Abstract
Macronutrient metabolism is a highly orchestrated process, with adipose tissue and liver each playing central roles in nutrient uptake, processing, transport, and storage. These 2 tissues form an important metabolic circuit, particularly as it relates to lipids as the primary storage form of excess energy. The function of the circuit is influenced by many factors, including the quantity and type of nutrients consumed and their impact on the overall health of the tissues. In this review we begin with a brief summary of the homeostatic disposition of lipids between adipose tissue and liver and how these processes can become dysregulated in obesity. We then explore how specific dietary nutrients and nutrient combinations can exert unique influences on the liver–adipose tissue axis.
Keywords: Carbohydrate, Fat, Metabolism, Diet, Fatty Liver Disease
Abbreviations used in this paper: DNL, de novo lipogenesis; ER, endoplasmic reticulum; FFA, free fatty acid; FGF21, fibroblast growth factor-21; LPL, lipoprotein lipase; MUFA, monounsaturated fatty acid; NAFLD, nonalcoholic fatty liver disease; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; VLDL, very-low-density lipoprotein
Summary.
The present review addresses the complex role of carbohydrates (simple and complex) and fats (saturated, unsaturated, polyunsaturated) in the pathogenesis of nonalcoholic fatty liver disease. Specifically the review focuses on the liver–adipose tissue axis in this disease and the role each nutrient class plays in the crosstalk between the liver and the adipose tissue and the pathophysiology of nonalcoholic fatty liver disease.
Macronutrient Flux Through Adipose Tissue and Liver
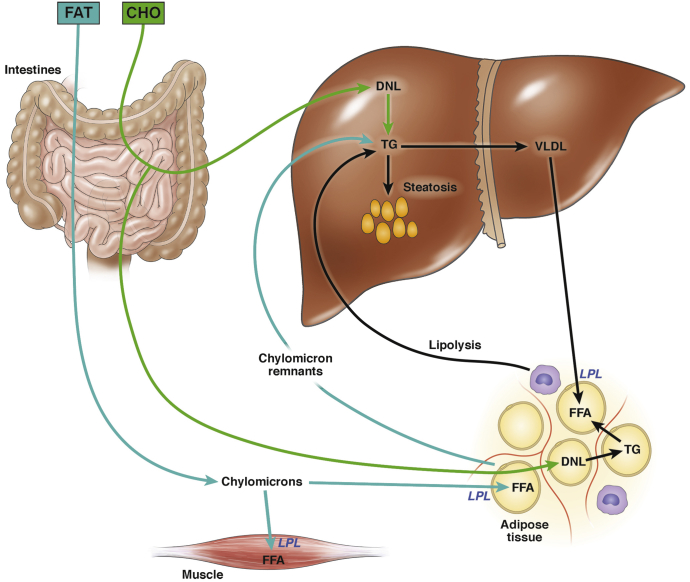
When a healthy individual consumes dietary fat, the lipids are converted to triglyceride within the intestine and packaged into chylomicrons for delivery to peripheral tissues (primarily muscle and adipose tissue) (Figure 1). When chylomicrons reach their target tissues, fatty acids are released through the local action of lipoprotein lipase (LPL). Adipose tissue is reasonably efficient at extracting free fatty acids (FFA) from chylomicrons for uptake and storage; however, there is some “spillover” of FFA into the circulation (33%–36% of the total delivered), which then become available for uptake by the liver.1 The chylomicron remnants that are left after LPL-mediated triglyceride lipolysis also contain a small proportion of their original triglyceride content. Spillover FFA and chylomicron remnants represent 2 routes by which dietary fat can gain direct access to the liver. Stable isotope studies indicate that in normal individuals, dietary fat accounts for approximately 15% of the triglyceride present in the liver at any given time.2
Figure 1.
Route of dietary carbohydrates and fats to the liver and adipose tissue. Dietary carbohydrate enters the portal circulation from the intestine and enters the liver. Excess substrate not needed for metabolism is converted to fatty acid via DNL and incorporated into triglyceride. Triglycerides are exported from the liver as VLDL, where they are delivered to adipose tissue, where they are broken down into FFA by the enzyme LPL and stored. Dietary fat is packaged into chylomicrons in the intestine and delivered initially to muscle and adipose tissue. Any lipid remaining in the chylomicron remnants are routed to the liver, as are “spillover” FFA not taken up by adipocytes. CHO, carbohydrate; TG, triglyceride.
When a healthy individual consumes carbohydrate, any substrate in excess of that needed to fulfill short-term metabolic need is converted into fatty acid through de novo lipogenesis (DNL). DNL takes place in both the liver and adipose tissue (reviewed in3, 4). The fatty acid products of DNL are esterified into triglyceride for storage; the primary reservoir for stored lipids is in adipose tissue, and thus the triglyceride produced in adipose tissue is stored directly. In the liver, some newly synthesized triglyceride is stored locally, but most is packaged into very low density lipoproteins (VLDL) for export to adipose tissue.5 Adipose tissue extracts lipid from VLDL in the same fashion as it does from chylomicrons, using LPL. When carbohydrates and lipids are consumed simultaneously, adipose tissue is called on to import glucose for DNL and take up lipids from both chylomicrons and VLDL. Insulin, induced by dietary carbohydrate, helps adipose tissue accommodate the substrate load by increasing cell-surface expression of the GLUT4 glucose transporter6 and increasing adipose tissue LPL activity.7
During fasting, adipose tissue becomes a net exporter rather than importer of lipid. When nutrients and insulin are sparse, adipocytes hydrolyze their intracellular triglycerides using hormone-sensitive lipase and release FFA for uptake by several tissues including the liver. Indeed, 59% of the triglyceride in a normal liver derives from FFA taken up from the circulation.2 In obesity, the situation in adipose tissue resembles fasting: although insulin levels are adequate or even high, adipocytes can no longer respond to the anabolic effects of the hormone, so they instead behave as though they are insulin-deficient, hydrolyzing intracellular triglyceride and releasing FFA into the circulation. To make matters worse, insulin resistance also suppresses the ability of adipocytes to take up lipid from chylomicrons and VLDL. This leads to further increases in circulating FFA, which are then diverted to other tissues including the liver, where they are stored as ectopic lipid. Overall, alterations in nutrient flux through the adipose-liver circuit play a key role in the pathogenesis of fatty liver disease. Dietary carbohydrates exert a direct influence on the liver through DNL; carbohydrates and fats can also contribute indirectly to fatty liver by increasing adipose tissue lipid stores that are subsequently mobilized through lipolysis.
The adipose tissue–liver metabolic circuit is sensitive not only to the total amount of calories consumed but also the distribution of calories across macronutrient classes (carbohydrates and fats) and even to individual types of macronutrients. The following sections explore the impact of specific nutrients and nutrient combinations on adipose tissue and liver health.
The Influence of Obesity on the Adipose-Liver Axis
Under normal physiological conditions the liver and adipose tissue strive to maintain metabolic homeostasis through the secretion of adipokines and growth factors.8, 9 Under conditions of nutrient excess this communication is disrupted, contributing to metabolic derangement and related injury to both organs.
Looking first at adipose tissue, obesity prompts an increase in tissue mass through a combination of adipocyte hypertrophy and hyperplasia. This enlargement increases oxygen consumption and strains oxygen delivery to the tissue,10, 11 which results in cell death and the associated recruitment of inflammatory cells from the circulation.12, 13, 14 Diseased adipocytes also undergo changes in adipokine production: for example, adiponectin, which promotes physiological lipid storage in fat and lipid oxidation in muscle and liver, is significantly decreased in obesity.15 In contrast, inflammatory cytokines and chemokines, such as tumor necrosis factor, interleukin-6, monocyte chemoattractant protein-1 (CCL2), and others, are produced in increased amounts by adipocytes (reviewed in8).16, 17, 18, 19, 20 Tumor necrosis factor and interleukin-6 impair responsiveness to insulin.21, 22 This interferes with the ability of adipocytes to take up fatty acids from the circulation, and furthermore induces the hydrolysis of intracellular triglycerides, which leads to the systemic release of more fatty acids. Cytokines also promote the recruitment of inflammatory cells to adipose tissue, which inflict further damage, thus perpetuating adipose tissue dysfunction.20, 23, 24 Overall, the dysregulation of metabolic and immunoregulatory adipokines in dysfunctional adipose tissue results in the rerouting of fatty acids to other “ectopic” tissues, including the liver.
The principal compound secreted by the liver that influences the adipose-liver axis is fibroblast growth factor-21 (FGF21). FGF21, produced by hepatocytes, promotes glucose uptake and energy expenditure in white adipose tissue.25 In response to FGF21, adipose tissue secretes adiponectin, which then circles back to the liver to facilitate insulin signaling.26 In obesity, hepatic production of FGF21 is upregulated.9 Its action toward white adipose tissue, however, is blunted.27 FGF21 rises even further with the development and progression of hepatic steatosis,28, 29 underscoring how obesity interferes with another fundamental circuit intended to maintain metabolic homeostasis.
The Influence of Specific Dietary Macronutrients on the Liver and Adipose Tissue
It is well established that consumption of excess calories is a major factor in the development of obesity and fatty liver disease, particularly when coupled with genetic predispositions and a sedentary lifestyle. There is also information indicating that the composition of a diet, independent of its caloric content, can exert a unique influence on the well-being and function of adipose tissue and liver. In this section we highlight the role of macronutrients in nonalcoholic fatty liver disease (NAFLD) pathogenesis, paying particular attention to their effect on adipose-liver interactions. The discussion is organized by macronutrient class, specifically highlighting carbohydrates and fats. Although we focus on macronutrients 1 group at a time, it is important to keep in mind that the human diet constitutes a mixture of carbohydrates, fats, and proteins. The standard human diet comprises 40%–50% carbohydrate, 30%–40% fat, and 20% protein. The interactions among macronutrient classes can themselves be biologically important, and we highlight these in select cases.
Dietary Carbohydrates
Carbohydrates are designated as simple or complex based on the number of sugar molecules they contain (monosaccharides and disaccharides vs polysaccharides). Individual carbohydrates differ in their abilities to induce DNL,30, 31, 32 which plays a role in their tendency to provoke fatty liver and more serious forms of liver injury. Numerous studies have investigated the role of dietary carbohydrates in NAFLD pathogenesis. Less information is available on the role of dietary carbohydrates on adipose tissue in the context of NAFLD.
Simple Carbohydrates
Simple carbohydrates (sugars) include the dietary sweeteners glucose, fructose, and sucrose. Glucose and fructose are monosaccharides, whereas sucrose is a disaccharide comprised of glucose and fructose. Glucose and fructose are readily absorbed by the small intestine. Until recently, both molecules were believed to be transported directly from the intestine to the portal circulation for delivery to the liver; however, new evidence indicates that fructose is metabolized by the intestine and enters the portal circulation only when consumed in amounts sufficient to saturate this metabolic capacity.33 Glucose and fructose molecules that do make their way into the portal circulation are taken up by the liver. Both sugars stimulate DNL, which under conditions of excess can lead to hepatic steatosis. Importantly, the DNL reaction yields palmitate, a toxic long-chain saturated fatty acid (SFA); once generated, palmitate must be promptly desaturated and incorporated into triglyceride, or it can cause hepatocellular injury (discussed in the saturated fat section). Fructose is a potent inducer of DNL because of the lack of feedback regulation of its metabolism by fructokinase (reviewed in 34). Fructose metabolism stimulates more hepatic lipid production than glucose; it also consumes ATP and generates uric acid as a by-product.35 Furthermore, fructose metabolism yields toxic carbonyls that can damage mitochondria.36 This combination of events, combined with the generation of SFA, can lead to liver injury through endoplasmic reticulum (ER) stress and hepatic mitochondrial dysfunction.37, 38, 39, 40 The heightened toxicity of fructose compared with glucose has been demonstrated in multiple studies involving animals and humans. Work from our laboratory showed that fructose, when used as the carbohydrate in a methionine-choline-deficient diet, induced twice the degree of liver injury than glucose.41 Others found that in mice fed a high-fat diet, fructose rather than glucose in the drinking water led to more microvesicular hepatic steatosis and more activation of the stress kinase Jun N-terminal kinase in the liver, which are both predictors of greater liver injury.42 In human subjects, consumption of fructose-sweetened beverages but not glucose-sweetened beverages for 10 weeks induced mild liver injury as evidenced by elevated serum γ-glutamyl transpeptidase along with elevations in several circulating inflammatory molecules.43, 44 Furthermore, epidemiologic studies confirm that long-term fructose consumption is associated with serious hepatic outcomes including steatohepatitis and liver fibrosis.45, 46, 47
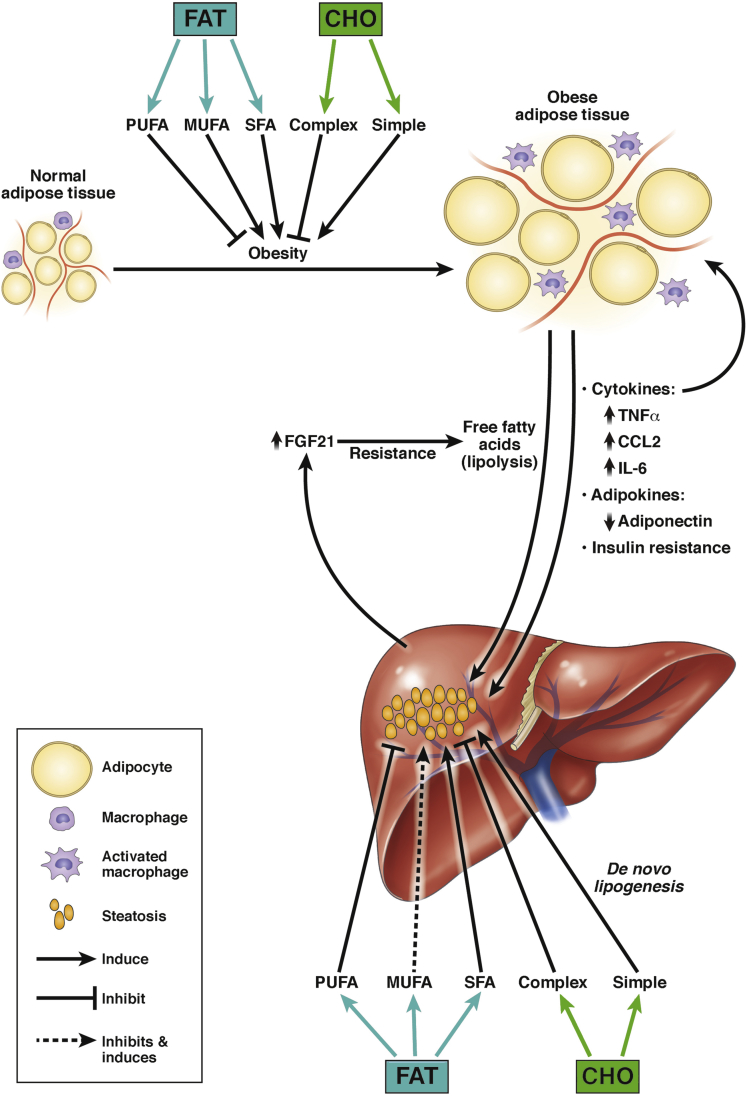
Although most ingested fructose is metabolized by the liver, fructose can also be used by other organs including adipose tissue. Studies of adipocytes in culture show that fructose, but not glucose, has a trophic effect on the cells, stimulating the expansion of adipocyte precursors.48 Not surprisingly, fructose metabolism by adipocytes also promotes lipogenesis, leading to storage of some of the fatty acids and the release of some as FFA.49 Studies have shown that fructose, but not glucose, consumption causes insulin resistance.32, 42 This acts as an ongoing stimulus to lipolysis in fructose-exposed adipose tissue. Moreover, the uric acid produced during fructose metabolism can inflict damage on adipocytes, stimulating oxidant stress and the production of inflammatory cytokines.50, 51 Overall, the adverse effects of fructose on adipocytes compound its adverse effects on the liver, by causing adipose inflammation and preventing proper adipose tissue lipid storage resulting in diversion of fatty acids to the liver (Figure 2).
Figure 2.
Endocrine interactions between liver and adipose tissue and the influence of macronutrients. Under conditions of dietary excess/obesity, adipose tissue adiponectin production declines and several proinflammatory cytokines and chemokines are upregulated. This can promote inflammation and insulin resistance in both tissues. FGF21 is upregulated in the liver, but its action is inhibited, preventing energy expenditure in adipose tissue and promoting lipolysis. The general contribution of individual macronutrients to the dysfunction of adipose tissue and liver in obesity and NAFLD is shown schematically (see text for details). CHO, carbohydrate; IL, interleukin; TNF, tumor necrosis factor.
The effects of glucose on liver and adipose tissue are generally milder than fructose, although overconsumption of glucose is not without consequence. Like fructose, glucose promotes DNL, which yields SFAs that are esterified and stored in adipose tissue and liver. Studies in mice indicate that glucose and fructose induce similar degrees of hepatic lipid accumulation when incorporated into isocaloric diets.41, 42 Similarly in humans, when identical amounts of glucose or fructose are fed to human subjects for periods up to 4 weeks, both sugars induce comparable increases in liver fat content.52, 53, 54 Because the lipogenic properties of glucose and fructose seem similar, the increased harm from fructose in humans is likely related to ATP depletion and uric acid production and their downstream consequences.
One important point to note about dietary sugars is that their ability to induce liver injury is modulated by the fat present in the diet. Specifically, saturated fat induces DNL independently of dietary sugar,55 so when sugar and saturated fat are consumed together, the DNL effect is magnified. This synergy is most evident when sugar (as the DNL substrate) is highly abundant in the diet. Our group showed that sugar + saturated fat, when fed to mice in a ratio of 60:20, induced significantly more DNL, hepatic steatosis, and liver injury than an equivalent combination of sugar + unsaturated fat.56, 57 This synergy was not seen when sugar + saturated fat were fed in a ratio of 40:40.58
Complex Carbohydrates
Complex carbohydrates (starches, glucans, fructans, and cellulose) are polysaccharides with a range of structures and physical properties. Glucans, fructans, and cellulose, along with a subset of starches that are highly resistant to enzymatic digestion, are often grouped into a single category under the term “dietary fiber.” The impact of starches and fiber on adipose tissue and liver is dependent in part on their metabolism by the host, but also on their metabolism by microbes residing in the gut.
Starches
Starches are polymers of glucose with different chain lengths and α-glycosidic linkages. Like all polysaccharides, they require enzymatic digestion before absorption. However, because of their size and structure they are not completely degraded to monosaccharides or disaccharides in the small intestine and as a result are less “glycemic” than simple sugars. Because starch yields lower blood concentrations of glucose than sugar, it is less likely to stimulate lipogenic genes in the liver; furthermore, it provides less substrate for fatty acid synthesis. The blunted lipogenic effect of dietary starch compared with sugar has been demonstrated in direct comparisons of the 2 carbohydrates in mice.59 Even within the category of dietary starches there are variations in the ability of individual polysaccharides to stimulate hepatic lipogenesis depending on the ease with which they are digested.60 The impact of high-glycemic (digestible) and low-glycemic (resistant) starches on adipose tissue have also been investigated in select studies.61, 62 As expected, high-glycemic starches induced more adipose tissue enlargement than low-glycemic starches. At present there is little information available about the effect of dietary starches on adipose tissue inflammation.
Fiber
Fiber is either very resistant to enzymatic digestion in the intestine, or in the case of β-linked plant polysaccharides (β-glucans and cellulose), cannot be digested at all by the intestine. These resistant polysaccharides make their way to the colon, where they undergo fermentation by colonic bacteria.63 Fermentation of fiber yields the short-chain fatty acids acetate, butyrate and propionate; some of these are used by the bacteria themselves, but some are also absorbed into the portal circulation where they have immediate access to the liver.64 Hepatic extraction of short-chain fatty acids is efficient but incomplete. This enables some fatty acids to enter the systemic circulation65 where they can exert independent influences on adipose tissue. Focusing on the liver first, it is important to note that short-chain fatty acids have several effects on hepatic metabolism that can either promote or prevent NAFLD. Butyrate stimulates fatty acid oxidation and thus should be beneficial to the liver.66 In contrast, acetate is a substrate for hepatic lipogenesis67 and propionate stimulates gluconeogenesis,68 which should have an overall adverse effect. A recent mouse study disputes the prospect of harm by demonstrating that supplemental short-chain fatty acids prevent, rather than promote, experimental fatty liver disease.69 Overall it remains unclear whether the net effect of short-chain fatty acids on the liver is beneficial or detrimental in the pathogenesis of NAFLD, although experts are leaning toward a salutary role for these compounds.70 With respect to adipose tissue, short-chain fatty acids are believed beneficial to metabolic homeostasis. Animals and cell culture studies demonstrate that short-chain fatty acids promote adipocyte development and fat accumulation, stimulate adipokine production, and decrease lipolysis.71, 72, 73, 74 Human studies, although correlative, indicate that diets that increase circulating levels of short-chain fatty acids also reduce systemic circulating fatty acid concentrations, suggesting a suppressive effect on adipose tissue lipolysis.75
Dietary Fats
Dietary fats are consumed largely as triglycerides. Fats are named for the dominant type of fatty acid within the triglyceride molecule: SFAs, monounsaturated fatty acids (MUFA) or polyunsaturated fatty acids (PUFA). Dietary fat is taken up by tissues in the form of fatty acids after lipolysis of triglycerides at the cell surface. Fats are primarily incorporated into adipose tissue; they have secondary access to the liver via chylomicron remnants or spillover of excess FFAs into the circulation (Figure 1). The biologic effects of dietary fats are attributable to their component fatty acids, which are the main focus of the following summary.
Saturated Fats
Saturated fats and their component SFA come primarily from animal sources (meat and dairy). The SFA present in these foods are typically long-chain species containing 16 or more carbon atoms. Long-chain SFA are considered the most harmful of dietary fats because they have toxic effects on many types of cells; this is in contrast to medium-chain SFA, which are more inert and can even be beneficial to metabolic health.76, 77 Although medium-chain SFA are less toxic than long-chain SFA they are only minor components of standard meat and dairy items. However, they are enriched in a limited number of natural foods, such as coconut and palm kernel oils. Focusing on long-chain SFA because of their prevalence in the standard human diet, these SFA can directly injure hepatocytes through a variety of mechanisms including death receptor signaling, the induction of ER stress leading to intrinsic mitochondrial apoptosis, stimulation of toll-like receptors, activation of inflammasomes, and impairment of autophagy (reviewed in78).79, 80, 81, 82, 83, 84, 85, 86, 87, 88 SFA are also detrimental to adipocytes. They enhance adipocyte oxygen consumption, which contributes to adipose tissue hypoxia in vivo,10, 11, 89 and in similar fashion to hepatocytes they cause ER stress, activation of toll-like receptor and nuclear factor-κB, which results in cell death and the production of proinflammatory cytokines.90, 91, 92, 93, 94, 95, 96, 97 The adverse effects of SFA-enriched diets on liver and adipose tissue have been documented in many studies in experimental animals.100, 101, 102, 11, 88, 98, 99 In contrast, relatively few research groups have challenged human subjects with saturated-fat diets in the context of a controlled clinical trial. The available data from short-term human studies comparing dietary saturated fats with polyunsaturated fats indicate that saturated fats have a greater tendency to induce insulin resistance, hepatic steatosis, and a proinflammatory state characterized by elevated serum concentrations of tumor necrosis factor and interleukin-1-receptor antagonist.103, 104, 105, 106
Although there is little doubt that SFA are cytotoxic, the toxicity of SFA toward liver cells in vivo seems dependent on their origin from the diet or DNL. This observation comes from studies from our laboratory investigating the hepatotoxicity of different combinations of dietary sugars and fats in mice. We reported that dietary tripalmitin, despite being comprised exclusively of SFA, caused only mild liver injury unless paired with sucrose.56 This suggests that DNL SFA are more toxic than dietary SFA, which corroborates evidence in humans that fatty liver disease is related to excessive DNL.2, 107
Unsaturated Fats
Unsaturated fats, which comprise MUFA and PUFA species, are the principal fats present in plants, seeds, nuts, and fish. The dominant dietary MUFA is oleic acid, which is abundant in olive oil. The dominant dietary PUFAs are linoleic acid (ω-6 PUFA) and α-linolenic acid (ω-3 PUFA) found in seeds and vegetables; other important dietary ω-3 PUFAs are the very long chain species eicosapentaenoic acid and docosahexaenoic acid, which can be produced from α-linolenic acid or obtained directly from a diet containing fish.108 In general, unsaturated fats are considered healthier than saturated fats for the liver and adipose tissue. Still, there are properties that distinguish MUFA from PUFA, so the 2 classes are summarized individually.
Monounsaturated Fatty Acid
MUFA, unlike SFA, exert little toxicity toward liver or adipose tissue cells.84, 88, 91, 97, 109, 110 In fact, MUFA have been reported to promote adipocyte hyperplasia rather than the less desirable cellular enlargement in vivo, in association with blunted expression of inflammasome components and activation of metabolic pathways that portend improved insulin sensitivity.99 Interestingly, despite the apparently benign nature of dietary MUFA toward hepatocytes and adipocytes in vitro, some studies indicate that MUFA-enriched diets (see Table 1 for ingredients) induce more hepatic steatosis than isocaloric SFA-enriched diets.58, 111, 112 Pertinent to this point, studies from our laboratory showed that mice fed diets containing 40% kcal MUFA in the form of high-oleate sunflower oil for 6 months developed substantial hepatic steatosis coincident with pronounced adipose tissue injury and inflammation.58 In 1 human study, feeding a high concentration of MUFA (44% kcal) for 12 weeks also induced monocyte chemoattractant protein-1 expression in adipose tissue.113 This raises questions as to whether MUFA are truly nontoxic in vivo, particularly when used in high concentrations in the diet. In humans with NAFLD, the effects of MUFA-enriched “Mediterranean” diets have been explored in a small number of carefully controlled clinical trials. In 2 studies, subjects were fed a MUFA-enriched diet (40% total kcal fat) or a lower-fat nonenriched diet (30% total kcal fat) for 6–8 weeks.114, 115 In both cases the MUFA-enriched diet substantially reduced hepatic steatosis and improved insulin sensitivity more than the comparison diet, but other measures were equivalent. A third study was recently published that tested similar diets for 12 weeks. Unlike the earlier studies, this one showed improvement in hepatic steatosis with both the MUFA-enriched diet and the low-fat diet, with no significant difference between the 2.116 Experts are now calling for further investigation of MUFA-enriched diets in patients with NAFLD that include extended treatment intervals and robust liver and adipose tissue outcome measures.117 One important caveat is that Mediterranean diets, although enriched in MUFA, may also contain higher levels of PUFA than comparison diets. This can make it difficult to assign any benefit of a Mediterranean diet specifically to MUFA. Until further evidence is collected, the specific benefit of MUFA for metabolic health remains uncertain.
Table 1.
Types of Monounsaturated Fatty Acid Used in Individual Animal and Human Studies
| Reference | Author | Species | Dietary monounsaturated fatty acid | Notes |
|---|---|---|---|---|
| 58 | Duwaerts et al | Animal | High-oleate sunflower oil | |
| 111 | Hoefel et al | Animal | Olive oil | |
| 112 | Sampath et al | Animal | Triolein | |
| 113 | Meneses et al | Human | Olive oil–enriched mayonnaise, nuts | LIPGENE Study |
| 114 | Bozzetto et al | Human | Olive oil | |
| 115 | Ryan et al | Human | Olive oil, nuts, olives, fish | Mediterranean |
| 116 | Properzi et al | Human | Olive oil, nuts, fish | Mediterranean |
Polyunsaturated Fatty Acid
PUFA are generally characterized as beneficial to metabolic health, particularly in relation to SFA. PUFA, however, are divided into 2 major subspecies (ω-3 and ω-6 PUFA) that can have different effects on tissue biology. For example, ω-3 PUFA can suppress inflammation through the generation of specialized proresolving mediators,118 whereas ω-6 PUFA can promote inflammation by conversion to arachidonic acid and other inflammatory eicosanoids119 (reviewed in120). The average Western diet contains far less ω-3 PUFA than ω-6 PUFA.108, 120, 121 Consequently, efforts are underway to encourage incorporation of more ω-3 PUFA into the diet or the use of ω-3 PUFA supplements, or both.121 Among the beneficial effects of PUFA, regardless of subspecies, are their ability to suppress lipogenesis and stimulate fatty acid oxidation by downregulating sterol regulatory element-binding protein-1, carbohydrate-responsive element-binding protein, and farnesoid X receptor and activating peroxisome proliferator-activated receptor-α.122, 123, 124, 125 In addition, ω-3 and ω-6 PUFA have both been shown to suppress nuclear factor-κB, inflammasome activation, and promote autophagy in hepatocytes.126, 127 Similarly, ω-3 and ω-6 PUFA are both capable of suppressing adipocyte hypertrophy and preventing inflammation and fibrosis in adipose tissue,91, 95, 128, 129 although there may be some selective benefit of ω-3 over ω-6 PUFA.129 In a study of mice in vivo, ω-3 PUFA supplementation could prevent and reverse high-fat diet-induced hepatic steatosis.130 Although in another study ω-6 PUFA did not achieve the same reduction in liver fat,100 ω-3 and ω-6 PUFA both seem capable of preventing diet-induced insulin resistance and avoiding the diet-induced ER stress and inflammatory changes in the liver that occur with SFA-enriched diets.100, 130 In humans, some trials have compared PUFA-enriched diets (ω-3 or ω-6) with diets with other fats,113, 131, 132 but many more have evaluated the effects of ω-3 PUFA supplements on subjects with fatty liver disease. The results of these trials are elegantly summarized in 2 recent reviews.108, 121 The consensus opinion is that although some studies report an advantage of ω-3 PUFA supplementation, overall outcomes are variable, and the promise of a therapeutic benefit is dampened by safety concerns related to bleeding and interactions with anticoagulant medications. Until further studies can verify the efficacy of ω-3 PUFA supplements as a therapeutic for NAFLD, experts still recommend incorporating more ω-3 PUFA into the diet in the form of fish and seafood to maintain or improve metabolic health.
Conclusions
Overnutrition in any form poses a risk for obesity and fatty liver disease. The specific consequences of overnutrition on adipose tissue and liver, however, depend not only on the amount of energy consumed but also on the type and distribution of macronutrients that make up the diet. Carefully controlled experiments dissecting the impact of individual macronutrients on adipose tissue and liver have enabled their placement into a general rank order on the spectrum of harmful-to-benign. For carbohydrates this is fructose > glucose > starch > fiber, based primarily on their bioavailability to and metabolism within the liver, and for fats the rank is saturated ≥ monounsaturated > polyunsaturated, based on their ability to induce cytotoxicity and their potential to regulate DNL and fatty acid oxidation. The challenge is how to translate the information gained from these experimental studies to real-world situations in which diets are complex and ever-changing. As nutritional research continues, these challenges can be addressed, culminating in the development of sound nutritional guidelines for metabolic health.
Acknowledgments
Caroline C. Duwaerts and Jacquelyn J. Maher are responsible for reviewing the literature, drafting of the manuscript, and critical revision of the manuscript.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by grants R01 DK068450 and P30 DK026743.
References
- 1.Fielding B. Tracing the fate of dietary fatty acids: metabolic studies of postprandial lipaemia in human subjects. Proc Nutr Soc. 2011;70:342–350. doi: 10.1017/S002966511100084X. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellerstein M.K., Schwarz J.M., Neese R.A. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr. 1996;16:523–557. doi: 10.1146/annurev.nu.16.070196.002515. [DOI] [PubMed] [Google Scholar]
- 4.Song Z., Xiaoli A.M., Yang F. Regulation and metabolic significance of de novo lipogenesis in adipose tissues. Nutrients. 2018;10 doi: 10.3390/nu10101383. :1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frayn K.N., Kingman S.M. Dietary sugars and lipid metabolism in humans. Am J Clin Nutr. 1995;62:250S–261S. doi: 10.1093/ajcn/62.1.250S. discussion 261S–263S. [DOI] [PubMed] [Google Scholar]
- 6.Klip A., Sun Y., Chiu T.T., Foley K.P. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol. 2014;306:C879–C886. doi: 10.1152/ajpcell.00069.2014. [DOI] [PubMed] [Google Scholar]
- 7.Chong M.F., Fielding B.A., Frayn K.N. Metabolic interaction of dietary sugars and plasma lipids with a focus on mechanisms and de novo lipogenesis. Proc Nutr Soc. 2007;66:52–59. doi: 10.1017/S0029665107005290. [DOI] [PubMed] [Google Scholar]
- 8.Deng Y., Scherer P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher F.M., Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 10.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y.S., Kim J.W., Osborne O., Oh D.Y., Sasik R., Schenk S., Chen A., Chung H., Murphy A., Watkins S.M., Quehenberger O., Johnson R.S., Olefsky J.M. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell. 2014;157:1339–1352. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alkhouri N., Gornicka A., Berk M.P., Thapaliya S., Dixon L.J., Kashyap S., Schauer P.R., Feldstein A.E. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010;285:3428–3438. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A.S., Obin M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Gornicka A., Fettig J., Eguchi A., Berk M.P., Thapaliya S., Dixon L.J., Feldstein A.E. Adipocyte hypertrophy is associated with lysosomal permeability both in vivo and in vitro: role in adipose tissue inflammation. Am J Physiol Endocrinol Metab. 2012;303:E597–E606. doi: 10.1152/ajpendo.00022.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu E., Liang P., Spiegelman B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 17.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kern P.A., Ranganathan S., Li C., Wood L., Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 19.Shimomura I., Funahashi T., Takahashi M., Maeda K., Kotani K., Nakamura T., Yamashita S., Miura M., Fukuda Y., Takemura K., Tokunaga K., Matsuzawa Y. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med. 1996;2:800–803. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K., Mizuarai S., Araki H., Mashiko S., Ishihara A., Kanatani A., Itadani H., Kotani H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem. 2003;278:46654–46660. doi: 10.1074/jbc.M309895200. [DOI] [PubMed] [Google Scholar]
- 21.Cheung A.T., Ree D., Kolls J.K., Fuselier J., Coy D.H., Bryer-Ash M. An in vivo model for elucidation of the mechanism of tumor necrosis factor-alpha (TNF-alpha)-induced insulin resistance: evidence for differential regulation of insulin signaling by TNF-alpha. Endocrinology. 1998;139:4928–4935. doi: 10.1210/endo.139.12.6336. [DOI] [PubMed] [Google Scholar]
- 22.Uysal K.T., Wiesbrock S.M., Marino M.W., Hotamisligil G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., Sole J., Nichols A., Ross J.S., Tartaglia L.A., Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J., Sandusky G.E., Hammond L.J., Moyers J.S., Owens R.A., Gromada J., Brozinick J.T., Hawkins E.D., Wroblewski V.J., Li D.S., Mehrbod F., Jaskunas S.R., Shanafelt A.B. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland W.L., Adams A.C., Brozinick J.T., Bui H.H., Miyauchi Y., Kusminski C.M., Bauer S.M., Wade M., Singhal E., Cheng C.C., Volk K., Kuo M.S., Gordillo R., Kharitonenkov A., Scherer P.E. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markan K.R., Naber M.C., Small S.M., Peltekian L., Kessler R.L., Potthoff M.J. FGF21 resistance is not mediated by downregulation of beta-klotho expression in white adipose tissue. Mol Metab. 2017;6:602–610. doi: 10.1016/j.molmet.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Fang Q., Gao F., Fan J., Zhou J., Wang X., Zhang H., Pan X., Bao Y., Xiang K., Xu A., Jia W. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53:934–940. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz Y., Eren F., Yonal O., Kurt R., Aktas B., Celikel C.A., Ozdogan O., Imeryuz N., Kalayci C., Avsar E. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest. 2010;40:887–892. doi: 10.1111/j.1365-2362.2010.02338.x. [DOI] [PubMed] [Google Scholar]
- 30.Hudgins L.C., Parker T.S., Levine D.M., Hellerstein M.K. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab. 2011;96:861–868. doi: 10.1210/jc.2010-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parks E.J., Skokan L.E., Timlin M.T., Dingfelder C.S. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138:1039–1046. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanhope K.L., Schwarz J.M., Keim N.L., Griffen S.C., Bremer A.A., Graham J.L., Hatcher B., Cox C.L., Dyachenko A., Zhang W., McGahan J.P., Seibert A., Krauss R.M., Chiu S., Schaefer E.J., Ai M., Otokozawa S., Nakajima K., Nakano T., Beysen C., Hellerstein M.K., Berglund L., Havel P.J. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang C., Hui S., Lu W., Cowan A.J., Morscher R.J., Lee G., Liu W., Tesz G.J., Birnbaum M.J., Rabinowitz J.D. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 2018;27:351–361. doi: 10.1016/j.cmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen T., Abdelmalek M.F., Sullivan S., Nadeau K.J., Green M., Roncal C., Nakagawa T., Kuwabara M., Sato Y., Kang D.H., Tolan D.R., Sanchez-Lozada L.G., Rosen H.R., Lanaspa M.A., Diehl A.M., Johnson R.J. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68:1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol. 1986;21:1–32. [PubMed] [Google Scholar]
- 36.Lee O., Bruce W.R., Dong Q., Bruce J., Mehta R., O'Brien P.J. Fructose and carbonyl metabolites as endogenous toxins. Chem Biol Interact. 2009;178:332–339. doi: 10.1016/j.cbi.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Choi Y.J., Shin H.S., Choi H.S., Park J.W., Jo I., Oh E.S., Lee K.Y., Lee B.H., Johnson R.J., Kang D.H. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab Invest. 2014;94:1114–1125. doi: 10.1038/labinvest.2014.98. [DOI] [PubMed] [Google Scholar]
- 38.Lanaspa M.A., Sanchez-Lozada L.G., Choi Y.J., Cicerchi C., Kanbay M., Roncal-Jimenez C.A., Ishimoto T., Li N., Marek G., Duranay M., Schreiner G., Rodriguez-Iturbe B., Nakagawa T., Kang D.H., Sautin Y.Y., Johnson R.J. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sapp V., Gaffney L., EauClaire S.F., Matthews R.P. Fructose leads to hepatic steatosis in zebrafish that is reversed by mechanistic target of rapamycin (mTOR) inhibition. Hepatology. 2014;60:1581–1592. doi: 10.1002/hep.27284. [DOI] [PubMed] [Google Scholar]
- 40.Wan X., Xu C., Lin Y., Lu C., Li D., Sang J., He H., Liu X., Li Y., Yu C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol. 2016;64:925–932. doi: 10.1016/j.jhep.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Pickens M.K., Ogata H., Soon R.K., Grenert J.P., Maher J.J. Dietary fructose exacerbates hepatocellular injury when incorporated into a methionine-choline-deficient diet. Liver Int. 2010;30:1229–1239. doi: 10.1111/j.1478-3231.2010.02285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Softic S., Gupta M.K., Wang G.X., Fujisaka S., O'Neill B.T., Rao T.N., Willoughby J., Harbison C., Fitzgerald K., Ilkayeva O., Newgard C.B., Cohen D.E., Kahn C.R. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest. 2017;127:4059–4074. doi: 10.1172/JCI94585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox C.L., Stanhope K.L., Schwarz J.M., Graham J.L., Hatcher B., Griffen S.C., Bremer A.A., Berglund L., McGahan J.P., Keim N.L., Havel P.J. Circulating concentrations of monocyte chemoattractant protein-1, plasminogen activator inhibitor-1, and soluble leukocyte adhesion molecule-1 in overweight/obese men and women consuming fructose- or glucose-sweetened beverages for 10 weeks. J Clin Endocrinol Metab. 2011;96:E2034–E2038. doi: 10.1210/jc.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox C.L., Stanhope K.L., Schwarz J.M., Graham J.L., Hatcher B., Griffen S.C., Bremer A.A., Berglund L., McGahan J.P., Keim N.L., Havel P.J. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond) 2012;9:68. doi: 10.1186/1743-7075-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdelmalek M.F., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R.J., Diehl A.M., Nonalcoholic Steatohepatitis Clinical Research N. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez-Lozada L.G., Mu W., Roncal C., Sautin Y.Y., Abdelmalek M., Reungjui S., Le M., Nakagawa T., Lan H.Y., Yu X., Johnson R.J. Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur J Nutr. 2010;49:1–9. doi: 10.1007/s00394-009-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanguesa G., Montanes J.C., Baena M., Sanchez R.M., Roglans N., Alegret M., Laguna J.C. Chronic fructose intake does not induce liver steatosis and inflammation in female Sprague-Dawley rats, but causes hypertriglyceridemia related to decreased VLDL receptor expression. Eur J Nutr. 2018 doi: 10.1007/s00394-018-1654-9. [DOI] [PubMed] [Google Scholar]
- 48.Zubiria M.G., Alzamendi A., Moreno G., Rey M.A., Spinedi E., Giovambattista A. Long-term fructose intake increases adipogenic potential: evidence of direct effects of fructose on adipocyte precursor cells. Nutrients. 2016;8:198. doi: 10.3390/nu8040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varma V., Boros L.G., Nolen G.T., Chang C.W., Wabitsch M., Beger R.D., Kaput J. Metabolic fate of fructose in human adipocytes: a targeted (13)C tracer fate association study. Metabolomics. 2015;11:529–544. doi: 10.1007/s11306-014-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldwin W., McRae S., Marek G., Wymer D., Pannu V., Baylis C., Johnson R.J., Sautin Y.Y. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258–1269. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sautin Y.Y., Nakagawa T., Zharikov S., Johnson R.J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 52.Johnston R.D., Stephenson M.C., Crossland H., Cordon S.M., Palcidi E., Cox E.F., Taylor M.A., Aithal G.P., Macdonald I.A. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology. 2013;145:1016–1025. doi: 10.1053/j.gastro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Ngo Sock E.T., Le K.A., Ith M., Kreis R., Boesch C., Tappy L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr. 2010;103:939–943. doi: 10.1017/S0007114509992819. [DOI] [PubMed] [Google Scholar]
- 54.Silbernagel G., Machann J., Unmuth S., Schick F., Stefan N., Haring H.U., Fritsche A. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr. 2011;106:79–86. doi: 10.1017/S000711451000574X. [DOI] [PubMed] [Google Scholar]
- 55.Lin J., Yang R., Tarr P.T., Wu P.H., Handschin C., Li S., Yang W., Pei L., Uldry M., Tontonoz P., Newgard C.B., Spiegelman B.M. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 56.Pierce A.A., Pickens M.K., Siao K., Grenert J.P., Maher J.J. Differential hepatotoxicity of dietary and DNL-derived palmitate in the methionine-choline-deficient model of steatohepatitis. BMC Gastroenterol. 2015;15:72. doi: 10.1186/s12876-015-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pierce A.A., Duwaerts C.C., Soon R.K., Siao K., Grenert J.P., Fitch M., Hellerstein M.K., Beysen C., Turner S.M., Maher J.J. Isocaloric manipulation of macronutrients within a high-carbohydrate/moderate-fat diet induces unique effects on hepatic lipogenesis, steatosis and liver injury. J Nutr Biochem. 2016;29:12–20. doi: 10.1016/j.jnutbio.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duwaerts C.C., Amin A.M., Siao K., Her C., Fitch M., Beysen C., Turner S.M., Goodsell A., Baron J.L., Grenert J.P., Cho S.J., Maher J.J. Specific macronutrients exert unique influences on the adipose-liver axis to promote hepatic steatosis in mice. Cell Mol Gastroenterol Hepatol. 2017;4:223–236. doi: 10.1016/j.jcmgh.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pickens M.K., Yan J.S., Ng R.K., Ogata H., Grenert J.P., Beysen C., Turner S.M., Maher J.J. Dietary sucrose is essential to the development of liver injury in the methionine-choline-deficient model of steatohepatitis. J Lipid Res. 2009;50:2072–2082. doi: 10.1194/jlr.M900022-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scribner K.B., Pawlak D.B., Ludwig D.S. Hepatic steatosis and increased adiposity in mice consuming rapidly vs. slowly absorbed carbohydrate. Obesity (Silver Spring) 2007;15:2190–2199. doi: 10.1038/oby.2007.260. [DOI] [PubMed] [Google Scholar]
- 61.Coate K.C., Huggins K.W. Consumption of a high glycemic index diet increases abdominal adiposity but does not influence adipose tissue pro-oxidant and antioxidant gene expression in C57BL/6 mice. Nutr Res. 2010;30:141–150. doi: 10.1016/j.nutres.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Lerer-Metzger M., Rizkalla S.W., Luo J., Champ M., Kabir M., Bruzzo F., Bornet F., Slama G. Effects of long-term low-glycaemic index starchy food on plasma glucose and lipid concentrations and adipose tissue cellularity in normal and diabetic rats. Br J Nutr. 1996;75:723–732. doi: 10.1079/bjn19960176. [DOI] [PubMed] [Google Scholar]
- 63.Flint H.J., Scott K.P., Duncan S.H., Louis P., Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bach Knudsen K.E. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv Nutr. 2015;6:206–213. doi: 10.3945/an.114.007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boets E., Gomand S.V., Deroover L., Preston T., Vermeulen K., De Preter V., Hamer H.M., Van den Mooter G., De Vuyst L., Courtin C.M., Annaert P., Delcour J.A., Verbeke K.A. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595:541–555. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M., Cefalu W.T., Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.den Besten G., Lange K., Havinga R., van Dijk T.H., Gerding A., van Eunen K., Muller M., Groen A.K., Hooiveld G.J., Bakker B.M., Reijngoud D.J. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol. 2013;305:G900–G910. doi: 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- 68.Perry R.J., Borders C.B., Cline G.W., Zhang X.M., Alves T.C., Petersen K.F., Rothman D.L., Kibbey R.G., Shulman G.I. Propionate increases hepatic pyruvate cycling and anaplerosis and alters mitochondrial metabolism. J Biol Chem. 2016;291:12161–12170. doi: 10.1074/jbc.M116.720631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weitkunat K., Stuhlmann C., Postel A., Rumberger S., Fankhanel M., Woting A., Petzke K.J., Gohlke S., Schulz T.J., Blaut M., Klaus S., Schumann S. Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Sci Rep. 2017;7:6109. doi: 10.1038/s41598-017-06447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu H., Duan Y., Yang L., Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2018;68 doi: 10.1136/gutjnl-2018-316307. 359. [DOI] [PubMed] [Google Scholar]
- 71.Ferchaud-Roucher V., Pouteau E., Piloquet H., Zair Y., Krempf M. Colonic fermentation from lactulose inhibits lipolysis in overweight subjects. Am J Physiol Endocrinol Metab. 2005;289:E716–E720. doi: 10.1152/ajpendo.00430.2004. [DOI] [PubMed] [Google Scholar]
- 72.Hong Y.H., Nishimura Y., Hishikawa D., Tsuzuki H., Miyahara H., Gotoh C., Choi K.C., Feng D.D., Chen C., Lee H.G., Katoh K., Roh S.G., Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- 73.Lee S.H., Hossner K.L. Coordinate regulation of ovine adipose tissue gene expression by propionate. J Anim Sci. 2002;80:2840–2849. doi: 10.2527/2002.80112840x. [DOI] [PubMed] [Google Scholar]
- 74.Xiong Y., Miyamoto N., Shibata K., Valasek M.A., Motoike T., Kedzierski R.M., Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robertson M.D., Bickerton A.S., Dennis A.L., Vidal H., Frayn K.N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82:559–567. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- 76.Lee J.Y., Zhao L., Youn H.S., Weatherill A.R., Tapping R., Feng L., Lee W.H., Fitzgerald K.A., Hwang D.H. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 77.Bhavsar N., St-Onge M.P. The diverse nature of saturated fats and the case of medium-chain triglycerides: how one recommendation may not fit all. Curr Opin Clin Nutr Metab Care. 2016;19:81–87. doi: 10.1097/MCO.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 78.Hirsova P., Ibrahim S.H., Gores G.J., Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758–1770. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barreyro F.J., Kobayashi S., Bronk S.F., Werneburg N.W., Malhi H., Gores G.J. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282:27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 80.Cazanave S.C., Mott J.L., Bronk S.F., Werneburg N.W., Fingas C.D., Meng X.W., Finnberg N., El-Deiry W.S., Kaufmann S.H., Gores G.J. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J Biol Chem. 2011;286:39336–39348. doi: 10.1074/jbc.M111.280420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cazanave S.C., Mott J.L., Elmi N.A., Bronk S.F., Werneburg N.W., Akazawa Y., Kahraman A., Garrison S.P., Zambetti G.P., Charlton M.R., Gores G.J. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284:26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Csak T., Ganz M., Pespisa J., Kodys K., Dolganiuc A., Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holzer R.G., Park E.J., Li N., Tran H., Chen M., Choi C., Solinas G., Karin M. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malhi H., Bronk S.F., Werneburg N.W., Gores G.J. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 85.Pfaffenbach K.T., Gentile C.L., Nivala A.M., Wang D., Wei Y., Pagliassotti M.J. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:E1027–E1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharma M., Urano F., Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol. 2012;56:192–198. doi: 10.1016/j.jhep.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A.M., Czaja M.J. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei Y., Wang D., Topczewski F., Pagliassotti M.J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 89.Pasarica M., Sereda O.R., Redman L.M., Albarado D.C., Hymel D.T., Roan L.E., Rood J.C., Burk D.H., Smith S.R. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ajuwon K.M., Spurlock M.E. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J Nutr. 2005;135:1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- 91.Bradley R.L., Fisher F.F., Maratos-Flier E. Dietary fatty acids differentially regulate production of TNF-alpha and IL-10 by murine 3T3-L1 adipocytes. Obesity (Silver Spring) 2008;16:938–944. doi: 10.1038/oby.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davis J.E., Gabler N.K., Walker-Daniels J., Spurlock M.E. The c-Jun N-terminal kinase mediates the induction of oxidative stress and insulin resistance by palmitate and toll-like receptor 2 and 4 ligands in 3T3-L1 adipocytes. Horm Metab Res. 2009;41:523–530. doi: 10.1055/s-0029-1202852. [DOI] [PubMed] [Google Scholar]
- 93.Guo W., Wong S., Xie W., Lei T., Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab. 2007;293:E576–E586. doi: 10.1152/ajpendo.00523.2006. [DOI] [PubMed] [Google Scholar]
- 94.Hunnicutt J.W., Hardy R.W., Williford J., McDonald J.M. Saturated fatty acid-induced insulin resistance in rat adipocytes. Diabetes. 1994;43:540–545. doi: 10.2337/diab.43.4.540. [DOI] [PubMed] [Google Scholar]
- 95.Yeop Han C., Kargi A.Y., Omer M., Chan C.K., Wabitsch M., O'Brien K.D., Wight T.N., Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59:386–396. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Youssef-Elabd E.M., McGee K.C., Tripathi G., Aldaghri N., Abdalla M.S., Sharada H.M., Ashour E., Amin A.I., Ceriello A., O'Hare J.P., Kumar S., McTernan P.G., Harte A.L. Acute and chronic saturated fatty acid treatment as a key instigator of the TLR-mediated inflammatory response in human adipose tissue, in vitro. J Nutr Biochem. 2012;23:39–50. doi: 10.1016/j.jnutbio.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schaeffler A., Gross P., Buettner R., Bollheimer C., Buechler C., Neumeier M., Kopp A., Schoelmerich J., Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Enos R.T., Velazquez K.T., Murphy E.A. Insight into the impact of dietary saturated fat on tissue-specific cellular processes underlying obesity-related diseases. J Nutr Biochem. 2014;25:600–612. doi: 10.1016/j.jnutbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Finucane O.M., Lyons C.L., Murphy A.M., Reynolds C.M., Klinger R., Healy N.P., Cooke A.A., Coll R.C., McAllan L., Nilaweera K.N., O'Reilly M.E., Tierney A.C., Morine M.J., Alcala-Diaz J.F., Lopez-Miranda J., O'Connor D.P., O'Neill L.A., McGillicuddy F.C., Roche H.M. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1beta secretion and insulin resistance despite obesity. Diabetes. 2015;64:2116–2128. doi: 10.2337/db14-1098. [DOI] [PubMed] [Google Scholar]
- 100.Gentile C.L., Weir T.L., Cox-York K.A., Wei Y., Wang D., Reese L., Moran G., Estrada A., Mulligan C., Pagliassotti M.J., Foster M.T. The role of visceral and subcutaneous adipose tissue fatty acid composition in liver pathophysiology associated with NAFLD. Adipocyte. 2015;4:101–112. doi: 10.4161/21623945.2014.978662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh R., Wang Y., Xiang Y., Tanaka K.E., Gaarde W.A., Czaja M.J. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Svegliati-Baroni G., Candelaresi C., Saccomanno S., Ferretti G., Bachetti T., Marzioni M., De Minicis S., Nobili L., Salzano R., Omenetti A., Pacetti D., Sigmund S., Benedetti A., Casini A. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. Am J Pathol. 2006;169:846–860. doi: 10.2353/ajpath.2006.050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bjermo H., Iggman D., Kullberg J., Dahlman I., Johansson L., Persson L., Berglund J., Pulkki K., Basu S., Uusitupa M., Rudling M., Arner P., Cederholm T., Ahlstrom H., Riserus U. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95:1003–1012. doi: 10.3945/ajcn.111.030114. [DOI] [PubMed] [Google Scholar]
- 104.de Wit N., Derrien M., Bosch-Vermeulen H., Oosterink E., Keshtkar S., Duval C., de Vogel-van den Bosch J., Kleerebezem M., Muller M., van der Meer R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303:G589–G599. doi: 10.1152/ajpgi.00488.2011. [DOI] [PubMed] [Google Scholar]
- 105.Rosqvist F., Iggman D., Kullberg J., Cedernaes J., Johansson H.E., Larsson A., Johansson L., Ahlstrom H., Arner P., Dahlman I., Riserus U. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–2368. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- 106.Summers L.K., Fielding B.A., Bradshaw H.A., Ilic V., Beysen C., Clark M.L., Moore N.R., Frayn K.N. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia. 2002;45:369–377. doi: 10.1007/s00125-001-0768-3. [DOI] [PubMed] [Google Scholar]
- 107.Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scorletti E., Byrne C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol Aspects Med. 2018;34 doi: 10.1016/j.mam.2018.03.001. :135. [DOI] [PubMed] [Google Scholar]
- 109.Akazawa Y., Cazanave S., Mott J.L., Elmi N., Bronk S.F., Kohno S., Charlton M.R., Gores G.J. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52:586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Granados N., Amengual J., Ribot J., Palou A., Bonet M.L. Distinct effects of oleic acid and its trans-isomer elaidic acid on the expression of myokines and adipokines in cell models. Br J Nutr. 2011;105:1226–1234. doi: 10.1017/S0007114510004885. [DOI] [PubMed] [Google Scholar]
- 111.Hoefel A.L., Hansen F., Rosa P.D., Assis A.M., Silveira S.L., Denardin C.C., Pettenuzzo L., Augusti P.R., Somacal S., Emanuelli T., Perry M.L., Wannmacher C.M. The effects of hypercaloric diets on glucose homeostasis in the rat: influence of saturated and monounsaturated dietary lipids. Cell Biochem Funct. 2011;29:569–576. doi: 10.1002/cbf.1789. [DOI] [PubMed] [Google Scholar]
- 112.Sampath H., Miyazaki M., Dobrzyn A., Ntambi J.M. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 113.Meneses M.E., Camargo A., Perez-Martinez P., Delgado-Lista J., Cruz-Teno C., Jimenez-Gomez Y., Paniagua J.A., Gutierrez-Mariscal F.M., Tinahones F.J., Vidal-Puig A., Roche H.M., Perez-Jimenez F., Malagon M.M., Lopez-Miranda J. Postprandial inflammatory response in adipose tissue of patients with metabolic syndrome after the intake of different dietary models. Mol Nutr Food Res. 2011;55:1759–1770. doi: 10.1002/mnfr.201100200. [DOI] [PubMed] [Google Scholar]
- 114.Bozzetto L., Prinster A., Annuzzi G., Costagliola L., Mangione A., Vitelli A., Mazzarella R., Longobardo M., Mancini M., Vigorito C., Riccardi G., Rivellese A.A. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care. 2012;35:1429–1435. doi: 10.2337/dc12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ryan M.C., Itsiopoulos C., Thodis T., Ward G., Trost N., Hofferberth S., O'Dea K., Desmond P.V., Johnson N.A., Wilson A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 116.Properzi C., O'Sullivan T.A., Sherriff J.L., Ching H.L., Jeffrey G.P., Buckley R.F., Tibballs J., MacQuillan G.C., Garas G., Adams L.A. Ad libitum Mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology. 2018;68:1741–1754. doi: 10.1002/hep.30076. [DOI] [PubMed] [Google Scholar]
- 117.Targher G., Byrne C.D. Ad libitum Mediterranean or low fat diets as treatments for non-alcoholic fatty liver disease? Hepatology. 2018;68 doi: 10.1002/hep.30142. :1668. [DOI] [PubMed] [Google Scholar]
- 118.Barden A.E., Mas E., Mori T.A. n-3 Fatty acid supplementation and proresolving mediators of inflammation. Curr Opin Lipidol. 2016;27:26–32. doi: 10.1097/MOL.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 119.Innes J.K., Calder P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 120.Scorletti E., Byrne C.D. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr. 2013;33:231–248. doi: 10.1146/annurev-nutr-071812-161230. [DOI] [PubMed] [Google Scholar]
- 121.Jump D.B., Lytle K.A., Depner C.M., Tripathy S. Omega-3 polyunsaturated fatty acids as a treatment strategy for nonalcoholic fatty liver disease. Pharmacol Ther. 2018;181:108–125. doi: 10.1016/j.pharmthera.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dentin R., Benhamed F., Pegorier J.P., Foufelle F., Viollet B., Vaulont S., Girard J., Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005;115:2843–2854. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kliewer S.A., Sundseth S.S., Jones S.A., Brown P.J., Wisely G.B., Koble C.S., Devchand P., Wahli W., Willson T.M., Lenhard J.M., Lehmann J.M. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu J., Nakamura M.T., Cho H.P., Clarke S.D. Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. A mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J Biol Chem. 1999;274:23577–23583. doi: 10.1074/jbc.274.33.23577. [DOI] [PubMed] [Google Scholar]
- 125.Zhao A., Yu J., Lew J.L., Huang L., Wright S.D., Cui J. Polyunsaturated fatty acids are FXR ligands and differentially regulate expression of FXR targets. DNA Cell Biol. 2004;23:519–526. doi: 10.1089/1044549041562267. [DOI] [PubMed] [Google Scholar]
- 126.Shen L., Yang Y., Ou T., Key C.C., Tong S.H., Sequeira R.C., Nelson J.M., Nie Y., Wang Z., Boudyguina E., Shewale S.V., Zhu X. Dietary PUFAs attenuate NLRP3 inflammasome activation via enhancing macrophage autophagy. J Lipid Res. 2017;58:1808–1821. doi: 10.1194/jlr.M075879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sui Y.H., Luo W.J., Xu Q.Y., Hua J. Dietary saturated fatty acid and polyunsaturated fatty acid oppositely affect hepatic NOD-like receptor protein 3 inflammasome through regulating nuclear factor-kappa B activation. World J Gastroenterol. 2016;22:2533–2544. doi: 10.3748/wjg.v22.i8.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bargut T.C., Mandarim-de-Lacerda C.A., Aguila M.B. A high-fish-oil diet prevents adiposity and modulates white adipose tissue inflammation pathways in mice. J Nutr Biochem. 2015;26:960–969. doi: 10.1016/j.jnutbio.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 129.Huber J., Loffler M., Bilban M., Reimers M., Kadl A., Todoric J., Zeyda M., Geyeregger R., Schreiner M., Weichhart T., Leitinger N., Waldhausl W., Stulnig T.M. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes (Lond) 2007;31:1004–1013. doi: 10.1038/sj.ijo.0803511. [DOI] [PubMed] [Google Scholar]
- 130.Kalupahana N.S., Claycombe K., Newman S.J., Stewart T., Siriwardhana N., Matthan N., Lichtenstein A.H., Moustaid-Moussa N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140:1915–1922. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
- 131.Aller R., de Luis D.A., Izaola O., de la Fuente B., Bachiller R. Effect of a high monounsaturated vs high polyunsaturated fat hypocaloric diets in nonalcoholic fatty liver disease. Eur Rev Med Pharmacol Sci. 2014;18:1041–1047. [PubMed] [Google Scholar]
- 132.Camargo A., Rangel-Zuniga O.A., Alcala-Diaz J., Gomez-Delgado F., Delgado-Lista J., Garcia-Carpintero S., Marin C., Almaden Y., Yubero-Serrano E.M., Lopez-Moreno J., Tinahones F.J., Perez-Martinez P., Roche H.M., Lopez-Miranda J. Dietary fat may modulate adipose tissue homeostasis through the processes of autophagy and apoptosis. Eur J Nutr. 2017;56:1621–1628. doi: 10.1007/s00394-016-1208-y. [DOI] [PubMed] [Google Scholar]