Abstract
A rapid method is proposed for the determination of selected H2SO4 stable organic compounds—eight organochlorines (OCs; hexachloro-1,3-butadiene, pentachlorobenzene, hexachlorobenzene, hexachlorocyclohexane—HCH—isomers, heptachlor) and six polybrominated diphenyl ethers (PBDEs; BDE-28, 47, 99, 100, 153, 154)—in fish samples. In the method, a modified QuEChERS (quick, easy, cheap, effective, rugged and safe) sample preparation using pH-tuned dispersive liquid–liquid microextraction (DLLME) and H2SO4 digestion fish extract clean-up is followed by gas chromatography–triple quadrupole tandem mass spectrometry (GC–QqQ-MS/MS) analysis. The method was validated in terms of linearity, limits of the method, recovery, accuracy, analysis of standard reference material (NIST SRM 1946), and estimation of combined uncertainty of the measurement (top-down approach). For validation, chub composite samples were used, and subsequently, the method was successfully applied to analysis of real samples of eight fish species. Finally, the method passed the analytical Eco-Scale evaluation as “an acceptable green analysis method”, and showed its advantages (simplicity, rapidity, low cost, high extract clean-up efficiency, good sensitivity) when compared to other reported QuEChERS based methods.
Keywords: QuEChERS, dispersive liquid–liquid microextraction, sulfuric acid treatment, gas chromatography, tandem mass spectrometry, priority substances, fish samples
1. Introduction
Anthropogenic halogenated organic compounds synthesized as pesticides, solvents or fire retardants have been found to pose a serious threat to aquatic environments, wildlife and humans due to their toxic, persistent and bioaccumulative properties [1]. Because of these harmful effects and impacts, the production and use of large number of organochlorine (OC) pesticides and certain brominated flame retardants was banned or severely restricted in the European Union (EU), and other parts of the world [2], but their presence and release in the environment can be expected over the next decades.
One of the ways human health can be endangered by these substances is through consuming fish living in contaminated waters and accumulating the toxic chemicals in their tissues. Therefore, it is necessary to monitor and analyze fish contamination to protect humans from the consumption of contaminated food. The regulatory limit applicable to residues of pesticides in fish and fishery products is the default maximum residue level (MRL) of 10 µg/kg set by the EU in Regulation 396/2005, which concerns public health and is relevant to the functioning of the internal market [3]. For the determination of PBDEs in fish and other seafood, the EU Commission recommends (recommendation 2014/118/EU) use of analytical methods with a limit of quantification of 0.01 µg/kg wet weight or lower [4].
This paper is focused on the determination of eight OC compounds (hexachloro-1,3-butadiene, pentachlorobenzene, hexachlorobenzene, hexachlorocyclohexane—HCH—isomers, heptachlor) and six polybrominated diphenyl ethers (PBDEs; BDE-28, 47, 99, 100, 153, 154) in fish that were selected from the EU list of priority substances in the field of water policy [5] and from the U.S. EPA (Environmental Protection Agency) priority pollutants list [6]. A great variety of extraction techniques have been applied in the analysis of organic halogenated compounds in fish samples. Among others, solid–liquid extraction (SLE), Soxhlet extraction, accelerated solvent extraction (ASE), supercritical fluid extraction (SFE), microwave-assisted extraction (MAE), matrix solid-phase dispersion (MSPD), and so-called QuEChERS method have been reported in literature [7,8,9,10]. The presented methods include both traditional extraction methods (SLE, Soxhlet) which are quite laborious, time-consuming (extraction duration up to 24 h), and require large amounts of organic solvents (up to few hundreds of mL), and novel methods with shortened extraction times (to 10–60 min), reduced solvent consumption, often with reduced cost, and that are amenable to automation. However, the disadvantage of ASE, SFE, and MAE methods lies in the cost of equipment setup.
In the last few decades, the QuEChERS method with the advantages summarized in its acronym (quick, easy, cheap, effective, rugged, and safe) has become a very attractive sample preparation method in food analysis [11]. Overall, this procedure consists of two main parts: extraction with a solvent (mostly acetonitrile, MeCN) and partitioning salts (MgSO4, NaCl), and extract clean-up using dispersive solid-phase extraction (dSPE) technique. However, the dSPE clean-up is not fully sufficient for the analysis of high fat matrices and, therefore, the clean-up part of the original QuEChERS method has gone through various modifications to enhance the co-extractives (mainly lipid) removal efficiency (use of freezing, dual dSPE, gel permeation chromatography, silica minicolumn, EMR-lipid sorbent) [9,12,13,14,15,16,17].
Currently, a novel method for clean-up of fatty MeCN extracts (after QuEChERS extraction), which is suitable for determination of H2SO4 stable organic compounds in complex biological samples, was developed in our laboratory [18]. The sample extract clean-up combines the pH-tuned dispersive liquid–liquid microextraction (DLLME) with conc. H2SO4 digestion. This clean-up offers many advantages, including high lipid removal efficiency, rapidity, analyte enrichment without evaporating solvent, low cost (cheap chemicals, no need for expensive sorbents), low chemicals and glassware usage, no need of special laboratory equipment, and less bench space. The lipid removal involves complete removal of fatty acids, which are partitioned from the organic phase into the alkaline aqueous phase in the DLLME clean-up step [18]. The disadvantage is the use of toxic and hazardous chemicals (CHCl3, hexane, MeCN, H2SO4), however, they are applied in small quantities.
The aim of this study was the validation including uncertainty estimation of a rapid and non-laborious method for determination of selected H2SO4 stable halogenated priority substances in fish employing modified QuEChERS sample preparation followed by gas chromatographic and triple quadrupole tandem mass spectrometric (GC–QqQ-MS/MS) analysis.
2. Materials and Methods
2.1. Standards and Reagents
Neat standards of pentachlorobenzene, hexachlorobenzene, alpha-HCH, beta-HCH, delta-HCH and heptachlor (purity: 98.1–99.5%) were obtained from Dr. Ehrenstorfer (Augsburg, Germany). Neat standards of hexachloro-1,3-butadiene (96%) and lindane (97%) were from Sigma–Aldrich (Steinheim, Germany). Standard of 2,4,5,6-tetrachloro-m-xylene (99.0%) in cyclohexane at 10 µg/mL was prepared by Dr. Ehrenstorfer. Individual PBDE standards BDE-28, BDE-47, BDE-77, BDE-99, BDE-100, BDE-153 and BDE-154, each at 50 µg/mL in nonane (≥98%), were produced by Cambridge Isotope Laboratories (CIL, Andover, MA, USA).
Anhydrous magnesium sulfate, sulfuric acid, acetone, chloroform and toluene, all Emsure grade, cyclohexane and ethyl acetate (SupraSolv), and n-hexane (UniSolv), were purchased from Merck (Darmstadt, Germany). Sodium chloride, anhydrous sodium acetate (both ReagentPlus) and MeCN (Chromasolv) were obtained from Sigma–Aldrich.
Sodium acetate solution at 0.5 M was prepared by dissolution of CH3COONa in Milli-Q water produced by a Direct-Q 3 water purification system (Millipore, Molsheim, France). Stock solutions of each OC compound obtained as a neat material were prepared in cyclohexane at a concentration of 5 mg/mL, with the exception of a solution of beta-HCH, which was prepared in a mixture of cyclohexane and acetone (4:1, v/v) at a concentration of 1 mg/mL. Standard working mixtures of eight OC compounds were prepared by dilution of their stock solutions with cyclohexane to obtain concentrations of 1 and 10 µg/mL. An internal standard (IS) solution of 2,4,5,6-tetrachloro-m-xylene at 1 µg/mL was prepared by dilution of the stock standard solution with cyclohexane. Standard working mixtures of six PBDEs (BDE-28, 47, 99, 100, 153 and 154) at concentrations of 5 and 0.5 µg/mL were obtained by dilution of the individual standard solutions with toluene. An IS solution of BDE-77 at 5 µg/mL was also prepared from the stock standard solution by dilution with toluene.
2.2. Fish Samples
The proposed method was validated and verified using samples of nine different fish species: European chub (Squalius cephalus), crucian carp (Carassius carassius), European perch (Perca fluviatilis), northern pike (Esox lucius), zander (Sander lucioperca), brown trout (Salmo trutta), Atlantic salmon (Salmo salar), Alaska pollock (Theragra chalcogramma), and lake trout (Salvelinus namaycush). The first six species were collected by electrofishing during a fish survey performed in Slovak water bodies in 2015 within the project: Monitoring and assessment of water body status (see Funding). The samples of salmon and pollock were purchased as frozen skinless fillets from a local supermarket. The samples were prepared as composite homogenates from several pieces (from 2 to 7) of the whole fish (chub, perch, pike, and trout) or homogenates from single fish (chub and remaining species). The samples were homogenized using a knife mill Grindomix GM 200 (Retsch, Haan, Germany) to give a wet weight of about 600 g and were stored in a freezer at −20 °C until extraction and analysis. The main part of the study was done using chub composite samples.
Accuracy of the method was demonstrated by the analysis of the standard reference material SRM 1946 (Lake Superior Fish Tissue) which was prepared from lake trout by the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA). This SRM was a frozen fish tissue homogenate with 10.2% of extractable fat and 71.4% of water.
2.3. Lipid and Moisture Determination
The lipid and moisture content of fish homogenate samples was determined by gravimetric methods according to our work [19]. For total lipid determination, 5 g of fish homogenate was extracted with 5 mL of acetone/ethyl acetate solvent mixture (6:4, v/v) by shaking with a vortex mixer (Stuart SA8, Bibby Scientific, Stone, UK) for 3 min and, after addition of 2 g of MgSO4 and 0.5 g NaCl and shaking for 3 min, the organic phase was separated by centrifugation (centrifuge Rotina 380, Hettich, Tuttlingen, Germany). An aliquot of the organic phase was dried to constant weight at 103 °C, and the percent lipid content was calculated from the mass of the final residue. The moisture content was determined from the mass difference of 2–3 g portions of fish homogenate before and after a 24 h drying at 60 °C. For all fish sample homogenates, the lipid content was determined in triplicates (results in the range 0.63–16%) and the moisture content in duplicates (58–81%).
2.4. Sample Preparation
An aliquot of 5 g of fish homogenate was weighed into a 50-mL polypropylene centrifuge tube (Corning, CentriStar, Sigma–Aldrich, Steinheim, Germany) and spiked with IS solutions and mixture of analytes (in case of standard addition). After 15 min, the spiked homogenate was mixed with 5 mL of MeCN and shaken by a vortex mixer at 800 rpm for 1 min. Then, a salt mixture of 2 g of anhydrous MgSO4 and 0.5 g of NaCl was added, and again, the tube was shaken vigorously for 1 min. Next, the sample was centrifuged at 5000 rpm for 5 min.
In the DLLME step, a 1-mL aliquot of supernatant was transferred to a 15-mL centrifuge tube with 4 mL of 0.5 M CH3COONa solution. Then, 50 µL of CHCl3 was injected rapidly into the mixture; the tube was vortexed for 1 min and centrifuged at 5000 rpm for 5 min.
Finally, for the H2SO4 clean-up step, the whole sedimented phase was placed in a 1.7-mL clickseal microcetrifuge tube (GoldenGate Bioscience, Claremont, CA, USA) and 1 mL of concentrated H2SO4 was added slowly. The tube was sealed, shortly shaken by hand and then 80 µL of hexane was added to the top of the solution. After short shaking, the tube was centrifuged in a microcentrifuge (Mikro 220R, Hettich) at 10,000 rpm for 5 min. The upper phase was transferred into a GC vial equipped with a 100-µL glass insert and was then ready for GC–MS/MS analysis.
2.5. Instrumental Analysis
Analyses were performed using an Agilent 7890B GC combined with a 7000D QqQ-MS/MS system (Agilent Technologies, Wilmington, DE, USA). The GC was equipped with a multimode inlet and for the injection of sample extracts a multipurpose sampler (MPS) from Gerstel (Mülheim a/d Ruhr, Germany) was used. Two identical Agilent HP-5MS UI capillary columns (15 m × 0.25 mm I.D., 0.25 µm film thickness) connected in series (via Agilent Purged Ultimate Union) were used for separation of the analytes, while a deactivated fused-silica tube (1 m × 0.32 mm I.D.) was used as a precolumn. Helium was used as the carrier gas at constant flow rates of 1.1 and 1.3 mL/min for the first and the second column, respectively.
Sample injection (1 µL) was carried in splitless mode (1 min) at 275 °C. The oven temperature was programmed from 60 °C (1 min hold) to 170 °C at a rate of 40 °C/min, and then to 300 °C (1.75 min hold) at a rate of 10 °C/min. After each run, a 3 min column clean-up was performed employing a mid-column backflush. The backflush was conducted at 305 °C, by applying helium to purged ultimate union at 320 kPa. This program resulted in a total run time of 21.5 min.
The mass selective detector (MSD) was operated using electron ionization at 70 eV in the multiple reaction monitoring (MRM) mode. The retention times (tR), quantifier and qualifier transitions for the selected analytes are listed in Table 1. Dwell times were in all cases set at 10 ms. The MSD transfer line was at 280 °C, ion source at 300 °C, and quadrupoles at 150 °C. The QqQ collision gas was nitrogen at 1.5 mL/min, and quench gas was helium at 2.25 mL/min. Agilent MassHunter software was used for instrument control and data analysis.
Table 1.
Analytes, retention times and MRM conditions.
| Analyte | tR (min) | MRM Transitions (m/z) | |||
|---|---|---|---|---|---|
| Quantifier | CE (V) | Qualifier | CE (V) | ||
| Hexachloro-1,3-butadiene | 4.42 | 225→190 | 15 | 260→225 | 15 |
| Pentachlorobenzene | 6.12 | 248→213 | 25 | 250→180 | 20 |
| Tetrachloro-m-xylene (IS-1) | 6.79 | 244→209 | 15 | 171→136 | 15 |
| alpha-HCH | 7.36 | 219→183 | 5 | 217→181 | 15 |
| Hexachlorobenzene | 7.50 | 284→214 | 35 | 284→249 | 20 |
| beta-HCH | 7.75 | 219→183 | 5 | 217→181 | 15 |
| Lindane | 7.86 | 219→183 | 5 | 217→181 | 15 |
| delta-HCH | 8.22 | 219→183 | 5 | 217→181 | 15 |
| Heptachlor | 9.02 | 272→237 | 25 | 272→117 | 35 |
| BDE-28 | 11.94 | 246→139 | 30 | 406→246 | 20 |
| BDE-47 | 14.02 | 326→217 | 30 | 486→326 | 20 |
| BDE-77 (IS-2) | 14.74 | 326→217 | 30 | 486→326 | 20 |
| BDE-100 | 15.55 | 564→404 | 20 | 404→297 | 30 |
| BDE-99 | 15.97 | 564→404 | 20 | 404→297 | 30 |
| BDE-154 | 17.19 | 644→484 | 20 | 484→324 | 40 |
| BDE-153 | 17.84 | 644→484 | 20 | 484→324 | 40 |
Abbreviations: tR—retention time; MRM—multiple reaction monitoring; CE—collision energy.
The quantification process was performed using a single point standard addition method applying Equation (1):
| (1) |
where ci is the determined analyte concentration, cad is the added concentration to the sample, Ai and AIS are the peak areas of the analyte and IS from the unknown sample analysis, Ai+ad and AISsa are the peak areas of the analyte and IS from the analysis with standard addition. For this purpose, the concentration of each added analyte and of IS tetrachloro-m-xylene was 10 µg/kg and of IS BDE-77 was 20 µg/kg. This was appropriate for the studied range and in agreement with the study of Frenich et al. [20]. In the whole work, the concentrations of the analytes are presented on a wet weight basis.
2.6. Matrix Effect Evaluation
The evaluation of the matrix effect (ME) on the GC–MS/MS analysis was based on comparing the analyte response measured in matrix-matched extracts spiked after QuEChERS extraction and processed by DLLME and H2SO4 clean-up procedure and the response measured in a corresponding neat solvent solution. The ME was calculated from replicate analyses as the average percent suppression or enhancement in the peak area using the following Equation (2):
| (2) |
A positive value of ME corresponds to a matrix-induced enhancement of analyte response, whereas a negative value corresponds to a suppression effect.
2.7. Measurement Uncertainty Calculation
The combined measurement uncertainty was estimated according to the top-down approach using quality control (QC) charts, validation data and the uncertainty of purity of analytical standards [21,22]. The random error contribution to the measurement uncertainty was characterized by the within-lab (intermediate) reproducibility (ur,repro), which was calculated as relative standard deviation (RSD%) from at least 20 independent consecutive measurement values taken from the QC charts (QC samples spiked at 5 µg/kg).
Systematic components of uncertainty were characterized as the relative bias (Br) and the uncertainty of the systematic error (ur,cm) and were determined by measuring QC samples at conditions of repeatability. The Br was quantified using the Equation (3):
| (3) |
where cm and cref are the mean measured concentration and reference concentration of the studied analyte, respectively. For determination of ur,cm, the QC samples were analyzed with minimum number of replicates of 10. For calculation, the following Equations (4) and (5) were used:
| (4) |
| (5) |
where ucm is the standard uncertainty of cm, SD is the standard deviation, and n is the number of replicates.
The uncertainty of purity of analytical standards (ur,ref) was determined by dividing the expanded combined uncertainty (Ur,ref) (given in manufacturer’s certificate) by the coverage factor k = 2, or was estimated on the basis of Equation (6) derived from rectangular distribution (in case of absence of the certificate):
| (6) |
where y (%) represents the purity of standard given in the manufacturer’s specification.
All the characterized uncertainty components were combined by the error propagation rule to obtain the relative combined measurement uncertainty (ur,tot) using Equation (7):
| (7) |
Finally, the expanded combined uncertainty (Ur,tot) was calculated by multiplying the ur,tot with the coverage factor of 2 (95% confidence level).
3. Results and Discussion
3.1. Instrumental Analysis
The instrumental analysis conditions summarized in Section 2.5 were selected based on our previous studies [9,18]. In contrast to study [18], in which sample loading for GC analysis was carried out by thermal desorption of fish extract from the insert placed in the thermal desorption tube, a liquid injection of sample extract was employed.
3.2. ME
The QuEChERS extracts from the chub homogenate sample with lipid content of 5.2% and water content of 74.6% spiked with test analytes at concentration level of 5 ng/mL and treated by DLLME and H2SO4 were used for ME evaluation according to 2.6. The MEs were calculated from five replicate analyses. The obtained ME values in the range from −5.1% to 10.5% (see Table 2) show very low enhancement or suppression of chromatographic response of the studied analytes. For comparison, MEs presented in the studies employing modified QuEChERS methods with dSPE clean-up were for selected OC pesticides incomparably higher. The ME values, in the studies [14] and [23], were 32 and 175.7% for beta-HCH, and 63 and 219.4% for delta-HCH, respectively. The MEs for alpha-HCH, hexachlorobenzene, lindane and heptachlor were in the study [23] calculated as 40.3, 27.4, 34.7 and 28.8%, respectively. For seven PBDEs, Sapozhnikova and Lehotay [24] observed matrix-induced suppression of chromatographic response with ME values in the range from −16% to −26% when using unbuffered QuEChERS method with dSPE clean-up for fish sample preparation. The low MEs obtained by the proposed method in this study indicate the high efficiency of fish extract clean-up.
Table 2.
Matrix effect (ME) evaluation for the studied analytes in the spiked QuEChERS extracts after DLLME and H2SO4 clean-up (n = 5).
| Analyte | ME (%) | RSD (%) |
|---|---|---|
| Hexachloro-1,3-butadiene | −5.1 | 11 |
| Pentachlorobenzene | −1.9 | 11 |
| Tetrachloro-m-xylene (IS-1) | −1.3 | 12 |
| alpha-HCH | −1.2 | 11 |
| Hexachlorobenzene | 3.8 | 13 |
| beta-HCH | 9.3 | 10 |
| Lindane | 2.3 | 10 |
| delta-HCH | 3.6 | 11 |
| Heptachlor | 1.0 | 12 |
| BDE-28 | 1.6 | 14 |
| BDE-47 | 5.7 | 16 |
| BDE-77 (IS-2) | 1.3 | 15 |
| BDE-100 | 3.9 | 16 |
| BDE-99 | 10 | 14 |
| BDE-154 | 7.0 | 14 |
| BDE-153 | 11 | 14 |
3.3. Method Validation
Within-laboratory validation of the proposed method was carried out using two chub composite samples with lipid and water content of 1.9% and 5.2%, and 80% and 75%, respectively, with absence or low levels of the analytes of interest. The validation was performed in terms of linearity, limits of detection (LOD), limits of quantification (LOQ), recovery, accuracy involving evaluation of precision and trueness and analysis of SRM and, finally, the combined uncertainty of the measurement was estimated.
3.3.1. Linearity
Response linearity was assessed by studying calibration curves from the analyses of matrix-matched standards of the test analytes. The standards were prepared by spiking the extract of chub composite sample (lipid content of 1.9%) with standard working mixtures to obtain seven concentration levels (0.1, 0.5, 1, 5, 15, 30 and 60 µg/kg). The response linearity was evaluated on the basis of coefficients of determination (R2) and RSDs of the relative response factors (RRF). The RRFs of the analytes were calculated relative to the internal standard at each concentration level applying a blank correction. In Table 3 it can be seen that the obtained calibration functions were linear for all the analytes, with R2 values above 0.999 and RSDs of the RRFs in the range of 5.6–13%.
Table 3.
Linearity, limits and accuracy of the proposed method for determination of test analytes in spiked fish matrix.
| Analyte | Linear Range (µg/kg) | R 2 | RRF | RRF_RSD (%) | LOD (µg/kg) | LOQ (µg/kg) | Accuracy | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Precision | Trueness | |||||||||
| Preintra | Preinter | R | Br | |||||||
| RSD (%) | RSD (%) | (%) | (%) | |||||||
| Hexachlorobutatadiene | 0.1–60 | 0.99997 | 1.7 | 6.7 | 0.028 | 0.092 | 3.0 | 5.7 | 95 | −4.7 |
| Pentachlorobenzene | 0.1–60 | 0.99975 | 1.0 | 5.8 | 0.036 | 0.12 | 2.3 | 4.9 | 95 | −5.2 |
| alpha-HCH | 0.1–60 | 0.99989 | 3.3 | 7.1 | 0.029 | 0.096 | 0.5 | 6.7 | 89 | −11 |
| Hexachlorobenzene | 0.1–60 | 0.99986 | 1.4 | 5.9 | 0.052 | 0.17 | 0.8 | 3.2 | 107 | 7.4 |
| beta-HCH | 0.1–60 | 0.99973 | 2.1 | 11 | 0.036 | 0.12 | 3.4 | 7.2 | 88 | −12 |
| Lindane | 0.1–60 | 0.99987 | 2.4 | 12 | 0.040 | 0.13 | 1.6 | 7.0 | 87 | −13 |
| delta-HCH | 0.1–60 | 0.99982 | 2.0 | 12 | 0.037 | 0.12 | 4.7 | 8.3 | 91 | −9.4 |
| Heptachlor | 0.1–60 | 0.99952 | 0.46 | 6.9 | 0.039 | 0.13 | 9.2 | 16 | 94 | −6.2 |
| BDE-28 | 0.1–60 | 0.99984 | 5.3 | 5.6 | 0.037 | 0.12 | 8.9 | 9.0 | 99 | −1.3 |
| BDE-47 | 0.1–60 | 0.99994 | 3.2 | 13 | 0.028 | 0.092 | 4.8 | 6.6 | 102 | 1.9 |
| BDE-100 | 0.1–60 | 0.99984 | 1.6 | 10 | 0.028 | 0.092 | 2.9 | 9.0 | 100 | 0.22 |
| BDE-99 | 0.1–60 | 0.99986 | 1.2 | 5.9 | 0.021 | 0.072 | 6.6 | 9.2 | 99 | −0.89 |
| BDE-154 | 0.1–60 | 0.99939 | 0.49 | 12 | 0.049 | 0.16 | 9.0 | 13 | 101 | 1.2 |
| BDE-153 | 0.1–60 | 0.99912 | 0.26 | 8.1 | 0.042 | 0.14 | 11 | 13 | 105 | 5.1 |
Abbreviations: R2—coefficient of determination; RRF—relative response factor; LOD—limit of detection; LOQ—limit of quantification; PREintra—intra-day precision; PREinter—inter-day precision; R—recovery; Br—relative bias.
3.3.2. Limits of the Method
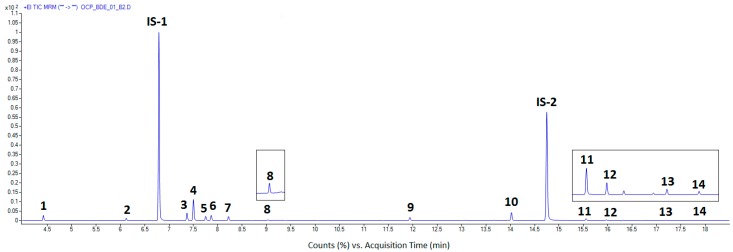
Ten replicate analysis of the blank chub composite sample spiked at 0.1 µg/kg were used for determination of the limits of the method. The LODs and LOQs were calculated as three and ten times the standard deviations (SD) of the results, respectively. As can be seen in Table 3, the LOQs for the test analytes were in the range from 0.07 to 0.17 µg/kg, much lower than the default MRL of 10 µg/kg applicable to pesticides in fish, and approaching the EU Commission recommended value of 0.01 µg/kg for LOQ of analytical methods for the determination of PBDEs in fish and other seafood [4]. In the published studies [14,23] employing similar GC–MS/MS instrumentation and applying the QuEChERS methodology with dSPE clean-up for determination of the test OC compounds in fish, the LOQs were in the ranges 2–13 µg/kg, and 1–5 µg/kg, respectively. In these studies, unlike with the employed SD calculation approach, a less/not appropriate (for QqQ-MS/MS detection, [22]) method based on signal to noise (S/N) estimation was used for LOQ evaluation, and therefore the obtained LOQs are hardly comparable. The lowest calibration levels (LCLs) of the test PBDEs obtained in the study [24] using original QuEChERS sample preparation and GC–MS/MS method were in the range 0.5–5 µg/kg. For illustration, Figure 1 shows a total MRM chromatogram from the analysis of the blank chub composite sample spiked with the test analytes at LOQ level of 0.1 µg/kg. It can be seen the baseline separation of all the analytes with no interferences from the matrix.
Figure 1.
Total ion MRM chromatogram from the GC–QqQ-MS/MS analysis of the blank chub composite sample spiked with the test analytes at LOQ level of 0.1 µg/kg, and internal standards at 10 (IS-1) and 20 µg/kg (IS-2), respectively. Peaks: 1—hexachloro-1,3-butadiene, 2—pentachlorobenzene, IS-1—tetrachloro-m-xylene, 3—alpha-HCH, 4—hexachlorobenzene, 5—beta-HCH, 6—lindane, 7—delta-HCH, 8—heptachlor, 9—BDE-28, 10—BDE-47, IS-2—BDE-77, 11—BDE-100, 12—BDE-99, 13—BDE-154, 14—BDE-153. Hexachlorobenzene (at 0.64 µg/kg), BDE-47 (0.35 µg/kg), BDE-100 (0.18 µg/kg), BDE-154 (0.23 µg/kg), and BDE-153 (0.15 µg/kg) were present in the sample before spiking.
3.3.3. Recovery
Recovery experiments were conducted with the chub composite sample (lipid content of 1.9%) spiked with the test analytes at levels of 1, 5, 15, 30 and 60 µg/kg, respectively, covering the linearity range of the method. A single-point standard addition method (see Section 2.5) was used for quantification, which enabled us to solve the problem of absence of suitable fish matrix free of analytes of interest that is necessary for matrix-matched calibration at low concentration levels, and also helped to overcome the negative effects of matrix components. From Table 4 it can be seen that the obtained recoveries are in the range of 57%–124% with RSDs in the range of 2%–18%. These results are acceptable according to the requirements of the EU guidance document SANTE/11813/2017 for pesticide residues analysis in food, because the recoveries outside the range of 70%–120% are consistent (RSDs ≤ 20%) and are not lower than 30% or above 140% [25].
Table 4.
Recoveries and RSDs of the test analytes from the spiked chub homogenate.
| Analyte | Recovery (RSD) a (%) | ||||
|---|---|---|---|---|---|
| 1 µg/kg | 5 µg/kg | 15 µg/kg | 30 µg/kg | 60 µg/kg | |
| Hexachloro-1,3-butadiene | 101 (6) | 96 (4) | 124 (5) | 98 (6) | 97 (6) |
| Pentachlorobenzene | 98 (4) | 88 (2) | 103 (10) | 103 (15) | 90 (13) |
| alpha-HCH | 99 (7) | 86 (5) | 101 (17) | 91 (17) | 62 (15) |
| Hexachlorobenzene | 103 (5) | 100 (4) | 101 (16) | 97 (6) | 94 (6) |
| beta-HCH | 95 (10) | 82 (7) | 86 (16) | 92 (18) | 58 (14) |
| Lindane | 94 (10) | 88 (5) | 96 (16) | 90 (18) | 59 (15) |
| delta-HCH | 91 (10) | 85 (16) | 89 (16) | 88 (18) | 57 (14) |
| Heptachlor | 91 (7) | 94 (8) | 89 (12) | 86 (9) | 83 (12) |
| BDE-28 | 101 (6) | 93 (10) | 94 (18) | 94 (3) | 93 (14) |
| BDE-47 | 98 (6) | 110 (2) | 95 (6) | 94 (3) | 92 (15) |
| BDE-100 | 100 (5) | 100 (5) | 100 (14) | 96 (5) | 96 (10) |
| BDE-99 | 101 (9) | 100 (8) | 101 (15) | 95 (12) | 96 (8) |
| BDE-154 | 105 (4) | 104 (12) | 99 (11) | 94 (6) | 94 (7) |
| BDE-153 | 98 (11) | 98 (12) | 102 (9) | 94 (12) | 93 (5) |
an = 5.
3.3.4. Accuracy
The accuracy of the method was studied in terms of two components—precision and trueness [26]. The precision was evaluated as intra-day (PREintra) and inter-day (PREinter) precision and expressed by RSD for the repeated analyses of QC samples (lipid content of 5.2%) spiked with the test analytes at 5 µg/kg. The PREintra was determined by the analysis of ten replicates QC samples on one day, while PREinter was calculated from measurements of four replicates QC samples per day analyzed on four consecutive days. The trueness of the method was evaluated on the basis of ten measurements from the determination of PREintra and expressed as a mean recovery (R) and a mean relative bias (Br). The results from the accuracy assessment are presented in Table 3. It can be seen that RSDs for PREinta and PREinter are in the ranges of 0.5%–11% and 3.2%–16%, respectively, showing a satisfactory precision of the method. The good trueness of the method is demonstrated by the values of Rec and Br in the ranges of 87%–107% and −13.0%–7.4%, respectively.
Finally, the method’s accuracy was studied by the analysis of the NIST SRM 1946 standard fish tissue reference material prepared from lake trout. Table 5 presents results obtained for those test analytes for which certified concentrations were available. According to obtained trueness and precision, acceptable results (trueness in the range 70%–120%, RSD ≤ 20%) were obtained for eight from nine analytes (except BDE-28). However, when comparing with the certified ranges, three results (lindane, BDE-28 and BDE-99) were outside and one result (BDE-154) was at the border of the certified range.
Table 5.
Results from determination of selected chlorinated pesticides and PBDEs in the standard reference material NIST SRM 1946 (Lake Superior Fish Tissue).
| Analyte | Certified Value a (μg/kg) | Determined Value a (μg/kg) | Trueness (RSD) (%) |
|---|---|---|---|
| Hexachlorobenzene | 7.25 ± 0.83 | 6.47 ± 1.5 | 89 (2) |
| alpha-HCH | 5.72 ± 0.65 | 5.44 ± 1.4 | 95 (7) |
| Lindane | 1.14 ± 0.18 | 0.89 ± 0.26 | 78 (5) |
| BDE-28 | 0.742 ± 0.027 | 0.467 ± 0.067 | 63 (5) |
| BDE-47 | 29.9 ± 2.3 | 30.2 ± 5.1 | 101 (5) |
| BDE-99 | 18.5 ± 2.1 | 22.0 ± 3.7 | 119 (16) |
| BDE-100 | 8.57 ± 0.52 | 9.04 ± 1.8 | 105 (9) |
| BDE-153 | 2.81 ± 0.41 | 3.16 ± 0.69 | 112 (9) |
| BDE-154 | 5.77 ± 0.80 | 6.57 ± 1.2 | 114 (12) |
a Mean value ± expanded combined measurement uncertainty (Ur,tot); n = 3.
3.3.5. Uncertainty of Measurement
To evaluate the uncertainty of measurement, a top-down approach has been used that utilizes data from validation and QC charts and is much simpler than the GUM (Generalized Uncertainty Method) bottom-up approach [27]. The combined measurement uncertainty was estimated according to Section 2.7 and the resulting values together with the individual uncertainty components are summarized in Table 6. As can be seen in Table 6, generally the most significant contribution to the measurement uncertainty was associated with the random error characterized by the within-lab reproducibility (ur,repro). In several cases, the highest uncertainty component was the relative bias (Br) representing the systematic error of the measurement. The Br values were in the broadest range among the evaluated uncertainty components from 0.2 to –13.0%. The resulting values of the expanded combined uncertainty (Ur,tot) for the test analytes were in the range between 14.4 and 28.7%, being in accordance with the requirement (50%) of the EU guidance document SANTE/11813/2017 [25]. For comparison, in the study combining the QuEChERS method with GC–MS analysis for the determination of OC compounds in fish, Olivares et al. [28] estimated the combined measurement uncertainty for hexachlorobenzene and lindane at levels of 29.9 and 20.0%, respectively. In the work dealing with determination of OC pesticides in meat employing ASE, mini-silica column purification and GC–ECD analysis, Dimitrova et al. [29] obtained for hexachlorobenzene and HCH isomers expanded uncertainties in the range of 14.6%–17.9%. In the currently published study [30] concerning the determination of halogenated flame retardants by GC–API-MS/MS and GC–EI-MS after ASE and multi column clean-up, the values of expanded measurement uncertainties for the PBDEs of our interest in fish fillet were below 50%.
Table 6.
Summary of uncertainties obtained for the test analytes using the top-down approach.
| Analyte | ur,repro (%) | Br (%) | ur,cm (%) | ur,ref (%) | ur,tot (%) | Ur,tot (%) |
|---|---|---|---|---|---|---|
| Hexachloro-1,3-butadiene | 8.01 | −4.66 | 0.909 | 1.15 | 9.38 | 18.8 |
| Pentachlorobenzene | 9.90 | −5.15 | 0.676 | 0.250 | 11.2 | 22.4 |
| alpha-HCH | 7.83 | −10.6 | 0.154 | 0.475 | 13.2 | 26.3 |
| Hexachlorobenzene | 8.42 | 7.38 | 0.274 | 1.00 | 11.2 | 22.5 |
| beta-HCH | 8.13 | −11.6 | 0.954 | 0.150 | 14.2 | 28.4 |
| Lindane | 6.04 | −13.0 | 0.451 | 0.866 | 14.4 | 28.7 |
| delta-HCH | 7.15 | −9.40 | 1.34 | 0.330 | 11.9 | 23.8 |
| Heptachlor | 7.60 | −6.23 | 2.74 | 0.250 | 10.2 | 20.4 |
| BDE-28 | 6.48 | −1.28 | 2.78 | 0.295 | 7.18 | 14.4 |
| BDE-47 | 7.98 | 1.90 | 1.56 | 0.300 | 8.36 | 16.7 |
| BDE-100 | 9.75 | 0.192 | 0.913 | 0.300 | 9.80 | 19.6 |
| BDE-99 | 8.01 | −0.879 | 2.06 | 0.300 | 8.32 | 16.6 |
| BDE-154 | 8.50 | 1.17 | 2.89 | 0.300 | 9.06 | 18.1 |
| BDE-153 | 9.00 | 5.08 | 3.50 | 0.300 | 10.9 | 21.8 |
Abbreviations: ur,repro—within-lab reproducibility; Br—relative bias; ur,cm—uncertainty of systematic error; ur,ref—uncertainty of purity of analytical standard; ur,tot—relative combined measurement uncertainty; Ur,tot—expanded combined measurement uncertainty.
3.4. Application of the Method to Real Samples
The applicability of the proposed method was evaluated by extraction and determination of the test analytes in homogenate samples of eight different fish species listed in Table 7. The lipid content of the analyzed fish (see Table 7) was in the range from 0.63% to 16% and the moisture content in the ranged from 58% to 81%, respectively. Table 7 presents the determined concentrations of the test analytes in fish homogenates and the relative recoveries (RR) of the analytes determined after their addition to the sample at concentration of 10 µg/kg. In general, the fish with the highest lipid content were the most contaminated (trout, salmon), while the least contaminated were those with the lowest lipid content (crucian carp, pollock). For all samples, hexachlorobenzene and BDE-47 were the most frequently detected analytes as well as the ones present at the highest concentrations. The RR values for the test analytes were in the range of 53%–128% with RSDs in the range of 1%–23%.
Table 7.
Analysis of samples of different fish species.
| Analyte | European chub Concentr. a/RR b (µg/kg/%) |
Crucian carp Concentr. a/RR b (µg/kg/%) |
European perch Concentr. a/RR b (µg/kg/%) |
Northern pike Concentr. a/RR b (µg/kg/%) |
Zander Concentr. a/RR b (µg/kg/%) |
Brown trout Concentr. a/RR b (µg/kg/%) |
Atlantic salmon Concentr. a/RR b (µg/kg/%) |
Alaska pollock Concentr. a/RR b (µg/kg/%) |
|---|---|---|---|---|---|---|---|---|
| Hexachloro-1,3-butadiene | <0.09/96 (2) | <0.09/86 (6) | <0.09/87 (1) | <0.09/95 (1) | <0.09/93 (1) | <0.09/116 (1) | 0.90 ± 0.01/98 (4) | 0.22 ± 0.01/104 (3) |
| Pentachlorobenzene | <0.12/92 (3) | <0.12/96 (6) | <0.12/90 (2) | <0.12/105 (4) | <0.12/87 (3) | <0.12/108 (3) | 0.22 ± 0.01/95 (1) | <0.12/104 (3) |
| alpha-HCH | <0.10/85 (2) | <0.10/98 (8) | <0.10/84 (2) | <0.10/92 (8) | <0.10/83 (6) | <0.10/105 (4) | 0.23 ± 0.01/86 (3) | <0.10/95 (2) |
| Hexachlorobenzene | 1.00 ± 0.01/95 (2) | 0.35 ± 0.01/92 (3) | 0.48 ± 0.01/93 (1) | 1.84 ± 0.02/96 (3) | 0.70 ± 0.01/95 (2) | 1.12 ± 0.01/118 (2) | 2.68 ± 0.05/96 (4) | 0.18 ± 0.01/99 (1) |
| beta-HCH | <0.12/82 (3) | <0.12/93 (8) | <0.12/82 (2) | <0.12/97 (11) | <0.12/76 (7) | 0.80 ± 0.02/113 (7) | 0.12 ± 0.01/81 (4) | <0.12/93 (3) |
| Lindane | <0.13/83 (3) | <0.13/93 (8) | <0.13/82 (2) | <0.13/91 (9) | <0.13/79 (6) | <0.13/101 (6) | <0.13/84 (4) | <0.13/91 (2) |
| delta-HCH | <0.12/82 (2) | <0.12/92 (8) | <0.12/82 (3) | <0.12/102 (10) | <0.12/78 (7) | 0.13 ± 0.003/100 (7) | <0.12/82 (4) | <0.12/90 (3) |
| Heptachlor | <0.13/78 (6) | <0.13/64 (12) | <0.13/98 (3) | <0.13/71 (6) | <0.13/114 (8) | <0.13/106 (8) | <0.13/83 (4) | <0.13/65 (10) |
| BDE-28 | <0.12/89 (7) | <0.12/128 (9) | <0.12/88 (2) | <0.12/110 (5) | <0.12/99 (8) | <0.12/87 (4) | <0.12/86 (4) | <0.12/114 (9) |
| BDE-47 | 0.69 ± 0.01/95 (2) | <0.09/100 (2) | 1.33 ± 0.04/103 (5) | 0.21 ± 0.01/104 (6) | 1.45 ± 0.01/103 (3) | 0.36 ± 0.01/94 (4) | 0.43 ± 0.02/97 (5) | <0.09/94 (0.4) |
| BDE-100 | 0.20 ± 0.002/100 (9) | <0.09/94 (8) | 0.26 ± 0.02/111 (5) | <0.09/105 (9) | 0.18 ± 0.01/96 (6) | 0.10 ± 0.01/97 (6) | 0.09 ± 0.01/105 (10) | <0.09/71 (12) |
| BDE-99 | <0.07/96 (9) | <0.07/91 (8) | 0.41 ± 0.02/113 (6) | <0.07/111 (14) | <0.07/96 (6) | 0.25 ± 0.01/94 (5) | <0.07/104 (9) | <0.07/67 (4) |
| BDE-154 | <0.16/103 (10) | <0.16/100 (21) | <0.16/120 (3) | <0.16/128 (15) | <0.16/99 (9) | <0.16/97 (7) | <0.16/112 (13) | <0.16/53 (15) |
| BDE-153 | <0.14/99 (14) | <0.14/103 (23) | <0.14/119 (3) | <0.14/114 (19) | <0.14/95 (7) | <0.14/95 (6) | <0.14/100 (2) | <0.14/53 (16) |
| Lipid content (%) | 3.5 | 0.96 | 3.0 | 2.4 | 1.5 | 8.2 | 16 | 0.63 |
| Moisture content (%) | 78 | 74 | 74 | 78 | 77 | 71 | 58 | 81 |
a For positive samples: mean value ± SD; n = 3. For negative samples: <LOQ value. b RR—relative recovery; in parenthesis: RSD value; n = 5.
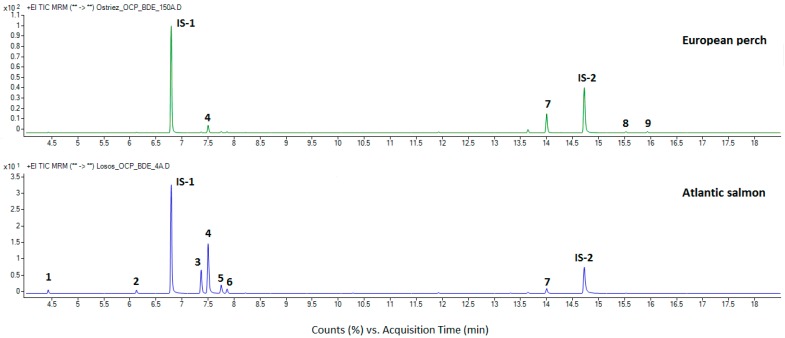
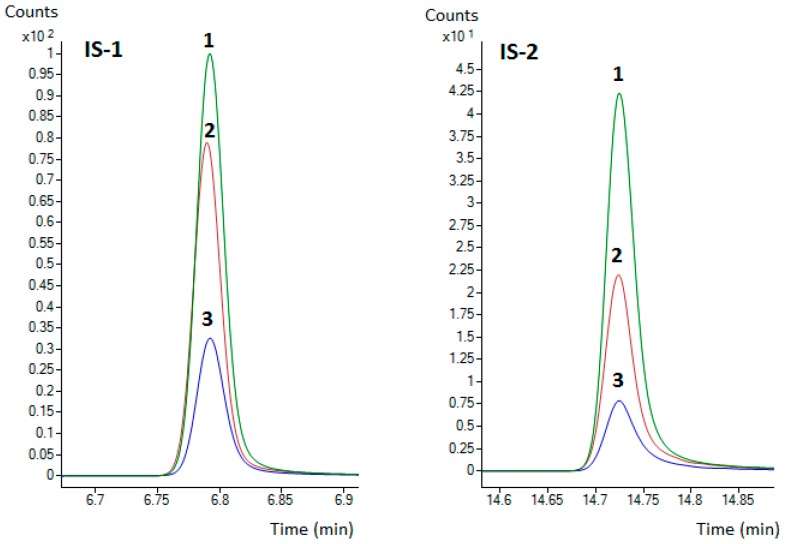
In Figure 2, chromatograms from the analysis of low fat (perch, 3.0%) and high fat (salmon, 16%) fish samples are shown. In both total MRM chromatograms a high selectivity for the detected analytes and absence of matrix components can be observed. A difference between the counts obtained for ISs in the first and second chromatograms can be seen that can be related to the effect of the sample lipid content on the analysis. This is demonstrated in Figure 3, where a dependence of the IS peak size on the lipid content of the analyzed fish is presented. Therefore, the ISs and analytes peak areas generally decreased with the increasing lipid content of the matrix.
Figure 2.
Total ion MRM chromatograms from the GC–QqQ-MS/MS analysis of extracts prepared from samples of European perch and Atlantic salmon. Peaks: 1—hexachloro-1,3-butadiene, 2—pentachlorobenzene, IS-1—tetrachloro-m-xylene, 3—alpha-HCH, 4—hexachlorobenzene, 5—beta-HCH, 6—lindane, 7—BDE-47, IS-2—BDE-77, 8—BDE-100, 9—BDE-99.
Figure 3.
Dependence of the IS peak size on the lipid content of analyzed fish matrix. 1—perch (3.0%), 2—trout (8.2%), 3—salmon (16%). Other parameters of these fish samples are presented in Table 7.
3.5. Method’s Analytical Eco-Scale Evaluation
The environmental impact of the proposed method was assessed using an analytical Eco-Scale approach that is based on assigning penalty points to parameters of the analytical process (depending on the use of hazardous chemicals, energy consumption, waste generation, etc.) that are not in agreement with the principles of green chemistry [31]. The analytical Eco-Scale analysis results in a score calculated by subtracting the penalty points from a value of 100, which represents an ideal green analysis. The assessment of the proposed method according to criteria set by Gałuszka et al. [31] is presented in Table 8. Due to obtained analytical Eco-Scale total score of 68, the current method can be classified as “an acceptable green analysis method” with low consumption of hazardous solvents.
Table 8.
Analytical Eco-Scale assessment of the proposed method according to Galuszka et al. [31].
| Penalty Points | |
|---|---|
| Reagents | |
| MeCN (5 mL) | 4 |
| CHCl3 (50 µL) | 2 |
| Hexane (80 µL) | 8 |
| Analytes standard solution | 4 |
| H2O (4 mL) | 0 |
| H2SO4 (1 mL) | 2 |
| MgSO4 (2 g) | 0 |
| NaCl (0.5 g) | 0 |
| CH3COONa | 0 |
| Instruments | |
| Vortex | 1 |
| Centrifuge | 1 |
| GC–MS/MS | 3 |
| Occupational hazard | 3 |
| Waste | 4 |
| Total penalty points | Σ 32 |
| Analytical Eco-Scale total score | 68 |
3.6. Comparison of the Proposed Method with Other Reported QuEChERS Based Methods
A comparison of the proposed method with other QuEChERS based methods with enhanced sample extract clean-up for determination of test analytes in fish is presented in Table 9. It can be seen that most of the applied clean-up procedures involve dual dSPE, which in several cases is combined with a freezing-out step for the low temperature lipid precipitation. The use of dSPE requires multiple weighing operations or purchase of custom-made sorbent blends and the freezing-out step significantly prolongs the sample preparation time. In the method used for determination of PBDEs (and other persistent organic pollutants) in salmon fillets [16], the ethyl acetate crude extract was purified applying gel permeation chromatography (GPC) and SPE. This clean-up procedure is rather laborious, requires a GPC instrument and is associated with high solvent consumption (ca. 150 mL per sample). The clean-up procedure described in the present paper is simple, fast, low cost, providing high co-extractives removal efficiency (involves complete removal of fatty acids), but it is only appropriate for the analysis of H2SO4 stable organic compounds. The LOQs of the presented method belong among the lowest listed in Table 9.
Table 9.
Comparison of the developed method with other QuEChERS based methods with enhanced sample extract clean-up for determination of test analytes in fish.
| Analytes | Extractant | Clean-Up | Analysis | Recoveries (%) | LOQs (µg/kg) | Reference |
|---|---|---|---|---|---|---|
| Pesticides | MeCN | Dual dSPE (1. PSA + C18 + MgSO4; 2. PSA + C18 + MgSO4) |
GC–ECD | 57–98 | 1.5–3.5 | [9] |
| Pesticides | MeCN or MeCN/THF (3:1) | Freezing (2 h), dual dSPE (1. CaCl2; 2. PSA + MgSO4) |
GC–MS | 43–113 | 1–10 | [12] |
| Pesticides | MeCN + CHCl3 (10:1) | Dual dSPE (1. PSA + SAX +NH2 + MgSO4; 2. C18), freezing (overnight) |
GC–MS | 61–102 | 4–6 | [13] |
| Pesticides | MeCN + hexane (15:2) | Freezing (20 min), dual dSPE (1. CaCl2 + MgSO4; 2. PSA + florisil + C18 +MgSO4) |
GC–MS/MS | 60–127 | 2–13 | [14] |
| PBDEs | MeCN (sonication) | Dual dSPE (1. PSA + C18 + MgSO4; 2. PSA + C18 + MgSO4) |
GC–MS | 60–107 | <15 | [15] |
| PBDEs | Ethyl acetate | GPC, SPE (silica + Na2SO4) | GC–MS | 88–140 | 0.09–2.2 | [16] |
| Pesticides | MeCN | Freezing (min. 4 h), dSPE (Z-Sep + MgSO4), filtration (0.2 µm PTFE filter) |
GC–MS/MS | 86–101 | 0.08–0.15 | [32] |
| PBDEs | MeCN + toluene (4:1) | Dual dSPE (1. EMR-Lipid; 2. Z-Sep + MgSO4) | GC–MS/MS | 79–116 (muscle) 89–107 (liver) |
0.015–0.065 0.85–1.1 |
[33] |
| Pesticides, PBDEs | MeCN | pH-tuned DLLME (0.5 M CH3COONa, CHCl3), H2SO4 clean-up |
GC–MS/MS | 57–124 (pesticides) 93–110 (PBDEs) |
0.09–0.17 0.07–0.16 |
This work |
Abbreviations: MeCN—acetonitrile; dSPE—dispersive solid-phase extraction; PSA—primary secondary amine; C18—octadecyl silica; GC—gas chromatography; ECD— electron-capture detector; THF—tetrahydrofurane; MS—mass spectrometry; SAX—strong anion exchange resin; GPC—gel permeation chromatography; SPE—solid-phase extraction cartridge; Z-Sep, EMR-Lipid—clean-up sorbents; PTFE—polytetrafluoroethylene (Teflon); DLLME— dispersive liquid–liquid microextraction.
4. Conclusions
In this work, a rapid and non-laborious method was proposed for the determination of selected H2SO4 stable OC compounds and PBDEs in fish samples. The method employing QuEChERS sample preparation with pH-tuned DLLME and H2SO4 digestion fish extract clean-up followed by GC–QqQ-MS/MS analysis has successfully passed the validation process. The results obtained from the analysis of nine different fish species samples show the applicability of the method for the determination of selected analytes in fish. According to analytical Eco-Scale evaluation, the proposed method can be classified as “an acceptable green analysis method” with low consumption of hazardous solvents. The comparison of the method with other reported QuEChERS based methods shows its advantages such as simplicity, rapidity, low cost, high extract clean-up efficiency and good sensitivity.
Author Contributions
Conceptualization, P.T.; methodology, P.T. and S.N.; validation, S.N.; formal analysis, S.N.; investigation, S.N.; data curation, S.N. and P.T.; writing—original draft preparation, P.T. and S.N.; writing—review and editing, P.T.; supervision, P.T.
Funding
The authors acknowledge the financial support from the EU Cohesion Funds within the project Monitoring and assessment of water body status (No. 310011A366 Phase III).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Haggblom M.M., Bossert I.D. Halogenated organic compounds: A global perspective. In: Haggblom M.M., Bossert I.D., editors. Dehalogenation: Microbial Processes and Environmental Applications. Kluwer Academic Publishers; Norwell, MA, USA: 2003. pp. 3–32. [Google Scholar]
- 2.United Nations Environment Program (UNEP) Stockholm Convention on Persistent Organic Pollutants . Adoption of Amendments of Annexes A, B and C. United Nations Environment Program; Geneva, Switzerland: 2009. [Google Scholar]
- 3.European Commission Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Offic. J. Eur. Commun. 2005;L 70:1–16. [Google Scholar]
- 4.European Commission 2014/118/EU: Commission Recommendation of 3 March 2014 on the monitoring of traces of brominated flame retardants in food. Offic. J. 2014;L 65:39–40. [Google Scholar]
- 5.European Commission Decision No 2455/2001/EC of the European Parliament and of the Council of 20 November 2001 establishing the list of priority substances in the field of water policy and amending Directive 2000/60/EC. Offic. J. Eur. Commun. 2001;L 331:1–5. [Google Scholar]
- 6.US EPA List of Priority Pollutants. [(accessed on 17 July 2018)]; Available online: http://water.epa.gov/scitech/methods/cwa/ pollutants.cfm.
- 7.Chung S.W.C., Chen B.L.S. Determination of organochlorine pesticide residues in fatty foods: A critical review on the analytical methods and their testing capabilities. J. Chromatogr. A. 2011;1218:5555–5567. doi: 10.1016/j.chroma.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 8.Berton P., Lana N.B., Ríos J.M., García-Reyes J.F., Altamirano J.C. State of the art of environmentally friendly sample preparation approaches for determination of PBDEs and metabolites in environmental and biological samples: A critical review. Anal. Chim. Acta. 2016;905:24–41. doi: 10.1016/j.aca.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Tölgyessy P., Miháliková Z., Matulová M. Determination of selected chlorinated priority substances in fish using QuEChERS method with dual dSPE clean-up and gas chromatography. Chromatographia. 2016;79:1561–1568. doi: 10.1007/s10337-016-3160-7. [DOI] [Google Scholar]
- 10.Pietroń W.J., Małagocki P. Quantification of polybrominated diphenyl ethers (PBDEs) in food. A review. Talanta. 2017;167:411–427. doi: 10.1016/j.talanta.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 11.Rejczak T., Tuzimski T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015;13:980–1010. doi: 10.1515/chem-2015-0109. [DOI] [Google Scholar]
- 12.Norli H.R., Christiansen A., Deribe E. Application of QuEChERS method for extraction of selected persistent organic pollutants in fish tissue and analysis by gas chromatography mass spectrometry. J. Chromatogr. A. 2011;1218:7234–7241. doi: 10.1016/j.chroma.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 13.Molina-Ruiz J.M., Cieslik E., Cieslik I., Walkowska I. Determination of pesticide residues in fish tissues by modified QuEChERS method and dual-d-SPE clean-up coupled to gas chromatography-mass spectrometry. Environ. Sci. Pollut. Res. Int. 2015;22:369–378. doi: 10.1007/s11356-014-3361-2. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee N.S., Utture S., Banerjee K., Ahammed Shabeer T.P., Kamble N., Mathew S., Ashok Kumar K. Multiresidue analysis of multiclass pesticides and polyaromatic hydrocarbons in fatty fish by gas chromatography tandem mass spectrometry and evaluation of matrix effect. Food Chem. 2016;196:1–8. doi: 10.1016/j.foodchem.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Morrison S.A., Sieve K.K., Ratajczak R.E., Bringolf R.B., Belden J.B. Simultaneous extraction and cleanup of high-lipid organs from white sturgeon (Acipenser transmontanus) for multiple legacy and emerging organic contaminants using QuEChERS sample preparation. Talanta. 2016;146:16–22. doi: 10.1016/j.talanta.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Cloutier P.L., Fortin F., Groleau P.E., Brousseau P., Fournier M., Desrosiers M. QuEChERS extraction for multi-residue analysis of PCBs, PAHs, PBDEs and PCDD/Fs in biological samples. Talanta. 2017;165:332–338. doi: 10.1016/j.talanta.2016.12.080. [DOI] [PubMed] [Google Scholar]
- 17.Han L., Matarrita J., Sapozhnikova Y., Lehotay S.J. Evaluation of a recent product to remove lipids and other matrix co-extractives in the analysis of pesticide residues and environmental contaminants in foods. J. Chromatogr. A. 2016;1449:17–29. doi: 10.1016/j.chroma.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 18.Tölgyessy P., Nagyová S. Rapid sample preparation method with high lipid removal efficiency for determination of sulphuric acid stable organic compounds in fish samples. Food Anal. Methods. 2018;11:2485–2496. doi: 10.1007/s12161-018-1241-y. [DOI] [Google Scholar]
- 19.Tölgyessy P., Miháliková Z. Rapid determination of total lipids in fish samples employing extraction/partitioning with acetone/ethyl acetate solvent mixture and gravimetric quantification. Food Control. 2016;60:44–49. doi: 10.1016/j.foodcont.2015.07.017. [DOI] [Google Scholar]
- 20.Frenich A.G., Martínez Vidal J.L., Fernández Moreno J.L., Romero-González R. Compensation for matrix effects in gas chromatography–tandem mass spectrometry using a single point standard addition. J. Chromatogr. A. 2009;1216:4798–4808. doi: 10.1016/j.chroma.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Suchánek M., Friedecký B., Kratochvíla J., Budina M., Bartoš V. Recommendations for the determination of uncertainties in the results of measurements/clinical tests in clinical laboratories. Klin. Biochem. Metab. 2006;14:43–53. (In Czech) [Google Scholar]
- 22.L’Homme B., Scholl G., Eppe G., Focant J.F. Validation of a gas chromatography–triple quadrupole mass spectrometry method for confirmatory analysis of dioxins and dioxin-like polychlorobiphenyls in feed following new EU Regulation 709/2014. J. Chromatogr. A. 2015;1376:149–158. doi: 10.1016/j.chroma.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Munaretto J.S., Ferronato G., Ribeiro L.C., Martins M.L., Adaime M.B., Zanella R. Development of a multiresidue method for the determination of endocrine disrupters in fish fillet using gas chromatography–triple quadrupole tandem mass spectrometry. Talanta. 2013;116:827–834. doi: 10.1016/j.talanta.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 24.Sapozhnikova Y., Lehotay S.J. Multi-class, multi-residue analysis of pesticides, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers and novel flame retardants in fish using fast, low-pressure gas chromatography–tandem mass spectrometry. Anal. Chim. Acta. 2013;758:80–92. doi: 10.1016/j.aca.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 25.European Commission Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed, SANTE/11813/2017. [(accessed on 16 November 2018)]; Supersedes SANTE/11945/2015, Implemented by 01/01/2018. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf.
- 26.ISO 5725-1 . Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 1: General Principles and Definitions. International Organization for Standardization; Geneva, Switzerland: 1994. [Google Scholar]
- 27.ConsultGLP Measurement Uncertainty—Comparing GUM and Top down Approaches. [(accessed on 2 March 2019)]; Available online: https://consultglp.com/2017/04/27/measurement-uncertainty-comparing-gum-and-top-down-approaches/
- 28.Olivares I.R.B., Costa S.P., Camargo R.S., Pacces V.H.P. Development of a rapid and sensitive routine method of analyses for organochlorine compounds in fish: A metrological approach. Pharm. Anal. Acta. 2016;7:502. [Google Scholar]
- 29.Dimitrova R.T., Stoykova I.I., Yankovska-Stefanova T.T., Yaneva S.A., Stoyanchev T.T. Development of analytical method for determination of organochlorine pesticides residues in meat by GC-ECD. Revue Méd. Vét. 2018;169:77–86. [Google Scholar]
- 30.Neugebauer F., Dreyer A., Lohmann N., Koschorreck J. Determination of halogenated flame retardants by GC-API-MS/MS and GC-EI-MS: A multi-compound multi-matrix method. Anal. Bioanal. Chem. 2018;410:1375–1387. doi: 10.1007/s00216-017-0784-x. [DOI] [PubMed] [Google Scholar]
- 31.Gałuszka A., Migaszewski Z.M., Konieczka P., Namieśnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures. Trends Anal. Chem. 2012;37:61–72. doi: 10.1016/j.trac.2012.03.013. [DOI] [Google Scholar]
- 32.Baduel C., Mueller J.F., Tsai H., Gomez Ramos M.J. Development of sample extraction and clean-up strategies for target and non-target analysis of environmental contaminants in biological matrices. J. Chromatogr. A. 2015;1426:33–47. doi: 10.1016/j.chroma.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 33.Cruz R., Marques A., Casal S., Cunha S.C. Fast and environmental-friendly methods for the determination of polybrominated diphenyl ethers and their metabolites in fish tissues and feed. Sci. Total Environ. 2019;646:1503–1515. doi: 10.1016/j.scitotenv.2018.07.342. [DOI] [PubMed] [Google Scholar]