Abstract
Increased fMRI food cue reactivity in obesity, i.e. higher responses to high- vs. low-calorie food images, is a promising marker of the dysregulated brain reward system underlying enhanced susceptibility to obesogenic environmental cues. Recently, it has also been shown that weight loss interventions might affect fMRI food cue reactivity and that there is a close association between the alteration of cue reactivity and the outcome of the intervention. Here we tested whether fMRI food cue reactivity could be used as a marker of diet-induced early changes of neural processing in the striatum that are predictive of the outcome of the weight loss intervention. To this end we investigated the relationship between food cue reactivity in the striatum measured one month after the onset of the weight loss program and weight changes obtained at the end of the six-month intervention. We observed a significant correlation between BMI change measured after six months and early alterations of fMRI food cue reactivity in the striatum, including the bilateral putamen, right pallidum, and left caudate. Our findings provide evidence for diet-induced early alterations of fMRI food cue reactivity in the striatum that can predict the outcome of the weight loss intervention.
Keywords: Obesity, Reward system, Striatum, Food-cue images, Body mass index
Highlights
-
•
fMRI food cue reactivity is associated with a six-month weight loss intervention outcome.
-
•
More weight loss is associated with lower fMRI response preference for high- vs. low-calorie food images in the striatum.
-
•
Food cue reactivity alterations in the striatum during the first month predict BMI changes at the end of the intervention.
1. Introduction
Obesity is a growing public health concern worldwide. In children and adolescents, the prevalence of obesity increased between 1975 and 2016 from 0.7% to 5.6% in girls and from 0.9% to 7.8% in boys (NCD Risk Factor Collaboration (NCD-RisC), 2017). In adults, the prevalence of obesity increased between 1975 and 2014 from 3.2% to 10.8% in women and from 6.4% to 14.9% in men (NCD Risk Factor Collaboration (NCD-RisC), 2016). Overweight and obesity are associated with a number of negative cognitive and health outcomes, including increased risk for heart disease (Eckel, 1997), cancer (Renehan et al., 2015), depression (Ha et al., 2017), and premature mortality (Fontana and Hu, 2014).
In developed countries, as a result of practically unrestricted access to high-calorie food, energy intake is controlled primarily by the hedonic aspects of food consumption rather than by the metabolic needs (Volkow and Baler, 2015; Ziauddeen et al., 2015). Because of this, recently obesity research has become focused more strongly on hedonic eating and the underlying dysregulation of the brain reward system (Richard, 2015; Ziauddeen et al., 2015). One of the most successful fields of research that emerged in this context is concerned with the brain processes that mediate increased susceptibility to obesogenic environmental cues (such as images of calorie-rich food) and thus lead to overeating and inability to maintain healthy weight (Berridge et al., 2010; Murdaugh et al., 2012; Stice et al., 2010; Stice and Yokum, 2016). Therefore, searching for reliable neural markers of brain processes underlying altered motivational saliency of food cues in obesity has become an important objective.
Neuroimaging has been extensively applied in research on the brain processes associated with obesity risk and responsiveness to weight loss interventions. For example, several studies have examined gray matter (GM) and white matter (WM) volumes as predictors of responsiveness to weight control interventions (Honea et al., 2016; Mokhtari et al., 2016). In addition to structural imaging, functional MRI (fMRI) has been used extensively to examine brain activation differences between obese/overweight individuals and those with normal weight. Obesity is related to altered resting-state functional connectivity (e.g., Avery et al., 2017) and associated with alterations in fMRI responses to both food intake and visual food cues (Stoeckel et al., 2008). Furthermore, fMRI activation and functional connectivity might also predict the outcome of weight loss interventions (Contreras-Rodríguez et al., 2017).
Importantly, neuroimaging has provided converging evidence for altered fMRI responses as well as resting-state functional connectivity of brain reward system in obesity (Avery et al., 2017; Richard, 2015; Ziauddeen et al., 2015; Carnell et al., 2012). In particular, investigation of fMRI responses to high- vs. low-calorie foods has been especially successful and has shown that food cue reactivity is increased in obese as compared to lean participants in brain areas implicated in reward computation and value-based action selection, such as ventral and dorsal striatum, insula, and the orbitofrontal cortex as well as in the frontoparietal network of brain areas involved in inhibitory control (Bruce et al., 2010; Martin et al., 2010; Pursey et al., 2014; Rothemund et al., 2007; Stice et al., 2010; Stoeckel et al., 2008). It has also been shown that initial food cue reactivity in these brain regions measured by fMRI can predict subsequent body weight trajectory and weight loss intervention outcome (Batterink et al., 2010; Demos et al., 2012; Murdaugh et al., 2012; Stice et al., 2010; Yokum et al., 2014). Furthermore, recently it has also been revealed that alteration of food cue reactivity in the reward system, especially in the dorsal striatum is closely associated with both body weight trajectory as well as the efficacy of the weight loss intervention and could also predict long-term ability to maintain weight loss in a follow-up period (Deckersbach et al., 2014; Murdaugh et al., 2012; Stice and Yokum, 2016). These results are in agreement with the food addiction model of obesity (Contreras-Rodríguez et al., 2017; García-García et al., 2014; Smith and Robbins, 2013; Volkow et al., 2013a, Volkow et al., 2013b), according to which obesity overlaps with addiction in terms of neurobiological alterations in the striatum leading to food craving and persistence of unhealthy food intake habits.
Taken together, these results suggest that alteration of food cue reactivity in the striatum might serve as an fMRI marker of weight loss intervention efficacy. Furthermore, based on previous findings (Elfhag and Rössner, 2010; Gripeteg et al., 2010; Gross et al., 2019; Handjieva-Darlenska et al., 2010; Nackers et al., 2010; Tronieri et al., 2019; Unick et al., 2014; Wadden et al., 1992) showing that initial weight loss—observed during the first 1–2 month of the intervention—might be closely associated with the weight loss program outcome, it is tempting to hypothesize that alterations in the striatum's fMRI food cue reactivity might take place already at the early stage of the intervention and could be used to predict its outcome. Here we addressed this possibility by investigating the relationship between food cue reactivity in the striatum measured one month after the onset of a weight loss program and weight changes obtained by the end of the six-month intervention. Our prospective neuroimaging study reveals that the difference in the fMRI responses to high- and low-calorie food images in the striatum—including the bilateral putamen, right pallidum, and left caudate—after one month of the weight loss intervention shows a close association with the changes in the participants' body mass index at the end of the six-month weight loss program.
2. Materials and methods
2.1. Participants
A total of 68 obese outpatients (58 female and 10 male) from the Metabolism Centre of Szent Imre Hospital (Budapest, Hungary) were recruited to participate in the study organized and sponsored by Gedeon Richter Plc. (Budapest, Hungary). Inclusion criteria were: age of 18–65 years, body mass index (BMI) > 30 kg/m2 and three-month weight stability (<5% body weight change) immediately prior to the study. In the case of two participants, BMI scores decreased slightly below 30 kg/m2 (29.44 and 29.92 kg/m2) during the time period between testing for inclusion criteria and the first baseline measurements. Major criteria for exclusion were body weight exceeding 130 kg, pregnancy, smoking, excess alcohol consumption, drug addiction within 1 year before the study, insulin-dependent diabetes mellitus, psychiatric disorders, claustrophobia, vision problems, metal implants, history of bariatric surgery, or conditions causing obesity, e.g., hypothyroidism. Out of 68 participants enrolled, only 31 (27 female and 4 male) completed the study with full dataset. The reasons for the high drop-out rate were the lack of compensation, low compliance with the diet/lifestyle changes and the relatively high time requirement of the visits. After image quality control, two additional participants had to be excluded due to the residual effect of motion artifacts on fMRI data after movement correction, leaving a total of 29 participants for data analysis.
The study was organized and conducted according to Hungarian regulations and laws and approved by the Hungarian Medical Research Council. Written informed consent was obtained from all participants prior to the study.
2.2. Study design
Participants were recommended to consume a low-calorie diet (1500 kcal/day) during the six-month-long intervention period. No specific exercise program was recommended. Dietary intervention involved the investigation of the patients' motivations, mapping the potential obstacles of weight loss, counseling about low calorie dieting, keeping a food diary, and encouragement for performing a voluntary intake restriction of approximately −500 kcal (to reach ~1500 kcal/day). Participants were asked to complete food diaries about full consumption three days per week (two workdays and a weekend day)—including the exact names of the foods; type and specifications (sugar and lipid content, etc.); place of consumption (at home, cantina, restaurant, etc.) and mode of preparation (raw, boiled, fried, etc.); size (a complete guideline was supplied to the participants). Patients were also asked to write down the thoughts and feelings about keeping the diary and how it influenced their eating habits. Major divergences from the daily habits (e.g., festivals, parties, and holidays) were also expected to be mentioned. In later visits, experiences and difficulties were addressed, and further dietary and exercise counseling was given on a personal basis.
Participants visited the hospital at weeks 0, 2, 4, 8, 12, and 24 during the intervention period for check-ups. At each scheduled visit, participants arrived at the hospital in fasted state, then underwent an evaluation of anthropometric parameters (body weight, height, and waist circumference). On the first, third (4 weeks), and last (24 weeks) visits, body composition was also measured using dual-energy X-ray absorptiometry (DXA; Lunar Prodigy Advance, GE Healthcare, Madison, WI, USA). For the first and third visits, participants stayed at the hospital overnight. On the second day of these visits, fMRI measurements were carried out 2–5 h after subjects consumed standard lunch at the hospital.
2.3. fMRI protocol
Participants were scheduled for two separate fMRI imaging sessions, one before starting the six-month weight loss intervention (baseline, Session 1) and another at the end of the first month of the six-month weight loss intervention (Session 2). Both imaging sessions included four ~6 min long experimental and two 10 min long resting-state runs as first and last runs. During the experimental runs images of neutral objects, high-calorie foods, and low-calorie foods were presented in a block design format. Each run consisted of four randomly presented 21 s long blocks for each image category in which 7 individual images were presented for 2 s followed by a 1 s gap. Each block was separated by 9 s and each run began with 15 s of blank screen with only a fixation cross present.
A total of 112 pictures for each image category were collected from the International Affective Picture System (IAPS) database (Lang et al., 2008) and Internet search engines. In the two sessions, the same 112 images were randomly presented in four runs of 28 images for each image category. High-calorie food images contained an equal number of sweet and savory food images. All images were equated for luminance and contrast and presented centrally, subtending 8 × 6°, on a uniform gray background. Stimuli were projected onto a translucent screen located at the back of the scanner bore using a Panasonic PT-D3500E DLP projector (Matsushita Electric Industrial Co., Ltd., Kadoma, Osaka, Japan) at a refresh rate of 60 Hz, and they were viewed through a mirror attached to the head coil at a viewing distance of 57 cm. Head motion was minimized using foam padding. Stimulus presentation was controlled by MATLAB R2010a (The MathWorks Inc., Natick, MA, USA) using PTB-3 (Brainard, 1997; Pelli, 1997; http://psychtoolbox.org/). Participants were instructed to pay attention to the images presented on the screen.
In order to control for attention to images during scanning, participants completed an old/new memory task outside the scanner following each session. During the task, 20 images for each image category were randomly presented for participants who had to decide whether the given image was seen (old) or not seen (new) in the scanner. Half of the images were a subset of those presented in the scanner, and the other half were novel.
2.4. MRI data acquisition
MRI data were collected on a 3 Tesla Philips Achieva MRI scanner (Philips Healthcare, Best, The Netherlands) equipped with an 8-channel SENSE head coil. High-resolution anatomical images were acquired for each subject using a T1-weighted 3D TFE sequence (repetition time (TR)/echo time (TE)/flip angle (FA) = 9.77 ms/4.60 ms/8°; field of view (FOV) = 240 × 240 × 180 mm3) yielding images with 1 × 1 × 1 mm3 resolution. Functional images were collected with an ascending acquisition order covering the whole brain with a BOLD-sensitive T2*-weighted 2.00-fold-accelerated SENSE-EPI sequence (36 slices, 3.25 mm slice thickness with 3 × 3 mm2 in-plane resolution; TR/TE/FA = 2000 ms/30 ms/90°).
2.5. fMRI data analysis
Preprocessing and analysis of the imaging data were performed using the SPM12 toolbox (Wellcome Trust Centre for Neuroimaging, London, UK) as well as custom-made scripts running on MATLAB R2015a. The functional images were spatially realigned to the first EPI image within a run for motion correction. All realigned functional images were coregistered with the 3D anatomical image which was then segmented and normalized to the MNI-152 space (2 × 2 × 2 mm3) using the unified segmentation-normalization tool of SPM12. To spatially normalize functional images to MNI-152 space, we applied the deformation field parameters that were obtained during the normalization of the 3D anatomical image. After the normalization procedure, functional images were spatially smoothed with an 8-mm full-width at half-maximum isotropic Gaussian kernel.
To calculate individual beta maps, we used a standard voxel-wise General Linear Model (GLM), with three regressors for neutral object, high-calorie food, and low-calorie food images as boxcar functions convolved with a hemodynamic response function (HRF; Worsley and Friston, 1995) and applied a temporal high-pass filter with a cutoff frequency of 1/128 Hz. Movement-related variance was accounted for by the spatial parameters resulting from the motion correction procedure. To calculate individual food cue reactivity maps, we contrasted the estimated beta weights of regressors for high- and low-calorie food images, serving as input for the second-level analyses.
2.6. Regression analysis
To investigate the relationship between food cue reactivity and BMI change following the six-month intervention, we performed a second-level voxel-wise linear regression analysis with the six-month BMI change as a covariate of interest on individual food cue reactivity maps measured at baseline vs. at the end of the first month of the six-month intervention (Session 2 − Session 1). In order to control for the interindividual differences in age, we included the participants' age as a nuisance covariate in the regression model. We focused our analysis on the striatal regions implicated in weight loss (Haber and Knutson, 2010; Pursey et al., 2014; Ziauddeen et al., 2015) using anatomically defined masks of the left and right accumbens, caudate, putamen, and pallidum based on the Harvard-Oxford Atlas (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). The union of these binary masks was taken and dilated by one voxel (2 × 2 × 2 mm3) in all directions to yield the striatum mask in which voxel-wise regression analyses were conducted. The resulting statistical t maps were small volume corrected for multiple comparisons using a two-tailed voxel-level False Discovery Rate (FDR) at q = 0.05. For visualization, statistical maps with FDR corrected p < .05 threshold were projected onto axial anatomical image slices averaged across participants using an in-house modified version of xjView 9.6 toolbox (http://www.alivelearn.net/xjview/). Stereotaxic coordinates are reported in Montreal Neurological Institute (MNI) space and regional labels were derived from the AAL2 atlas (Rolls et al., 2015; Tzourio-Mazoyer et al., 2002).
2.7. Correlation analysis
When testing associations across anthropometric measurements (BMI, BFP, age), we used the Robust Correlation Toolbox (Pernet et al., 2013) in MATLAB R2015a. Robust correlation methods have been shown to provide better estimates of the true association with high power and accurate false positive control by down-weighting or removing outliers and accounting for them in significance testing. Skipped Pearson's correlation coefficients were calculated where bivariate outliers were detected using an adjusted box-plot rule and removed in the computation of skipped correlations. The number of outliers (NO) detected and removed is reported for each correlation. For correlation coefficients (r), 95% confidence intervals (CI) were calculated based on 1000 samples with the percentile bootstrap method implemented in the toolbox.
3. Results
3.1. Weight change and behavioral assessments
The experiments presented in this study were successfully completed by 29 obese adults (26 women, mean age ± SD: 47.59 ± 12.64, min/max age: 21/66) with average (±SD) initial body mass index (BMI) of 36.88 (± 5.50) kg/m2 (Fig. 1.). Importantly, despite the fact that there was a large interindividual variability in participants' age, the baseline BMI scores were not correlated with the participants' age (skipped Pearson r(27) = −0.03, p = .868, CI = [−0.38 0.28], NO = 0).
Fig. 1.
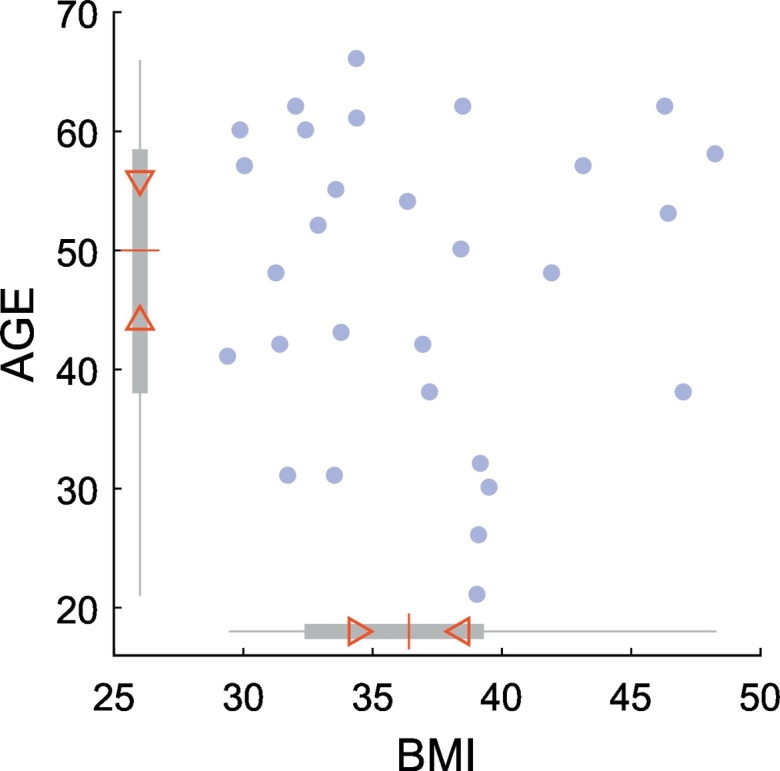
Scatter plot of participants' age (y-axis) and BMI scores (x-axis) at baseline. Blue circles represent individual data points. In boxplots along the x- and y-axes, the “central box” represents the interquartile range (IQR) with lower and upper boundary lines at the 25th and 75th percentile of the data, respectively. Whiskers indicate the full range, the red central line indicates the median of the data. Triangles depict the 95% confidence interval of the median value (median ± 1.57 × IQR/n0.5).
We found that participants had significant weight loss following the six-month weight loss intervention: relative to their initial magnitude, BMI scores were significantly reduced after the six-month intervention (t(28) = −2.57, p = .016). Importantly, there were no correlations between BMI scores at baseline and their reduction after the six-month weight loss intervention (r(26) = 0.03, p = .449, CI = [−0.24 0.33], NO = 1), suggesting that diet-induced body mass reduction did not depend on the initial body mass index.
After the first month of the six-month intervention there was also a significant reduction in BMI (t(28) = −5.18, p = 1.69 × 10−5) which did not differ significantly in magnitude from the six-month BMI reduction (t(28) = 0.89, p = .379). The between-subject variance, however, was significantly higher for the six-month relative to the one-month BMI change (F(28,28) = 7.43, p = 4.77 × 10−7) indicating that individual differences in weight loss were more pronounced after the six-month intervention (Fig. 2). In addition, individual differences in six-month BMI change were predicted by one-month BMI changes, since there was a significant positive correlation between one-month and six-month BMI changes (r(26) = 0.73, p = 5.95 × 10−7, CI = [0.55 0.85], NO = 1).
Fig. 2.
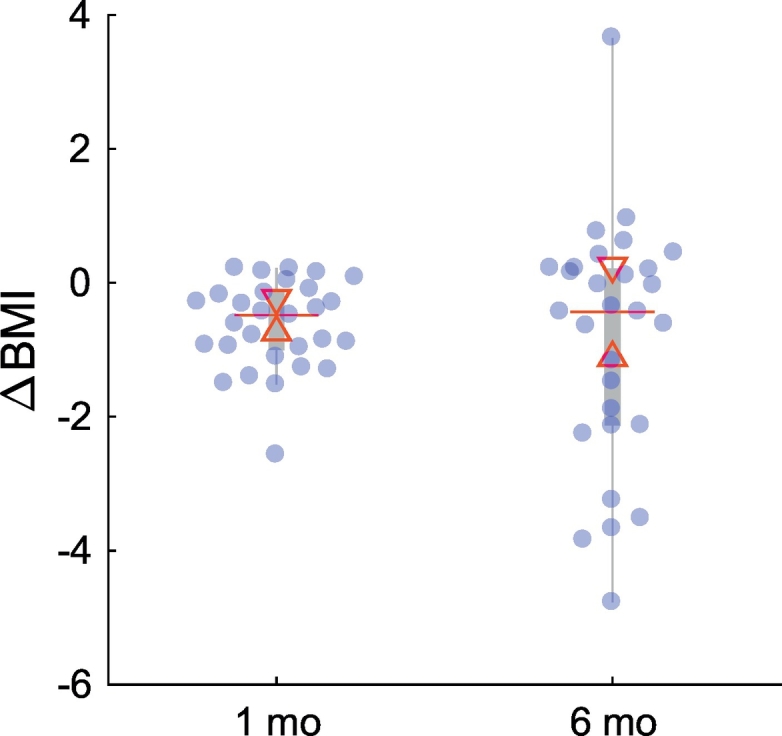
Distributions of participants' weight change (∆BMI) at the end of the first (1 mo) and last month (6 mo) of the six-month weight loss intervention. Median weight change did not differ significantly between sessions at the 5% significance level, however, interindividual variability at 6 months was larger, as shown by the larger interquartile range (IQR). Blue circles represent individual data points jittered to prevent overlap. In each boxplot the “central box” represents the IQR with lower and upper boundary lines at the 25th and 75th percentile of the data, respectively. Whiskers indicate the non-outlier range (1.5 × IQR), the red central line indicates the median of the data. Triangles depict the 95% confidence interval of the median value (median ± 1.57 × IQR/n0.5).
In order to control for attention to food images during scanning, participants performed an old/new recognition memory task outside the scanner. Data of four participants were missing due to technical difficulties. Participants did in fact attend to the food images, as evidenced by high average accuracy rates of 75.60 ± 2% (mean percent correct ± SEM) in this task. There were no differences in memory performance scores (d-prime) across food images (main effect of food cue: t(24) = −0.58, p = .567) and sessions (main effect of session: t(24) = 0.25, p = .804). The non-significant interaction of food cue and session (t(24) = −0.88, p = .388) suggests that memory performance was similar for the high- and low-calorie food images in the two sessions.
3.2. Brain regions showing elevated fMRI responses to high-calorie food images
First, we performed a whole brain analysis to confirm that our experimental approach was successful in activating brain areas that were shown to respond more strongly to high-calorie food images as compared to low-calorie ones. In agreement with previous results (Bruce et al., 2010; Martin et al., 2010; Pursey et al., 2014; Rothemund et al., 2007; Stice et al., 2010; Stoeckel et al., 2008) our analysis revealed significantly larger fMRI responses to high-calorie food images, i.e. significant food cue reactivity in several regions of the striatum, frontal, orbitofrontal, and visual cortex (p < .05, two-tailed FDR whole brain corrected at voxel level) (see Table 1).
Table 1.
Brain regions showing increased fMRI responses to high- vs. low-calorie food images.
| Region | Hemisphere | x | y | z | t |
|---|---|---|---|---|---|
| Lingual gyrus | R | 12 | −82 | −8 | 6.42 |
| Lingual gyrus | R | 26 | −48 | −10 | 5.68 |
| Fusiform | L | −26 | −60 | −10 | 5.39 |
| Posterior orbital gyrus | L | −28 | 20 | −16 | 5.22 |
| Superior frontal gyrus, medial | L | −10 | 54 | 30 | 5.17 |
| Pallidum | L | −24 | −8 | −8 | 5.07 |
| Calcarine | L | −4 | −84 | −8 | 4.93 |
| Superior frontal gyrus, medial orbital | R | 2 | 50 | −2 | 4.69 |
| Putamen | R | 28 | −16 | −4 | 4.52 |
| Inferior frontal gyrus, triangular part | L | −42 | 26 | 6 | 4.44 |
| Inferior frontal gyrus, opercular part | R | 46 | 8 | 8 | 4.42 |
| Middle occipital gyrus | R | 38 | −78 | 16 | 4.39 |
| IFG pars orbitalis | R | 30 | 26 | −12 | 4.24 |
The peak MNI coordinates (x, y, z in mm) and t values (df = 28) are reported. Anatomical labels are derived from the AAL2 atlas using an in-house modified version of xjView 9.6. Results are shown at p < .05, two-tailed FDR whole brain corrected at voxel level. Abbreviations: L = left; R = right.
3.3. Effect of weight loss intervention on the fMRI responses in the striatum
Our analysis was focused on testing whether food cue reactivity in striatum measured after only 30 days of a six-month weight loss intervention could predict the outcome of the weight loss program. Neural (i.e. fMRI) responses to high- vs. low-calorie food images were measured at baseline (Session 1) and 30 days after the onset of the intervention (Session 2) in the striatum and regressed on BMI change following the six-month intervention. BMI change was calculated by subtracting baseline BMI from BMI measured at the end of the six-month intervention.
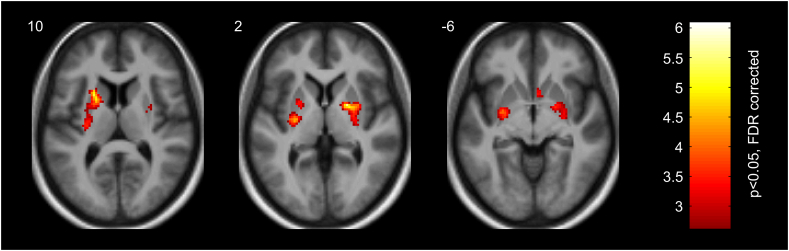
Random-effects regression analysis (p < .05, two-tailed FDR small volume corrected at voxel level) of the fMRI data obtained at the end of the first month of the six-month intervention (Session 2) revealed the close association of fMRI responses in the dorsal and ventral regions of the bilateral putamen, as well as in the right pallidum and caudate with the six-month BMI change (see Fig. 3 and Table 2): the higher the magnitude of the fMRI responses to high- relative to low-calorie food images in these regions at the end of the first month, the more positive the BMI score change (i.e. less weight loss) at the end of the last month of the six-month intervention.
Fig. 3.
Striatal regions (left dorsal putamen (z = 10 mm), right pallidum and dorsal putamen (z = 2 mm), bilateral ventral putamen and right caudate (z = −6 mm)) showing positive relationship between fMRI responses to high- vs. low-calorie food images measured after one month and BMI loss at the end of the intervention. Color bar represents t values (df = 26). Statistical maps are displayed with p < .05, two-tailed FDR small volume corrected at voxel level on axial slices of a mean structural image from all participants. MNI z-coordinates in mm are indicated in the upper left corner of each slice.
Table 2.
Striatal regions showing associations between fMRI responses to high- vs. low-calorie food images measured after one month and BMI loss at the end of the intervention.
| Region | Hemisphere | x | y | z | t |
|---|---|---|---|---|---|
| Putamen (dorsal anterior) | L | −22 | 8 | 14 | 6.07 |
| Putamen (dorsal posterior) | L | −28 | −14 | 4 | 5.78 |
| Pallidum | R | 20 | 0 | 2 | 5.54 |
| Putamen (dorsal middle) | R | 30 | −2 | 4 | 4.57 |
| Putamen (ventral posterior) | R | 26 | −6 | −10 | 4.05 |
| Putamen (ventral posterior) | L | −28 | −8 | −4 | 4.02 |
| Putamen (dorsal middle) | L | −24 | 4 | 2 | 3.42 |
| Caudate (head) | R | 4 | 10 | −6 | 3.41 |
| Putamen (dorsal posterior) | R | 30 | −18 | 6 | 3.08 |
The peak MNI coordinates (x, y, z in mm) and t values (df = 26) are reported. Anatomical labels are derived from the AAL2 atlas using an in-house modified version of xjView 9.6. Results are shown at p < .05, two-tailed FDR small volume corrected at voxel level. Abbreviations: L = left; R = right.
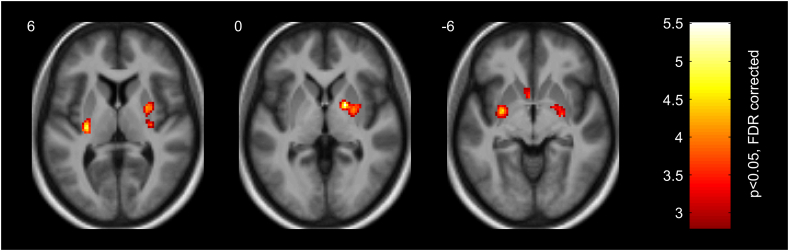
Next, we tested whether the observed relationship between food cue reactivity measured in the striatum 30 days after diet onset and BMI changes at the end of the six-month intervention is mediated by the diet-induced alteration of striatal responses. To this end, we performed a linear regression analysis for estimating the relationship between the six-month BMI loss and the difference of fMRI responses to high- vs. low-calorie food images between Session 2 and Session 1. There was a close association between the diet-induced change of fMRI response modulation and BMI loss primarily in the posterior part of the dorsal and ventral putamen, as well as in the right pallidum, and left caudate (see Fig. 4 and Table 3): the lower the fMRI response preference for high- compared to low-calorie food images in these regions at the end of the first month of the six-month intervention relative to baseline, the more negative the BMI score change (i.e. more weight loss) at the end of the last month of the six-month intervention.
Fig. 4.
Striatal regions (bilateral dorsal putamen (z = 6 mm), right pallidum and dorsal putamen (z = 0 mm), bilateral ventral putamen and left caudate (z = −6 mm)) showing positive relationship between diet-induced alterations in fMRI responses to high- vs. low-calorie food images from baseline to one month and BMI loss at the end of the intervention. Color bar represents t values (df = 26). Statistical maps are displayed with p < .05, two-tailed FDR small volume corrected at voxel level on axial slices of a mean structural image from all participants. MNI z-coordinates in mm are indicated in the upper left corner of each slice.
Table 3.
Striatal regions showing associations between diet-induced alterations in fMRI responses to high- vs. low-calorie food images from baseline to one month and BMI loss at the end of the intervention.
| Region | Hemisphere | x | y | z | t |
|---|---|---|---|---|---|
| Pallidum | R | 18 | 0 | 0 | 5.48 |
| Putamen (dorsal posterior) | L | −28 | −20 | 8 | 4.94 |
| Putamen (dorsal middle) | R | 26 | −4 | 4 | 4.73 |
| Putamen (ventral posterior) | L | −28 | −4 | −6 | 4.52 |
| Putamen (ventral posterior) | R | 26 | −8 | −8 | 3.76 |
| Putamen (dorsal posterior) | R | 30 | −18 | 6 | 3.75 |
| Caudate (body) | L | −20 | 16 | 14 | 3.68 |
| Caudate (head) | L | −4 | 10 | −6 | 3.55 |
The peak MNI coordinates (x, y, z in mm) and t values (df = 26) are reported. Anatomical labels are derived from the AAL2 atlas using an in-house modified version of xjView 9.6. Results are shown at p < .05, two-tailed FDR small volume corrected at voxel level. Abbreviations: L = left; R = right.
Although, previous research has also shown an association between the initial food cue reactivity in the striatum and the efficacy of the weight loss program (Murdaugh et al., 2012), our analysis failed to reveal a significant relationship between fMRI responses to high- vs. low-calorie food images measured at baseline (Session 1) and BMI change after the six-month intervention using a two-tailed FDR corrected threshold of p < .05.
In addition to BMI, body fat percentage (BFP) (Gallagher et al., 2000) is also a commonly used measure of overweight and obesity as well as weight status during weight loss intervention (Shah and Braverman, 2012). Consistent with previous results showing a close association between BMI and BFP (Jackson et al., 2002), in our study there was a significant correlation between the diet-induced BMI and BFP loss after the six-month intervention (skipped Pearson r(26) = 0.76, p = 2.04 × 10−6, CI = [0.55 0.89], NO = 1) leading to similar relationship between fMRI responses to high- vs. low-calorie food images and BFP loss as observed in the case of BMI. In the left (t(26) = 4.62; x, y, z = −18, 2, 10 and t(26) = 4.30; x, y, z = −26, −12, 4) and right (t(26) = 4.87; x, y, z = 30, −2, 4) dorsal putamen food cue dependent modulation of fMRI responses at the end of the first month of the diet was positively associated with BFP loss following the six-month intervention (p < .05, two-tailed FDR small volume corrected at voxel level). The relationship of diet-induced alterations in putamen food cue reactivity (Session 2 − Session 1) with six-month BFP loss, however, was only revealed when using an uncorrected threshold of p < .001.Taken together, these results revealed that diet-induced alteration of food cue reactivity in the bilateral putamen, right pallidum, and left caudate of obese participants measured 30 days after the onset of a six-month weight loss program can predict the magnitude of BMI changes at the end of the intervention. Importantly, these results were not confounded by the interindividual variability in age, since participants' age was included as a nuisance covariate in all regression analyses.
4. Discussion
We examined the relationship between early weight loss intervention effects on food cue reactivity in the striatum and participants' BMI in a six-month prospective study. Participants' food cue reactivity was measured at baseline by means of fMRI and again after one month participating in a weight loss program. BMI was tracked in all participants a total of six months after beginning the intervention. In agreement with previous findings (Gross et al., 2019; Nackers et al., 2010; Tronieri et al., 2019; Unick et al., 2014; Wadden et al., 1992) we found that weight loss during the first month can predict the outcome of the intervention. Importantly, the fMRI results revealed that after just one month in the weight loss intervention there were alterations in neural reactivity to food cues in the brain's reward network that showed a close association with BMI loss after six months in the program. In particular, we found that the change in food cue reactivity in the bilateral putamen, right pallidum, and left caudate between baseline and just one month in the weight loss program predicted BMI change after six months. These findings are in line with previous research showing that initial food cue reactivity in striatum measured by fMRI can predict subsequent body weight trajectory and weight loss intervention outcome (Demos et al., 2012; Murdaugh et al., 2012; Stice et al., 2010; Yokum et al., 2014). Furthermore, recently it has also been revealed that alteration of food cue reactivity in the reward system, especially in the dorsal striatum is closely associated with both body weight trajectory as well as the efficacy of the weight loss intervention and could also predict long-term ability to maintain weight loss in a follow-up period (Deckersbach et al., 2014; Murdaugh et al., 2012; Stice and Yokum, 2016).
Our results revealed a close association between weight loss and diet-induced modulation of food cue reactivity in several regions of the striatum, including the bilateral putamen, right pallidum, and left caudate. Although the specific role of the different striatal regions remains to be explored, it is the putamen that has been implicated in food-cue related reward processing most consistently in previous studies. In a meta-analysis of fMRI findings, García-García et al. (2014) found that overweight/obese individuals had altered putamen responses relative to healthy-weight individuals for both high-calorie food cues, but also for general reward processing. This altered processing is also present in individuals with an obesity risk allele for the fat mass and obesity associated (FTO) gene (Wiemerslage et al., 2016) and in adolescents at risk for obesity (Stice et al., 2011). Furthermore, increased food cue reactivity in the putamen has been shown to correlate with weight gain (Stice and Yokum, 2016) while decreased reactivity has been shown to correlate with subsequent weight decreases (Murdaugh et al., 2012). Changes in putamen activation likely do not reflect changes in BMI or weight per se, as they were observed after a 12-week intervention but predicted weight change at a 9-month delay (Murdaugh et al., 2012). Finally, it appears that putamen responses to food cue reward potential may be modulated by context. For example, Masterson et al. (2016) found that the putamen responded to visual food cues (both high- and low-calorie foods) but that the level of activation depended on time of day (with greater activation in the morning than the evening). Our results are in line with these previous findings and provide further evidence that diet-induced alterations of neural processes within the putamen—as reflected in changes of food cue reactivity—are closely associated with weight loss and might be used as an fMRI marker of intervention efficacy.
Previous neuroimaging studies investigating the relationship between neural food cue reactivity and weight loss have examined changes over longer time periods than that used here (e.g., 12 weeks, Murdaugh et al., 2012). An important novel aspect of the current findings is that we revealed a close association between fMRI food cue reactivity changes in the putamen over the first month of the diet and the weight loss at the end of the six-months intervention. These findings are in agreement with previous studies in which alterations in putamen food cue reactivity has been identified in other weight control interventions such as gastric bypass surgery (Ochner et al., 2011), or behavioral interventions (Deckersbach et al., 2014), suggesting that it might serve as a robust marker of intervention effectiveness.
According to a recent study providing precise functional maps of regional specialization within the human striatum (Pauli et al., 2016), the posterior part of the dorsal putamen where food cue reactivity alterations predicted weight loss in the current study might be primarily associated with somatosensory processing including taste and might be implicated in habits processing. This is compatible with the food addiction model of the obesity, which proposes that obesity overlaps with addiction in terms of neurobiological alterations in the dorsal striatum leading to dominance of habit-based control of eating behavior (Contreras-Rodríguez et al., 2017; García-García et al., 2014; Smith and Robbins, 2013; Volkow et al., 2013a, Volkow et al., 2013b).
Based on previous findings (Demos et al., 2012; Murdaugh et al., 2012; Stice et al., 2010; Yokum et al., 2014) one could have expected an association also between the baseline fMRI food cue reactivity and intervention outcome. However, there might be several explanations for the lack of significant correlation between these measures in the current study. First, most previous studies finding an association between baseline food cue reactivity and weight loss contrasted fMRI responses to (high-calorie) food images with responses to non-food control images (Murdaugh et al., 2012; Yokum et al., 2014) or simply used brain responses to food images (Demos et al., 2012). In contrast, in the current study we compared high- and low-calorie food images in order to isolate food-reward-related neural processes selectively. Second, it is also important to note that most of these studies (Demos et al., 2012; Stice et al., 2010; Yokum et al., 2014) used baseline food cue reactivity to predict spontaneous weight change, not treatment-related weight change, as in our study. Third, in many cases, the strongest association between food cue reactivity and weight loss was found in the ventral striatum (nucleus accumbens in particular, Demos et al., 2012; Murdaugh et al., 2012) which brain region is strongly affected by susceptibility artifacts and thus would require application of specially designed scanning protocols, which was not feasible in the current study. Further, our population was very heterogeneous in physical and also motivational terms, which is typical in general clinical practice. This might also explain why diet-induced alterations in food cue reactivity, rather than its baseline values, were found to be associated with the intervention outcome. This issue is further complicated by recent findings showing that the presence of DRD2 TaqIA A1 allele significantly moderates the relation between baseline food cue reactivity and weight loss (Stice et al., 2010).
Our findings suggest a new direction in neuroimaging research of obesity focusing on diet-induced early alterations in brain processes that are predictive of weight loss intervention outcome. Strong additional support for such an approach has been provided by a recent study showing that change in fMRI food cue reactivity within the fronto-parietal network underlying self-control of food intake during the first month of the intervention is closely associated with successful weight loss as well as subsequent weight maintenance (Neseliler et al., 2019). The converging evidence provided by the Neseliler et al. (2019) and the current study for the utility of diet-induced early (1-month) alterations of brain fMRI food cue reactivity in prediction of weight loss intervention outcome is especially notable, considering that there were substantial differences in the experimental design between the two studies, including the weight loss intervention protocol and measurement of food cue reactivity (high-calorie vs. non-food images and high-calorie vs. low-calorie food images in the Neseliler et al. (2019) and present study, respectively). Taken together, these findings hold great potential from a translational neuroscience perspective, by suggesting ways by which the process of target identification for weight loss intervention could be shortened and made more efficient.
In this study, there are potential limitations which should be acknowledged. Although the relatively small number of participants tested in this study is common for neuroimaging research on obesity, it could limit the statistical power and generalizability of our findings. Furthermore, given the large interindividual variability in our obese sample, age and baseline body weight might be potential confounding factors affecting our findings. The heterogeneous sample we investigated in this study, however, was more reflective of the general population, not limiting the scope of these results to a specific group. It is important to note, however, that we did not find any correlations between age, baseline BMI, and BMI loss. In addition, to control for the age-related changes in fMRI activity, we used the participants' age as a nuisance covariate in all regression analyses. Another limitation is the lack of a control group. Further studies are justified, and could include larger cohorts, a control group, monitoring of daily food intake and activity.
5. Conclusions
Our findings provide evidence that the efficacy of weight loss intervention can be predicted one month after its onset based on the alteration of fMRI food cue reactivity in the striatum, including the bilateral putamen, right pallidum, and left caudate.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by Gedeon Richter Plc., Budapest, Hungary. ZV was supported by a grant from the Hungarian Brain Research Program (KTIA_13_NAP–A–I/18). CBK was supported by a grant from the Hungarian Academy of Sciences (MTA Mobility Grant FBM2017-6/2017/NKF) and a Fulbright Research Grant (2217201). The authors thank Gábor Simonyi, MD, PhD, FAHA, for hosting and organizing the weight loss program, Ildikó Takács, MD, for DXA support, Gergő Csitári, MD, and Réka Kollár, MD, for their clinical supervision of patient care, and all the employees of the Metabolism Centre of Szent Imre Hospital (Budapest, Hungary) who took part in the clinical program. We also would like to thank all the patients who participated in the study.
Contributor Information
Petra Hermann, Email: hermann.petra@ttk.mta.hu.
Zoltán Vidnyánszky, Email: vidnyanszky.zoltan@ttk.mta.hu.
References
- Avery J.A., Powell J.N., Breslin F.J., Lepping R.J., Martin L.E., Patrician T.M., Donnelly J.E., Savage C.R., Simmons W.K. Obesity is associated with altered mid-insula functional connectivity to limbic regions underlying appetitive responses to foods. J. Psychopharmacol. (Oxf.) 2017;31:1475–1484. doi: 10.1177/0269881117728429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink L., Yokum S., Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. NeuroImage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Ho C.-Y., Richard J.M., DiFeliceantonio A.G. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. Neural Mechanisms of Ingestive Behaviour and Obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D.H. The psychophysics toolbox. Spat. Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Bruce A.S., Holsen L.M., Chambers R.J., Martin L.E., Brooks W.M., Zarcone J.R., Butler M.G., Savage C.R. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int. J. Obes. 2010;2005(34):1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnell S., Gibson C., Benson L., Ochner C.N., Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2012;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Rodríguez O., Martín-Pérez C., Vilar-López R., Verdejo-Garcia A. Ventral and dorsal striatum networks in obesity: link to food craving and weight gain. Biol. Psychiatry. 2017;81:789–796. doi: 10.1016/j.biopsych.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Deckersbach T., Das S.K., Urban L.E., Salinardi T., Batra P., Rodman A.M., Arulpragasam A.R., Dougherty D.D., Roberts S.B. Pilot randomized trial demonstrating reversal of obesity-related abnormalities in reward system responsivity to food cues with a behavioral intervention. Nutr. Diabetes. 2014;4:e129. doi: 10.1038/nutd.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos K.E., Heatherton T.F., Kelley W.M. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel R.H. Obesity and heart disease: a statement for healthcare professionals from the nutrition committee, American Heart Association. Circulation. 1997;96:3248–3250. doi: 10.1161/01.cir.96.9.3248. [DOI] [PubMed] [Google Scholar]
- Elfhag K., Rössner S. Initial weight loss is the best predictor for success in obesity treatment and sociodemographic liabilities increase risk for drop-out. Patient Educ. Couns. 2010;79:361–366. doi: 10.1016/j.pec.2010.02.006. Changing obesity: Theories, facts and interventions. [DOI] [PubMed] [Google Scholar]
- Fontana L., Hu F.B. Optimal body weight for health and longevity: bridging basic, clinical, and population research. Aging Cell. 2014;13:391–400. doi: 10.1111/acel.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D., Heymsfield S.B., Heo M., Jebb S.A., Murgatroyd P.R., Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- García-García I., Horstmann A., Jurado M.A., Garolera M., Chaudhry S.J., Margulies D.S., Villringer A., Neumann J. Reward processing in obesity, substance addiction and non-substance addiction. Obes. Rev. 2014;15:853–869. doi: 10.1111/obr.12221. [DOI] [PubMed] [Google Scholar]
- Gripeteg L., Karlsson J., Torgerson J., Lindroos A.K. Predictors of very-low-energy diet outcome in obese women and men. Obes. Facts. 2010;3:159–165. doi: 10.1159/000314655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A.C., Kaizer A.M., Kelly A.S., Rudser K.D., Ryder J.R., Borzutzky C.R., Santos M., Tucker J.M., Yee J.K., Fox C.K. Long and short of it: early response predicts longer-term outcomes in pediatric weight management. Obesity. 2019;27:272–279. doi: 10.1002/oby.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H., Han C., Kim B. Can obesity cause depression? A pseudo-panel analysis. J. Prev. Med. Pub. Health. 2017;50:262–267. doi: 10.3961/jpmph.17.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handjieva-Darlenska T., Handjiev S., Larsen T.M., van Baak M.A., Jebb S., Papadaki A., Pfeiffer A.F.H., Martinez J.A., Kunesova M., Holst C., Saris W.H.M., Astrup A. Initial weight loss on an 800-kcal diet as a predictor of weight loss success after 8 weeks: the Diogenes study. Eur. J. Clin. Nutr. 2010;64:994–999. doi: 10.1038/ejcn.2010.110. [DOI] [PubMed] [Google Scholar]
- Honea R.A., Szabo-Reed A.N., Lepping R.J., Perea R., Breslin F., Martin L.E., Brooks W.M., Donnelly J.E., Savage C.R. Voxel-based morphometry reveals brain gray matter volume changes in successful dieters. Obes. Silver Spring Md. 2016;24:1842–1848. doi: 10.1002/oby.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A.S., Stanforth P.R., Gagnon J., Rankinen T., Leon A.S., Rao D.C., Skinner J.S., Bouchard C., Wilmore J.H. The effect of sex, age and race on estimating percentage body fat from body mass index: the heritage family study. Int. J. Obes. 2002;26:789–796. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. University of Florida; Gainesville, FL: 2008. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A-8. [Google Scholar]
- Martin L.E., Holsen L.M., Chambers R.J., Bruce A.S., Brooks W.M., Zarcone J.R., Butler M.G., Savage C.R. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obes. Silver Spring Md. 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- Masterson T.D., Kirwan C.B., Davidson L.E., LeCheminant J.D. Neural reactivity to visual food stimuli is reduced in some areas of the brain during evening hours compared to morning hours: an fMRI study in women. Brain Imaging Behav. 2016;10:68–78. doi: 10.1007/s11682-015-9366-8. [DOI] [PubMed] [Google Scholar]
- Mokhtari F., Paolini B.M., Burdette J.H., Marsh A.P., Rejeski W.J., Laurienti P.J. Baseline gray- and white-matter volume predict successful weight loss in the elderly. Obes. Silver Spring Md. 2016;24:2475–2480. doi: 10.1002/oby.21652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh D.L., Cox J.E., Cook E.W., Weller R.E. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackers L.M., Ross K.M., Perri M.G. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int. J. Behav. Med. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet Lond. Engl. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet Lond. Engl. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neseliler S., Hu W., Larcher K., Zacchia M., Dadar M., Scala S.G., Lamarche M., Zeighami Y., Stotland S.C., Larocque M., Marliss E.B., Dagher A. Neurocognitive and hormonal correlates of voluntary weight loss in humans. Cell Metab. 2019;29:39–49. doi: 10.1016/j.cmet.2018.09.024. e4. [DOI] [PubMed] [Google Scholar]
- Ochner C.N., Kwok Y., Conceição E., Pantazatos S.P., Puma L.M., Carnell S., Teixeira J., Hirsch J., Geliebter A. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann. Surg. 2011;253:502–507. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli W.M., O'Reilly R.C., Yarkoni T., Wager T.D. Regional specialization within the human striatum for diverse psychological functions. Proc. Natl. Acad. Sci. U. S. A. 2016;113:1907–1912. doi: 10.1073/pnas.1507610113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli D.G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Pernet C.R., Wilcox R., Rousselet G.A. Robust correlation analyses: false positive and power validation using a new open source matlab toolbox. Front. Psychol. 2013;3 doi: 10.3389/fpsyg.2012.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursey K.M., Stanwell P., Callister R.J., Brain K., Collins C.E., Burrows T.L. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front. Nutr. 2014;1 doi: 10.3389/fnut.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan A.G., Zwahlen M., Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat. Rev. Cancer. 2015;15:484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- Richard D. Cognitive and autonomic determinants of energy homeostasis in obesity. Nat. Rev. Endocrinol. 2015;11:489–501. doi: 10.1038/nrendo.2015.103. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Joliot M., Tzourio-Mazoyer N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. NeuroImage. 2015;122:1–5. doi: 10.1016/j.neuroimage.2015.07.075. [DOI] [PubMed] [Google Scholar]
- Rothemund Y., Preuschhof C., Bohner G., Bauknecht H.-C., Klingebiel R., Flor H., Klapp B.F. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Shah N.R., Braverman E.R. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 2012;7:e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.G., Robbins T.W. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol. Psychiatry. 2013;73:804–810. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Stice E., Yokum S. Gain in body fat is associated with increased striatal response to palatable food cues, whereas body fat stability is associated with decreased striatal response. J. Neurosci. 2016;36:6949–6956. doi: 10.1523/JNEUROSCI.4365-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S., Bohon C., Marti N., Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S., Burger K.S., Epstein L.H., Small D.M. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J. Neurosci. 2011;31:4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel L.E., Weller R.E., Cook E.W., Twieg D.B., Knowlton R.C., Cox J.E. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Tronieri J.S., Wadden T.A., Chao A.M., Pearl R.L., Alamuddin N., Berkowitz R.I. Early weight loss in behavioral treatment predicts later rate of weight loss and response to pharmacotherapy. Ann. Behav. Med. 2019;53:290–295. doi: 10.1093/abm/kay036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Unick J.L., Hogan P.E., Neiberg R.H., Cheskin L.J., Dutton G.R., Evans-Hudnall G., Jeffery R., Kitabchi A.E., Nelson J.A., Pi-Sunyer F.X., West D.S., Wing R.R. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity. 2014;22:1608–1616. doi: 10.1002/oby.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Baler R.D. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci. 2015;38:345–352. doi: 10.1016/j.tins.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Tomasi D., Baler R.D. The addictive dimensionality of obesity. Biol. Psychiatry. 2013;73:811–818. doi: 10.1016/j.biopsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Tomasi D., Baler R.D. Obesity and addiction: neurobiological overlaps. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden T.A., Foster G.D., Wang J., Pierson R.N., Yang M.U., Moreland K., Stunkard A.J., VanItallie T.B. Clinical correlates of short- and long-term weight loss. Am. J. Clin. Nutr. 1992;56:271S–274S. doi: 10.1093/ajcn/56.1.271S. [DOI] [PubMed] [Google Scholar]
- Wiemerslage L., Nilsson E.K., Solstrand Dahlberg L., Ence-Eriksson F., Castillo S., Larsen A.L., Bylund S.B.A., Hogenkamp P.S., Olivo G., Bandstein M., Titova O.E., Larsson E.-M., Benedict C., Brooks S.J., Schiöth H.B. An obesity-associated risk allele within the FTO gene affects human brain activity for areas important for emotion, impulse control and reward in response to food images. Eur. J. Neurosci. 2016;43:1173–1180. doi: 10.1111/ejn.13177. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Friston K.J. Analysis of fMRI time-series revisited—again. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yokum S., Gearhardt A.N., Harris J.L., Brownell K.D., Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obes. Silver Spring Md. 2014;22:2544–2551. doi: 10.1002/oby.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H., Alonso-Alonso M., Hill J.O., Kelley M., Khan N.A. Obesity and the neurocognitive basis of food reward and the control of intake. Adv. Nutr. Bethesda Md. 2015;6:474–486. doi: 10.3945/an.115.008268. [DOI] [PMC free article] [PubMed] [Google Scholar]