., Adra N., Hwalla N. Trends in nutritional intakes and nutrition-related cardiovascular disease risk factors in Lebanon: The need for immediate action. J. Med. Liban. 2014;62:83–91. doi: 10.12816/0004102. [DOI] [PubMed] [Google Scholar]
- 9.Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J., Singh G.M., Gutierrez H.R., Lu Y., Bahalim A.N., et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doo M., Kim Y. Obesity: Interactions of genome and nutrients intake. Prev. Nutr. Food Sci. 2015;20:1–7. doi: 10.3746/pnf.2015.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang T., Hu F.B. Gene-environment interactions and obesity: Recent developments and future directions. BMC Med. Genom. 2015;8:S2. doi: 10.1186/1755-8794-8-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC Public Health Genomics Knowledge Base (v5.0). Phenopedia. Obesity. [(accessed on 10 January 2018)]; Available online: https://phgkb.cdc.gov/PHGKB/phenoPedia.action?firstQuery=Obesity&cuiID=C0028754&typeSubmit=GO&check=y&which=2&pubOrderType=pubD.
- 13.Hunt S.C., Stone S., Xin Y., Scherer C.A., Magness C.L., Iadonato S.P., Hopkins P.N., Adams T.D. Association of the FTO gene with BMI. Obesity. 2008;16:902–904. doi: 10.1038/oby.2007.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang L., Wu H., Pan A., Patel B., Xiang G., Qi L., Kaplan R.C., Hu F., Wylie-Rosett J., Qi Q. FTO genotype and weight loss in diet and lifestyle interventions: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016;103:1162–1170. doi: 10.3945/ajcn.115.123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., Qi Q., Zhang C., Smith S.R., Hu F.B., Sacks F.M., Bray G.A., Qi L. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: The POUNDS LOST Trial. Diabetes. 2012;61:3005–3011. doi: 10.2337/db11-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips C.M., Kesse-Guyot E., McManus R., Hercberg S., Lairon D., Planells R., Roche H.M. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J. Nutr. 2012;142:824–831. doi: 10.3945/jn.111.153460. [DOI] [PubMed] [Google Scholar]
- 17.Sonestedt E., Roos C., Gullberg B., Ericson U., Wirfalt E., Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am. J. Clin. Nutr. 2009;90:1418–1425. doi: 10.3945/ajcn.2009.27958. [DOI] [PubMed] [Google Scholar]
- 18.Andreasen C.H., Stender-Petersen K.L., Mogensen M.S., Torekov S.S., Wegner L., Andersen G., Nielsen A.L., Albrechtsen A., Borch-Johnsen K., Rasmussen S.S., et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- 19.Grau K., Cauchi S., Holst C., Astrup A., Martinez J.A., Saris W.H., Blaak E.E., Oppert J.M., Arner P., Rössner S., et al. TCF7L2 rs7903146-macronutrient interaction in obese individuals’ responses to a 10-wk randomized hypoenergetic diet. Am. J. Clin. Nutr. 2010;91:472–479. doi: 10.3945/ajcn.2009.27947. [DOI] [PubMed] [Google Scholar]
- 20.Mattei J., Qi Q., Hu F.B., Sacks F.M., Qi L. TCF7L2 genetic variants modulate the effect of dietary fat intake on changes in body composition during a weight-loss intervention. Am. J. Clin. Nutr. 2012;96:1129–1136. doi: 10.3945/ajcn.112.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseini-Esfahani F., Mirmiran P., Daneshpour M.S., Mehrabi Y., Hedayati M., Zarkesh M., Azizi F. Western dietary pattern interaction with APOC3 polymorphism in the risk of metabolic syndrome: Tehran Lipid and Glucose Study. J. Nutrigenet. Nutrigenom. 2014;7:105–117. doi: 10.1159/000365445. [DOI] [PubMed] [Google Scholar]
- 22.Hosseini-Esfahani F., Mirmiran P., Daneshpour M.S., Mehrabi Y., Hedayati M., Soheilian-Khorzoghi M., Azizi F. Dietary patterns interact with APOA1/APOC3 polymorphisms to alter the risk of the metabolic syndrome: The Tehran Lipid and Glucose Study. Br. J. Nutr. 2015;113:644–653. doi: 10.1017/S0007114514003687. [DOI] [PubMed] [Google Scholar]
- 23.Khalilitehrani A., Qorbani M., Hosseini S., Pishva H. The association of MC4R rs17782313 polymorphism with dietary intake in Iranian adults. Gene. 2015;563:125–129. doi: 10.1016/j.gene.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Zgheib N.K., Sleiman F., Nasreddine L., Nasrallah M., Nakhoul N., Isma’eel H., Tamim H. Short Telomere Length is Associated with Aging, Central Obesity, Poor Sleep and Hypertension in Lebanese Individuals. Aging Dis. 2018;9:77–89. doi: 10.14336/AD.2017.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craig C.L., Marshall A.L., Sjostrom M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 26.Lee R.D., Nieman D.C. Nutritional Assessment. 4th ed. McGraw-Hill; New York, NY, USA: 2007. [Google Scholar]
- 27.Naja F., Nasreddine L., Itani L., Chamieh M.C., Adra N., Sibai A.M., Hwalla N. Dietary patterns and their association with obesity and sociodemographic factors in a national sample of Lebanese adults. Public Health Nutr. 2011;14:1570–1578. doi: 10.1017/S136898001100070X. [DOI] [PubMed] [Google Scholar]
- 28.Millen B.E., Morgan J.L. The 2D Food Portion Visual. Nutrition Consulting Enterprises; Framingham, MA, USA: 1996. [Google Scholar]
- 29.Pellet P., Shadarevian S. Food Composition: Tables for Use in the Middle East. American University of Beirut; Beirut, Lebanon: 1970. [Google Scholar]
- 30.CSHL-HAPMAP CEU. [(accessed on 10 January 2018)]; Available online: https://www.ncbi.nlm.nih.gov/projects/SNP/snp_viewTable.cgi?pop=1409.
- 31.Tanaka T., Ngwa J.S., van Rooij F.J., Zillikens M.C., Wojczynski M.K., Frazier-Wood A.C., Houston D.K., Kanoni S., Lemaitre R.N., Luan J., et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am. J. Clin. Nutr. 2013;97:1395–1402. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speliotes E.K., Willer C.J., Berndt S.I., Monda K.L., Thorleifsson G., Jackson A.U., Lango Allen H., Lindgren C.M., Luan J., Mägi R., et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinney A., Nguyen T.T., Scherag A., Friedel S., Bronner G., Muller T.D., Grallert H., Illig T., Wichmann H.E., Rief W., et al. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS ONE. 2007;2:e1361. doi: 10.1371/journal.pone.0001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyre D., Delplanque J., Chevre J.C., Lecoeur C., Lobbens S., Gallina S., Durand E., Vatin V., Degraeve F., Proença C., et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat. Genet. 2009;41:157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 35.Willer C.J., Speliotes E.K., Loos R.J., Li S., Lindgren C.M., Heid I.M., Berndt S.I., Elliott A.L., Jackson A.U., Lamina C., et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagg S., Ganna A., Van Der Laan S.W., Esko T., Pers T.H., Locke A.E., Berndt S.I., Justice A.E., Kahali B., Siemelink M.A., et al. Gene-based meta-analysis of genome-wide association studies implicates new loci involved in obesity. Hum. Mol. Genet. 2015;24:6849–6860. doi: 10.1093/hmg/ddv379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J., Loos R.J., Powell J.E., Medland S.E., Speliotes E.K., Chasman D.I., Rose L.M., Thorleifsson G., Steinthorsdottir V., Mägi R., et al. FTO genotype is associated with phenotypic variability of body mass index. Nature. 2012;490:267–272. doi: 10.1038/nature11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer J., Koch L., Emmerling C., Vierkotten J., Peters T., Bruning J.C., Rüther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 39.Church C., Lee S., Bagg E.A., McTaggart J.S., Deacon R., Gerken T., Lee A., Moir L., Mecinović J., Quwailid M.M., et al. A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet. 2009;5:e1000599. doi: 10.1371/journal.pgen.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X., Yang Y., Sun B.F., Zhao Y.L., Yang Y.G. FTO and obesity: Mechanisms of association. Curr. Diabetes Rep. 2014;14:486. doi: 10.1007/s11892-014-0486-0. [DOI] [PubMed] [Google Scholar]
- 41.Tan L.J., Zhu H., He H., Wu K.H., Li J., Chen X.D., Zhang J.G., Shen H., Tian Q., Krousel-Wood M., et al. Replication of 6 obesity genes in a meta-analysis of genome-wide association studies from diverse ancestries. PLoS ONE. 2014;9:e96149. doi: 10.1371/journal.pone.0096149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livingstone K.M., Celis-Morales C., Papandonatos G.D., Erar B., Florez J.C., Jablonski K.A., Razquin C., Marti A., Heianza Y., Huang T., et al. FTO genotype and weight loss: Systematic review and meta-analysis of 9563 individual participant data from eight randomised controlled trials. BMJ. 2017;356:j263. doi: 10.1136/bmj.i4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merritt D.C., Jamnik J., El-Sohemy A. FTO genotype, dietary protein intake, and body weight in a multiethnic population of young adults: A cross-sectional study. Genes Nutr. 2018;13:4. doi: 10.1186/s12263-018-0593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi F., Brubaker P.L., Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J. Biol. Chem. 2005;280:1457–1464. doi: 10.1074/jbc.M411487200. [DOI] [PubMed] [Google Scholar]
- 45.Schafer S.A., Tschritter O., Machicao F., Thamer C., Stefan N., Gallwitz B., Holst J.J., Dekker J.M., ’t Hart L.M., Nijpels G., et al. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia. 2007;50:2443–2450. doi: 10.1007/s00125-007-0753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cauchi S., El Achhab Y., Choquet H., Krempler F., Weitgasser R., Nejjari C., Patsch W., Chikri M., Meyre D., Froguel P. TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: A global meta-analysis. J. Mol. Med. 2007;85:777–782. doi: 10.1007/s00109-007-0203-4. [DOI] [PubMed] [Google Scholar]
- 47.Povel C.M., Boer J.M., Reiling E., Feskens E.J. Genetic variants and the metabolic syndrome: A systematic review. Obes. Rev. 2011;12:952–967. doi: 10.1111/j.1467-789X.2011.00907.x. [DOI] [PubMed] [Google Scholar]
- 48.Fisher E., Meidtner K., Angquist L., Holst C., Hansen R.D., Halkjaer J., Masala G., Ostergaard J.N., Overvad K., Palli D., et al. Influence of dietary protein intake and glycemic index on the association between TCF7L2 HapA and weight gain. Am. J. Clin. Nutr. 2012;95:1468–1476. doi: 10.3945/ajcn.111.014670. [DOI] [PubMed] [Google Scholar]
- 49.Roswall N., Angquist L., Ahluwalia T.S., Romaguera D., Larsen S.C., Ostergaard J.N., Halkjaer J., Vimaleswaran K.S., Wareham N.J., Bendinelli B., et al. Association between Mediterranean and Nordic diet scores and changes in weight and waist circumference: Influence of FTO and TCF7L2 loci. Am. J. Clin. Nutr. 2014;100:1188–1197. doi: 10.3945/ajcn.114.089706. [DOI] [PubMed] [Google Scholar]
- 50.Georgiadi A., Kersten S. Mechanisms of gene regulation by fatty acids. Adv. Nutr. 2012;3:127–134. doi: 10.3945/an.111.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosqvist F., Iggman D., Kullberg J., Cedernaes J., Johansson H.E., Larsson A., Johansson L., Ahlström H., Arner P., Dahlman I., et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–2368. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- 52.Norris L.E., Collene A.L., Asp M.L., Hsu J.C., Liu L.F., Richardson J.R., Li D., Bell D., Osei K., Jackson R.D., et al. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2009;90:468–476. doi: 10.3945/ajcn.2008.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization (WHO) Healthy Diet: Key Facts. [(accessed on 28 December 2018)];2018 Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet.
- 54.Naja F., Hwalla N., Itani L., Baalbaki S., Sibai A., Nasreddine L. A novel Mediterranean diet index from Lebanon: Comparison with Europe. Eur. J. Nutr. 2015;54:1229–1243. doi: 10.1007/s00394-014-0801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willett W. Nutritional Epidemiology. 2nd ed. Oxford University Press; New York, NY, USA: 1998. [Google Scholar]
- 56.Caan B.J., Lanza E., Schatzkin A., Coates A.O., Brewer B.K., Slattery M.L., Marshall J.R., Bloch A. Does nutritionist review of a self-administered food frequency questionnaire improve data quality? Public Health Nutr. 1999;2:565–569. doi: 10.1017/S1368980099000750. [DOI] [PubMed] [Google Scholar]
- 57.Nasreddine L., Hwalla N., Sibai A., Hamze M., Parent-Massin D. Food consumption patterns in an adult urban population in Beirut, Lebanon. Public Health Nutr. 2006;9:194–203. doi: 10.1079/PHN2005855. [DOI] [PubMed] [Google Scholar]
- 58.Naja F., Nasreddine L., Itani L., Adra N., Sibai A.M., Hwalla N. Association between dietary patterns and the risk of metabolic syndrome among Lebanese adults. Eur. J. Nutr. 2013;52:97–105. doi: 10.1007/s00394-011-0291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chamieh M.C., Moore H.J., Summerbell C., Tamim H., Sibai A.M., Hwalla N. Diet, physical activity and socio-economic disparities of obesity in Lebanese adults: Findings from a national study. BMC Public Health. 2015;15:279. doi: 10.1186/s12889-015-1605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okamoto K., Ohsuka K., Shiraishi T., Hukazawa E., Wakasugi S., Furuta K. Comparability of epidemiological information between self- and interviewer-administered questionnaires. J. Clin. Epidemiol. 2002;55:505–511. doi: 10.1016/S0895-4356(01)00515-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.