Abstract
Mycobacterium tuberculosis (Mtb) remains an important human pathogen. The Mtb cell envelope is a critical bacterial structure that contributes to virulence and pathogenicity. Mycobacterial membrane protein large (MmpL) proteins export bulky, hydrophobic substrates that are essential for the unique structure of the cell envelope and directly support the ability of Mtb to infect and persist in the host. This review summarizes recent investigations that have enabled insight into the molecular mechanisms underlying MmpL substrate export and the role that these substrates play during Mtb infection.
Keywords: Mycobacterium tuberculosis, lipids, MmpL, lipid transport, cell envelope
1. Introduction
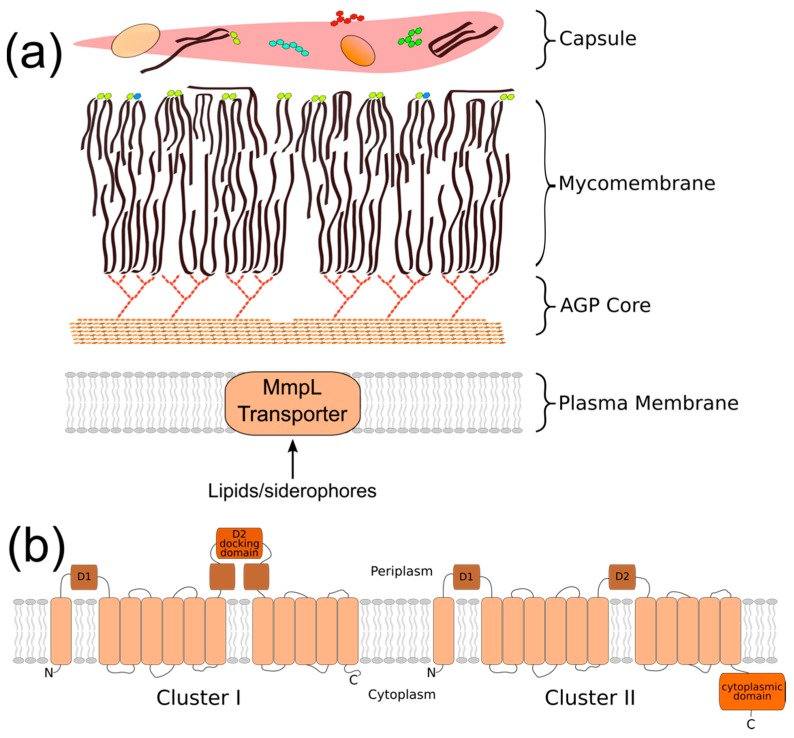
The cell envelope of Mycobacterium tuberculosis (Mtb) is a key attribute of this bacterial pathogen that promotes survival and persistence in the face of host immune responses. This unique structure consists of four regions that define the border between the bacterial cytoplasm and the extracellular environment (Figure 1a). These are the: (1) plasma membrane, (2) arabinogalactan/peptidoglycan cell wall (AGP) core, (3) mycobacterial outer membrane (MOM, or mycomembrane), and 4) “outer layer” (OL, or capsule) [1]. Together, they form a robust physical barrier that contributes to the intrinsic resistance of Mtb against host antimicrobial defenses and limits the access of chemotherapeutics to the bacterial cytoplasm [2]. Additionally, certain cell envelope components directly interfere with aspects of host immunity to potentiate bacterial survival [3,4,5,6]. Thus, the cell envelope is a critical aspect of Mtb physiology and virulence. Investigating the biological processes that generate the cell envelope has the potential to greatly improve the treatment of TB disease.
Figure 1.
Mycobacterial membrane protein large (MmpL) proteins are topologically complex membrane proteins that export cell envelope components across the plasma membrane. (a) Cartoon representation of the mycobacterial cell envelope; (b) M. tuberculosis (Mtb) MmpL can be classified into two clusters based on the complexity of the D2 periplasmic domain and the presence of a cytoplasmic C-terminal domain. Cluster I = MmpL1/2/4/5/6/7/8/9/10/12; cluster II = MmpL3/11/13a/b.
The MmpL (mycobacterial membrane protein large) proteins are increasingly recognized for their importance in establishing the mycobacterial cell envelope. The primary role of the MmpL is to translocate complex, virulence-associated envelope lipids and siderophores across the plasma membrane to the periplasmic space. In the H37Rv strain of Mtb, there are 13 MmpL proteins (MmpL1-13) [7,8]. The majority of MmpL family members are predicted to be >100-kDa, and to consist of 11–12 transmembrane domains (TMD) and two periplasmic loop domains (D1/2) [8,9,10]. Exceptions include MmpL6, which is truncated to 42-kDa (five TMD), and MmpL13, which comprised two adjacent open reading frames, mmpL13a and mmpL13b, that are predicted to encode proteins of 32-kDa (four TMD) and 50-kDa (seven TMD), respectively [8,9].
Other species of mycobacteria harbor different numbers of MmpL proteins. M. leprae, often considered to possess a “minimal” mycobacterial genome as a result of reductive evolution, has five intact mmpL genes (mmpL3/4/7/10/11) as well as a similarly split mmpL13a/b locus [11,12]. These six MmpL proteins likely represent the core set of MmpL transporters, given that they exhibit a high degree of syntenic conservation in both slow growing (Mtb/M. leprae/M. avium/M. marinum) and rapidly growing mycobacteria (M. smegmatis/M. abscessus) [13]. On the other hand, M. abscessus possesses up to 31 putative MmpL transporters [11]. This illustrates the increased prevalence of genes coding for MmpL transporters in the genomes of rapidly growing, compared to slow growing, mycobacteria species [13]. Mtb is a pernicious mycobacterial human pathogen and H37Rv is the most widely used Mtb reference strain; therefore, we strive, where possible, to consider the MmpL and their contributions to virulence and pathogenicity in this context.
A subset of H37Rv mmpL genes (mmpL1,2,4,5) are accompanied by accessory mmpS (mycobacterial membrane protein small; mmpS1,2,4,5) open reading frames, which are each predicted to contain one to two TMD [7,10]. MmpS4 and -S5 proteins have been shown to interact with their cognate MmpL transporters to permit substrate extrusion [14]. Little is known about the other MmpS proteins and their potential roles in the transport of MmpL substrates. rv2198c was annotated as mmpS3 due to partial homology with other mmpS genes, though it was not located in an operon with a cognate mmpL gene. Recently, it was shown to be a component of the mycobacterial division machinery and renamed lamA [15].
2. Phylogeny/Structure/Bioenergetics
The MmpL family members are traditionally categorized as belonging to the RND (resistance, nodulation, and cell division) superfamily of integral membrane permeases based on their topological organization [8,16]. Phylogenetic comparison has further classified the 10 “full-length” MmpL into two hydrophobe/amphiphile efflux (HAE) subfamilies of the RND superfamily. The MmpL1/2/4/5/8/10/12 belong to the HAE2 family of Gram-positive efflux pumps, while the MmpL3/7/11 are instead categorized into the HAE3 archaeal family [9,17]. Chim et al. proposed a two-cluster MmpL classification scheme based on predicted structural motifs (Figure 1b) [18]. MmpL cluster I comprises MmpL1/2/4/5/6/7/8/9/10/12, and is distinguished by the presence of a predicted docking domain in the periplasmic D2 loop region. MmpL cluster II comprises MmpL3/11 and the predicted MmpL13a/b fusion protein. It lacks the D2 periplasmic docking domain and possesses a substantial C-terminal cytoplasmic domain. Both classification schemes classify MmpL3 and MmpL11 separate from the other MmpL transporters.
Many RND family proteins contribute to antibiotic resistance and stress-response in Gram-negative bacteria (reviewed in Reference [19]). The best characterized RND protein is AcrB (E. coli), which, together with a membrane fusion protein (MFP) and an outer membrane factor (OMF), functions as a multidrug efflux pump to extrude substrates through the periplasm to the extracellular environment [20,21,22,23,24]. The association of RND-MFP-OMF proteins to form an extrusion channel through the periplasm is paradigmatic in Gram-negative bacteria, with notable examples found in P. aeruginosa (MexAB/OprM) [25,26] and C. metallidurans (ZneABC) [27,28]. While Mtb exhibits pseudo-Gram-negative membrane organization, it remains to be determined if the MmpL interact with periplasmic/outer membrane proteins to form a similar tripartite export apparatus that would enable efficient translocation and proper localization of their substrates. Periplasmic lipoproteins such as LprG and LppX are required for the proper surface expression of some cell envelope components, suggesting that these proteins may be functioning as MFPs [29,30]. However, these lipoproteins have not been shown to interact with any MmpL transporters. MmpS proteins may be candidate MFPs given that MmpS4/5 interact with MmpL4/5 and are required for the transport of their siderophore substrates. However, only some MmpL transporters have cognate MmpS proteins, and MmpL-MmpS protein–protein interactions have not been demonstrated for MmpS1/2. CpnT has been described as an Mtb outer membrane porin, and may be a putative OMF [31,32]. How MmpL substrates traverse the periplasmic space and cell wall core en route to the mycomembrane remains an area of active inquiry.
The crystal structures of the apo- and inhibitor-bound M. smegmatis MmpL3 orthologue were recently reported, representing an important advancement in the field [33]. Notably, MmpL3 was observed to be monomeric, a finding that was corroborated by native protein electrophoresis. This differs from most known bacterial RND permeases, which typically associate as either homodimers or homotrimers [26,34,35]. Indeed, a previous structural study of the purified Corynebacterial MmpL3 orthologue CmpL1, via size exclusion chromatography and negative staining electron microscopy (EM), indicated homotrimeric association [36]. A subsequent MmpL3 homology structure, modeled on the EM-refined CmpL1 homology model, suggested that Mtb MmpL3 also associates as a homotrimer. Additional structures and analyses are needed to reconcile these conflicting observations and determine whether the functional MmpL transporter units differ by species and if all MmpL proteins are monomeric.
The transmembrane region of apo-MmpL3 exhibited pseudo-two-fold symmetry, with the central TM4 and TM10 domains hydrogen-bonded to each other via a pair of Asp-Tyr dyads [33]. The periplasmic region was observed to contain three openings leading to a central cavity, which are presumably important for substrate extrusion. Individually, the structure of each periplasmic MmpL3 D1 and D2 subdomain broadly resembled that of the isolated Mtb MmpL11 D2 domain, obtained by Chim et al. [18]. This work demonstrated a homology between the MmpL11 D2 domain and the periplasmic transporter/porter subdomains of the RND permeases MexB and ZneA. Furthermore, the MmpL11 D1 and D2 periplasmic domains were shown to interact with each other. This was also the case for the MmpL3 D1 and D2 periplasmic domains, suggesting that intra- or inter-MmpL interactions occur in the periplasm [18].
The proton motive force (PMF) is understood to be the underlying energetic basis for most RND-mediated substrate transport [24,37,38]. RND proteins generally function as antiporters, coupling proton translocation across the inner membrane to conformational changes that result in substrate extrusion [39,40]. A suite of conserved residues found in TM4 and TM10 are critical for proton transfer down the membrane electrochemical gradient [38,41,42]. Multiple sequence alignments of the Mtb H37Rv MmpL identified similarly conserved regions in MmpL TM4/10 [43]. These include the central Asp/Tyr-containing TM4/10 segments observed in the M. smegmatis MmpL3 crystal structure, strongly suggesting that the core of each MmpL monomer is responsible for proton translocation [33]. Indeed, mutation of either the TM10 D640 or Y641 residues completely abrogated MmpL3 function [43], while mutation of the homologous Y610 residue likewise resulted in cessation of MmpL11-mediated lipid transport (Purdy Lab, unpublished data). Furthermore, several ostensible chemical inhibitors of MmpL3 appear to function by non-specifically disrupting the PMF [44]. Others, including the developmental therapeutic drug SQ109, directly bind MmpL3 in the putative proton translocation channel defined by TM4/10 [33]. However, the observation that SQ109 failed to inhibit MmpL3-mediated substrate transport in a spheroplast-based assay raises questions about whether this interaction is sufficient to abrogate MmpL3 transporter activity [45]. Regardless of the exact mechanism of action of SQ109, the data strongly suggest that MmpL substrate transport is energetically dependent on the PMF, like that of most other RND permeases.
3. Roles in Virulence
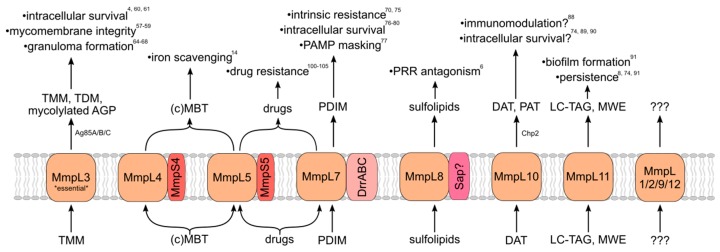
MmpL proteins indirectly contribute to virulence via the transport of substrates that directly influence bacterial survival in the human host when properly localized. Most, but not all, identified MmpL substrates are virulence-associated envelope lipids (Figure 2). MmpL-transported lipids are incorporated into both leaflets of the mycomembrane, from which Mtb derives much of its intrinsic resistance and immunomodulatory capacity. Other MmpL substrates are important for responding to environmental and nutrient stress. How MmpL substrates influence Mtb virulence is therefore essential for understanding the importance of the MmpL protein family to the pathogenic lifestyle of Mtb.
Figure 2.
MmpL transporters export substrates that are important for virulence and pathogenicity. MmpL proteins and membrane-associated transport partners, exported substrates, and contributions to virulence/pathogenicity of the mature exported substrates. TMM/TDM—trehalose mono/di-mycolate; AGP—arabinogalactan/peptidoglycan complex; (c)MBT—(carboxy)mycobactin; PDIM—phthiocerol dimycocerosate; PAMP—pathogen-associated molecular pattern; PRR—pattern recognition receptor; DAT/PAT—di/poly-acyl trehalose; LC-TAG—long-chain triacylglycerol; and MWE—mycolate wax ester.
3.1. MmpL3 (TMM)
MmpL3 (Rv0206c) is responsible for transporting trehalose monomycolate (TMM), and is the only MmpL for whom transporter activity has been directly determined via biochemical assay [45]. TMM transport by MmpL3 has also been demonstrated indirectly, based on the observation that genetic or chemical ablation of MmpL3 activity reduces cell envelope mycolylation [46,47]. Mycolic acids are unique, long chain (C60-90) α-alkyl β-hydroxy fatty acids that are synthesized in the bacterial cytoplasm and esterified to the disaccharide trehalose to generate TMM (reviewed in References [48,49]). Once exported, mycolic acid transfer in the cell envelope is catalyzed by the members of the secreted antigen 85 mycolyltransferase complex (Ag85A/B/C, Rv3804c/1886c/0129c) [50,51,52]. When esterified to the non-reducing ends of the cell wall AGP core, mycolic acids constitute the inner leaflet of the mycomembrane [53,54,55]. Additionally, mycolic acid transfer between molecules of TMM generates trehalose dimycolate (TDM), an abundant and important virulence-associated free lipid [50,51,52].
Genetic approaches to knock out mmpL3 have been uniformly unsuccessful; however, conditional knockdown and chemical inhibition strategies revealed that reduction or inhibition of MmpL3 caused accumulation of TMM in the cytoplasm and a reduction of cell envelope mycolylation [8,46,56,57,58]. MmpL3 is therefore the only essential MmpL protein, underscoring the importance of mycolic acid export to Mtb physiology. These observations also highlight MmpL3 as an attractive drug target [57,59].
Apart from their importance to the covalently-anchored inner leaflet of the mycomembrane, mycolic acids are also an essential component of TDM, perhaps the quintessential Mtb virulence-associated glycolipid [60,61]. TDM was originally described as a “cord factor,” referring to the tendency of mycobacteria harboring TDM to aggregate about their long axes to form “cords” of bacteria [62,63]. It plays an extremely important role in the infectious context by promoting Mtb survival in the macrophage through inhibiting phagosomal maturation via recruitment of the host pattern recognition receptor Mincle during Fcγ-R mediated phagocytosis [4]. In the host, purified TDM induces granuloma formation, also in a Mincle-dependent manner [64,65,66,67,68].
3.2. MmpL7 (PDIM)
MmpL7 (Rv2942) is associated with transport of phthiocerol dimycocerosates (PDIM), a family of long-chain β-diols esterified with polymethyl-branched fatty acids [69]. Interestingly, PDIM translocation is also dependent on the presence of the DrrABC (Rv2936-8) transporter in addition to MmpL7, a unique requirement for the transport of MmpL substrates [70]. Once exported into the periplasm, the proper surface localization of PDIM is mediated by the lipoprotein LppX (Rv2945c), potentially via a direct protein–lipid interaction [29].
The genes responsible for PDIM biosynthesis and transport are clustered together in the Mtb genome [71]. PDIM production occurs in the cytoplasm, and is likely spatially and temporally coordinated with MmpL7-mediated transport [72,73]. This model was suggested by an observed protein–protein interaction between the D2 domain of MmpL7 and the PDIM biosynthetic polyketide synthase PpsE (Rv2935) in a two-hybrid system [73]. This result was surprising since MmpL topology predictions strongly suggest that the D2 domain projects into the periplasm. Because PpsE is localized in the cytoplasm, this interaction could suggest that MmpL topology is fundamentally dissimilar to that of other RND family proteins, or, as suggested by the authors, that the D2 domain is able to access the cytoplasm, possibly through a pore formed by the MmpL7 TM domains. Whether this activity might be a shared feature of the Mtb MmpL or unique to MmpL7 is not known.
MmpL7 is required for virulence in mouse models of infection due to its role in PDIM transport [8,74]. PDIM plays multiple roles in the virulence of Mtb. The highly hydrophobic nature of PDIM limits the permeability of the Mtb cell envelope and contributes to intrinsic resistance against antimicrobial compounds [70,75]. PDIM also promotes the uptake of Mtb by permissive macrophages [76,77], and appears to enable phagosomal escape upon internalization [76,77,78,79,80]. Additionally, PDIM contributes to Mtb virulence by masking the recognition of pathogen associated molecular patterns by host innate immune receptors [77].
3.3. MmpL8 (Sulfolipids)
MmpL8 (Rv3823c) transports cell envelope sulfolipids [81,82]. Sulfolipids consist of a sulfated trehalose tetra-acylated with straight chain and multiple polymethyl-branched fatty acids [83,84]. The diacylated SL1278 precursor is synthesized in the cytoplasm through the sequential esterification of sulfated trehalose with palmitate and (hydroxy)phthioceranoate by the polyketide synthase associated proteins PapA2 (Rv3820c) and PapA1 (Rv3824c), respectively [84]. Two additional (hydroxy)phthioceranoate additions are performed by the cytoplasmic membrane-associated acyltransferase Chp1 (Rv3822) to generate mature tetra-acylated sulfolipid-1 (SL-1), which is subsequently translocated by MmpL8 [85]. The integral membrane protein Sap (Rv3821), while not absolutely required for SL-1 transport, appears to enhance surface expression of SL-1. It is suggested that Sap interacts with MmpL8 and the sulfolipid biosynthetic enzymes to coordinate biosynthesis and transport [85].
Sulfolipids contribute to Mtb virulence by inhibiting activation of the host pattern recognition receptor toll-like receptor 2, thereby limiting the innate immune response to Mtb [6]. This immunomodulation is contingent upon proper localization of sulfolipids in the mycobacterial cell envelope. MmpL8-deficient Mtb exhibits attenuated growth and pathogenesis in mouse infection models [8,74,81,82].
3.4. MmpL10 (Di/Poly-Acyltrehaloses)
MmpL10 (Rv1183) translocates diacyltrehaloses (DAT) across the plasma membrane, where they are further acylated to generate penta-acyltrehaloses (PAT) [86]. DAT biosynthesis is initiated in the cytoplasm by the esterification of a straight chain fatty acid to the 2-position of trehalose by the acyltransferase PapA3 (Rv1182), which is likely also responsible for incorporation of a polymethyl-branched fatty acid at the 3-position [87]. MmpL10-mediated transport of DAT to the periplasm allows the transfer of additional polymethyl-branched fatty acid moieties between DATs by the periplasmic acyltransferase Chp2 (Rv1184c) to generate PAT [86]. The topological discontinuity of PAT biosynthesis is reminiscent of TDM biosynthesis, with transport of a cytoplasmic precursor to the periplasm where additional biosynthetic enzymes operate.
The contribution of DAT/PAT to Mtb virulence is not completely understood. Purified DAT inhibited T-cell proliferation and production of proinflammatory cytokines by macrophages in vitro [88]. MmpL10 did not appear to contribute to Mtb virulence in a mouse aerosol infection model, implying that its substrates are dispensable for a typical Mtb infection [8]. However, another study found that an mmpL10 mutant was attenuated in an intravenous mouse infection [74]. Perplexingly, a different study showed that a DAT/PAT-deficient strain of Mtb more readily infected macrophages and was hypervirulent in an intravenous, but not a respiratory, mouse infection model [89]. Finally, a recent study found that an Mtb DAT/PAT mutant was attenuated only in the absence of PDIM, leading the researchers to conclude that these methyl-branched cell envelope lipids may be functionally redundant [90].
3.5. MmpL11 (Long-Chain TAG/Mycolate Wax Esters)
MmpL11 (Rv0202c) transports long-chain triacylglycerols (LC-TAG) and mycolate wax esters (MWE) in Mtb [91]. These lipids are of particular importance during biofilm formation. Since Mtb in biofilms is phenotypically drug tolerant, LC-TAG and MWE may contribute to the extensive drug treatment regimens necessary to cure TB disease [92]. Furthermore, these lipids may play a role in persistence in the host. Mtb mmpL11 mutants are attenuated for survival in an in vitro granuloma model and during long-term infections in mice, suggesting that MmpL11 lipid substrates are required for the maintenance of long-term Mtb infection [8,74,91].
3.6. MmpL4/5 (Mycobactin/Carboxymycobactin)
MmpL4 and MmpL5 are unique in that they do not transport a cell envelope lipid, but instead export mycobacterial siderophores. Iron is critical for many cellular processes and iron restriction is a common strategy used by hosts to combat infection. Mtb produces two siderophores to scavenge iron from the environment: lipophilic mycobactin (MBT) and hydrophilic carboxymycobactin (cMBT). The MmpL4/5 transporters (Rv0450c/0676c) and their cognate MmpS4/5 accessory proteins (Rv0451c/0677c) coordinate biosynthesis and transport of these siderophore substrates [14]. MmpL4 and MmpL5 are at least partially redundant, given that single mutants of either mmpS4 or mmpS5 are not affected in iron-limited culture conditions. However, an mmpS4/5 double mutant exhibited a significant growth defect in low-iron culture, failed to produce and export siderophores, and was markedly less virulent in a mouse infection model compared to wild-type Mtb [14]. Interestingly, a genetic method showed that MmpL5 was capable of interacting with either MmpS4 or MmpS5, whereas MmpL4 was only capable of interacting with MmpS4 [14].
Siderophore export likely depends on MmpL/MmpS interactions in the periplasm. For MmpL4, this was suggested by the fact that the MmpL4 D1 periplasmic region co-precipitates with the periplasmic region of MmpS4 [14]. Another group demonstrated, via molecular dynamics simulations, that MBT is taken up from the cytoplasm by MmpL5, and that the periplasmic interaction between MmpL5 and MmpS5 was likely required for MBT release into the periplasm [93]. While in vitro models of iron sensitivity have suggested that the MmpL/S4 and S5 systems are functionally redundant, an mmpL4 single mutant was attenuated in a mouse model of infection [8]. However, another group observed growth attenuation of mmpL5 mutants in the lungs of infected mice after intravenous inoculation [74]. Interestingly, the same study reported a reduction in mmpL4 mutant growth in the spleen compared to wild-type Mtb. Combined, these observations imply that the two different siderophore export systems have differing levels of importance depending on the infection context.
3.7. Other MmpL Transporters
The substrates and functions of the Mtb MmpL1/2/6/9/12/13a/b transporters have not yet been conclusively identified. However, these transporters are not as critical as the aforementioned MmpL for virulence of Mtb as assessed by the mouse model of infection [8]. Nevertheless, some clues as to the functions of these transporters can be derived from the genomic contexts of their loci, and through comparison with orthologous proteins in other mycobacterial species.
The genes encoding MmpL1 (Rv0402c) and MmpL12 (Rv1522c) are both located near pks and fad genes, implying that they transport complex glyco- or polyketide lipids similar to TMM or PDIM [11]. Indeed, the exported substrate of MAB_0855, a putative M. abscessus orthologue of MmpL12, was recently identified as a glycosyl diacylated nonadecyl diol, suggesting that the substrate of MmpL12 in Mtb is a heretofore undescribed complex cell envelope glycolipid [94].
Very little is known about MmpL2 (Rv0507). The genomic context of the mmpL2 gene does not give any clues as to the nature of its putative substrate. MmpL2 is dispensable during pulmonary models of infection, and there are conflicting reports regarding its relevance during intravenous infection [8,71,74]. MmpL9 (Rv2339) is similarly mysterious. It appears to be involved in the inhibition of phagosomal maturation; however, loss of this capability did not impact the survival of a ∆mmpL9 mutant strain of Mtb in ex vivo infections [95]. Another study observed increased susceptibility of an mmpL9 mutant to in vitro oxidative stress [96]. Despite these observations, mmpL9-deficient Mtb is still able to replicate effectively in mouse infection models [8,74].
MmpL13a/b (Rv1145/1146) is a unique case. An intact full-length MmpL13 orthologue was found in both M. bovis and M. canettii, suggesting that the gene split occurred during the evolution of modern Mtb [9]. Interestingly, these genes appear to be separate, functional open reading frames in H37Rv Mtb, whereas they are pseudogenes in M. leprae, implying that the mmpL13 gene is currently undergoing a pseudogenization process in modern Mtb.
As mentioned, MmpL6 (Rv1557) is truncated relative to the other MmpL in H37Rv Mtb. This likely occurred during the evolution of modern circulating Mtb strains, during which its cognate mmpS6 gene was completely lost [97]. A recent paper demonstrated that an un-truncated “ancestral” version of the mmpL/S6 operon conferred oxidative stress resistance to “modern” Mtb lineages [98]. However, it is not known whether this increased resistance is linked to substrate export or to some other mechanism. Production of reactive oxygen species is a well-described defense mechanism of innate immunity (reviewed in Reference [99]). Thus, it is somewhat perplexing how this ostensibly beneficial means of intrinsic resistance was lost during Mtb-host co-evolution.
3.8. MmpL as Drug Exporters
In addition to transporting their endogenous cell envelope substrates, certain MmpL transporters may also act as drug efflux pumps. This has been most convincingly shown for the MmpL5/S5 transporter system in both Mtb and nontuberculous mycobacteria (NTM). Increased resistance to azole drugs, as well as to clofazimine and bedaquiline, can arise through mutations in rv0678, a transcriptional repressor that inhibits expression of mmpL5/S5 [100,101]. Altering the repressive ability of Rv0678 results in over-expression of mmpL5/S5 and resistance to the aforementioned drugs via drug efflux. Similarly, mutations in the transcriptional repressor of an MmpL5/S5 orthologue also augmented resistance to clofazimine and bedaquiline in the clinically important NTM species M. abscessus [102]. De-repression of a different MmpL5/S5 orthologue in M. abscessus likewise conferred increased resistance to thioacetazone-based chemotherapeutics [103]. In addition to MmpL5/S5, MmpL7 has also been implicated in drug efflux. The upregulated expression of mmpL7 in response to isoniazid treatment increases mycobacterial resistance to this important frontline TB antibiotic through direct export [104,105].
4. MmpL Regulation
4.1. Transcriptional Regulation
A number of transcriptional regulators that bind to mmpL genomic regions were identified via ChIP-seq [106]. These include Rv0302, Rv0678, Rv1816, and Rv3249c. Further studies revealed that these transcriptional regulators modulate their activity by binding fatty acid ligands, implying that Mtb alters its cell wall in response to metabolic cues [107,108,109,110,111]. This is particularly relevant during infection because Mtb metabolizes fatty acids in the host [107,112]. Rv0302 is a TetR-family transcriptional regulator that binds in the intragenic regions of mmpL1/2/7/9, upstream of mmpL3 and in the promoters of mmpL6 and mmpL11 [106,109]. Upon binding palmitic acid, the Rv0302 dimer dissociates from its target DNA [109]. Rv3249 is also a TetR-like transcription factor that binds the promoters of mmpS1 and mmpL3, and inside mmpL11. It also releases from its target DNA sequences upon binding palmitic acid [110]. Rv1816 binds to promoter and intergenic regions of mmpL3, mmpL7, and mmpL11 [110]. Additionally, it binds kasA, which is involved in mycolic acid biosynthesis [113]. Thus, Rv1816 represents a single point of control at which production and export of cell envelope components may be transcriptionally regulated. Rv1816 binds two fatty acid ligands, lauric acid and palmitic acid; however, palmitic acid was the only ligand that reduced DNA binding activity [110]. Rv0678 is a member of the MarR family of transcriptional regulators that binds the promoter regions of mmpL2, mmpL4/S4, and mmpS5 as a dimer [111]. The binding of 2-stearoylglycerol by Rv0678 reduced its ability to interact with target sequences [111].
Combined, these observations suggest that Mtb monitors its metabolic state and adjusts its cell wall composition in response to metabolic cues. This would presumably enable Mtb to better persist in the face of the host immune response during the course of establishing an infection.
4.2. Post-Translational Regulation
MmpL transporter function may also be regulated through post-translational modification. A likely means of MmpL regulation is via phosphorylation by serine/threonine protein kinases (STPKs). Mtb has 11 STPKs, nine of which contain a cytoplasmic kinase domain, a transmembrane domain, and an extracellular sensor domain (reviewed in Reference [114]). STPK-mediated phospho-regulation of protein function is therefore an immediate way to alter cellular processes in response to external stimuli.
MmpL7 is a potential substrate of the PknD kinase [115]. Surprisingly, the identified phospho-residue lies in the predicted D2 periplasmic loop region, which would not be expected to interact with the cytoplasmic kinase domain of PknD. However, as mentioned above, the MmpL7 D2 domain may access the cytoplasm [73]. How phosphorylation affects the MmpL7 transporter function has not been demonstrated.
MmpL3 and MmpL11 have substantial C-terminal cytoplasmic domains that are likely sites of phospho-regulation by mycobacterial STPKs. Indeed, both MmpL3 and MmpL11 were found to be phosphorylated in a comprehensive survey of Mtb phospho-proteins [116]. MmpL3 had extensive C-terminal phosphorylation, with multiple phospho-residues identified in a variety of culture and in vitro stress conditions. However, the MmpL11 C-terminus was only phosphorylated at a single residue when grown with acetate as the sole carbon source, suggesting that phosphorylation of MmpL proteins occurs in response to metabolic cues [116]. Future studies should investigate the effects of phosphorylation on MmpL transporter activity.
5. Conclusions
The MmpL transporters are an important family of inner membrane transporters in Mtb. Their substrates make immense contributions to Mtb virulence and pathogenicity. MmpL3-transported mycolic acids are an essential part of the mycomembrane and confer much of Mtb’s intrinsic resistance to the antimicrobial defenses of innate immunity. Other MmpL substrates such as PDIM, sulfolipids, and the acylated trehaloses modulate host immunity in subtler ways. Storage lipids exported by MmpL11 enable long-term persistence in the face of the adaptive immune response. Siderophore transport by MmpL4 and MmpL5 and their cognate MmpS proteins permits Mtb to acquire essential iron in the restrictive environment of the host. Therefore, this relatively small family of transporters plays a substantial role in supporting Mtb’s specialization as a devastating human pathogen.
Author Contributions
Writing—original draft preparation, G.M.; writing—review and editing, G.E.P.
Funding
Research in the Purdy Laboratory is funded by the National Institutes of Health, R01 AI087840, R01 AI123148, R21 AI113074 to GEP. GM was funded in part by T32 AI007472.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chiaradia L., Lefebvre C., Parra J., Marcoux J., Burlet-Schiltz O., Etienne G., Tropis M., Daffe M. Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane. Sci. Rep. 2017;7:12807. doi: 10.1038/s41598-017-12718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan P.J., Nikaido H. The Envelope of Mycobacteria. Annu. Rev. Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 3.Indrigo J. Cord factor trehalose 6,6’-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology. 2003;149:2049–2059. doi: 10.1099/mic.0.26226-0. [DOI] [PubMed] [Google Scholar]
- 4.Patin E.C., Geffken A.C., Willcocks S., Leschczyk C., Haas A., Nimmerjahn F., Lang R., Ward T.H., Schaible U.E. Trehalose dimycolate interferes with FcγR-mediated phagosome maturation through Mincle, SHP-1 and FcγRIIB signalling. PLoS ONE. 2017;12:e0174973. doi: 10.1371/journal.pone.0174973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vergne I., Chua J., Lee H.-H., Lucas M., Belisle J., Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanc L., Gilleron M., Prandi J., Song O.-R., Jang M.-S., Gicquel B., Drocourt D., Neyrolles O., Brodin P., Tiraby G., et al. Mycobacterium tuberculosis inhibits human innate immune responses via the production of TLR2 antagonist glycolipids. Proc. Natl. Acad. Sci. USA. 2017;114:11205–11210. doi: 10.1073/pnas.1707840114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole S.T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S.V., Eiglmeier K., Gas S., Barry C.E., et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 8.Domenech P., Reed M.B., Barry C.E. Contribution of the Mycobacterium tuberculosis MmpL Protein Family to Virulence and Drug Resistance. Infect. Immun. 2005;73:3492–3501. doi: 10.1128/IAI.73.6.3492-3501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandhu P., Akhter Y. The internal gene duplication and interrupted coding sequences in the MmpL genes of Mycobacterium tuberculosis: Towards understanding the multidrug transport in an evolutionary perspective. Int. J. Med. Microbiol. 2015;305:413–423. doi: 10.1016/j.ijmm.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Krogh A., Larsson B., von Heijne G., Sonnhammer E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 11.Kapopoulou A., Lew J.M., Cole S.T. The MycoBrowser portal: A comprehensive and manually annotated resource for mycobacterial genomes. Tuberculosis. 2011;91:8–13. doi: 10.1016/j.tube.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Eiglmeier K., Parkhill J., Honoré N., Garnier T., Tekaia F., Telenti A., Klatser P., James K.D., Thomson N.R., Wheeler P.R., et al. The decaying genome of Mycobacterium leprae. Lepr. Rev. 2001;72:387–398. [PubMed] [Google Scholar]
- 13.Viljoen A., Dubois V., Girard-Misguich F., Blaise M., Herrmann J.-L., Kremer L. The diverse family of MmpL transporters in mycobacteria: From regulation to antimicrobial developments. Mol. Microbiol. 2017;104:889–904. doi: 10.1111/mmi.13675. [DOI] [PubMed] [Google Scholar]
- 14.Wells R.M., Jones C.M., Xi Z., Speer A., Danilchanka O., Doornbos K.S., Sun P., Wu F., Tian C., Niederweis M. Discovery of a Siderophore Export System Essential for Virulence of Mycobacterium tuberculosis. PLoS Pathog. 2013;9:e1003120. doi: 10.1371/journal.ppat.1003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rego E.H., Audette R.E., Rubin E.J. Deletion of a mycobacterial divisome factor collapses single-cell phenotypic heterogeneity. Nature. 2017;546:153–157. doi: 10.1038/nature22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tekaia F., Gordon S.V., Garnier T., Brosch R., Barrell B.G., Cole S.T. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber. Lung Dis. 1999;79:329–342. doi: 10.1054/tuld.1999.0220. [DOI] [PubMed] [Google Scholar]
- 17.Tseng T.T., Gratwick K.S., Kollman J., Park D., Nies D.H., Goffeau A., Saier M.H., Jr. The RND permease superfamily: An ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1999;1:107–125. [PubMed] [Google Scholar]
- 18.Chim N., Torres R., Liu Y., Capri J., Batot G., Whitelegge J.P., Goulding C.W. The Structure and Interactions of Periplasmic Domains of Crucial MmpL Membrane Proteins from Mycobacterium tuberculosis. Chem. Biol. 2015;22:1098–1107. doi: 10.1016/j.chembiol.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delmar J.A., Su C.C., Yu E.W. Bacterial Multidrug Efflux Transporters. Biophysics. 2014;43:93–117. doi: 10.1146/annurev-biophys-051013-022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okusu H., Ma D., Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma D., Cook D.N., Alberti M., Pon N.G., Nikaido H., Hearst J.E. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J. Bacteriol. 1993;175:6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinh T., Paulsen I.T., Saier M.H. A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J. Bacteriol. 1994;176:3825–3831. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulsen I.T., Park J.H., Choi P.S., Saier M.H. A family of Gram-negative bacterial outer membrane factors that function in the export of proteins, carbohydrates, drugs and heavy metals from Gram-negative bacteria. FEMS Microbiol. Lett. 1997;156:1–8. doi: 10.1016/S0378-1097(97)00379-0. [DOI] [PubMed] [Google Scholar]
- 24.Zgurskaya H.I., Nikaido H. Bypassing the periplasm: Reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1999;96:7190–7195. doi: 10.1073/pnas.96.13.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole K., Krebes K., McNally C., Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: Evidence for involvement of an efflux operon. J. Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sennhauser G., Bukowska M.A., Briand C., Grütter M.G. Crystal Structure of the Multidrug Exporter MexB from Pseudomonas aeruginosa. J. Mol. Biol. 2009;389:134–145. doi: 10.1016/j.jmb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 27.De Angelis F., Lee J.K., O’Connell J.D., Miercke L.J.W., Verschueren K.H., Srinivasan V., Bauvois C., Govaerts C., Robbins R.A., Ruysschaert J.-M., et al. Metal-induced conformational changes in ZneB suggest an active role of membrane fusion proteins in efflux resistance systems. Proc. Natl. Acad. Sci. USA. 2010;107:11038–11043. doi: 10.1073/pnas.1003908107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pak J.E., Ekendé E.N., Kifle E.G., O’Connell J.D., De Angelis F., Tessema M.B., Derfoufi K.-M., Robles-Colmenares Y., Robbins R.A., Goormaghtigh E., et al. Structures of intermediate transport states of ZneA, a Zn(II)/proton antiporter. Proc. Natl. Acad. Sci. USA. 2013;110:18484–18489. doi: 10.1073/pnas.1318705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sulzenbacher G., Canaan S., Bordat Y., Neyrolles O., Stadthagen G., Roig-Zamboni V., Rauzier J., Maurin D., Laval F., Daffe M., et al. LppX is a lipoprotein required for the translocation of phthiocerol dimycocerosates to the surface of Mycobacterium tuberculosis. EMBO J. 2006;25:1436–1444. doi: 10.1038/sj.emboj.7601048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaur R.L., Ren K., Blumenthal A., Bhamidi S., Gibbs S., Jackson M., Zare R.N., Ehrt S., Ernst J.D., Banaei N. LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of Mycobacterium tuberculosis. PLoS Pathog. 2014;10:e1004376-14. doi: 10.1371/journal.ppat.1004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danilchanka O., Sun J., Pavlenok M., Maueröder C., Speer A., Siroy A., Marrero J., Trujillo C., Mayhew D.L., Doornbos K.S., et al. An outer membrane channel protein of Mycobacterium tuberculosis with exotoxin activity. Proc. Natl. Acad. Sci. USA. 2014;111:6750–6755. doi: 10.1073/pnas.1400136111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danilchanka O., Pires D., Anes E., Niederweis M. The Mycobacterium tuberculosis outer membrane channel protein CpnT confers susceptibility to toxic molecules. Antimicrob. Agents Chemother. 2015;59:2328–2336. doi: 10.1128/AAC.04222-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B., Li J., Yang X., Wu L., Zhang J., Yang Y., Zhao Y., Zhang L., Yang X., Yang X., et al. Crystal Structures of Membrane Transporter MmpL3, an Anti-TB Drug Target. Cell. 2019;176:636–648. doi: 10.1016/j.cell.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Murakami S., Nakashima R., Yamashita E., Yamaguchi A. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature. 2002;419:587–593. doi: 10.1038/nature01050. [DOI] [PubMed] [Google Scholar]
- 35.Kumar N., Su C.C., Chou T.H., Radhakrishnan A., Delmar J.A., Rajashankar K.R., Yu E.W. Crystal structures of the Burkholderia multivorans hopanoid transporter HpnN. Proc. Natl. Acad. Sci. USA. 2017;114:6557–6562. doi: 10.1073/pnas.1619660114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belardinelli J.M., Yazidi A., Yang L., Fabre L., Li W., Jacques B., Angala S.K., Rouiller I., Zgurskaya H.I., Sygusch J., et al. Structure–Function Profile of MmpL3, the Essential Mycolic Acid Transporter from Mycobacterium tuberculosis. ACS Infect. Dis. 2016;2:702–713. doi: 10.1021/acsinfecdis.6b00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thanassi D.G., Cheng L.W., Nikaido H. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su C.C., Li M., Gu R., Takatsuka Y., McDermott G., Nikaido H., Yu E.W. Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. J. Bacteriol. 2006;188:7290–7296. doi: 10.1128/JB.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seeger M.A., Schiefner A., Eicher T., Verrey F., Diederichs K., Pos K.M. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science. 2006;313:1295–1298. doi: 10.1126/science.1131542. [DOI] [PubMed] [Google Scholar]
- 40.Murakami S., Nakashima R., Yamashita E., Matsumoto T., Yamaguchi A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature. 2006;443:173–179. doi: 10.1038/nature05076. [DOI] [PubMed] [Google Scholar]
- 41.Guan L., Nakae T. Identification of Essential Charged Residues in Transmembrane Segments of the Multidrug Transporter MexB of Pseudomonas aeruginosa. J. Bacteriol. 2001;183:1734–1739. doi: 10.1128/JB.183.5.1734-1739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami S., Yamaguchi A. Multidrug-exporting secondary transporters. Curr. Opin. Struct. Biol. 2003;13:443–452. doi: 10.1016/S0959-440X(03)00109-X. [DOI] [PubMed] [Google Scholar]
- 43.Bernut A., Viljoen A., Dupont C., Sapriel G., Blaise M., Bouchier C., Brosch R., de Chastellier C., Herrmann J.-L., Kremer L. Insights into the smooth-to-rough transitioning in Mycobacterium bolletii unravels a functional Tyr residue conserved in all mycobacterial MmpL family members. Mol. Microbiol. 2015;99:866–883. doi: 10.1111/mmi.13283. [DOI] [PubMed] [Google Scholar]
- 44.Li W., Upadhyay A., Fontes F.L., North E.J., Wang Y., Crans D.C., Grzegorzewicz A.E., Jones V., Franzblau S.G., Lee R.E., et al. Novel Insights into the Mechanism of Inhibition of MmpL3, a Target of Multiple Pharmacophores in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014;58:6413–6423. doi: 10.1128/AAC.03229-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z., Meshcheryakov V.A., Poce G., Chng S.S. MmpL3 is the flippase for mycolic acids in mycobacteria. Proc. Natl. Acad. Sci. USA. 2017;114:7993–7998. doi: 10.1073/pnas.1700062114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grzegorzewicz A.E., Pham H., Gundi V.A.K.B., Scherman M.S., North E.J., Hess T., Jones V., Gruppo V., Born S.E.M., Korduláková J., et al. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat. Chem. Biol. 2012;8:334–341. doi: 10.1038/nchembio.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tahlan K., Wilson R., Kastrinsky D.B., Arora K., Nair V., Fischer E., Barnes S.W., Walker J.R., Alland D., Barry C.E., et al. SQ109 Targets MmpL3, a Membrane Transporter of Trehalose Monomycolate Involved in Mycolic Acid Donation to the Cell Wall Core of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2012;56:1797–1809. doi: 10.1128/AAC.05708-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marrakchi H., Lanéelle M.-A., Daffe M. Mycolic Acids: Structures, Biosynthesis, and Beyond. Chem. Biol. 2014;21:67–85. doi: 10.1016/j.chembiol.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Quémard A. New Insights into the Mycolate-Containing Compound Biosynthesis and Transport in Mycobacteria. Trends Microbiol. 2016;24:725–738. doi: 10.1016/j.tim.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Belisle J.T., Vissa V.D., Sievert T., Takayama K., Brennan P.J., Besra G.S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 51.Kremer L., Maughan W.N., Wilson R.A., Dover L.G., Besra G.S. The M. tuberculosis antigen 85 complex and mycolyltransferase activity. Lett. Appl. Microbiol. 2002;34:233–237. doi: 10.1046/j.1472-765x.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- 52.Backus K.M., Dolan M.A., Barry C.S., Joe M., McPhie P., Boshoff H.I.M., Lowary T.L., Davis B.G., Barry C.E. The three Mycobacterium tuberculosis antigen 85 isoforms have unique substrates and activities determined by non-active site regions. J. Biol. Chem. 2014;289:25041–25053. doi: 10.1074/jbc.M114.581579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minnikin D.E. Chemical principles in the organization of lipid components in the mycobacterial cell envelope. Res. Microbiol. 1991;142:423–427. doi: 10.1016/0923-2508(91)90114-P. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann C., Leis A., Niederweis M., Plitzko J.M., Engelhardt H. Disclosure of the mycobacterial outer membrane: Cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl. Acad. Sci. USA. 2008;105:3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foley H.N., Stewart J.A., Kavunja H.W., Rundell S.R., Swarts B.M. Bioorthogonal Chemical Reporters for Selective In Situ Probing of Mycomembrane Components in Mycobacteria. Angew. Chem. Int. Ed. 2016;55:2053–2057. doi: 10.1002/anie.201509216. [DOI] [PubMed] [Google Scholar]
- 56.Varela C., Rittmann D., Singh A., Krumbach K., Bhatt K., Eggeling L., Besra G.S., Bhatt A. MmpL Genes Are Associated with Mycolic Acid Metabolism in Mycobacteria and Corynebacteria. Chem. Biol. 2012;19:498–506. doi: 10.1016/j.chembiol.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W., Obregón-Henao A., Wallach J.B., North E.J., Lee R.E., Gonzalez-Juarrero M., Schnappinger D., Jackson M. Therapeutic Potential of the Mycobacterium tuberculosis Mycolic Acid Transporter, MmpL3. Antimicrob. Agents Chemother. 2016;60:5198–5207. doi: 10.1128/AAC.00826-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Degiacomi G., Benjak A., Madacki J., Boldrin F., Provvedi R., Palù G., Korduláková J., Cole S.T., Manganelli R. Essentiality of mmpL3 and impact of its silencing on Mycobacterium tuberculosis gene expression. Sci. Rep. 2017;7:43495. doi: 10.1038/srep43495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li W., Yazidi A., Pandya A.N., Hegde P., Tong W., Calado Nogueira de Moura V., North E.J., Sygusch J., Jackson M. MmpL3 as a Target for the Treatment of Drug-Resistant Nontuberculous Mycobacterial Infections. Front. Microbiol. 2018;9:1547. doi: 10.3389/fmicb.2018.01547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hunter R.L., Olsen M.R., Jagannath C., Actor J.K. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann. Clin. Lab. Sci. 2006;36:371–386. [PubMed] [Google Scholar]
- 61.Welsh K.J., Hunter R.L., Actor J.K. Trehalose 6,6′-dimycolate—A coat to regulate tuberculosis immunopathogenesis. Tuberculosis. 2013;93:S3–S9. doi: 10.1016/S1472-9792(13)70003-9. [DOI] [PubMed] [Google Scholar]
- 62.Bloch H. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J. Exp. Med. 1950;91:197–218. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noll H., Bloch H., Asselineau J., Lederer E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim. Biophys. Acta. 1956;20:299–309. doi: 10.1016/0006-3002(56)90289-X. [DOI] [PubMed] [Google Scholar]
- 64.Baba T., Natsuhara Y., Kaneda K., Yano I. Granuloma formation activity and mycolic acid composition of mycobacterial cord factor. Cell. Mol. Life Sci. 1997;53:227–232. doi: 10.1007/PL00000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamasaki N., Isowa K.-I., Kamada K., Terano Y., Matsumoto T., Arakawa T., Kobayashi K., Yano I. In Vivo Administration of Mycobacterial Cord Factor (Trehalose 6,6′-Dimycolate) Can Induce Lung and Liver Granulomas and Thymic Atrophy in Rabbits. Infect. Immun. 2000;68:3704–3709. doi: 10.1128/IAI.68.6.3704-3709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunter R.L., Olsen M., Jagannath C., Actor J.K. Trehalose 6,6’-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. Am. J. Pathol. 2006;168:1249–1261. doi: 10.2353/ajpath.2006.050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishikawa E., Ishikawa T., Morita Y.S., Toyonaga K., Yamada H., Takeuchi O., Kinoshita T., Akira S., Yoshikai Y., Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bowdish D.M.E., Sakamoto K., Kim M.-J., Kroos M., Mukhopadhyay S., Leifer C.A., Tryggvason K., Gordon S., Russell D.G. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cox J.S., Chen B., McNeil M., Jacobs W.R. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999;402:79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- 70.Camacho L.R., Constant P., Raynaud C., Laneelle M.A., Triccas J.A., Gicquel B., Daffe M., Guilhot C. Analysis of the Phthiocerol Dimycocerosate Locus of Mycobacterium tuberculosis. J. Biol. Chem. 2001;276:19845–19854. doi: 10.1074/jbc.M100662200. [DOI] [PubMed] [Google Scholar]
- 71.Camacho L.R., Ensergueix D., Perez E., Gicquel B., Guilhot C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol. Microbiol. 1999;34:257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 72.Trivedi O.A., Arora P., Vats A., Ansari M.Z., Tickoo R., Sridharan V., Mohanty D., Gokhale R.S. Dissecting the Mechanism and Assembly of a Complex Virulence Mycobacterial Lipid. Mol. Cell. 2005;17:631–643. doi: 10.1016/j.molcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 73.Jain M., Cox J.S. Interaction between Polyketide Synthase and Transporter Suggests Coupled Synthesis and Export of Virulence Lipid in M. tuberculosis. PLoS Pathog. 2005;1:e2–e8. doi: 10.1371/journal.ppat.0010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamichhane G., Tyagi S., Bishai W.R. Designer Arrays for Defined Mutant Analysis to Detect Genes Essential for Survival of Mycobacterium tuberculosis in Mouse Lungs. Infect. Immun. 2005;73:2533–2540. doi: 10.1128/IAI.73.4.2533-2540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rousseau C., Winter N., Pivert E., Bordat Y., Neyrolles O., Avé P., Huerre M., Gicquel B., Jackson M. Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection. Cell Microbiol. 2004;6:277–287. doi: 10.1046/j.1462-5822.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- 76.Astarie Dequeker C., Le Guyader L., Malaga W., Seaphanh F.-K., Chalut C., Lopez A., Guilhot C. Phthiocerol Dimycocerosates of M. tuberculosis Participate in Macrophage Invasion by Inducing Changes in the Organization of Plasma Membrane Lipids. PLoS Pathog. 2009;5:e1000289. doi: 10.1371/journal.ppat.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cambier C.J., Takaki K.K., Larson R.P., Hernandez R.E., Tobin D.M., Urdahl K.B., Cosma C.L., Ramakrishnan L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Augenstreich J., Arbues A., Simeone R., Haanappel E., Wegener A., Sayes F., Chevalier F.L., Chalut C., Malaga W., Guilhot C., et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol. 2017;19:e12726. doi: 10.1111/cmi.12726. [DOI] [PubMed] [Google Scholar]
- 79.Quigley J., Hughitt V.K., Velikovsky C.A., Mariuzza R.A., El-Sayed N.M., Briken V. The Cell Wall Lipid PDIM Contributes to Phagosomal Escape and Host Cell Exit of Mycobacterium tuberculosis. mBio. 2017;8:e00148-17. doi: 10.1128/mBio.00148-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lerner T.R., Queval C.J., Fearns A., Repnik U., Griffiths G., Gutierrez M.G. Phthiocerol dimycocerosates promote access to the cytosol and intracellular burden of Mycobacterium tuberculosis in lymphatic endothelial cells. BMC Biol. 2018;16:1. doi: 10.1186/s12915-017-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Converse S.E., Mougous J.D., Leavell M.D., Leary J.A., Bertozzi C.R., Cox J.S. MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence. Proc. Natl. Acad. Sci. USA. 2003;100:6121–6126. doi: 10.1073/pnas.1030024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Domenech P., Reed M.B., Dowd C.S., Manca C., Kaplan G., Barry C.E. The Role of MmpL8 in Sulfatide Biogenesis and Virulence of Mycobacterium tuberculosis. J. Biol. Chem. 2004;279:21257–21265. doi: 10.1074/jbc.M400324200. [DOI] [PubMed] [Google Scholar]
- 83.Goren M.B., Brokl O., Roller P., Fales H.M., Das B.C. Sulfatides of Mycobacterium tuberculosis: The structure of the principal sulfatide (SL-I) Biochemistry. 1976;15:2728–2735. doi: 10.1021/bi00658a003. [DOI] [PubMed] [Google Scholar]
- 84.Kumar P., Schelle M.W., Jain M., Lin F.L., Petzold C.J., Leavell M.D., Leary J.A., Cox J.S., Bertozzi C.R. PapA1 and PapA2 are acyltransferases essential for the biosynthesis of the Mycobacterium tuberculosis virulence factor sulfolipid-1. Proc. Natl. Acad. Sci. USA. 2007;104:11221–11226. doi: 10.1073/pnas.0611649104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seeliger J.C., Holsclaw C.M., Schelle M.W., Botyanszki Z., Gilmore S.A., Tully S.E., Niederweis M., Cravatt B.F., Leary J.A., Bertozzi C.R. Elucidation and Chemical Modulation of Sulfolipid-1 Biosynthesis in Mycobacterium tuberculosis. J. Biol. Chem. 2012;287:7990–8000. doi: 10.1074/jbc.M111.315473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Belardinelli J.M., Larrouy-Maumus G., Jones V., Sorio de Carvalho L.P., McNeil M.R., Jackson M. Biosynthesis and translocation of unsulfated acyltrehaloses in Mycobacterium tuberculosis. J. Biol. Chem. 2014;289:27952–27965. doi: 10.1074/jbc.M114.581199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hatzios S.K., Schelle M.W., Holsclaw C.M., Behrens C.R., Botyanszki Z., Lin F.L., Carlson B.L., Kumar P., Leary J.A., Bertozzi C.R. PapA3 is an acyltransferase required for polyacyltrehalose biosynthesis in Mycobacterium tuberculosis. J. Biol. Chem. 2009;284:12745–12751. doi: 10.1074/jbc.M809088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee K.-S., Dubey V.S., Kolattukudy P.E., Song C.-H., Shin A.-R., Jung S.-B., Yang C.-S., Kim S.-Y., Jo E.-K., Park J.-K., et al. Diacyltrehalose of Mycobacterium tuberculosis inhibits lipopolysaccharide- and mycobacteria-induced proinflammatory cytokine production in human monocytic cells. FEMS Microbiol. Lett. 2007;267:121–128. doi: 10.1111/j.1574-6968.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 89.Rousseau C., Neyrolles O., Bordat Y., Giroux S., Sirakova T.D., Prevost M.-C., Kolattukudy P.E., Gicquel B., Jackson M. Deficiency in mycolipenate- and mycosanoate-derived acyltrehaloses enhances early interactions of Mycobacterium tuberculosis with host cells. Cell Microbiol. 2003;5:405–415. doi: 10.1046/j.1462-5822.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- 90.Passemar C., Arbues A., Malaga W., Mercier I., Moreau F., Lepourry L., Neyrolles O., Guilhot C., Dequeker C.A. Multiple deletions in the polyketide synthase gene repertoire of Mycobacterium tuberculosis reveal functional overlap of cell envelope lipids in host–pathogen interactions. Cell Microbiol. 2014;16:195–213. doi: 10.1111/cmi.12214. [DOI] [PubMed] [Google Scholar]
- 91.Wright C.C., Hsu F.F., Arnett E., Dunaj J.L., Davidson P.M., Pacheco S.A., Harriff M.J., Lewinsohn D.M., Schlesinger L.S., Purdy G.E. The Mycobacterium tuberculosis MmpL11 Cell Wall Lipid Transporter Is Important for Biofilm Formation, Intracellular Growth, and Nonreplicating Persistence. Infect. Immun. 2017;85:e00131-17. doi: 10.1128/IAI.00131-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ojha A.K., Baughn A.D., Sambandan D., Hsu T., Trivelli X., Guerardel Y., Alahari A., Kremer L., Jacobs W.R., Jr., Hatfull G.F. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 2008;69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sandhu P., Akhter Y. Siderophore transport by MmpL5-MmpS5 protein complex in Mycobacterium tuberculosis. J. Inorgan. Biochem. 2017;170:75–84. doi: 10.1016/j.jinorgbio.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 94.Dubois V., Viljoen A., Laencina L., Le Moigne V., Bernut A., Dubar F., Blaise M., Gaillard J.-L., Guerardel Y., Kremer L., et al. MmpL8MAB controls Mycobacterium abscessus virulence and production of a previously unknown glycolipid family. Proc. Natl. Acad. Sci. USA. 2018;115:E10147–E10156. doi: 10.1073/pnas.1812984115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacGurn J.A., Cox J.S. A Genetic Screen for Mycobacterium tuberculosis Mutants Defective for Phagosome Maturation Arrest Identifies Components of the ESX-1 Secretion System. Infect. Immun. 2007;75:2668–2678. doi: 10.1128/IAI.01872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mestre O., Hurtado-Ortiz R., Dos Vultos T., Namouchi A., Cimino M., Pimentel M., Neyrolles O., Gicquel B. High Throughput Phenotypic Selection of Mycobacterium tuberculosis Mutants with Impaired Resistance to Reactive Oxygen Species Identifies Genes Important for Intracellular Growth. PLoS ONE. 2013;8:e53486. doi: 10.1371/journal.pone.0053486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brosch R., Gordon S.V., Marmiesse M., Brodin P., Buchrieser C., Eiglmeier K., Garnier T., Gutierrez C., Hewinson G., Kremer K., et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA. 2002;99:3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arumugam P., Shankaran D., Bothra A., Gandotra S., Rao V. The MmpS6-MmpL6 Operon Is an Oxidative Stress Response System Providing Selective Advantage to Mycobacterium tuberculosis in Stress. J. Infect. Dis. 2018;367:850. doi: 10.1093/infdis/jiy526. [DOI] [PubMed] [Google Scholar]
- 99.Ehrt S., Schnappinger D. Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell Microbiol. 2009;11:1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milano A., Pasca M.R., Provvedi R., Lucarelli A.P., Manina G., Ribeiro A.L., Manganelli R., Riccardi G. Azole resistance in Mycobacterium tuberculosis is mediated by the MmpS5-MmpL5 efflux system. Tuberculosis. 2009;89:84–90. doi: 10.1016/j.tube.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 101.Hartkoorn R.C., Uplekar S., Cole S.T. Cross-Resistance between Clofazimine and Bedaquiline through Upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014;58:2979–2981. doi: 10.1128/AAC.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richard M., Gutiérrez A.V., Viljoen A., Rodriguez-Rincon D., Roquet-Baneres F., Blaise M., Everall I., Parkhill J., Floto R.A., Kremer L. Mutations in the MAB_2299c TetR Regulator Confer Cross-Resistance to Clofazimine and Bedaquiline in Mycobacterium abscessus. Antimicrob. Agents Chemother. 2019;63:e01316-18. doi: 10.1128/AAC.01316-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Halloum I., Viljoen A., Khanna V., Craig D., Bouchier C., Brosch R., Coxon G., Kremer L. Resistance to Thiacetazone Derivatives Active against Mycobacterium abscessus Involves Mutations in the MmpL5 Transcriptional Repressor MAB_4384. Antimicrob. Agents Chemother. 2017;61:e02509-16. doi: 10.1128/AAC.02509-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pasca M.R., Guglierame P., De Rossi E., Zara F., Riccardi G. mmpL7 gene of Mycobacterium tuberculosis is responsible for isoniazid efflux in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 2005;49:4775–4777. doi: 10.1128/AAC.49.11.4775-4777.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodrigues L., Machado D., Couto I., Amaral L., Viveiros M. Contribution of efflux activity to isoniazid resistance in the Mycobacterium tuberculosis complex. Infect. Genet. Evol. 2012;12:695–700. doi: 10.1016/j.meegid.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 106.Galagan J.E., Minch K., Peterson M., Lyubetskaya A., Azizi E., Sweet L., Gomes A., Rustad T., Dolganov G., Glotova I., et al. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature. 2013;499:178–183. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee W., VanderVen B.C., Fahey R.J., Russell D.G. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J. Biol. Chem. 2013;288:6788–6800. doi: 10.1074/jbc.M112.445056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jain M., Petzold C.J., Schelle M.W., Leavell M.D., Mougous J.D., Bertozzi C.R., Leary J.A., Cox J.S. Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proc. Natl. Acad. Sci. USA. 2007;104:5133–5138. doi: 10.1073/pnas.0610634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chou T.H., Delmar J.A., Wright C.C., Kumar N., Radhakrishnan A., Doh J.K., Licon M.H., Bolla J.R., Lei H.T., Rajashankar K.R., et al. Crystal structure of the Mycobacterium tuberculosis transcriptional regulator Rv0302. Protein Sci. 2015;24:1942–1955. doi: 10.1002/pro.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Delmar J.A., Chou T.H., Wright C.C., Licon M.H., Doh J.K., Radhakrishnan A., Kumar N., Lei H.T., Bolla J.R., Rajashankar K.R., et al. Structural Basis for the Regulation of the MmpL Transporters of Mycobacterium tuberculosis. J. Biol. Chem. 2015;290:28559–28574. doi: 10.1074/jbc.M115.683797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Radhakrishnan A., Kumar N., Wright C.C., Chou T.H., Tringides M.L., Bolla J.R., Lei H.T., Rajashankar K.R., Su C.C., Purdy G.E., et al. Crystal Structure of the Transcriptional Regulator Rv0678 of Mycobacterium tuberculosis. J. Biol. Chem. 2014;289:16526–16540. doi: 10.1074/jbc.M113.538959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McKinney J.D., Bentrup K.H.Z., Muñoz-Elías E.J., Miczak A., Chen B., Chan W.-T., Swenson D., Sacchettini J.C., Jacobs W.R., Jr., Russell D.G. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 113.Slayden R.A., Barry C.E., 3rd The role of KasA and KasB in the biosynthesis of meromycolic acids and isoniazid resistance in Mycobacterium tuberculosis. Tuberculosis. 2002;82:149–160. doi: 10.1054/tube.2002.0333. [DOI] [PubMed] [Google Scholar]
- 114.Prisic S., Husson R.N. Mycobacterium tuberculosis Serine/Threonine Protein Kinases. Microbiol. Spectrum. 2014;2:1–26. doi: 10.1128/microbiolspec.MGM2-0006-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pérez J., Garcia R., Bach H., de Waard J.H., Jacobs W.R., Av-Gay Y., Bubis J., Takiff H.E. Mycobacterium tuberculosis transporter MmpL7 is a potential substrate for kinase PknD. Biochem. Biophys. Res. Commun. 2006;348:6–12. doi: 10.1016/j.bbrc.2006.06.164. [DOI] [PubMed] [Google Scholar]
- 116.Prisic S., Dankwa S., Schwartz D., Chou M.F., Locasale J.W., Kang C.-M., Bemis G., Church G.M., Steen H., Husson R.N. Extensive phosphorylation with overlapping specificity by Mycobacterium tuberculosis serine/threonine protein kinases. Proc. Natl. Acad. Sci. USA. 2010;107:7521–7526. doi: 10.1073/pnas.0913482107. [DOI] [PMC free article] [PubMed] [Google Scholar]