Abstract
Background: The study’s aim was to summarize the incidence and impacts of post-liver transplant (LTx) acute kidney injury (AKI) on outcomes after LTx. Methods: A literature search was performed using the MEDLINE, EMBASE and Cochrane Databases from inception until December 2018 to identify studies assessing the incidence of AKI (using a standard AKI definition) in adult patients undergoing LTx. Effect estimates from the individual studies were derived and consolidated utilizing random-effect, the generic inverse variance approach of DerSimonian and Laird. The protocol for this systematic review is registered with PROSPERO (no. CRD42018100664). Results: Thirty-eight cohort studies, with a total of 13,422 LTx patients, were enrolled. Overall, the pooled estimated incidence rates of post-LTx AKI and severe AKI requiring renal replacement therapy (RRT) were 40.7% (95% CI: 35.4%–46.2%) and 7.7% (95% CI: 5.1%–11.4%), respectively. Meta-regression showed that the year of study did not significantly affect the incidence of post-LTx AKI (p = 0.81). The pooled estimated in-hospital or 30-day mortality, and 1-year mortality rates of patients with post-LTx AKI were 16.5% (95% CI: 10.8%–24.3%) and 31.1% (95% CI: 22.4%–41.5%), respectively. Post-LTx AKI and severe AKI requiring RRT were associated with significantly higher mortality with pooled ORs of 2.96 (95% CI: 2.32–3.77) and 8.15 (95%CI: 4.52–14.69), respectively. Compared to those without post-LTx AKI, recipients with post-LTx AKI had significantly increased risk of liver graft failure and chronic kidney disease with pooled ORs of 3.76 (95% CI: 1.56–9.03) and 2.35 (95% CI: 1.53–3.61), respectively. Conclusion: The overall estimated incidence rates of post-LTx AKI and severe AKI requiring RRT are 40.8% and 7.0%, respectively. There are significant associations of post-LTx AKI with increased mortality and graft failure after transplantation. Furthermore, the incidence of post-LTx AKI has remained stable over the ten years of the study.
Keywords: Acute renal failure, Acute kidney injury, Epidemiology, Incidence, Meta-analysis, Liver Transplantation, Transplantation, Systematic reviews
1. Introduction
Acute kidney injury (AKI) is associated with high mortality worldwide (1.7 million deaths per year) [1,2,3,4]. Patients who survive AKI are at increased risk for significant morbidities such as hypertension and progressive chronic kidney disease (CKD) [5]. The incidence of AKI has steadily increased in recent years [2]. It has been suggested that AKI’s global burden is 13.3 million cases a year [6]. In the United States, hospitalizations for AKI have been steeply rising, and data from national inpatient sample shows that the number of hospitalizations due to AKI increased from 953,926 in 2000 to 1,823,054 in 2006 and 3,959,560 in 2014, which accounts for one hospitalization associated with AKI every 7.5 minutes [7,8].
AKI is a common and significant complication after liver transplantation (LTx), and is associated with increased mortality, hospital length of stay, utilization of resources, and health care costs [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Although the survival of LTx recipients has improved substantially over the past five decades, mortality rates related to post-LTx AKI and subsequent progressive CKD remain high and are of increasing concern [14,15,28,29,30,31]. The underlying mechanisms for post-LTx AKI appear to be complex and differ from other medical or surgery-associated AKI [11,23,24,25,32,33,34,35]. Recent studies have suggested several important factors that influence post-LTx AKI, including hepatic ischemia-reperfusion injury (HIRI) [36,37,38], increased use of high-risk or marginal grafts, and transplantation of liver grafts to sicker patients with higher Model For End-Stage Liver Disease (MELD) score or with more comorbidities [23,39,40,41,42,43,44,45,46,47,48,49,50,51]. In our literature review, the reported incidences are a farrago, having a range between 5% to 94% [10,11,14,15,16,17,18,19,20,21,22,23,24,25,28,29,30,31,32,33,34,35,39,40,41,42,43,44,45,46,47,48,49,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80]. These wide variabilities are possibly due to non-uniform definitions of AKI [10,11,14,15,16,17,18,19,20,21,22,23,24,25,28,29,30,31,32,33,34,35,39,40,41,42,43,44,45,46,47,48,49,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80]. In addition, despite progress in transplant medicine, the incidence, risk factors, and mortality associated with AKI in post-LTx patients and their trends remain unclear [10,11,14,15,16,17,18,19,20,21,22,23,24,25,28,29,30,31,32,33,34,35,39,40,41,42,43,44,45,46,47,48,49,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83].
Thus, we performed a systematic review to summarize the incidence (using standard AKI definitions of Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE), Acute Kidney Injury Network (AKIN), and Kidney Disease: Improving Global Outcomes (KDIGO) classifications), risk factors, and mortality and their trends for AKI in patients undergoing LTx.
2. Methods
2.1. Search Strategy and Literature Review
The protocol for this systematic review was registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42018100664). A systematic literature search of MEDLINE (1946 to December 2018), EMBASE (1988 to December 2018) and the Cochrane Database of Systematic Reviews (database inception to December 2018) was performed to evaluate the incidence of AKI in adult patients undergoing LTx. The systematic literature review was conducted independently by two investigators (C.T. and W.C.) using the search strategy that consolidated the terms “acute kidney injury” OR “renal failure” AND “liver transplantation," which is provided in online supplementary data 1. No language limitation was implemented. A manual search for conceivably related studies using references of the included articles was also performed. This study was conducted by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [84] and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [85].
2.2. Selection Criteria
Eligible studies must be clinical trials or observational studies (cohort, case-control, or cross-sectional studies) that reported the incidence of post-LTx AKI in adult patients (age >/= 18 years old). Included studies must provide data to estimate the incidence of post-LTx AKI with 95% confidence intervals (CI). Retrieved articles were individually reviewed for eligibility by the two investigators (C.T. and W.C.). Discrepancies were addressed and solved by mutual consensus. Inclusion was not limited by the size of study.
2.3. Data Abstraction
A structured data collecting form was used to obtain the following information from each study, including title, name of the first author, year of the study, publication year, country where the study was conducted, post-LTx AKI definition, incidence of AKI post-LTx, risk factors for post-LTx AKI, and impact of post-LTx AKI on patient outcomes.
2.4. Statistical Analysis
Analyses were performed utilizing the Comprehensive Meta-Analysis 3.3 software (Biostat Inc, Englewood, NJ, USA). Adjusted point estimates from each study were consolidated by the generic inverse variance approach of DerSimonian and Laird, which designated the weight of each study based on its variance [86]. Given the possibility of between-study variance, we used a random-effect model rather than a fixed-effect model. Cochran’s Q test and I2 statistic were applied to determine the between-study heterogeneity. A value of I2 of 0%–25% represents insignificant heterogeneity, 26%–50% low heterogeneity, 51%–75% moderate heterogeneity and 76–100% high heterogeneity [87]. The presence of publication bias was assessed by the Egger test [88].
3. Results
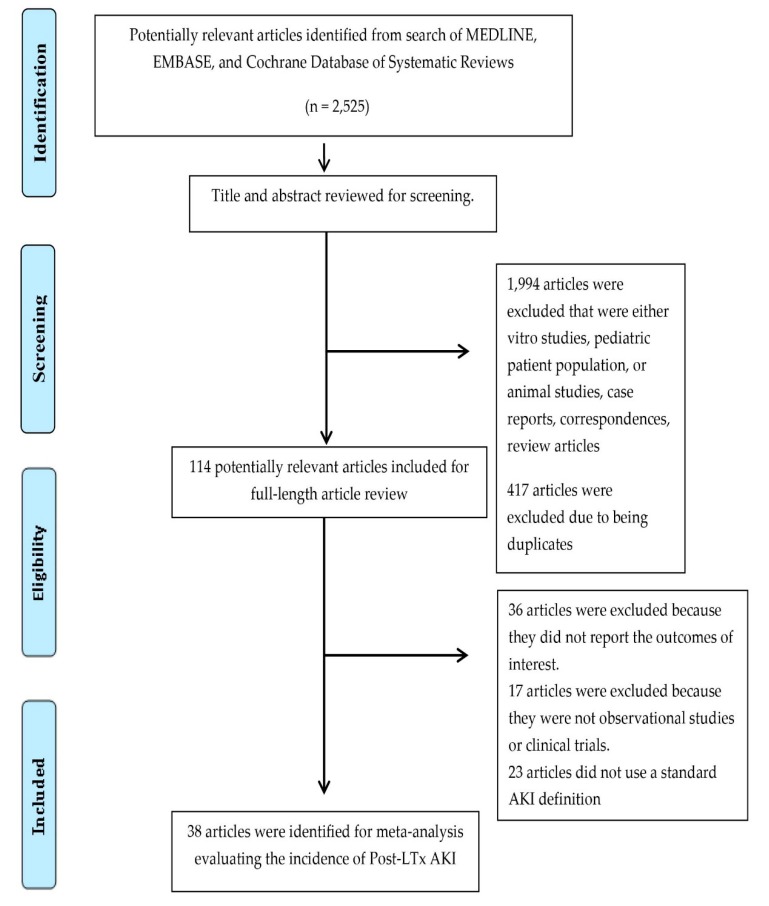
A total of 2525 potentially eligible articles were identified using our search strategy. After the exclusion of 1994 articles based on title and abstract for clearly not fulfilling inclusion criteria on the basis of type of article, patient population, study design, or outcome of interest, and 417 due to being duplicates, 114 articles were left for full-length review. Thirty-six of them were excluded from the full-length review as they did not report the outcome of interest, while 17 were excluded because they were not observational studies or clinical trials. Twenty-three studies were subsequently excluded because they did not use a standard AKI definition. Thus, we included 38 cohort studies [14,18,19,21,28,29,30,31,32,39,41,42,43,44,48,49,55,56,57,58,59,60,62,63,64,65,66,69,70,72,73,74,75,76,77,78,79,80] in the meta-analysis of post-LTx AKI incidence with 13,422 patients enrolled. The literature retrieval, review, and selection process are demonstrated in Figure 1. The characteristics of the included studies are presented in Table 1.
Figure 1.
Outline of our search methodology.
Table 1.
Main characteristics of studies included in meta-analysis of AKI in patients undergoing LTx [14,18,19,21,28,29,30,31,32,39,41,42,43,44,48,49,55,56,57,58,59,60,62,63,64,65,66,69,70,72,73,74,75,76,77,78,79,80].
| Study | Year | Country | Procedure/Patients | Number | Deceased Donor | AKI Definition | Incidence | Mortality in AKI |
|---|---|---|---|---|---|---|---|---|
| O’riordan et al. [32] | 2007 | Ireland | Deceased donor orthotopic liver transplant | 350 | 350 (100%) | ARI/ARF; RIFLE Injury and Failure stage within 2 weeks after transplant | ARI/ARF 129/350 (36.9%) Dialysis 68/350 (19.4%) |
1-year mortality 56/129 (43%) |
| Kundakci et al. [41] | 2010 | Turkey | Orthotopic liver transplant | 112 | 75 (67%) | AKI; RIFLE criteria | AKI 64/112 (57.1%) |
1-year mortality 23/64 (36%) |
| Portal et al. [55] | 2010 | UK | Liver transplant | 80 | N/A | AKI; AKIN criteria within 48 hours after transplants | AKI 30/80 (37.5%) |
N/A |
| Zhu et al. [42] | 2010 | China | Deceased donor orthotopic liver transplant | 193 | 193 (100%) | AKI; AKIN criteria within 28 days after transplants | AKI 116/193 (60.1%) Dialysis 10/193 (5.2%) |
1-year mortality 30/116 (26%) |
| Lee et al. [56] | 2010 | Korea | Liver transplant | 431 | 99 (23%) | AKI; RIFLE criteria | AKI 118/431 (27.4%) Dialysis 14/431 (3.2%) |
N/A |
| Ferreira et al. [57] | 2010 | Portugal | Orthotopic liver transplant | 708 | N/A | AKI; RIFLE criteria within 21 days after transplant | AKI 235/708 (33.2%) Dialysis 73/708 (10.3%) |
Mortality 43/235 (18%) |
| Tinti et al. [58] | 2010 | Italy | Deceased donor orthotopic liver transplant | 24 | 24 (100%) | AKI; RIFLE criteria within 15 days after transplant | AKI 9/24 (37.5%) |
N/A |
| Chen et al. (1) [18] | 2011 | USA | Liver transplant | 334 | N/A | ARI/ARF; RIFLE Injury and Failure stage within 2 weeks after transplant within 7 days after transplant | ARI/ARF 118/334 (38.3%) |
Mortality 13/118 (11%) |
| Umbro et al. [59] | 2011 | Italy | Deceased donor liver transplant | 46 | 46 (100%) | AKI; RIFLE criteria within 7 days after transplant | AKI 26/46 (56.5%) |
N/A |
| Karapanagiotou et al. (1) [43] | 2012 | Greece | Orthotopic liver transplant | 75 | N/A | AKI; an increase in SCr 1.5 times above baseline or value > 2.0 mg/dL within 7 days after transplant | AKI 22/75 (29.3%) Dialysis 7/75 (9.3%) |
1-year mortality 11/22 (50%) |
| Utsumi et al. [44] | 2013 | Japan | Living donor liver transplant | 200 | 0 (0%) | AKI; RIFLE criteria within 28 days after transplants | AKI 121/200 (60.5%) ARI/ARF 74/200 (37%) |
Hospital mortality AKI 14/121 (12%) ARI/ARF 12/74(16%) 1-year mortality AKI 24/121 (20%) ARI/ARF 22/74 (30%) |
| Narciso et al. [60] | 2013 | Brazil | Liver transplant | 315 | 181 (57%) | AKI; AKIN criteria within 48 hours after transplants | AKI 48 hours: 101/315 (32.1%) 1 week: 255/315 (81%) Hospitalization: 293/315 (93%) Dialysis Any: 48/315 (15.2%) 1 week: 31/315 (9.8%) |
Dialysis 28/48 (58%) |
| Leithead et al. [39] | 2014 | UK | Liver transplant | 1152 | 1152 (100%) DCD 112 (10%) |
AKI; KDIGO criteria within 7 days after transplants | AKI 381/1152 (33.1%) Dialysis 238/1152 (20.7%) |
AKI 152/381 (40%) |
| Karapanagiotou et al. (2) [48] | 2014 | Greece | Liver transplant | 71 | N/A | AKI; RIFLE within 7 days or AKIN criteria within 48 hours | RIFLE AKI 28/71 (39.4%) AKIN AKI 37/71 (52.1%) |
6-month mortality RIFLE AKI 15/28 (54%) AKIN AKI 17/37 (46%) |
| Nadeem et al. [49] | 2014 | Saudi Arabia | Liver transplant | 158 | N/A | AKI; RIFLE criteria within 72 hours after transplants | AKI 57/158 (36.1%) |
N/A |
| Lewandowska et al. [62] | 2014 | Poland | Orthotopic liver transplant | 63 | N/A | AKI; RIFLE criteria within 72 hours after transplant | AKI 35/63 (55.6%) |
N/A |
| Barreto et al. [63] | 2015 | Brazil | Orthotopic liver transplant | 134 | N/A | AKI; AKIN criteria 2 or 3 within 72 hours after transplants | AKIN stage 2 or 3 64/134 (47.8%) Dialysis 33/134 (24.6%) |
N/A |
| Hilmi et al. [19] | 2015 | USA | Deceased donor liver transplant | 424 | 424 (100%) EDC 257 (61%) |
AKI; KDIGO criteria within 72 hours after transplant | AKI 221/424 (52.1%) |
30-day mortality 3/221 (1%) |
| Park et al. [64] | 2015 | Korea | Living donor liver transplant | 538 | 0 (0%) | AKI; RIFLE criteria within 30 days after transplant | AKI 147/538 (27.3%) Dialysis 34/538 (6.3%) |
Hospital mortality 26/147 (18%) 1-year mortality 29/147 (20%) |
| Mukhtar et al. [65] | 2015 | Egypt | Living donor liver transplant | 303 | 0 (0%) | AKI; AKIN criteria within 96 hours after transplant | AKI 115/303 (38%) Dialysis 28/303 (9.2%) |
N/A |
| Sang et al. [66] | 2015 | Korea | Living donor liver transplant | 998 | 0 (0%) | AKI; RIFLE or AKIN criteria within 7 days after transplant | RIFLE AKI 709/998 (71.0%) AKIN AKI 593/998 (59.4%) |
RIFLE AKI 79/709 (11%) AKIN AKI 66/593 (11%) |
| Biancofiore et al. [69] | 2015 | Italy | Deceased donor liver transplant | 295 | 295 (100%) | AKI; AKIN criteria within 7 days after transplant | AKIN stage 2 AKI 51/295 (17.3%) |
N/A |
| Jun et al. [70] | 2016 | Korea | Living donor liver transplant | 1617 | 0 (0%) | AKI; KDIGO criteria within 7 days after transplant | AKI 999/1617 (61.8%) Dialysis 9/448 (2%) |
N/A |
| Erdost et al. [72] | 2016 | Turkey | Liver transplant | 440 | 194 (44%) | AKI; RIFLE, AKIN, KDIGO criteria within 7 days after transplant | RIFLE AKI 35/440 (8.0%) AKIN AKI 63/440 (14.3%) KDIGO AKI 64/440 (14.5%) |
30-day mortality RIFLE AKI 8/35 (23%) AKIN AKI 34/63 (54%) KDIGO AKI 35/64 (55%) |
| Kamei et al. [73] | 2016 | Japan | Liver transplant | 62 | DBD 4 (6%) | AKI; RIFLE injury or failure stage within 4 weeks after transplant | AKI 13/62 (21%) Dialysis 4/62 (6.5%) |
N/A |
| Mizota et al. (1) [74] | 2016 | Japan | Living donor liver transplant | 320 | 0 (0%) | AKI; KDIGO criteria within 7 days after transplant | AKI 199/320 (62.2%) |
Hospital mortality 39/199 (20%) |
| Sun et al. [21] | 2017 | USA | Liver transplant | 1037 | N/A | AKI; AKIN criteria within 48 hours after transplant | AKI 549/1037 (54.9%) |
N/A |
| Chae et al. [75] | 2017 | Korea | Living donor liver transplant | 334 | 0 (0%) | AKI; AKIN criteria within 48 hours after transplant | AKI 76/334 (22.7%) |
Hospital mortality 10/76 (13.2%) |
| Mizota et al. (2) [76] | 2017 | Japan | Living donor liver transplant | 231 | 0 (0%) | Severe AKI; KDIGO stage 2 or 3 criteria within 7 days after transplant | Severe AKI 71/231 (30.7%) |
Hospital mortality 23/71 (32.4%) |
| Trinh et al. [77] | 2017 | Canada | Deceased donor liver transplant | 491 | 491 (100%) | AKI; KDIGO criteria within 7 days after transplant | AKI 278/491 (56.6%) |
N/A |
| Kalisvaart et al. [78] | 2017 | Netherlands | Donation after brain death liver transplant | 155 | 155 (100%) DBD 155 (100%) |
AKI; AKIN criteria within 7 days after transplant | AKI 61/155 (39.4%) Dialysis 5/155 (3.2%) |
Hospital mortality 9/61 (15%) |
| Chen et al. (2) [79] | 2017 | China | Liver transplant in hepatocellular carcinoma | 566 | N/A | AKI; AKIN criteria within 48 hours after transplant | AKI 109/566 (19.3%) Dialysis 13/566 (2.3%) |
30-day mortality 9/109 (8%) |
| Baron-Stefaniak et al. [80] | 2017 | Austria | Orthotopic liver transplant | 45 | N/A | AKI; KDIGO criteria within 48 hours after transplant | AKI 34/45 (75.6) |
N/A |
| Zhou et al. [30] | 2017 | China | Donation after circulatory death orthotopic liver transplant | 103 | 103 (100%) DCD 103 (100%) |
AKI; KDIGO criteria within 7 days after transplant | AKI 42/103 (40.8%) CRRT 7/103 (6.8%) |
N/A |
| Yoo et al. [31] | 2017 | Korea | Liver transplant | 304 | 84 (28%) | AKI; RIFLE criteria within 7 days after transplant | AKI 132/304 (43.4%) |
N/A |
| Jochmans [29] | 2017 | Belgium | Orthotopic liver transplant | 80 | 80 (100%) DCD 13 (16%) DBD 67 (84%) |
AKI; RIFLE criteria within 5 days after reperfusion | AKI 21/80 (26.3%) Dialysis 4/80 (5%) |
1-year mortality 2/21 (10%) |
| Kandil et al. [28] | 2017 | Egypt | Living donor liver transplant | 50 | 0 (0%) | AKI; AKIN criteria within 48 hours | AKI 23/50 (46%) |
N/A |
| Kim et al. [14] | 2018 | Korea | Living donor liver transplant | 583 | 0 (0%) | AKI; KDIGO criteria within 7 days after transplant | AKI 205/583 (35.2%) |
N/A |
Abbreviations: AKIN, Acute Kidney Injury Network; DCD, donation after circulatory death; EDC, extended donor criteria liver allografts; KDIGO, Kidney Disease Improving Global Outcomes; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; UK, United Kingdom; USA, United States of America.
3.1. Incidence of Post-LTx AKI
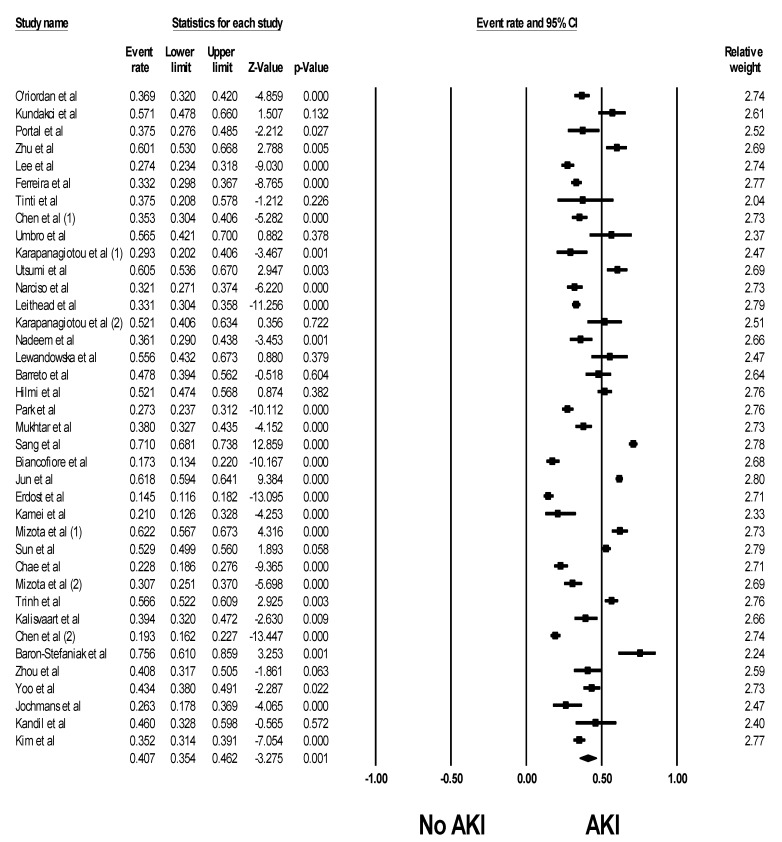
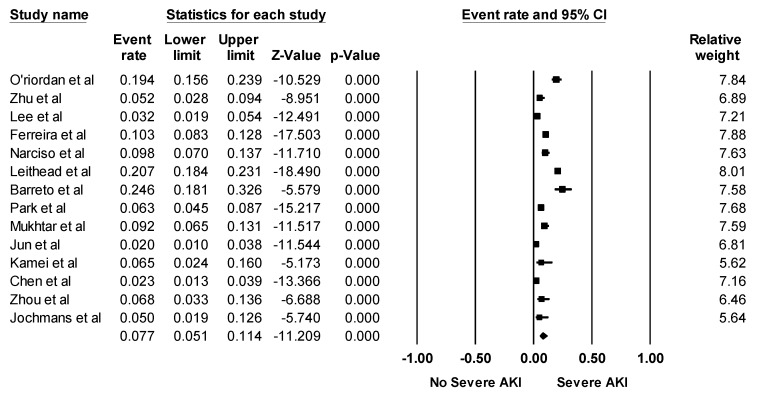
Overall, the pooled estimated incidence rates of post-LTx AKI and severe AKI requiring RRT following LTx were 40.7% (95% CI: 35.4%–46.2%, I2 = 97%, Figure 2) and 7.7% (95% CI: 5.1%–11.4%, I2 = 95%, Figure 3), respectively.
Figure 2.
Forest plots of the included studies assessing incidence rates of post-LTx AKI. A diamond data marker represents the overall rate from each included study (square data marker) and 95% confidence interval.
Figure 3.
Forest plots of the included studies assessing incidence rates of severe AKI requiring RRT following LTx. A diamond data marker represents the overall rate from each included study (square data marker) and 95% confidence interval.
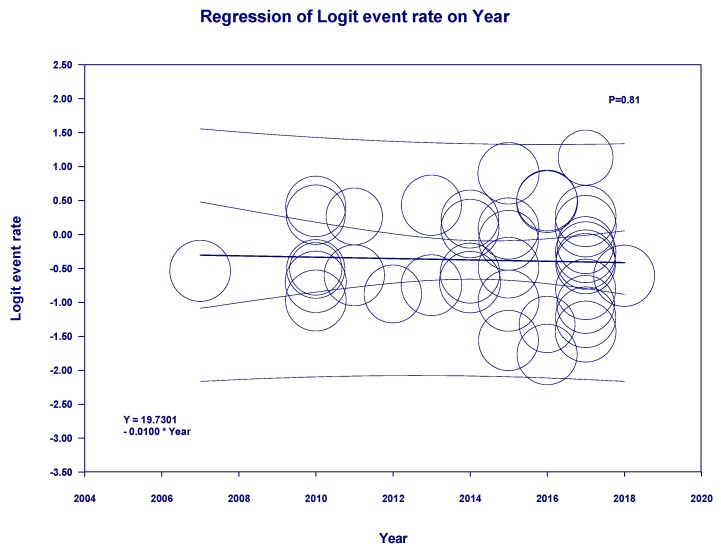
Meta-regression showed no significant impact of type of donor (deceased vs living donors) (p = 0.33) on the incidence of post-LTx AKI. In addition, the year of study (p = 0.81) did not significantly affect the incidence of post-LTx AKI (Figure 4).
Figure 4.
Meta-regression analyses showed no significant impact of year of study on the incidence of post-LTx AKI (p = 0.81). The solid black line represents the weighted regression line based on variance-weighted least squares. The inner and outer lines show the 95% confidence interval and prediction interval around the regression line. The circles indicate log event rates in each study.
3.2. Risk Factors for Post-LTx AKI
Reported risk factors for post-LTx AKI are demonstrated in Table 2. Higher pretransplant SCr [11,23,24,25,32,33,34,35], high body mass index (BMI) [39,64,66,67], high MELD/MELD-Na score [23,39,40,41,42,43,44,45,46,47,48,49], intraoperative blood loss and perioperative blood transfusion [18,25,39,48,54,65], high APACHE II score [25,43,48,55], hypotension and vasopressor requirement [18,24,48,54], cold and warm ischemia time [14,35,78], graft dysfunction [11,40,53], post-reperfusion syndrome [20,64,66,75,78], infection prior to transplant [25,45,48], and hypoalbuminemia [18,64,66] were consistently identified as important risk factors for Post-LTx AKI.
Table 2.
Reported Potential Predictors/Associated-Risk Factors of Post-LTx AKI.
| Donor and Graft Factors | Recipient Factors | Surgical and Postoperative Factors |
|---|---|---|
| Cold ischemia time [14,35,78], warm ischemic time [35,39,63,64,66] Small-for-size graft/Graft-recipient body weight ratio [40,44,65,66] Deceased donor [20,47] Graft dysfunction [11,53] DCD [39] ABO incompatibility [70] Lower donor BMI [39] Older donor age [39] |
Higher MELD score/MELD-Na [23,39,40,41,42,43,44,45,46,47,48,49,64,67,89] APACHE II25 [43,48,55], Preoperative SCr11 [23,24,25,32,33,34,35] Preoperative BUN [23,24] Preoperative renal dysfunction/ARF [40,43,53] Child-Pugh score [19] SOFA [48] Male sex [42], female sex [19,31] Preoperative hepatic encephalopathy [47] Infection [25,48,71] Hypoalbuminemia [18,53,64,66] Preoperative low hemoglobin [14,72] High body weight, BMI [14,19,39,44,64,66,67,75] Pretransplant hypertension [32,54] Preoperative DM [19,44] Alcoholic liver disease [32] Pretransplant hepatitis B and/or C [54,63] Tumor as indication for transplant [47] Elevated lactate [54,63] Elevated plasma NGAL [55] Hyponatremia [39] Pulmonary hypertension [31] |
Intra-operative hypotension, low MAP [24,33,34,54,66,79] Inotrope/vasopressor requirement [18,30,32,48,65], dopamine [35], intra-operative need of noradrenaline [33,67] Duration of treatment with dopamine [53] Blood loss [35,44,47,64,70,71], RBC transfusion [14,18,25,33,39,48,54,65,66,72,89] Need of cryoprecipitate [64] Anesthetic/Operation time [30,64,66,70] Post-reperfusion syndrome [20,64,66,78] SvO2 reduction with oliguria [14], Oxygen content 5 min after graft reperfusion [75] Terlipressin (protective) [65] Venovenous bypass (protective) [21] Postoperative ICU days [23,48] Duration of ventilator support [48] Aminoglycoside use [32] Duration of anhepatic phase [41,79] Intra-operative acidosis [41] Intra-operative urine output [14,24,30,33] Overexposure to calcineurin inhibitor [35,44,64] Need of diuretics [46,75] Chloride-liberal fluid received within the 24 h posttransplant [49] Crystalloid administration [14] Use of 6% HES [89] Mean blood glucose during the day of surgery [64], glucose variability [31] Peak AST occurring at 6 h [29] |
Abbreviations:: ABO incompatibility, incompatibility of the ABO blood group; AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; ALP, alkaline phosphatase; APACHE, Acute Physiology and Chronic Health Evaluation; ARI, acute renal injury; ARF, acute renal failure; AST, aspartate aminotransferase; ATG, Anti-thymocyte globulin; BMI, body mass index; BUN, blood urea nitrogen; CMV, cytomegalovirus; DBD, graft donated after brain death; DCD, donation after circulatory death; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FFP, fresh frozen plasma; HCV, hepatitis C virus; HES, hydroxyethyl starch; ICU, intensive care unit; KDIGO, Kidney Disease Improving Global Outcomes; SCr, serum creatinine; MAP, mean arterial pressure; MELD, Model For End-Stage Liver Disease; MMF, mycophenolate mofetil; N/A, not available; NGAL, neutrophil gelatinase-associated lipocalin; PBC, primary biliary cirrhosis; RBC, red blood cell; RRT, renal replacement therapy; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; SOFA, Sequential Organ Failure Assessment; SvO2, mixed venous oxygen saturation.
3.3. Impacts of Post-LTx AKI on Patient Outcomes
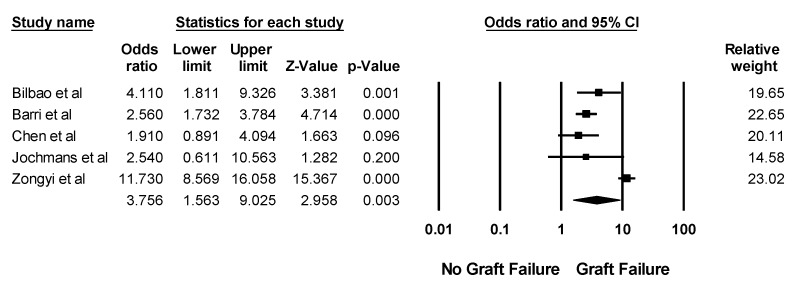
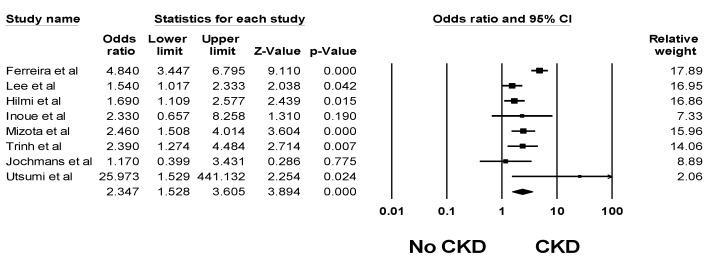
The impacts of post-LTx AKI on patient outcomes are demonstrated in Table 3. Overall, the pooled estimated in-hospital or 30-day mortality, and 1-year mortality rates of patients with post-LTx AKI were 16.5% (95% CI: 10.8%–24.3%, I2 = 94%) and 31.1% (95% CI: 22.4%–41.5%, I2 = 78%), respectively. Post-LTx AKI was associated with significantly higher mortality with a pooled OR of 2.96 (95% CI: 2.32–3.77, I2 = 59%). In addition, severe post-LTx AKI requiring RRT was associated with significantly higher mortality with a pooled OR of 8.15 (95% CI: 4.52–14.69, I2 = 90%). Compared to those without post-LTx AKI, recipients with post-LTx AKI had significantly increased risks of liver graft failure and CKD with pooled ORs of 3.76 (95% CI: 1.56–9.03, I2 = 91%, Figure 5) and 2.35 (95% CI: 1.53–3.61, I2 = 75%, Figure 6), respectively. AKI was associated with prolonged intensive care (ICU) and hospital stay [17,18,23,24,29,32,35,40,42,44,48,49,53,61,64,75,78] (Table 3).
Table 3.
Reported Outcomes of Post-LTx AKI.
| Study | Outcomes | Confounder Adjustment |
|---|---|---|
| Bilbao et al. [11] | Mortality Dialysis: 6.47 (2.73–15.35) Graft failure Dialysis: 4.11 (1.81–9.32) |
None |
| Contreras et al. [24] | Hospital mortality Dialysis: 9.91 (3.45–28.51) ICU LOS Dialysis: 15 ± 13 vs. 7 ± 11 days Hospital LOS Dialysis: 34 ± 27 vs. 19 ± 20 days |
None |
| Lebrón Gallardo et al. [25] | Mortality Early renal dysfunction: 2.47 (1.29–4.72) Dialysis: 8.80 (3.65–21.23) |
None |
| Sanchez et al. [23] | 1-year mortality Dialysis: 9.07 (5.49–14.97) ICU LOS 2.1 ± 3.0 in no dialysis vs. 8.6 ± 11.6 in hemodialysis vs. 10.5 ± 12.8 days in CRRT |
None |
| Wyatt et al. [22] | Mortality ARF without RRT: 8.69 (3.25–23.19) ARF with RRT: 12.07 (3.90–37.32) |
Age, sex, race, DM, transplant centers |
| Cabezuelo et al. [53] | ICU LOS ARF: 12.9 ± 7.4 vs. 7.2 ± 4.0 days |
N/A |
| O’Riordan et al. [32] | 1-year mortality ARF: 2.6 (1.5–4.5) Hospital LOS 39.3 ± 79.5 in no ARI/ARF vs. 53.3 ± 72.8 in ARI vs. 73.0 ± 129.8 days in ARF |
DM, pretransplant, SCr, PBC, inotrope use, CMV infection/disease, rejection |
| Lee et al. [40] | Hospital LOS Renal dysfunction: 75 ± 144 vs. 45.2 ± 34.5 days |
N/A |
| Rueggeberg et al. [54] | 1-year mortality AKI: 10.93 (3.64–32.83) |
None |
| Barri et al. [17] | 2-year mortality AKI: 2.33 (1.53–3.53) 2-year graft failure AKI: 2.56 (1.73–3.78) ICU LOS AKI: 8 ± 19 vs. 3 ± 5 days Hospital LOS AKI: 20 ± 24 vs. 11 ± 10 days |
None |
| Kundakci et al. [41] | 1-year mortality AKI: 6.73 (2.15–21.06) |
None |
| Zhu et al. [42] | 1-year mortality AKI: 12.1 (1.57–93.54) ICU LOS AKI: 6 (4–9) vs. 4 (3–5) days Hospital LOS AKI: 29 (16–47) vs. 29 (20–48) days |
Hypertension, infection and APACHE II |
| Ferreira et al. [57] | Mortality AKI: 0.73 (0.59–1.08) CKD AKI: 4.84 (3.45–6.80) |
None |
| Lee et al. [56] | CKD AKI: 1.54 (1.02–2.34) |
Age, sex, period of transplant, BMI, pretransplant DM, pretransplant hypertension, history of cardiovascular disease, donor type, underlying liver disease, HBV-related liver disease, hepatocellular carcinoma, use of adefovir, calcineurin inhibitors, purine metabolism inhibitors, acute rejection, pretransplant hemoglobin, pretransplant GFR, pretransplant proteinuria, hepatorenal syndrome, Child-Pugh score, MELD score |
| Chen et al. [18] | 1-year mortality ARI/ARF: 2.79 (0.96–8.12) 1-year graft failure ARI/ARF: 1.91 (0.89–4.09) Hospital LOS 21.8 ± 22.1 in no ARI/ARF vs. 24 ± 25 in ARI and 37 ± 49 days in ARF |
None |
| Karapanagiotou et al. [43] | 1-year mortality 9.61 (1.48–62.55) |
Infection, hemorrhage, MELD, APACHE score |
| Utsumi et al. [44] | Hospital mortality AKI: 5.04 (1.11–22.81) ARI/ARF: 5.90 (1.83–19.06) 1-year mortality AKI: 9.53 (2.18–41.56) ARI/ARF: 12.90 (4.24–39.30) CKD AKI: 15/107 (14%) vs. 0/77 (0%) ARI/ARF: 35.29 (4.51–275.82) Hospital LOS ARI/ARF: 101.5 ± 68.8 vs. 69.7 ± 48.5 days |
None |
| Narciso et al. [60] | Mortality Dialysis: 6.7 (3.49–12.96) |
None |
| Romano et al. [45] | Hospital mortality AKI: 1.88 (0.76–4.65) |
None |
| Leithead et al. [39] | Mortality 1.71 (1.35–2.17) |
Age, sex, MELD score, eGFR, DM |
| Klaus et al. [46] | Mortality AKI: 5.11 (1.39–18.71) Dialysis:14.4 (4.60–45.09) |
None |
| Kim et al. [47] | 1-year mortality Dialysis: 56.5 (12.32–259.20) |
None |
| Karapanagiotou et al. [48] | 6-month mortality RIFLE: 3.08 (1.09–1.95) AKIN: 9.34 (1.20–15.69) ICU LOS RIFLE: 15.44 ± 15.41 vs. 8.65 ± 12.59 days AKIN: 13.75 ± 14.53 vs. 9.1 ± 13.08 days |
Vasopressor use, RBC transfusion |
| Nadeem et al. [49] | ICU LOS AKI: 13.4 ± 19 vs. 5.5 ± 4.7 days |
N/A |
| Kirnap et al. [61] | Mortality AKI: 1.85 (0.65–5.23) ICU LOS AKI: 10 ± 8 vs. 3 ± 2 days Hospital LOS AKI: 26 ± 70 vs. 16 ± 7 days |
None |
| Barreto et al. [63] | Hospital mortality AKIN stage 2 or 3: 4.3 (1.3–14.6) |
None |
| Hilmi et al. [19] | 30-day mortality AKI: 3/221(1.4%) vs. 0/203 (0%) CKD AKI: 1.69 (1.11–2.58) |
None |
| Park et al. [64] | Hospital mortality 3.44 (1.89–6.25) 1-year mortality AKI: 1.57 (0.95–2.58) ICU LOS 6 (6–7) in no AKI vs. 6 (6–9) in Risk vs. 7 (6–18) in Injury vs. 11 (10–85) in Failure group Hospital LOS 29 (23–42) in no AKI vs. 31 (21–43) in Risk vs. 33 (26–47) in Injury vs. 46 (16–108) in Failure group |
None |
| Mukhtar et al. [65] | Mortality AKI: 2.1 (1.18–4.0) |
Graft weight to recipient body weight ratio, baseline creatinine, MELD score, DM, Terlipressin use, massive transfusion, vasopressor use |
| Sang et al. [66] | Mortality RIFLE AKI: 2.29 (1.29–4.05) AKIN AKI: 1.69 (1.06–2.67) |
None |
| Wyssusek et al. [67] | Mortality AKI: 3.23 (0.43–24.27) |
None |
| Jun et al. [70] | Mortality AKI: 0.36 (0.09–1.43) |
ABO incompatibility, MELD score, hypertension, coronary artery disease, age, post-reperfusion syndrome, vasopressor, crystalloid, RBC transfusion, FFP transfusion, operation time, cold ischemic time |
| Inoue et al. [71] | 1-year mortality AKI: 4.54 (1.27–16.32) CKD AKI: 2.33 (0.66–8.29) |
None |
| Mizota et al. [74] | Hospital mortality AKI: 2.53 (1.23–5.22) CKD AKI: 2.46 (1.51–4.02) |
Age, MELD score, blood type incompatibility, re-transplantation |
| Erdost et al. [72] | 30-day mortality RIFLE AKI: 4.15 (1.72–10.00) AKIN AKI: 440.83 (58.24–3336.87) KDIGO AKI: 35/64 (55%) vs. 0/376 |
None |
| Chae et al. [75] | Hospital mortality AKI: 1.63 (0.73–3.60) ICU LOS AKI: 7 (6–8) vs. 7 (5–7) days Hospital LOS AKI: 28 (22–39) vs. 23 (21–31) days |
None |
| Mizota et al. [76] | Hospital mortality Severe AKI: 3.56 (1.78–7.09) |
None |
| Trinh et al. [77] | Mortality AKI: 1.41 (1.03–1.92) CKD stage 4–5 AKI: 2.39 (1.27–4.47) |
Age, sex, MELD score, baseline eGFR, ATG induction, pretransplant hypertension and DM |
| Kalisvaart et al. [78] | Hospital mortality AKI: 7.96 (1.66–38.25) ICU LOS AKI: 3 (2–5) vs. 2 (2–3) days Hospital LOS AKI: 24 (19–35) vs. 17 (14–27) days |
None |
| Nadkarni et al. [16] | Hospital mortality Dialysis: 2.00 (1.55–2.59) |
Not specified |
| Chen et al. [79] | 30-day mortality AKI: 4.05 (1.02–16.18) |
ALP, MELD score, operation time, blood transfusion |
| Zongyi et al. [35] | 1-year mortality RIFLE failure stage AKI: 12.25 (8.99–16.70) 1-year graft failure RIFLE failure stage AKI: 11.73 (8.57–16.06) Hospital LOS RIFLE failure stage AKI: 16 (6–34.5) vs. 25 (18–35) days |
None |
| Zhou et al. [30] | 14-day mortality AKI: 3.35 (0.94–11.98) Hospital LOS AKI: 28.13 ± 20.04 vs. 26.16 ± 11.91 days |
None |
| Jochmans et al. [29] | 1-year mortality AKI: 6.11 (0.52–71.16) 1-year graft failure AKI: 2.54 (0.61–10.55) CKD AKI:1.17 (0.40–3.44) ICU LOS AKI: 4 (3–9) vs. 2 (2–4) Hospital LOS AKI: 23 (17–46) vs. 16 (13–26) |
None |
Abbreviations:: ABO incompatibility, incompatibility of the ABO blood group; AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; ALP, alkaline phosphatase; APACHE, Acute Physiology and Chronic Health Evaluation; ARI, acute renal injury; ARF, acute renal failure; AST, aspartate aminotransferase; ATG, Anti-thymocyte globulin; BMI, body mass index; BUN, blood urea nitrogen; CMV, cytomegalovirus; DCD, donation after circulatory death; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FFP, fresh frozen plasma; HCV, hepatitis C virus; HES, hydroxyethyl starch; ICU, intensive care unit; KDIGO, Kidney Disease Improving Global Outcomes; SCr, serum creatinine; MAP, mean arterial pressure; MELD, Model For End-Stage Liver Disease; MMF, mycophenolate mofetil; N/A, not available; NGAL, neutrophil gelatinase-associated lipocalin; PBC, primary biliary cirrhosis; RBC, red blood cell; RRT, renal replacement therapy; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; SOFA, Sequential Organ Failure Assessment; SvO2, mixed venous oxygen saturation.
Figure 5.
Forest plots of the included studies assessing liver graft failure among patients with post-LTx AKI. A diamond data marker represents the overall rate from each included study (square data marker) and 95% confidence interval.
Figure 6.
Forest plots of the included studies assessing CKD risk among patients with post-LTx AKI. A diamond data marker represents the overall rate from each included study (square data marker) and 95% confidence interval.
3.4. Evaluation for Publication Bias
Funnel plot (Supplementary Figure S1) and Egger’s regression asymmetry test were performed to evaluate for publication bias in the analysis evaluating incidence of post-LTx AKI and mortality risk of post-LTx AKI. There was no significant publication bias in meta-analysis assessing the incidence of post-LTx AKI, p-value = 0.12.
4. Discussion
In this meta-analysis, we found that AKI and severe AKI requiring RRT after LTx are common, with an incidence of 40.8% and 7.0%, respectively. In addition, our findings showed no significant correlation between the incidence of post-LTx AKI and study year for the ten years of the study. Furthermore, compared to patients without post-LTx AKI, those with post-LTx AKI carry a 2.96-fold increased risk of mortality and a 3.76-fold higher risk of liver graft failure.
The development of post-LTx AKI appears to be multifactorial with a number of preoperative, intraoperative and postoperative factors involved [90]. Pre-LTx factors include high MELD/MELD-Na score, high APACHE II score, hypoalbuminemia, and reduced eGFR [11,23,24,25,32,33,34,35]. Preexisting renal impairment is common among patients with end-stage liver disease [91]. Although cirrhotic patients with significant CKD are eligible to receive a combined liver-kidney transplantation [92], a lower baseline GFR among those who received LTx alone remained an important risk factor for post-operative AKI [11,23,24,25,32,33,34,35]. Studies have demonstrated that hepatorenal syndrome before LTx can also lead to renal insufficiency and render LTx recipients more susceptible to post-LTx AKI [22,90,93]. In addition, sepsis, graft dysfunction, thrombotic microangiopathy, and calcineurin inhibitor nephrotoxicity may all contribute to AKI [22,37,94,95,96].
Studies have shown that higher MELD scores were associated with post-LTx AKI [23,39,40,41,42,43,44,45,46,47,48,49]. In patients with high MELD scores >30, the majority required RRT post LTx [44,97]. Although SCr is an important determinant of the MELD score, other components of MELD such as pre-LTx INR has also been demonstrated to be strongly associated with post-LT AKI, suggesting that the severity of the liver disease itself, as reflected by the MELD score, is associated with post-LT AKI [45]. Identified perioperative factors for post-LTx AKI include cardiopulmonary failure, vasopressor requirement, hemodynamic effects of prolonged surgery, and blood loss/RBC transfusion [18,24,25,39,48,54,65]. Moreover, it has been hypothesized that HIRI is an important cause of post-LTx AKI [37,38]. Aspartate aminotransferase (AST), as a surrogate marker for HIRI, has been shown to be correlated with post-LTx AKI. [38,78] HIRI has a close relationship with the systemic inflammatory response, which in turn is related to AKI and multiorgan dysfunction in similar settings such as sepsis [37]. Early hepatic graft dysfunction has also been shown to be associated to post-LTx AKI [98]. In addition, recipients of donation after circulatory death (DCD) grafts are reported to have a higher incidence of post-LTx AKI compared to donation after brain death (DBD grafts). After DCD LTx, peak AST levels were an independent predictor of post-LTx AKI [99]. Other known factors that influence HIRI such as donor age, cold and warm ischemia times and graft steatosis have also been associated with post-LTx AKI [37].
As demonstrated in our study, post-LTx AKI is associated with an increased risk of death and liver graft failure. Several pharmacological and non-pharmacological interventions have been studied, but so far these have failed to demonstrate any significant benefit in the prevention of post-LTx AKI [37,100,101]. To continue efforts to mitigate post-LTx AKI, it is important to identify those who are at high-risk for post-LTx AKI in order to develop earlier protective strategies [37]. There have been many attempts to develop predictive models for post-LTx AKI [37]. Seven published predictive models addressing a diverse range of AKI definitions for post-LT AKI have been developed [19,23,24,33,47,54,55]. However, the numbers of patients in these studies were limited [19,23,24,33,47,54,55], and future prospective external validation, ideally with multi-center studies with large number of patients, is required.
Several limitations in our meta-analysis are worth mentioning. First, there were statistical heterogeneities present in our study. Possible sources for heterogeneities were the differences in the patient characteristics in the individual studies. However, we performed a meta-regression analysis which demonstrated that the type of donor (deceased vs. living donors); the year of study did not significantly affect the incidence of post-LTx AKI. Second, there is a lack of data from included studies on novel AKI biomarkers. Novel biomarkers for AKI are emerging and could be useful for the early identification and characterization of AKI. Thus, future studies evaluating predictive models with novel biomarkers are needed. Lastly, this is a systematic review and meta-analysis of cohort studies. Thus, it can demonstrate associations of post-LTx AKI with increased risk of mortality and liver graft failure, but not a causal relationship.
5. Conclusions
In conclusion, there are overall high incidence rates of post-LTx AKI and severe AKI requiring RRT of 40.8% and 7.0%. Post-LTx AKI is significantly associated with increased mortality and liver graft failure. In addition, the incidence of post-LTx AKI has remained stable over time. This study provides an epidemiological perspective to support the need for future large-scale multi-center studies to identify preventive strategies for post-LTx AKI.
Acknowledgments
None. All authors had access to the data and played essential roles in writing of the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/8/3/372/s1, Figure S1: Funnel plot evaluating for publication bias evaluating incidence of post-LTx AKI.
Author Contributions
Conceptualization, M.A.M. and W.C.; Data curation, C.T., N.T., and K.W. Formal analysis, C.T. and W.C.; Investigation, C.T., N.T., T.B., P.L., K.S., S.A.S., P.U. and W.C.; Methodology, C.T., W.K., T.B., P.U., K.W., P.T.K., N.R.A. and W.C.; Project administration, W.K., T.B., K.W., P.L., K.S. and S.A.S.; Resources, T.B., K.W., P.L., K.S. and S.A.S. and P.U.; Software, K.W.; Supervision, W.K., M.A.M. and W.C.; Validation, P.U., M.A.M. and W.C.; Visualization, W.C.; Writing—original draft, C.T.; Writing—review & editing, C.T., W.K., N.T., T.B., K.W., P.L., K.S. and S.A.S., P.U., K.W., P.T.K., N.R.A., M.A.M. and W.C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gameiro J., Agapito Fonseca J., Jorge S., Lopes J.A. Acute Kidney Injury Definition and Diagnosis: A Narrative Review. J. Clin. Med. 2018;7:307. doi: 10.3390/jcm7100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoste E.A.J., Kellum J.A., Selby N.M., Zarbock A., Palevsky P.M., Bagshaw S.M., Goldstein S.L., Cerdá J., Chawla L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018;14:607–625. doi: 10.1038/s41581-018-0052-0. [DOI] [PubMed] [Google Scholar]
- 3.Mehta R.L., Burdmann E.A., Cerdá J., Feehally J., Finkelstein F., García-García G., Godin M., Jha V., Lameire N.H., Levin N.W., et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: A multinational cross-sectional study. Lancet. 2016;387:2017–2025. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 4.Mehta R.L., Burdmann E.A., Cerdá J., Feehally J., Finkelstein F., García-García G., Godin M., Jha V., Lameire N.H., Levin N.W., et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 5.Sawhney S., Marks A., Fluck N., Levin A., McLernon D., Prescott G., Black C. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Kidney Int. 2017;92:440–452. doi: 10.1016/j.kint.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponce D., Balbi A. Acute kidney injury: Risk factors and management challenges in developing countries. Int. J. Nephrol. Renov. Dis. 2016;9:193–200. doi: 10.2147/IJNRD.S104209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavkov M.E., Harding J.L., Burrows N.R. Trends in Hospitalizations for Acute Kidney Injury—United States, 2000–2014. Morb. Mortal. Wkly. Rep. 2018;67:289. doi: 10.15585/mmwr.mm6710a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Renal Data System. [(accessed on 15 February 2019)];2017 Available online: https://www.usrds.org/2017/download/v1_c05_AKI_17.pdf.
- 9.Mokdad A.A., Lopez A.D., Shahraz S., Lozano R., Mokdad A.H., Stanaway J., Murray C.J., Naghavi M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCauley J., Van Thiel D.H., Starzl T.E., Puschett J.B. Acute and chronic renal failure in liver transplantation. Nephron. 1990;55:121–128. doi: 10.1159/000185938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilbao I., Charco R., Balsells J., Lazaro J.L., Hidalgo E., Llopart L., Murio E., Margarit C. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin. Transplant. 1998;12:123–129. [PubMed] [Google Scholar]
- 12.Carmona M., Álvarez M., Marco J., Mahíllo B., Domínguez-Gil B., Núñez J.R., Matesanz R. Global Organ Transplant Activities in 2015. Data from the Global Observatory on Donation and Transplantation (GODT) Transplantation. 2017;101:S29. doi: 10.1097/01.tp.0000525015.43613.75. [DOI] [Google Scholar]
- 13.White S.L., Hirth R., Mahillo B., Dominguez-Gil B., Delmonico F.L., Noel L., Chapman J., Matesanz R., Carmona M., Alvarez M., et al. The global diffusion of organ transplantation: Trends, drivers and policy implications. Bull. World Health Organ. 2014;92:826–835. doi: 10.2471/BLT.14.137653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim W.H., Lee H.C., Lim L., Ryu H.G., Jung C.W. Intraoperative Oliguria with Decreased SvO Predicts Acute Kidney Injury after Living Donor Liver Transplantation. J. Clin. Med. 2018;8:29. doi: 10.3390/jcm8010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamada M., Matsukawa S., Shimizu S., Kai S., Mizota T. Acute kidney injury after pediatric liver transplantation: Incidence, risk factors, and association with outcome. J. Anesth. 2017;31:758–763. doi: 10.1007/s00540-017-2395-2. [DOI] [PubMed] [Google Scholar]
- 16.Nadkarni G.N., Chauhan K., Patel A., Saha A., Poojary P., Kamat S., Patel S., Ferrandino R., Konstantinidis I., Garimella P.S., et al. Temporal trends of dialysis requiring acute kidney injury after orthotopic cardiac and liver transplant hospitalizations. BMC Nephrol. 2017;18:244. doi: 10.1186/s12882-017-0657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barri Y.M., Sanchez E.Q., Jennings L.W., Melton L.B., Hays S., Levy M.F., Klintmalm G.B. Acute kidney injury following liver transplantation: Definition and outcome. Liver Transplant. 2009;15:475–483. doi: 10.1002/lt.21682. [DOI] [PubMed] [Google Scholar]
- 18.Chen J., Singhapricha T., Hu K.Q., Hong J.C., Steadman R.H., Busuttil R.W., Xia V.W. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: A matched study. Transplantation. 2011;91:348–353. doi: 10.1097/TP.0b013e31820437da. [DOI] [PubMed] [Google Scholar]
- 19.Hilmi I.A., Damian D., Al-Khafaji A., Planinsic R., Boucek C., Sakai T., Chang C.C., Kellum J.A. Acute kidney injury following orthotopic liver transplantation: Incidence, risk factors, and effects on patient and graft outcomes. Br. J. Anaesth. 2015;114:919–926. doi: 10.1093/bja/aeu556. [DOI] [PubMed] [Google Scholar]
- 20.Hilmi I.A., Damian D., Al-Khafaji A., Sakai T., Donaldson J., Winger D.G., Kellum J.A. Acute kidney injury after orthotopic liver transplantation using living donor versus deceased donor grafts: A propensity score-matched analysis. Liver Transplant. 2015;21:1179–1185. doi: 10.1002/lt.24166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun K., Hong F., Wang Y., Agopian V.G., Yan M., Busuttil R.W., Steadman R.H., Xia V.W. Venovenous Bypass Is Associated With a Lower Incidence of Acute Kidney Injury After Liver Transplantation in Patients with Compromised Pretransplant Renal Function. Anesth. Analg. 2017;125:1463–1470. doi: 10.1213/ANE.0000000000002311. [DOI] [PubMed] [Google Scholar]
- 22.Wyatt C.M., Arons R.R. The burden of acute renal failure in nonrenal solid organ transplantation. Transplantation. 2004;78:1351–1355. doi: 10.1097/01.TP.0000140848.05002.B8. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez E.Q., Gonwa T.A., Levy M.F., Goldstein R.M., Mai M.L., Hays S.R., Melton L.B., Saracino G., Klintmalm G.B. Preoperative and perioperative predictors of the need for renal replacement therapy after orthotopic liver transplantation. Transplantation. 2004;78:1048–1054. doi: 10.1097/01.TP.0000137176.95730.5B. [DOI] [PubMed] [Google Scholar]
- 24.Contreras G., Garces G., Quartin A.A., Cely C., LaGatta M.A., Barreto G.A., Roth D., Gomez E. An epidemiologic study of early renal replacement therapy after orthotopic liver transplantation. J. Am. Soc. Nephrol. 2002;13:228–233. doi: 10.1681/ASN.V131228. [DOI] [PubMed] [Google Scholar]
- 25.Lebron Gallardo M., Herrera Gutierrez M.E., Seller Perez G., Curiel Balsera E., Fernandez Ortega J.F., Quesada Garcia G. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transplant. 2004;10:1379–1385. doi: 10.1002/lt.20215. [DOI] [PubMed] [Google Scholar]
- 26.Alvares-da-Silva M.R., Waechter F.L., Francisconi C.F., Barros E., Thome F., Traiber C., Fonseca D.L., Zingani J.M., Sampaio J.A., Pinto R.D., et al. Risk factors for postoperative acute renal failure at a new orthotopic liver transplantation program. Transplant. Proc. 1999;31:3050–3052. doi: 10.1016/S0041-1345(99)00666-1. [DOI] [PubMed] [Google Scholar]
- 27.Rossi A.P., Vella J.P. Acute Kidney Disease After Liver and Heart Transplantation. Transplantation. 2016;100:506–514. doi: 10.1097/TP.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 28.Kandil M.A., Abouelenain K.M., Alsebaey A., Rashed H.S., Afifi M.H., Mahmoud M.A., Yassen K.A. Impact of terlipressin infusion during and after live donor liver transplantation on incidence of acute kidney injury and neutrophil gelatinase-associated lipocalin serum levels: A randomized controlled trial. Clin. Transplant. 2017;31:e13019. doi: 10.1111/ctr.13019. [DOI] [PubMed] [Google Scholar]
- 29.Jochmans I., Meurisse N., Neyrinck A., Verhaegen M., Monbaliu D., Pirenne J. Hepatic ischemia/reperfusion injury associates with acute kidney injury in liver transplantation: Prospective cohort study. Liver Transplant. 2017;23:634–644. doi: 10.1002/lt.24728. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Z.Q., Fan L.C., Zhao X., Xia W., Luo A.L., Tian Y.K., Wang X.R. Risk factors for acute kidney injury after orthotopic liver transplantation: A single-center data analysis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017;37:861–863. doi: 10.1007/s11596-017-1818-5. [DOI] [PubMed] [Google Scholar]
- 31.Yoo S., Lee H.J., Lee H., Ryu H.G. Association Between Perioperative Hyperglycemia or Glucose Variability and Postoperative Acute Kidney Injury After Liver Transplantation: A Retrospective Observational Study. Anesth. Analg. 2017;124:35–41. doi: 10.1213/ANE.0000000000001632. [DOI] [PubMed] [Google Scholar]
- 32.O’Riordan A., Wong V., McQuillan R., McCormick P.A., Hegarty J.E., Watson A.J. Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am. J. Transplant. 2007;7:168–176. doi: 10.1111/j.1600-6143.2006.01602.x. [DOI] [PubMed] [Google Scholar]
- 33.Xu X., Ling Q., Wei Q., Wu J., Gao F., He Z.L., Zhou L., Zheng S.S. An effective model for predicting acute kidney injury after liver transplantation. Hepatobiliary Pancreat. Dis. Int. 2010;9:259–263. [PubMed] [Google Scholar]
- 34.De Ataide E.C., Perales S.R., Bortoto J.B., Peres M.A.O., Filho F.C., Stucchi R.S.B., Udo E., Boin I. Immunomodulation, Acute Renal Failure, and Complications of Basiliximab Use After Liver Transplantation: Analysis of 114 Patients and Literature Review. Transplant. Proc. 2017;49:852–857. doi: 10.1016/j.transproceed.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 35.Zongyi Y., Baifeng L., Funian Z., Hao L., Xin W. Risk factors of acute kidney injury after orthotopic liver transplantation in China. Sci. Rep. 2017;7:41555. doi: 10.1038/srep41555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croome K.P., Lee D.D., Croome S., Chadha R., Livingston D., Abader P., Keaveny A.P., Taner C.B. The impact of post-reperfusion syndrome during liver transplantation using livers with significant macrosteatosis. Am. J. Transplant. 2019 doi: 10.1111/ajt.15330. [DOI] [PubMed] [Google Scholar]
- 37.De Haan J.E., Hoorn E.J., de Geus H.R.H. Acute kidney injury after liver transplantation: Recent insights and future perspectives. Best Pract. Res. Clin. Gastroenterol. 2017;31:161–169. doi: 10.1016/j.bpg.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Leithead J.A., Armstrong M.J., Corbett C., Andrew M., Kothari C., Gunson B.K., Muiesan P., Ferguson J.W. Hepatic ischemia reperfusion injury is associated with acute kidney injury following donation after brain death liver transplantation. Transpl. Int. 2013;26:1116–1125. doi: 10.1111/tri.12175. [DOI] [PubMed] [Google Scholar]
- 39.Leithead J.A., Rajoriya N., Gunson B.K., Muiesan P., Ferguson J.W. The evolving use of higher risk grafts is associated with an increased incidence of acute kidney injury after liver transplantation. J. Hepatol. 2014;60:1180–1186. doi: 10.1016/j.jhep.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 40.Lee S.K., Park J.B., Kim S.J., Choi G.S., Kim D.J., Kwon C.H., Joh J.W. Early postoperative renal dysfunction in the adult living donor liver transplantation. Transplant. Proc. 2007;39:1517–1519. doi: 10.1016/j.transproceed.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Kundakci A., Pirat A., Komurcu O., Torgay A., Karakayali H., Arslan G., Haberal M. Rifle criteria for acute kidney dysfunction following liver transplantation: Incidence and risk factors. Transplant. Proc. 2010;42:4171–4174. doi: 10.1016/j.transproceed.2010.09.137. [DOI] [PubMed] [Google Scholar]
- 42.Zhu M., Li Y., Xia Q., Wang S., Qiu Y., Che M., Dai H., Qian J., Ni Z., Axelsson J., et al. Strong impact of acute kidney injury on survival after liver transplantation. Transplant. Proc. 2010;42:3634–3638. doi: 10.1016/j.transproceed.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 43.Karapanagiotou A., Kydona C., Dimitriadis C., Sgourou K., Giasnetsova T., Fouzas I., Imvrios G., Gritsi-Gerogianni N. Acute kidney injury after orthotopic liver transplantation. Transplant. Proc. 2012;44:2727–2729. doi: 10.1016/j.transproceed.2012.09.096. [DOI] [PubMed] [Google Scholar]
- 44.Utsumi M., Umeda Y., Sadamori H., Nagasaka T., Takaki A., Matsuda H., Shinoura S., Yoshida R., Nobuoka D., Satoh D., et al. Risk factors for acute renal injury in living donor liver transplantation: Evaluation of the RIFLE criteria. Transpl. Int. 2013;26:842–852. doi: 10.1111/tri.12138. [DOI] [PubMed] [Google Scholar]
- 45.Romano T.G., Schmidtbauer I., Silva F.M., Pompilio C.E., D’Albuquerque L.A., Macedo E. Role of MELD score and serum creatinine as prognostic tools for the development of acute kidney injury after liver transplantation. PLoS ONE. 2013;8:e64089. doi: 10.1371/journal.pone.0064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klaus F., Keitel da Silva C., Meinerz G., Carvalho L.M., Goldani J.C., Cantisani G., Zanotelli M.L., Duro Garcia V., Keitel E. Acute kidney injury after liver transplantation: Incidence and mortality. Transplant. Proc. 2014;46:1819–1821. doi: 10.1016/j.transproceed.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 47.Kim J.M., Jo Y.Y., Na S.W., Kim S.I., Choi Y.S., Kim N.O., Park J.E., Koh S.O. The predictors for continuous renal replacement therapy in liver transplant recipients. Transplant. Proc. 2014;46:184–191. doi: 10.1016/j.transproceed.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 48.Karapanagiotou A., Dimitriadis C., Papadopoulos S., Kydona C., Kefsenidis S., Papanikolaou V., Gritsi-Gerogianni N. Comparison of RIFLE and AKIN criteria in the evaluation of the frequency of acute kidney injury in post-liver transplantation patients. Transplant. Proc. 2014;46:3222–3227. doi: 10.1016/j.transproceed.2014.09.161. [DOI] [PubMed] [Google Scholar]
- 49.Nadeem A., Salahuddin N., El Hazmi A., Joseph M., Bohlega B., Sallam H., Sheikh Y., Broering D. Chloride-liberal fluids are associated with acute kidney injury after liver transplantation. Crit. Care. 2014;18:625. doi: 10.1186/s13054-014-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi Z., Mayorga M.E., Orman E.S., Wheeler S.B., Hayashi P.H., Barritt A.S.T. Trends in Characteristics of Patients Listed for Liver Transplantation Will Lead to Higher Rates of Waitlist Removal Due to Clinical Deterioration. Transplantation. 2017;101:2368–2374. doi: 10.1097/TP.0000000000001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orman E.S., Barritt A.S.T., Wheeler S.B., Hayashi P.H. Declining liver utilization for transplantation in the United States and the impact of donation after cardiac death. Liver Transplant. 2013;19:59–68. doi: 10.1002/lt.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chuang F.R., Lin C.C., Wang P.H., Cheng Y.F., Hsu K.T., Chen Y.S., Lee C.H., Chen C.L. Acute renal failure after cadaveric related liver transplantation. Transplant. Proc. 2004;36:2328–2330. doi: 10.1016/j.transproceed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Cabezuelo J.B., Ramirez P., Rios A., Acosta F., Torres D., Sansano T., Pons J.A., Bru M., Montoya M., Bueno F.S., et al. Risk factors of acute renal failure after liver transplantation. Kidney Int. 2006;69:1073–1080. doi: 10.1038/sj.ki.5000216. [DOI] [PubMed] [Google Scholar]
- 54.Rueggeberg A., Boehm S., Napieralski F., Mueller A.R., Neuhaus P., Falke K.J., Gerlach H. Development of a risk stratification model for predicting acute renal failure in orthotopic liver transplantation recipients. Anaesthesia. 2008;63:1174–1180. doi: 10.1111/j.1365-2044.2008.05604.x. [DOI] [PubMed] [Google Scholar]
- 55.Portal A.J., McPhail M.J., Bruce M., Coltart I., Slack A., Sherwood R., Heaton N.D., Shawcross D., Wendon J.A., Heneghan M.A. Neutrophil gelatinase--associated lipocalin predicts acute kidney injury in patients undergoing liver transplantation. Liver Transplant. 2010;16:1257–1266. doi: 10.1002/lt.22158. [DOI] [PubMed] [Google Scholar]
- 56.Lee J.P., Heo N.J., Joo K.W., Yi N.J., Suh K.S., Moon K.C., Kim S.G., Kim Y.S. Risk factors for consequent kidney impairment and differential impact of liver transplantation on renal function. Nephrol. Dial. Transplant. 2010;25:2772–2785. doi: 10.1093/ndt/gfq093. [DOI] [PubMed] [Google Scholar]
- 57.Ferreira A.C., Nolasco F., Carvalho D., Sampaio S., Baptista A., Pessegueiro P., Monteiro E., Mourao L., Barroso E. Impact of RIFLE classification in liver transplantation. Clin. Transplant. 2010;24:394–400. doi: 10.1111/j.1399-0012.2009.01087.x. [DOI] [PubMed] [Google Scholar]
- 58.Tinti F., Umbro I., Mecule A., Rossi M., Merli M., Nofroni I., Corradini S.G., Poli L., Pugliese F., Ruberto F., et al. RIFLE criteria and hepatic function in the assessment of acute renal failure in liver transplantation. Transplant. Proc. 2010;42:1233–1236. doi: 10.1016/j.transproceed.2010.03.128. [DOI] [PubMed] [Google Scholar]
- 59.Umbro I., Tinti F., Mordenti M., Rossi M., Ianni S., Pugliese F., Ruberto F., Ginanni Corradini S., Nofroni I., Poli L., et al. Model for end-stage liver disease score versus simplified acute physiology score criteria in acute renal failure after liver transplantation. Transplant. Proc. 2011;43:1139–1141. doi: 10.1016/j.transproceed.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 60.Narciso R.C., Ferraz L.R., Mies S., Monte J.C., dos Santos O.F., Neto M.C., Rodrigues C.J., Batista M.C., Durao M.S., Jr. Impact of acute kidney injury exposure period among liver transplantation patients. BMC Nephrol. 2013;14:43. doi: 10.1186/1471-2369-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirnap M., Colak T., Baskin E., Akdur A., Moray G., Arslan G., Haberal M. Acute renal injury in liver transplant patients and its effect on patient survival. Exp. Clin. Transplant. 2014;12(Suppl. 1):156–158. [PubMed] [Google Scholar]
- 62.Lewandowska L., Matuszkiewicz-Rowinska J., Jayakumar C., Oldakowska-Jedynak U., Looney S., Galas M., Dutkiewicz M., Krawczyk M., Ramesh G. Netrin-1 and semaphorin 3A predict the development of acute kidney injury in liver transplant patients. PLoS ONE. 2014;9:e107898. doi: 10.1371/journal.pone.0107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barreto A.G., Daher E.F., Silva Junior G.B., Garcia J.H., Magalhaes C.B., Lima J.M., Viana C.F., Pereira E.D. Risk factors for acute kidney injury and 30-day mortality after liver transplantation. Ann. Hepatol. 2015;14:688–694. [PubMed] [Google Scholar]
- 64.Park M.H., Shim H.S., Kim W.H., Kim H.J., Kim D.J., Lee S.H., Kim C.S., Gwak M.S., Kim G.S. Clinical Risk Scoring Models for Prediction of Acute Kidney Injury after Living Donor Liver Transplantation: A Retrospective Observational Study. PLoS ONE. 2015;10:e0136230. doi: 10.1371/journal.pone.0136230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukhtar A., Mahmoud I., Obayah G., Hasanin A., Aboul-Fetouh F., Dabous H., Bahaa M., Abdelaal A., Fathy M., El Meteini M. Intraoperative terlipressin therapy reduces the incidence of postoperative acute kidney injury after living donor liver transplantation. J. Cardiothorac. Vasc. Anesth. 2015;29:678–683. doi: 10.1053/j.jvca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Sang B.H., Bang J.Y., Song J.G., Hwang G.S. Hypoalbuminemia within Two Postoperative Days Is an Independent Risk Factor for Acute Kidney Injury Following Living Donor Liver Transplantation: A Propensity Score Analysis of 998 Consecutive Patients. Crit. Care Med. 2015;43:2552–2561. doi: 10.1097/CCM.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 67.Wyssusek K.H., Keys A.L., Yung J., Moloney E.T., Sivalingam P., Paul S.K. Evaluation of perioperative predictors of acute kidney injury post orthotopic liver transplantation. Anaesth. Intensive Care. 2015;43:757–763. doi: 10.1177/0310057X1504300614. [DOI] [PubMed] [Google Scholar]
- 68.Aksu Erdost H., Ozkardesler S., Ocmen E., Avkan-Oguz V., Akan M., Iyilikci L., Unek T., Ozbilgin M., Meseri Dalak R., Astarcioglu I. Acute Renal Injury Evaluation After Liver Transplantation: With RIFLE Criteria. Transplant. Proc. 2015;47:1482–1487. doi: 10.1016/j.transproceed.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 69.Biancofiore G., Bindi M.L., Miccoli M., Cerutti E., Lavezzo B., Pucci L., Bisa M., Esposito M., Meacci L., Mozzo R., et al. Intravenous fenoldopam for early acute kidney injury after liver transplantation. J. Anesth. 2015;29:426–432. doi: 10.1007/s00540-014-1951-2. [DOI] [PubMed] [Google Scholar]
- 70.Jun I.G., Lee B., Kim S.O., Shin W.J., Bang J.Y., Song J.G., Song G.W., Lee S.G., Hwang G.S. Comparison of acute kidney injury between ABO-compatible and ABO-incompatible living donor liver transplantation: A propensity matching analysis. Liver Transplant. 2016;22:1656–1665. doi: 10.1002/lt.24634. [DOI] [PubMed] [Google Scholar]
- 71.Inoue Y., Soyama A., Takatsuki M., Hidaka M., Kinoshita A., Natsuda K., Baimakhanov Z., Kugiyama T., Adachi T., Kitasato A., et al. Does the development of chronic kidney disease and acute kidney injury affect the prognosis after living donor liver transplantation? Clin. Transplant. 2016;30:518–527. doi: 10.1111/ctr.12715. [DOI] [PubMed] [Google Scholar]
- 72.Erdost H.A., Ozkardesler S., Akan M., Iyilikci L., Unek T., Ocmen E., Dalak R.M., Astarcioglu I. Comparison of the RIFLE, AKIN, and KDIGO Diagnostic Classifications for Acute Renal Injury in Patients Undergoing Liver Transplantation. Transplant. Proc. 2016;48:2112–2118. doi: 10.1016/j.transproceed.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 73.Kamei H., Onishi Y., Nakamura T., Ishigami M., Hamajima N. Role of cytokine gene polymorphisms in acute and chronic kidney disease following liver transplantation. Hepatol. Int. 2016;10:665–672. doi: 10.1007/s12072-016-9721-x. [DOI] [PubMed] [Google Scholar]
- 74.Mizota T., Minamisawa S., Imanaka Y., Fukuda K. Oliguria without serum creatinine increase after living donor liver transplantation is associated with adverse post-operative outcomes. Acta Anaesthesiol. Scand. 2016;60:874–881. doi: 10.1111/aas.12722. [DOI] [PubMed] [Google Scholar]
- 75.Chae M.S., Lee N., Park D.H., Lee J., Jung H.S., Park C.S., Choi J.H., Hong S.H. Influence of oxygen content immediately after graft reperfusion on occurrence of postoperative acute kidney injury in living donor liver transplantation. Medicine. 2017;96:e7626. doi: 10.1097/MD.0000000000007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mizota T., Hamada M., Matsukawa S., Seo H., Tanaka T., Segawa H. Relationship Between Intraoperative Hypotension and Acute Kidney Injury After Living Donor Liver Transplantation: A Retrospective Analysis. J. Cardiothorac. Vasc. Anesth. 2017;31:582–589. doi: 10.1053/j.jvca.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Trinh E., Alam A., Tchervenkov J., Cantarovich M. Impact of acute kidney injury following liver transplantation on long-term outcomes. Clin. Transplant. 2017;31:e12863. doi: 10.1111/ctr.12863. [DOI] [PubMed] [Google Scholar]
- 78.Kalisvaart M., de Haan J.E., Hesselink D.A., Polak W.G., Hansen B.E., JNM I.J., Gommers D., Metselaar H.J., de Jonge J. The postreperfusion syndrome is associated with acute kidney injury following donation after brain death liver transplantation. Transpl. Int. 2017;30:660–669. doi: 10.1111/tri.12891. [DOI] [PubMed] [Google Scholar]
- 79.Chen X., Ding X., Shen B., Teng J., Zou J., Wang T., Zhou J., Chen N., Zhang B. Incidence and outcomes of acute kidney injury in patients with hepatocellular carcinoma after liver transplantation. J. Cancer Res. Clin. Oncol. 2017;143:1337–1346. doi: 10.1007/s00432-017-2376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baron-Stefaniak J., Schiefer J., Miller E.J., Berlakovich G.A., Baron D.M., Faybik P. Comparison of macrophage migration inhibitory factor and neutrophil gelatinase-associated lipocalin-2 to predict acute kidney injury after liver transplantation: An observational pilot study. PLoS ONE. 2017;12:e0183162. doi: 10.1371/journal.pone.0183162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paramesh A.S., Roayaie S., Doan Y., Schwartz M.E., Emre S., Fishbein T., Florman S., Gondolesi G.E., Krieger N., Ames S., et al. Post-liver transplant acute renal failure: Factors predicting development of end-stage renal disease. Clin. Transplant. 2004;18:94–99. doi: 10.1046/j.1399-0012.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 82.Lima E.Q., Zanetta D.M., Castro I., Massarollo P.C., Mies S., Machado M.M., Yu L. Risk factors for development of acute renal failure after liver transplantation. Ren. Fail. 2003;25:553–560. doi: 10.1081/JDI-120022546. [DOI] [PubMed] [Google Scholar]
- 83.Leithead J.A., Armstrong M.J., Corbett C., Andrew M., Kothari C., Gunson B.K., Mirza D., Muiesan P., Ferguson J.W. Split liver transplant recipients do not have an increased frequency of acute kidney injury. Transpl. Int. 2014;27:1125–1134. doi: 10.1111/tri.12376. [DOI] [PubMed] [Google Scholar]
- 84.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 87.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Easterbrook P.J., Berlin J.A., Gopalan R., Matthews D.R. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-Y. [DOI] [PubMed] [Google Scholar]
- 89.Hand W.R., Whiteley J.R., Epperson T.I., Tam L., Crego H., Wolf B., Chavin K.D., Taber D.J. Hydroxyethyl starch and acute kidney injury in orthotopic liver transplantation: A single-center retrospective review. Anesth. Analg. 2015;120:619–626. doi: 10.1213/ANE.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caragata R., Wyssusek K.H., Kruger P. Acute kidney injury following liver transplantation: A systematic review of published predictive models. Anaesth. Intensive Care. 2016;44:251–261. doi: 10.1177/0310057X1604400212. [DOI] [PubMed] [Google Scholar]
- 91.Agarwal B., Shaw S., Shankar Hari M., Burroughs A.K., Davenport A. Continuous renal replacement therapy (CRRT) in patients with liver disease: Is circuit life different? J. Hepatol. 2009;51:504–509. doi: 10.1016/j.jhep.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 92.Asch W.S., Bia M.J. New Organ Allocation System for Combined Liver-Kidney Transplants and the Availability of Kidneys for Transplant to Patients with Stage 4-5 CKD. Clin. J. Am. Soc. Nephrol. 2017;12:848–852. doi: 10.2215/CJN.08480816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ojo A.O. Renal disease in recipients of nonrenal solid organ transplantation. Semin. Nephrol. 2007;27:498–507. doi: 10.1016/j.semnephrol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 94.Biancofiore G., Pucci L., Cerutti E., Penno G., Pardini E., Esposito M., Bindi L., Pelati E., Romanelli A., Triscornia S., et al. Cystatin C as a marker of renal function immediately after liver transplantation. Liver Transplant. 2006;12:285–291. doi: 10.1002/lt.20657. [DOI] [PubMed] [Google Scholar]
- 95.Huen S.C., Parikh C.R. Predicting acute kidney injury after cardiac surgery: A systematic review. Ann. Thorac. Surg. 2012;93:337–347. doi: 10.1016/j.athoracsur.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clajus C., Hanke N., Gottlieb J., Stadler M., Weismuller T.J., Strassburg C.P., Brocker V., Bara C., Lehner F., Drube J., et al. Renal comorbidity after solid organ and stem cell transplantation. Am. J. Transplant. 2012;12:1691–1699. doi: 10.1111/j.1600-6143.2012.04047.x. [DOI] [PubMed] [Google Scholar]
- 97.Schlegel A., Linecker M., Kron P., Gyori G., De Oliveira M.L., Mullhaupt B., Clavien P.A., Dutkowski P. Risk Assessment in High- and Low-MELD Liver Transplantation. Am. J. Transplant. 2017;17:1050–1063. doi: 10.1111/ajt.14065. [DOI] [PubMed] [Google Scholar]
- 98.Wadei H.M., Lee D.D., Croome K.P., Mai M.L., Golan E., Brotman R., Keaveny A.P., Taner C.B. Early Allograft Dysfunction After Liver Transplantation Is Associated With Short- and Long-Term Kidney Function Impairment. Am. J. Transplant. 2016;16:850–859. doi: 10.1111/ajt.13527. [DOI] [PubMed] [Google Scholar]
- 99.Leithead J.A., Tariciotti L., Gunson B., Holt A., Isaac J., Mirza D.F., Bramhall S., Ferguson J.W., Muiesan P. Donation after cardiac death liver transplant recipients have an increased frequency of acute kidney injury. Am. J. Transplant. 2012;12:965–975. doi: 10.1111/j.1600-6143.2011.03894.x. [DOI] [PubMed] [Google Scholar]
- 100.Jo S.K., Rosner M.H., Okusa M.D. Pharmacologic treatment of acute kidney injury: Why drugs haven’t worked and what is on the horizon. Clin. J. Am. Soc. Nephrol. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 101.Valentino K.L., Gutierrez M., Sanchez R., Winship M.J., Shapiro D.A. First clinical trial of a novel caspase inhibitor: Anti-apoptotic caspase inhibitor, IDN-6556, improves liver enzymes. Int. J. Clin. Pharmacol. Ther. 2003;41:441–449. doi: 10.5414/CPP41441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.